3.1. FTIR Analyses
Figure 1 presents the FTIR analyses of GS and MGS-10, respectively, before and after diclofenac biosorption. In the case of unmodified guava seeds (GS in
Figure 1), the main functional groups contained in this biosorbent material can be observed. Firstly, it should be considered that GS are a rich source of lignocellulosic material, which is why these seeds have a high content of total dietary fiber, with cellulose, hemicellulose, and lignin being the fundamental structural biopolymers of plant cell walls. Most of this fiber is insoluble, with an approximate content of 63.5 g/100 g [
34]. Guava seeds, rich in proteins, lipids, and functional molecules like pectin and phenolic compounds, offer long-term stability due to their chemically heterogeneous surface, which can serve as active sites for contaminant adsorption. For instance, the absorption band from 3600 to 3000 cm
−1, dominated by a single, composite band, is a hallmark of lignocellulosic materials, resulting from the overlapping stretching vibrations of hydroxyl groups involved in a complex hydrogen bond network. These OH groups are abundant in cellulose, hemicellulose, lignin, and residual adsorbed water molecules. Furthermore, the stretching vibrations of amine (N-H) groups from the seed’s protein fraction, particularly near 3300 cm
−1 (
Figure 1), also contribute to this band [
35]. The broadness of this peak indicates the presence of hydroxyl groups and the surface’s energetic heterogeneity, indicating a wide range of hydrogen bond strengths and environments due to the different locations of OH groups. These facts suggest the existence of multiple types of adsorption sites with a spectrum of affinities for hydrogen bonding, a factor with significant implications for the adsorption process. Moreover, the sharp and well-defined bands observed at ~2925 cm
−1 and ~2855 cm
−1 are characteristic of aliphatic carbon-hydrogen (C-H) stretching vibrations. The peak at approximately 2925 cm
−1 corresponds to the asymmetric stretching of methylene groups (-CH
2), while the peak near 2855 cm
−1 is attributed to the symmetric stretching of methylene and methyl groups (-CH
3) [
36]. These aliphatic groups are ubiquitous structural components of cellulose, hemicellulose, lignin, and fatty acid chains within the lipid content of the seed. The shoulder peak present at 1749 cm
−1 is of critical diagnostic importance. It is assigned to the C=O stretching vibration of carbonyl groups in ester linkages and non-ionized carboxylic acids [
37]. These linkages are also significant in the context of lignin’s role in the structural integrity and chemical reactivity of plant cell walls [
38]. Guava seed contains a band originating from acetyl and uronic ester groups in hemicellulose and galacturonic acid units of pectin, distinguishing them from cellulose. The intensity of this band can indicate the content of hemicellulose and pectin, revealing carboxyl and ester functional groups on the biosorbent surface, which is crucial for pH-dependent adsorption. The absorption band of 1639 cm
−1 is attributed to the C=C stretching vibrations in the aromatic rings of lignin, a compound composed of phenylpropane units. These aromatic rings contribute to the absorption band and may also reflect C=C stretching in aliphatic chains, as well as in lignin and hemicellulose structures, in lignocellulosic materials [
39,
40]. The peak at 1554 cm
−1 is also associated with the aromatic skeletal vibrations of lignin. The region around 1460–1370 cm
−1 contains a series of overlapping peaks of medium intensity. These are primarily due to various C-H bending (deformation) vibrations, including scissoring and wagging modes of -CH
2 and -CH
3 groups within the lignocellulosic and lipid structures [
41] Vibrations of the polysaccharide framework dominate the fingerprint region (1300–900 cm
−1) and are thus crucial for characterizing lignocellulosic materials. This region is characterized by strong C-O stretching vibrations from alcohols and ethers, as well as C-C stretching vibrations along the pyranose ring structures of cellulose and hemicellulose [
41]. The most prominent feature is the intense, broad band centered around 1030–1050 cm
−1, which is a classic signature of C-O stretching in primary and secondary alcohols, as well as C-O-C stretching of ether linkages, collectively representing the carbohydrate backbone [
37]. The bands near 601–667 cm
−1 are linked to the out-of-plane bending vibrations of aromatic C-H bonds, which are common in lignin’s structure [
42].
To analyze the changes in the spectrum following adsorption (
Figure 1), it is essential first to understand the molecular structure of the adsorbate, diclofenac. Diclofenac (molecular formula C
14H
11Cl
2NO
2) is an organic compound distinguished by multiple significant functional groups that exhibit activity in the infrared spectrum [
43]. These include a carboxylic acid (-COOH) group attached to a phenylacetic acid core, a secondary amine (-NH-) group that bridges the two aromatic rings, two aromatic (phenyl) rings, and two chlorine atoms attached to one of the aromatic rings, creating a dichlorophenyl moiety. The acidity of the carboxylic acid group, with a pKa value of approximately 4.15 [
44], significantly influences its speciation. At pH values below 4.15, the molecule is predominantly in its neutral, protonated form (R-COOH), while at pH values above 4.15, it deprotonates to become an anion, with the carboxyl group existing as a carboxylate (R-COO
−). Accordingly, the FTIR spectrum of the diclofenac-loaded guava seed (GSC), shown as the red line in
Figure 1, exhibits significant changes compared to the raw GS spectrum. These changes include the appearance of new peaks characteristic of diclofenac and shifts in the bands of the GS biosorbent. For instance, the C=C band in the GS spectra shifted to 1506 cm
−1. This shift suggests that the group associated with lignin content plays a significant role in the adsorption of diclofenac (DCF) in GS. This finding indicates that lignin’s complex aromatic structure provides multiple sites for interaction with diclofenac, thereby facilitating its adsorption, in addition to the C=C stretching of the aromatic rings of the diclofenac itself. Pure diclofenac sodium is known to exhibit a characteristic C=C ring stretching peak at 1557 cm
−1 [
45]. The intensity of these new diclofenac peaks completely overwhelms and masks the original, weaker composite band from the guava seed’s protein and lignin at ~1558 cm
−1. This is why the original peak appears to “disappear.” In summary, the phenomenon is best described not as a simple shift but as the masking of the original biosorbent’s C=C (lignin) and Amide II (protein) bands by the new, strong C=C and COO
− bands of diclofenac, accompanied by the emergence of a second, distinct C=C band from diclofenac at a lower wavenumber (~1506 cm
−1). Moreover, after diclofenac was adsorbed (as shown in the GSC spectra in
Figure 1), the presence of C–Cl bonding was confirmed in the 600–800 cm
−1 region. This range is well-known for indicating carbon–chlorine (C–Cl) stretching vibrations. Notable peaks were observed at approximately 774 cm
−1 and 730 cm
−1. These bands are consistent with C–Cl functional groups as reported in the literature across a range of chlorinated organic compounds [
46,
47]. Additionally, peaks in the 1096–1089 cm
−1 range, typically associated with chlorobenzenes, were also observed, further supporting the presence of aromatic C–Cl stretching [
48]. The presence of these bands in the FTIR spectrum indicates that the chlorinated aromatic structure of diclofenac remains intact post-adsorption, making these bands reliable diagnostic markers for monitoring such interactions. In the GSC spectrum, a small band at 1687 cm
−1 is also noted, which is associated with the C=O group, as mentioned above. This new band indicates that this group also plays a significant role in the adsorption of DCF, as these groups can form hydrogen bonds and other interactions with diclofenac molecules, thereby stabilizing the drug within the material matrix [
49,
50]. The presence of these functional groups is fundamental for the effective loading and controlled release of diclofenac, as they provide active sites for binding and interaction with the DCF [
51].
Finally, regarding the changes in the band associated with OH and NH (from 3580 to 3250 cm
−1), the disappearance of the band related to NH seems to be observed; however, it appears to be rather a significant change in the shape and position of the entire broad absorption band that dominates this region. A composite signal, centered around 3400 cm
−1, is observed in the spectrum of the diclofenac-loaded seed. After DCF adsorption, the entire broadband shifts to a lower wavenumber and alters shape, indicating hydrogen bonding as a primary adsorption mechanism, as evidenced by the modified wavenumber. The N-H group of diclofenac (a secondary amino group -NH-), whose stretching vibration is located in this same region, typically appears between 3325 cm
−1 and 3387 cm
−1 [
52]. Therefore, when diclofenac adsorbs, its own N-H signal is added to this complex spectral region. This key event involves the formation of new hydrogen bonds between diclofenac molecules and functional groups on the surface of the guava seed. Diclofenac’s electronegative atoms, including oxygen and nitrogen, act as hydrogen bond acceptors by interacting with hydroxyl and amide groups on the biosorbent surface. Conversely, the amine group’s hydrogen atom can donate hydrogen bonds by interacting with oxygen atoms on the biosorbent surface [
44,
53]. The formation of new hydrogen bonds between the adsorbate and the biosorbent disrupts and reorganizes the original hydrogen bonding network of the biosorbent. This alteration changes the vibrational energy of the O-H and N-H bonds involved, causing the entire broad peak to shift and change shape. In summary, the observed shift in the N-H region is not a disappearance but a perturbation caused by the formation of hydrogen bonds between diclofenac and the guava seed surface. These facts confirm that the amine groups of the native proteins in the biosorbent and the diclofenac molecule, along with the abundant hydroxyl groups, are directly involved in the binding process, providing substantial evidence in support of a multimodal adsorption mechanism.
