Electrochemical and Computational Analyses of Thiocolchicoside as a New Corrosion Inhibitor for Biomedical Ti6Al4V Alloy in Saline Solution: DFT, NBO, and MD Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electrochemical Testing
2.3. Surface Characterization
2.4. Computational Studies
2.4.1. Quantum Theoretical Computations
2.4.2. Molecular Simulation Dynamics
2.4.3. NBO Analysis
3. Results and Discussion
3.1. TCC as an Efficient Inhibitor for Ti6Al4V Alloy
3.1.1. PPCs
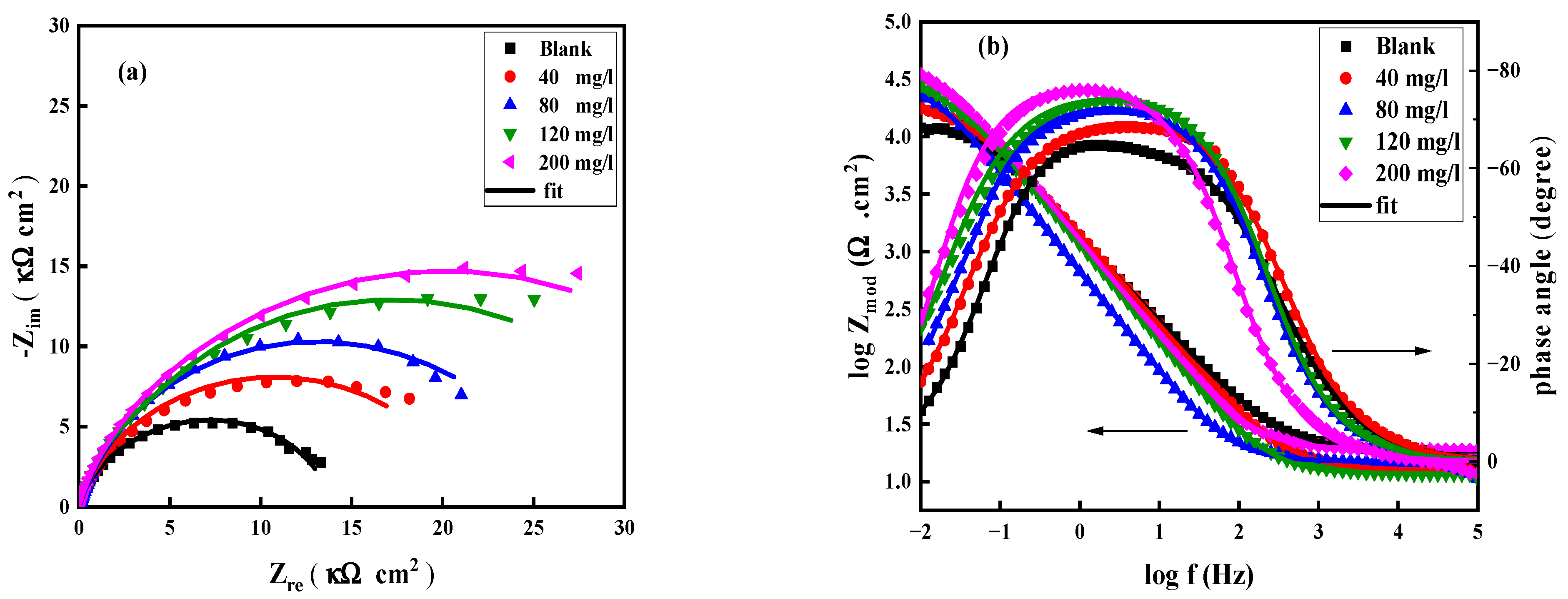
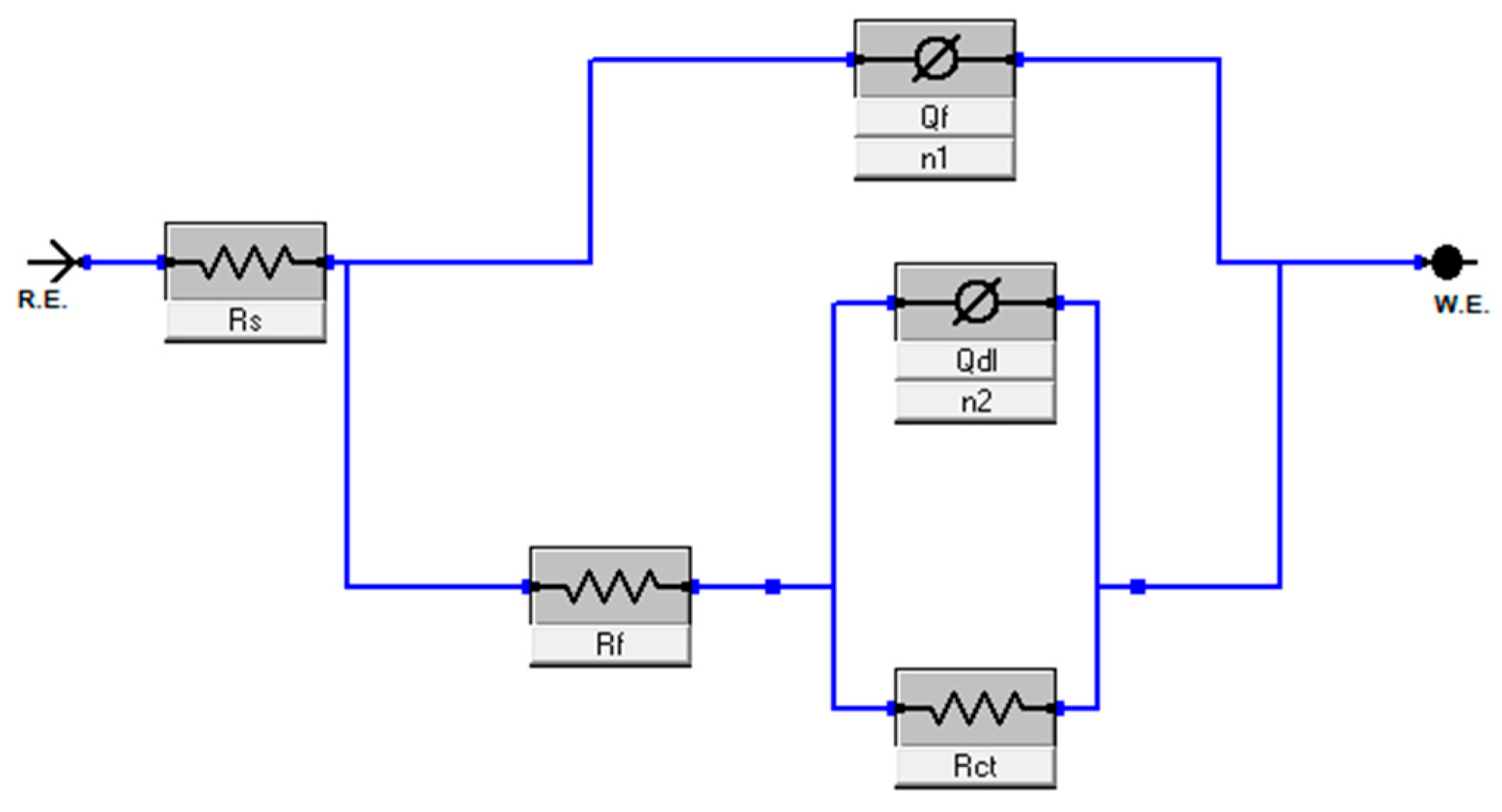
3.1.2. Impedance Analysis
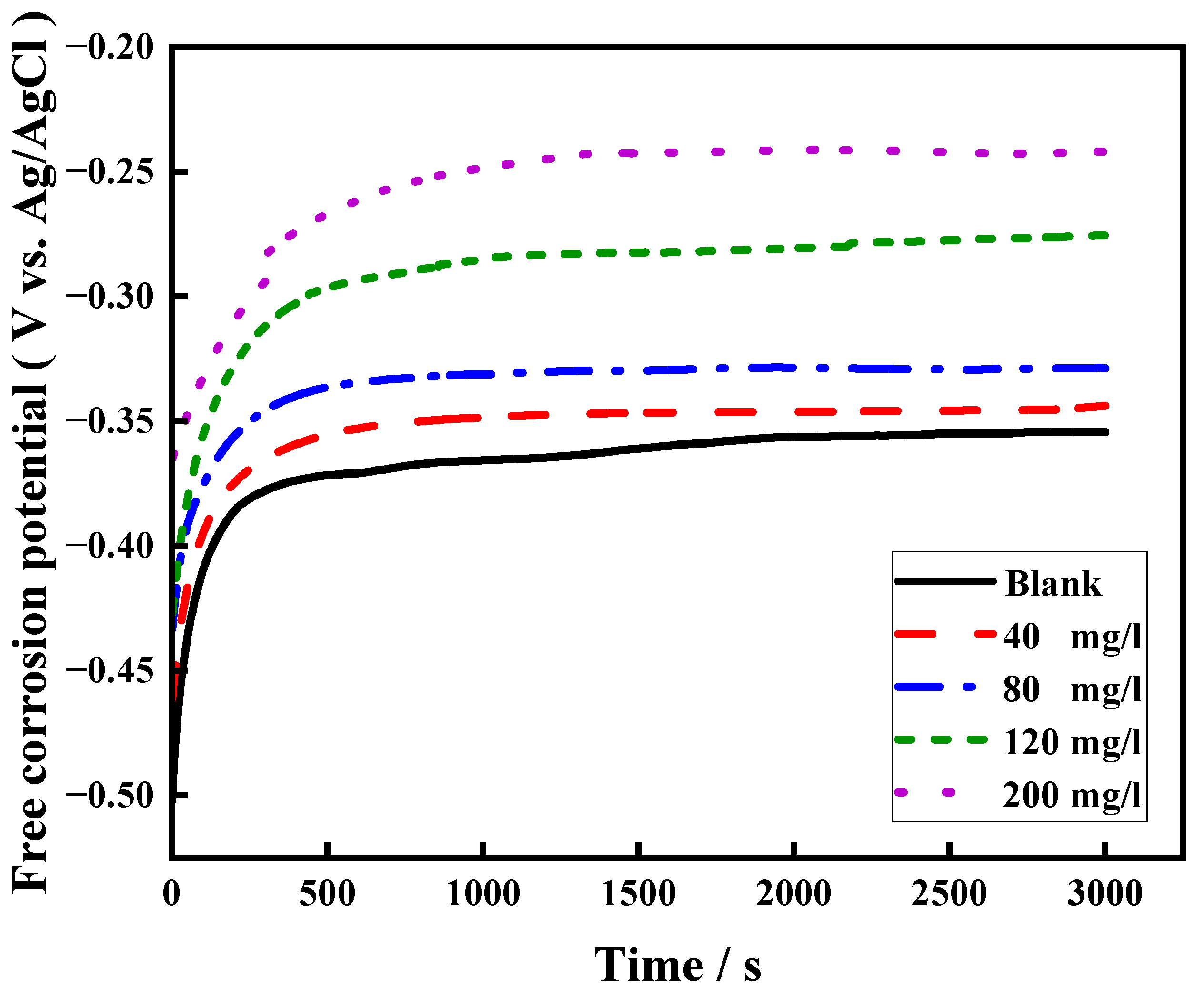
3.1.3. OCP Measurements
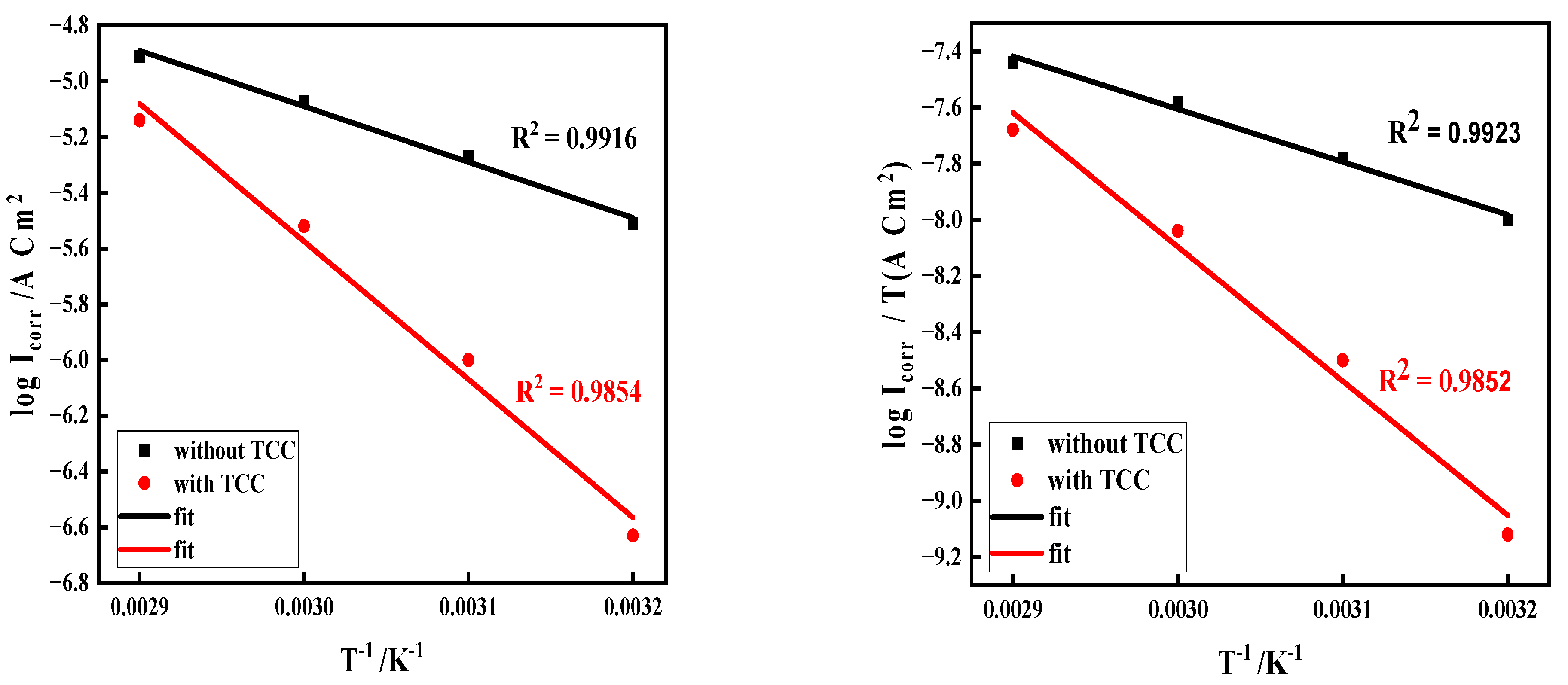
3.2. The Impact of Temperature and Thermodynamic Investigations
3.2.1. PPCs
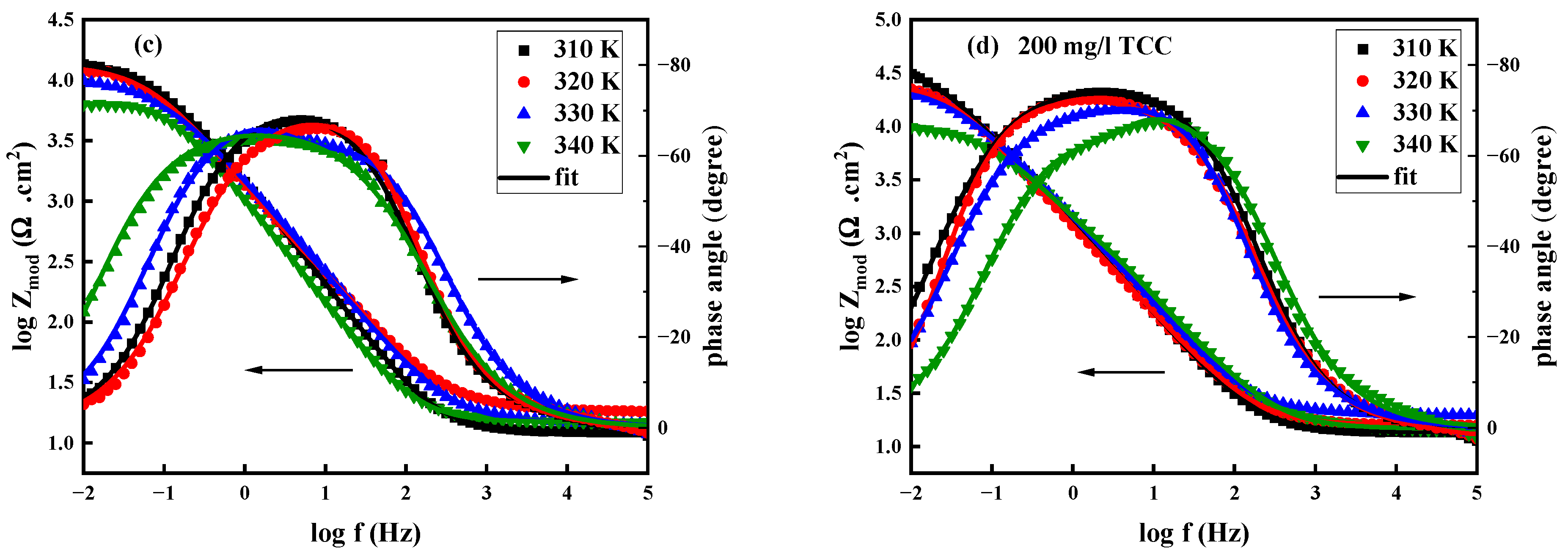
3.2.2. Impedance Analysis
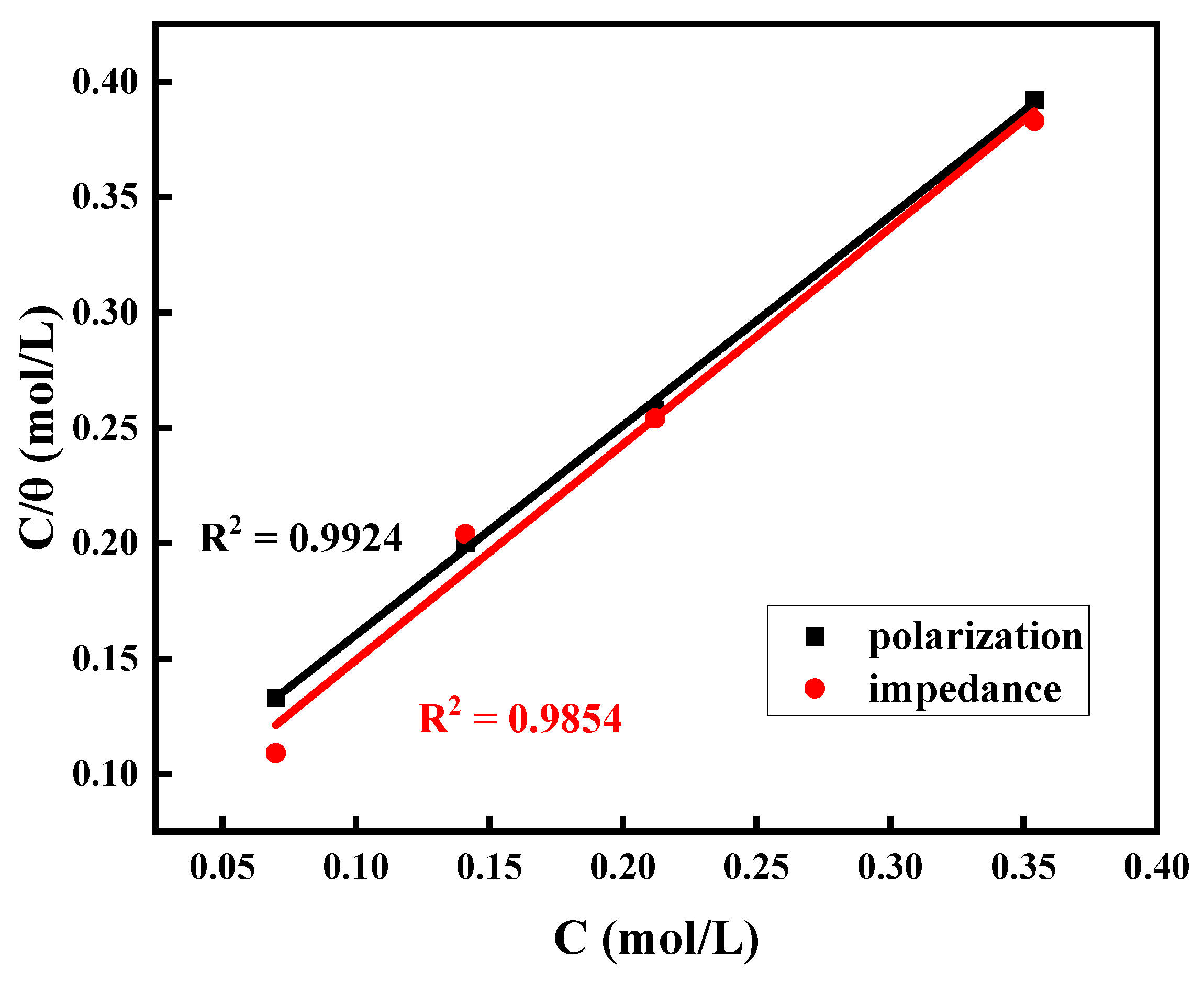
3.3. Adsorption Isotherm
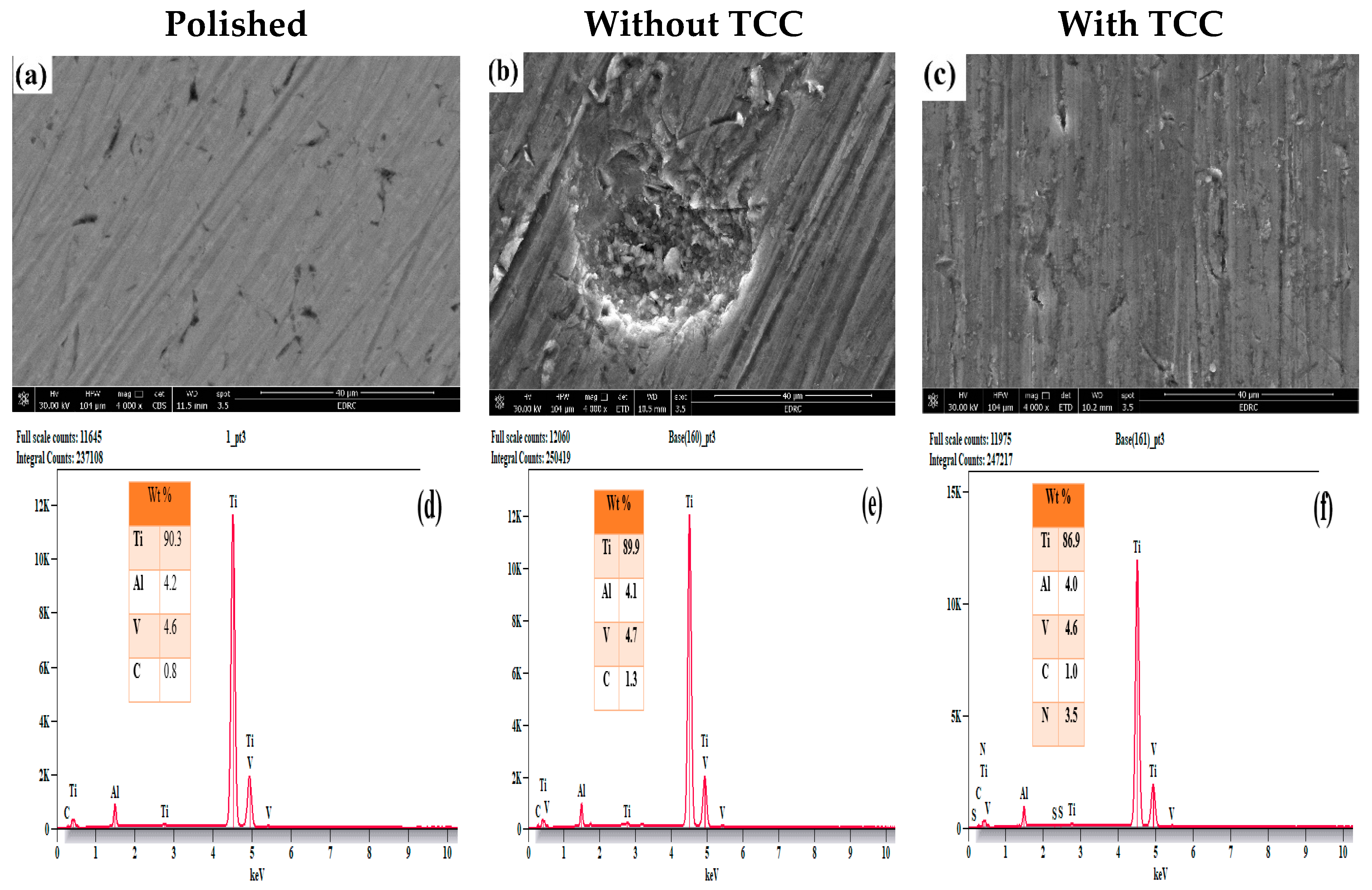
3.4. Surface Morphology
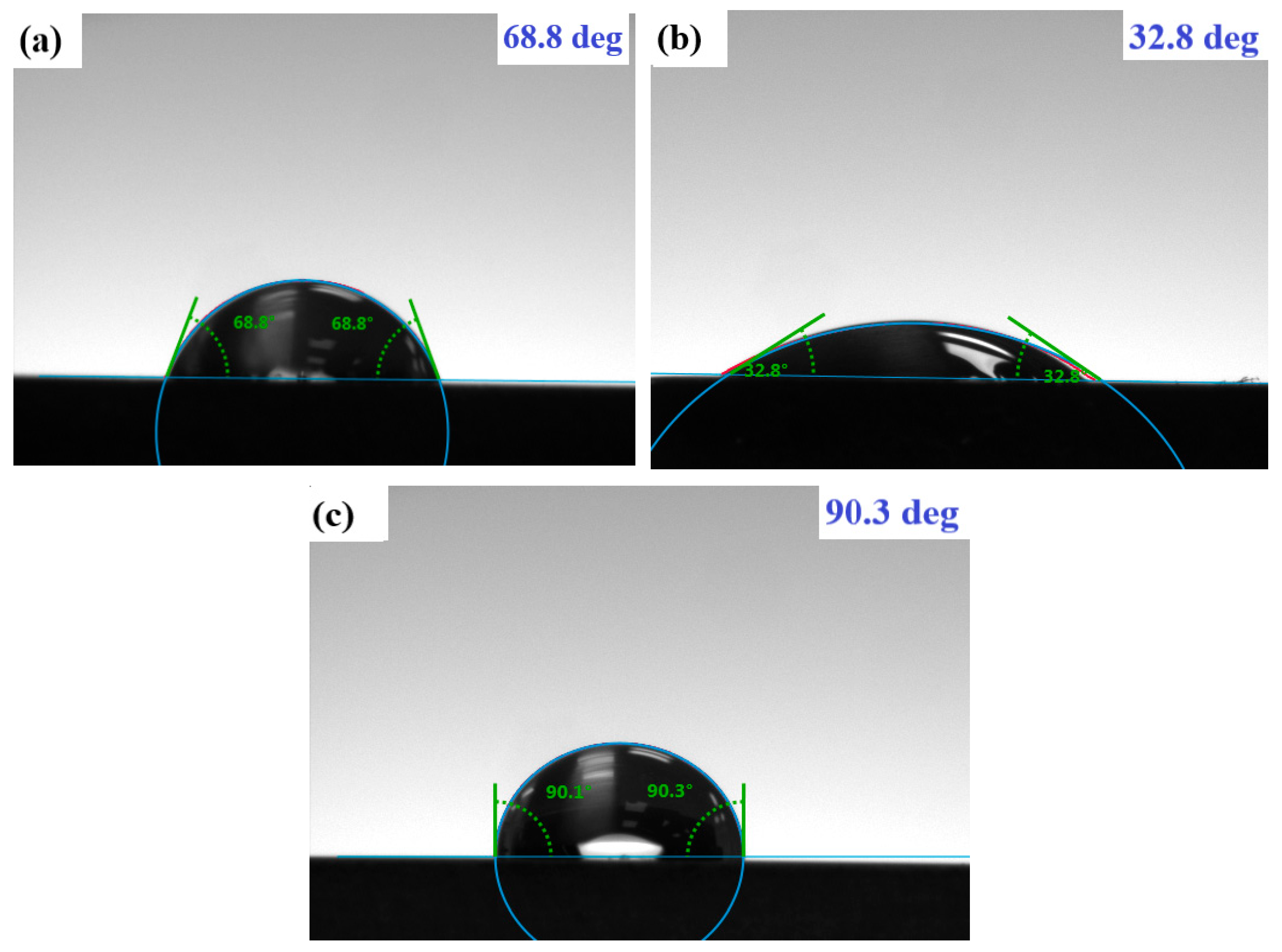
3.5. Contact Angle (CA) Approach
3.6. Atomic Force Microscopy (AFM)
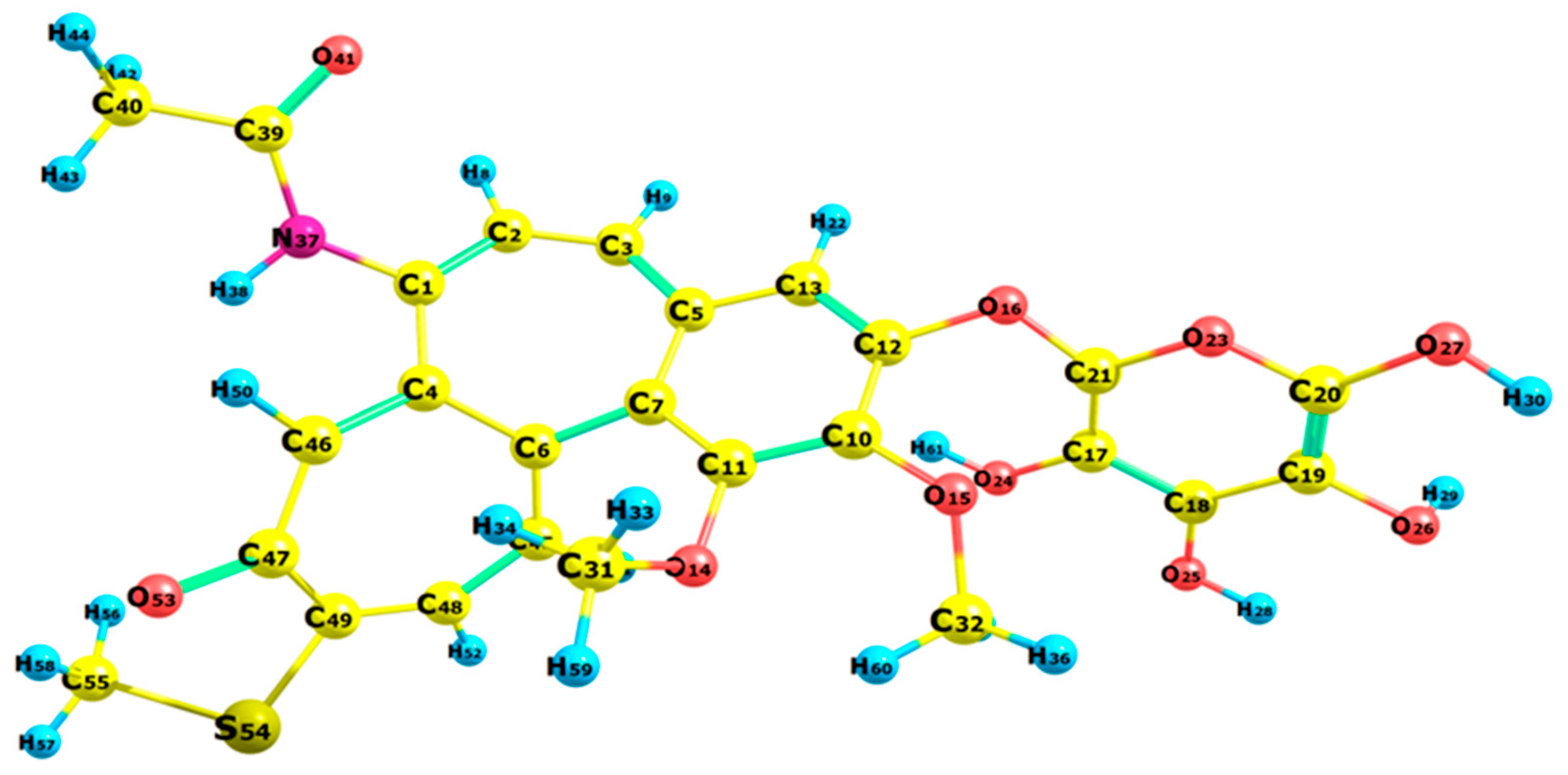
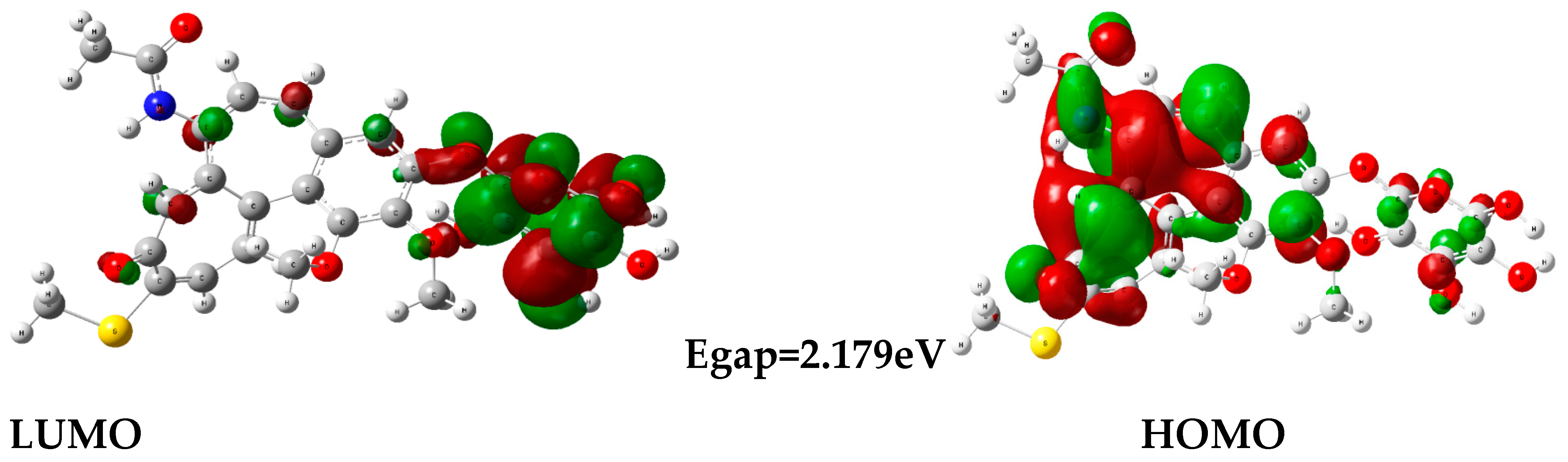
3.7. DFT Approach
3.7.1. Natural Bond Orbital (NBO) Analysis
3.7.2. Molecular Simulation Dynamics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cvijović-Alagić, I.; Cvijović, Z.; Bajat, J.; Rakin, M. Composition and processing effects on the electrochemical characteristics of biomedical titanium alloys. Corros. Sci. 2014, 83, 245–254. [Google Scholar] [CrossRef]
- Su, C.; Yu, H.; Wang, Z.; Yang, J.; Zeng, X. Controlling the tensile and fatigue properties of selective laser melted Ti–6Al–4V alloy by post treatment. J. Alloys Compd. 2021, 857, 157552. [Google Scholar] [CrossRef]
- Okazaki, Y.; Tateishi, T.; Ito, Y. Corrosion resistance of implant alloys in pseudo physiological solution and role of alloying elements in passive films. Mater. Trans. JIM 1997, 38, 78–84. [Google Scholar] [CrossRef]
- Tsai, M.-T.; Chen, Y.-W.; Chao, C.-Y.; Jang, J.S.; Tsai, C.-C.; Su, Y.-L.; Kuo, C.-N. Heat-treatment effects on mechanical properties and microstructure evolution of Ti-6Al-4V alloy fabricated by laser powder bed fusion. J. Alloys Compd. 2020, 816, 152615. [Google Scholar] [CrossRef]
- Choi, Y.R.; Sun, S.D.; Liu, Q.; Brandt, M.; Qian, M. Influence of deposition strategy on the microstructure and fatigue properties of laser metal deposited Ti-6Al-4V powder on Ti-6Al-4V substrate. Int. J. Fatigue 2020, 130, 105236. [Google Scholar] [CrossRef]
- Zhou, X.; Leng, A.; Li, C.; Tang, T.; Xu, J.; Wei, B.; Liao, B.; Sun, C. The role of oral microbiota in accelerating corrosion of Ti6Al4V: An electrochemical study. Mater. Chem. Phys. 2025, 340, 130836. [Google Scholar] [CrossRef]
- Torbati-Sarraf, H.; Ding, L.; Khakpour, I.; Daviran, G.; Poursaee, A. Unraveling the corrosion of the Ti–6Al–4V orthopedic alloy in phosphate-buffered saline (PBS) solution: Influence of frequency and potential. Corros. Mater. Degrad. 2024, 5, 276–288. [Google Scholar] [CrossRef]
- Yu, F.; Addison, O.; Davenport, A.J. A synergistic effect of albumin and H2O2 accelerates corrosion of Ti6Al4V. Acta Biomater. 2015, 26, 355–365. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Varaprasad, K.; Jayaramudu, T. Biomaterials: Design, development and biomedical applications. In Nanotechnology Applications for Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 21–44. [Google Scholar] [CrossRef]
