Enhancing Water Condensation on Hybrid Surfaces by Optimizing Wettability Contrast
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
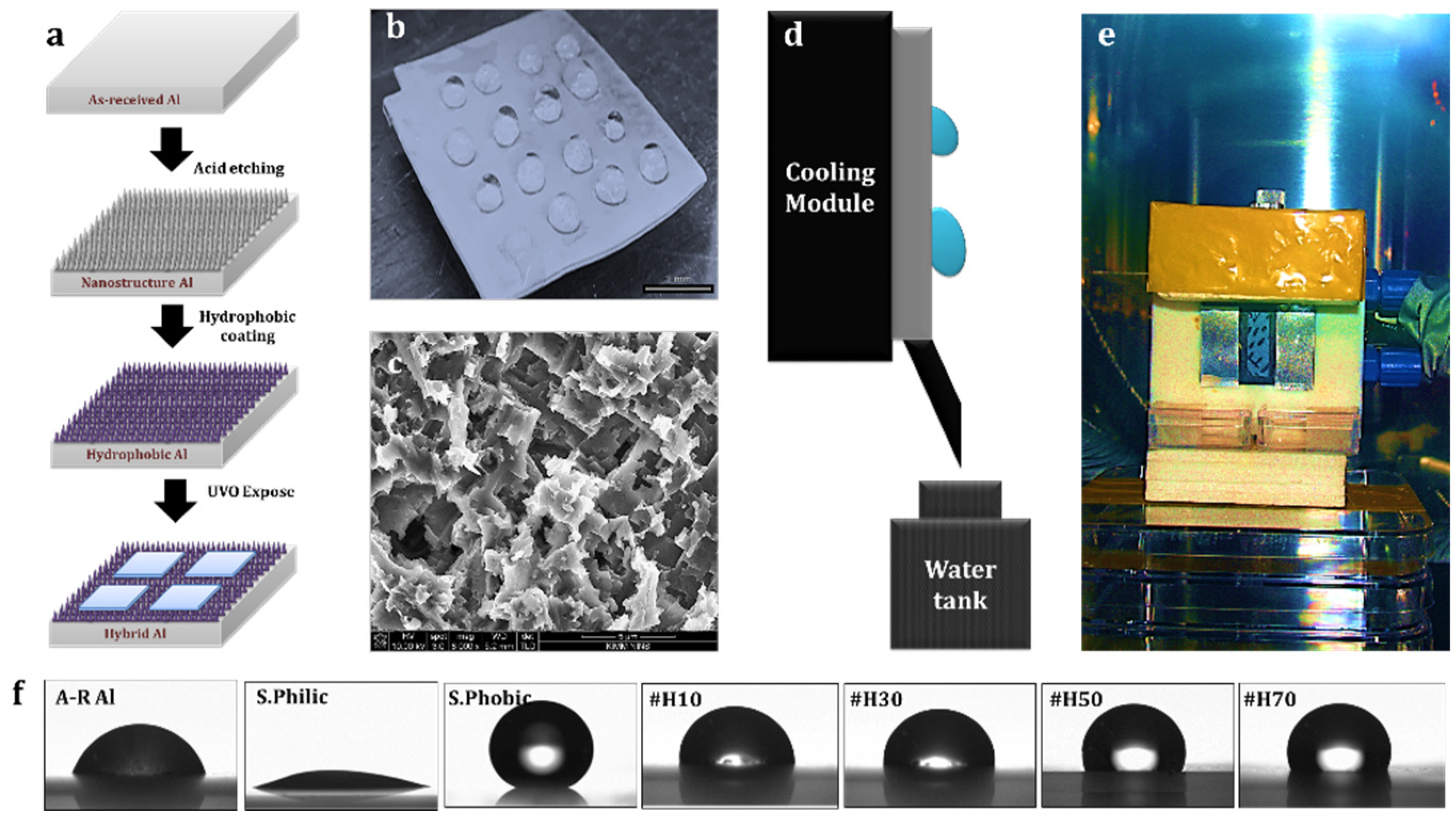
2.2. Method
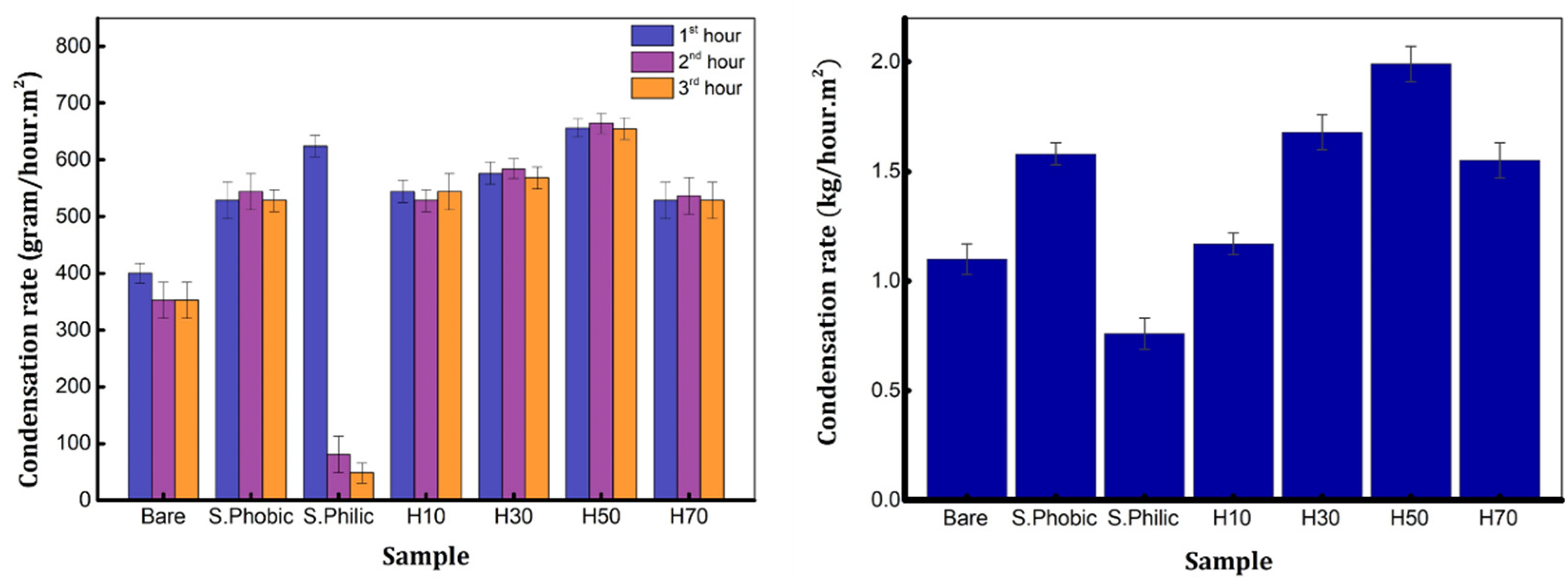
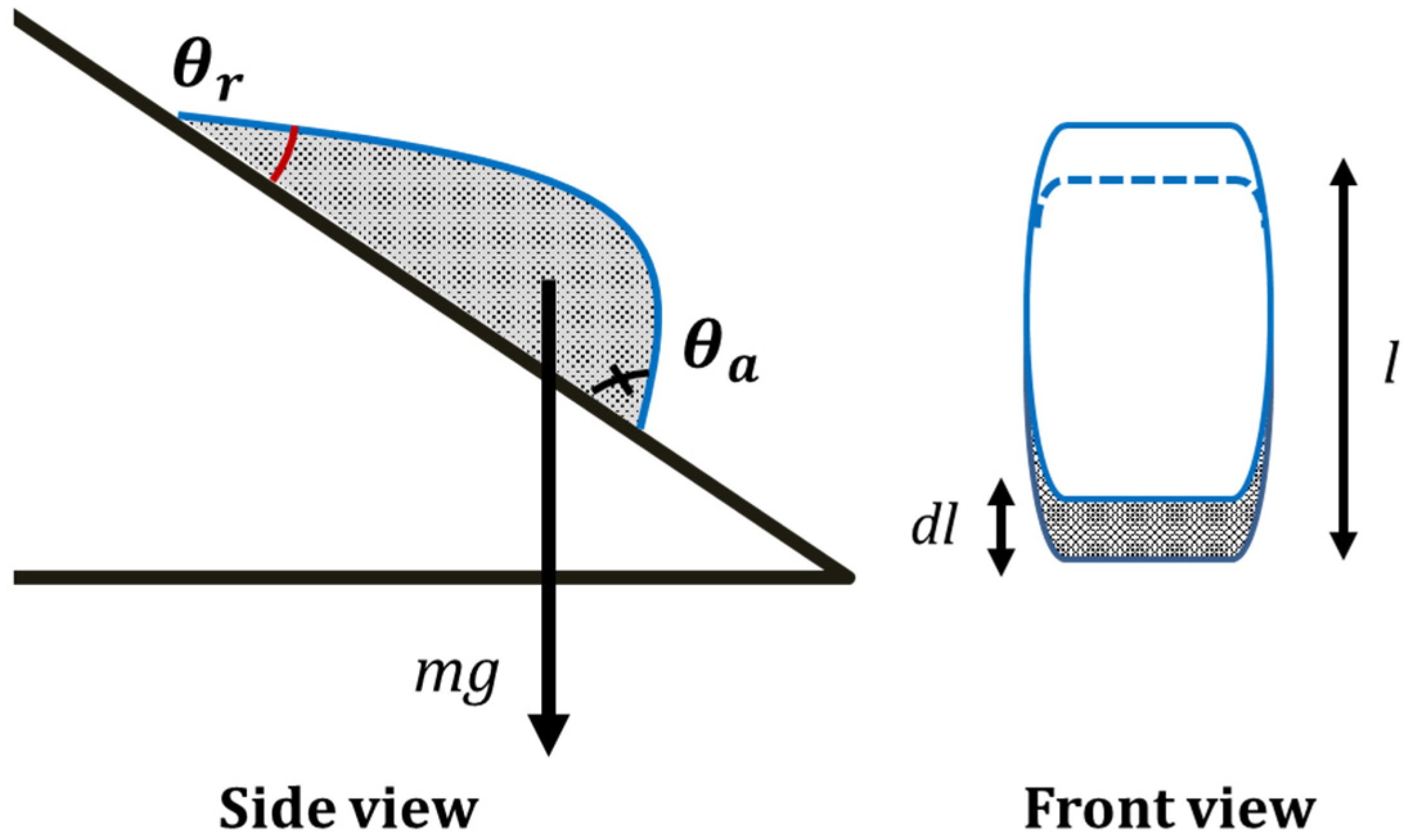
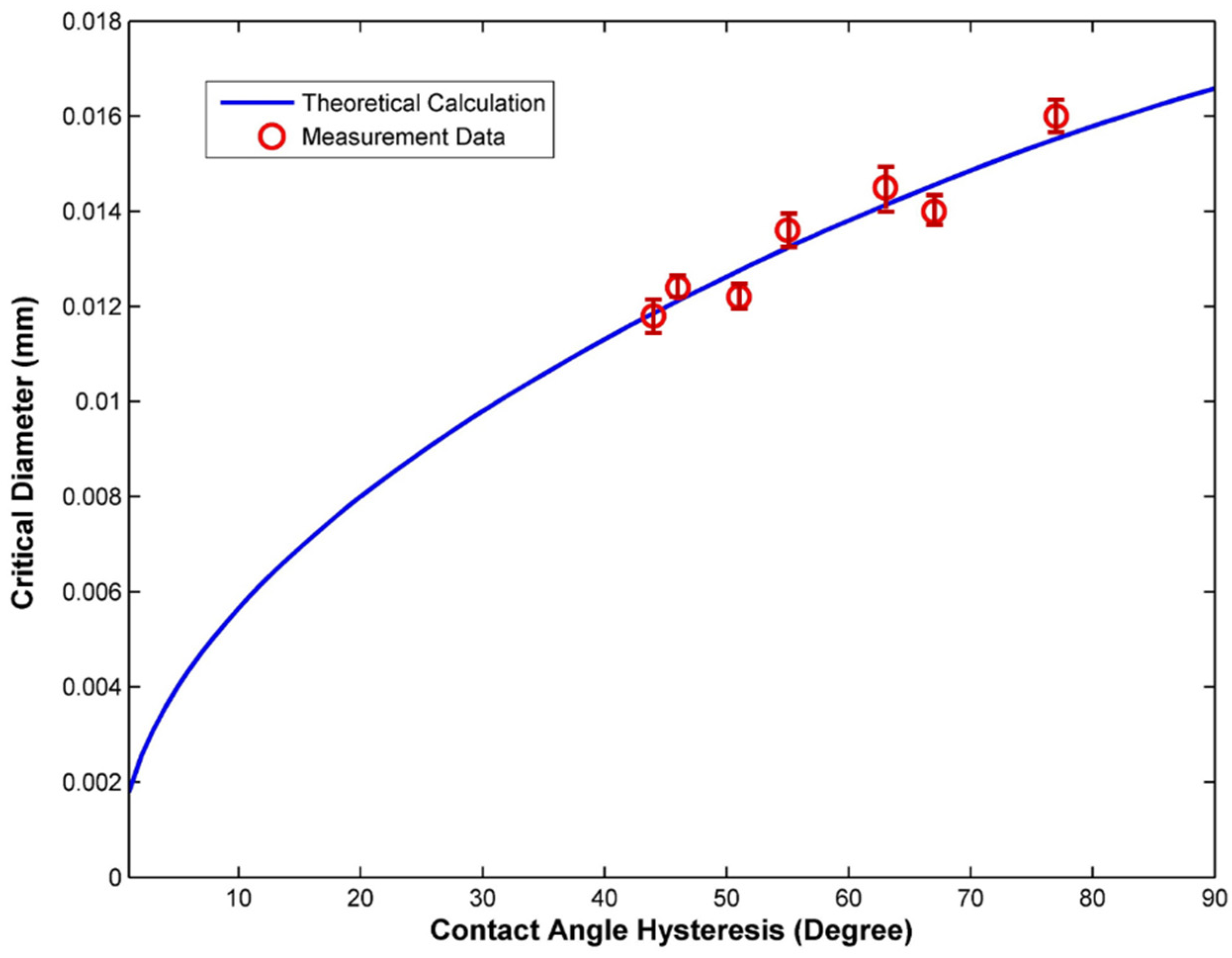
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Zhang, Z. Recent Progress in Beetle-Inspired Superhydrophilic-Superhydrophobic Micropatterned Water-Collection Materials. Water Sci. Technol. 2020, 82, 207–226. [Google Scholar] [CrossRef]
- Snustad, I.; Røe, I.T.; Brunsvold, A.; Ervik, Å.; He, J.; Zhang, Z. A Review on Wetting and Water Condensation—Perspectives for CO2 Condensation. Adv. Colloid. Interface Sci. 2018, 256, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Danilo, S.; Dominique, C.; Frédéric, P. Experimental Dropwise Condensation of Unsaturated Humid Air—Influence of Humidity Level on Latent and Convective Heat Transfer for Fully Developed Turbulent Flow. Int. J. Heat. Mass. Transf. 2016, 102, 846–855. [Google Scholar] [CrossRef]
- Pinheiro, R.A.; Silva, A.A.; Trava-Airoldi, V.J.; Corat, E.J. Water Vapor Condensation and Collection by Super-Hydrophilic and Super-Hydrophobic VACNTs. Diam. Relat. Mater. 2018, 87, 43–49. [Google Scholar] [CrossRef]
