Effect of Hydrodynamic Conditions on the Corrosion Mechanism of HSLA X100 Steel by EIS and EN Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mechanical Characterization of X100 Steel
2.2. Microstructural Characterization of X100 Steel
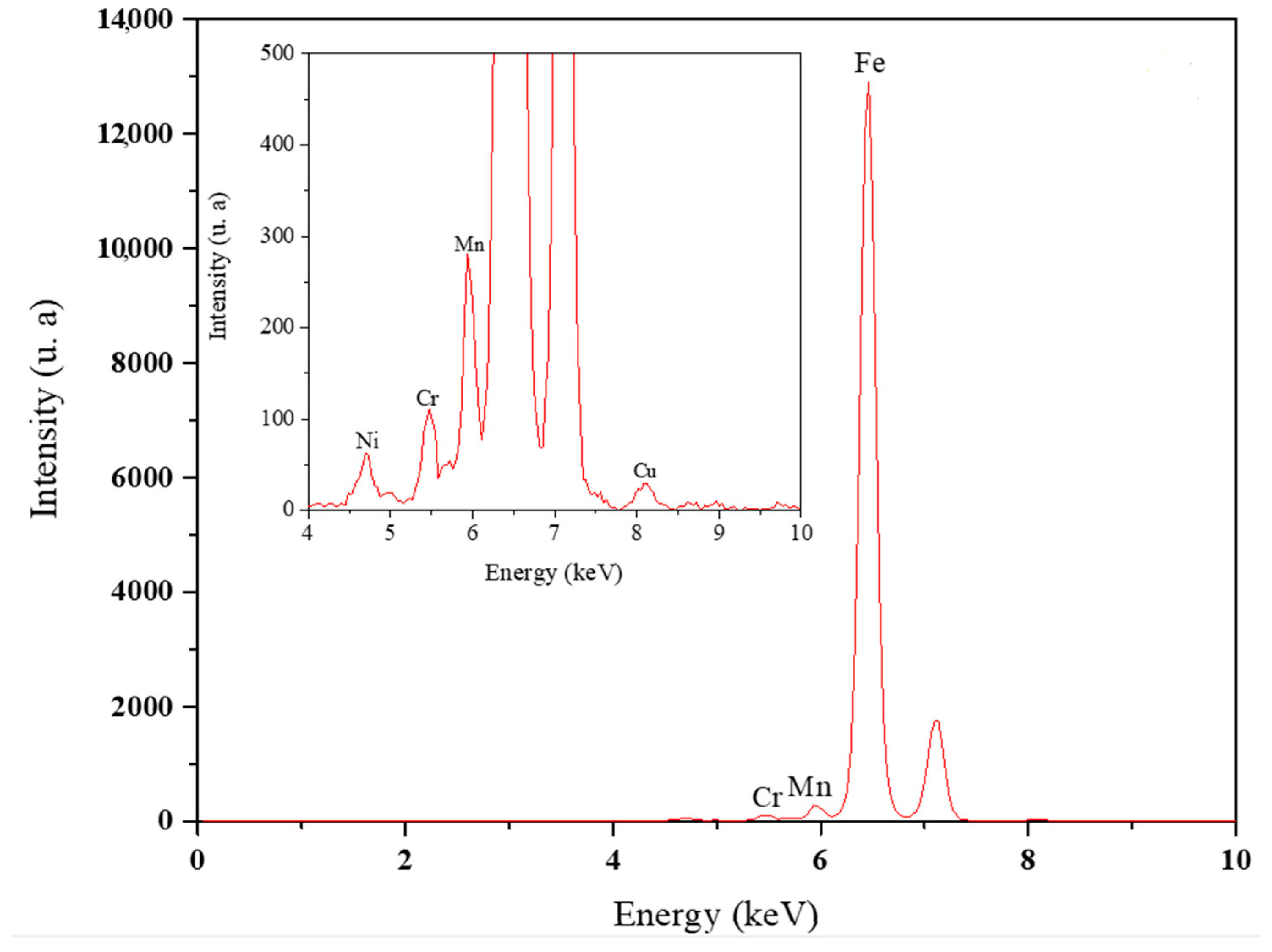
2.3. Surface and Elemental Characterization of X100 Steel
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Mechanical Characterization
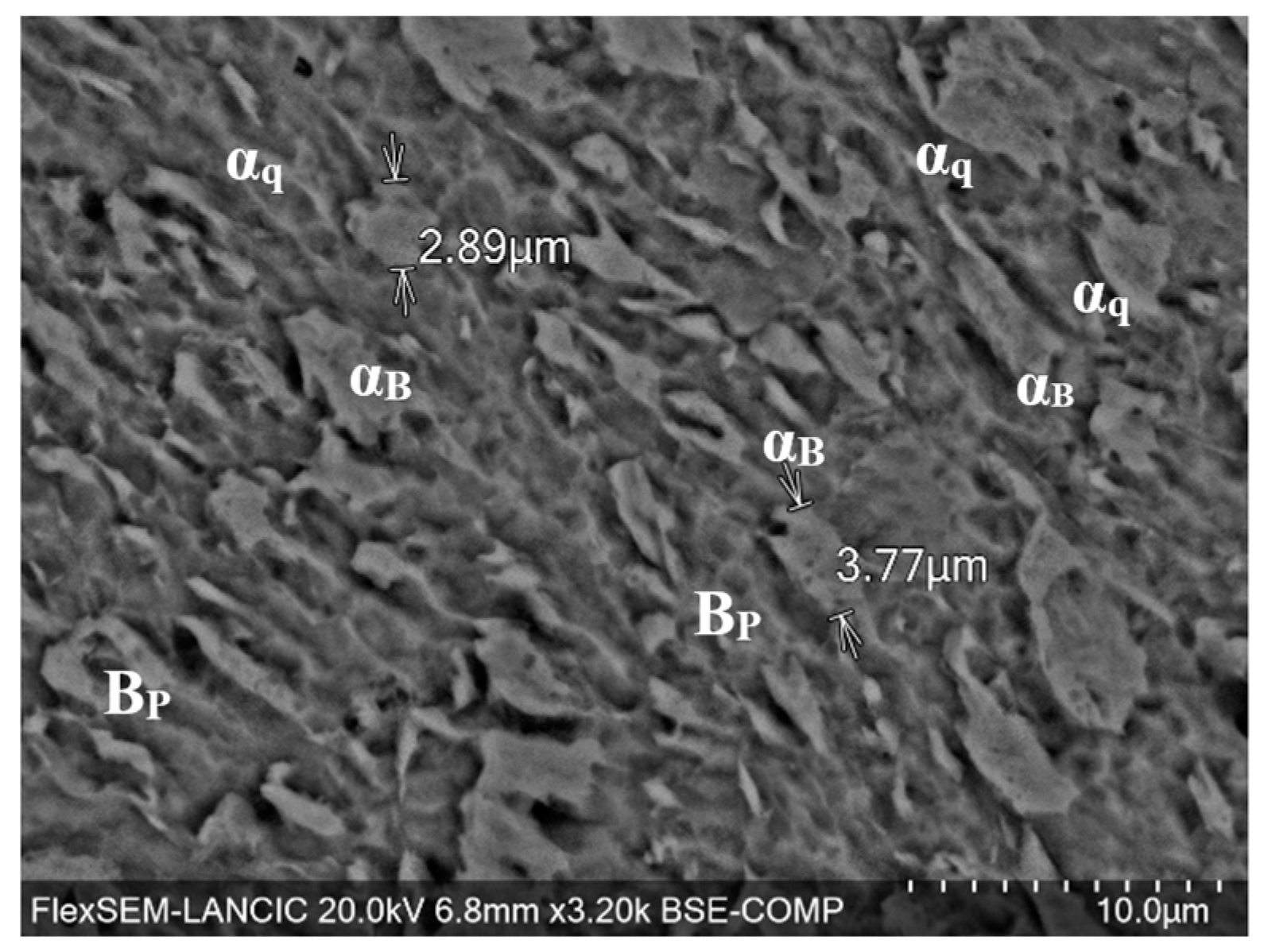
3.2. Elemental and Microstructural Analysis
3.3. Electrochemical Evaluation of X100 Steel: Hydrodynamics Parameters and Ecorr
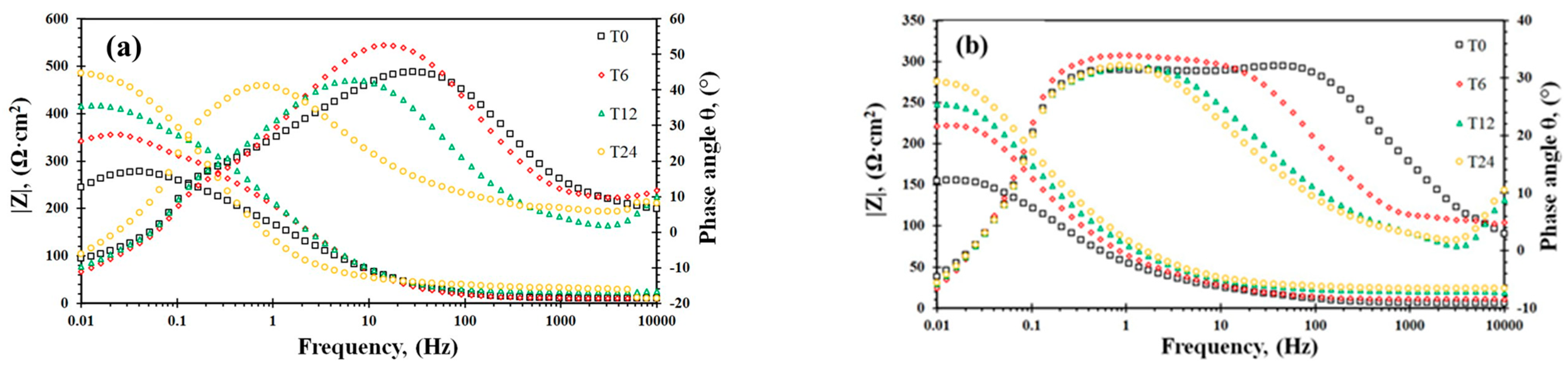
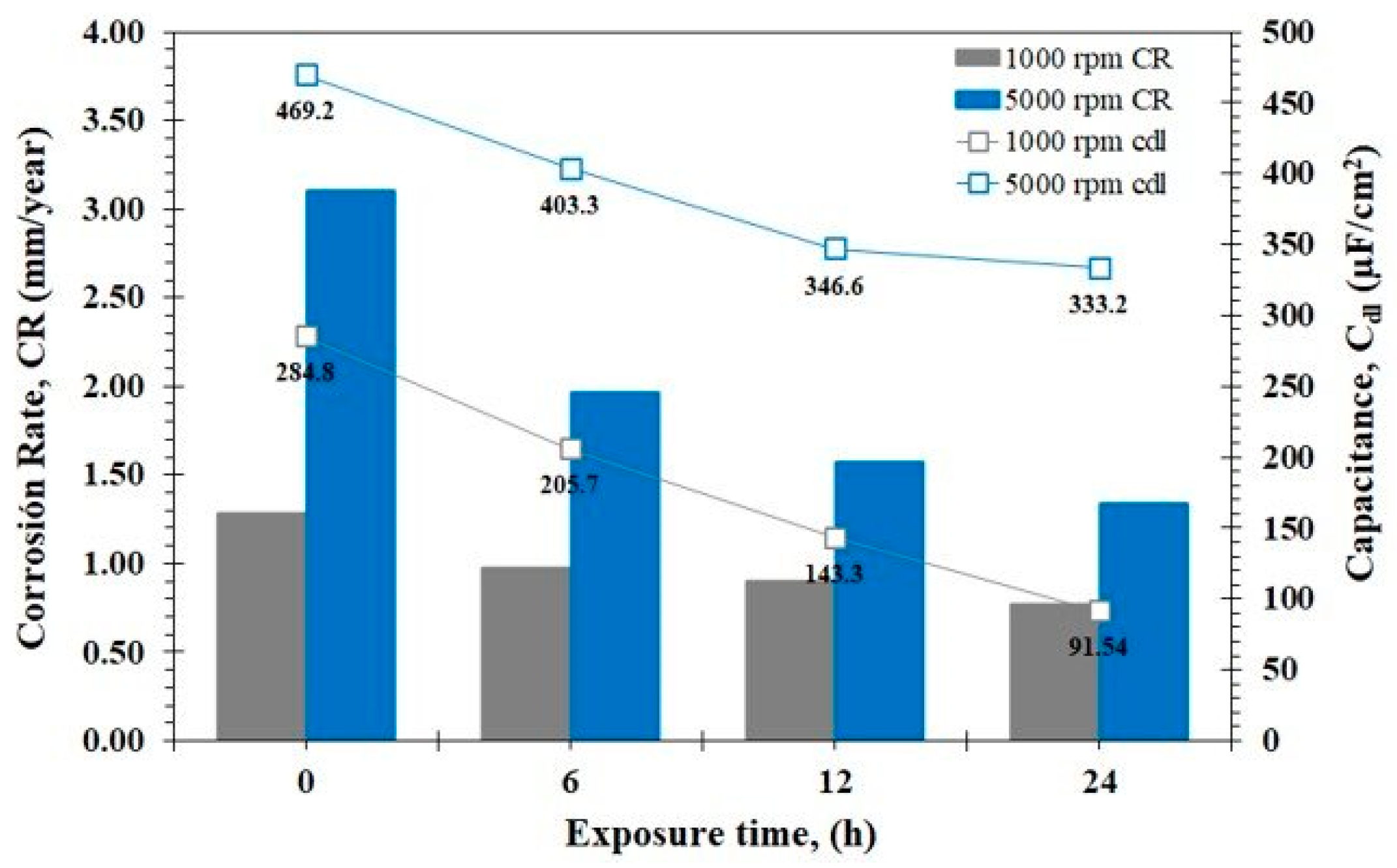
3.4. EIS Analysis
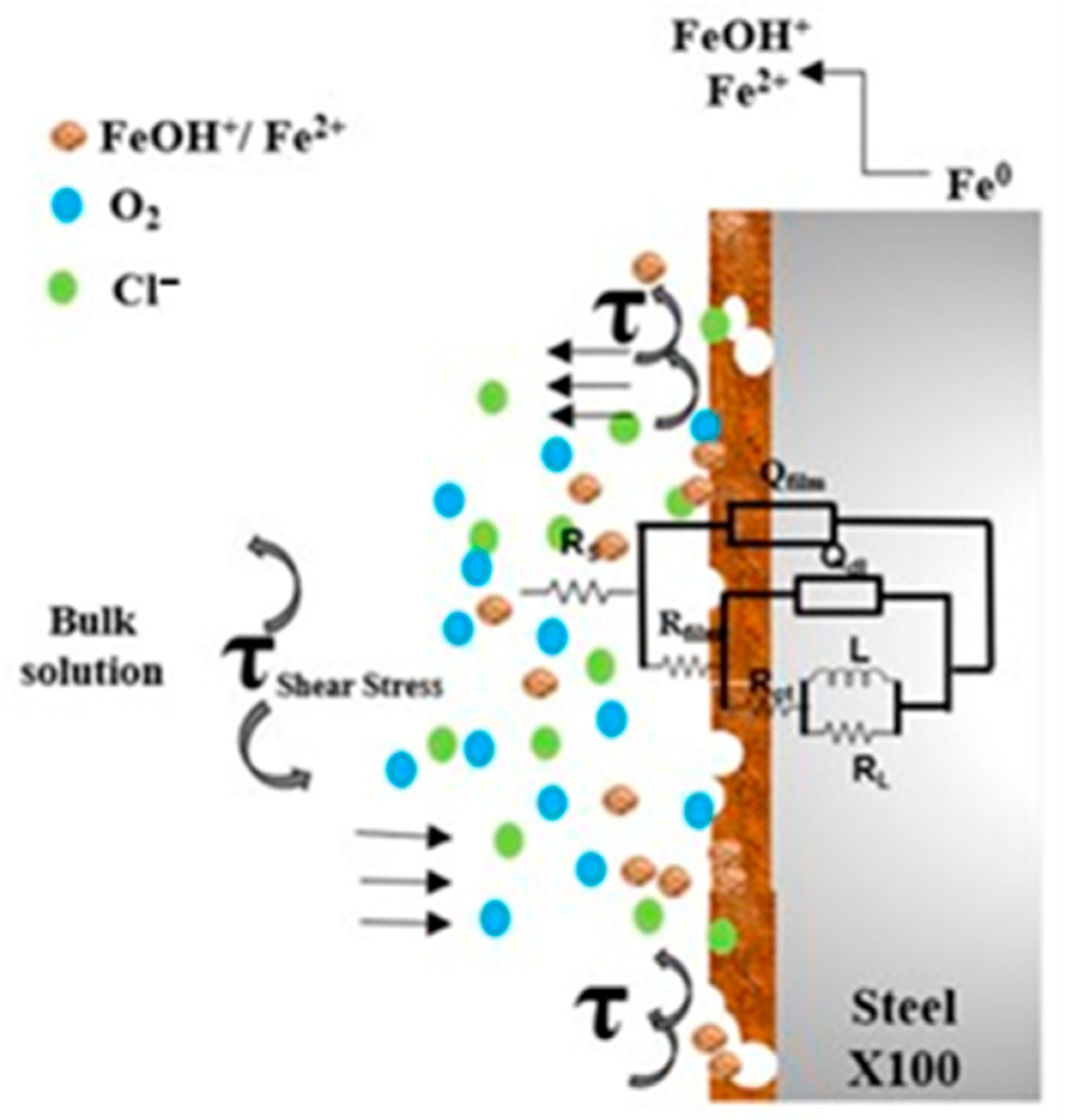
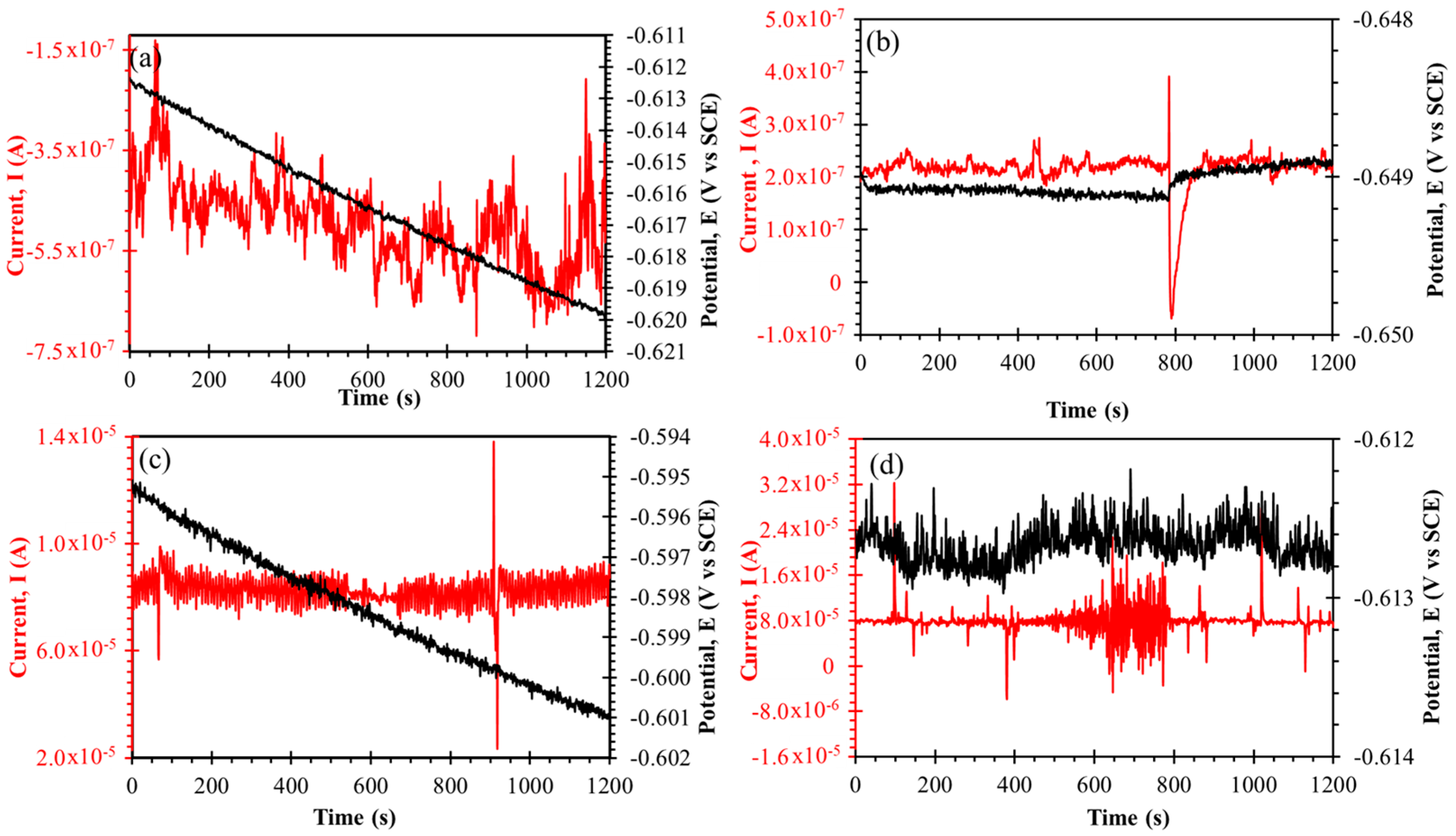
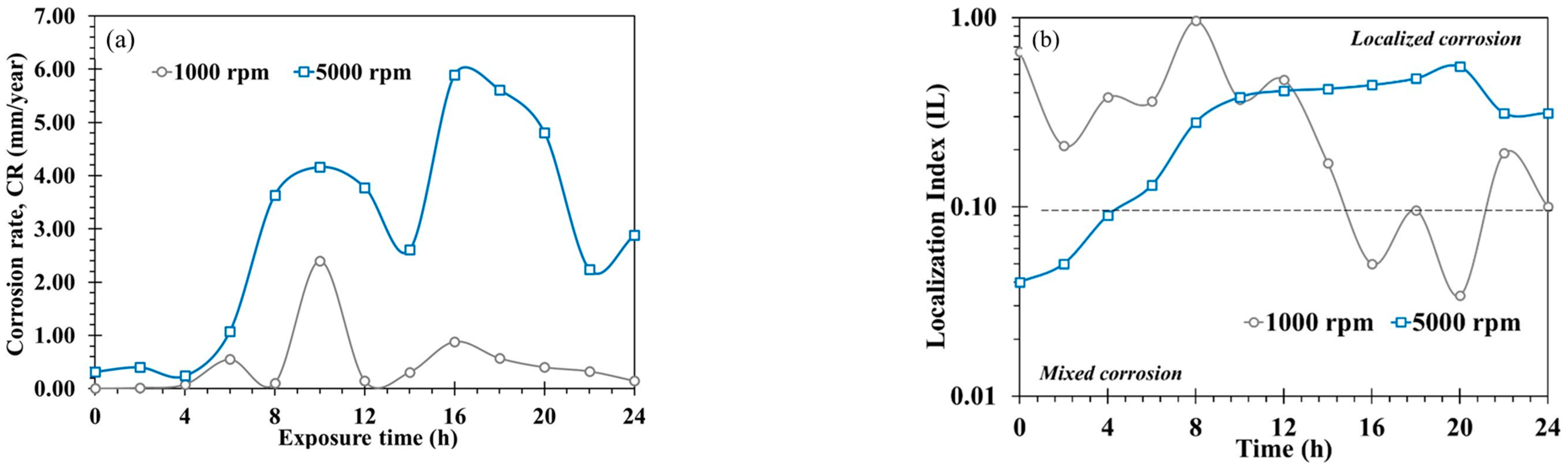
3.5. Electrochemical Noise (EN)
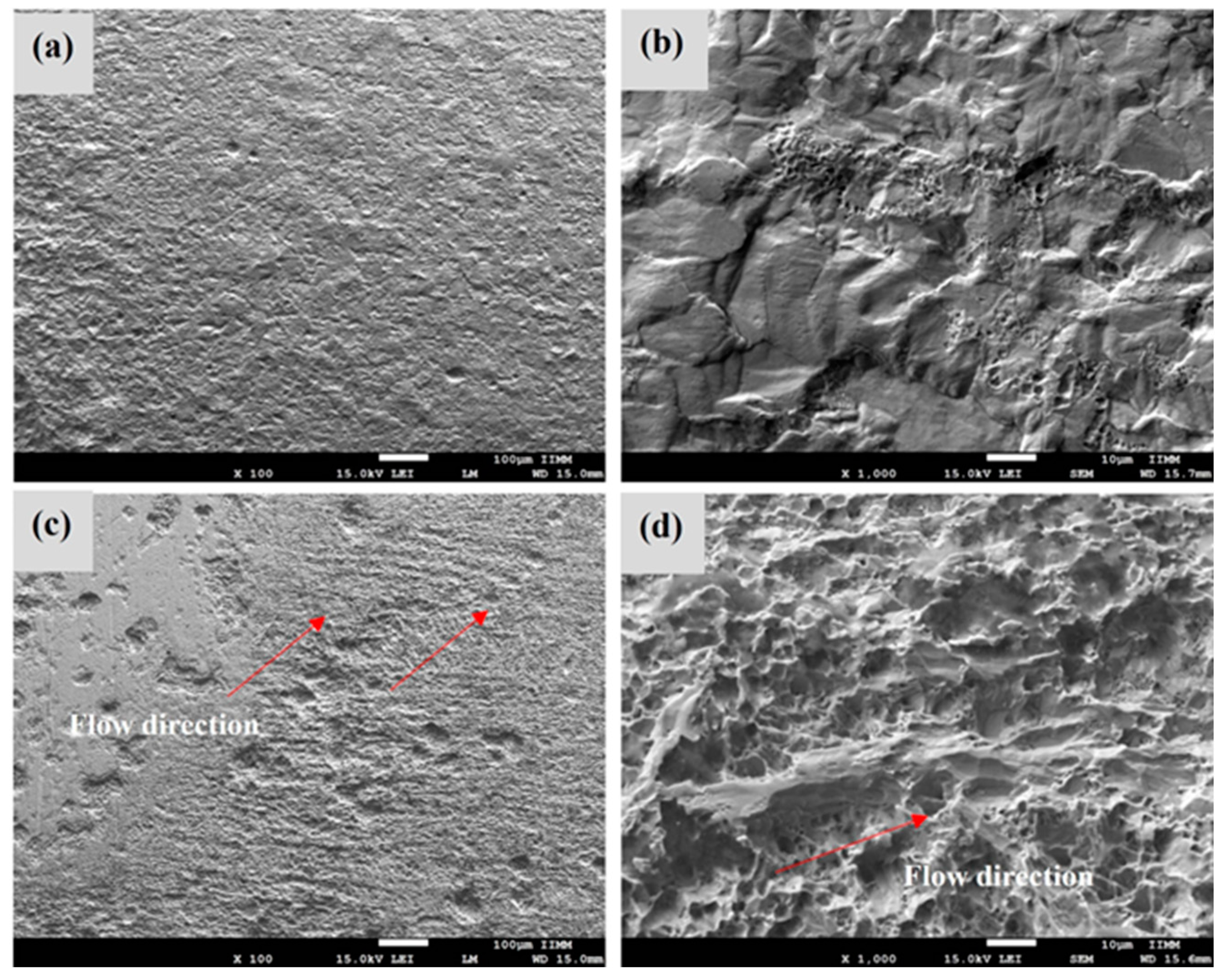
3.6. SEM Analysis of Corrosion Morphology
4. Conclusions
- The hydrodynamic parameters of the medium had a significant influence on the electrochemical behavior of the steel. The shear stress increased with increasing rotation rate and the thickness of the boundary layer decreased allowing mass transfer from bulk solution to the metal surface through diffusion and convection phenomena.
- The analysis of EIS revealed that there was an increase in CR of almost double at 5000 rpm with respect to the 1000 rpm system, Cdl showed a behavior directly proportional to CR.
