1. Introduction
Ion irradiation is a technique used in materials science to control the physical properties of a material. This process involves bombarding the material’s surface with a beam of ions, triggering a series of physical interactions at the atomics level that can induce structural, morphological, optical, electrical, and mechanical changes [
1,
2].
When the ions penetrate the material, they transfer their energy to the atoms in the crystal lattice through elastic and inelastic collisions. This process can generate point defects, vacancies, interstitial defects, and localized amorphization, resulting in modifications to the crystal structure [
3,
4].
The density of these defects depends on the type of ion, its energy, the applied fluence, and the nature of the target material [
5]. Irradiation with various ions and energies finds applications in microelectronics, nanotechnology, and surface modification. Its most significant role, however, is in tailoring physical properties and creating versatile materials [
6,
7,
8].
Zinc oxide (ZnO) is an inorganic compound with unique properties that has been studied for industrial, medical, and technological applications [
9]. ZnO is an n-type semiconductor, with a wide direct bandgap of 3.37 eV and a high exciton energy of 60 meV, which commonly crystallizes in a hexagonal structure known as wurtzite. In this way, ZnO is an ideal material for the development of various devices, including gas sensors, light-emitting diodes (LEDs), thin-film transistors (TFTs), and solar cells [
10,
11,
12,
13].
Spray pyrolysis is a highly effective and versatile method for synthesizing zinc oxide thin films, enabling precise control over their properties. This technique begins with the generation of a fine aerosol using an ultrasonic atomizer that creates a mist of precursor solution. A stream of gas then carries the aerosol to a deposition chamber, where thermal pyrolysis takes place on a preheated substrate. This heating process promotes the decomposition of the precursor material, leading to the formation and deposition of homogeneous, compact, and uniform ZnO thin films on the substrate surface. One of the primary advantages of spray pyrolysis is its cost-effectiveness, making it accessible for both research and industrial applications. The process operates at atmospheric pressure, which simplifies equipment requirements and enhances safety. Additionally, it can be performed at relatively low temperatures, thereby reducing energy consumption and enabling the use of temperature-sensitive substrates. Furthermore, spray pyrolysis is capable of efficiently covering large surface areas, making it suitable for various applications in electronics, optics, and sensors. This method provides a promising pathway for the scalable production of high-quality ZnO thin films tailored for specific optoelectronic applications [
14,
15].
Various techniques exist for depositing ZnO thin films, but achieving the desired properties for specific applications can be challenging. One method of modifying the material is through thermal treatments; however, this approach does not allow for control over the size of the synthesized particles. A more promising alternative is ion irradiation, which enables the manipulation of material properties by varying irradiation parameters such as energy, fluence, ion mass, and implantation angle. The irradiation of ZnO with ions of different energies and fluences is a controlled process typically performed at room temperature. Using accelerators, energy and fluence can be precisely tuned to generate specific defects in ZnO in order to modify its physical properties [
16].
Ion irradiation induces point defects, including Zn and O vacancies, as well as interstitials. For instance, Sarkar et al. [
17] observed clusters of Zn and O vacancies after irradiating ZnO with 1.2 MeV Ar
+ and 800 keV O
+ ions. They also noticed that the band-edge photoemission intensity decreases with increasing ion fluence. In contrast, emission at 2.4 eV (associated with oxygen vacancies) increases more with Ar
+ ions than with O
+ ions. Dee et al. [
18] reported that ZnO nanowires converted from a crystalline wurtzite structure to a disordered amorphous state after irradiation with 70 keV H
+ at 2 × 10
17 ions/cm
−2. Studies on nanosized ZnO also reveal lattice rearrangement. Wu et al. [
19] observed that upon irradiation of nanorods with 180 keV H
+ ions, the lattice constant
c contracts, and the crystallinity improves slightly, suggesting that electronic and thermal shock waves induce compressive stresses that rearrange the lattice. They also found a blue shift in the excitonic emission of ZnO nanorods after irradiation with H
+ at 180 keV, which is also attributed to compressive stresses that increase the band gap.
This study investigated the deposition of ZnO thin films on soda–lime glass using the spray pyrolysis technique at a temperature of 500 °C. The samples were implanted with 10 keV H+ and Ar+ ions at room temperature using a Colutron ion gun (Colutron Research Corporation, Boulder, CO, USA), with a fluence of 1 × 1014 ions/cm2. Subsequently, variations in the structural, morphological, and optical properties of the films were examined.
This article explores the effects of various irradiation conditions on morphological deformation and shaping characteristics, as well as the impact of different ion types on the optical, structural, and morphological properties of ZnO thin films.
