Metal–Perovskite Interfacial Engineering to Boost Activity in Heterogeneous Catalysis
Abstract
1. Introduction: Simple Exsolution vs. Activity of the Metal–Perovskite Interface in Heterogeneous Catalysis
2. Metal–Perovskite Interfaces in Heterogeneous Catalysis
2.1. Hydrocarbon Dry Reforming
2.1.1. Nickelates
2.1.2. Ferrites
2.1.3. Niobates
2.1.4. Titanates
2.1.5. Cuprates
2.1.6. Cobaltites
2.1.7. Aluminates
2.1.8. Zirconates
2.1.9. Manganites
2.1.10. Chromates

2.2. Methane (Partial) Oxidation
2.2.1. Nickelates
2.2.2. Ferrites
2.2.3. Titanates
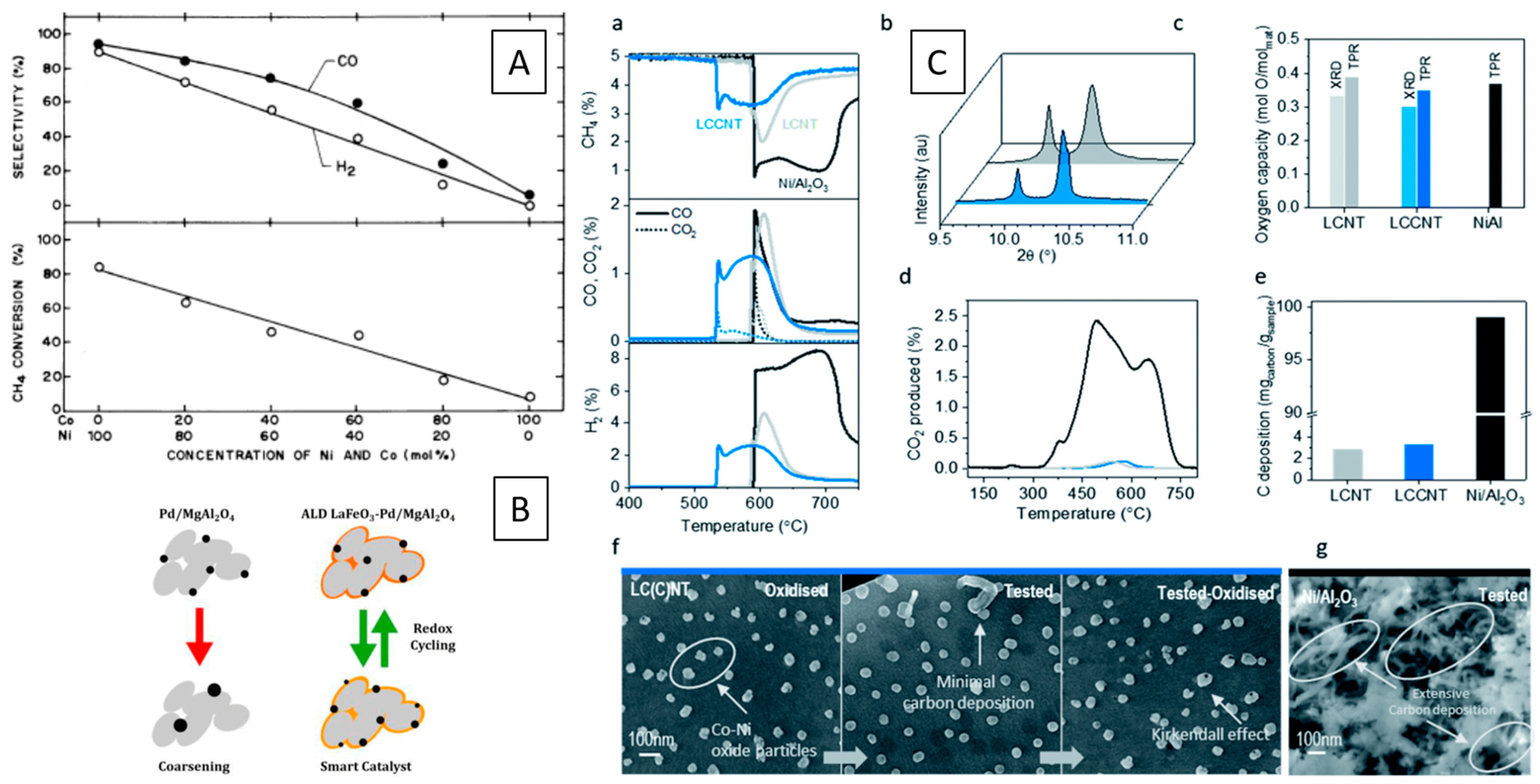
2.3. (Reverse) Water–Gas Shift Reaction
2.3.1. Ferrites
2.3.2. Nickelates
2.3.3. Titanates
2.3.4. Cobaltites
2.3.5. Yttrates
2.3.6. Molybdates
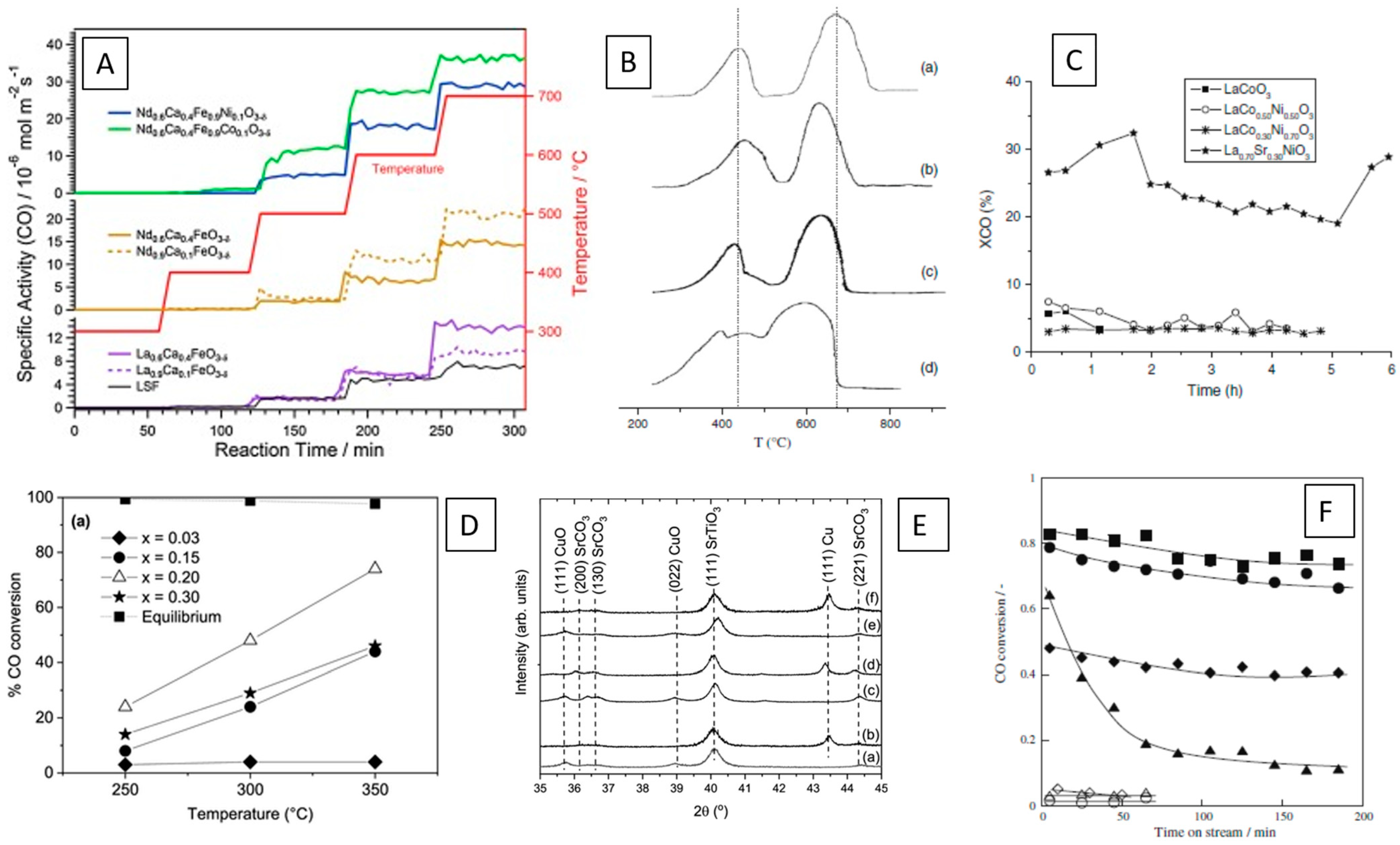
2.4. Alcohol and Hydrocarbon Steam Reforming
2.4.1. Ferrites
2.4.2. Titanates
2.4.3. Nickelates
2.4.4. Aluminates
2.4.5. Cobaltites