On the other hand,
Figure 1 shows MGS-10 spectra before (MGS-10) and after (MGSC-10) DCF adsorption. The MGS-2 spectra exhibited similar spectral behavior to the MGS-10 sorbent, with characteristic peaks and shifts upon DCF binding; these spectra are presented in
Figure S1. New bands in the MGS-10 material, compared with the GS biosorbent, appeared around 1490 to 1550 cm
−1; this region contains a key diagnostic peak for CTAB. It corresponds to the asymmetric and symmetric C-H bending (scissoring) vibrations of the methyl groups directly bonded to the positively charged nitrogen atom (H
3C-N
+). It has also been associated with N-H bending vibrations. The presence and intensity of absorption in this specific region provide strong evidence of the surfactant’s headgroup adsorbed onto the guava seeds’ surface [
54,
55]. The weaker bands in the MGS-10 spectrum around 1026 cm
−1 and 1057 cm
−1, in the context of quaternary ammonium cationic headgroups, are likely associated with specific molecular vibrations, particularly C-N stretching vibrations within the headgroup. These bands correspond to symmetric and asymmetric stretching vibrations of the C–N bonds, which are sensitive to the conformation of the O–C–C–N
+ backbone [
56]. Also, a slight shift and a change in the shape of the band at 2924 cm
−1 were detected. This intense, sharp peak is a composite signal arising from the asymmetric stretching of -CH
2 groups. It includes contributions from the aliphatic chains in the seed’s native lipids and polysaccharides. Still, its higher intensity and sharpness are characteristic of the long, well-ordered hexadecyl tail of the CTAB molecule, confirming successful modification.
The CTAB modifier and the vibrational signatures of the guava seed matrix combine to create the complex MGSC-10 spectrum (green line in
Figure 1), which reveals the chemical state of the biosorbent after it has contacted the DCF solution. This spectrum, which highlights new peaks and shifts in existing ones, is crucial for understanding the adsorption mechanism. For instance, a new shoulder or weak peak emerging around 1273 cm
−1 corresponds to the C-N stretching vibration of the secondary aromatic amine group in the diclofenac molecule [
52]. Additionally, specific band shifts or changes are also observed in the MGSC-10 spectrum after DCF adsorption (
Figure 1). The most direct evidence of the interaction between these two oppositely charged groups is the perturbation of the vibrational band of the CTAB headgroup, since, when comparing the MGS-10 (blue line) and MGSC-10 (green line) spectra, a notable shift is observed in the region around 1470 cm
−1. Upon DFC adsorption, this peak, which contains the signature of the H
3C-N
+ group, appears to shift slightly and become part of a broader and more complex absorption structure. This perturbation occurs because the short-range electrostatic pairing of the positively charged CTAB headgroup with the negatively charged carboxylate group of diclofenac alters the local chemical and electronic environment of the H
3C-N
+ bond. The electrostatic environment alters the vibrational frequency of MGSC-10, resulting in a shift in the absorption band shape and subsequent alterations due to DCF adsorption into the GS structural matrix, as previously discussed. Based on the above, the modification of the GS biosorbent with CTAB leads to significant changes in the overall adsorption mechanism of DCF.
3.2. SEM-EDS Analyses
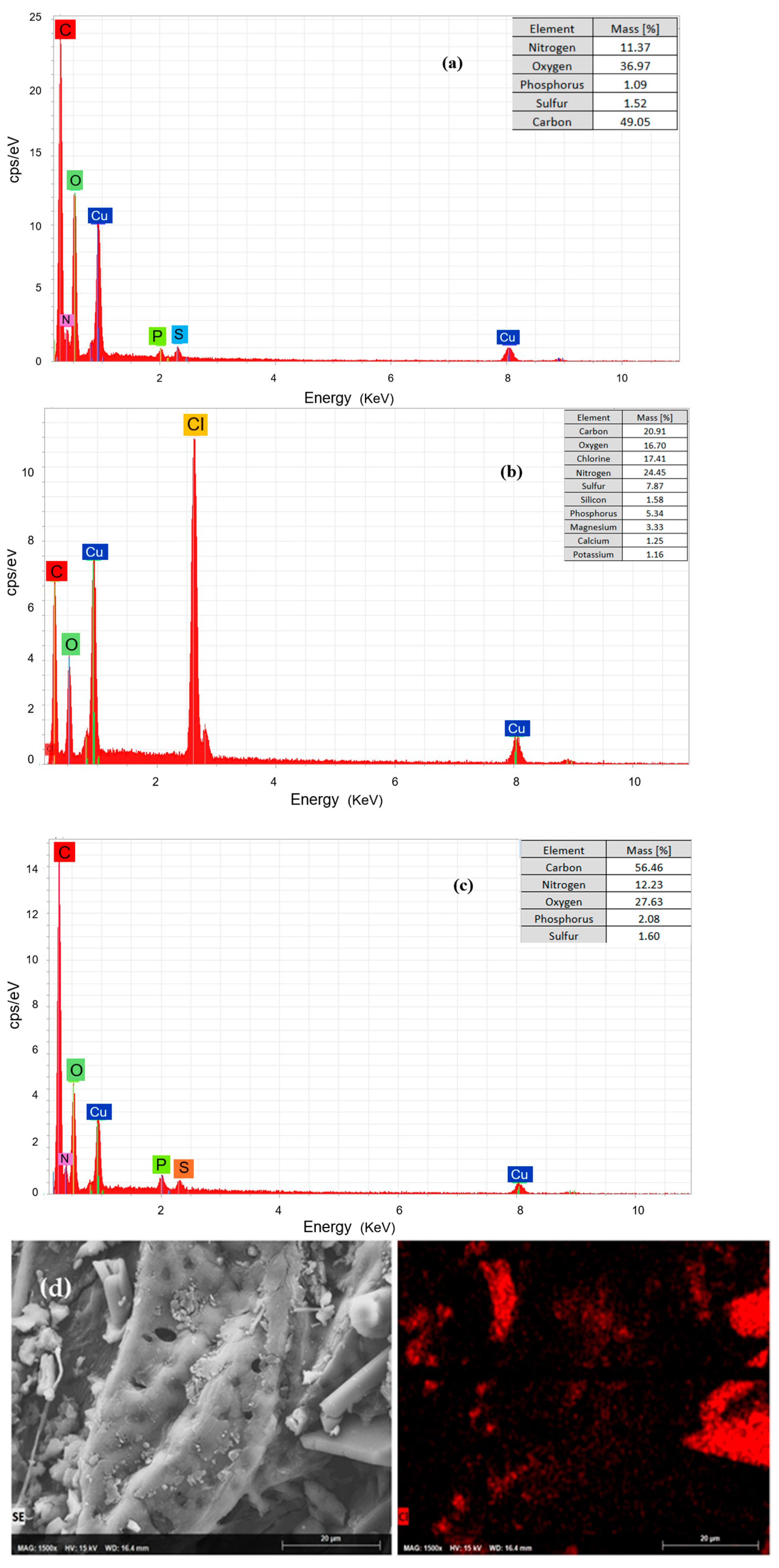
Figure 2 and
Figure 3 show the SEM micrographs and EDS analyses, respectively, of the biosorbents under study. In the case of SEM micrographs for GS material, images labeled as
Figure 3a–c provide a complete visual assessment of the surface morphology of the GS biosorbent. These micrographs reveal a highly irregular, heterogeneous structure, a characteristic trait of lignocellulosic biosorbents derived from agricultural residues. At low magnification (
Figure 2a), the GS surface appears coarse, rugged, and heterogeneous, with notable fibrous and flaky structures. The fibrous structures in GS cell walls are due to cellulose polymer chains, while the amorphous binding regions and ridges are due to the complex lignin network. Similar structures have been observed in SEM studies of biosorbents such as date pits, orange peels, and almond shells, all of which share a lignocellulosic origin [
57]. Besides, these rough surfaces suggest the presence of a substantial number of potential sorption sites for interaction with adsorbates, particularly for DCF. Intermediate magnification (
Figure 2b) reveals elongated voids, cracks, and longitudinal channels traversing the surface. These characteristics are indicative of mesoporous and macroporous features, which are beneficial for the transport and diffusion of larger organic molecules, such as DCF. The random distribution of these channels suggests a hierarchical pore network; such channeling features facilitate increased surface contact and accessibility of adsorbates [
58]. Moreover, in
Figure 2c, a highly porous, cavitated surface with a distinct “honeycombed” or sponge-like architecture can be observed. The structure is composed of a network of large, interconnected cavities. The SEM micrographs provide an illustrative, qualitative view of the surface porosity. In
Figure 2c, for example, macropores with diameters in the approximate range of 1.0 to 9.0 µm can be observed, which confirms the material has a macroporous structure. This observation is consistent with literature reports [
59], which confirm that guava seeds possess a hierarchical pore structure, containing both macropores and a significant network of smaller, internal mesopores. The large macropores observed here serve as the primary transport channels for DCF molecules, while the underlying mesoporous network, though not resolvable by our SEM, provides the high surface area necessary for the active adsorption sites. The presence of such “large” pores is new evidence of the diverse pore size distribution of the GS biosorbent.