2. Materials and Methods
Zinc oxide (ZnO) thin films were synthesized on a borosilicate glass substrate using the ultrasonic spray pyrolysis technique [
20]. The precursor solution was prepared by dissolving 10.97 g of zinc acetate in 250 mL of deionized water at 40 °C. To stabilize the solution and achieve complete dissolution of the salts, 50 mL of acetic acid was added to the solution, followed by 700 mL of methanol. Of this solution, 50 mL was atomized onto the substrate, which was heated to 500 °C. After obtaining the samples, one substrate was kept pristine, while the others were irradiated with H
+ and Ar
+ ions. This irradiation was conducted using a 10 keV energy and a fluence of 1 × 10
14 ions/cm
2, utilizing a Colutron-type ion gun at the Science Faculty of UNAM. The samples were analyzed both before and after irradiation to investigate the changes in the semiconductor’s structural, optical, and morphological properties. The structural properties of the films were analyzed using X-ray diffraction (XRD) with Cu K
α radiation on a Bruker D8 Advance diffractometer (Bruker AXS, Karlsruhe, Germany). The morphology and variations in particle size of the ZnO films were examined using Scanning Electron Microscopy (SEM) with a FEG JEOL JSM-7800 microscope (JEOL USA, Inc., Peabody, MA, USA). Additionally, the optical properties of the ZnO films were measured at room temperature using an Agilent HP 8453 UV-Vis spectrophotometer (Agilent, Santa Clara, CA, USA), which covers a spectral range of 300–1100 nm.
3. Results and Discussion
3.1. RBS Results
Once the ZnO film was deposited on the Borosilicate glass substrate, we determined the film thickness using the Rutherford Backscattering Spectrometry (RBS) technique [
21,
22]. The measurement was performed using the 3 MV Pelletron-type accelerator (NEC 9SDH-2, National Electrostatics Corporation, Middleton, WI, USA) at the Physics Institute of the National Autonomous University of Mexico (UNAM), with a 2.5 MeV alpha beam used as the projectile. Alpha particles collide with the sample and scatter at an angle of 167° before being detected by a surface barrier detector. A multichannel analyzer processes the analog signals produced by the system to create the Rutherford Backscattering spectrum. In this spectrum, the target element is identified by the energy of the backscattered particles resulting from specific reactions between the incident ions and the target element.
Figure 1 displays the RBS spectrum of the ZnO thin film, highlighting the elements that compose the material.
To calculate the thickness of the thin film, the FWHM of the Zn peak and the energy loss values of the alpha particles interacting with the Zn atoms were used. These values were obtained using the SRIM code [
23]. When conducting an RBS experiment, alpha particles lose energy in various ways depending on the type of atom they collide with and the depth at which the atom is located. By calculating the stopping power of the alpha particles for each element in the material, we can determine the thickness of the film using the following equation [
21]:
In this equation, X denotes the thickness of the zinc layer, ΔE represents the energy lost by alpha particles during their interaction with zinc atoms, and [ε] indicates the stopping power of the alpha particles in zinc. The stopping power is a crucial parameter in determining the thickness of the zinc film, and reflects how effectively the material absorbs the energy of the incoming alpha particles. As alpha particles penetrate the zinc layer, their energy diminishes due to interactions such as ionization and scattering. According to Equation (1), the thickness of the ZnO thin film is measured to be 93 ± 5 nm.
3.2. SRIM Simulations
The SRIM (Stopping and Range of Ions in Matter) code utilizes the Monte Carlo method to investigate how particles interact with matter [
23]. This approach relies on probabilistic processes, generating random numbers and running multiple simulations to explore various scenarios. By considering initial conditions, such as the type of projectile, its energy, angle of incidence, and the elemental composition of the material, SRIM can simulate the different processes that occur during the interaction between the projectile and the material. It provides reliable outputs, including the depth profile of implanted ions, the distribution of damage (vacancies per ion), energy loss profiles, and ionization processes.
Electron stopping power (
Se) and nuclear stopping power (
Sn) are the two fundamental mechanisms through which charged ions dissipate energy as they penetrate a solid material. These processes influence not only the depth of ion penetration but also the modifications of structural damage inflicted and the subsequent alterations to the physical properties of the irradiated materials. Electronic stopping power occurs when the incoming ion transfers energy to the electrons in the target material. This interaction with the electron cloud triggers a cascade of excitations and ionizations, which can potentially lead to the formation of electronic defects. In some instances, these processes may result in significant modifications in the material’s optical characteristics or electrical conductivity [
24].
On the other hand, nuclear stopping power results from elastic collisions between the ion and the nuclei of the target material. These energetic interactions induce atomic displacements within the crystal lattice, creating defects such as vacancies and interstitials, as well as cascading damage. The direct energy transfer between the ion and the target nucleus generates structural disorder, which may lead to a reduction in crystallite size or even lead to the amorphization of the material. Through these interactions, the material’s microstructure undergoes profound changes, influencing its overall performance and durability [
25].
