Exploring Bismuth Oxide Supported Kaolinite for Photocatalytic Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Bi2O3 Powders by Microwave-Assisted Method
2.2. Synthesis of Bi2O3/Kaolin Composite by Microwave-Assisted Method
2.3. Characterization
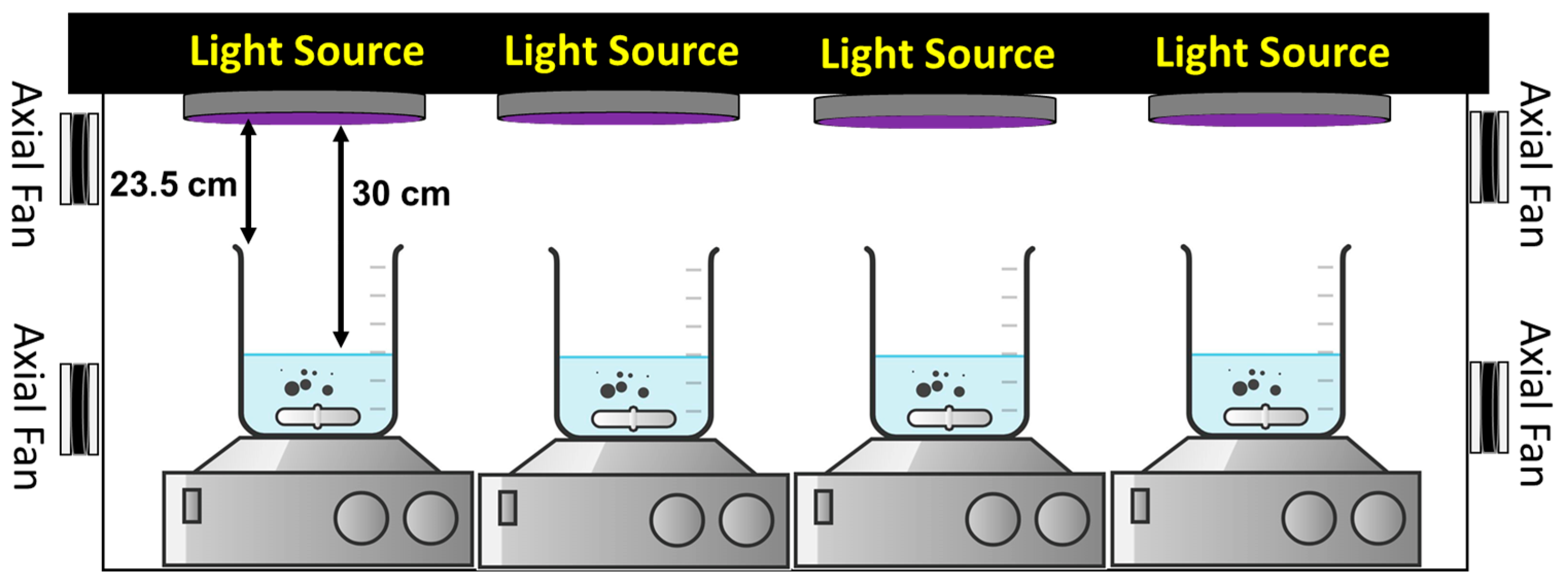
2.4. Photocatalytic Activity Measurement
2.5. Detection of Hydroxyl Radical (•OH)
3. Results
