Mapping Acid–Base Sites on Anatase Titania (100) and (101) Surfaces by Density Functional Theory: The Link Between Lewis Acidity and the Surface Ability to Flex
Abstract
1. Introduction
2. Experimental Section and Computational Methods
3. Results
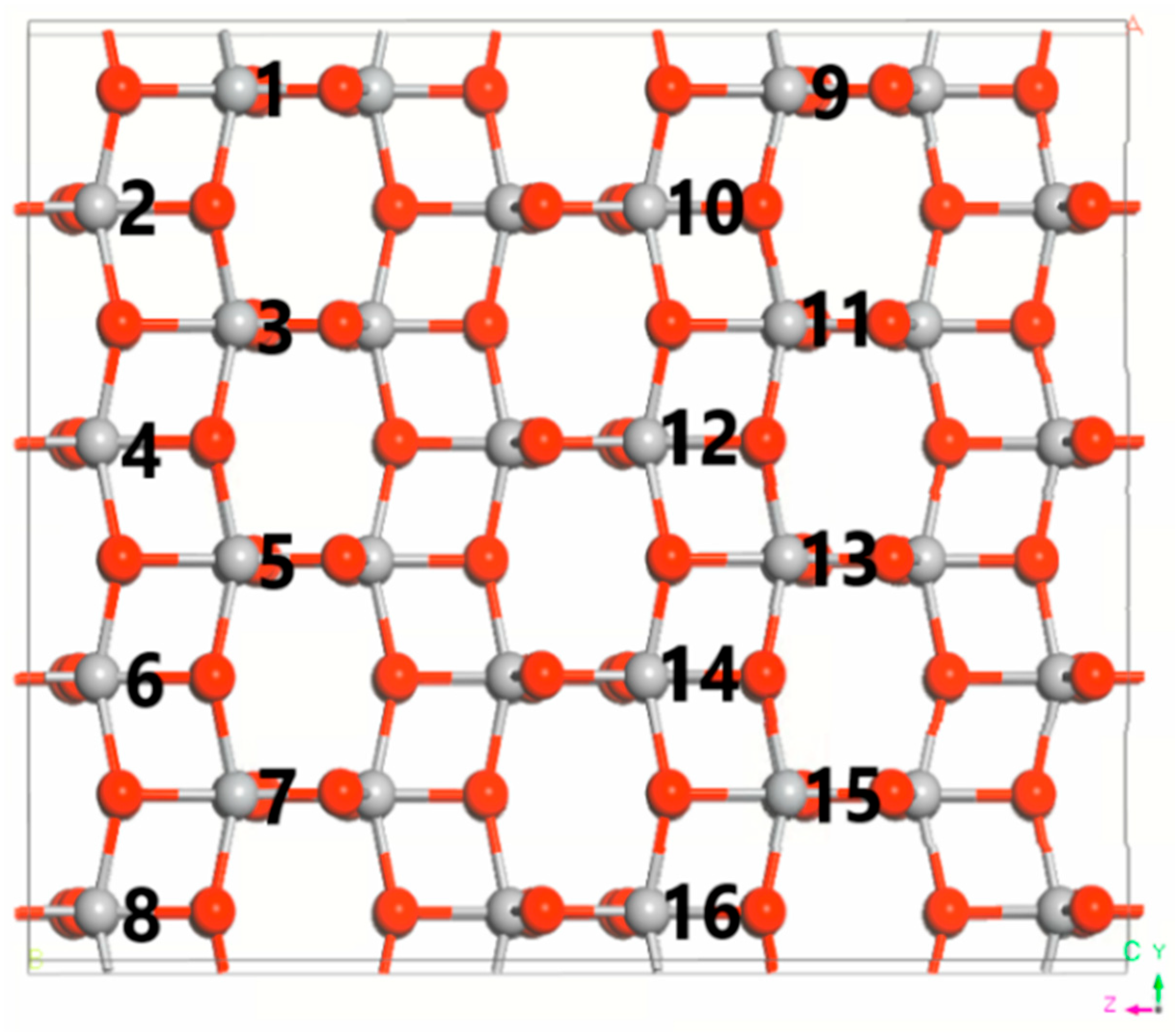
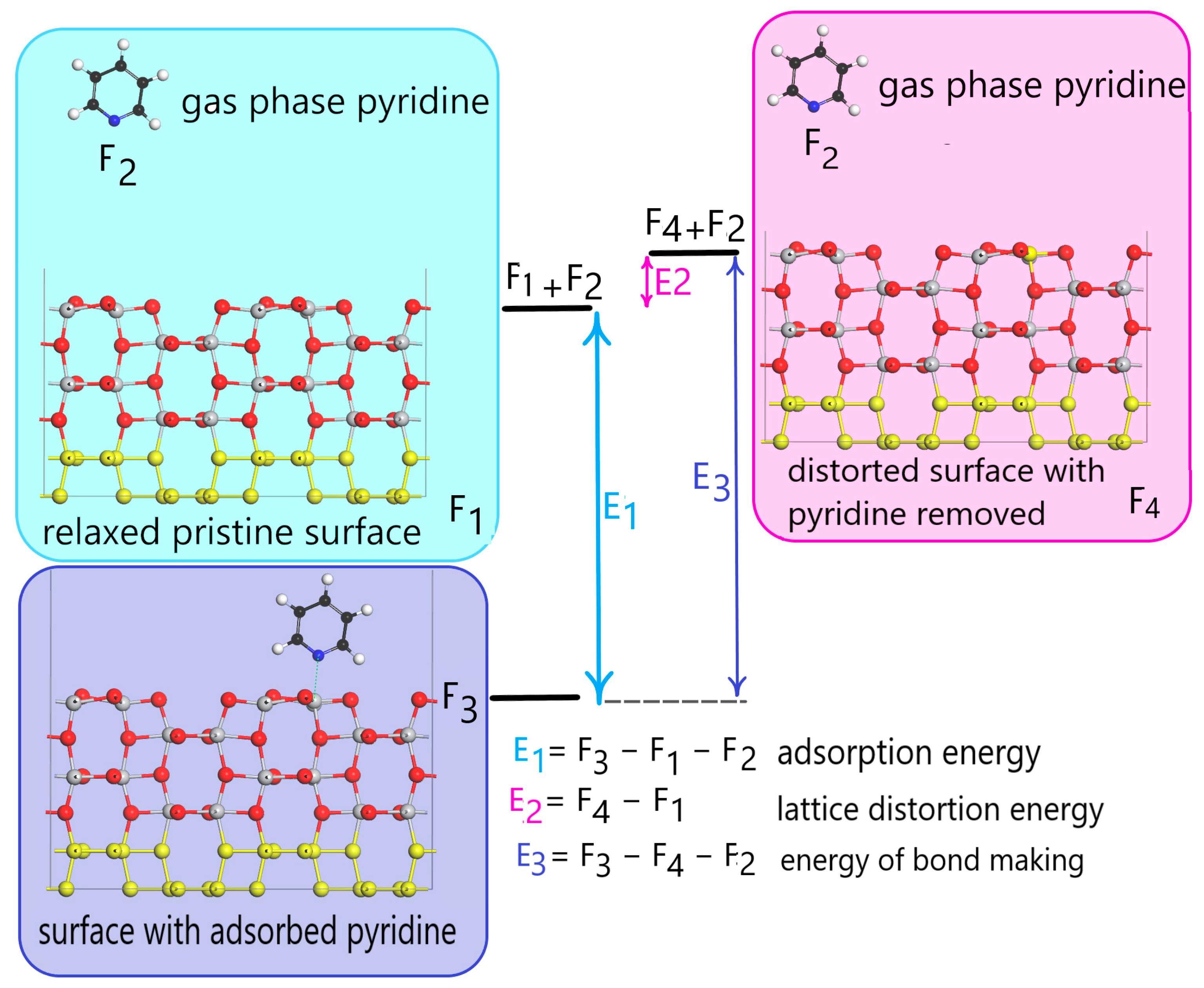
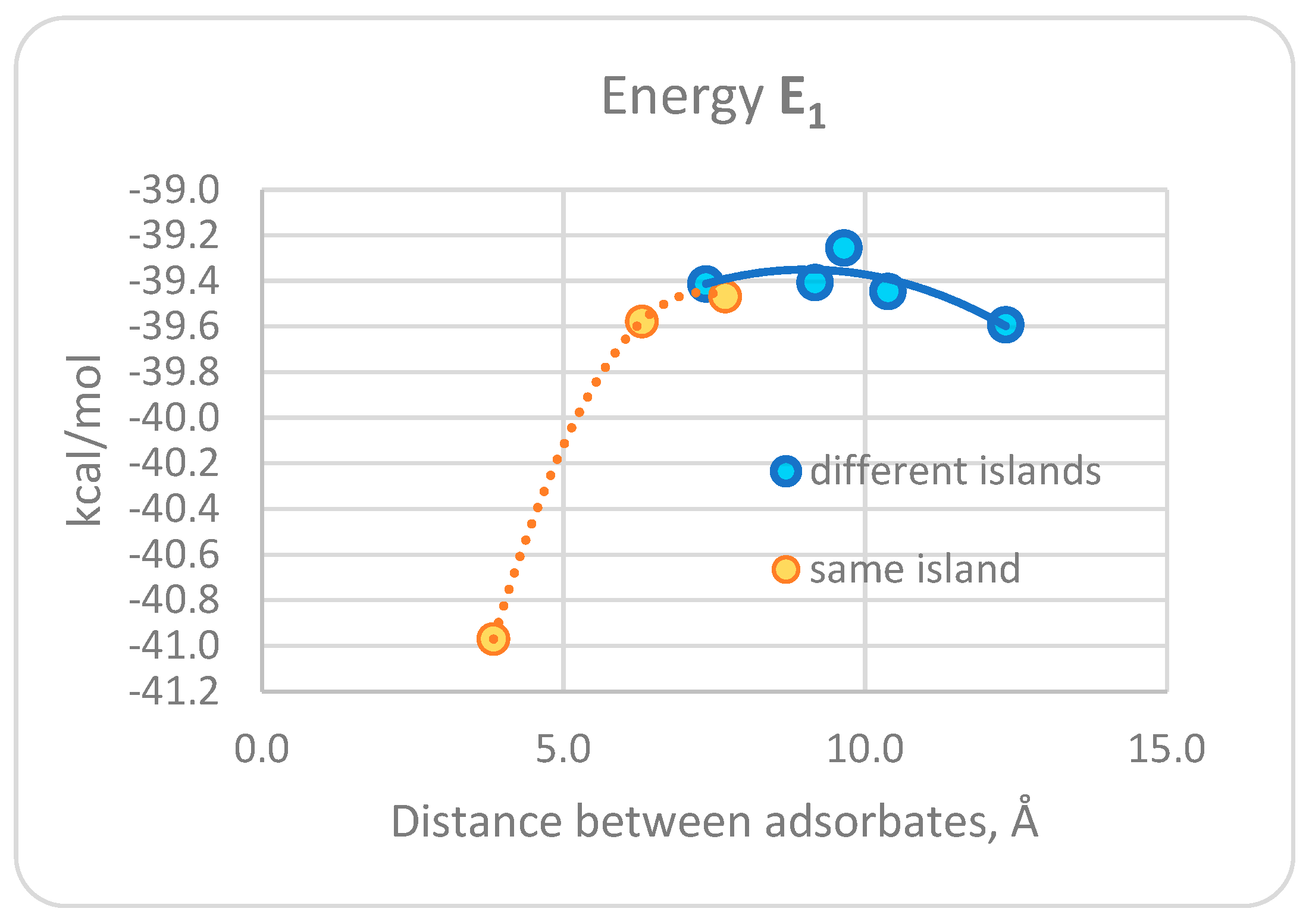
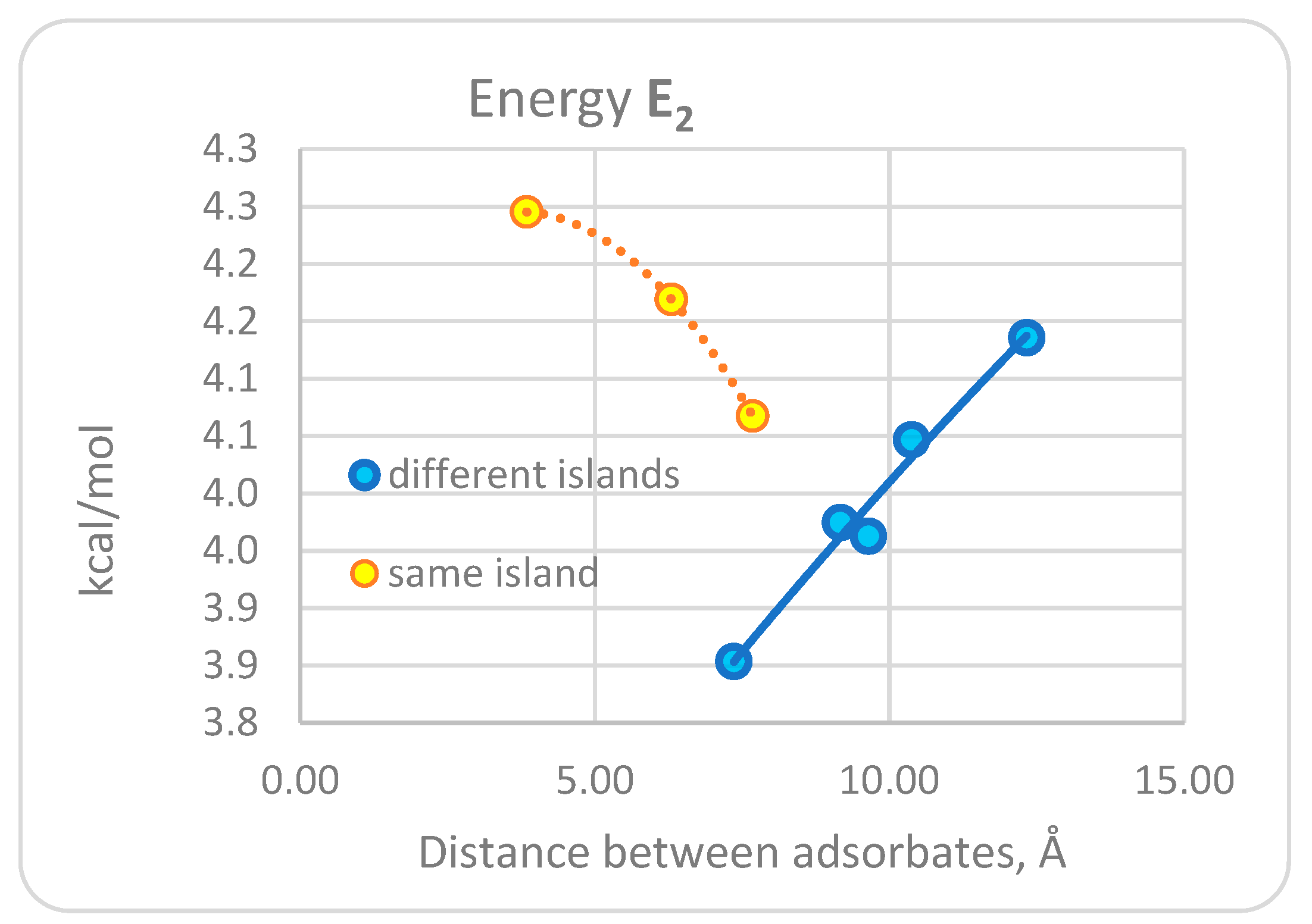
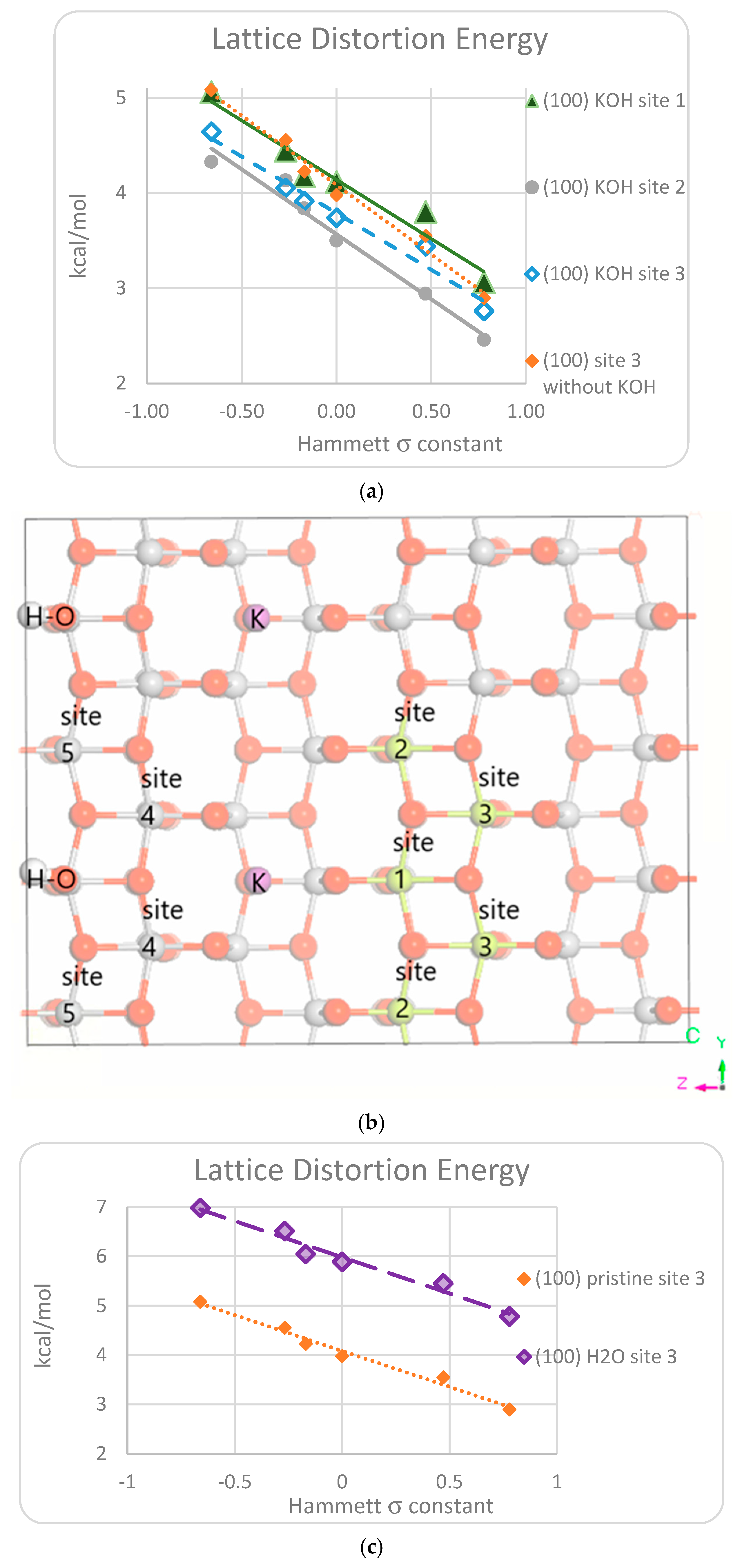
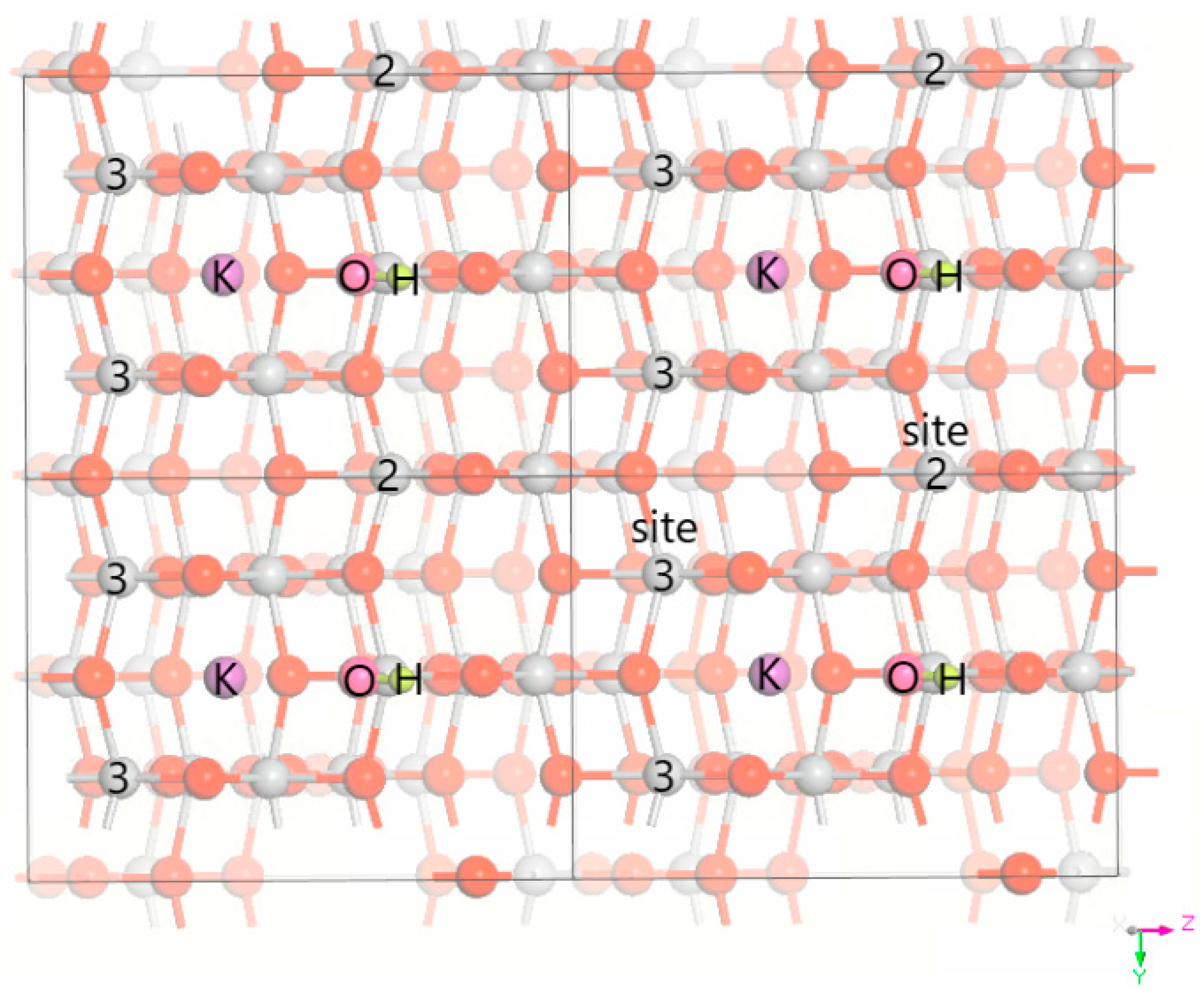
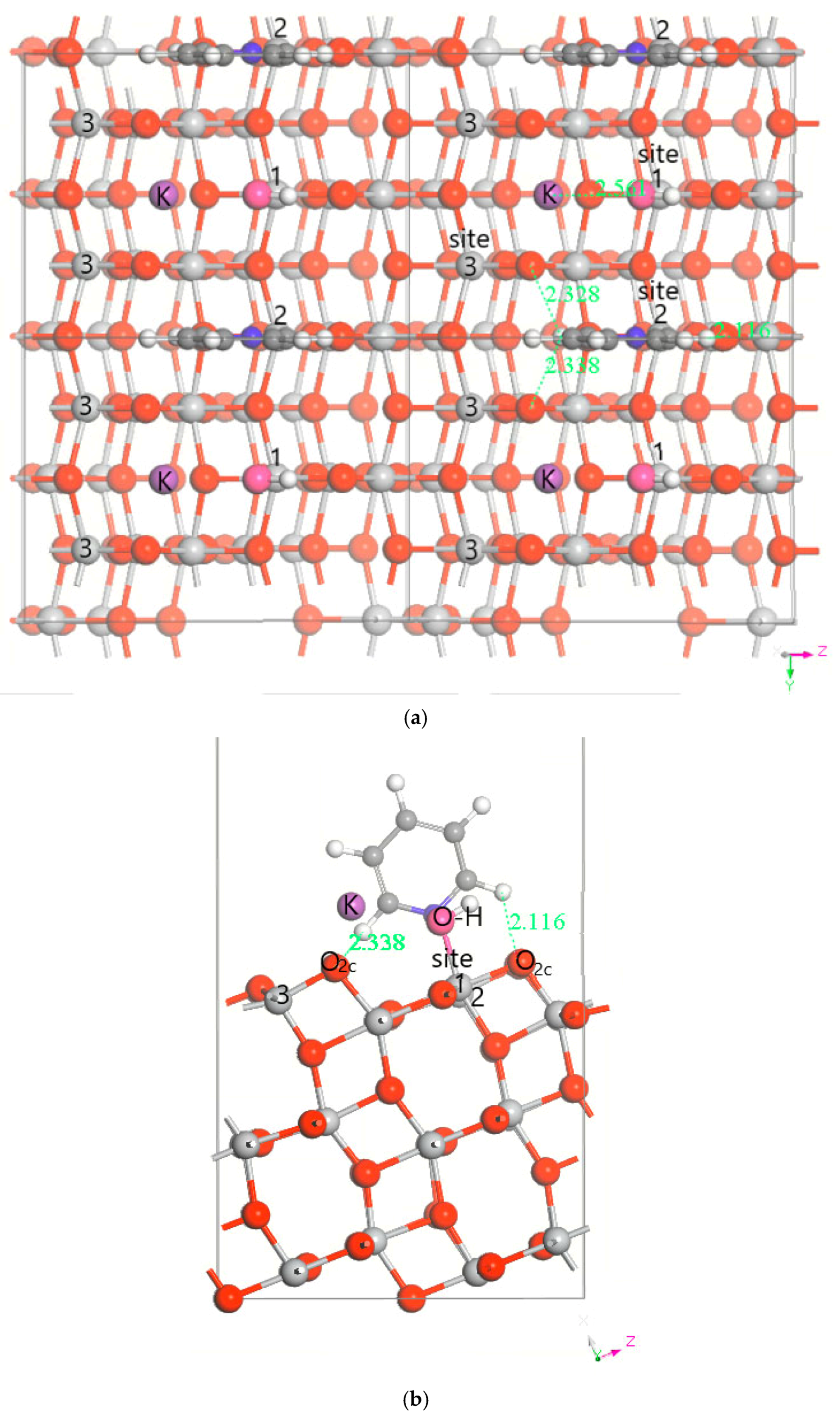
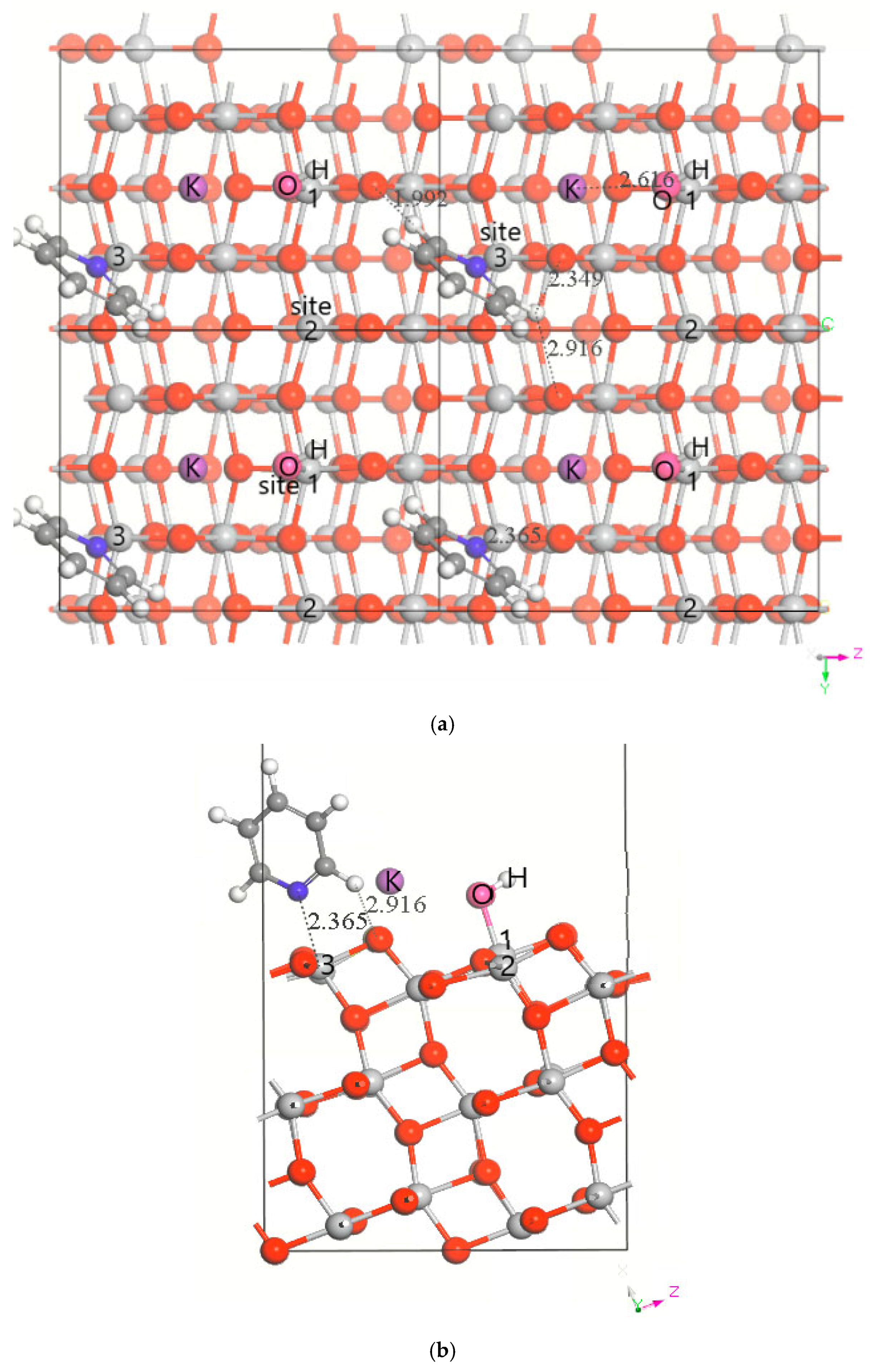
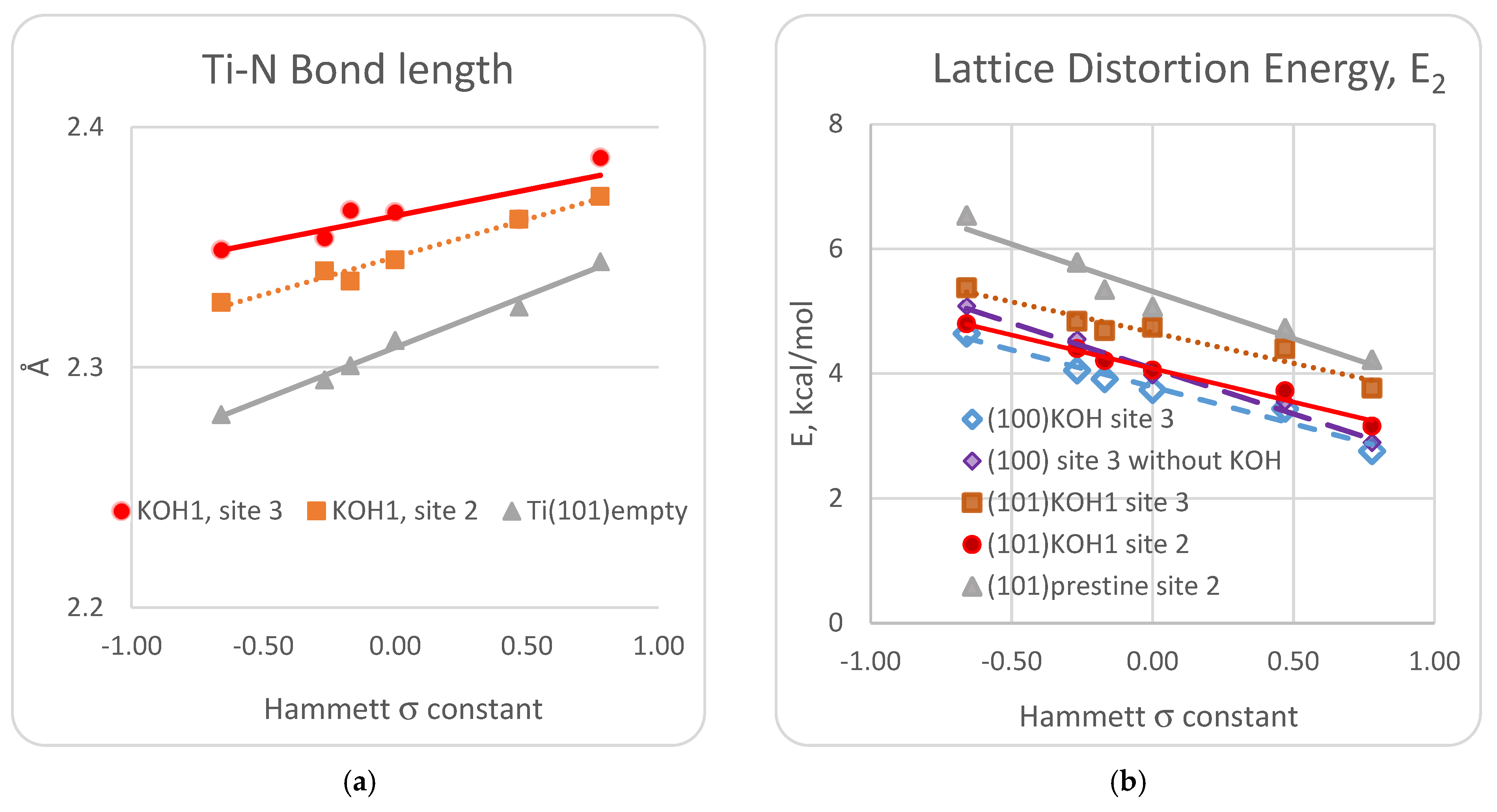
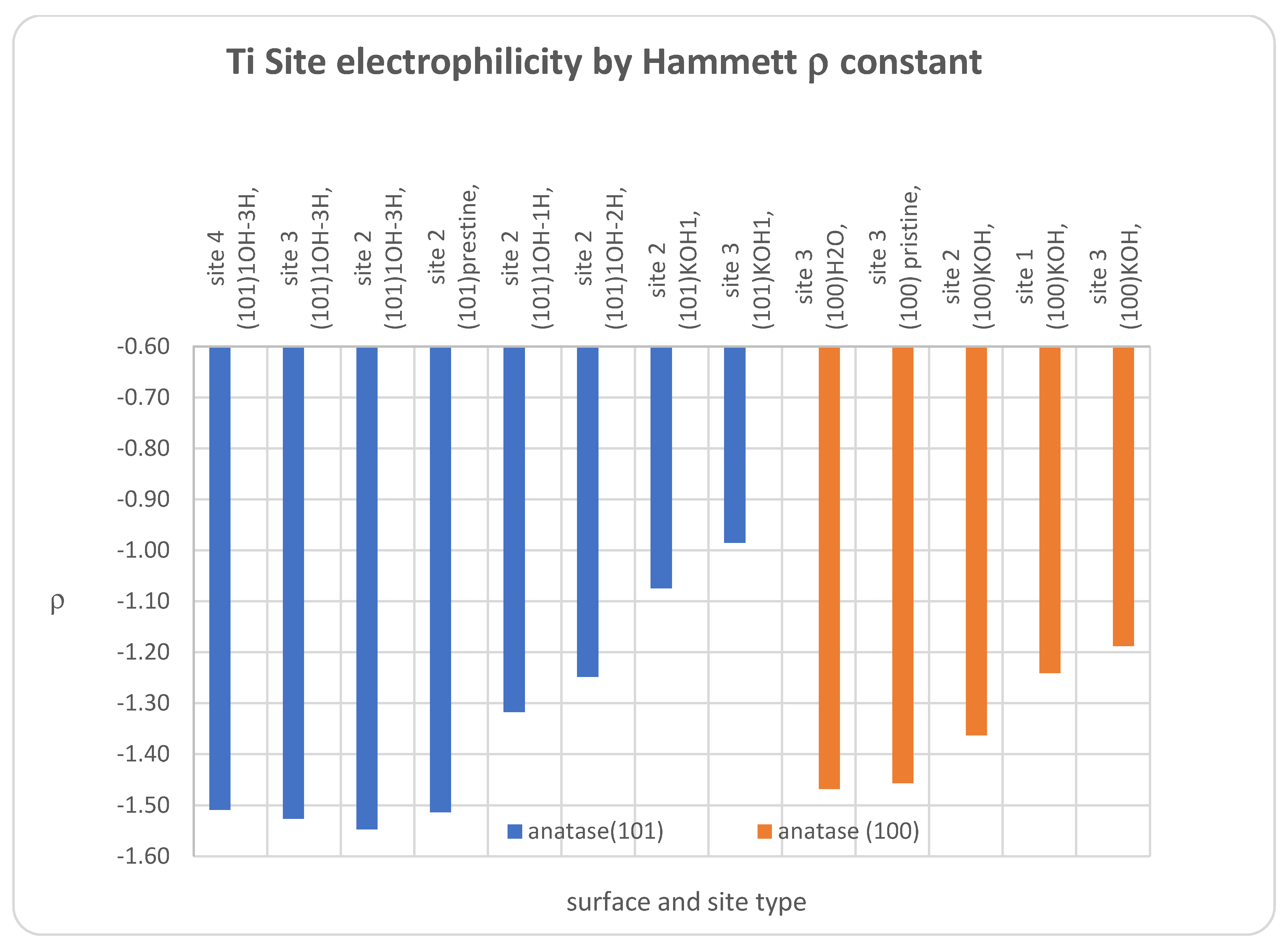
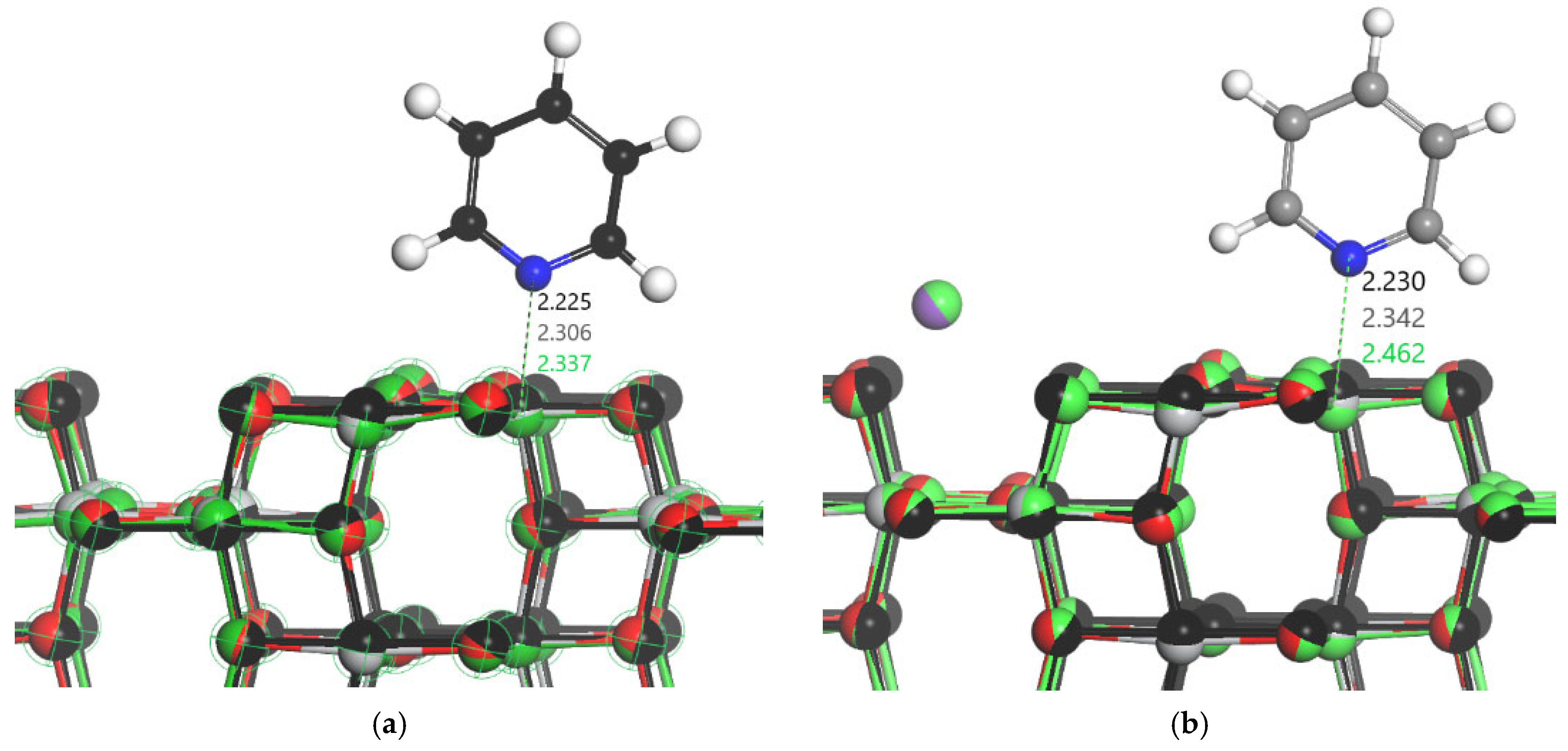
3.1. DFT Computational Study of Adsorption
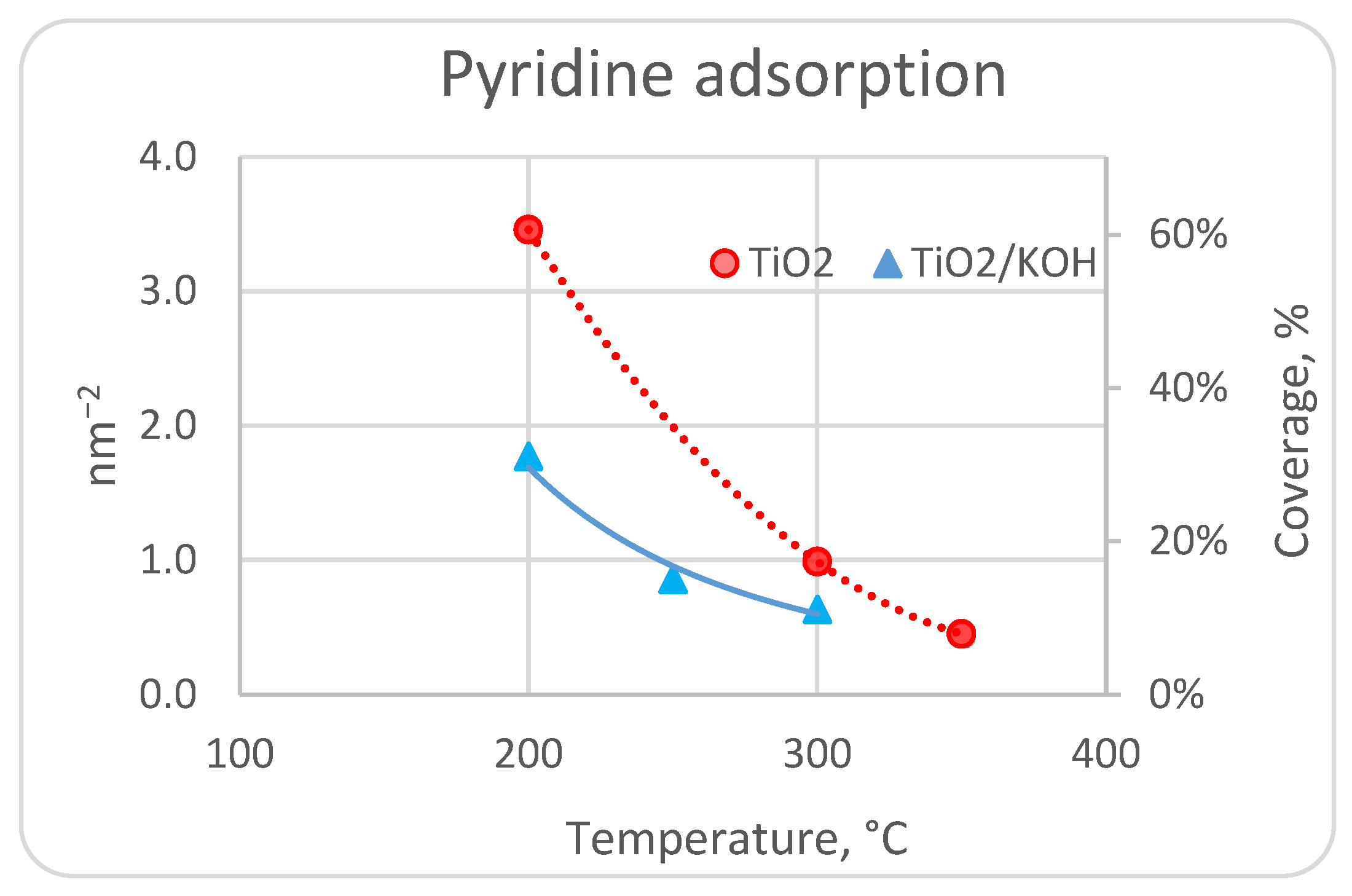
3.2. Experimental Study of Pyridine Adsorption in a Pulse Microreactor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tsang, C.H.A.; Li, K.; Zeng, Y.; Zhao, W.; Zhang, T.; Zhan, Y.; Xie, R.; Leung, D.Y.C.; Huang, H. Titanium Oxide Based Photocatalytic Materials Development and Their Role of in the Air Pollutants Degradation: Overview and Forecast. Environ. Int. 2019, 125, 200–228. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, A. Titanium-Doped Alumina for Catalytic Dehydration of Methanol to Dimethyl Ether at Relatively Low Temperatures. Fuel 2011, 90, 2422–2427. [Google Scholar] [CrossRef]