As previously discussed, when incident ions traverse a material, they collide with the atoms in the matrix, resulting in a gradual loss of their energy. This energy transfer continues until the ions come to a stop within the material. As they collide, the recoiling atoms gain enough energy to induce further recoils, creating a dynamic cascade of collisions. These interactions can lead to either the ionization or excitation of surrounding atoms, leaving a trail of energized atoms in the ion’s path. Consequently, the temperature within this localized region increases rapidly, creating a narrow cylindrical zone known as a columnar defect or ion track. The intense transfer of energy results in the swift melting of the target material, generating a thermal spike that significantly raises the local temperature. This localized increase in temperature during irradiation has the potential to anneal the material’s defects [
19,
26,
27].
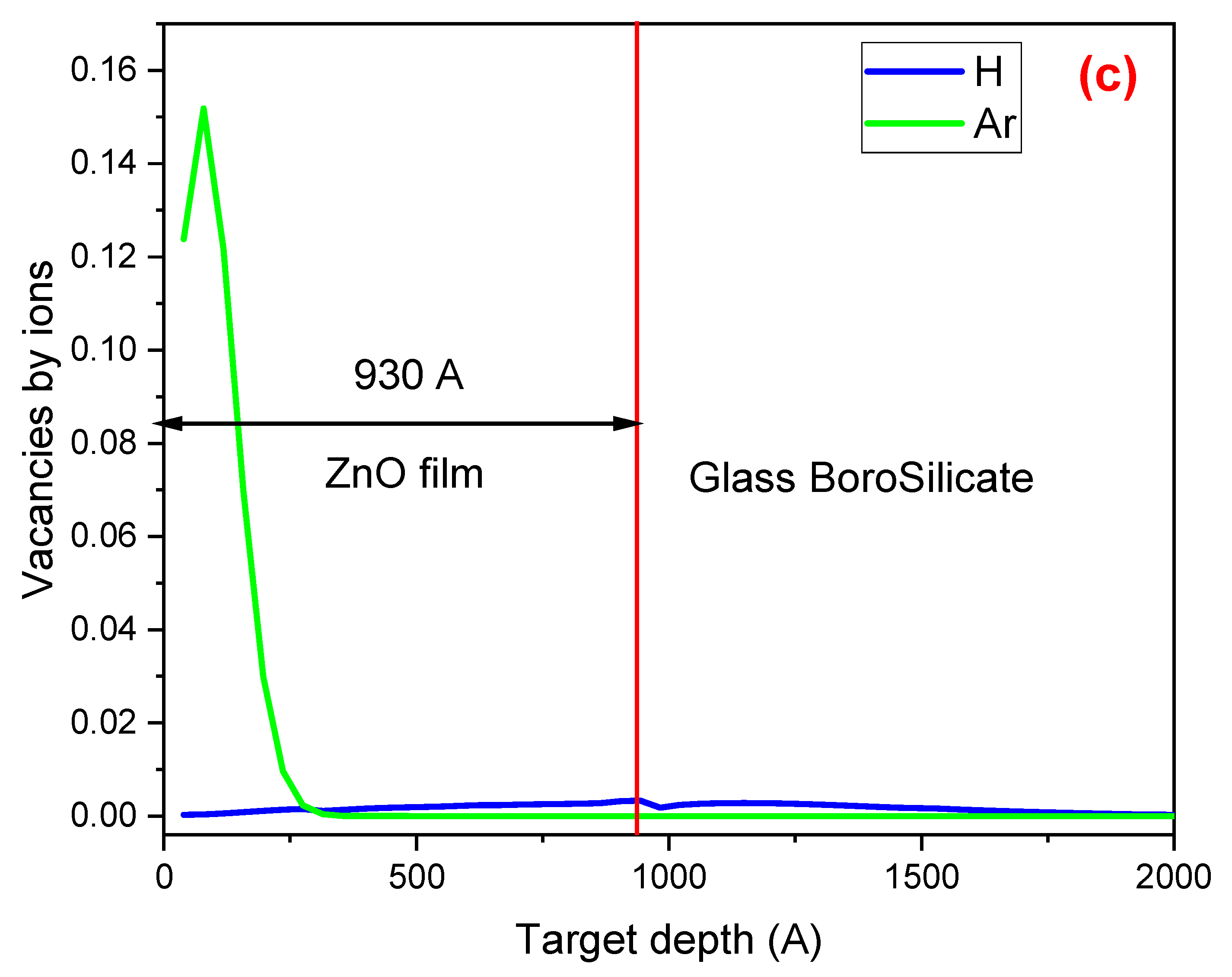
Figure 2 presents a comparison of the SRIM simulation results for (a) the concentration of H and Ar atoms in the ZnO thin film, (b) the electronic energy loss (ionization events) of H and Ar as they interact with the electrons of the atoms in the ZnO film, and (c) the nuclear energy loss (resulting in vacancies and defects) due to the interaction of H and Ar with the nuclei of the atoms comprising the film and the matrix. The vertical red line in the three figures indicates the boundary between the film (on the left side) and the substrate (on the right side). These results were obtained using the SRIM code with the following data: a 93 ± 5 nm ZnO film deposited on a borosilicate glass matrix and irradiated with H
+ and Ar
+ ions at an energy of 10 keV and a fluence of 1 × 10
14 ions/cm
2.
