Application of Piper betle Leaf Extract as a Bioactive Additive in Eco-Friendly Antifouling Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of P. betle Extracts
2.2. Extracts Characterization
2.2.1. Fourier Transform Infrared (FTIR) Analysis
2.2.2. Determination of the Presence of P. betle Extract Compounds by Phytochemical Screening
2.2.3. Determination of the Total Phenolic Content (TPC) and the Total Flavonoid Content (TFC)
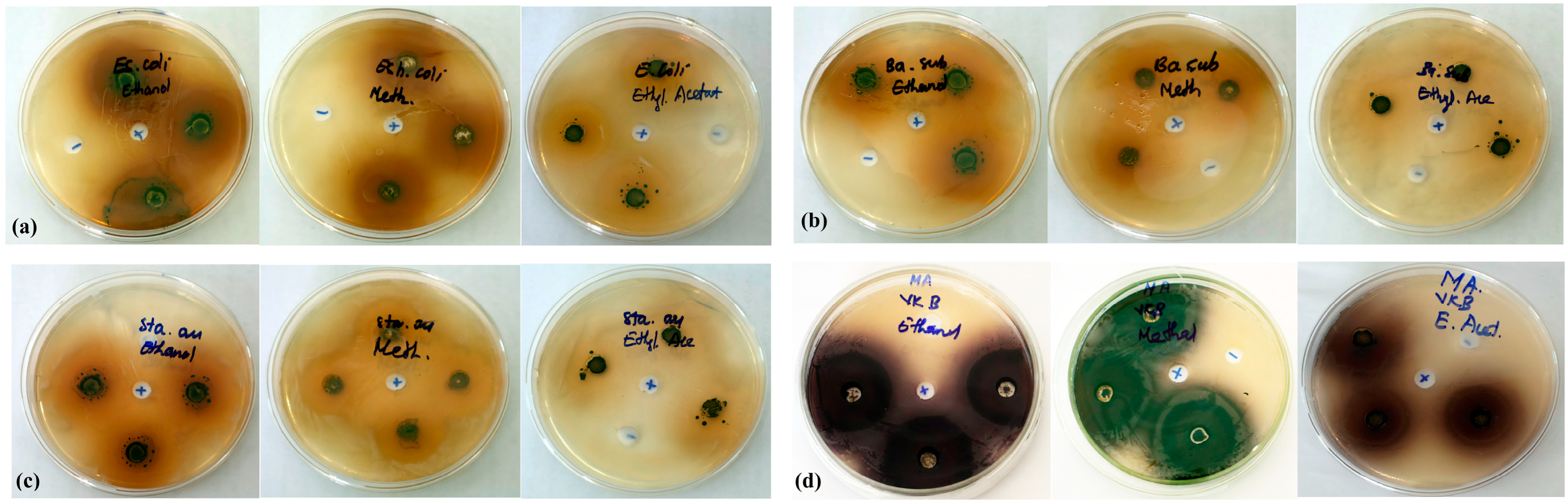
2.2.4. Antibacterial Activity Test
2.3. Preparation and Characterization of Coatings
2.3.1. Paint Preparation
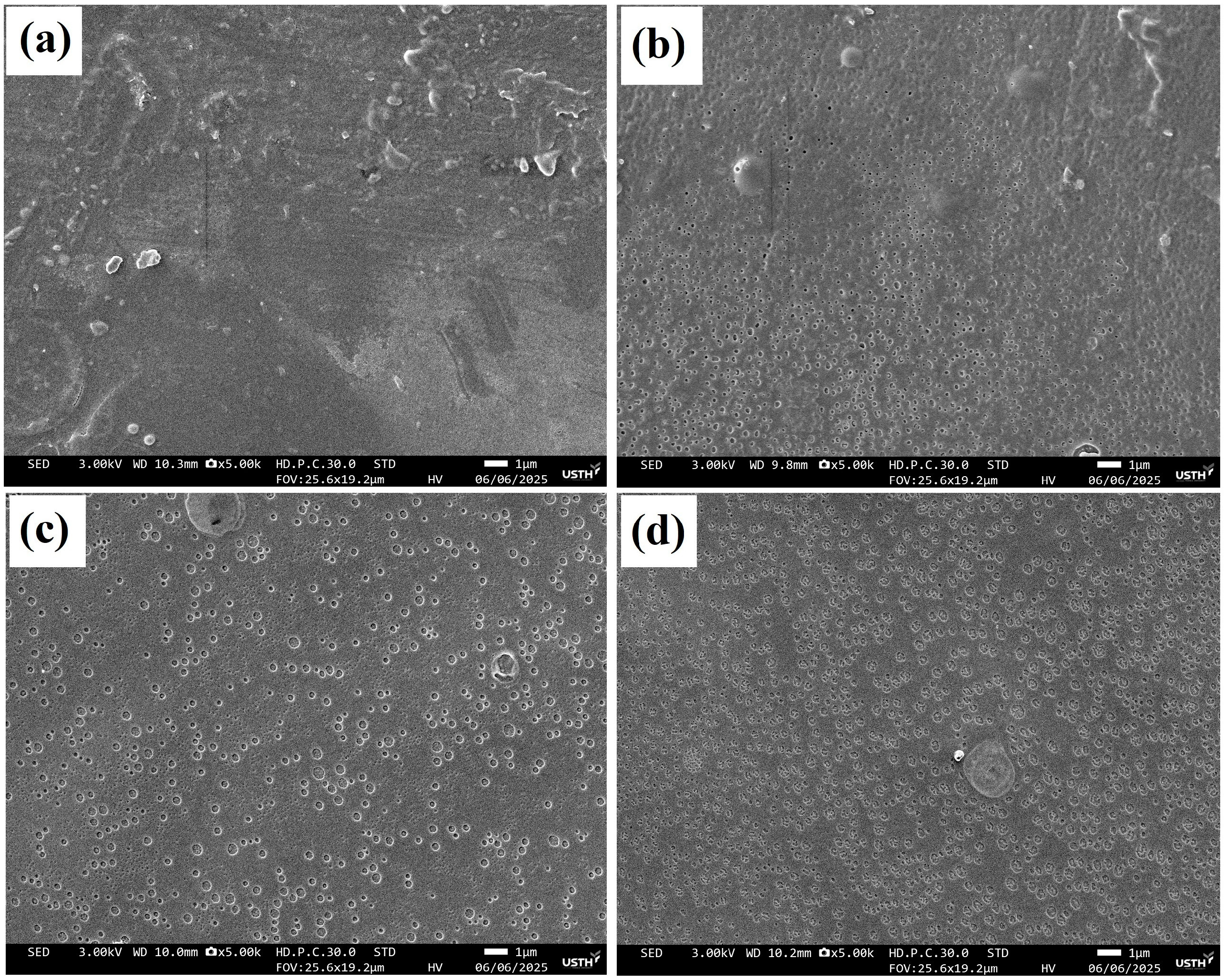
2.3.2. Surface Morphology Analysis
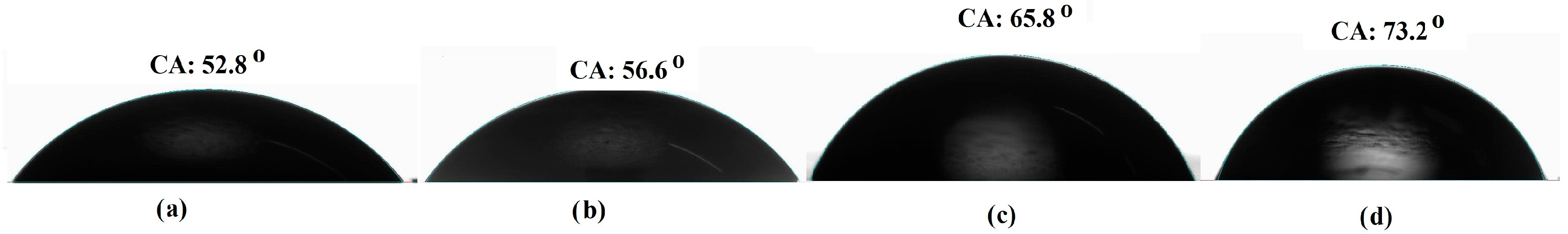
2.3.3. Surface Wettability Analysis
2.3.4. Determination of the Self-Polishing Rate (SPR) of Coatings
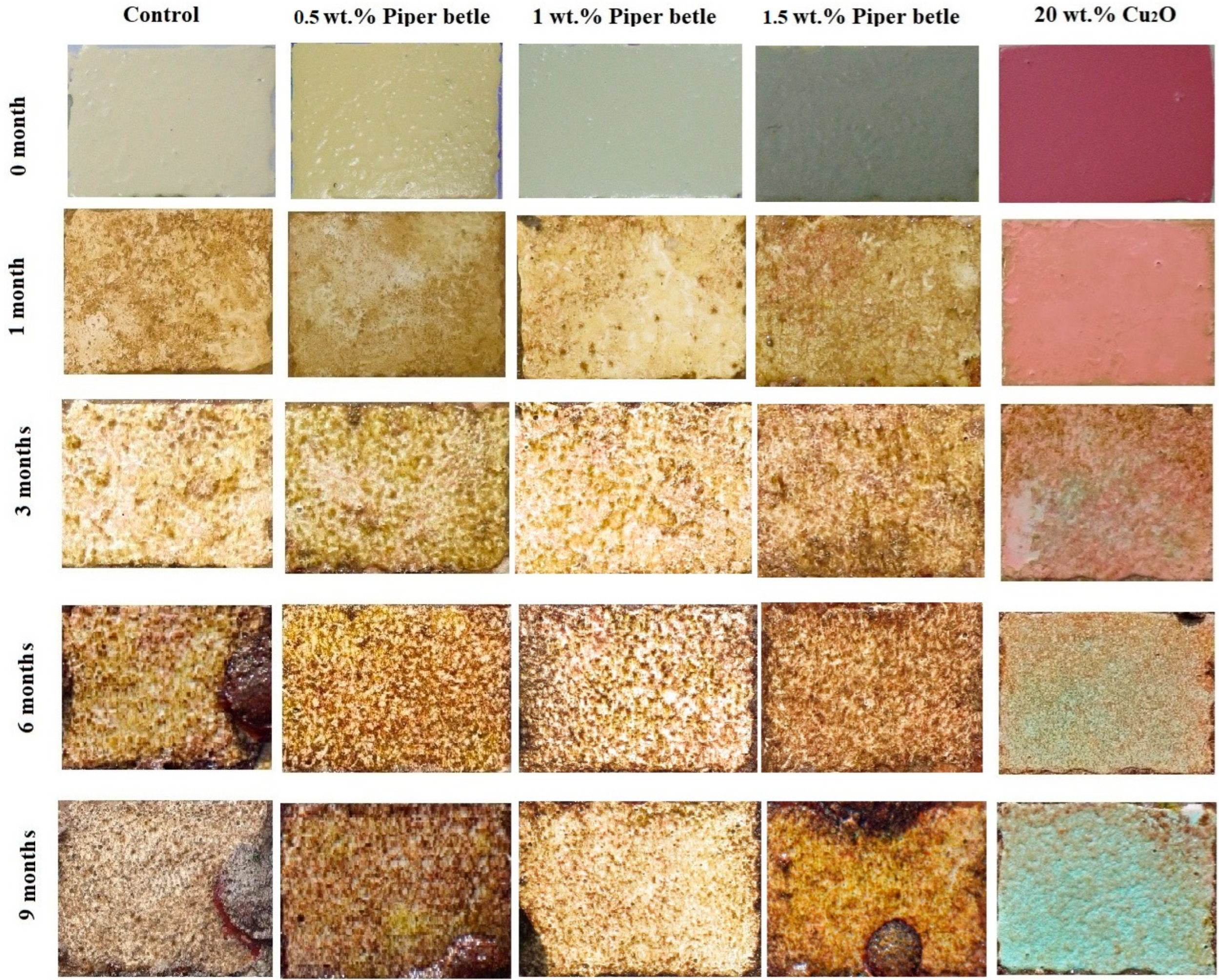
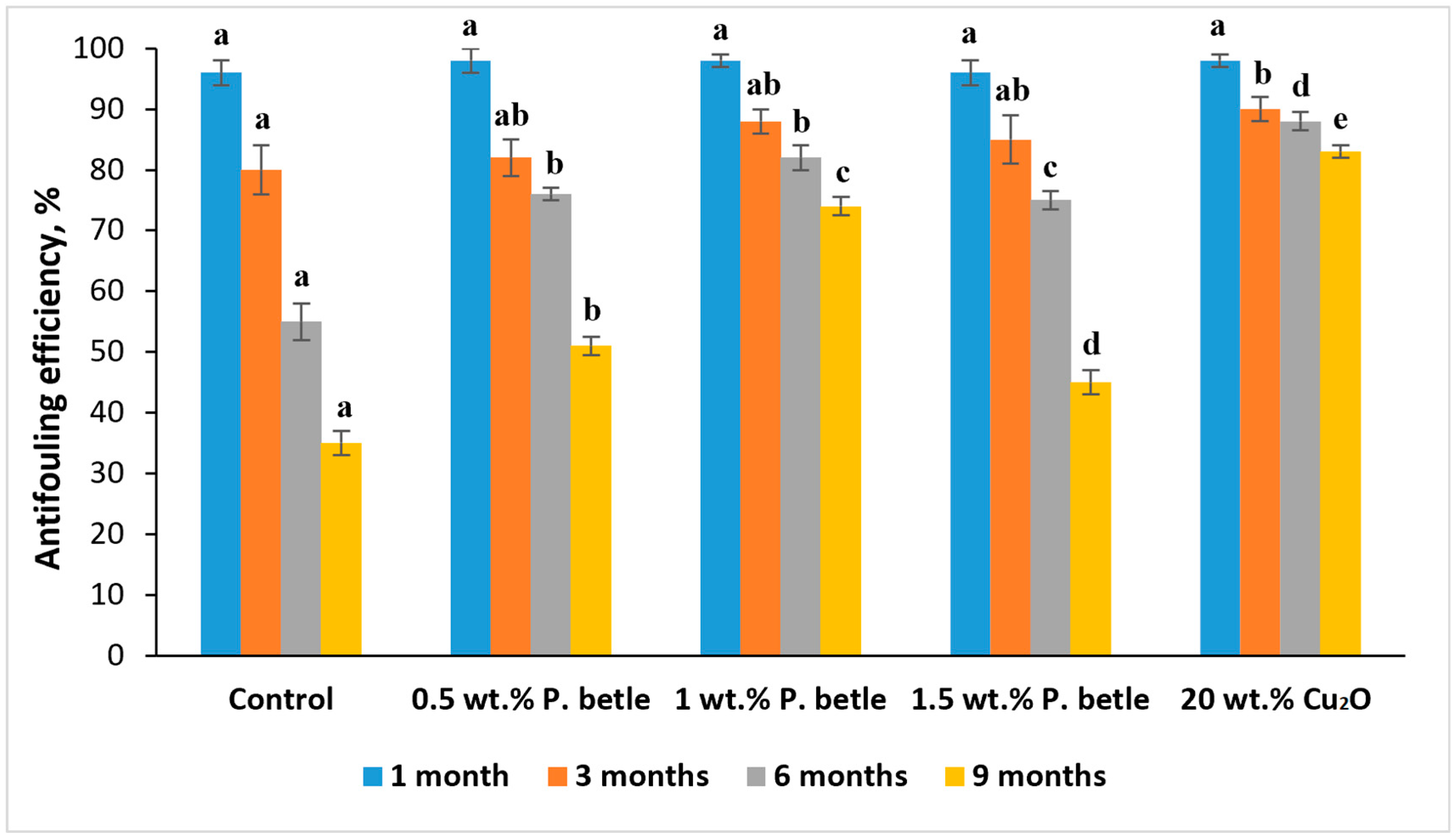
2.3.5. Field Test of Coatings
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, X.; Wei, H.; Zhou, W.; Rodriguez, P.E.D.S.; Lin, C.; Wang, L.; Zhang, Z. Advanced strategies for marine antifouling based on nanomaterial-enhanced functional PDMS coatings. Nano Mater. Sci. 2024, 6, 375–395. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Chen, S.; Liu, X.; Yu, Y.; Li, T.; Wan, Y.; Tang, N.; Liu, Y.; Gu, Y. Bioinspired marine antifouling coatings: Antifouling mechanisms, design strategies and application feasibility studies. Eur. Polym. J. 2023, 190, 111997. [Google Scholar] [CrossRef]
- Zang, X.; Ni, Y.; Wang, Q.; Cheng, Y.; Huang, J.; Cao, X.; Carmalt, C.J.; Lai, Y.; Kim, D.H.; Liu, Y.; et al. Non-toxic evolution: Advances in multifunctional antifouling coatings. Mater. Today 2024, 75, 210–243. [Google Scholar] [CrossRef]
- Srinivasan, M.; Swain, G.W. Managing the Use of Copper-Based Antifouling Paints. Environ. Manag. 2007, 39, 423–441. [Google Scholar] [CrossRef]
- Ding, T.; Xu, L.; Liu, X.; Ma, L.; Cui, Y.; Li, D.; Sun, X.; Gao, C. A Cu2O-based marine antifouling coating with controlled release of copper ion mediated by amphiphilic PLMA-b-PDMAEMA copolymers. Prog. Org. Coat. 2022, 170, 107003. [Google Scholar] [CrossRef]