- Ignatchenko, A.V. Multiscale Approach for the Optimization of Ketones Production from Carboxylic Acids by the Decarboxylative Ketonization Reaction. Catal. Today 2019, 338, 3–17. [Google Scholar] [CrossRef]
- Parida, K.; Mishra, H.K. Catalytic Ketonisation of Acetic Acid over Modified Zirconia. J. Mol. Catal. A Chem. 1999, 139, 73–80. [Google Scholar] [CrossRef]
- Ignatchenko, A.V.; King, M.M. Catalyst for the Production of Methyl Isopropyl Ketone. U.S. Patent 7,501,379, 10 March 2009. [Google Scholar]
- Beavers, W.A.; Ignatchenko, A.V.; Liu, Z.; Ashcroft, C.D.; White, T.M. Catalyst and Process for the Preparation of Unsymmetrical Ketones. U.S. Patent 2007/0100166, 3 May 2007. [Google Scholar]
- Pham, T.N.; Sooknoi, T.; Crossley, S.P.; Resasco, D.E. Ketonization of Carboxylic Acids: Mechanisms, Catalysts, and Implications for Biomass Conversion. ACS Catal. 2013, 3, 2456–2473. [Google Scholar] [CrossRef]
- Ignatchenko, A.V.; DeRaddo, J.S.; Marino, V.J.; Mercado, A. Cross-Selectivity in the Catalytic Ketonization of Carboxylic Acids. Appl. Catal. A Gen. 2015, 498, 10–24. [Google Scholar] [CrossRef][Green Version]
- Ignatchenko, A.V.; Springer, M.E.; Walker, J.D.; Brennessel, W.W. Alkyl Substituted Beta-Keto Acids: Molecular Structure and Decarboxylation Kinetics in Aqueous Solution and on the Surface of Metal Oxides. J. Phys. Chem. C 2021, 125, 3368–3384. [Google Scholar] [CrossRef]