Figure 2a depicts the distribution of H (blue curve) and Ar (green curve) ions when they interact with the material composed of a 93 ± 5 nm ZnO layer and a glass borosilicate substrate. The distinct values of electronic and nuclear energy loss for H
+ and Ar
+ ions lead to their different behavior. We observed that some H
+ ions remain implanted in the film when they interact with Zn or O atoms, while the rest pass through the ZnO and stop in the substrate. In the case of Ar ion interaction with the material, the scene is different. Due to their higher mass, Ar ions lose more energy when interacting with the elements that compose the film and come to a complete stop within it at 300 Å. On the other hand, the H
+ ions pass through the film and stop inside the substrate at 2200 Å. The implications of these stopping points are significant, as they can influence the performance of materials in various applications.
Figure 2b illustrates the ionization events resulting from the electronic energy loss of H
+ and Ar
+ ions as they interact with the atoms that form the films and the substrate. The SRIM simulation indicates that the elements forming the ZnO film become highly ionized when irradiated with Ar
+ ions. In this case, ionization of the atoms forming the film is high at the surface and declines sharply at 200 Å, reaching nearly zero by 270 Å. On the other hand, the electronic energy loss of H
+ ions is distributed almost uniformly throughout the ZnO film, continuing the ionization process inside the substrate.
Figure 2c shows the defects created during irradiation with H
+ and Ar
+. The number of vacancies produced during Ar
+ irradiation ranges from the surface to 270 Å and is significantly higher than that produced during H
+ irradiation.
3.3. Optical Properties
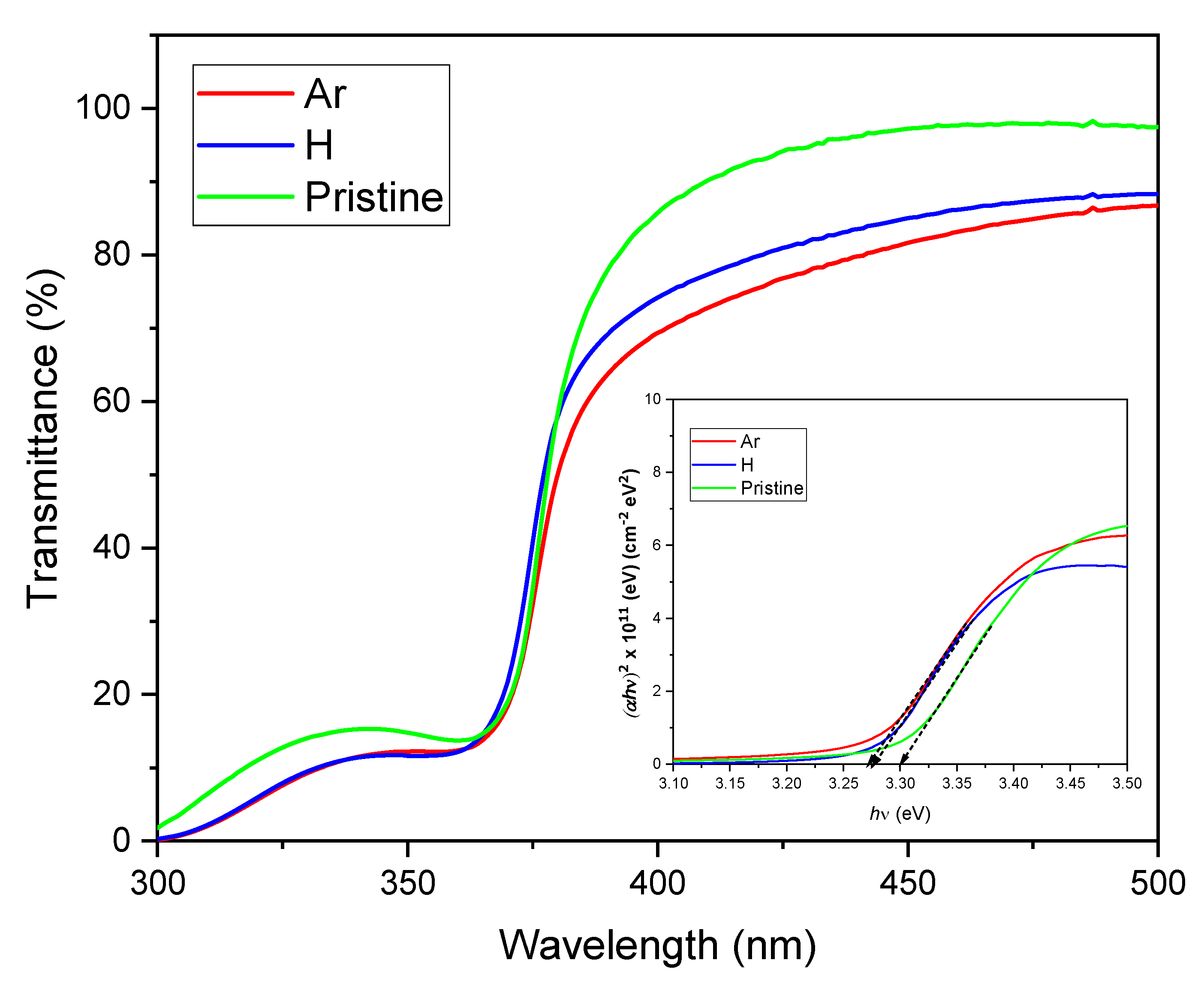
Figure 3 presents the transmittance measurements for a pristine ZnO sample as well as samples irradiated with H
+ and Ar
+ ions, covering the wavelength range of 300 to 1100 nm. The spectra reveal a decrease in transmittance in the UV region, particularly between 360 and 370 nm, which can be attributed to fundamental bandgap absorption. However, beyond 370 nm, the transmittance increases to between 80% and 95% in the visible range. The unirradiated sample exhibits the highest transmittance value with 95%. In contrast, the irradiated samples show a reduction in transmittance within the visible range, especially the Ar-irradiated material, which has a transmittance of 81%. The same behavior has been observed by Sarkar et al. [
17]. They attribute it to new defect levels that absorb light or to the increase in scattering due to changes in grain size, which results from ionization effects and defects created during irradiation. This observation also aligns with our SRIM simulations presented in
Figure 2a,b.