The pore architecture visible in the SEM micrographs plays a crucial role in determining the adsorption performance of GS. Although SEM does not quantify pore sizes with high precision, the visible mesoporous and macroporous structures, combined with literature reports indicating the presence of some micropores in GS, suggest that this biosorbent contains interconnected porosity conducive to sorption phenomena. In this case, DCF has a molecular size of approximately 0.6–1.2 nm and a log Kow of 4.51, indicating moderate hydrophobicity [
60,
61]. The adsorption of such molecules generally benefits from mesoporous materials, where pore sizes exceed the molecular size of the adsorbate, allowing for efficient transport and access to inner surfaces [
62]. Furthermore, the rough and heterogeneous morphology could improve adsorption via hydrogen bonding between hydroxyl/carboxyl groups on GS and polar groups on DCF, π-π interactions due to aromatic rings present in both lignin residues and the DCF molecule, and electrostatic interactions, particularly at pH values near or above the pKa of diclofenac (4.15), when it exists predominantly in an anionic form. This is in agreement with the facts discussed in the FTIR spectra. Therefore, GS’s morphology, as evident in
Figure 3a–c, positions it as a promising biosorbent for removing diclofenac from aqueous systems.
EDS is a technique used to examine the elemental composition of a material’s surface, providing a qualitative and semi-quantitative profile of elements present. In this study, EDS was performed on the biosorbents before and after surfactant modification, as well as after DCF sorption experiments, to verify the baseline composition of the guava seed biosorbent, confirm the success of surfactant modification, and track pollutant adsorption. The results are shown in
Figure 3, displaying the EDS spectra of GS (
Figure 3a), DCF-loaded GS (GSC,
Figure 3b), MGS-10 (
Figure 3c), and the chlorine mapping of a DCF-loaded MGS-10 sample (
Figure 3d). In addition to the major C and O signals, smaller peaks corresponding to mineral elements are observed (
Figure 3a). The presence of elements such as Calcium (Ca), Potassium (K), and Magnesium (Mg) is typical for plant-based materials, as these are essential macronutrients accumulated during growth [
63]. The detection of a distinct Ca peak is particularly noteworthy, as it corroborates literature reports on the presence of crystalline calcium carbonate within the guava seed matrix, which may influence surface properties and reactivity [
63]. This baseline spectrum serves as the essential control against which all subsequent modifications and adsorption experiments are compared.
Similarly, the EDS spectrum of the surfactant-modified guava seeds (MGS-10), shown in
Figure 3c, provides compelling evidence of a successful surface functionalization with CTAB. The most significant change, compared to the GS spectrum, is an increase in the relative intensity of the carbon peak. This is the expected outcome of coating the lignocellulosic substrate with the highly carbon-rich CTAB molecule (C
19H
42BrN). The 19 carbon atoms of the surfactant molecule add substantial carbon mass to the surface. Concurrently, the relative intensity of the Oxygen peak is markedly reduced. This occurs because the dense layer of surfactant molecules effectively masks the underlying oxygen-rich lignocellulosic substrate from the probing electron beam. The addition of an oxygen-free, carbon-dense CTAB layer significantly increased the surface C/O ratio of the raw lignocellulose in GS, indicating a fundamental change in surface chemistry from an oxygen-rich biopolymer to a carbon-rich organo-functionalized material, providing semi-quantitative proof. Furthermore, in the MGS-10 spectrum, it is important to note that a weak or absent Br peak does not necessarily indicate failure of the modification. Instead, it could suggest an ion-exchange mechanism where the bromide counter-ion is released into the solution as the cationic CTAB headgroup forms a direct electrostatic bond with the negatively charged sites on the biosorbent surface. A similar behavior has been previously reported [
64].
Conversely, the EDS spectrum of the unmodified guava seeds after exposure to DCF (designated as GSC), shown in
Figure 3b, provides direct evidence of pollutant uptake. When compared to the baseline spectrum of the native GS (
Figure 3a), the GSC spectrum exhibits a new, clearly distinguishable peak corresponding to Chlorine (Cl). The molecular formula of DCF contains two chlorine atoms per molecule. Since chlorine is not an intrinsic component of the guava seed biomass or the aqueous solution used, its appearance in the EDS spectrum is an unambiguous elemental fingerprint confirming the presence of DCF on the biosorbent surface. The Cl peak in the GSC spectrum, representing 17.4% of the mass percentage, indicates DCF adsorption despite the expected electrostatic repulsion between the biosorbent and DCF molecule at the experimental pH. This signal suggests that non-electrostatic forces, such as hydrogen bonding, van der Waals forces, and hydrophobic interactions, are responsible for the adsorption process, which overcomes the energy barrier and drives the uptake of DCF.
On the other hand, the evidence of the efficacy of the modified biosorbent is provided by the elemental mapping of chlorine on a DCF-loaded MGS-10 sample (
Figure 3d). In this map, the red spots correspond to regions of high chlorine concentration, which directly correlate with areas of high diclofenac accumulation. Two critical observations emerge from this map. First, the intensity of the chlorine signal in these bright spots appears significantly higher than the general signal observed in the GSC spectrum, providing strong qualitative support for the conclusion that the surfactant modification substantially enhances the overall loading of DCF. This enhancement is visually confirmed by a direct comparison of the EDS elemental maps for the DCF-loaded GSC and MGSC-10 (see
Supplementary Materials, Figure S2) and is quantitatively proven by the adsorption isotherm data presented in
Section 3.7. Second, and more importantly, the distribution of chlorine is highly non-uniform. Rather than a homogeneous coating, DCF is concentrated in discrete, localized “hotspots” across the biosorbent surface. This heterogeneous distribution is a pivotal finding. It demonstrates that DCF adsorption does not occur evenly but is instead directed to preferential sites. This fact directly implies that the underlying CTAB modification is not uniform itself. The regions of high DCF concentration must correspond to areas where the CTAB layer is most densely packed or most effectively organized, creating localized zones of high positive charge density and hydrophobicity. This observation shifts the focus from the total surface area of the biosorbent to the nature and distribution of these highly active adsorption domains. Furthermore, the observation from the chlorine map (
Figure 3d) that DCF is localized in specific “crystal-like” structures within the guava seed provides a critical insight into the nature of the biosorbent. The term “crystal-like” in this biological context can refer to two distinct types of structures. Guava seeds show inorganic crystallinity, derived from crystalline forms of calcium carbonate or calcium oxalate [
65,
66], which can act as preferential nucleation sites for CTAB admicelles. These mineral-anchored surfactant islands become active hotspots for DCF adsorption, which explains the localized signals. Guava seeds also contain sclereids, or “stone cells,” which are specialized cells with thick, heavily lignified secondary walls [
67]. These sclereids are micro-scale composites of highly ordered, crystalline cellulose microfibrils embedded within a dense, amorphous matrix of lignin and hemicellulose, responsible for the gritty texture of the guava fruit pulp. Since no calcium is significantly present in such crystal-like forms in MGS-10, it can be inferred that sclereids play a significant role in DCF removal using this biosorbent.
The study determined the BET surface area of all materials using water vapor adsorption, yielding values of 85 m2/g for GS, 47 m2/g for MGS-2, and 32 m2/g for MGS-10, revealing an inverse relationship between the measured surface area and the surfactant modification. This suggests that the adsorption mechanism is not controlled by the physical surface area but is dominated by the engineered surface chemistry. However, these values may underestimate the actual surface area of the hydrophobically modified materials. More detailed N2 physisorption studies are planned for future work to fully characterize the pore structure.
3.3. Thermogravimetric Analyses (TGA)
The thermal stability of the biosorbents was assessed through Thermogravimetric Analysis (TGA), which revealed distinct thermal degradation stages for guava seeds (GS) and their surfactant-modified variants, MGSC-2 and MGSC-10. The results are shown in
Figure 4. The first significant mass loss, associated with the evaporation of moisture and surface volatiles, occurred at temperatures below 120 °C for all samples, with initial losses beginning around 60 °C. The decomposition of organic components, such as cellulose, hemicellulose, and lignin, was observed between 200 °C and 450 °C. The maximum degradation temperature, representing the peak of this decomposition, was found to be 365 °C for GS, 358 °C for MGSC-2, and 355 °C for MGSC-10, suggesting a slight decrease in thermal stability with the modification of guava seeds by surfactants. Such temperatures were determined from the Derivative Thermogravimetry (DTG) Spectra of each material, as depicted in
Figure 4 (DTG data were normalized for a better visualization). The final stage, occurring above 500 °C, corresponded to the formation of mineral residues (ash) and carbon structure of the guava seeds, where the residual mass stabilized, reflecting the inorganic content of the biosorbents. These findings indicate that surfactant modification enhances the thermal stability of guava seeds, as evidenced by the higher maximum degradation temperatures observed in MGSC-2 and MGSC-10 compared to the unmodified GS.