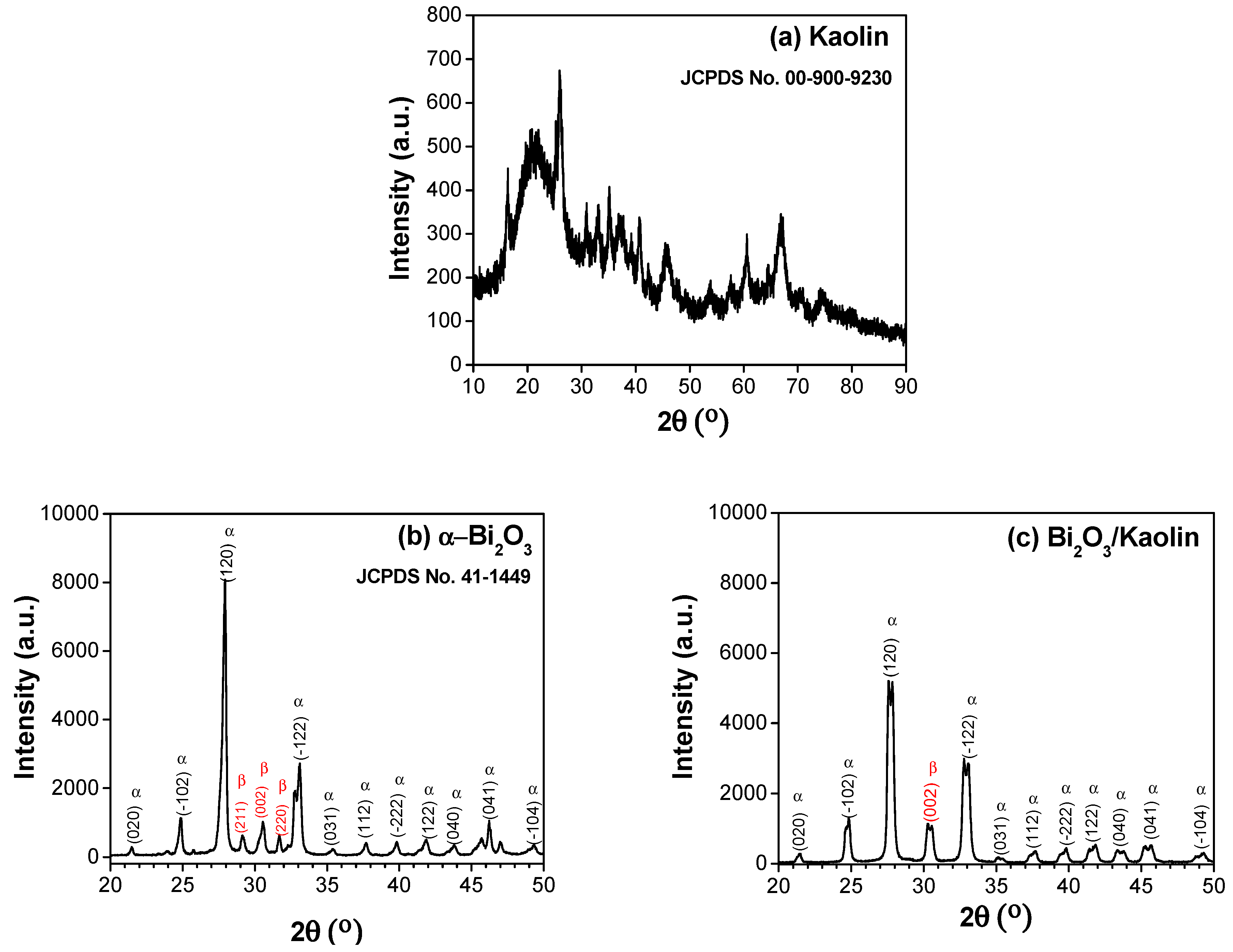
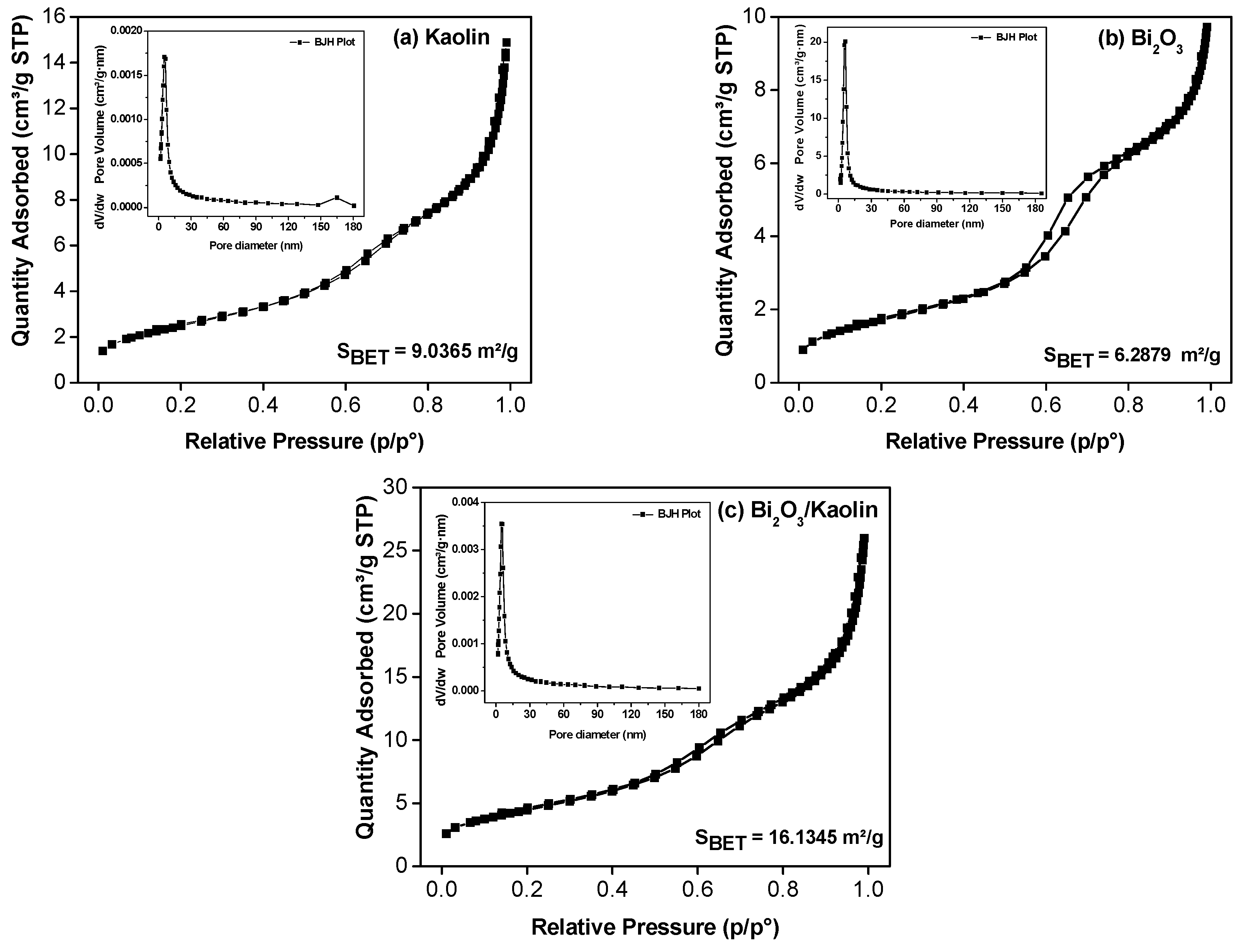
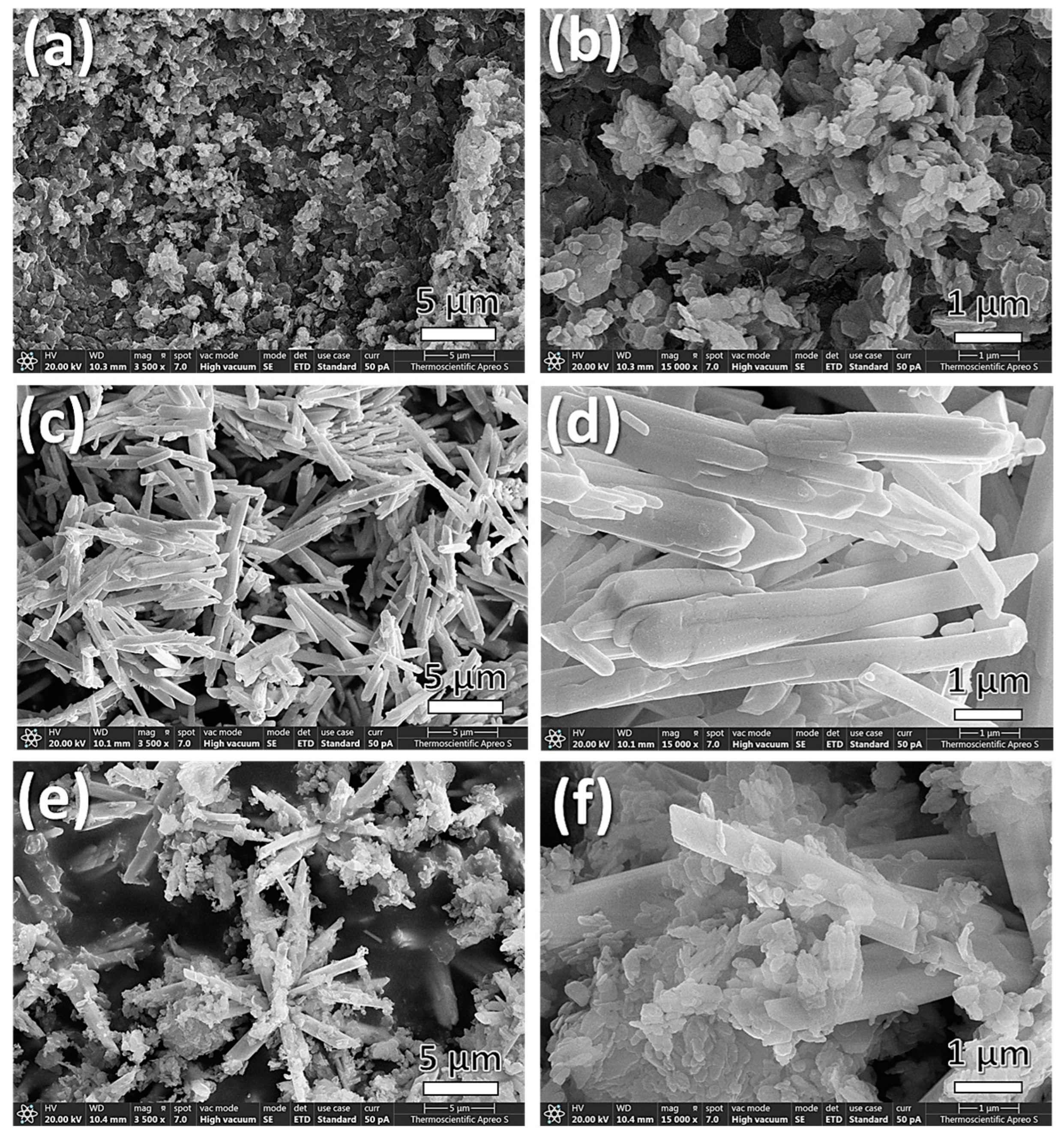
3.1. Microstructure Analysis
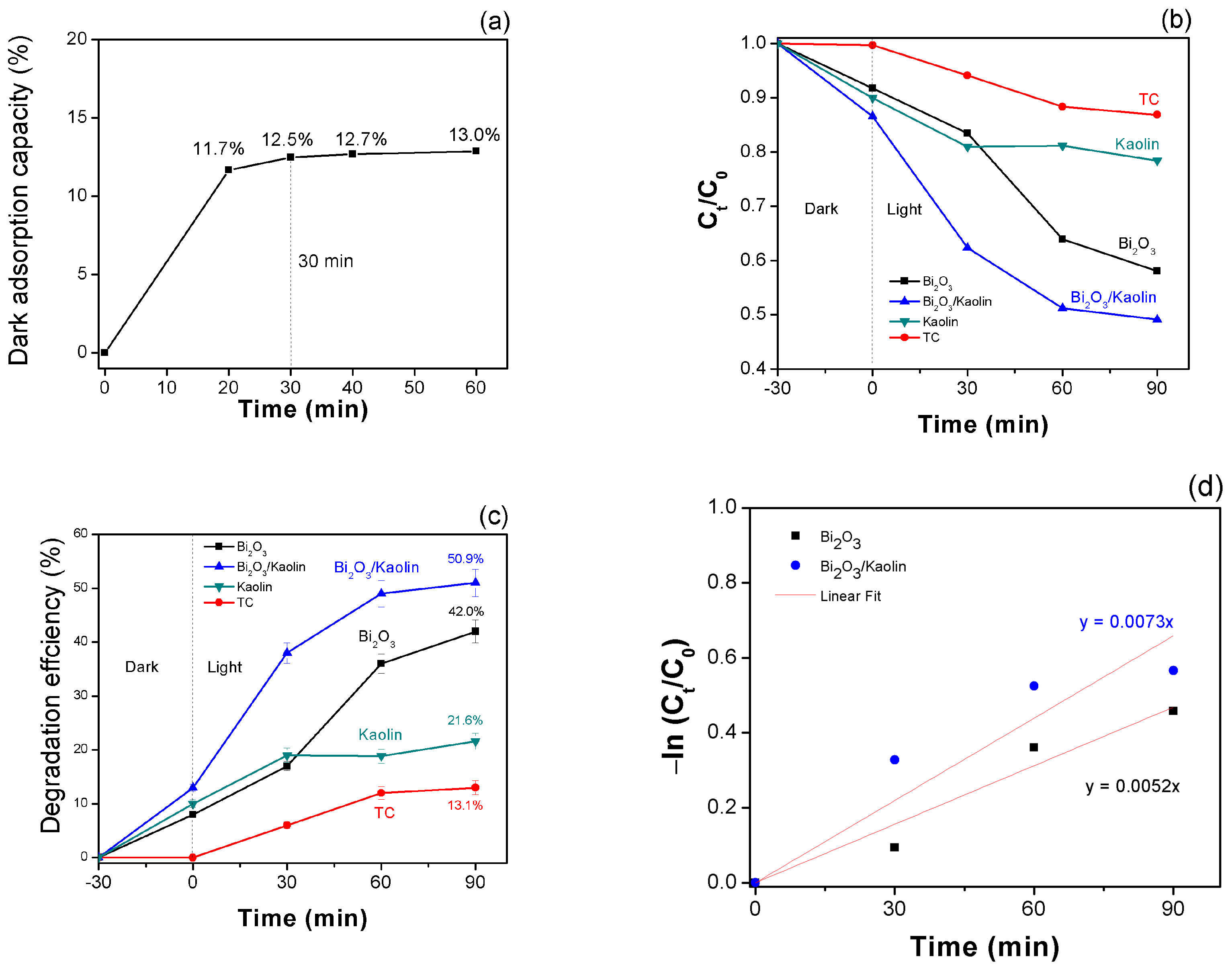