- Marie, O.; Ignatchenko, A.V.; Renz, M. Methyl Ketones from Carboxylic Acids as Valuable Target Molecules in the Biorefinery. Catal. Today 2020, 367, 258–267. [Google Scholar] [CrossRef]
- Ignatchenko, A.V.; Diprospero, T.J.; Patel, H.; Lapenna, J.R. Equilibrium in the Catalytic Condensation of Carboxylic Acids with Methyl Ketones to 1,3-Diketones and the Origin of the Reketonization Effect. ACS Omega 2019, 4, 11032–11043. [Google Scholar] [CrossRef]
- Ding, S.; Wang, H.; Han, J.; Zhu, X.; Ge, Q. Ketonization of Propionic Acid to 3-Pentanone over CexZr1−XO2 Catalysts: The Importance of Acid-Base Balance. Ind. Eng. Chem. Res. 2018, 57, 17086–17096. [Google Scholar] [CrossRef]
- Boekaerts, B.; Sels, B.F. Catalytic Advancements in Carboxylic Acid Ketonization and Its Perspectives on Biomass Valorisation. Appl. Catal. B 2021, 283, 119607. [Google Scholar] [CrossRef]
- Ignatchenko, A.V. Density Functional Theory Study of Carboxylic Acids Adsorption and Enolization on Monoclinic Zirconia Surfaces. J. Phys. Chem. C 2011, 115, 16012–16018. [Google Scholar] [CrossRef]
- Wang, S.; Iglesia, E. Experimental and Theoretical Evidence for the Reactivity of Bound Intermediates in Ketonization of Carboxylic Acids and Consequences of Acid–Base Properties of Oxide Catalysts. J. Phys. Chem. C 2017, 121, 18030–18046. [Google Scholar] [CrossRef]