- Boraei, N.F.E.; Ibrahim, M.A.; Rehim, S.S.A.E.; Elshamy, I.H. Electrochemical corrosion behavior of β-Ti alloy in a physiological saline solution and the impact of H2O2 and albumin. J. Solid State Electrochem. 2024, 28, 2243–2256. [Google Scholar] [CrossRef]
- Boraei, N.F.E.; Elshamy, I.H.; Ibrahim, M.A. A comparative study on the electrochemical corrosion behaviour of biomedical β-titanium alloy with TiAlV and titanium in hank’s physiological solution and the impact of reactive oxygen species and immersion time. J. Bio-Tribo-Corros. 2024, 10, 38. [Google Scholar] [CrossRef]
- Shivaram, M.; Arya, S.B.; Nayak, J.; Panigrahi, B.B. Electrochemical Corrosion and Impedance Studies of Porous Ti–x Nb–Ag Alloy in Physiological Solution. Trans. Indian Inst. Met. 2020, 73, 921–928. [Google Scholar] [CrossRef]
- El Boraei, N.F.; Ibrahim, M.A.; El Rehim, S.S.A.; Elshamy, I.H. The Effect of Annealing Temperature and Immersion Time on the Active–Passive Dissolution of Biomedical Ti70Zr20Nb7. 5Ta2. 5 Alloy in Ringer’s Solution. J. Bio-Tribo-Corros. 2023, 9, 62. [Google Scholar] [CrossRef]
- Höhn, S.; Braem, A.; Neirinck, B.; Virtanen, S. Albumin coatings by alternating current electrophoretic deposition for improving corrosion resistance and bioactivity of titanium implants. Mater. Sci. Eng. C 2017, 73, 798–807. [Google Scholar] [CrossRef]
- González, J.; Mirza-Rosca, J. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. J. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Ghoneim, A.; Mogoda, A.; Awad, K. Electrochemical behaviour of Ti–6Al–4V alloy and Ti in azide and halide solutions. Corros. Sci. 2011, 53, 2728–2737. [Google Scholar] [CrossRef]
- Qu, Q.; Wang, L.; Chen, Y.; Li, L.; He, Y.; Ding, Z. Corrosion behavior of titanium in artificial saliva by lactic acid. Materials 2014, 7, 5528–5542. [Google Scholar] [CrossRef] [PubMed]
- Mareci, D.; Ungureanu, G.; Aelenei, D.; Rosca, J.M. Electrochemical characteristics of titanium based biomaterials in artificial saliva. Mater. Corros. 2007, 58, 848–856. [Google Scholar] [CrossRef]
- Elshamy, I.H.; Ibrahim, M.A.; Abdel Rehim, S.S.; El Boraei, N.F. Electrochemical characteristics of a biomedical Ti70Zr20Nb7. 5Ta2. 5 refractory high entropy alloy in an artificial saliva solution. J. Bio-Tribo-Corros. 2023, 9, 10. [Google Scholar] [CrossRef]
- Barril, S.; Mischler, S.; Landolt, D. Electrochemical effects on the fretting corrosion behaviour of Ti6Al4V in 0.9% sodium chloride solution. Wear 2005, 259, 282–291. [Google Scholar] [CrossRef]
- Cao, L.; Wan, Y.; Yang, S.; Pu, J. The tribocorrosion and corrosion properties of thermally oxidized Ti6Al4V alloy in 0.9 wt.% NaCl physiological saline. Coatings 2018, 8, 285. [Google Scholar] [CrossRef]
- Hrir, H.; Ait Layachi, O.; Boudouma, A.; El Bouari, A.; Ait Sidimou, A.; El Marrakchi, M.; Khoumri, E. Electrochemical corrosion behavior of α-titanium alloys in simulated biological environments (comparative study). RSC Adv. 2024, 14, 38110–38119. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, Y.; Liu, Y.; Wei, B.; Guo, J.; Jiao, W.; Zhang, Z.; Zhang, Q. Titanium dioxide nanoparticles modified by salicylic acid and arginine: Structure, surface properties and photocatalytic decomposition of p-nitrophenol. Appl. Surf. Sci. 2016, 363, 627–635. [Google Scholar] [CrossRef]
- Almashhadani, H.A.; Alshujery, M.K.; Khalil, M.; Kadhem, M.M.; Khadom, A.A. Corrosion inhibition behavior of expired diclofenac Sodium drug for Al 6061 alloy in aqueous media: Electrochemical, morphological, and theoretical investigations. J. Mol. Liq. 2021, 343, 117656. [Google Scholar] [CrossRef]