- Yang, K.-S.; Lin, K.-H.; Tu, C.-W.; He, Y.-Z.; Wang, C.-C. Experimental Investigation of Moist Air Condensation on Hydrophilic, Hydrophobic, Superhydrophilic, and Hybrid Hydrophobic-Hydrophilic Surfaces. Int. J. Heat. Mass. Transf. 2017, 115, 1032–1041. [Google Scholar] [CrossRef]
- Starostin, A.; Valtsifer, V.; Barkay, Z.; Legchenkova, I.; Danchuk, V.; Bormashenko, E. Drop-Wise and Film-Wise Water Condensation Processes Occurring on Metallic Micro-Scaled Surfaces. Appl. Surf. Sci. 2018, 444, 604–609. [Google Scholar] [CrossRef]
- Yao, C.-W.; Alvarado, J.L.; Marsh, C.P.; Jones, B.G.; Collins, M.K. Wetting Behavior on Hybrid Surfaces with Hydrophobic and Hydrophilic Properties. Appl. Surf. Sci. 2014, 290, 59–65. [Google Scholar] [CrossRef]
- Chung, S.; Kadala, K.; Taylor, H. Stable Dropwise Condensation Observed on a Hierarchically Structured Superhydrophobic Surface Incorporating Micro-Domes. Microelectron. Eng. 2020, 225, 111252. [Google Scholar] [CrossRef]
- Zarei, S.; Talesh Bahrami, H.R.; Saffari, H. Effects of Geometry and Dimension of Micro/Nano-Structures on the Heat Transfer in Dropwise Condensation: A Theoretical Study. Appl. Therm. Eng. 2018, 137, 440–450. [Google Scholar] [CrossRef]