- Statistical analysis of EN indicated that localized attack was more severe as rotation rate increased from 1000 to 5000 rpm. SEM micrograph confirmed this fact, the attacked metal surface showed a localized corrosion process, with deep pits nucleating, and greater wear was observed at 5000 rpm due to turbulent flow and shear stress.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadala, I.M.; Alfantazi, A. Electrochemical Behavior of API-X100 Pipeline Steel in NS4, near-Neutral, and Mildly Alkaline PH Simulated Soil Solutions. Corros. Sci. 2014, 82, 45–57. [Google Scholar] [CrossRef]
- Javidi, M.; Bahalaou Horeh, S. Investigating the Mechanism of Stress Corrosion Cracking in Near-Neutral and High PH Environments for API 5L X52 Steel. Corros. Sci. 2014, 80, 213–220. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B. External Corrosion of Oil and Gas Pipelines: A Review of Failure Mechanisms and Predictive Preventions. J. Nat. Gas. Sci. Eng. 2022, 100, 104467. [Google Scholar] [CrossRef]
- Villalobos, J.; Del-Pozo, A.; Campillo, B.; Mayen, J.; Serna, S. Microalloyed Steels through History until 2018: Review of Chemical Composition, Processing and Hydrogen Service. Metals 2018, 8, 351. [Google Scholar] [CrossRef]
- Al-Mansour, M.; Alfantazi, A.M.; El-boujdaini, M. Sulfide Stress Cracking Resistance of API-X100 High Strength Low Alloy Steel. Mater. Des. 2009, 30, 4088–4094. [Google Scholar] [CrossRef]