- Hamza, M.; Abd El Rehim, S.; Ibrahim, M.A. Inhibition effect of hexadecyl pyridinium bromide on the corrosion behavior of some austenitic stainless steels in H2SO4 solutions. Arab. J. Chem. 2013, 6, 413–422. [Google Scholar] [CrossRef]
- Abd El Rehim, S.; Ibrahim, M.A.; Khalid, K. The inhibition of 4-(2′-amino-5′-methylphenylazo) antipyrine on corrosion of mild steel in HCl solution. Mater. Chem. Phys. 2001, 70, 268–273. [Google Scholar] [CrossRef]
- Nathiya, R.; Perumal, S.; Murugesan, V.; Raj, V. Expired drugs: Environmentally safe inhibitors for aluminium corrosion in 1 M H2SO4. J. Bio-Tribo-Corros. 2018, 4, 4. [Google Scholar] [CrossRef]
- Schmitt, G. Application of inhibitors for acid media: Report prepared for the European federation of corrosion working party on inhibitors. Br. Corros. J. 1984, 19, 165–176. [Google Scholar] [CrossRef]
- Sastri, V.S. Corrosion Inhibitors: Principles and Applications; Wiley: New York, NY, USA, 1998; Volume 1. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Elshamy, I.H.; Elshamy, M.I.; Elaraby, A. An experimental and computational investigation on the performance of cefotaxime in mitigating Ti6Al4V alloy corrosion. Corros. Eng. Sci. Technol. 2025, 1478422X251348976. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Abdel-Azeim, S.; Chauhan, D.S.; Quraishi, M.A. Electrochemical and computational insights on the application of expired Metformin drug as a novel inhibitor for the sweet corrosion of C1018 steel. ACS Omega 2020, 6, 65–76. [Google Scholar] [CrossRef]
- Karthikeyan, S. Drugs/antibiotics as potential corrosion inhibitors for metals—A review. Int. J. ChemTech Res. 2016, 9, 251–259. [Google Scholar]
- Abdallah, M.; Alfakeer, M.; El Guesmi, N.; Felaly, R.N.; Al-Juaid, S.S.; Al-abdali, F.H.; Sobhi, M. Appraisal of expired linezolid and piperacillin as potent and harmless inhibitors for mitigation of copper corrosion in 1.0 M HNO3. Green Chem. Lett. Rev. 2025, 18, 2474131. [Google Scholar] [CrossRef]
- Radovanović, M.B.; Tasić, Z.a.Z.; Simonović, A.T.; Petrović Mihajlović, M.B.; Antonijević, M.M. Corrosion behavior of titanium in simulated body solutions with the addition of biomolecules. ACS Omega 2020, 5.22, 12768–12776. Available online: http://pubs.acs.org/journal/acsodf?ref=pdf (accessed on 22 May 2025). [CrossRef] [PubMed]
- Abdallah, M.; Al-Rashidi, A.; Al-Gorair, A.S.; Al-Juaid, S.S.; Soliman, K.A. Experimental and theoretical analysis of expired doxycycline and Klacid as aluminum corrosion inhibitors in HCl. Corros. Eng. Sci. Technol. 2024, 1478422X251351692. [Google Scholar] [CrossRef]
- Kotabagi, S.D.; Rajappa, S.; Minagalavar, R.L.; Rathod, M.R.; Suma, J.; Sajjan, A.M. Expired Lircetam drug as a corrosion inhibitor for low-carbon steel in 1 M HCl: Experimental, theoretical, and quantum chemical insights. Results Surf. Interfaces 2025, 18, 100459. [Google Scholar] [CrossRef]
- Gece, G. Drugs: A review of promising novel corrosion inhibitors. Corros. Sci. 2011, 53, 3873–3898. [Google Scholar] [CrossRef]
- Bhamburkar, S.; Khandare, S.; Patharkar, S.; Thakare, S. Thiocolchicoside: An updated review. Asian J. Res. Pharm. Sci. 2022, 12, 213–218. [Google Scholar] [CrossRef]
- Soonawalla, D.F.; Joshi, N. Efficacy of thiocolchicoside in Indian patients suffering from low back pain associated with muscle spasm. J. Indian Med. Assoc. 2008, 106, 331–335. [Google Scholar]
- Ketenci, A.; Ozcan, E.; Karamursel, S. Assessment of efficacy and psychomotor performances of thiocolchicoside and tizanidine in patients with acute low back pain. Int. J. Clin. Pract. 2005, 59, 764–770. [Google Scholar] [CrossRef]