- Pulido, A.; Oliver-Tomas, B.; Renz, M.; Boronat, M.; Corma, A. Ketonic Decarboxylation Reaction Mechanism: A Combined Experimental and DFT Study. ChemSusChem 2013, 6, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Ignatchenko, A.V.; McSally, J.P.; Bishop, M.D.; Zweigle, J. Ab Initio Study of the Mechanism of Carboxylic Acids Cross-Ketonization on Monoclinic Zirconia via Condensation to Beta-Keto Acids Followed by Decarboxylation. Mol. Catal. 2017, 441, 35–62. [Google Scholar] [CrossRef]
- Zecchina, A.; Lamberti, C.; Bordiga, S. Surface Acidity and Basicity: General Concepts. Catal. Today 1998, 41, 169–177. [Google Scholar] [CrossRef]
- Mekki-Berrada, A.; Auroux, A. Thermal Methods. In Characterization of Solid Materials and Heterogeneous Catalysts: From Structure to Surface Reactivity; Che, M., Vedrine, J.C., Eds.; Wiley-VCH: Paris, France, 2012; Volume 1, pp. 777–782. [Google Scholar]
- Strunk, J.; Bañares, M.A.; Wachs, I.E. Vibrational Spectroscopy of Oxide Overlayers. Top. Catal. 2017, 60, 1577–1617. [Google Scholar] [CrossRef]
- Halawy, S.A.; Osman, A.I.; Abdelkader, A.; Nasr, M.; Rooney, D.W. Assessment of Lewis-Acidic Surface Sites Using Tetrahydrofuran as a Suitable and Smart Probe Molecule. ChemistryOpen 2022, 11, e202200021. [Google Scholar] [CrossRef]
- Scaranto, J.; Giorgianni, S. A Quantum-Mechanical Study of CO Adsorbed on TiO2: A Comparison of the Lewis Acidity of the Rutile (110) and the Anatase (101) Surfaces. J. Mol. Struct. THEOCHEM 2008, 858, 72–76. [Google Scholar] [CrossRef]