The initial mass loss observed in the TGA curve is attributed to the release of physically adsorbed water, as well as to a lesser extent, some highly volatile organic compounds [
68]. For air-dried guava biomass, the initial mass loss is expected to be around 3–7%, as it falls within the typical range for lignocellulosic air-dried biomass [
68]. For the comparative analysis of GS, MGSC-2, and MGSC-10, this stage provides an initial point of interest, as slight differences in mass loss were noted in this stage for the modified samples, particularly for MGSC-2 (
Figure 4b), suggesting that the surfactant treatment has altered the material’s surface hygroscopicity. For instance, the introduction of hydrophilic surfactant head-groups could increase water retention, while the orientation of hydrophobic tails at the surface could decrease it. This final reason may explain the slightly higher mass loss of MGS-10 (
Figure 4c), where a more effective surfactant layer (or multilayer) is predicted to be present on the surface of the biosorbent.
Furthermore, as mentioned above, the reduction in thermal stability is CTAB dose-dependent, suggesting a catalytic effect where the surfactant promotes the onset of pyrolytic decomposition at lower temperatures. This interpretation is supported by the derived thermogravimetric (DTG) curves, which show an increasingly prominent shoulder in the 220–315 °C range for the modified samples (
Figure 4), indicating that surfactant decomposition overlaps with that of the native hemicellulose component. Interestingly, despite initiating decomposition earlier, the sample with the highest modification level (MGS-10) yielded a slightly greater final char residue at 800 °C (~24%) compared to both GS and MGS-2 (both ~22%). This indicates a dual role for the surfactant: it reduces overall thermal stability, but at higher concentrations, it may also change the pyrolysis pathway to enhance the formation of a more stable carbonaceous residue.
The thermal stability of surfactant-modified lignocellulosic materials is enhanced through two primary mechanisms. The adsorbed CTAB layer acts as a physical barrier, insulating the biopolymer and slowing heat transfer, preventing pyrolytic degradation. The surfactant’s interaction with the biosorbent surface passivates thermally labile functional groups, creating a stable surface complex. This effect has been observed in other CTAB-modified cellulosic materials, confirming the surfactant coating’s contribution to the thermal integrity of the composite biosorbent [
55,
69].
While TGA can be used to quantify surface coatings, a quantitative estimation of the adsorbed CTAB mass from this data is not feasible for this specific system. The literature reports that pure CTAB undergoes a multi-stage thermal degradation, with an initial decomposition occurring between 250 °C and 350 °C, followed by a final degradation that is completed by approximately 500 °C [
70]. This entire range significantly overlaps with the main decomposition region of the lignocellulosic components in guava seeds (200–450 °C), as shown in
Figure 4. Because these major mass loss events occur concurrently, a deconvolution of the TGA curves cannot be used to accurately isolate the mass loss attributable solely to the surfactant.
3.4. Biosorption Kinetics
Figure 5 shows the DCF biosorption as a function of time for GS, MGS-2, and MGS-10. It is worth noting that the performance of GS is modest. The initial adsorption rate is relatively slow, achieving approximately 30% DCF removal within the first 60 min of contact time. The process proceeds to a distinct equilibrium plateau, which is reached approximately 180 min later, with a maximum removal efficiency of 63%. This limited capacity and slower rate are consistent with an adsorption mechanism hindered by electrostatic repulsion, likely relying on weaker, non-electrostatic forces such as van der Waals interactions or π-π stacking between the DCF molecules and the GS biomass surface. For the case of MGS-2, a considerable enhancement of adsorption performance was observed. The initial rate is significantly faster than that of GS, with approximately 45% of the DCF being removed within the first 60 min. The total removal capacity is also significantly improved, reaching an equilibrium of approximately 70%. Interestingly, the time required to achieve this final equilibrium is longer than for GS, extending to around 300 min. The kinetic profile exhibits a rapid initial phase, followed by a much slower, prolonged approach to the final plateau. The MGS-10 biosorbent showed a superior performance in DCF biosorption kinetics. The initial adsorption rate is significantly high, as evidenced by the near-vertical slope of the curve at the beginning of the experiment. A 70% removal was achieved in the first 60 min of contact time. The system reaches equilibrium at 240 min, showcasing both the highest removal capacity and the fastest kinetics of the three materials tested. Furthermore, this material achieves nearly complete removal of DCF, achieving approximately 90% removal.
A direct comparison between the three materials reveals a concentration-dependent effect of CTAB modification on key kinetic parameters. For instance, MGS-10, with a higher CTAB concentration, exhibits a steep initial slope, indicating favorable surface chemistry and rapid mass transfer kinetics, likely driven by strong electrostatic attractions. The equilibrium removal percentage follows the same trend, with MGS-10 (~90%) > MGS-2 (~70%) > GS (~60%), indicating that increasing CTAB concentration directly increases the number of effective binding sites available for DCF. The time to reach equilibrium does not follow a simple linear trend with performance, with MGS-10 being the fastest (~120 min), followed by GS (~240 min), and MGS-2 being the slowest (~300 min). This suggests a more complex adsorption process for MGS-2, with the initial rapid uptake driven by the newly introduced positive sites and the subsequent slow, extended phase suggesting a diffusion-limited process. This kinetic behavior hints at fundamental differences in the surface chemistry between the MGS-2 and MGS-10 materials. This difference in kinetic behavior is a direct reflection of their distinct surface structures: the bilayer on MGS-2 facilitates a complex, two-stage mechanism of electrostatic attraction followed by slower hydrophobic partitioning, whereas the dense multilayer on MGS-10 promotes a more direct, single-stage electrostatic adsorption.
To quantify the impact of the modification on the biosorption kinetics of DCF and to compare the removal rates of the biosorbents, the biosorption kinetic data were fitted to established empirical models: the Lagergren model, pseudo-second-order model, and the Elovich equation [
71,
72,
73], utilizing non-linear regression to derive the global reaction kinetic constants for each tested model. The equations for these empirical models are provided in the
Supplementary Materials (see Equations (S1)–(S3)). First, the kinetic data for removal (% removal) were converted to the concentration of DCF in the sorbent (
qt) to enable fitting to these models using the following equation:
where
Co is the initial concentration of DCF in the solution (mg/L),
Cf is the DCF concentration in the solution at time
t (mg/L),
V is the volume of solution used in the batch tests (L),
m is the used mass of the biosorbent (g), and
qt is the DCF adsorption capacity of the biosorbent (mg/g). The results of these fittings are presented in
Table 1 for all biosorbents, including the resulting model parameters and their respective determination coefficients (R
2). Model plots are presented in
Figure S3.
Based on the results presented in
Table 1, a comparative evaluation using the correlation coefficients (R
2) indicates the effectiveness of each model in describing the biosorption process across different materials. For instance, in the cases of GS and MGS-10, the Lagergren model provided the best fit, with the highest R
2. In contrast, for MGS-2, the Elovich model yielded the highest correlation coefficient (R
2 = 0.8765), closely followed by the pseudo-second-order model (R
2 = 0.8739). While the pseudo-second-order model is frequently reported to describe biosorption processes best, particularly those governed by chemisorption, its subordinate fit for GS and MGS-10 in this study is noteworthy. The superior fit of the Lagergren model for MGS-10 may suggest that, despite the strong electrostatic driving force, the rate is primarily dependent on the concentration of DCF and the vast number of homogenous, readily available binding sites created by the CTAB multilayer. For MGS-2, the better fit of the Elovich model, which is characteristic of chemisorption on heterogeneous surfaces, aligns perfectly with the previously hypothesized surface structure of such material, leading to a more complex kinetic profile. The suitability of this model supports the hypothesis of a chemically heterogeneous surface for MGS-2, characterized by a mosaic of active sites with varying adsorption energies.
Additionally, when comparing the kinetic parameters, a clear and consistent trend directly attributable to the CTAB modification is revealed. The rate constants from all three models (KL, K2, and α) demonstrate a consistent and significant increase in the order of MGS-10 > MGS-2 > GS. Specifically, the initial adsorption rate parameter α from the Elovich model shows a more than six-fold increase from 0.1762 mg/g·min for GS to 1.0862 mg/g·min for MGS-10; this behavior provides robust quantitative evidence for the acceleration of the biosorption process, confirming the visual interpretation of the steep initial slopes in the kinetic plots, discussed above. This rate enhancement is a direct consequence of the surface charge reversal induced by the cationic surfactant, which creates a strong electrostatic attraction for the anionic DCF molecules, thereby overcoming the inherent repulsive forces present in the GS biosorbent.