3.2. Photocatalytic Performance
- (i)
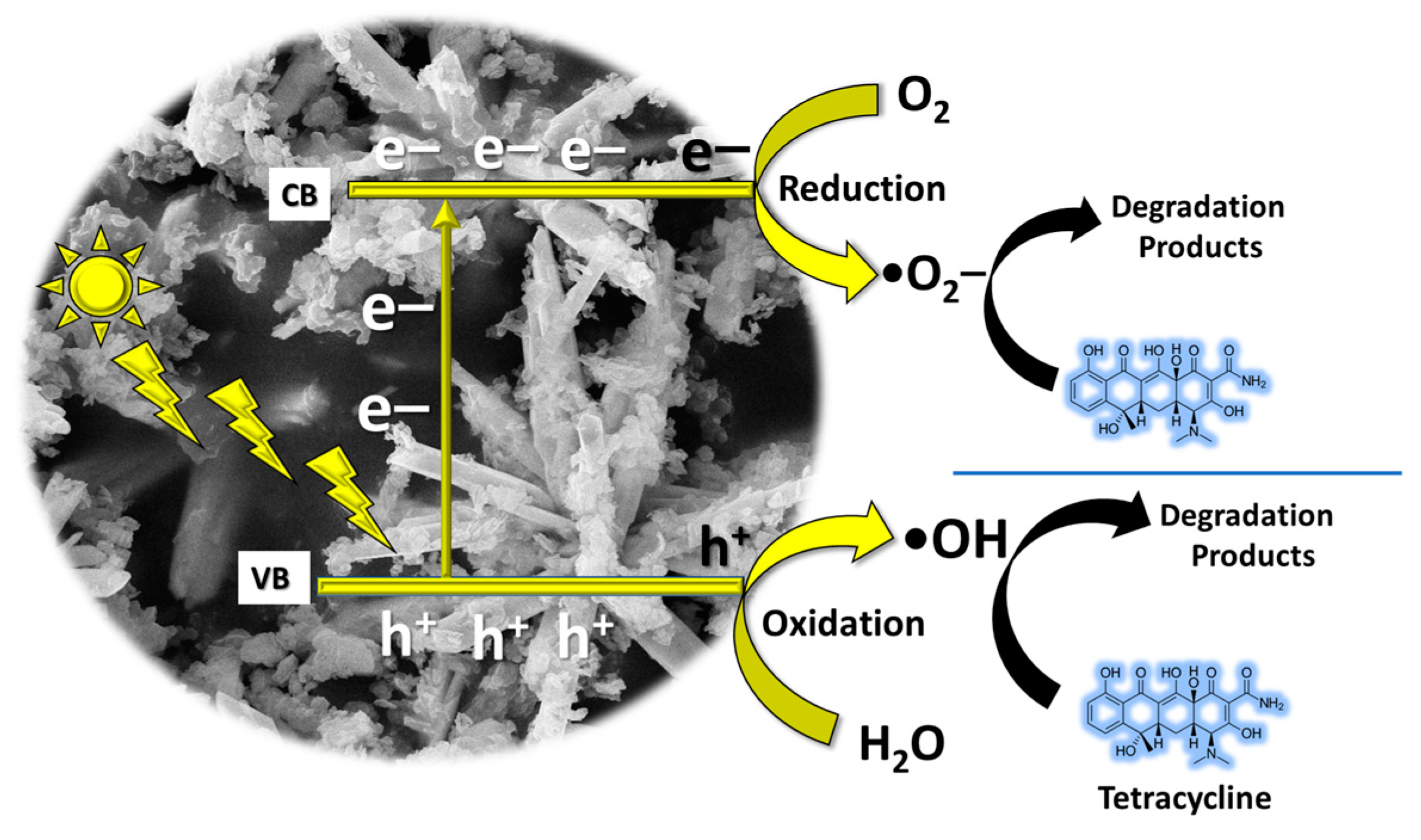
- Light absorption: the process initiates when the Bi2O3 photocatalyst absorbs photons from incident light.