Based on the transmittance results of
Figure 3 and the Tauc relationship (Equation (2)), we can calculate the optical band gap value of the semiconductor [
28].
In Equation (2), α represents the absorption coefficient, hv denotes the photon energy, Eg is the optical band gap, and A refers to a constant related to the material’s refractive index.
The absorption coefficient is determined using the following equation, which connects transmittance (
T) and sample thickness (
x). The last variable was obtained through the RBS technique as outlined in Equation (1).
The optical band gap of the material can be determined by plotting (
αhν)
2 against the energy of the incident radiation, as illustrated in the inset of
Figure 3. The optical band gap energy is defined as the point at which the linear portion of the curve intersects the energy axis [
29].
The results presented in
Table 1 indicate that ion irradiation results in a slight decrease in the optical band gap. We propose that this reduction is due to the quantum confinement effect, which results from changes in crystallite size after irradiation. This interpretation is supported by the findings in
Table 2, which indicate that the crystallite size decreases post-irradiation. The decrease in the band gap can be attributed to both electronic energy loss and nuclear energy loss (see
Figure 2), which introduce defects within the band gap. Additionally, ion irradiation triggers strain relaxation within the crystalline structure. These changes in the crystalline lattice affect the material’s electronic properties, resulting in a decrease in the energy band gap. Research conducted by Abdel-Galil et al. [
30], Soubhik et al. [
31], Singh et al. [
32], and Khawal et al. [
33] supports these findings, demonstrating a consistent decrease in the band gap with ion irradiation.
3.4. Structural Properties
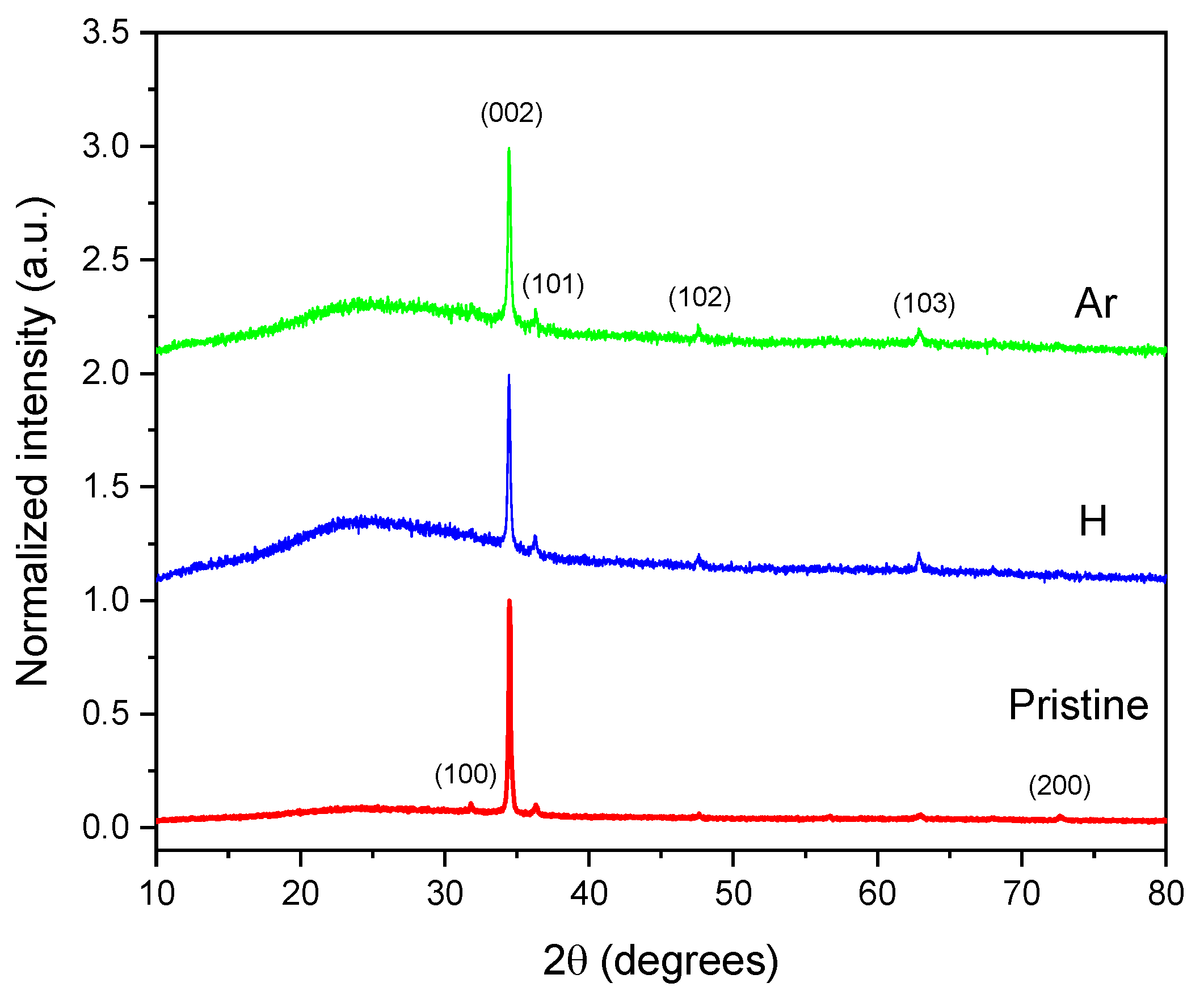
The crystalline structure of the ZnO thin films was thoroughly analyzed using X-ray diffraction (XRD), as illustrated in
Figure 4. The diffractogram reveals distinct diffraction peaks corresponding to the (100), (002), (101), (102), and (103) planes, characteristic of the hexagonal wurtzite phase of ZnO. The relative intensities and sharpness of these peaks indicate that the films are polycrystalline, with a noticeable preferential orientation along the c-axis.
Additionally, a slight protuberance can be seen at low diffraction angles in the irradiated samples. This feature may be due to structural defects induced by ion irradiation, possibly related to ionization or excitation events that occur during the interaction of radiation with the film.
It is important to note that irradiation does not significantly alter the positions of the diffraction peaks. Instead, it increases their intensities compared to the pristine sample, indicating an improvement in crystallinity, particularly evident in the (103) peak. This phenomenon can be explained by the thermal spike theory [