- Ansanelli, G.; Manzo, S.; Parrella, L.; Massanisso, P.; Chiavarini, S.; Landa, G.D.; Ubaldi, C.; Cannarsa, S.; Cremisini, C. Antifouling biocides (Irgarol, Diuron and dichlofluanid) along the Italian Tyrrhenian coast: Temporal, seasonal and spatial threats. Reg. Stud. Mar. Sci. 2017, 16, 254–266. [Google Scholar] [CrossRef]
- Montemor, M.F.B. Smart Composite Coatings and Membranes: Transport, Structural, Environmental and Energy Applications, 1st ed.; Wood. Pub.: Cambridge, UK, 2016; 490p. [Google Scholar]
- Chen, L.; Duan, Y.; Cui, M.; Huang, R.; Su, R.; Qi, W.; He, Z. Biomimetic surface coatings for marine antifouling: Natural antifoulants, synthetic polymers and surface microtopography. Sci. Total. Environ. 2021, 766, 144469. [Google Scholar] [CrossRef]
- Carteau, D.; Vallée-Réhel, K.; Linossier, I.; Quiniou, F.; Davy, R.; Compère, C.; Delbury, M.; Faÿ, F. Development of environmentally friendly antifouling paints using biodegradable polymer and lower toxic substances. Prog. Org. Coat. 2014, 77, 485–493. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Zhang, G.; Qian, P. Environmentally Friendly Antifouling Coatings Based on Biodegradable Polymer and Natural Antifoulant. Sustain. Chem. Eng. 2017, 5, 6304–6309. [Google Scholar] [CrossRef]
- Sultana, H.; Chetia, A.; Saikia, A.; Khan, N. An Updated Review on Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Sch. Acad. J. Pharm. 2023, 12, 2320–4206. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants. 2021, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Etoh, H.; Kondoh, T.; Noda, R.; Singh, I.P.; Sekiwa, Y.; Morimitsu, K.; Kubota, K. Shogaols from Zingiber ocinale as Promising Antifouling Agents. Biosci. Biotechnol. Biochem. 2002, 66, 1748–1750. [Google Scholar] [CrossRef]
- Liu, H.; Chen, S.Y.; Guo, J.Y.; Su, P.; Qiu, Y.K.; Ke, C.H.; Feng, D.Q. Effective natural antifouling compounds from the plant Nerium oleander and testing. Int. Biodeterior. Biodegrad. 2018, 127, 170–177. [Google Scholar] [CrossRef]
- Qian, P.Y.; Xu, Y.; Fusetani, N. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling 2010, 26, 223–234. [Google Scholar] [CrossRef]