- Monschein, S.; Kapp, M.; Zügner, D.; Fasching, J.; Landefeld, A.; Schnitzer, R. Influence of Microalloying Elements and Deformation Parameters on the Recrystallization and Precipitation Behavior of Two Low-Alloyed Steels. Steel Res. Int. 2021, 92, 2100065. [Google Scholar] [CrossRef]
- Qian, L.-Y.; Fang, G.; Zeng, P.; Wang, L.-X. Correction of Flow Stress and Determination of Constitutive Constants for Hot Working of API X100 Pipeline Steel. Int. J. Press. Vessel. Pip. 2015, 132–133, 43–51. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, P.; Shi, Y.; Zhang, Y.; Yang, T. The Pitting Susceptibility Investigation of Passive Films Formed on X70, X80, and X100 Pipeline Steels by Electrochemical Noise and Mott-Schottky Measurements. Int. J. Corros. 2015, 2015, 298584. [Google Scholar] [CrossRef]
- Zhao, J.; Xiong, D.; Gu, Y.; Zeng, Q.; Tian, B. A Comparative Study on the Corrosion Behaviors of X100 Steel in Simulated Oilfield Brines under the Static and Dynamic Conditions. J. Pet. Sci. Eng. 2019, 173, 1109–1120. [Google Scholar] [CrossRef]
- Jin, T.Y.; Cheng, Y.F. In Situ Characterization by Localized Electrochemical Impedance Spectroscopy of the Electrochemical Activity of Microscopic Inclusions in an X100 Steel. Corros. Sci. 2011, 53, 850–853. [Google Scholar] [CrossRef]