2.4.6. Manganates
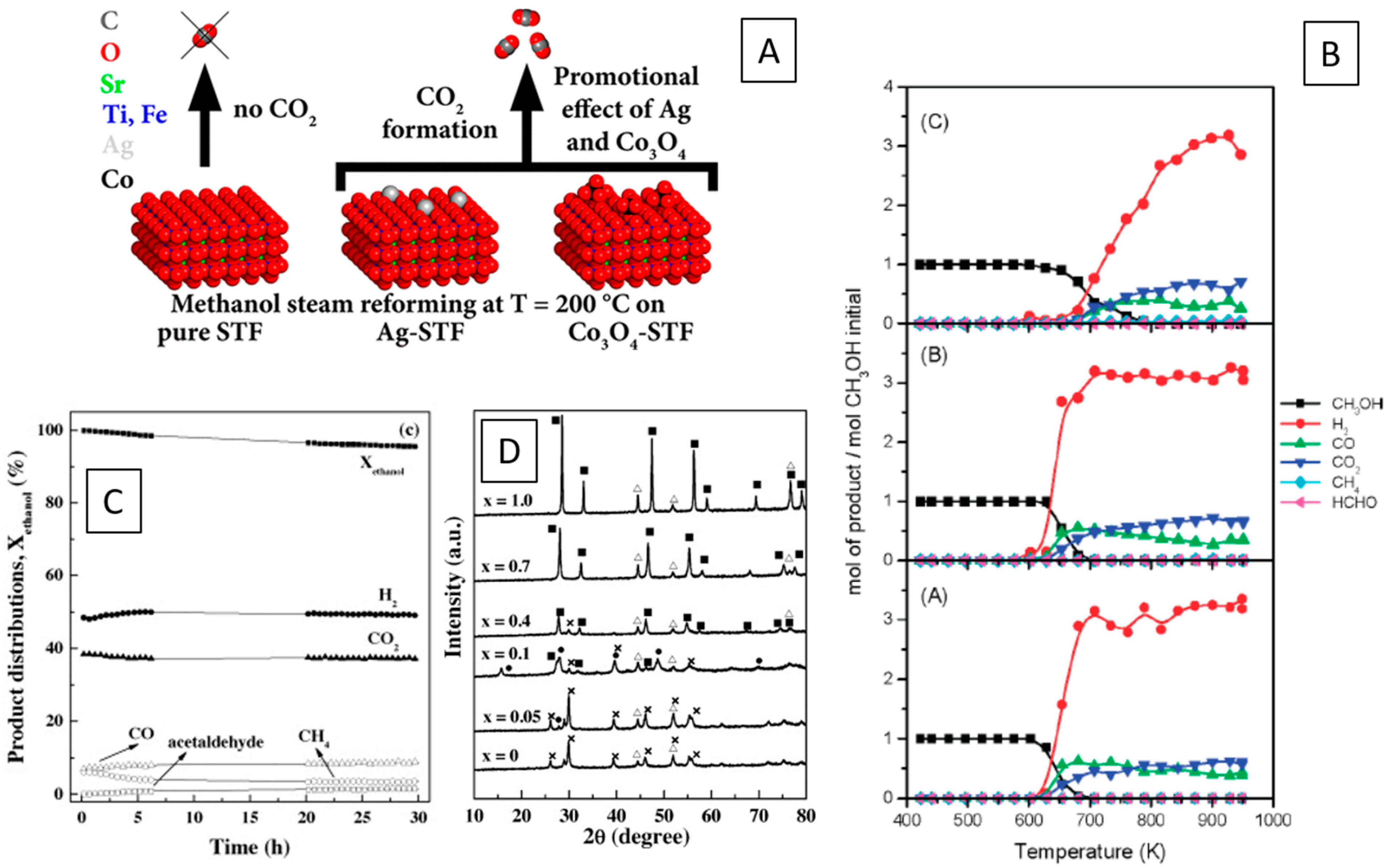
2.5. Methanation and Hydrogenation Reactions
2.5.1. Ferrites
2.5.2. Titanates
2.5.3. Aluminates
2.5.4. Rhodates and Platinates
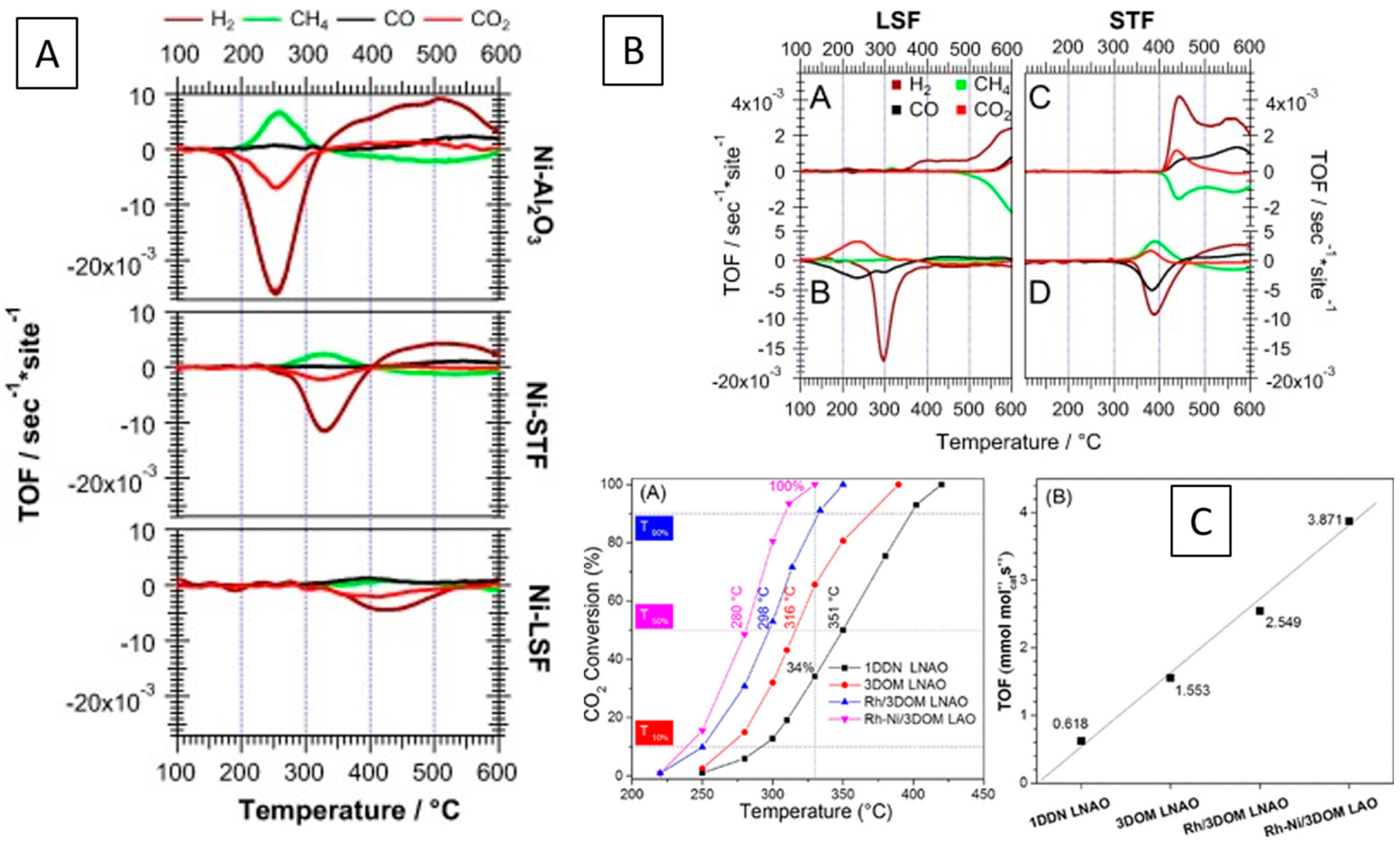
2.6. Car Exhaust Catalysis
2.6.1. Ferrites
2.6.2. Titanates and Zirconates
2.6.3. Cobaltites
2.7. CO Oxidation
2.7.1. Titanates
2.7.2. Manganites
2.8. deNOx NO+CO
2.8.1. Manganites
2.8.2. Cobaltites
2.8.3. Aluminates
2.8.4. Titanates
3. Strategies for the In Situ Steering of the Extent of Metal–Perovskite Interface and Correlation to Activity in Heterogeneous Catalysis
3.1. Formation by Metal Exsolution from Structure-Pure (Doped) Perovskites
3.1.1. Methane Dry Reforming
3.1.2. Methane Oxidation
3.1.3. Water–Gas Shift Reaction
3.1.4. Steam Reforming of Hydrocarbons and Alcohols
3.2. Impregnation Strategies
3.3. Deliberate Over-Doping
3.4. Combination of Exsolution and Impregnation