- An, X.; Yang, X.; Dong, W.; Ni, C.; Jiang, X. Synthesis and fouling resistance of capsaicin derivatives containing amide groups. Chem. Phys. Lett. 2022, 808, 139824. [Google Scholar] [CrossRef]
- Qin, Y.; Xue, J.; Wang, S.; Fan, Y.; Wang, L.; Xu, J.; Zhao, J. Capsaicin-based silicone antifouling coating with enhanced interlocking adhesion via SIPN. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 667, 132346. [Google Scholar] [CrossRef]
- Mohd Ramzi, M.; Rahman, N.I.A.; Rawi, N.N.; Bhubalan, K.; Ariffin, F.; Mazlan, N.W.; Saidin, J.; Danish-Daniel, M.; Siong, J.Y.F.; Bakar, K.; et al. Antifouling Potential of Diadema setosum and Sonneratia lanceolata Extracts for Marine Applications. J. Mar. Sci. Eng. 2023, 11, 602. [Google Scholar] [CrossRef]
- Madhumita, M.; Guha, P.; Nag, A.B. Processing and potential health benefits of betel leaf (Piper betle L.). In Herbal Medicine in India, 2nd ed.; Sen, S., Chakraborty, A., Eds.; Springer: Singapore, 2020; pp. 237–246. [Google Scholar]
- Syahidah, A.; Saad, C.R.; Hassan, M.D.; Rukayadi, Y.; Norazian, M.H.; Kamarudin, M.S. Phytochemical analysis, identification and quantification of antibacterial active compounds in betel leaves, piper betle methanolic extract. Pak. J. Biol. Sci. 2017, 20, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Biswal, S. Phytochemical analysis and a study on the antiestrogenic antifertility effect of leaves of Piper betel in female albino rat. Anc. Sci. Life. 2014, 34, 16. [Google Scholar] [CrossRef]
- Rashida, M.; Islam, I.; Haque, A.; Rahman, A.; Hossain, T.; Hamid, A. Antibacterial Activity of Polyaniline Coated Silver Nanoparticles Synthesized from Piper Betle Leaves Extract. Iran. J. Pharm. Res. 2016, 15, 591–597. [Google Scholar]
- Phumat, P.; Khongkhunthian, S.; Wanachantararak, P.; Okonogi, S. Potential of Piper Betle Extracts on Inhibition of Oral Pathogens. Drug Discov. 2017, 11, 307–331. [Google Scholar] [CrossRef]