- El-Mokadem, T.; Hashem, A.; Abd El-Sattar, N.E.; Dawood, E.; Abdelshafi, N. Green synthesis, electrochemical, DFT studies and MD simulation of novel synthesized thiourea derivatives on carbon steel corrosion inhibition in 1.0 M HCl. J. Mol. Struct. 2023, 1274, 134567. [Google Scholar] [CrossRef]
- Abdelshafi, N.; Farag, A.A.; Heakal, F.E.-T.; Badran, A.-S.; Abdel-Azim, K.; El Sayed, A.-R.M.; Ibrahim, M.A. In-depth experimental assessment of two new aminocoumarin derivatives as corrosion inhibitors for carbon steel in HCl media combined with AFM, SEM/EDX, contact angle, and DFT/MDs simulations. J. Mol. Struct. 2024, 1304, 137638. [Google Scholar] [CrossRef]
- Hadisaputra, S.; Purwoko, A.A.; Savalas, L.R.T.; Prasetyo, N.; Yuanita, E.; Hamdiani, S. Quantum chemical and Monte Carlo simulation studies on inhibition performance of caffeine and its derivatives against corrosion of copper. Coatings 2020, 10, 1086. [Google Scholar] [CrossRef]
- Arrousse, N.; Salim, R.; Kaddouri, Y.; Zahri, D.; El Hajjaji, F.; Touzani, R.; Taleb, M.; Jodeh, S. The inhibition behavior of two pyrimidine-pyrazole derivatives against corrosion in hydrochloric solution: Experimental, surface analysis and in silico approach studies. Arab. J. Chem. 2020, 13, 5949–5965. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, Y.; Cang, H.; Xu, J.; Lu, G.; Jing, W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part II: Theoretical studies. Corros. Sci. 2014, 83, 292–298. [Google Scholar] [CrossRef]
- Khaled, K.; Hamed, M.N.; Abdel-Azim, K.; Abdelshafi, N. Inhibition of copper corrosion in 3.5% NaCl solutions by a new pyrimidine derivative: Electrochemical and computer simulation techniques. J. Solid State Electrochem. 2011, 15, 663–673. [Google Scholar] [CrossRef]
- Pinto, G.M.; Nayak, J.; Shetty, A.N. Corrosion inhibition of 6061 Al–15 vol. pct. SiC (p) composite and its base alloy in a mixture of sulphuric acid and hydrochloric acid by 4-(N, N-dimethyl amino) benzaldehyde thiosemicarbazone. Mater. Chem. Phys. 2011, 125, 628–640. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Zuo, X.; Tan, B.; Xu, C.; Zhang, S. Investigating the inhibitive effect of Davidia involucrata leaf extract as a biological eco-friendly inhibitor for copper in acidic medium. J. Mol. Liq. 2021, 325, 115214. [Google Scholar] [CrossRef]
- Chocholoušová, J.; Špirko, V.; Hobza, P. First local minimum of the formic acid dimer exhibits simultaneously red-shifted O–H⋯ O and improper blue-shifted C–H⋯ O hydrogen bonds. Phys. Chem. Chem. Phys. 2004, 6, 37–41. [Google Scholar] [CrossRef]
- Szafran, M.; Komasa, A.; Bartoszak-Adamska, E. Crystal and molecular structure of 4-carboxypiperidinium chloride (4-piperidinecarboxylic acid hydrochloride). J. Mol. Struct. 2007, 827, 101–107. [Google Scholar] [CrossRef]
- Yadav, D.K.; Chauhan, D.; Ahamad, I.; Quraishi, M. Electrochemical behavior of steel/acid interface: Adsorption and inhibition effect of oligomeric aniline. RSC Adv. 2013, 3, 632–646. [Google Scholar] [CrossRef]
- Yadav, D.K.; Quraishi, M. Electrochemical investigation of substituted pyranopyrazoles adsorption on mild steel in acid solution. Ind. Eng. Chem. Res. 2012, 51, 8194–8210. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Espinoza-Vázquez, A.; Rodríguez-Gómez, F.; González-Olvera, R.; Angeles-Beltrán, D.; Mendoza-Espinosa, D.; Negrón-Silva, G. Electrochemical assessment of phenol and triazoles derived from phenol (BPT) on API 5L X52 steel immersed in 1 M HCl. RSC Adv. 2016, 6, 72885–72896. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Kluza, K.; Makowska-Janusik, M.; Olasunkanmi, L.O.; Ebenso, E.E. Corrosion inhibition of mild steel in 1M HCl by D-glucose derivatives of dihydropyrido [2, 3-d: 6, 5-d′] dipyrimidine-2, 4, 6, 8 (1H, 3H, 5H, 7H)-tetraone. Sci. Rep. 2017, 7, 44432. [Google Scholar] [CrossRef] [PubMed]