4. Further Development and Current Understanding of the Metal–Perovskite Interface in Heterogeneous Catalysis
- Knowledge-based steering of the metal–perovskite interface:
- Striking is the fact that, upon screening the literature, it is evident that, in the vast majority of cases, the formation of the interface is “by accident”, rather than the result of a knowledge-based approach. This is mostly the case if perovskite precursors are used. In this respect, one should be careful to discriminate between the understanding of the exsolution process itself and the interpretation of the synergistic action of the formed interface. While the former has been scrutinized in almost every aspect already, a deeper understanding of the latter is still mostly missing. To develop this understanding, a two-pronged approach is necessary: correlation of differently prepared interfaces, allowing for varying the extent of the interface, and in situ formation and analysis of the interfaces.
- Different approaches allow altering the extent of the metal–perovskite interface:
- There is a high need for detailed studies of hetero-interfaces with varying metal–perovskite or metal–oxide (if started from perovskite precursors) extent prepared by different approaches. This can for example achieved by varying the composition in the parent doped perovskite and steering the decomposition process, or by entirely different preparation approaches. The combination of impregnation, exsolution, and/or a combination of those appears particularly promising. A comparison of interfaces prepared by impregnation in comparison with exsolved ones is the absolute minority in the discussed cases studies, but it is still imperative for a deeper understanding.
- Mind the difference between a metal–perovskite and metal–oxide interface:
- Speaking about the knowledge-based approach to such interfaces, a clear discrimination must be made between the interfaces gained from a deliberate support of the metal particles on the perovskite surface, the metal–perovskite interface arising from a partial decomposition of a perovskite precursor and a metal–oxide interface developing from a total decomposition of such a perovskite precursor. While it is evident that, usually, only a comparison of such interfaces reveals the true mechanistic understanding, the literature-reported cases fail to a large extent in this respect. In connection with this deficiency, it has to be stated that, unfortunately, the bad habit of starting from structurally impure initial perovskite structures has wormed itself into research. While the appearance of parasitic stray phases might not have a strong effect on the decomposition process, it cannot be ruled out at will.