- Sivareddy, B.; Reginald, B.A.; Sireesha, D.; Samatha, M.; Reddy, K.H.; Subrahamanyam, G. Antifungal Activity of Solvent Extracts of Piper Betle and Ocimum Sanctum Linn on Candida Albicans: An in Vitro Comparative Study. J. Oral Maxillofac. Pathol. 2019, 23, 333–337. [Google Scholar] [CrossRef]
- Valle, D.L.; Andrade, J.I.; Puzon, J.J.M.; Cabrera, E.C.; Rivera, W.L. Antibacterial Activities of Ethanol Extracts of Philippine Medicinal Plants against Multidrug-Resistant Bacteria. Asian Pac. J. Trop. Biomed. 2015, 5, 532–540. [Google Scholar] [CrossRef]
- Kurnia, D.; Hutabarat, G.S.; Windaryanti, D.; Herlina, T.; Herdiyati, Y.; Satari, M.H. Potential Allylpyrocatechol Derivatives as Antibacterial Agent Against Oral Pathogen of S. Sanguinis ATCC 10,556 and as Inhibitor of MurA Enzymes: In Vitro and in Silico Study. Drug Des. Dev. Ther. 2020, 14, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Pavarish, J.; Varomyalin, T.; Warapond, W.; Nuvee, P.; Monton, V.; Dennapa, S.S. Potential natural antimicrobial and antibiofilm properties of Piper betle L. against Staphylococcus pseudintermedius and methicillin-resistant strains. J. Ethnopharmacol. 2023, 317, 116820. [Google Scholar] [CrossRef] [PubMed]
- Kulnanan, P.; Chuprom, J.; Thomrongsuwannakij, T.; Romyasamit, C.; Sangkanu, S.; Manin, N.; Nissapatorn, V.; Pereira, M.d.L.; Wilairatana, P.; Kitpipit, W.; et al. Antibacterial, antibiofilm, and anti-adhesion activities of Piper betle leaf extract against Avian pathogenic Escherichia coli. Arch. Microbiol. 2022, 204, 49. [Google Scholar] [CrossRef]
- Jaradat, N.; Hussen, F.; Al Ali, A. Preliminary phytochemical screening, quantitative estimation of total flavonoids, total phenols and antioxidant activity of Ephedra alata Decne. J. Mater. Environ. Sci. 2015, 6, 1771–1778. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós Rosa, M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Wang, D.; Xu, J.; Yang, J.; Zhou, S. Preparation and synergistic antifouling effect of self-renewable coatings containing quaternary ammonium-functionalized SiO2 nanoparticles. J. Colloid Interface Sci. 2020, 563, 261–271. [Google Scholar] [CrossRef]