Moreover, the adsorption capacities predicted by models, including the Lagergren model and pseudo-second-order model, demonstrate the efficacy of the modification. The Lagergren model showed a clear progression in adsorption capacity, with values ranging from 4.65 mg/g to 6.68 mg/g. This trend aligns with observed removal percentages from kinetic curves, confirming that increasing the CTAB loading concentration from 2 mmol to 10 mmol accelerates the biosorption process and increases the number of effective binding sites for DCF capture. The proposed mechanism involves forming a partial bilayer on MGS-2 and a densely packed surfactant bilayer (or multilayer) on MGS-10. Another interesting piece of evidence emerges when analyzing the desorption constant (b) values, which relate to surface coverage and activation energy for chemisorption [
72]. The data reveal notable trends: 0.8961 g/mg for GS, 1.0012 g/mg for MGS-2, and 0.8235 g/mg for MGS-10. The slightly lower b value for MGS-10 suggests a reduced activation energy for chemisorption at higher CTAB concentrations, potentially indicating more favorable adsorption thermodynamics due to the presence of additional heterogeneous sites with favorable adsorption characteristics. This phenomenon can be attributed to the formation of hemimicelles and admicelles on the surface. In summary, the kinetic modeling provides a quantitative framework that corroborates the mechanistic hypotheses, confirming that the CTAB modification engineers the guava seed surface to create a superior biosorbent characterized by both rapid kinetics and enhanced capacity for diclofenac removal.
3.5. Effect of Biosorbent Dosage
Figure 6 shows the effect of biosorbent dosage on the removal efficiency of diclofenac (DCF) using GS, MGS-2, and MGS-10. These biosorbents were tested at varying dosages (ranging from 1 to 10 g/L) to identify the optimal dosage for maximum DCF removal efficiency. For the removal of DCF using GS, it can be observed that, at lower dosages (1–3 g/L), the DCF removal efficiency is relatively low, but it increases steadily as the dosage increases. The maximum removal is achieved at around 8–9 g/L, where the removal rate plateaus. This behavior suggests that higher biosorbent dosages lead to increased surface area for adsorption, allowing more DCF molecules to bind to the available active sites on GS. However, beyond this optimal dosage, no significant improvement in removal efficiency is observed, indicating that the adsorption sites become saturated.
On the other hand, the removal of DCF by MGS-2 increases in a slow and nearly linear fashion across the entire dosage range tested. The removal efficiency begins at a negligible level and only reaches a maximum of approximately 51% at a dosage of 9.0 g/L. A slight decrease can be observed at higher dosages, probably due to MGS-2 particle aggregation, which reduces the effective surface area available for the adsorption process (Tapia Quiroz).
The MGS-10 material displays a profoundly different and significantly more effective dosage-response profile compared to the unmodified GS. The removal of DCF increases significantly faster as the dosage is raised, achieving approximately 68% removal at a low dosage of 3.0 g/L and continuing to climb to a maximum removal of 98.7% at a dosage of 9.0 g/L (
Figure 6). A particularly noteworthy feature of this curve is the slight but distinct decrease in removal efficiency, which drops to approximately 85% when the dosage is increased from its peak at 9.0 g/L to 10.0 g/L. This well-defined peak at 9.0 g/L unambiguously identifies it as the optimal dosage for MGS-10 under these experimental conditions. The subsequent decline strongly suggests that at very high particle concentrations, the effects of particle aggregation begin to dominate, overriding the benefits of introducing additional biosorbent mass to the biosorption system.
The superior performance of MGS-10 is a direct consequence of the radical transformation of the biosorbent’s surface chemistry by the CTAB modification. As mentioned above, the high surfactant concentration is well above its CMC, so the CTAB molecules are expected to form aggregate structures known as admicelles, or bilayers, on the biosorbent surface. This admicelle structure effectively inverts the surface charge of the biosorbent, presenting a dense, uniform layer of positive charges to the surrounding aqueous solution. Thus, the primary adsorption mechanism shifts to electrostatic attraction, resulting in high removal efficiency. Hydrophobic interactions with the surfactant multilayer further enhance this mechanism, with the non-polar phenyl rings and alkyl chain of the DCF molecule partitioning into the dense environment created by the CTAB admicelle. The dosage-effect curve for MGS-10 reflects this efficient process, with an initial steep increase in removal efficiency due to the high density and strong affinity of the newly created binding sites. However, at 10.0 g/L, excessive particle agglomeration occurs, which reduces the effective surface area of the biosorbent and decreases the overall system efficiency. This fact illustrates the limitations of particle-particle interactions in concentrated suspensions, where increasing the biosorbent mass may not be beneficial. Besides, the biosorption process involves multiple mass transfer steps, including external film diffusion, intraparticle diffusion, and surface reaction. Thus, at higher biosorbent dosages, the increased particle density can create mass transfer limitations through several mechanisms, particularly when the biosorbent has been modified on its surface. First, the increased competition for DCF molecules in the bulk solution can reduce the concentration gradient driving force for mass transfer [
74]. Second, particle crowding can impede bulk solution mixing and create stagnant zones around biosorbent particles, enhancing external mass transfer resistance [
75]. These diffusion resistances can also lead to low removal at high dosages.
3.6. pH Effect on DCF Biosorption
Figure 7 illustrates the influence of pH on the DCF removal using GS, MGS-2, and MGS-10as biosorbents. To understand these results, it is crucial to consider the charge of both the biosorbent surface and the DCF molecule at a given pH. At pH values above its pKa of ~4.15, DCF exists as an anion (DCF
−), while the respective PZC values dictate the surface charge of the biosorbents. The GS bar plot exhibits a clear trend in DCF removal as a function of solution pH, with the highest adsorption efficiency occurring at acidic pH values ranging from 3.5 to 4.0. In this acidic range, the GS surface is positively charged due to protonation of amino and carboxyl functional groups [
76]. At the same time, DCF predominantly exists in its neutral or slightly anionic form near its pKa. This condition facilitates favorable interactions, including hydrogen bonding, hydrophobic interactions, and some non-electrostatic attraction [
77]. As the pH increases above the point of zero charge (PZC ~3.5 [
29]), the GS surface progressively acquires a negative charge through deprotonation of acidic groups (e.g., –COOH to –COO
−). Simultaneously, diclofenac predominantly ionizes to its anionic form. The resulting electrostatic repulsion between the negatively charged biosorbent surface and anionic diclofenac leads to a slight decrease in removal efficiency at near-neutral and alkaline pH values. However, it can be established that the GS biosorbent performs relatively well over the entire pH range studied, demonstrating the suitability of guava seeds for removing DCF from water.
MGS-10 exhibits similar behavior to GS, with even higher removal values across the entire acidic range, including neutral and slightly alkaline pH levels. This is consistent with its PZC value (6) [
29], which is below the point at which the biosorbent surface is positively charged. However, at higher pH values (8.5 to 9), a significant decrease in DCF removal is observed on MGS-10. This phenomenon is primarily due to changes in the electrostatic interactions between the biosorbent surface and the DCF molecules, which become less favorable at higher pH levels; furthermore, competition with OH- ions for adsorption sites on MGS-10 can also contribute to this decrease in removal. On the other hand, it has been reported that while hydrophobic interactions within the surfactant multilayer can enhance the adsorption capacity on MGS-10 compared to GS, the dominant mechanism for many pharmaceuticals is electrostatic attraction, which weakens at higher pH levels due to the factors mentioned above.
As shown in
Figure 7, the performance of MGS-2 in removing DCF is significantly influenced by the solution’s pH. In this case, the highest removal values are observed at acidic values (3.5 and 4), while they decrease drastically and steadily as the pH increases. As shown in the plot (MGS-2 in
Figure 7), the removal efficiency reaches a maximum of approximately 77% at a pH of around 3.5. It then gradually decreases to less than 10% under highly alkaline conditions (pH 8.5–9). This trend suggests that the primary adsorption mechanism is not electrostatic attraction, but rather non-electrostatic interactions, specifically hydrophobic partitioning or interactions with functional groups present in the guava seed structure. This further corroborates the different DCF removal mechanisms of the different biosorbents under study. To explain this behavior, it is worth noting that the removal efficiency of polar adsorbates on surfactant-modified adsorbents decreases significantly with increasing solution pH due to several interrelated factors. These include the nature of the surfactant-adsorbate interactions and the solubility of the adsorbate in the surfactant. With increasing pH, the electrostatic interactions and hydrogen bonding that facilitate adsorption weaken, reducing the adsorption capacity. Furthermore, at pH values well below diclofenac’s pKa of ~4.15, the molecule exists predominantly in its protonated neutral form (DCFH). This neutral species exhibits limited water solubility and a high affinity for nonpolar environments. The CTAB bilayer on the surface of MGS-2, with its dense layer of long hydrophobic alkyl tails, provides an ideal nonpolar domain for the adsorption of neutral DCFH molecules. The high removal efficiency at low pH is therefore attributed to the hydrophobic driving force that separates the neutral drug from the aqueous phase and draws it into the surfactant layer. However, as the pH exceeds 4.15, DCF transforms into its deprotonated anionic form (DCF
−). While this creates the potential for electrostatic attraction to the positively charged MGS-2 surface, the anionic form is also significantly more soluble in water [
78]. The experimental data (
Figure 7), showing a marked decrease in adsorption in this pH range, suggest that the loss of the potent hydrophobic partitioning mechanism (due to the higher solubility of the adsorbate) far outweighs any gain from electrostatic attraction. Furthermore, as the pH approaches the alkaline region, the concentration of hydroxide ions increases, introducing a competitive effect in which these small anions compete with DCF
− for positive binding sites, thereby further suppressing adsorption. Therefore, for MGS-2, the adsorption process is paradoxically most effective when the target molecule is neutral, highlighting the predominance of hydrophobic interactions over electrostatic forces or chemical bonding in this specific system. Finally, although higher removal was observed under acidic conditions, a pH of 6 was selected for the subsequent isotherm and thermodynamic studies as it is more representative of real wastewater conditions and ensures the evaluation of the biosorbents’ performance in a practical, environmentally relevant scenario.