26] discussed in
Section 3.2. According to this theory, as ions travel through the material, the electronic energy loss excites electrons and ionizes the atoms that make up the material. This process creates a trail of excited atoms. The energy is then released as heat, resulting in a substantial increase in temperature. This rise in temperature leads to the annealing of the defects present in the crystal structure [
27]. Additionally, there is no shift observed in the position of the (002) diffraction peak, suggesting that the preferential growth orientation of the ZnO structures remains unchanged under ion irradiation. Consequently, irradiation plays a crucial role in creating favorable conditions for nucleation and crystal growth.
To investigate the effects of ion irradiation on ZnO thin films, we calculated the lattice parameters (
a and
c) of the hexagonal wurtzite structure, along with the average crystallite size (
D). These calculations were performed using Equation (4), which relates the interplanar spacing to the Miller indices, and Equation (5), known as the Scherrer formula [
34]. The values obtained from these calculations are summarized in
Table 2.
In the last equation, k represents the shape factor, which is 0.9. The symbol λ denotes the wavelength of Cu Kα radiation, while β indicates the full width at half maximum (FWHM) of the most intense peak in the XRD curves. Finally, θ refers to the Bragg angle.
According to JCPDS data (File 36-1451) [
35], the lattice constants for ZnO are
a = 3.2498 Å and
c = 5.2066 Å, with a c/a ratio of 1.60. The data presented in
Table 2 indicate slight variations in the lattice parameters
a and
c for the irradiated samples. The increase in the c/a ratio suggests an expansion of the crystalline structure due to the incorporation of H and Ar atoms into the hexagonal structure, as has been observed by Kim et al. [
36] and Bharati et al. [
37].
A decrease in the average crystallite size was observed in the samples that underwent ion irradiation, with a more pronounced reduction corresponding to an increase in the mass of the irradiated ions. Specifically, as the ion mass increased (from lighter ions such as H
+ to heavier ions like Ar
+), the extent of crystallite fragmentation became more significant. This observed trend can be attributed to the mechanisms of sputtering and collision cascades that occur during ion irradiation. These processes involve the ejection of atoms from the surface and the creation of localized damage within the crystalline structure, resulting in increased fragmentation of the crystallites. As heavier ions impact the material, they impart more energy, resulting in the formation of a greater number of defects and dislocations (see
Figure 2c). This, in turn, contributes to the reduction in crystallite size [
38,
39].