- Bahgat Radwan, A.; Sliem, M.H.; Okonkwo, P.C.; Shibl, M.F.; Abdullah, A.M. Corrosion Inhibition of API X120 Steel in a Highly Aggressive Medium Using Stearamidopropyl Dimethylamine. J. Mol. Liq. 2017, 236, 220–231. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, C.; Lu, M.; Chai, C.; Wu, Y. Evaluation of Inhibition Efficiency of an Imidazoline Derivative in CO2-Containing Aqueous Solution. Mater. Chem. Phys. 2007, 105, 331–340. [Google Scholar] [CrossRef]
- Inman, S.B.; Han, J.; Wischhusen, M.A.; Qi, J.; Agnew, S.R.; Ogle, K.; Scully, J.R. Passivation and Localized Corrosion Resistance of Al0.3Cr0.5Fe2MoxNi1.5Ti0.3 Compositionally Complex Alloys: Effect of Mo Content. Corros. Sci. 2024, 227, 111692. [Google Scholar] [CrossRef]
- Nešić, S. Key Issues Related to Modelling of Internal Corrosion of Oil and Gas Pipelines—A Review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Askari, M.; Aliofkhazraei, M.; Ghaffari, S.; Hajizadeh, A. Film Former Corrosion Inhibitors for Oil and Gas Pipelines—A Technical Review. J. Nat. Gas. Sci. Eng. 2018, 58, 92–114. [Google Scholar] [CrossRef]
- El-Gammal, M.; Mazhar, H.; Cotton, J.S.; Shefski, C.; Pietralik, J.; Ching, C.Y. The Hydrodynamic Effects of Single-Phase Flow on Flow Accelerated Corrosion in a 90-Degree Elbow. Nucl. Eng. Des. 2010, 240, 1589–1598. [Google Scholar] [CrossRef]
- Kain, V. Flow Accelerated Corrosion: Forms, Mechanisms and Case Studies. Procedia Eng. 2014, 86, 576–588. [Google Scholar] [CrossRef]