- Hrimla, M.; Bahsis, L.; Boutouil, A.; Laamari, M.R.; Julve, M.; Stiriba, S.-E. Corrosion inhibition performance of a structurally well-defined 1, 2, 3-triazole derivative on mild steel-hydrochloric acid interface. J. Mol. Struct. 2021, 1231, 129895. [Google Scholar] [CrossRef]
- Olasunkanmi, L.O.; Kabanda, M.M.; Ebenso, E.E. Quinoxaline derivatives as corrosion inhibitors for mild steel in hydrochloric acid medium: Electrochemical and quantum chemical studies. Phys. E Low-Dimens. Syst. Nanostructures 2016, 76, 109–126. [Google Scholar] [CrossRef]
- Rouifi, Z.; Rbaa, M.; Abousalem, A.S.; Benhiba, F.; Laabaissi, T.; Oudda, H.; Lakhrissi, B.; Guenbour, A.; Warad, I.; Zarrouk, A. Synthesis, characterization and corrosion inhibition potential of newly benzimidazole derivatives: Combining theoretical and experimental study. Surf. Interfaces 2020, 18, 100442. [Google Scholar] [CrossRef]
- Shalabi, K.; Abdel-Galil, E.; El-Askalany, A.; Abdallah, Y. Adsorption, electrochemical behavior, and theoretical studies for copper corrosion inhibition in 1 M nitric acid medium using triazine derivatives. J. Mol. Liq. 2022, 348, 118420. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.; Khamis, E.; Abo-Eldahab, H.; Adeel, S. Novel package for inhibition of aluminium corrosion in alkaline solutions. Mater. Chem. Phys. 2010, 124, 773–779. [Google Scholar] [CrossRef]
- Aouine, Y.; Sfaira, M.; Touhami, M.E.; Alami, A.; Hammouti, B.; Elbakri, M.; El Hallaoui, A.; Touir, R. Temperature and time investigations on the adsorption behavior of isoindoline, tetrazole and isoindoline-tetrazole on corrosion of mild steel in acidic medium. Int. J. Electrochem. Sci. 2012, 7, 5400–5419. [Google Scholar] [CrossRef]
- Amin, M.A.; Ibrahim, M.M. Corrosion and corrosion control of mild steel in concentrated H2SO4 solutions by a newly synthesized glycine derivative. Corros. Sci. 2011, 53, 873–885. [Google Scholar] [CrossRef]
- Kokalj, A. Corrosion inhibitors: Physisorbed or chemisorbed? Corros. Sci. 2022, 196, 109939. [Google Scholar] [CrossRef]
- Fawzy, A.; Abdallah, M.; Zaafarany, I.; Ahmed, S.; Althagafi, I. Thermodynamic, kinetic and mechanistic approach to the corrosion inhibition of carbon steel by new synthesized amino acids-based surfactants as green inhibitors in neutral and alkaline aqueous media. J. Mol. Liq. 2018, 265, 276–291. [Google Scholar] [CrossRef]
- Hegazy, M. Novel cationic surfactant based on triazole as a corrosion inhibitor for carbon steel in phosphoric acid produced by dihydrate wet process. J. Mol. Liq. 2015, 208, 227–236. [Google Scholar] [CrossRef]
- Machnikova, E.; Whitmire, K.H.; Hackerman, N. Corrosion inhibition of carbon steel in hydrochloric acid by furan derivatives. Electrochim. Acta 2008, 53, 6024–6032. [Google Scholar] [CrossRef]
- Saliyan, V.R.; Adhikari, A.V. Quinolin-5-ylmethylene-3-{[8-(trifluoromethyl) quinolin-4-yl] thio} propanohydrazide as an effective inhibitor of mild steel corrosion in HCl solution. Corros. Sci. 2008, 50, 55–61. [Google Scholar] [CrossRef]