- Silva, E.R.; Ferreira, O.; Ramalho, P.A.; Azevedo, N.F.; Bayón, R.; Igartua, A.; Bordado, J.C.; Calhorda, M.J. Eco-friendly non-biocide-release coatings for marine biofouling prevention. Sci. Total. Environ. 2019, 650, 2499–2511. [Google Scholar] [CrossRef]
- Asharani, I.V.; Badmapriya, D. Dye degradation studies catalysed by green sythesized Iron oxide nanoparticles. Int. J. ChemTech Res. 2016, 9, 409–416. [Google Scholar]
- Kaveti, B.; Tan, L.; Sarnnia, K.T.S.; Baig, M. Antibacterial Activity of Piper Betel Leaves. Int. J. Pharm. Teach. Pract. 2011, 2, 129–132. [Google Scholar]
- Taukoorah, U.; Lall, N.; Mahomoodally, F. Piper betle L. (betel quid) shows bacteriostatic, additive, and synergistic antimicrobial action when combined with conventional antibiotics. S. Afr. J. Bot. 2016, 105, 133–140. [Google Scholar] [CrossRef]
- Periyanayagam, K.; Jagadeesan, M.; Kavimani, S.; Vetriselvan, T. Pharmacognostical and Phyto-physicochemical profile of the leaves of Piper betle L. var Pachaikodi (Piperaceae)-Valuable assessment of its quality. Asian Pac. J. Trop. Biomed. 2012, 2, 506–510. [Google Scholar] [CrossRef]
- Nayaka, N.M.D.M.W.; Sasadara, M.M.V.; Sanjaya, D.A.; Yuda, P.E.S.K.; Dewi, N.L.K.A.A.; Cahyaningsih, E.; Hartati, R. Piper betle L.: Recent review of antibacterial and antifungal properties, safety profiles, and commercial applications. Molecules 2021, 26, 2321. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Nguyen, T.T.; Nguyen, H.N.; Bui, T.Q.P. Simultaneous determination of active compounds in Piper betle Linn. leaf extract and effect of extracting solvents on bioactivity. Eng. Rep. 2020, 2, e12246. [Google Scholar] [CrossRef]
- Prakash, B.; Shukla, R.; Singh, P.; Kumar, A.; Mishra, P.K.; Dubey, N.K. Efficacy of Chemically Characterized Piper betle L. Essential Oil against Fungal and Aflatoxin Contamination of Some Edible Commodities and Its Antioxidant Activity. Int. J. Food Microbiol. 2010, 142, 114–119. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwar, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Tan, Y.P.; Chan, E.W.C. Antioxidant, antityrosinase and antibacterial properties of fresh and processed leaves of Anacardium occidentale and Piper betle. Food Biosci. 2014, 6, 17–23. [Google Scholar] [CrossRef]