- Wang, L.; Xing, Y.; Liu, Z.; Zhang, D.; Du, C.; Li, X. Erosion–Corrosion Behavior of 2205 Duplex Stainless Steel in Wet Gas Environments. J. Nat. Gas Sci. Eng. 2016, 35, 928–934. [Google Scholar] [CrossRef]
- Ajmal, T.S.; Arya, S.B.; Thippeswamy, L.R.; Quraishi, M.A.; Haque, J. Influence of Green Inhibitor on Flow-Accelerated Corrosion of API X70 Line Pipe Steel in Synthetic Oilfield Water. Corros. Eng. Sci. Technol. 2020, 55, 487–496. [Google Scholar] [CrossRef]
- Tan, Z.; Yang, L.; Zhang, D.; Wang, Z.; Cheng, F.; Zhang, M.; Jin, Y. Development Mechanism of Internal Local Corrosion of X80 Pipeline Steel. J. Mater. Sci. Technol. 2020, 49, 186–201. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Gu, Y.; Xiong, D.; Zeng, Q.; Tian, B. Experimental and Numerical Investigation into the Corrosion Performance of X100 Pipeline Steel under a Different Flow Rate in CO2-Saturated Produced Water. J. Solid State Electrochem. 2021, 25, 993–1006. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Liu, J.; Yin, C.; Qi, Y.; Zhou, J. Internal Localized Corrosion of X100 Pipeline Steel under Simulated Flow Conditions. J. Electroanal. Chem. 2023, 945, 117680. [Google Scholar] [CrossRef]
- Walsh, F.C.; Kear, G.; Nahlé, A.H.; Wharton, J.A.; Arenas, L.F. The Rotating Cylinder Electrode for Studies of Corrosion Engineering and Protection of Metals—An Illustrated Review. Corros. Sci. 2017, 123, 1–20. [Google Scholar] [CrossRef]