3.5. Morphological Properties
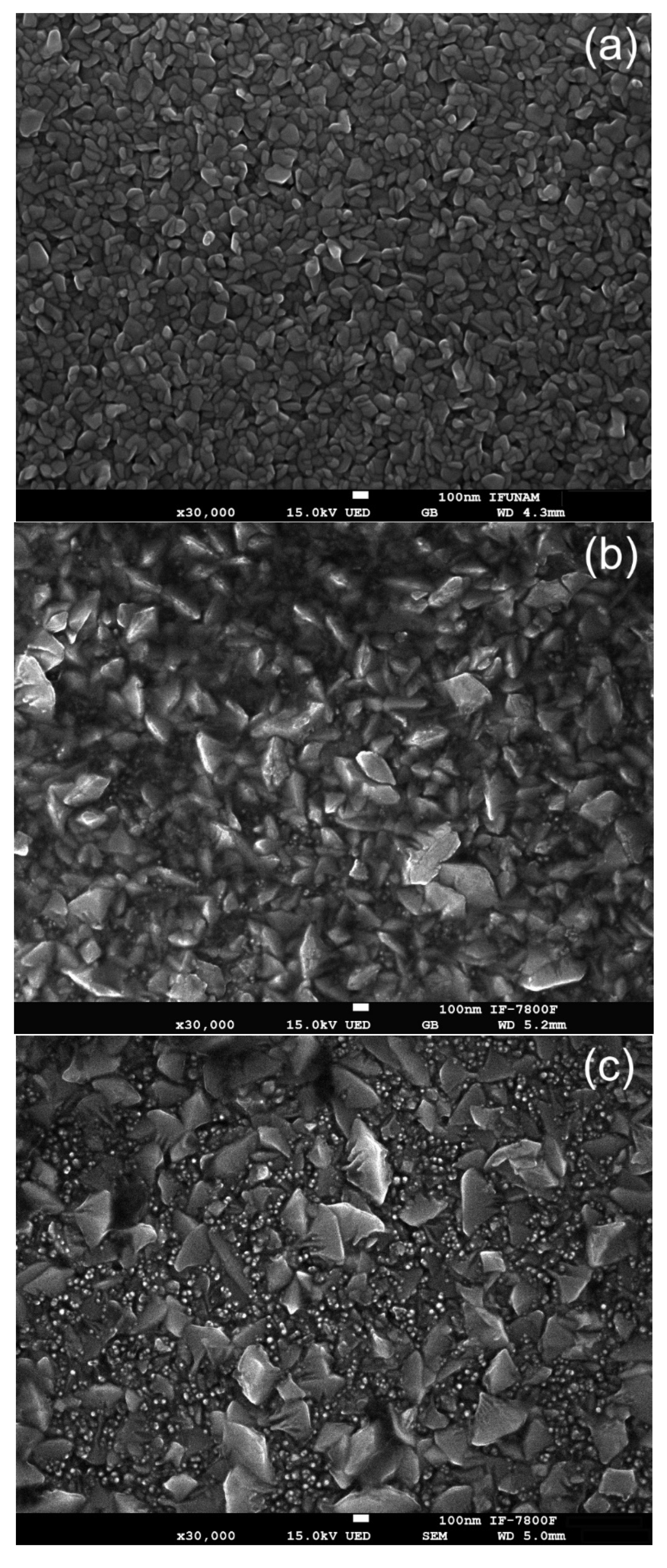
Figure 5 shows the SEM micrographs of three samples: (a) the pristine ZnO thin film, (b) the film irradiated with H
+ ions, and (c) the film irradiated with Ar
+ ions. The pristine ZnO thin films exhibit a granular and uniform surface morphology, characterized by a compact microstructure composed of grain sizes in the nanometer range. In contrast, the surfaces of the H
+ ion and Ar
+ ion irradiated films reveal significant morphological changes, highlighting the effects of ion irradiation on the material’s microstructure. The analysis of these modifications is crucial for understanding the implications of ion bombardment in enhancing or degrading the functional properties of ZnO thin films.
Image J 1.45e3 software was used to measure the grain size both before and after irradiation. The results show that the mean grain size increased from 125.7 nm in the pristine sample to 295.3 nm after exposure to H+ ions, and further to 329.8 nm following Ar+ ion irradiation. Notably, the sample irradiated with Ar+ ions exhibited a mixture of small and large particles, with the smaller particles averaging 23.7 nm in size.