- (ii)
- Generation of electron–hole pairs: when exposed to light, electrons within the valence band of the Bi2O3 photocatalyst become energized to the conduction band, creating positively charged holes (known as electron–hole pairs), in the process.
- (iii)
- Redox reactions: the electrons in the conduction band and the holes in the valence band are both highly reactive species. They can participate in redox (reduction–oxidation) reactions with other molecules adsorbed onto the surface of the photocatalyst. The electron–hole pairs generated on the surface of the photocatalyst can react with O2 molecules adsorbed from the surrounding environment, producing reactive oxygen species (ROS) such as superoxide radicals (O2•−) and hydroxyl radicals (•OH).
- (iv)
- Adsorption of contaminants: organic contaminants, such as the target molecule TC in this study, adsorb onto the surface of the Bi2O3 photocatalyst with the assistance of Kaolin.
- (v)
- Degradation of contaminants: the generated ROS, particularly hydroxyl radicals, are highly oxidative and can react with the adsorbed organic contaminants, including TC, breaking down their molecular structure into smaller, less harmful molecules such as carbon dioxide, water, and other byproducts.
4. Conclusions
- -
- The addition of Kaolin to Bi2O3 did not alter its monoclinic crystal structure; however, it significantly reduced the band gap and created sub-band states that acted as non–radiative recombination centers.
- -
- The addition of kaolin significantly increased the surface area of Bi2O3, enhancing its photocatalytic degradation efficiency, which is the primary focus of this study (from 6.2879 to 16.1345 m2/g).
- -
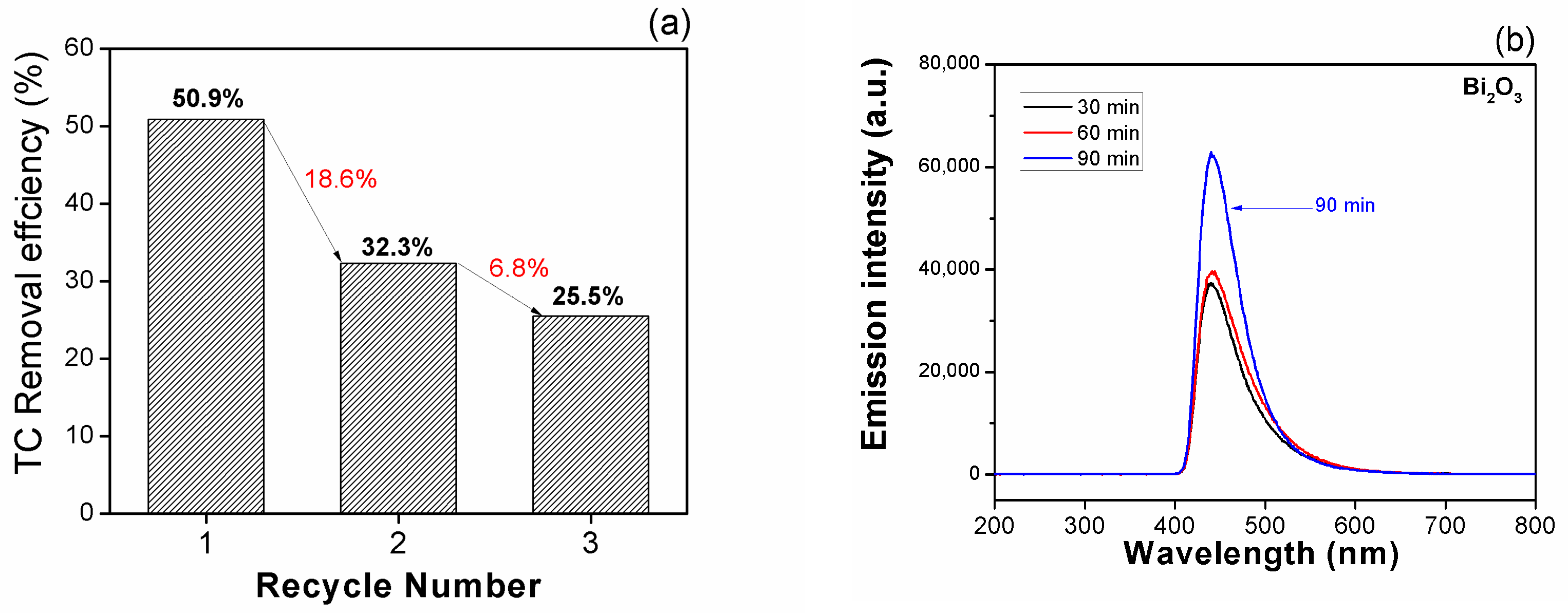
- Regarding photocatalytic performance, after an additional 90 min of visible light exposure, the Bi2O3/Kaolin composite exhibited the highest degradation efficiency at 50.9%, compared to pure Bi2O3, which achieved approximately 42.0%. For the recycled Bi2O3/Kaolin composite, the degradation efficiency decreased from 50.9% to 32.3% and further declined to 25.5% after three recycling cycles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dharma, H.N.C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Othman, M.H.D.; Rahman, M.A.; Jafri, N.N.M.; Suhaimin, N.S.; Nasir, A.M.; et al. A review of titanium dioxide (tio2)–based photocatalyst for oilfield–produced water treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Apopei, P.; Catrinescu, C.; Teodosiu, C.; Royer, S. Mixed–phase TiO2 photocatalysts: Crystalline phase isolation and reconstruction, characterization and photocatalytic activity in the oxidation of 4–chlorophenol from aqueous effluents. Appl. Catal. B Environ. 2014, 160–161, 374–382. [Google Scholar] [CrossRef]
- Lv, X.; Lam Frank, L.Y.; Hu, X. A Review on bismuth oxyhalide (BiOX, X=Cl, Br, I) based photocatalysts for wastewater remediation. Front. Catal. 2022, 2, 839072. [Google Scholar] [CrossRef]
- Chang, A.M.; Chen, Y.H.; Lai, C.C.; Pu, Y.C. Synergistic effects of surface passivation and charge separation to improve photo–electrochemical performance of BiOI nanoflakes by Au nanoparticle decoration. ACS Appl. Mater. Inter. 2021, 13, 5721–5730. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Huang, J.; Cheng, C.; Sui, Q.; Sha, W.; Ji, G.; Deng, S.; Yu, G. BiOX (X = Cl, Br, I) photocatalysts prepared using NaBiO3 as the Bi Source: Characterization and catalytic performance. Catal. Commun. 2010, 11, 460–464. [Google Scholar] [CrossRef]
- Ren, X.; Wu, K.; Qin, Z.; Zhao, X.; Yang, H. The construction of type II heterojunction of Bi2WO6/BiOBr Photocatalyst with Improved Photocatalytic Performance. J. Alloys Compd. 2019, 788, 102–109. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Zhao, X.; Zhu, Y. Controllable synthesis of Bi2MoO6 and effect of morphology and variation in local structure on photocatalytic activities. Appl. Catal. B Environ. 2010, 98, 138–146. [Google Scholar] [CrossRef]