- Barmatov, E.; Hughes, T.; Nagl, M. Efficiency of Film-Forming Corrosion Inhibitors in Strong Hydrochloric Acid under Laminar and Turbulent Flow Conditions. Corros. Sci. 2015, 92, 85–94. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Obot, I.B.; Adesina, A.Y. Green Corrosion Inhibitor for Oilfield Application II: The Time–Evolution Effect on the Sweet Corrosion of API X60 Steel in Synthetic Brine and the Inhibition Performance of 2-(2-Pyridyl) Benzimidazole under Turbulent Hydrodynamics. Corros. Sci. 2020, 168, 108589. [Google Scholar] [CrossRef]
- Campechano-Lira, C.; Orozco-Cruz, R.; Bedolla-Jacuinde, A.; Contreras-Cuevas, A.; Galvan-Martinez, R. Electrochemical Analysis of an API X100 Steel Immersed in NACE Brine Solution with Addition of Corrosion Inhibitor. ECS Trans. 2019, 94, 81–89. [Google Scholar] [CrossRef]
- ASTM E-23-23a; Test Methods for Notched Bar Impact Testing of Metallic Materials. ASTM: West Conshohocken, PA, USA, 2023.
- ASTM E8; Test Methods for Tension Testing of Metallic Materials. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM E407-07; Practice for Microetching Metals and Alloys. ASTM: West Conshohocken, PA, USA, 2015.
- ASTM G1-03; Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM: West Conshohocken, PA, USA, 2017.
- NACE 1D182; Wheel Test Method Used for Evaluation of Film-Persistent Corrosion Inhibitors for Oilfield Applications. NACE International: Houston, TX, USA, 2017.