- Md, S.A.; Byung, S.C. Characterization of chitosan-based packaging film incorporating betel leaves phenolic compounds recovered via subcritical water extraction. Food Biosci. 2025, 65, 106125. [Google Scholar] [CrossRef]
- Qian, P.Y.; Li, Z.; Xu, Y.; Li, Y.; Fusetani, N. Mini-review: Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101–122. [Google Scholar] [CrossRef]
- Wang, K.L.; Wu, Z.H.; Wang, Y.; Wang, C.Y.; Xu, Y. Mini-review: Antifouling natural products from marine microorganisms and their synthetic analogs. Mar. Drugs 2017, 15, 266. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Wu, C.H.; Qian, P.Y. Marine natural products as antifouling molecules—A mini-review (2014–2020). Biofouling 2020, 36, 1210–1226. [Google Scholar] [CrossRef]
- Sjögren, M.; Dahlström, M.; Göransson, U.; Jonsson, P.R.; Bohlin, L. Recruitment in the field of Balanus improvisus and Mytilus edulis in response to the antifouling cyclopeptides barettin and 8,9-dihydrobarettin from the marine sponge Geodia barretti. Biofouling 2004, 20, 291–297. [Google Scholar] [CrossRef]
- Stupak, M.E.; Garcia, M.T.; Perez, M.C. Non-toxic alternative compounds for marine antifouling paints. Int. Biodeter. Biodegrad. 2003, 52, 49–52. [Google Scholar] [CrossRef]
- Zmozinski, A.V.; Peres, R.S.; Brust, F.R.; Macedo, A.J.; Becker, E.M.; Napp, A.P.; de Brito, H.A.; Vainstein, M.H.; Schrank, A.; Ferreira, C.A. The effect of rue (Ruta graveolens) and ginger (Zingiber officinale) extracts as antifouling agents in silicone matrix coatings. J. Coat. Technol. Res. 2021, 18, 1013–1025. [Google Scholar] [CrossRef]
- Feng, D.Q.; He, J.; Chen, S.Y.; Su, P.; Ke, C.H.; Wang, W. The plant alkaloid camptothecin as a novel antifouling compound for marine paints: Laboratory bioassays and field trials. Mar. Biotechnol. 2018, 20, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Peres, R.S.; Armelin, E.; Alemán, C.; Ferreira, C.A. Modified tannin extracted from black wattle tree as an environmentally friendly antifouling pigment. Ind. Crop. Prod. 2015, 65, 506–514. [Google Scholar] [CrossRef]