- Chen, S.H.; Jiang, Y.S.; Lin, H.Y. Easy Synthesis of BiVO4 for photocatalytic overall water splitting. ACS Omega. 2020, 5, 8927–8933. [Google Scholar] [CrossRef]
- Channei, D.; Thammaacheep, P.; Kerdphon, S.; Jannoey, P.; Khanitchaidecha, W.; Nakaruk, A. Domestic microwave–assisted synthesis of Pd doped–BiVO4 photocatalysts. Inorg. Chem. Commun. 2023, 150, 110478. [Google Scholar] [CrossRef]
- Qiu, T.; Liu, S.; Cai, H.; Zhou, Y.; Chen, K.; Huang, Y.; Feng, Q. One step solid–state reaction synthesis, characterization, and catalytic performance of n–p SnO2/Bi2O3 composite. J. Mater. Sci. Mater. Electron. 2018, 29, 17463–17472. [Google Scholar] [CrossRef]
- Sohail, A.; Shah, M.A.; Majid, K. Ultrathin α–Bi2O3 Nanosheets prepared via hydrothermal method for electrochemical supercapacitor applications. ECS J. Solid. State Sci. Technol. 2023, 12, 011001. [Google Scholar] [CrossRef]
- Mallahi, M.; Shokuhfar, A.; Vaezi, M.R.; Esmaeilirad, A.; Mazinani, V. Synthesis and characterization of bismuth oxide nanoparticles via sol–gel method. Am. J. Eng. Res. 2014, 3, 162–165. [Google Scholar]
- Senthamilselvi, R.; Velavan, R. Microstructure and photocatalytic properties of bismuth oxide (Bi2O3) nanocrystallites. Malaya J. Math. 2020, 2, 4870–4874. [Google Scholar]
- Azizian–Kalandaragh, Y.; Sedaghatdoust–Bodagh, F.; Habibi–Yangjeh, A. Ultrasound–assisted preparation and characterization of β–Bi2O3 nanostructures: Exploring the photocatalytic activity against rhodamine B. Superlattices Microstruct. 2015, 81, 151–160. [Google Scholar] [CrossRef]
- Yahyazadehfar, M.; Sheikhhosseini, E.; Ahmadi, S.A.; Ghazanfari, D. Microwave–assisted synthetic method of novel Bi2O3 nanostructure and its application as a high–performance nano–catalyst in preparing benzylidene barbituric acid derivatives. Front. Chem. 2022, 10, 951229. [Google Scholar] [CrossRef]
- Lu, H.; Hao, Q.; Chen, T.; Zhang, L.; Chen, D.; Ma, C.; Yao, W.; Zhu, Y. A high–performance Bi2O3/Bi2SiO5 p–n heterojunction photocatalyst induced by phase transition of Bi2O3. Appl. Catal. B Environ. 2018, 273, 59–97. [Google Scholar] [CrossRef]
- Hou, J.; Yang, C.; Wang, Z.; Zhou, W.; Jiao, S.; Zhu, H. In situ synthesis of α–β phase heterojunction on Bi2O3 nanowires with exceptional visible– light photocatalytic performance. Appl. Catal. B Environ. 2013, 142–143, 504–511. [Google Scholar] [CrossRef]
- Elizarraras–Peñaloza, A.; Estrada–Flores, M.; Reza–San Germán, C.M.; Manríquez Ramírez, M.E.; Díaz Barriga-Arceo, L.G.; Santiago-Jacinto, P. Change of phase from α–Bi2O3 to β–Bi2O3 using the ceramic microwave–assisted approach and its increase of capacitance. Superficies y Vacío. 2019, 32, 14–21. [Google Scholar] [CrossRef]
- Guo, X.; Liang, T.T.; Rager, M.; Cui, X. Low–temperature controlled synthesis of novel bismuth oxide (Bi2O3) with microrods and microflowers with great photocatalytic activities. Mater. Lett. 2018, 228, 427–430. [Google Scholar] [CrossRef]
- Rao, S.S.; Saptami, K.; Venkatesan, J.; Rekha, P.D. Microwave–assisted rapid synthesis of silver nanoparticles using fucoidan: Characterization with assessment of biocompatibility and antimicrobial activity. Int. J. Biol. Macromol. 2020, 163, 745–755. [Google Scholar] [CrossRef]
- Sreeju, N.; Rufus, A.; Philip, D. Microwave–assisted rapid synthesis of copper nanoparticles with exceptional stability and their multifaceted applications. J. Mol. Liq. 2016, 221, 1008–1021. [Google Scholar] [CrossRef]
- Papoulis, D.; Panagiotaras, D.; Tsigrou, P.; Christoforidis, K.C.; Petit, C.; Apostolopoulou, A. Halloysite and sepiolite –TiO2 nanocomposites: Synthesis characterization and photocatalytic activity in three aquatic wastes. Mater. Sci. Semicond. Process. 2018, 85, 1–8. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay–TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Kasumba, A.; Buyondo, H.K.; Kirabira, J.B. A comprehensive review on kaolin as pigment for paint and coating: Recent trends of chemical–based paints, their environmental impacts and regulation. Case Stud. Chem. Environ. Eng. 2022, 6, 100244. [Google Scholar]