- Her, E.K.; Ko, T.-J.; Lee, K.-R.; Oh, K.H.; Moon, M.-W. Bioinspired Steel Surfaces with Extreme Wettability Contrast. Nanoscale 2012, 4, 2900–2905. [Google Scholar] [CrossRef]
- Chatterjee, A.; Derby, M.M.; Peles, Y.; Jensen, M.K. Condensation Heat Transfer on Patterned Surfaces. Int. J. Heat. Mass. Transf. 2013, 66, 889–897. [Google Scholar] [CrossRef]
- Baba, S.; Sawada, K.; Tanaka, K.; Okamoto, A. Dropwise Condensation on a Hierarchical Nanopillar Structured Surface. Langmuir 2020, 36, 10033–10042. [Google Scholar] [CrossRef]
- Im, H.; Ji, S.; Moon, D.-I.; Lim, H.; Choi, Y.-K. Enhanced Water Droplet Mobility on Superhydrophobic Rippled Nanoshell Array. Appl. Phys. Lett. 2016, 109, 151601. [Google Scholar] [CrossRef]
- Mahapatra, P.S.; Ghosh, A.; Ganguly, R.; Megaridis, C.M. Key Design and Operating Parameters for Enhancing Dropwise Condensation through Wettability Patterning. Int. J. Heat. Mass. Transf. 2016, 92, 877–883. [Google Scholar] [CrossRef]
- Choo, S.; Choi, H.-J.; Lee, H. Water-Collecting Behavior of Nanostructured Surfaces with Special Wettability. Appl. Surf. Sci. 2015, 324, 563–568. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.J. Dropwise Condensation Modeling Suitable for Superhydrophobic Surfaces. J. Heat. Transf. 2011, 133, 081502. [Google Scholar] [CrossRef]