- Plata, J.; Collico, V.; Márquez, A.; Fdez Sanz, J. Analysis of the Origin of Lateral Interactions in the Adsorption of Small Organic Molecules on Oxide Surfaces. Theor. Chem. Acc. 2012, 132, 1311. [Google Scholar] [CrossRef]
- Guermeur, R.; Jacolin, C. Influence of Surface Silanols on the Dielectric Properties of Nitrogen Adsorbed on Activated Silica. Surf. Sci. 1994, 315, 323–336. [Google Scholar] [CrossRef]
- González-Torres, J.C.; Cipriano, L.A.; Poulain, E.; Domínguez-Soria, V.; García-Cruz, R.; Olvera-Neria, O. Optical Properties of Anatase TiO2: Synergy between Transition Metal Doping and Oxygen Vacancies. J. Mol. Model. 2018, 24, 276. [Google Scholar] [CrossRef]
- Verma, P.; Truhlar, D.G. Does DFT+U Mimic Hybrid Density Functionals? Theor. Chem. Acc. 2016, 135, 182. [Google Scholar] [CrossRef]
- Long, R.; English, N.J. Electronic Properties of Anatase-TiO2 Codoped by Cation-Pairs from Hybrid Density Functional Theory Calculations. Chem. Phys. Lett. 2011, 513, 218–223. [Google Scholar] [CrossRef]
- Ignatchenko, A.V.; Kozliak, E.I. Distinguishing Enolic and Carbonyl Components in the Mechanism of Carboxylic Acid Ketonization on Monoclinic Zirconia. ACS Catal. 2012, 2, 1555–1562. [Google Scholar] [CrossRef]