3.7. DCF Biosorption Isotherms
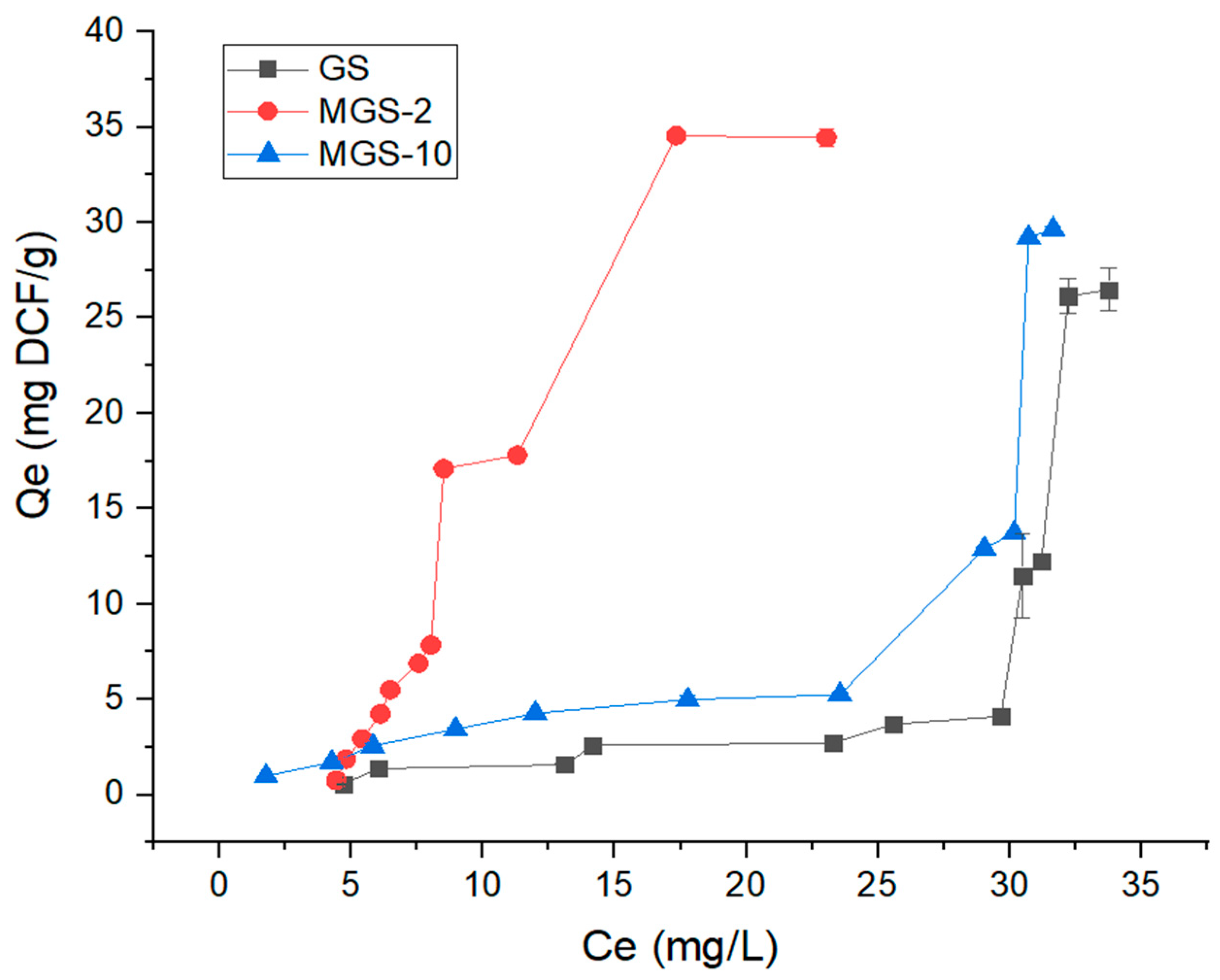
Adsorption isotherms are crucial for understanding the molecular interactions between solid surfaces and adsorbates (contaminants) present in liquids or gases, as they describe how a substance adsorbs to a solid material as a function of its concentration in the fluid phase, at a constant temperature. They can also be used to predict the stability of the adsorbate-adsorbent system, identify the maximum adsorption capacity, and aid in selecting suitable adsorbent materials. In this study, DCF biosorption isotherms at three different temperatures are presented in
Figure 8,
Figure 9 and
Figure 10 for 25 °C, 35 °C, and 45 °C, respectively, using GS, MGS-2, and MGS-10 as biosorbents. Generally, it can be observed from these plots that the unmodified GS biosorbent demonstrates a limited affinity for DCF, particularly at lower equilibrium concentrations. In contrast, both MGS-2 and MGS-10 exhibit significantly greater adsorption capacities, underscoring the important role of the CTAB modification in transforming the surface properties of the biosorbent to favor the uptake of DCF. The overall performance, in terms of the equilibrium adsorption capacity (Qe), follows the order MGS-2 > MGS-10 > GS. This preliminary observation immediately highlights a non-linear relationship between the degree of surfactant loading and the ultimate adsorption performance, a central theme for subsequent mechanistic discussion. By analyzing the first part of the isotherms (low concentrations), there are apparent differences in initial adsorption affinity, which focuses on the low-concentration Henry regime: the steep, near-vertical slope for MGS-2 confirms its exceptionally high affinity, followed by the moderately high affinity of MGS-10, and the significantly lower affinity of the unmodified GS. Furthermore, when performing a quantitative analysis of the experimental maximum adsorption capacity (Qe
max), defined throughout this study as the highest Qe value observed within the tested concentration range, it provides a precise measure of the biosorbents’ performance. At 25 °C (
Figure 8), it is evident that GS exhibits low DCF uptake across a wide range of equilibrium concentrations (Ce), with a final observed capacity of approximately 26.5 mg/g. This value is reached following a sharp increase in uptake at Ce values exceeding 30 mg/L, suggesting a complex adsorption behavior. In the case of MGS-10 (
Figure 8), the isotherm exhibits a slightly improved performance, achieving an observed Qe
max of approximately 29.8 mg/g. Most notably, the MGS-2 material, prepared with an intermediate level of surfactant, demonstrates the highest adsorption capacity. Its isotherm culminates in a distinct and stable plateau, indicating surface saturation, with a Qe
max of approximately 34.5 mg/g. This counterintuitive result, that an intermediate modification level (MGS-2) yields superior performance to a higher one (MGS-10), is a critical finding. It suggests that while surfactant modification is beneficial, an excessive concentration introduces inhibitory effects that diminish the overall capacity of the biosorbent. This study suggests an optimal surfactant coverage threshold, with MGS-2 showing superior performance at or near this level, resulting in a well-organized bilayer structure for multi-stage DCF uptake. However, MGS-10’s capacity diminishes as the 10 mmol/L concentration surpasses this threshold, resulting in a less efficient surface architecture due to inhibitory effects such as steric hindrance, pore blockage, or excessive surfactant aggregation.
Analyzing the shape of equilibrium isotherms enhances the understanding of adsorption processes, functioning as a diagnostic tool for interpreting interactions between adsorbents and adsorbates. The classification system proposed by Giles et al. [
79] provides a robust framework for correlating isotherm shapes to underlying molecular mechanisms. The intricate forces governing the adsorption of DCF onto natural and modified guava seed surfaces can be interpreted using this classification. For instance, the shape of the GS isotherm at 25 °C, characterized by a shallow slope at low equilibrium concentrations, is an L-type isotherm (subgroup 3) with relatively low affinity, suggesting adsorption occurs on a finite number of sites. The initial curvature near the origin suggests a weak driving force for adsorption, with minor adsorption occurring through weaker, non-electrostatic interactions. A notable feature of the GS isotherm is the sharp, nearly vertical ascent in Qe at a Ce value of approximately 31 mg/L. The observed behavior is not typical of standard isotherm types. It is likely due to the surface-induced aggregation of DCF, a hydrophobic molecule with a high octanol-water partition coefficient, which dominates electrostatic forces. The MGS-10 isotherm shows a similar shape to the MGS-2 isotherm (
Figure 8), and can also be classified as an L-type isotherm. This behavior indicates that the adsorbate has good affinity for the biosorbent surface, but the interaction is not as intense as observed in H-type isotherms.