- Only in situ and operando analysis gives insight into the structure–activity correlation of metal–perovskite and metal–oxide interfaces derived from (partial) perovskite decomposition:
- A thorough understanding of the interface developing under operational reaction conditions is equally imperative. This is, sadly, still under-represented in most of the studies, despite the fact that such in situ and operando characterization exists for many methods nowadays. In addition, in the vast majority of the discussed cases, the interface is triggered by a pre-reduction step in hydrogen and not by the reaction mixture itself. This is a particular pity, as it has been shown, e.g., for methane dry reforming or the reduction of NO by CO, that the nature and reactivity in terms of, e.g., the size of exsolved metal particles (hence, extent of the interface), the structural pre-steps to the formation of the interface, and the reactivity of the interface itself, is strongly depending on the gas-phase chemical potential. It is the rule, rather than the exception, that the physico-chemical and catalytic properties of interfaces formed by reduction in hydrogen or in the reaction mixture are different. The archetypical material in methane dry reforming, LaNiO3, serves as the prime example: upon decomposition in the reaction mixture, the reactivity of the interface is governed by the intermediate appearance of the Ruddlesden-Popper structure La2NiO4. However, upon hydrogen reduction, this phase does not appear during perovskite decomposition. In consequence, the Ni particle size and the reactivity of the interface are different.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Lassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef] [PubMed]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, H.; Won, B.; Jeon, H.; Park, C.; Park, D.; Kim, Y.; Lee, S.; Myung, J. Nanoparticle exsolution on perovskite oxides: Insights into mechanism, chareristics and novel strategies. Nano Micro Lett. 2024, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Sun, Y. The metal/oxide heterointerface delivered by solid-based exsolution strategy: A review. Chem. Eng. J. 2022, 440, 135868. [Google Scholar] [CrossRef]
- Sharif, R.; Khalid, A.; Ahmad, S.; Rehman, A.; Qutab, H.; Akhtar, H.; Mahmood, K.; Afzal, S.; Saleem, F. A comprehensive review of the current progresses and material advances in perovskite solar cells. Nanoscale Adv. 2023, 5, 3803–3833. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Joo, S.; Choi, S.; Sengodan, S.; Kim, G. Review on exsolution and its driving forces in perovskites. J. Phys. Energy 2020, 2, 032001. [Google Scholar] [CrossRef]
- Sun, Z.; Hao, C.; Toan, S.; Zhang, R.; Li, H.; Wu, Y.; Liu, H.; Sun, Z. Recent advances in exsolved perovskite oxide construction: Exsolution theory, modulation, challenges, and prospects. J. Mater. Chem. A 2023, 11, 17961–19776. [Google Scholar] [CrossRef]
- Ruh, T.; Berkovec, D.; Schrenk, F.; Rameshan, C. Exsolution on perovskite oxides: Morphology and anchorage of nanoparticles. Chem. Commun. 2023, 59, 3948–3956. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Rao, R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 7751–7756. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, J.; Gong, Y.; Lin, N.; Yang, Q.; Zhang, X.; Wang, Y. Perovskite catalysts with different dimensionalities for environmental and energy applications: A review. Sep. Purif. Technol. 2023, 307, 122716. [Google Scholar] [CrossRef]