- Rouifi, Z.; Rbaa, M.; Benhiba, F.; Laabaissi, T.; Oudda, H.; Lakhrissi, B.; Guenbour, A.; Warad, I.; Zarrouk, A. Preparation and anti-corrosion activity of novel 8-hydroxyquinoline derivative for carbon steel corrosion in HCl molar: Computational and experimental analyses. J. Mol. Liq. 2020, 307, 112923. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor. Chem. Acc. 2002, 108, 134–142. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Sayed, A.R.; Gomha, S.M.; Bakir, E.M.; Shalabi, K. Synthesis and study of poly [(hydrazinylazo)] thiazoles as potent corrosion inhibitors for cast iron-carbon alloy in molar HCl: A collective computational and experiential methods. J. Mol. Liq. 2021, 337, 116555. [Google Scholar] [CrossRef]
- Fouda, A.; Ismail, M.; Abousalem, A.S.; Elewady, G. Experimental and theoretical studies on corrosion inhibition of 4-amidinophenyl-2, 2′-bifuran and its analogues in acidic media. RSC Adv. 2017, 7, 46414–46430. [Google Scholar] [CrossRef]
- Mert, B.D.; Yüce, A.O.; Kardaş, G.; Yazıcı, B. Inhibition effect of 2-amino-4-methylpyridine on mild steel corrosion: Experimental and theoretical investigation. Corros. Sci. 2014, 85, 287–295. [Google Scholar] [CrossRef]
- Guerrab, W.; Chung, I.-M.; Kansiz, S.; Mague, J.T.; Dege, N.; Taoufik, J.; Salghi, R.; Ali, I.H.; Khan, M.I.; Lgaz, H. Synthesis, structural and molecular characterization of 2, 2-diphenyl-2H, 3H, 5H, 6H, 7H-imidazo [2, 1-b][1, 3] thiazin-3-one. J. Mol. Struct. 2019, 1197, 369–376. [Google Scholar] [CrossRef]
- Guerrab, W.; Lgaz, H.; Kansiz, S.; Mague, J.T.; Dege, N.; Ansar, M.; Marzouki, R.; Taoufik, J.; Ali, I.H.; Chung, I.-M. Synthesis of a novel phenytoin derivative: Crystal structure, Hirshfeld surface analysis and DFT calculations. J. Mol. Struct. 2020, 1205, 127630. [Google Scholar] [CrossRef]
- Abdallah, M.; Al Bahir, A.; Altass, H.; Fawzy, A.; El Guesmi, N.; Al-Gorair, A.S.; Benhiba, F.; Warad, I.; Zarrouk, A. Anticorrosion and adsorption performance of expired antibacterial drugs on Sabic iron corrosion in HCl solution: Chemical, electrochemical and theoretical approach. J. Mol. Liq. 2021, 330, 115702. [Google Scholar] [CrossRef]
- Abdelshafi, N.; Ibrahim, M.A.; Badran, A.-S.; Halim, S.A. Experimental and theoretical evaluation of a newly synthesized quinoline derivative as corrosion inhibitor for iron in 1.0 M hydrochloric acid solution. J. Mol. Struct. 2022, 1250, 131750. [Google Scholar] [CrossRef]
- Jucai, W.; Ke, T.; Xiaodi, S.; Xin, H. Theoretical calculations of pyridine adsorption on the surfaces of Ti, Zr, N doped graphene. J. Fuel Chem. Technol. 2024, 52, 1162–1172. [Google Scholar] [CrossRef]
- Tshwane, D.M.; Modiba, R.; Govender, G.; Ngoepe, P.; Chauke, H. The adsorption of halogen molecules on Ti (110) surface. J. Mater. Res. 2021, 36, 592–601. [Google Scholar] [CrossRef]
- Boda, A.; Chandorkar, N.; Ali, S.M. Density functional theoretical assessment of titanium metal for adsorption of hydrogen, deuterium and tritium isotopes. Theor. Chem. Acc. 2023, 142, 46. [Google Scholar] [CrossRef]
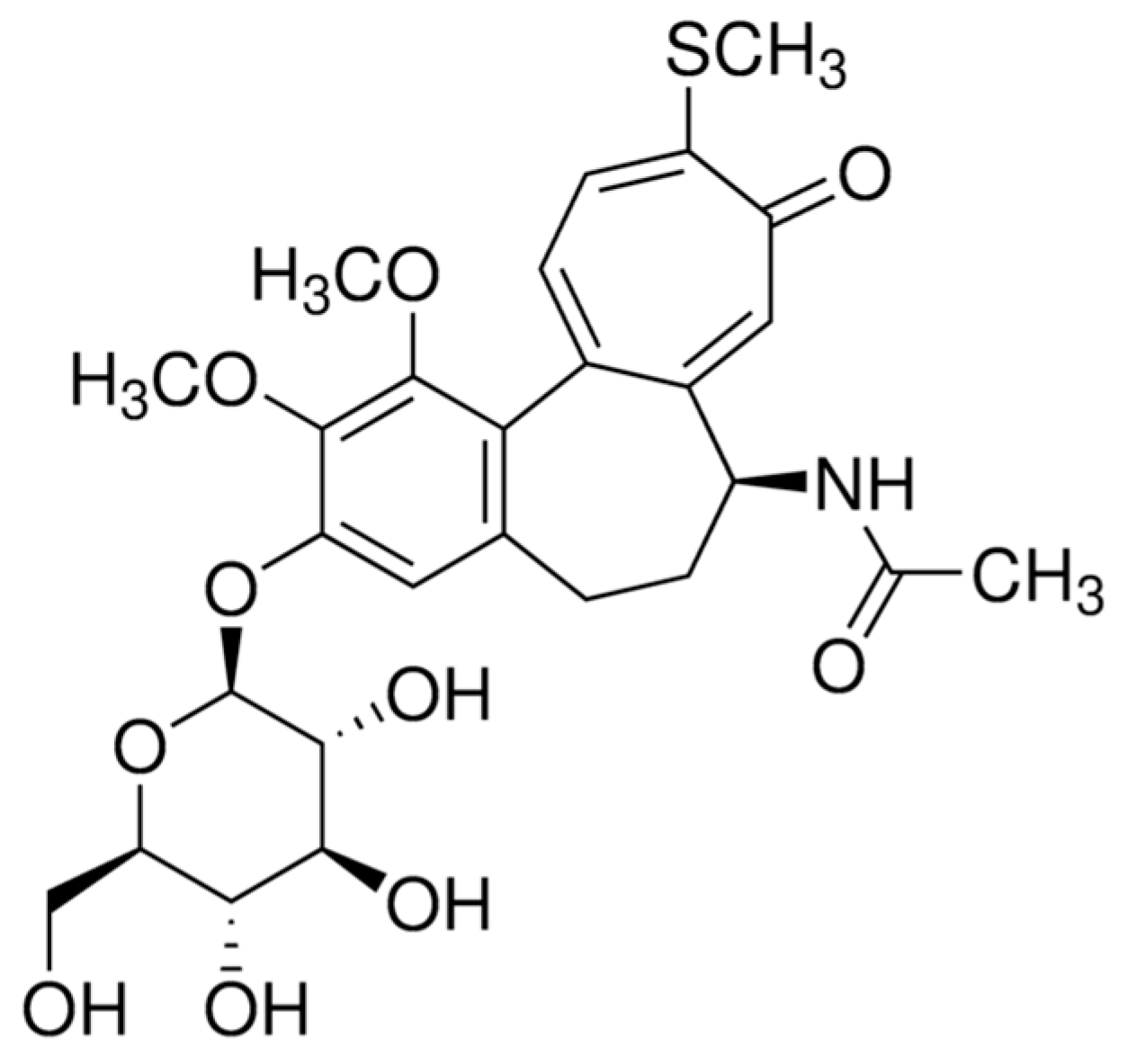





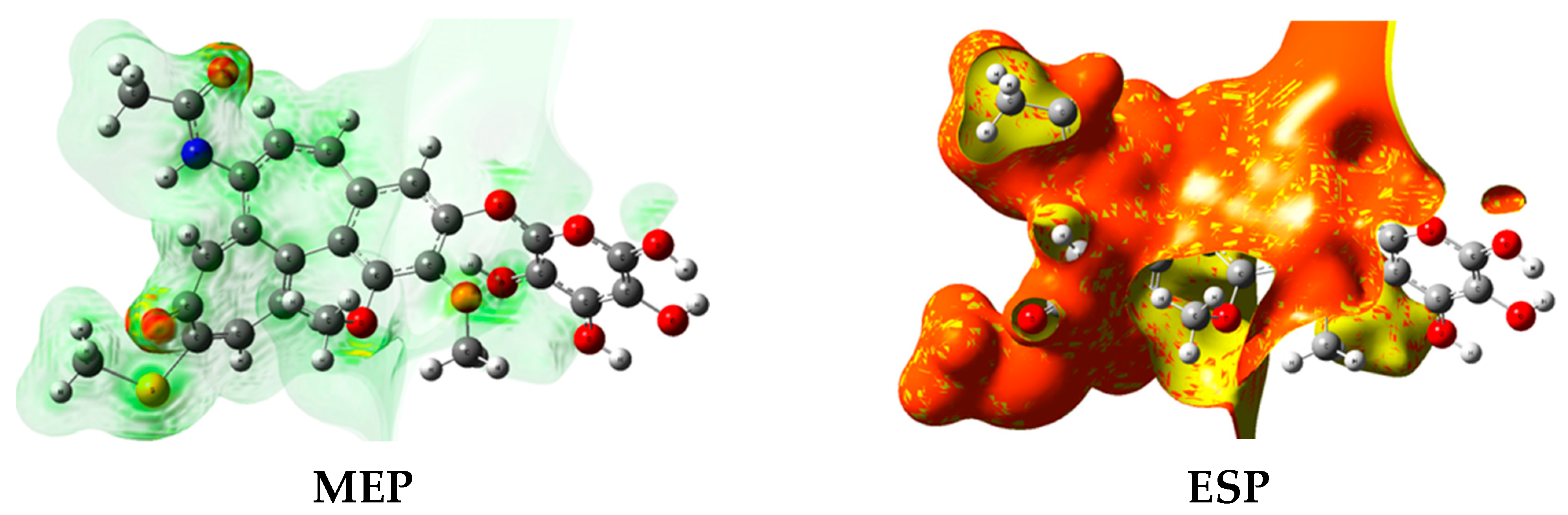
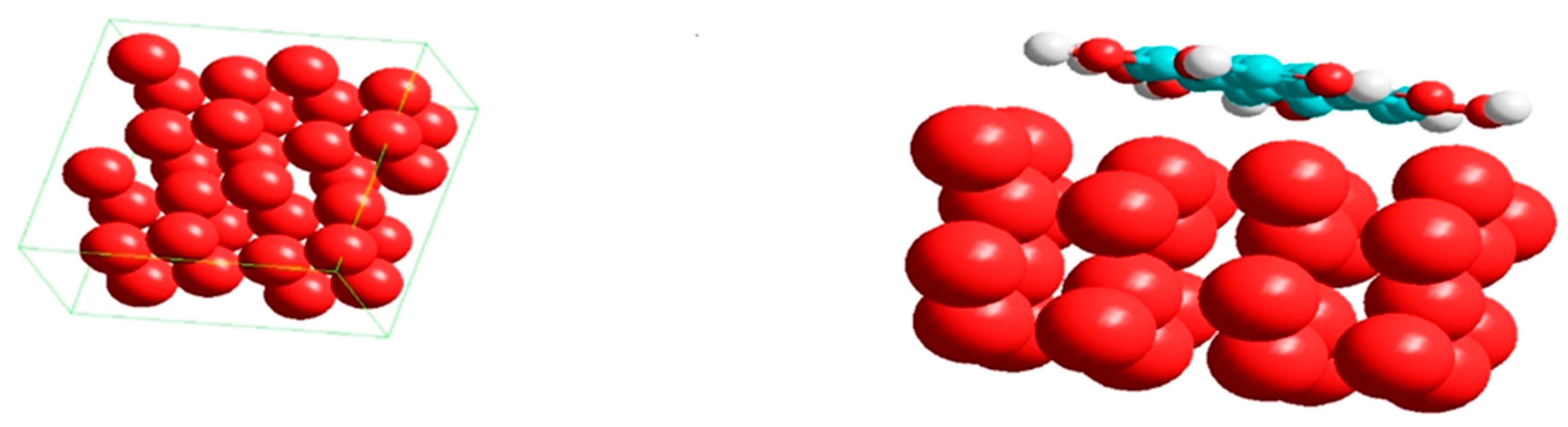
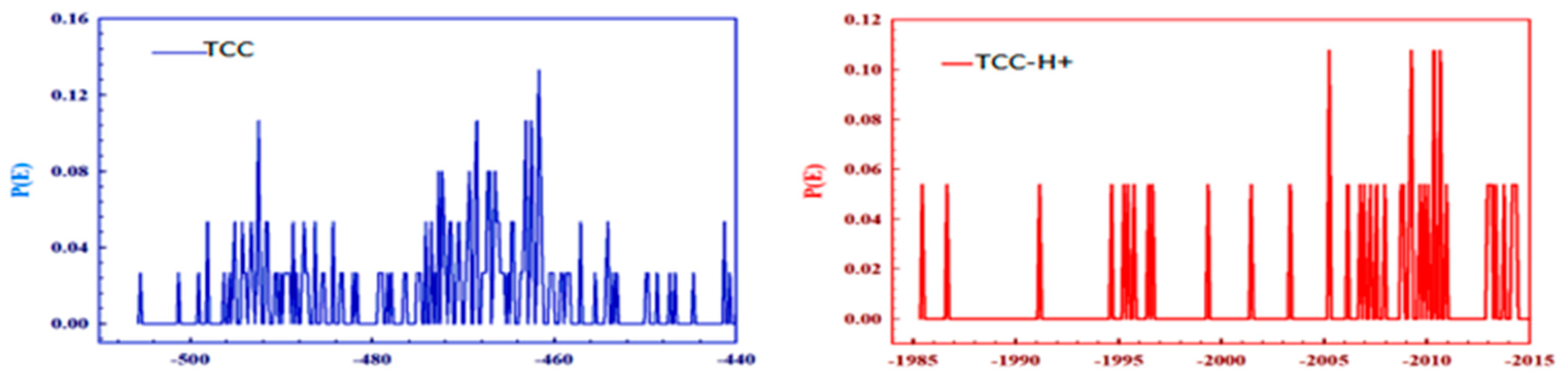
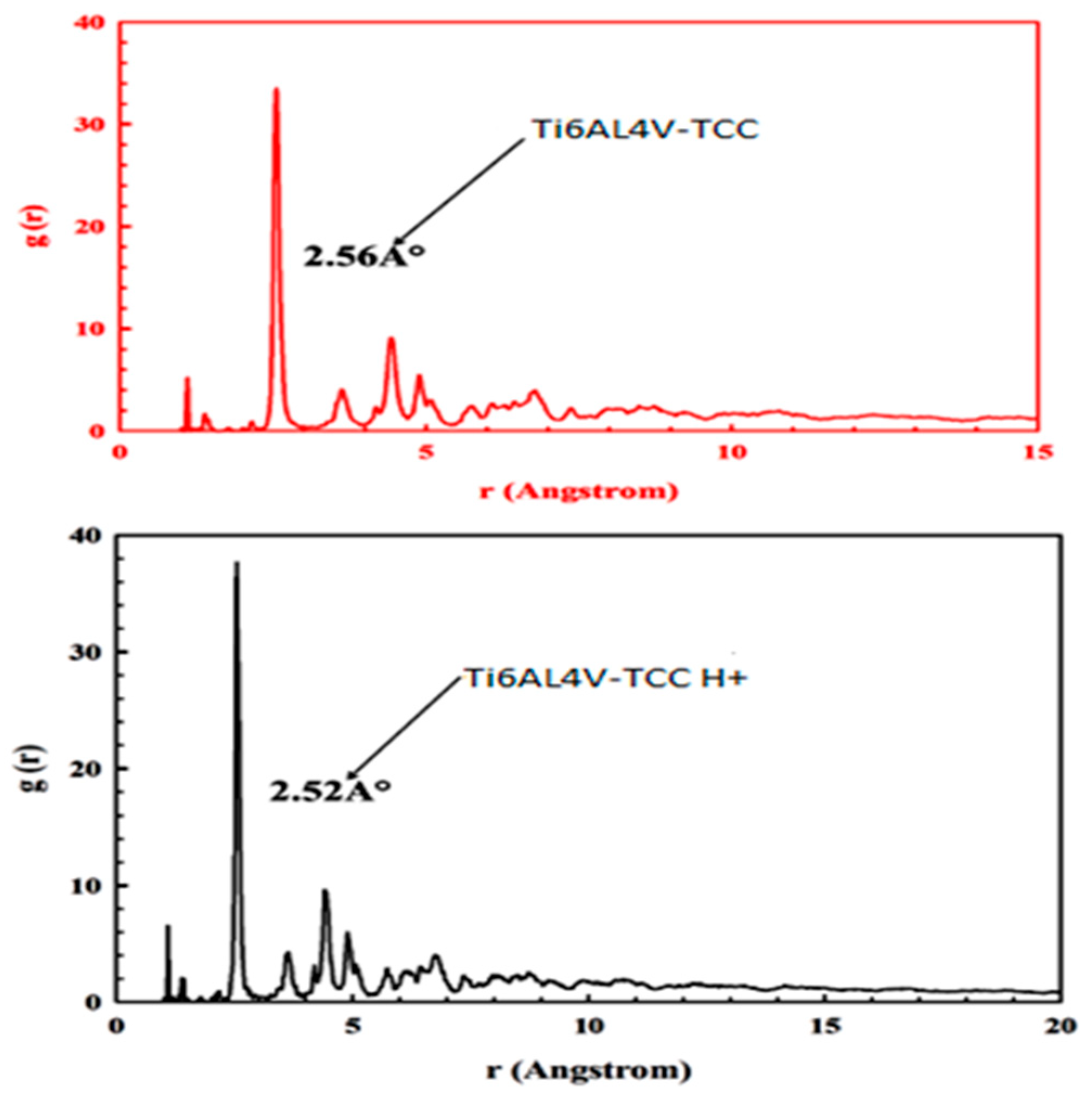
| [TCC] mg/L | ICorr A cm−2 | −ECorr /V | βa (V dec−1) | βc (V dec−1) | CR Mpy | ղPol (%) | |
|---|---|---|---|---|---|---|---|
| 0.0 | 3.08 × 10−6 | 0.297 ± 0.04 | 0.210 ± 0.03 | 0.294 ± 0.03 | 0.396 ± 0.07 | - | - |
| 40 | 1.46 × 10−6 | 0.255 ± 0.03 | 0.656 ± 0.04 | 0.238 ± 0.04 | 0.188 ± 0.04 | 0.525 ± 0.05 | 52.50 ± 3 |
| 80 | 9.10 × 10−7 | 0.226 ± 0.02 | 0.128 ± 0.02 | 0.108 ± 0.06 | 0.117 ± 0.03 | 0.704 ± 0.03 | 70.40 ± 2.5 |