- Dorrer, C. Mimicking the Stenocara Beetle s Dewetting of Drops from a Patterned Superhydrophobic Surface. Langmuir 2008, 24, 6154–6158. [Google Scholar] [CrossRef]
- Caldona, E.B.; Brown, H.O.; Smith, D.W.J.; Wipf, D.O. Superhydrophobic/Superoleophilic Surfaces by Electroless Nanoparticle Deposition and Perfluorinated Polymer Modification. Ind. Eng. Chem. Res. 2021, 60, 14239–14250. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, C.; Shen, C.; Cao, J.; Chen, Y. Self-Propelled Dropwise Condensation on a Gradient Surface. Int. J. Heat. Mass. Transf. 2017, 114, 419–429. [Google Scholar] [CrossRef]
- Li, H.; You, Z.; Zhang, H. Experimental Investigation on the Heat Transfer Enhancement of Steam Condensation on Tube with Hydrophilic-Hydrophobic Hybrid Surface. J. Phys. Conf. Ser. 2022, 2280, 12059. [Google Scholar] [CrossRef]
- Parker, A.R.; Lawrence, C.R. Water Capture by a Desert Beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, T.; Dacke, M. Fog-Basking Behaviour and Water Collection Efficiency in Namib Desert Darkling Beetles. Front. Zool. 2010, 7, 23. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, X.; Li, Y.; Zhu, D. Design of Hybrid Superwetting Surfaces with Self-Driven Droplet Transport Feature for Enhanced Condensation. Adv. Mater. Interfaces 2021, 8, 2100284. [Google Scholar] [CrossRef]