- Bonmassar, N.; Bekheet, M.F.; Schlicker, L.; Gili, A.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; Bernardi, J.; Klötzer, B.; et al. In Situ-Determined Catalytically Active State of LaNiO3 in Methane Dry Reforming. ACS Catal. 2020, 10, 1102–1112. [Google Scholar] [CrossRef]
- Bekheet, M.F.; Nezhad, P.D.K.; Bonmassar, N.; Schlicker, L.; Gili, A.; Praetz, S.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; et al. Steering the Methane Dry Reforming Reactivity of Ni/La2O3 Catalysts by Controlled In Situ Decomposition of Doped La2NiO4 Precursor Structures. ACS Catal. 2021, 11, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, P.D.K.; Bekheet, M.F.; Bonmassar, N.; Schlicker, L.; Gili, A.; Kamutzki, F.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; et al. Mechanistic in situ insights into the formation, structural and catalytic aspects of the La2NiO4 intermediate phase in the dry reforming of methane over Ni-based perovskite catalysts. Appl. Catal. A 2021, 612, 117984. [Google Scholar] [CrossRef]
- Neagu, D.; Oh, T.; Miller, D.; Menard, H.; Bukhari, S.; Gamble, S.; Gorte, R.; Vohs, J.; Irvine, J.T.S. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Commun. 2015, 6, 8120. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Park, J.; Nam, S.; Kim, K.; Choi, G.; Parkin, S.; Jang, H.; Irvine, J.T.S. Lattice strain-enhanced exsolution of nanoparticles in thin films. Nat. Commun. 2019, 10, 1471. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, G.; Goldwasser, M.; de Navarro, C.; Barrault, J.T.J.; Batiot-Dupeyrat, C.; Martinez, F. Dry reforming of methane over Ni perovskite type oxides. Catal. Today 2005, 107, 785–791. [Google Scholar] [CrossRef]
- Gallego, G.; Mondragon, F.; Tatibouet, J.; Barrault, J.; Batiot-Dupeyrat, C. Carbon dioxide reforming of methane over La2NiO4 as catalyst precursor-characterization of carbon deposition. Catal. Today 2008, 133, 200–209. [Google Scholar] [CrossRef]
- Das, S.; Bhattar, S.; Liu, L.; Wang, Z.; Xi, S.; Spivey, J.J.; Kawi, S. Effect of partial Fe substitution in La0.9Sr0.1NiO3 perovskite-derived catalysts on the reaction mechanism of methane dry reforming. ACS Catal. 2020, 10, 12466–12486. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Li, M.; Wang, H. Perovskite La2(NiCu)O4 catalyst precursors for dry reforming of methane: Effects of Cu-substitution on carbon resistance. RSC Adv. 2017, 7, 41847. [Google Scholar] [CrossRef]
- Batiot-Dupeyrat, C.; Valderrama, G.; Meneses, A.; Martinez, F.; Barrault, J.; Tatibouet, J. Pulse study of CO2 reforming of methane over LaNiO3. Appl. Catal. A 2003, 248, 143–151. [Google Scholar] [CrossRef]
- Hayakawa, T.; Suzuki, S.; Nakamura, J.; Uchijima, T.; Hamakawa, S.; Suzuki, K.; Shishido, T.; Takehira, K. CO2 reforming of CH4 over Ni/perovskite catalysts prepared by solid phase crystallization method. Appl. Catal. A 1999, 183, 273–285. [Google Scholar] [CrossRef]
- Urasaki, K.; Sekine, Y.; Kawabe, S.; Kikuchi, E.; Matsukata, M. Catalytic activities and coking resistance of Ni/perovskites in steam reforming of methane. Appl. Catal. A 2005, 286, 23–29. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Imtiaz, Q.; Kim, S.; Abdala, P.; Yoon, S.; Müller, C.R. Dry reforming of methane over bimetallic Ni-N/La2O3 (M=Co, Fe): The effect of the rate of La2O2CO3 formation and phase stability on the catalytic activity and stability. J. Catal. 2016, 343, 208–214. [Google Scholar] [CrossRef]
- Song, X.; Dong, X.; Yin, S.; Weng, M.; Li, M.; Wang, H. Effects of Fe partial substitution of La2NiO4/LaNiO3 catalyst precursors prepared by wet impregnation method for the dry reforming of methane. Appl. Catal. A 2016, 526, 132–138. [Google Scholar] [CrossRef]