| 120 | 5.51 × 10−7 | 0.189 ± 0.02 | 0.077 ± 0.05 | 0.067 ± 0.04 | 0.070 ± 0.02 | 0.821 ± 0.04 | 82.10 ± 2.0 |
| 200 | 2.32 × 10−7 | 0.170 ± 0.02 | 0.035 ± 0.06 | 0.050 ± 0.03 | 0.029 ± 0.02 | 0.924 ± 0.02 | 92.40 ± 1.5 |
| Concentration mg/L | Rs (Ω cm2) | Rct (kΩ cm2) | Qdl (F cm2 HZ1−n1) | Rf (KΩ cm2) | Qf (F cm2 HZ1−n2) | Rp (Rf + Rct) (kΩ cm2) | χ2 ×10−9 | ղEIS (%) | |
|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 16.79 ± 0.67 | 7.80 ± 0.55 | 79.61 × 10−6 | 0.045 ± 0.005 | 197.0 × 10−6 | 7.85 ± 0.55 | 0.227 | - | - |
| 40 | 18.30 ± 0.73 | 21.77 ± 1.5 | 59.02 × 10−6 | 0.067 ± 0.007 | 182.9 × 10−6 | 21.83 ± 1.5 | 0.552 | 0.641 | 64.1 ± 3 |
| 80 | 20.83 ± 0.83 | 25.01 ± 1.8 | 47.54 × 10−6 | 0.073 ± 0.007 | 166.2 × 10−6 | 25.08 ± 1.8 | 0.027 | 0.688 | 68.8 ± 3 |
| 120 | 33.25 ± 1.7 | 47.12 ± 3.3 | 33.29 × 10−6 | 0.088 ± 0.009 | 140.3 × 10−6 | 47.20 ± 3.3 | 0.732 | 0.834 | 83.4 ± 2.5 |
| 200 | 55.53 ± 2.8 | 78.79 ± 5.5 | 22.64 × 10−6 | 0.096 ± 0.010 | 77.60 × 10−6 | 78.88 ± 5.5 | 0.856 | 0.901 | 90.1 ± 2.0 |
| Temperature K | ICorr A cm−2 | −ECorr /V | βa (V dec−1) | βc (V dec−1) | Corr Rate mpy | Θ | ηpol% |
|---|---|---|---|---|---|---|---|
| 0.00 mg/L | |||||||
| 310 | 3.08 × 10−6 | 0.297 | 0.110 | 0.094 | 0.396 | - | - |
| 320 | 5.29 × 10−6 | 0.334 | 0.313 | 0.262 | 0.681 | - | - |
| 330 | 8.50 × 10−6 | 0.345 | 0.221 | 0.120 | 1.098 | - | - |
| 340 | 1.22 × 10−5 | 0.362 | 0.425 | 0.238 | 1.574 | - | - |
| 200 mg/L | |||||||
| 310 | 2.32 × 10−7 | 0.170 | 0.035 | 0.050 | 0.029 | 0.924 | 92.4 |
| 320 | 1.00 × 10−6 | 0.223 | 0.144 | 0.154 | 0.084 | 0.825 | 82.5 |
| 330 | 2.96 × 10−6 | 0.261 | 0.219 | 0.175 | 0.380 | 0.651 | 65.1 |
| 340 | 7.09 × 10−6 | 0.333 | 0.518 | 0.314 | 0.913 | 0.418 | 41.8 |
| Solution | (kJ mol−1) | (J mol−1 K−1) | Ea (kJ mol−1) |
|---|---|---|---|
| Without TCC | 13.59 | −64.57 | 14.46 |
| With 200 mg/L TCC | 34.56 | −33.62 | 35.79 |
| Temperature K | Rs (Ω cm2) | Rct (kΩ cm2) | CPEdl (F cm2 HZ1−n1) | n | ղEIS (%) | |
|---|---|---|---|---|---|---|
| 0.00 mg/L | ||||||
| 310 | 16.79 | 7.80 | 79.61 × 10−6 | 0.843 | - | - |
| 320 | 22.36 | 6.12 | 123.80 × 10−6 | 0.829 | - | - |
| 330 | 44.19 | 5.43 | 235.11 × 10−6 | 0.788 | - | - |
| 340 | 76.15 | 4.49 | 310.74 × 10−6 | 0.769 | - | - |
| 200 mg/L | ||||||
| 310 | 55.53 | 78.79 | 22.64 × 10−6 | 0.855 | 0.901 | 90.10 |
| 320 | 23.14 | 32.15 | 57.20 × 10−6 | 0.812 | 0.809 | 80.90 |
| 330 | 75.04 | 15.90 | 75.42 × 10−6 | 0.755 | 0.658 | 65.80 |
| 340 | 14.06 | 7.57 | 94.12 × 10−6 | 0.787 | 0.406 | 40.60 |
| Alloy | Kads (L mol−1) | ΔGoads (kJ mol−1) | ||
|---|---|---|---|---|
| Polarization | Impedance | Polarization | Impedance | |
| Ti6Al4V | 10.87 × 104 | 11.50 × 104 | −49.02 | −49.20 |
| Descriptors | Equations | TCC | TCC H+ |
|---|---|---|---|
| Energy of highest occupied molecular orbital (EHOMO), (eV) | −4.291 | −3.981 | |
| Energy of lowest unoccupied molecular orbital (ELUMO), (eV) | −2.112 | −3.601 | |
| Energy Gap ΔE | (LUMO-HOMO) | 2.179 | 0.380 |
| Dipole moment, (µ), (Debye) | 1.983 | 19.758 | |
| Ionization energy (I) (ev) | 4.291 | 3.981 | |
| Electron affinity () (ev) | 2.112 | 3.601 | |
| Electronegativity (ϕ) | ϕ = | 3.201 | 3.791 |
| Global hardness ψ | ψ = | 1.089 | 0.190 |
| Global softness (S) | s = | 0.918 | 5.263 |
| Global electrophilicity (ω) | ω = ϕ2/2ψ | 4.705 | 37.820 |
| Global nucleophilicity (ε) | 0.212 | 0.026 | |
| Electroaccepting (ω+) power | 3.239 | 35.948 | |
| Electrodonating (ω−) power | 6.441 | 39.739 | |
| Net electrophilicity (Δω± = ω+ +ω−) | (Δω± = ω+ + ω−) | 9.680 | 75.687 |