- Ignatchenko, A.; Hill, J.; Gray, J.; Nealon, D.; Dushane, R. Innovative Use of a Gas Chromatograph for Heterogeneous Catalytic Studies. In Proceedings of the 18th NACS Meeting, Cancun, Mexico, 1–6 June 2003; Volume 7444, p. 169. Available online: https://www.nacatsoc.org/18nam/Posters/P169-Innovative%20use%20of%20a%20Gas%20Chromatograph%20for%20heterogeneo.pdf (accessed on 5 November 2024).
- Ignatchenko, A.V.; Nealon, D.G.; Dushane, R.; Humphries, K. Interaction of Water with Titania and Zirconia Surfaces. J. Mol. Catal. A Chem. 2006, 256, 57–74. [Google Scholar] [CrossRef]
- Wang, S.; Iglesia, E. Experimental and Theoretical Assessment of the Mechanism and Site Requirements for Ketonization of Carboxylic Acids on Oxides. J. Catal. 2017, 345, 183–206. [Google Scholar] [CrossRef]
- Delley, B. An All-Electron Numerical Method for Solving the Local Density Functional for Polyatomic Molecules. J. Phys. Chem. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From Molecules to Solids with the DMol3 Approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Burdett, J.K.; Hughbanks, T.; Miller, G.J.; Richardson, J.W.; Smith, J.V. Structural-Electronic Relationships in Inorganic Solids: Powder Neutron Diffraction Studies of the Rutile and Anatase Polymorphs of Titanium Dioxide at 15 and 295 K. J. Am. Chem. Soc. 1987, 109, 3639–3646. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102, 73005. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Für Krist. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the Exchange Screening Parameter on the Performance of Screened Hybrid Functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef]