- Carretero Olalla, V.; Bliznuk, V.; Sanchez, N.; Thibaux, P.; Kestens, L.A.I.; Petrov, R.H. Analysis of the Strengthening Mechanisms in Pipeline Steels as a Function of the Hot Rolling Parameters. Mater. Sci. Eng. A 2014, 604, 46–56. [Google Scholar] [CrossRef]
- Nking’wa, A.A.; Gao, K. Study on Stress Corrosion Cracking of X100 Pipeline Steel in NS4 Solution. J. Fail. Anal. Prev. 2024, 24, 1934–1944. [Google Scholar] [CrossRef]
- Wang, Z.X.; Zhang, R.F.; Chao, Y.J.; Lam, P.S. Elastic-Plastic Constraint Analysis of Semi-Elliptic Surface Cracks in X100 Pipeline Steel. In Proceedings of the ASME 2011 Pressure Vessels and Piping Conference. Volume 6: Materials and Fabrication, Parts A and B, Baltimore, MD, USA, 17–21 July 2011; pp. 1325–1334. [Google Scholar]
- Xu, L.Y.; Cheng, Y.F. Corrosion of X100 Pipeline Steel under Plastic Strain in a Neutral PH Bicarbonate Solution. Corros. Sci. 2012, 64, 145–152. [Google Scholar] [CrossRef]
- Yang, X.-L.; Xu, Y.-B.; Tan, X.-D.; Wu, D. Relationships among Crystallographic Texture, Fracture Behavior and Charpy Impact Toughness in API X100 Pipeline Steel. Mater. Sci. Eng. A 2015, 641, 96–106. [Google Scholar] [CrossRef]
- Nafisi, S.; Arafin, M.A.; Collins, L.; Szpunar, J. Texture and Mechanical Properties of API X100 Steel Manufactured under Various Thermomechanical Cycles. Mater. Sci. Eng. A 2012, 531, 2–11. [Google Scholar] [CrossRef]
- Bianchi, G.L.; Acosta, V.; Seijas, C. A Combination of Laboratory Testing, RCE, and Corrosion Loop for Inhibitor Selection. Appl. Sci. 2023, 13, 4586. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Li, J.; Song, L.; Zhang, X.; Liu, Z. Flow-Accelerated Corrosion Behavior of 13Cr Stainless Steel in a Wet Gas Environment Containing CO2. Int. J. Miner. Metall. Mater. 2018, 25, 779–787. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, J.; Wang, Z.M. Exploring the Dynamic Mechanism of Water Wetting Induced Corrosion on Differently Pre-Wetted Surfaces in Oil–Water Flows. J. Colloid Interface Sci. 2024, 664, 284–298. [Google Scholar] [CrossRef]
- Gabe, D.R.; Walsh, F.C. The Rotating Cylinder Electrode: A Review of Development. J. Appl. Electrochem. 1983, 13, 3–21. [Google Scholar] [CrossRef]
- Yabuki, A.; Murakami, M. Breakaway Properties of Film Formed on Copper and Copper Alloys in Erosion–Corrosion by Mass Transfer Equation. Mater. Corros. 2008, 59, 25–31. [Google Scholar] [CrossRef]
- Córdoba-Torres, P.; Keddam, M.; Nogueira, R.P. On the Intrinsic Electrochemical Nature of the Inductance in EIS. Electrochim. Acta 2008, 54, 518–523. [Google Scholar] [CrossRef]
- Du, C.; Zhao, T.; Liu, Z.; Li, X.; Zhang, D. Corrosion Behavior and Characteristics of the Product Film of API X100 Steel in Acidic Simulated Soil Solution. Int. J. Miner. Metall. Mater. 2016, 23, 176–183. [Google Scholar] [CrossRef]
- Schrader, L.U.; Amin, S.; Brandt, L. Transition to Turbulence in the Boundary Layer over a Smooth and Rough Swept Plate Exposed to Free-Stream Turbulence. J. Fluid. Mech. 2010, 646, 297–325. [Google Scholar] [CrossRef]
- Nesic, S. Effects of Multiphase Flow on Internal CO2 Corrosion of Mild Steel Pipelines. Energy Fuels 2012, 26, 4098–4111. [Google Scholar] [CrossRef]
- Heitz, E. Chemo-Mechanical Effects of Flow on Corrosion. Corrosion 1991, 47, 135–145. [Google Scholar] [CrossRef]
- Farelas, F.; Galicia, M.; Brown, B.; Nesic, S.; Castaneda, H. Evolution of Dissolution Processes at the Interface of Carbon Steel Corroding in a CO2 Environment Studied by EIS. Corros. Sci. 2010, 52, 509–517. [Google Scholar] [CrossRef]
- Xu, Y.; Tan, M.Y. Probing the Initiation and Propagation Processes of Flow Accelerated Corrosion and Erosion Corrosion under Simulated Turbulent Flow Conditions. Corros. Sci. 2019, 151, 163–174. [Google Scholar] [CrossRef]
- Bommersbach, P.; Alemany-Dumont, C.; Millet, J.-P.; Normand, B. Hydrodynamic Effect on the Behaviour of a Corrosion Inhibitor Film: Characterization by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2006, 51, 4011–4018. [Google Scholar] [CrossRef]
- Orazem, M.E.; Pébère, N.; Tribollet, B. Enhanced Graphical Representation of Electrochemical Impedance Data. J. Electrochem. Soc. 2006, 153, B129. [Google Scholar] [CrossRef]
- Markhali, B.P.; Naderi, R.; Mahdavian, M.; Sayebani, M.; Arman, S.Y. Electrochemical Impedance Spectroscopy and Electrochemical Noise Measurements as Tools to Evaluate Corrosion Inhibition of Azole Compounds on Stainless Steel in Acidic Media. Corros. Sci. 2013, 75, 269–279. [Google Scholar] [CrossRef]
- Córdoba-Torres, P.; Keddam, M.; Nogueira, R.P. On the Intrinsic Electrochemical Nature of the Inductance in EIS—A Monte Carlo Simulation of the Two-Consecutive Steps Mechanism: The Rough 3D Case and the Surface Relaxation Effect. Electrochim. Acta 2009, 54, 6779–6787. [Google Scholar] [CrossRef]
- Umoren, S.A.; Madhankumar, A. Effect of Addition of CeO2 Nanoparticles to Pectin as Inhibitor of X60 Steel Corrosion in HCl Medium. J. Mol. Liq. 2016, 224, 72–82. [Google Scholar] [CrossRef]
- Ortega-Toledo, D.M.; Gonzalez-Rodriguez, J.G.; Casales, M.; Caceres, A.; Martinez, L. Hydrodynamic Effects on the CO2 Corrosion Inhibition of X-120 Pipeline Steel by Carboxyethyl-Imidazoline. Int. J. Electrochem. Sci. 2011, 6, 778–792. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The Analysis of Electrode Impedances Complicated by the Presence of a Constant Phase Element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- ASTM G102; Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM: West Conshohocken, PA, USA, 2015.