- Ekosse, G.I.E. Kaolin deposits and occurrences in Africa: Geology, mineralogy and utilization. Appl. Clay Sci. 2010, 50, 212–236. [Google Scholar] [CrossRef]
- Oliveira, W.V.; Morais, A.S.; Honorio, L.M.C.; Trigueiro, P.A.; Almeida, L.C.; Garcia, R.R.P.; Viana, B.C.; Furtini, M.B.; Silva–Filho, E.C.; Osajima, J.A. TiO2 Immobilized on Fibrous Clay as Strategies to Photocatalytic Activity. Mater. Res. 2020, 23, 20190463. [Google Scholar] [CrossRef]
- Zhang, Y.; Gan, H.; Zhang, G. A novel mixed–phase TiO2/kaolinite composites and their photocatalytic activity for degradation of organic contaminants. Chem. Eng. J. 2011, 172, 936–943. [Google Scholar] [CrossRef]
- Li, X.; Peng, K.; Chen, H. TiO2 nanoparticles assembled on kaolinites with different morphologies for efficient photocatalytic performance. Sci. Rep. 2018, 8, 11663. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Olatunji, O.S. Photocatalytic degradation of tetracycline in aqueous systems under visible light irridiation using needle–like SnO2 nanoparticles anchored on exfoliated g–C3N4. Environ. Sci. Eur. 2022, 34, 5. [Google Scholar] [CrossRef]
- Bui, T.S.; Bansal, P.; Lee, B.K.; Mahvelati–Shamsabadi, T.; Soltani, T. Facile fabrication of novel Ba–doped g–C3N4 photocatalyst with remarkably enhanced photocatalytic activity towards tetracycline elimination under visible–light irradiation. Appl. Surf. Sci. 2020, 506, 144184. [Google Scholar] [CrossRef]
- Rosario, D. Misuse of Beer–Lambert Law and other calibration curves. R. Soc. Open Sci. 2022, 9, 211103. [Google Scholar]
- Balakrishnan, A.; Gopalram, K.; Appunni, S. Photocatalytic degradation of 2,4–dichlorophenoxyacetic acid by TiO2 modified catalyst: Kinetics and operating cost analysis. Environ. Sci. Pollut. Res. 2021, 28, 33331–33343. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Jana, N.R. Plasmonic photocatalysis: Complete degradation of bisphenol A by a gold nanoparticle–reduced graphene oxide composite under visible light. Photochem. Photobiol. Sci. 2018, 17, 17628–17637. [Google Scholar] [CrossRef]
- Essawy, A.A.; El-Massry, K.F.; Alsohaimi, I.H.; El-Ghorab, A. Managing Encapsulated Oil Extract of Date Seed Waste for High Hydroxyl Radical Scavenging Assayed via Hybrid Photo-Mediated/Spectrofluorimetric Probing. Molecules 2023, 28, 5160. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Garcia-Pinilla, M.Á.; Pillai, S.C.; O'Shea, K. UV and Visible Light-Driven Production of Hydroxyl Radicals by Reduced Forms of N, F, and P Codoped Titanium Dioxide. Molecules 2019, 24, 2147. [Google Scholar] [CrossRef] [PubMed]
- Belachew, N.; Hinsene, H. Preparation of cationic surfactant−modified kaolin for enhanced adsorption of hexavalent chromium from aqueous solution. Appl. Water. Sci. 2020, 10, 38. [Google Scholar] [CrossRef]
- Bish, D.L.; Von Dreele, R.B. Rietveld Refinement of Non−Hydrogen Atomic Positions in Kaolinite. Clays Miner. 1989, 37, 289–296. [Google Scholar] [CrossRef]
- Cheng, H.; Baibiao, H.; Jibao, L.; Zeyan, W.; Bing, X.; Xiaoyan, Q.; Xiaoyang, Z.; Ying, D. Synergistic effect of crystal and electronic structures on the visible–light–driven photocatalytic performances of Bi2O3 polymorphs. Phys. Chem. Chem. Phys. 2010, 12, 15468. [Google Scholar] [CrossRef]
- Eberl, J.; Kisch, H. Mineralization of Phenol and 4–Chlorophenol Induced by Visible Light and Assisted by Semiconducting β–Bi2O3. Z. Naturforsch B. 2010, 65, 399–404. [Google Scholar] [CrossRef]
- Choudhury, B.; Borah, B.; Choudhury, A. Ce–Nd codoping effect on the structural and optical properties of TiO2 nanoparticles. Mater. Sci. Eng. B. 2013, 178, 239–247. [Google Scholar] [CrossRef]
- Choudhury, B.; Dey, M.; Choudhury, A. Defect generation, d–d transition, and band gap reduction in Cu–doped TiO2 nanoparticles. Int. Nano Lett. 2013, 3, 25. [Google Scholar] [CrossRef]
- Buckeridge, J.; Butler, K.T.; Catlow, C.R.A.; Logsdail, A.J.; Scanlon, D.O.; Shevlin, S.A.; Woodley, S.M.; Sokol, A.A.; Walsh, A. Polymorph engineering of TiO2: Demonstrating how absolute reference potentials are determined by local coordination. Chem. Mat. 2015, 27, 3844–3851. [Google Scholar] [CrossRef]