After exposure to ion irradiation, the choice of ion mass, whether H
+ or Ar
+, plays a crucial role in inducing significant changes to the surface topography. Ion irradiation enhances crystallinity and alters surface morphology by reducing structural defects and increasing grain size [
40]. Additionally, it modifies the surface texture, leading to the formation of nanostructures such as flakes or tiny spheres, as illustrated in
Figure 5b,c. During this interaction, energy is transferred to the atoms within the lattice, promoting phenomena such as atomic displacements and surface diffusion. As the ions penetrate the material, they create a cascade of collisions that induce the reconfiguration of surface atoms, contributing to the diverse range of nano-features observed after irradiation. This detailed understanding of ion mass effects is crucial for tailoring material properties for specific applications in fields such as electronics, photonics, and advanced coatings [
32].
In
Section 3.2, we noted that both the electronic energy loss (
Figure 2b) and the nuclear energy loss (
Figure 2c) of H and Ar during their interaction with the film can lead to structural modifications. Depending on the conditions, one phenomenon may dominate over the other, or both phenomena may contribute to changes in grain size. The increase in grain size and the change in their shape in samples irradiated with H
+ and Ar
+ can be explained using
Figure 2b. This figure illustrates that the electronic energy loss of the H and Ar atoms during their interaction with the electrons in the ZnO film is substantial. Interactions can produce excited or ionized target atoms, leading to the formation of a hot electron gas around the ion’s trajectory. The interaction between this electron gas and the solid atoms can cause a local increase in temperature, which may result in localized melting and rapid resolidification of the material. The process of track formation, which enlarges and alters the shape of grains, can be explained using the inelastic thermal spike model [
41]. This thermal process occurs in electronic systems, where the energy deposited dissipates through electron-electron interactions and is subsequently transferred to the lattice system via electron-phonon coupling. The observed deformation can be understood as a relaxation effect within a hot ion track at temperatures approaching ~10
4 K over a timescale of ~10
−12 s [
41]. This involves a rise in temperature induced by the ion compared to the sample’s baseline temperature along the ion’s path. This temperature increase can facilitate the nucleation of particles within the film, leading to the clustering of grains into larger ones, which results in an overall increase in grain size, similar to processes that occur during thermal treatments.
The presence of tiny grains in the film irradiated with Ar
+ ions can be attributed to several factors [
42]. Heavier ions, such as Ar
+, typically cause more significant surface damage compared to lighter ions like H
+. This is due to their greater mass and momentum transfer capabilities, which result in more localized damage and lattice distortions. Such effects lead to the fragmentation of existing grains into smaller ones, a mechanism known as grain fragmentation or grain splitting, which occurs as a result of nuclear energy loss [
43]. As illustrated in
Figure 2c, the number of defects produced during Ar
+ irradiation is significantly higher than those generated during H
+ irradiation. Consequently, the sample irradiated with Ar
+ ions shows a considerable number of fragmented grains, as confirmed by the SEM technique in
Figure 5c. The reduction in average grain size can be explained as follows: when ions impact the material, they transfer energy to the lattice, creating localized stress and elevated temperatures within it. This energy transfer leads to lattice vibrations and the formation of point defects. When these areas of localized damage overlap, they cause the grains to fracture into smaller pieces.
4. Conclusions
ZnO thin films were synthesized on soda–lime glass substrates using the chemical spray pyrolysis technique, at a substrate temperature of 500 °C. After deposition, the samples were irradiated with H+ and Ar+ ions at an energy of 10 keV. This study focused on investigating the effects of irradiation on the optical, structural, and morphological properties of the ZnO thin films.
The optical properties of the samples, particularly beyond 370 nm, showed an increase in transmittance within the visible spectrum. The irradiated samples, especially those exposed to Ar+ irradiation, exhibited a reduction in transmittance across the entire visible range. This reduction can mainly be attributed to an increase in light scattering from the surface of the ZnO film, which is caused by ionization effects and the introduction of defects during the irradiation process. Regarding structural properties, a slight increase in the c/a ratio was observed, indicating an expansion of the crystalline structure due to the incorporation of H and Ar atoms into the hexagonal lattice. Additionally, a decrease in the average crystallite size was noted in the samples subjected to ion irradiation, which can be attributed to sputtering and collision cascades that occur during the ion irradiation process. Morphological characterization revealed a significant increase in average grain size and a change in their shape after irradiation. This behavior can be attributed to a thermal spike process, which involves a rapid temperature rise over a short period.
The ability to control and adjust the structure of ZnO films through ion irradiation offers valuable opportunities to enhance their functional properties. For instance, in optoelectronics, tailoring the structure of ZnO films can lead to improved light emission and detection. In gas sensing applications, it can enhance the sensitivity and selectivity of ZnO thin films. Additionally, in photocatalysis, it can improve the efficiency of ZnO films in degrading organic pollutants.
In conclusion, our results are consistent with previous studies on ion irradiation conducted at high energies. This suggests that it is feasible to reduce the energy used in ion irradiation while still tailoring the structural, morphological, and optical properties of ZnO thin films, without compromising their efficiency.