| Fraction of transferred electrons (ΔN) | −0.087 | −0.108 | |
| Back-donation energy ΔE back-donation (ev) | −0.272 | −0.047 | |
| Metal/inhibitor interaction energy ΔETi6Al4V/inhibitor (ev) | 0.593 | 216.352 |
| Atom | f(+) | f(−) | ∆fk | Mulliken Atomic Charges |
|---|---|---|---|---|
| C(1) | 0.008 | 0.010 | 0.002 | −0.091 |
| N(2) | 0.046 | 0.040 | 0.006 | −0.115 |
| C(3) | −0.019 | −0.021 | 0.002 | 0.013 |
| S(4) | 0.101 | 0.360 | 0.259 | −0.144 |
| C(5) | −0.020 | −0.022 | 0.002 | −0.091 |
| C(6) | 0.111 | −0.004 | 0.107 | −0.022 |
| N(7) | 0.129 | 0.032 | 0.097 | 0.275 |
| O(8) | 0.047 | −0.004 | 0.043 | −0.035 |
| C(9) | −0.036 | −0.012 | 0.024 | −0.338 |
| C(10) | 0.042 | 0.000 | 0.042 | 0.469 |
| O(11) | 0.081 | 0.001 | 0.080 | −0.355 |
| N(12) | 0.021 | 0.005 | 0.016 | 0.404 |
| N(13) | 0.009 | 0.003 | 0.006 | −0.136 |
| C(14) | −0.002 | −0.004 | 0.002 | −0.479 |
| S(15) | −0.008 | 0.023 | 0.015 | −0.373 |
| C(16) | −0.001 | −0.006 | 0.005 | 0.042 |
| C(17) | 0.008 | 0.020 | 0.012 | −0.203 |
| C(18) | −0.001 | −0.004 | 0.003 | −0.192 |
| C(19) | 0.005 | 0.002 | 0.003 | −0.075 |
| O(20) | 0.022 | 0.008 | 0.014 | −0.192 |
| C(21) | −0.018 | −0.005 | 0.013 | 0.309 |
| H(22) | 0.024 | 0.017 | 0.007 | −0.39 |
| C(23) | −0.004 | 0.009 | 0.005 | 0.308 |
| O(24) | 0.011 | 0.047 | 0.036 | −0.448 |
| O(25) | 0.009 | 0.043 | 0.034 | −0.448 |
| C(26) | 0.002 | 0.000 | 0.002 | −0.192 |
| O(27) | 0.001 | 0.002 | 0.001 | −0.075 |
| C(28) | 0.005 | 0.000 | 0.005 | −0.192 |
| O(29) | 0.009 | 0.002 | 0.007 | 0.309 |
| N(30) | 0.013 | 0.060 | 0.047 | −0.39 |
| Compound | Donor | Acceptor | E(2) a (kcal/mol) | Occupancy |
|---|---|---|---|---|
| TCC | πC12–O41 | π*C13–O40 | 22.52 | 1.67 |
| πC19–O15 | π*C11–C13 | 21.13 | 1.65 | |
| LP (1) N37 | π*S54–N37 | 13.19 | 1.87 | |
| LP (1) O23 | π*O14–C6 | 50.05 | 1.74 | |
| LP (1) O24 | π*O23–O15 | 16.74 | 1.75 | |
| LP (1) O25 | π*C13–O26 | 45.21 | 1.88 | |
| LP (1) O26 | π*C32–O15 | 36.58 | 1.69 | |
| LP (1) O27 | π*C10–O24 | 26.47 | 1.57 | |
| LP (3) S54 | π*O14–N37 | 31.04 | 1.35 | |
| LP (2) O14 | π*S54–H57 | 29.83 | 1.82 | |
| LP (2) O15 | π*C10–C7 | 18.81 | 1.94 | |
| LP (2) O16 | π*C13–C11 | 28.83 | 1.82 | |
| LP (2) O53 | π*C13–O16 | 19.81 | 1.94 | |
| LP (2) O41 | π*C10–O15 | 33.04 | 1.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, I.M.A.; Elshamy, I.H.; Halim, S.A.; Ibrahim, M.A.M. Electrochemical and Computational Analyses of Thiocolchicoside as a New Corrosion Inhibitor for Biomedical Ti6Al4V Alloy in Saline Solution: DFT, NBO, and MD Approaches. Surfaces 2025, 8, 77. https://doi.org/10.3390/surfaces8040077
Omar IMA, Elshamy IH, Halim SA, Ibrahim MAM. Electrochemical and Computational Analyses of Thiocolchicoside as a New Corrosion Inhibitor for Biomedical Ti6Al4V Alloy in Saline Solution: DFT, NBO, and MD Approaches. Surfaces. 2025; 8(4):77. https://doi.org/10.3390/surfaces8040077
Chicago/Turabian StyleOmar, Inam M. A., Ibrahim H. Elshamy, Shimaa Abdel Halim, and Magdy A. M. Ibrahim. 2025. "Electrochemical and Computational Analyses of Thiocolchicoside as a New Corrosion Inhibitor for Biomedical Ti6Al4V Alloy in Saline Solution: DFT, NBO, and MD Approaches" Surfaces 8, no. 4: 77. https://doi.org/10.3390/surfaces8040077
APA StyleOmar, I. M. A., Elshamy, I. H., Halim, S. A., & Ibrahim, M. A. M. (2025). Electrochemical and Computational Analyses of Thiocolchicoside as a New Corrosion Inhibitor for Biomedical Ti6Al4V Alloy in Saline Solution: DFT, NBO, and MD Approaches. Surfaces, 8(4), 77. https://doi.org/10.3390/surfaces8040077