The MGS-2 isotherm is a complex, composite isotherm that demonstrates the transformation of adsorption behavior. The initial phase of the curve is steep, indicating an H-type (High-affinity) isotherm, driven by the powerful electrostatic attraction between the positively charged surface of MGS-2 and the anionic DCF molecules in solution. However, the isotherm is not a simple, single-step H-type curve but a more complex composite that delineates two distinct adsorption mechanisms operating at different concentration regimes. The first mechanism (Low Ce, H-type behavior) governs adsorption at low equilibrium concentrations, resulting in the rapid filling of primary positive sites created by the CTAB headgroups. This initial phase corresponds to the steep rise and subsequent “knee” of the curve, transitioning into an intermediate plateau-like region between Ce values of approximately 8 and 12 mg/L. The second mechanism (High Ce, S- or C-type behavior) activates a new, powerful adsorption mechanism, admicellar solubilization, a form of hydrophobic partitioning. This process could involve the formation of surface aggregates known as hemimicelles or admicelles, which create a nonpolar, “oily” pseudo-phase on the biosorbent surface. This cooperative process accounts for the significant additional uptake of DCF. Thus, MGS-2 could be classified as an H-type (subgroup 4) isotherm. The role of increasing the temperature from 25 °C to 35 °C extends beyond simply favoring an endothermic process; it fundamentally alters the adsorption mechanisms for the modified biosorbents. The added thermal energy appears to modulate the physical state of the surfactant layers, thereby changing the balance of the dominant adsorption forces. For MGS-2, this enhancement facilitates the secondary, diffusion-limited step of hydrophobic partitioning into the CTAB bilayer, making the characteristic “step” of its H-type isotherm more pronounced. For MGS-10, the temperature increase induces a more dramatic shift to cooperative adsorption (S-type isotherm), likely by increasing the mobility of the surfactant chains, which allows adsorbed DCF molecules to create favorable sites for subsequent adsorption.
For 35 °C, the biosorption isotherms data of the three biosorbents (
Figure 9) revealed that their adsorption behavior was significantly altered compared to the data at 25 °C. The MGS-10 isotherm exhibits a sigmoidal or “S” shape, with a low initial slope indicating low adsorption at low concentrations. As the equilibrium concentration increases, the slope steepens, passing through an inflection point (around Ce = 37 mg/L), indicating a rapid uptake. At higher concentrations, the curve levels off, approaching a new saturation plateau. This sigmoidal profile is a classic example of an S-type isotherm, a phenomenon known as cooperative adsorption. The mechanistic implication is that the affinity of the biosorbent for the adsorbate increases as the surface becomes more covered, facilitating the adsorption of subsequent molecules from the solution.
The GS isotherm data at 35 °C (
Figure 9) can be classified as type L, subgroup 3, based on its shape. The initial portion of the curve displays a relatively steep slope that gradually decreases as the concentration of DCF in the solution increases. This change results in a plateau at intermediate concentrations, specifically around Ce = 11 mg/L. Then, DCF adsorption increases again exponentially and reaches a slight plateau at the end of the concentration range studied until a Qe
max is reached at approximately Qe = 28.7 mg/g. This type of isotherm is typically associated with systems where the adsorbate has a high affinity for the biosorbent, leading to a steep initial rise in the adsorption curve. These facts indicate that the DCF biosorption on GS was enhanced with the increase in temperature. Furthermore, this system is defined by the interaction between a negatively charged biosorbent surface (GS) and a negatively charged adsorbate molecule (DCF anion). Despite classical electrostatic theory suggesting significant electrostatic repulsion, experimental data demonstrate substantial uptake of DCF. This apparent contradiction indicates that the adsorption process is not governed by electrostatics, but rather by other, more potent non-electrostatic attractive forces that can overcome the repulsive energy barrier. Under this context, the L-type isotherm shape is a net outcome of a competition between dominant non-electrostatic attractions and persistent electrostatic repulsion. Adsorption is initially favorable at lignin-rich regions, where attractive forces are most significant. As these sites become saturated, subsequent DCF molecules must adsorb at less favorable locations or approach negatively charged surfaces. These diminishing returns characteristic of the L-type curve explain the small plateau at approximately 28 mg/g, where attractive forces overcome the repulsive barrier.
The adsorption isotherm for MGS-2 at 35 °C is again markedly different from the other two. This curve is characterized by a highly steep initial rise, where a substantial quantity of DCF is adsorbed at low equilibrium concentrations. The initial portion of the isotherm is nearly vertical on the plot. Following this high-affinity region, the curve exhibits a short, distinct plateau, which is then followed by a second, less steep rise, creating a “step” in the isotherm. According to Giles et al.’s classification [
79], the quite steep initial slope, which indicates a strong affinity between the adsorbent and adsorbate, qualifies this as an H-type (High-affinity) isotherm. The presence of a subsequent step after the initial plateau places it into a more specific subgroup, likely H3 or H4, which are characterized by inflections or steps. Such stepped isotherms are often associated with adsorption on highly uniform surfaces, where each step can correspond to the formation of a complete molecular layer, or they can indicate a surface phase transition or the activation of a secondary adsorption mechanism. The Qe
max shown on the plot, at the end of the second adsorption stage, is approximately 36.5 mg/g. The stepped nature of the adsorption process is a key feature, indicating a multi-stage process involving at least two distinct mechanisms. The first stage, the initial plateau, corresponds to the saturation of the most accessible and energetically favorable binding sites, likely due to the strong electrostatic binding of a monolayer of anionic DCF molecules onto the cationic outer surface of the complete CTAB bilayer. The second stage, characterized by a less steep rise in adsorption, represents the second step due to a secondary, less favorable mechanism, which may include adsolubilization, surface phase transition, or multilayer adsorption [
80]. The two-stage mechanism involving initial high-affinity electrostatic binding followed by secondary, lower-affinity adsolubilization into the bilayer core provides the most coherent and physically sound explanation for the observed stepped H-type isotherm (a schematic representation of this proposed mechanism is provided in
Figure S4, Supplementary Materials).
The isotherm plots presented in
Figure 10 depict the DFC equilibrium biosorption onto the three biosorbents at 45 °C. Both GS and MGS-10 biosorbents show a Type L (subgroup 3) isotherm characterized by an initial concave curvature, indicating a relatively high affinity between the DCF molecules and the GS surface. This L-type behavior suggests that the adsorbate-adsorbent interactions are stronger than adsorbate-adsorbate interactions, with adsorption occurring primarily through monolayer formation on relatively homogeneous surface sites [
81]. The resurgence of this isotherm pattern for GS at 45 °C can be ascribed to a temperature-induced alteration in the prevailing adsorption process. The 35 °C isotherm indicates an optimal binding process predominantly influenced by hydrogen bonding; however, the elevated thermal energy at 45 °C may initiate the disruption of these particular interactions. Concurrently, the hydrophobic effect is markedly enhanced at elevated temperatures, facilitating a vigorous hydrophobic expulsion of DCF molecules from the aqueous phase [
82]. This transition results in the primary mechanism shifting from individual site binding to a cooperative, aggregation-like process on the nonpolar domains of the GS surface, hence elucidating the alteration in the isotherm shape. In contrast, the MGS-2 isotherm shows an S-type behavior. S-type isotherms, especially subgroup three, exhibit an initial convex shape, indicating cooperative adsorption where the interactions between adsorbates become increasingly significant. This cooperative behavior suggests that once initial DCF molecules are adsorbed, they facilitate the adsorption of additional molecules through lateral interactions [
83].
The maximum experimental adsorption capacities (Qemax) for all biosorbents were estimated from the highest Qe value measured for each material within the tested concentration range. At this experimental temperature (45 °C), for biosorbent GS, the isotherm does not reach a defined plateau, indicating that saturation was not reached; Qemax is approximately 29.7 mg/g. For MGS-10, although its steep trajectory suggests a potentially high final capacity, this was reached at Qemax = 32.7 mg/g within the tested range. In contrast, MGS-2 reached a maximum adsorption capacity (Qemax) of nearly 38.0 mg/g, which is higher than that of the other biosorbents. It is also important to note that this adsorbate-biosorbent system did not reach saturation, suggesting that it can still effectively remove DCF even at higher concentrations under the operating temperature. Furthermore, a direct comparison between the three biosorbents highlights the success of the modification strategy, particularly for MGS-2, which shows an improvement of approximately 28% in observed capacity compared to the unmodified GS. On the other hand, while the final data point for MGS-10 is numerically lower than that of MGS-2, the multistage nature of its isotherm implies a different operating behavior, as its high-capacity mode is activated only at higher concentrations.
The systematic investigation successfully determined that the optimal conditions for the removal operation were achieved using the intermediately modified MGS-2 biosorbent at a dosage of 9.0 g/L, a temperature of 45 °C, and an acidic to near-neutral pH.
To contextualize the performance of the developed biosorbents, their adsorption capacities were compared with values reported in the literature for DCF removal using various other biosorbents derived from agricultural waste. This comparison, presented in
Table 2, is crucial for assessing the relative efficacy and potential of the guava seed-based biosorbents.