- Schmitt, H.G.; Bakalli, M. Flow Assisted Corrosion. In Shreir’s Corrosion; Elsevier: Amsterdam, The Netherlands, 2010; pp. 954–987. [Google Scholar]
- Cheng, Y.F.; Luo, J.L.; Wilmott, M. Spectral Analysis of Electrochemical Noise with Different Transient Shapes. Electrochim. Acta 2000, 45, 1763–1771. [Google Scholar] [CrossRef]
- Montoya-Rangel, M.; Garza-Montes de Oca, N.; Gaona-Tiburcio, C.; Colás, R.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Electrochemical Noise Measurements of Advanced High-Strength Steels in Different Solutions. Metals 2020, 10, 1232. [Google Scholar] [CrossRef]
- Mansfeld, F.; Sun, Z. Technical Note: Localization Index Obtained from Electrochemical Noise Analysis. Corrosion 1999, 55, 915–918. [Google Scholar] [CrossRef]


| Element | Fe | C | Mn | Si | Cu | Ni | Cr | Mo | Nb | V | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 96.39 | 0.05 | 1.75 | 0.36 | 0.51 | 0.29 | 0.36 | 0.19 | 0.03 | 0.03 | 0.04 |
| Stress-Strength | Charpy Impact | Vickers Hardness | |
|---|---|---|---|
| YS-0.02 % | UTS | Impact energy | HV |
| (MPa) | (MPa) | (J) | (kgf·mm2) |
| 641 | 891.5 | 120 | 220 |
| Rotation Velocity (rpm) | Reynolds Number ReRCE | Shear Stress τRCE (N/m2) | Thickness Boundary Layer δBL (m) | Corrosion Potential Ecorr (V vs. SCE) |
|---|---|---|---|---|
| 0 | - | - | - | −0.680 |
| 1000 | 8157.8 | 2.18 | 2.18 × 10−10 | −0.600 |
| 5000 | 40,789.1 | 33.72 | 7.074 × 10−11 | −0.550 |
| Rotation Velocity | Exposure Time | χ2 | Rs | Qfilm | Rfilm | Qdl | Rct | L | RL | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (rpm) | (h) | (Ω·cm2) | Yo | n | (Ω·cm2) | Yo | n | (Ω·cm2) | (H) | (Ω·cm2) | |
| 1000 | 0 | 2.11 × 10−3 | 9.20 | 8.32 × 10−4 | 0.73 | 28.10 | 3.35 × 10−4 | 0.94 | 235 | 1174 | 91.70 |
| 6 | 4.15 × 10−3 | 9.70 | 7.83 × 10−4 | 0.71 | 30.50 | 3.57 × 10−4 | 0.80 | 309 | 1527 | 309.00 | |
| 12 | 2.89 × 10−3 | 23.10 | 7.42 × 10−4 | 0.72 | 35.00 | 2.33 × 10−4 | 0.84 | 335 | 1889 | 314.10 | |
| 24 | 6.25 × 10−3 | 29.70 | 6.88 × 10−4 | 0.701 | 52.00 | 1.34 × 10−4 | 0.89 | 392 | 1941 | 340.0 | |
| 5000 | 0 | 1.85 × 10−3 | 5.10 | 2.24 × 10−3 | 0.80 | 49.56 | 5.28 × 10−3 | 0.85 | 97 | 880 | 96.00 |
| 6 | 2.50 × 10−3 | 10.37 | 1.84 × 10−3 | 0.71 | 49.36 | 4.55 × 10−3 | 0.75 | 153 | 954 | 76.00 | |
| 12 | 2.52 × 10−3 | 20.11 | 1.39 × 10−3 | 0.80 | 28.00 | 3.76 × 10−3 | 0.80 | 192 | 786 | 132.20 | |
| 24 | 2.46 × 10−4 | 23.84 | 1.24 × 10−3 | 0.80 | 30.00 | 3.54 × 10−3 | 0.80 | 225 | 765 | 178.90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galván-Martínez, R.; Campechano-Lira, C.; Orozco-Cruz, R.; Hernández-Pérez, M.Á.; López-Huerta, F.; Mejía-Sánchez, E.; Ramírez-Cano, J.A.; Carmona-Hernández, A. Effect of Hydrodynamic Conditions on the Corrosion Mechanism of HSLA X100 Steel by EIS and EN Analysis. Surfaces 2025, 8, 10. https://doi.org/10.3390/surfaces8010010
Galván-Martínez R, Campechano-Lira C, Orozco-Cruz R, Hernández-Pérez MÁ, López-Huerta F, Mejía-Sánchez E, Ramírez-Cano JA, Carmona-Hernández A. Effect of Hydrodynamic Conditions on the Corrosion Mechanism of HSLA X100 Steel by EIS and EN Analysis. Surfaces. 2025; 8(1):10. https://doi.org/10.3390/surfaces8010010
Chicago/Turabian StyleGalván-Martínez, Ricardo, Clarisa Campechano-Lira, Ricardo Orozco-Cruz, Miguel Ángel Hernández-Pérez, Francisco López-Huerta, Edgar Mejía-Sánchez, Jorge Alberto Ramírez-Cano, and Andres Carmona-Hernández. 2025. "Effect of Hydrodynamic Conditions on the Corrosion Mechanism of HSLA X100 Steel by EIS and EN Analysis" Surfaces 8, no. 1: 10. https://doi.org/10.3390/surfaces8010010
APA StyleGalván-Martínez, R., Campechano-Lira, C., Orozco-Cruz, R., Hernández-Pérez, M. Á., López-Huerta, F., Mejía-Sánchez, E., Ramírez-Cano, J. A., & Carmona-Hernández, A. (2025). Effect of Hydrodynamic Conditions on the Corrosion Mechanism of HSLA X100 Steel by EIS and EN Analysis. Surfaces, 8(1), 10. https://doi.org/10.3390/surfaces8010010