- Cho, H.; Lee, J.; Lee, S.; Hwang, W. Durable Superhydrophilic/Phobic Surfaces Based on Green Patina with Corrosion Resistance. Phys. Chem. Chem. Phys. 2015, 17, 6786–6793. [Google Scholar] [CrossRef]
- Zhou, L.; He, W.; Wang, M.; Hou, X. Enhanced Phase-Change Heat Transfer by Surface Wettability Control. ChemSusChem 2022, 15, e202102531. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Gao, S.; Xu, Z.; Wu, S.; Deng, Z. Experimental Study on the Condensation Heat Transfer on a Wettability-Interval Grooved Surface. Appl. Sci. 2023, 13, 10518. [Google Scholar] [CrossRef]
- Upot, N.V.; Fazle Rabbi, K.; Khodakarami, S.; Ho, J.Y.; Kohler Mendizabal, J.; Miljkovic, N. Advances in Micro and Nanoengineered Surfaces for Enhancing Boiling and Condensation Heat Transfer: A Review. Nanoscale Adv. 2023, 5, 1232–1270. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Moon, M.-W.; Lim, H.; Kim, W.-D.; Kim, H.-Y. Water Harvest via Dewing. Langmuir 2012, 28, 10183–10191. [Google Scholar] [CrossRef]
- Seo, D.; Lee, J.; Lee, C.; Nam, Y. The Effects of Surface Wettability on the Fog and Dew Moisture Harvesting Performance on Tubular Surfaces. Sci. Rep. 2016, 6, 24276. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Nguyen, B.D.; Pham, H.T.; Lam, S.S.; Vo, D.