- Noor, I.M.S.; Ferry, M.; Wan, N.W.B.; Jasnizat, S. Evaluation of tannin from Rhizophora apiculata as natural antifouling agents in epoxy paint for marine application. Prog. Org. Coat. 2015, 81, 125–131. [Google Scholar] [CrossRef]
- Pérez, M.; García, M.; Sánchez, M.; Stupak, M.; Mazzuca, M.; Palermo, J.A.; Blustein, G. Effect of secochiliolide acid isolated from the Patagonian shrub Nardophyllum bryoides as active component in antifouling paints. Int. Biodeter. Biodegrad. 2014, 89, 37–44. [Google Scholar] [CrossRef]
- Wang, X.; Yu, L.; Liu, Y.; Jiang, X. Synthesis and fouling resistance of capsaicin derivatives containing amide groups. Sci. Total Environ. 2020, 710, 136361. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Guo, Z.; Wang, P.; Wang, A. Preparation of Microcapsules Coating and the Study of Their Bionic Anti-Fouling Performance. Materials 2020, 13, 1669. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, Z.; Guo, Y.; Ju, Y.; Liu, Y.; Feng, R.; Xiong, C.; Ober, C.K.; Dong, L. Flexible Hydrophobic Antifouling Coating with Oriented Nanotopography and Nonleaking Capsaicin. ACS Appl. Mater. Interfaces 2018, 10, 9718–9726. [Google Scholar] [CrossRef] [PubMed]

| Phytochemical Group | Presence (+/−) 1 |
|---|---|
| Phenolics | + |
| Tannins | + |
| Proteins | + |
| Carbohydrates | + |
| Flavonoids | + |
| Component | Results |
|---|---|
| Total phenolic content, mg GAE/g dry weight | 260.3 ± 5.22 |
| Total flavonoid content, mg QE/g dry weight | 52.56 ± 1.26 |
| Extracts | Diameter of Inhibition Zone (mm) | |||
|---|---|---|---|---|
| E. coli | B. subtilis | S. aureus | Marine bacteria | |
| Positive control | 15.0 ± 0.5 | 11.4 ± 0.2 | 40.1 ± 0.6 | 42.3 ± 0.4 |
| Ethanol extract | 28.7 ± 0.5 | 27.0 ± 1.6 | 22.1 ± 0.6 | 35.1 ± 0.5 |
| Methanol extract | 23.3 ± 0.4 | 20.0 ± 0.2 | 18.1 ± 1.0 | 33.0 ± 0.5 |
| Ethyl acetate extract | 15.7 ± 1.8 | 12.3 ± 0.7 | 9.1 ± 1.0 | 20.0 ± 1.5 |
| Extract Concentration (wt.%) | Contact Angle (o) | Self-Polishing Rate (mg/cm2·day) |
|---|---|---|