- Ambrosetti, A.; Reilly, A.M.; DiStasio, R.A., Jr.; Tkatchenko, A. Long-Range Correlation Energy Calculated from Coupled Atomic Response Functions. J. Chem. Phys. 2014, 140, 18A508. [Google Scholar] [CrossRef] [PubMed]
- Scaranto, J.; Giorgianni, S. DFT Calculations of Carbon Monoxide Adsorbed on Anatase TiO2 (101) and (001) Surfaces: Correlation between the Binding Energy and the CO Stretching Frequency. Mol. Simul. 2013, 39, 245–249. [Google Scholar] [CrossRef]
- Koch, D.; Manzhos, S. On the Charge State of Titanium in Titanium Dioxide. J. Phys. Chem. Lett. 2017, 8, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Hammett, L.P. The Effect of Structure upon the Reactions of Organic Compounds. Benzene Derivatives. J. Am. Chem. Soc. 1937, 59, 96–103. [Google Scholar] [CrossRef]


| CO Adsorption | C=O IR Band, cm−1 | C=O Bond Length, Å | Ti-C Bond Length, Å | Pyridine Adsorption | |||||
| E2 | E1 | E3 | E2 | E1 | E3 | ||||
| K, OH site 1, Adsorbate site 2 | 0.7 | −17.2 | −17.9 | 2184 | 1.1391 | 2.5100 | 4.3 | −42.4 | −46.7 |
| H site 1, OH site 1, Adsorbate site 2 | 1.3 | −18.2 | −19.5 | 2178 | 1.1346 | 2.4912 | 5.4 | −43.5 | −48.9 |
| H site 2, OH site 1, Adsorbate site 2 | 1.7 | −19.1 | −20.8 | 2155 | 1.1346 | 2.4624 | 7.7 | −46.6 | −54.4 |
| H site 3, OH site 1, Adsorbate site 2 | 1.2 | −18.0 | −19.2 | 2171 | 1.1352 | 2.4724 | 5.4 | −44.3 | −49.6 |