- Peng, H.; Guo, R.T.; Lin, H.; Liu, X.Y. Synthesis of Bi2O3/g–C3N4 for enhanced photocatalytic CO2 reduction with a Z–scheme mechanism. RSC Adv. 2019, 9, 37162–37170. [Google Scholar] [CrossRef]
- Thao, L.T.S.; Dang, T.T.T.; Khanitchaidecha, W.; Channei, D.; Nakaruk, A. Photocatalytic Degradation of Organic Dye under UV–A Irradiation Using TiO2–Vetiver Multifunctional Nano Particles. Materials 2017, 10, 122. [Google Scholar] [CrossRef]
- Poorsajadi, F.; Sayadi, M.H.; Hajiani, M.; Rezaei, M.R. Synthesis of CuO/Bi2O3 nanocomposite for efficient and recycling photodegradation of methylene blue dye. J. Environ. Anal. Chem. 2020, 102, 7165–7178. [Google Scholar] [CrossRef]
- Bouziani, A.; Yahya, M.; Bianchi, C.L.; Falletta, E.; Celik, G. Ternary Polyaniline@Bi2O3-BiOCl Nanocomposites as Innovative Highly Active Photocatalysts for the Removal of the Dye under Solar Light Irradiation. Nanomaterials 2023, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, C.; Liu, B.; Qin, W.; Xie, Y. Facile Synthesis of Nano-Flower β-Bi2O3/TiO2 Heterojunction as Photocatalyst for Degradation RhB. Molecules 2023, 28, 882. [Google Scholar] [CrossRef] [PubMed]


| Synthesis Method | Steps of Synthesis | Disadvantages | Ref. |
|---|---|---|---|
| Solid-State Reaction | Mixing of precursors, calcination at elevated temperatures followed by cooling and grinding. | Requires high temperatures and longer reaction times. | [10] |
| Hydrothermal Method | Aqueous reaction at high temperature and pressure. | High-pressure conditions and longer reaction times. | [11] |
| Sol–Gel Method | Solution preparation, gel formation through controlled hydrolysis, and heat treatment (drying and calcination). | Complex process and multiple processing steps. | [12] |
| Co-precipitation | Precipitation of bismuth hydroxide (Bi(OH)3), washing, drying followed by calcination to produce Bi2O3. | Challenges in controlling particle size, agglomeration issues, and impurity incorporation. | [13] |
| Ultrasound-assisted method | Dissolving a bismuth precursor in a suitable solvent, applying ultrasound irradiation to induce formation and precipitation of Bi2O3 nanoparticles, followed by washing and drying. | High heat production during sonication, leading to the agglomeration of nanoparticles. | [14] |
| Microwave-assisted method | Preparing a precursor solution, subjecting to microwave irradiation for rapid heating, leading to the formation of Bi2O3 particles, followed by washing and drying. | Dependency on specialized microwave equipment and limited scalability for large-scale production. | [15] |
| Component | Unit (%wt) |
|---|---|
| SiO2 | 49.6 |
| Al2O3 | 48.8 |
| TiO2 | 0.53 |
| Fe2O3 | 0.46 |
| P2O5 | 0.22 |
| MgO | 0.18 |
| CaO | 0.06 |
| Other compounds | 0.15 |
| Samples | BET Surface Area (SBET, m2/g) | BJH Pore Size (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| Bi2O3 | 6.2879 | 8.2698 | 0.01099 |
| Kaolin | 9.0365 | 9.6641 | 0.01382 |
| Bi2O3-supported Kaolin | 16.1345 | 9.3547 | 0.02454 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thammaacheep, P.; Phetthai, P.; Suchai, S.; Jannoey, P.; Channei, D. Exploring Bismuth Oxide Supported Kaolinite for Photocatalytic Application. Surfaces 2024, 7, 698-713. https://doi.org/10.3390/surfaces7030045
Thammaacheep P, Phetthai P, Suchai S, Jannoey P, Channei D. Exploring Bismuth Oxide Supported Kaolinite for Photocatalytic Application. Surfaces. 2024; 7(3):698-713. https://doi.org/10.3390/surfaces7030045
Chicago/Turabian StyleThammaacheep, Punyanuch, Pornpraphatson Phetthai, Suthitra Suchai, Panatda Jannoey, and Duangdao Channei. 2024. "Exploring Bismuth Oxide Supported Kaolinite for Photocatalytic Application" Surfaces 7, no. 3: 698-713. https://doi.org/10.3390/surfaces7030045
APA StyleThammaacheep, P., Phetthai, P., Suchai, S., Jannoey, P., & Channei, D. (2024). Exploring Bismuth Oxide Supported Kaolinite for Photocatalytic Application. Surfaces, 7(3), 698-713. https://doi.org/10.3390/surfaces7030045