| 0 | 52.8 ± 1.95 | 0.470 ± 0.012 |
| 0.5 | 56.6 ± 1.57 | 0.351 ± 0.011 |
| 1.0 | 65.8 ± 1.03 | 0.393 ± 0.015 |
| 1.5 | 73.2 ± 1.49 | 0.560 ± 0.018 |
| Source of Natural Compounds | Test Duration and Location | References |
|---|---|---|
| Barettein and 8,9-dehydrobarettein were isolated from a marine sponge | 2 months in seawater (near Sweden) | [47] |
| Tannins from plant extracts (chestnut, mimosa, quebracho) | 4 months (Atlantic Ocean, near Argentina) | [48] |
| Rue (Ruta graveolens) and ginger (Zingiber officinale) extracts | 6 months (Atlantic Ocean, near Brazil) | [49] |
| Plant alkaloid camptothecin from Camptotheca acuminata | 11 months (near Dalipuyu Islet in Xiamen Bay) | [50] |
| Plant extract of Nerium oleander | 1 month (Lingshui Bay, China) | [14] |
| Modified tannin derived from black acacia | 7 months (Mediterranean Sea, Spain) | [51] |
| Tannins from mangrove trees (Rhizophora apiculata) | 3 months (Chendering Port, Malaysia) | [52] |
| Plant extract of Nardophyllum bryoides | 1.5 months (Mar del Plata harbor, Argentina) | [53] |
| Capsaicin and its derivatives from Capsicum annuum | 3 months (Qingdao Bay, China) | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anh, N.D.; Linh, C.N.; Hiep, L.T.M.; Van Kien, D. Application of Piper betle Leaf Extract as a Bioactive Additive in Eco-Friendly Antifouling Coatings. Surfaces 2025, 8, 72. https://doi.org/10.3390/surfaces8040072
Anh ND, Linh CN, Hiep LTM, Van Kien D. Application of Piper betle Leaf Extract as a Bioactive Additive in Eco-Friendly Antifouling Coatings. Surfaces. 2025; 8(4):72. https://doi.org/10.3390/surfaces8040072
Chicago/Turabian StyleAnh, Nguyen Duc, Cao Nhat Linh, Le Thi My Hiep, and Dong Van Kien. 2025. "Application of Piper betle Leaf Extract as a Bioactive Additive in Eco-Friendly Antifouling Coatings" Surfaces 8, no. 4: 72. https://doi.org/10.3390/surfaces8040072
APA StyleAnh, N. D., Linh, C. N., Hiep, L. T. M., & Van Kien, D. (2025). Application of Piper betle Leaf Extract as a Bioactive Additive in Eco-Friendly Antifouling Coatings. Surfaces, 8(4), 72. https://doi.org/10.3390/surfaces8040072