The comparative analysis reveals several key points (
Table 2). Firstly, unmodified agricultural wastes, such as citrus, olive, and artichoke residues, generally exhibit low adsorption capacities for DCF (from 4 to 9 mg/g). In this context, the natural guava seeds (GS) with a capacity of 29.7 mg/g perform remarkably well. Secondly, the CTAB-modified guava seed (MGS-2), at 38.0 mg/g, shows a capacity that is competitive with some processed biosorbents such as pine bark biochar (54.64 mg/g). However, the performance of the MGS materials is surpassed by biosorbents that have undergone more intensive modification, such as activation to produce high-surface-area carbons (e.g., argan nutshell AC, 126.16 mg/g) or modification with other surfactants (e.g., TTAB-modified Cuminum cyminum, 93.65 mg/g); despite this fact, it can be established that the biosorbents evaluated in this work could be considered competitive, economical, and sustainable, as they do not require high-energy processes such as pyrolysis or carbonization.
3.8. Thermodynamic Parameters
Thermodynamic parameters were calculated using biosorption data for DCF using the three biosorbents across different temperatures. The variations in standard Gibbs free energy (∆G°), standard enthalpy (∆H°), and standard entropy (∆S°) were assessed utilizing the Van’t Hoff equation. This technique involves plotting the natural logarithm of the equilibrium constant (ln Kc) against the inverse of temperature (1/T) to determine the enthalpy change (ΔH°) and entropy change (ΔS°) from the slope and intercept of the resulting graph, respectively [
29]. Equilibrium constants (Kc) were determined at various temperatures for the adsorption of DCF by biosorbent materials. The constants are essential for utilizing the Van’t Hoff equation to ascertain thermodynamic parameters. Our earlier work documented the equations pertinent to this method [
89].
Figures S5–S7 present the plots of ln Kc versus 1/T for DCF biosorption for all biosorbents. The plots exhibit high determination coefficients (ranging from 0.8085 to 0.9986) for the data across all systems in linear regression, indicating a predictable pattern in the adsorption process with respect to temperature variations. This predictability enhances the method’s reliability and facilitates the accurate calculation of thermodynamic parameters from the slope and intercept of the linear regression equations.
Table 3 shows the calculated parameters for the three evaluated biosorbents. These results reveal that all three biosorbent systems exhibit endothermic adsorption processes, as evidenced by the positive enthalpy values (ΔH°) shown in this Table. These positive ΔH° values indicate that heat absorption occurs during the adsorption process. The magnitude of these enthalpy values places all three systems within the physisorption regime, as they are below or near the threshold of 40 kJ/mol typically used to distinguish between physical adsorption (ΔH° < 40 kJ/mol) and chemical adsorption (ΔH° > 80 kJ/mol). This classification is further supported by the reversible nature of the adsorption process and the relatively weak intermolecular interactions involved in the binding mechanism.
The spontaneity of the adsorption process varies significantly among the three systems and is highly temperature-dependent. For the GS system, the Gibbs free energy values transition from positive (0.564 kJ/mol at 298.15 K) to negative (−1.209 kJ/mol at 318.15 K), indicating that the process becomes spontaneous only at elevated temperatures. Similarly, MGS-10 exhibits a transition from non-spontaneous (1.20 kJ/mol at 298.15 K) to spontaneous (−0.591 kJ/mol at 318.15 K) behavior with increasing temperature. Conversely, the MGS-2 system demonstrates spontaneous behavior across all tested temperatures, with increasingly negative ΔG° values (
Table 3), indicating more favorable thermodynamic conditions for diclofenac adsorption.
The entropy changes (ΔS°) for all three systems are positive, indicating an increase in randomness and disorder at the solid-liquid interface during the adsorption process [
90]. The significantly higher entropy change observed for MGS-2 suggests more extensive structural reorganization during adsorption, likely related to the optimal surfactant loading that creates favorable microenvironments for diclofenac molecules. The positive entropy changes serve as the primary driving force for these endothermic processes, with the −TΔS° term becoming increasingly favorable at higher temperatures and ultimately overcoming the unfavorable enthalpy contribution to render the overall process spontaneous [
91].
The CTAB modification demonstrates a profound impact on the thermodynamic behavior of the biosorbent systems. The MGS-2 system exhibits the highest enthalpy requirement (46.985 kJ/mol) and entropy increase (161.03 J/mol·K), suggesting that the 2 mmol CTAB concentration creates an optimal surface environment that promotes significant molecular reorganization during adsorption [
92]. This enhanced thermodynamic activity translates to superior adsorption performance, as evidenced by the consistently negative ΔG° values across all temperatures. On the other hand, the MGS-10 system, despite having a higher surfactant concentration, shows thermodynamic parameters similar to those of the unmodified GS system, indicating that excessive surfactant loading may not provide additional thermodynamic advantages and could potentially create steric hindrance or aggregation effects that limit adsorption efficiency.
These thermodynamic findings align with previous works reporting that entropy-driven endothermic processes are characteristic of many pharmaceutical compound adsorption systems onto natural and modified adsorbents [
93,
94]. The results from
Table 3 provide fundamental insights into the molecular-level interactions governing diclofenac removal, confirming that CTAB modification at appropriate concentrations can significantly enhance the thermodynamic favorability of the biosorption process while maintaining the physically reversible nature of the adsorption mechanism.
3.9. Biosorbent Regeneration Tests
Figure 11 shows the desorption efficiency data for GS and MGS-10, which were calculated according to Equation (S4). Such data across four consecutive regeneration cycles revealed critical insights into their reusability potential for DCF removal, with both materials exhibiting comparable initial adsorption capacities (
Figure 8), yet demonstrating different regeneration behaviors; specifically, GS displayed marginally higher initial desorption efficiency (3.47%) compared to MGS-10 (2.46%) in the first cycle, but experienced a steep decline in subsequent cycles, descending to 0.42%, 0.14%, and 0.06% in cycles 2, 3, and 4, respectively. At the same time, MGS-10 maintained a relatively stable, though still low, desorption efficiency, decreasing gradually to 1.03%, 0.43%, and 0.30% across the same cycles. Both materials exhibited consistently low desorption percentages throughout all regeneration attempts, suggesting that the 0.01 N NaOH regenerant was limited in its effectiveness in reversing DCF binding under the experimental conditions used in these tests. However, these desorption data provide critical validation of the adsorption mechanisms previously established for both biosorbent systems, with the near-complete loss of desorption capacity in GS after the first regeneration cycle (and the subsequent decline until cycle 4) strongly corroborate the proposed non-electrostatic adsorption mechanism involving hydrogen bonding and hydrophobic interactions, as evidenced by the FTIR analysis (
Figure 1) which revealed characteristic shifts in the N-H stretching region associated with DCF binding. This high resistance to desorption is consistent with the fundamental nature of these interactions. While individually reversible, their cumulative effect creates a strong binding that is inherently resistant to pH-driven regeneration, a finding supported by the pH-dependent adsorption studies (
Figure 7), where GS performance was not significantly influenced by solution pH. In these tests, data for GS and MGS-10 were presented as representative examples of the overall trend, as the MGS-2 data were similar to those of MGS-10.
In contrast, the unexpectedly low desorption efficiency observed for MGS-10 across all cycles differs from the theoretical expectations for electrostatically driven systems. This indicates that while the CTAB modification successfully created a positively charged surface, the structural integrity of the surfactant layer was insufficient to withstand repeated alkaline treatments. The gradual decline in desorption efficiency suggests that there was progressive degradation or displacement of CTAB, which compromised the electrostatic binding mechanism. Additionally, this observation supports the alternative hypothesis that admicellar solubilization—which was identified through isotherm analysis as a secondary adsorption mechanism at higher DCF concentrations—may have dominated the binding process. This created hydrophobic interactions that proved to be just as resistant to NaOH regeneration as the non-electrostatic mechanisms governing the adsorption of GS.
Despite both biosorbents showing poor regeneration performance, a comparative analysis reveals that MGS-10 consistently outperforms GS in later cycles, maintaining 2–3 times higher desorption efficiency by the fourth cycle. This suggests that while the CTAB modification reduces initial desorption efficiency, it provides greater stability against degradation induced by regeneration. Moreover, studies on CTAB-modified biosorbents report better desorption efficiencies using mild organic solvents (e.g., ethanol) for hydrophobic contaminants [
95]. The use of mixed regenerants may enhance desorption while preserving surfactant integrity.
This study employs regeneration tests to evaluate the practical reusability of a biosorbent, serving also as a powerful form of mechanistic validation. The low desorption efficiencies observed for both biosorbents provide critical insight into the strength and nature of the DCF-biosorbent interactions. The study uses the regeneration test as a diagnostic tool, transforming a practical limitation into a powerful, independent line of evidence.
The limited success with NaOH highlights that strong non-electrostatic forces, such as hydrophobic interactions, play a key role in the binding mechanism. More promising regeneration strategies could involve mild organic solvents, such as ethanol, which can effectively disrupt these hydrophobic bonds; however, potential surfactant leaching over repeated cycles must be considered. Another viable alternative is a controlled pH swing using a mild acid, such as acetic acid, to neutralize the adsorbed DCF and weaken its bond to the surface. Further investigation into these pathways is essential to improve the biosorbent’s reusability and overall sustainability.