| H site 3, OH site 1, Adsorbate site 3 | 1.6 | −17.7 | −19.3 | 2152 | 1.1350 | 2.4661 | 7.1 | −45.1 | −52.2 |
| H site 3, OH site 1, Adsorbate site 4 | 1.0 | −16.3 | −17.4 | 2144 | 1.1362 | 2.5170 | 5.5 | −42.3 | −47.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignatchenko, A.V.; Denman, P.E. Mapping Acid–Base Sites on Anatase Titania (100) and (101) Surfaces by Density Functional Theory: The Link Between Lewis Acidity and the Surface Ability to Flex. Surfaces 2024, 7, 1060-1078. https://doi.org/10.3390/surfaces7040070
Ignatchenko AV, Denman PE. Mapping Acid–Base Sites on Anatase Titania (100) and (101) Surfaces by Density Functional Theory: The Link Between Lewis Acidity and the Surface Ability to Flex. Surfaces. 2024; 7(4):1060-1078. https://doi.org/10.3390/surfaces7040070
Chicago/Turabian StyleIgnatchenko, Alexey V., and Paige E. Denman. 2024. "Mapping Acid–Base Sites on Anatase Titania (100) and (101) Surfaces by Density Functional Theory: The Link Between Lewis Acidity and the Surface Ability to Flex" Surfaces 7, no. 4: 1060-1078. https://doi.org/10.3390/surfaces7040070
APA StyleIgnatchenko, A. V., & Denman, P. E. (2024). Mapping Acid–Base Sites on Anatase Titania (100) and (101) Surfaces by Density Functional Theory: The Link Between Lewis Acidity and the Surface Ability to Flex. Surfaces, 7(4), 1060-1078. https://doi.org/10.3390/surfaces7040070