-V.N.; Shokouhimehr, M.; Vu, T.H.H.; Nguyen, T.-B.; Kim, S.Y.; Le, Q.V. Anti-Icing Performance on Aluminum Surfaces and Proposed Model for Freezing Time Calculation. Sci. Rep. 2021, 11, 3641. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Jin, J.; Liu, J.; Yan, Y.; Han, Z.; Ren, L. Anti-Icing Property of Bio-Inspired Micro-Structure Superhydrophobic Surfaces and Heat Transfer Model. Appl. Surf. Sci. 2017, 400, 498–505. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Park, S.; Lim, H. Effects of Morphology Parameters on Anti-Icing Performance in Superhydrophobic Surfaces. Appl. Surf. Sci. 2018, 435, 585–591. [Google Scholar] [CrossRef]
- Garimella, M.M.; Koppu, S.; Kadlaskar, S.S.; Pillutla, V.; Abhijeet; Choi, W. Difference in Growth and Coalescing Patterns of Droplets on Bi-Philic Surfaces with Varying Spatial Distribution. J. Colloid. Interface Sci. 2017, 505, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Weisensee, P.B.; Wang, Y.; Qian, H.; Schultz, D.; King, W.P.; Miljkovic, N. Condensate Droplet Size Distribution on Lubricant-Infused Surfaces. Int. J. Heat. Mass. Transf. 2017, 109, 187–199. [Google Scholar] [CrossRef]
- Furmidge, C.G.L. Studies at Phase Interfaces. I. The Sliding of Liquid Drops on Solid Surfaces and a Theory for Spray Retention. J. Colloid. Sci. 1962, 17, 309–324. [Google Scholar] [CrossRef]


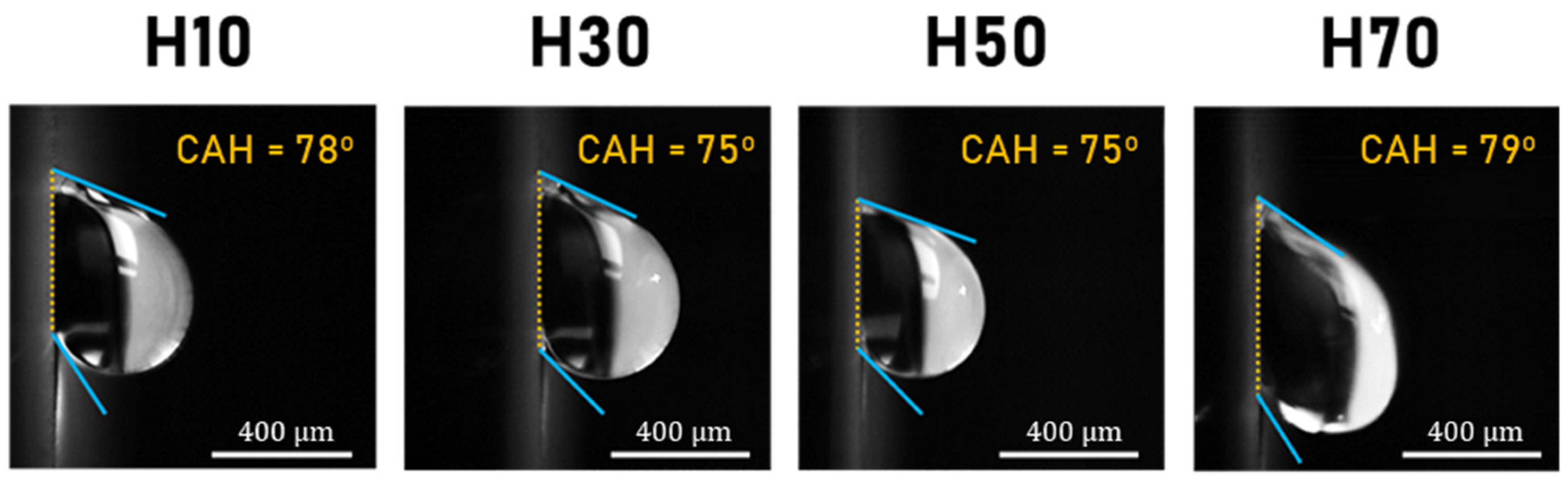
| Sample | CA | CAH | SA | Vc | Amount (L/h·m2) | Note | ||
|---|---|---|---|---|---|---|---|---|
| A-R Al | 81 | 113 | 64 | 3.8 | ||||
| S.Philic | 9 | -- | -- | -- | -- | |||
| S.Phobic | ||||||||
| #H10 | to mimic the Stenocara beetle’s back morphology | |||||||
| #H30 | ||||||||
| #H50 | ||||||||
| #H70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, D.-T.; Nguyen, T.-B. Enhancing Water Condensation on Hybrid Surfaces by Optimizing Wettability Contrast. Surfaces 2024, 7, 508-516. https://doi.org/10.3390/surfaces7030033
Chi D-T, Nguyen T-B. Enhancing Water Condensation on Hybrid Surfaces by Optimizing Wettability Contrast. Surfaces. 2024; 7(3):508-516. https://doi.org/10.3390/surfaces7030033
Chicago/Turabian StyleChi, Do-Thuy, and Thanh-Binh Nguyen. 2024. "Enhancing Water Condensation on Hybrid Surfaces by Optimizing Wettability Contrast" Surfaces 7, no. 3: 508-516. https://doi.org/10.3390/surfaces7030033
APA StyleChi, D.-T., & Nguyen, T.-B. (2024). Enhancing Water Condensation on Hybrid Surfaces by Optimizing Wettability Contrast. Surfaces, 7(3), 508-516. https://doi.org/10.3390/surfaces7030033