- Kim, W.; Jang, J.; Ra, E.; Kim, K.; Kim, E.; Lee, J. Reduced perovskite LaNiO3 catalysts modified with Co and Mn for low coke formation in dry reforming of methane. Appl. Catal. A 2019, 575, 198–203. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Maneerung, T.; Kathiraser, Y.; Kawi, S. CO2 dry reforming of methane over La0.8Sr0.2Ni0.8M0.2O3 perovskite (M=Bi, Co, Cr, Cu, Fe): Roles of lattice oxygen on C-H activation and carbon suppression. Int. J. Hydrogen Energy 2012, 37, 11195–11207. [Google Scholar] [CrossRef]
- Araujo, G.; Lima, S.; Assaf, J.; Pena, M.; Fierro, J.; Rangel, M. Catalytic evaluation of perovskite-type oxide LaNi1-xRuxO3 in methane dry reforming. Catal. Today 2008, 133, 129–135. [Google Scholar] [CrossRef]
- Lima, S.; Assaf, J. Ni-Fe catalysts based on perovskite-type oxides for dry reforming of methane to syngas. Catal. Lett. 2006, 108, 63–70. [Google Scholar] [CrossRef]
- Provendier, H.; Petit, C.; Estournes, C.; Kiennemann, A. Dry reforming of methane. Interest of La-Ni-Fe solid solutions compared to LaNiO3 and LaFeO3, Natural Gas conversions V. Stud. Surf. Sci. Catal. 1998, 119, 741–746. [Google Scholar]
- Su, Y.; Pan, K.; Chang, M. Modifying perovskite-type oxide catalyst LaNiO3 with Ce for carbon dioxide reforming of methane. Int. J. Hydrogen Energy 2014, 39, 4917–4925. [Google Scholar] [CrossRef]
- Moradi, G.R.; Rahmanzadeh, M.; Khosravian, F. The effects of pertial substitution of Ni by Zn in LaNiO3 perovskite catalyst for methane dry reforming. J. CO2 Util. 2014, 6, 7–11. [Google Scholar] [CrossRef]
- Rabelo-Nieto, R.C.; Sales, H.; Inocencio, C.; Varga, E.; Osko, A.; Erdohelyi, A.; Noronha, F.; Mattos, L. CO2 reforming of methane over supported LaNiO3 perovskite-type oxides. Appl. Catal. B 2018, 221, 349–361. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Dong, X.; Li, M.; Wang, H. Effect of Ce substitution at the A-site of LaNi0.5Fe0.5O3 perovskite on the enhanced catalytic activity for dry reforming of methane. Appl. Catal. B 2018, 224, 214–221. [Google Scholar] [CrossRef]
- Nezhad, P.D.K.; Bekheet, M.F.; Bonmassar, N.; Gili, A.; Kamutzki, F.; Gurlo, A.; Doran, A.; Schwarz, S.; Bernardi, J.; Praetz, S.; et al. Elucidating the role of earth alkaline doping in perovskite-based methane dry reforming catalysts. Catal. Sci. Technol. 2022, 12, 1229–1244. [Google Scholar] [CrossRef]
- Kühl, S.; Düdder, H.; Girgsdies, F.; Kähler, K.; Muhler, M.; Behrens, M. Perovskites as precursors for Ni/La2O3 catalysts in the dry reforming of methane: Synthesis by constant pH co-precipitation, reduction mechanism and effect of Ru-doping. Z. Für Anorg. Und Allg. Chem. 2017, 643, 1088–1095. [Google Scholar] [CrossRef]
- Gao, J.; Jia, L.; Fang, W.; Li, Q.; Song, H. Methanation of carbon dioxide over the LaNiO3 perovskite catalysts activaed under the reactant stream. J. Fuel Chem. Technol. 2009, 37, 573–577. [Google Scholar] [CrossRef]
- Wang, H.; Dong, X.; Zhao, T.; Yu, H.; Li, M. Dry reforming of methane over bimetallic Ni-Co catalyst prepared from La(CoxNi1-x)0.5Fe0.5O3 perovskite precursor: Catalytic activity and coking resistance. Appl. Catal. B 2019, 245, 302–313. [Google Scholar] [CrossRef]
- Zhao, B.; Yan, B.; Yao, S.; Xie, Z.; Wu, Q.; Ran, R.; Weng, D.; Zhang, C.; Chen, J. LaFe0.9Ni0.1O3 perovskite catalyst with enhanced activity and coke-resistance for dry reforming of ethane. J. Catal. 2018, 358, 168–178. [Google Scholar] [CrossRef]
- Alvarez, J.; Valderrama, G.; Pietri, E.; Perez-Zurita, M.; Navarro, C.; Sousa-Aguiar, E.; Goldwasser, M. Ni-Nb-based mixed oxide precursors for the dry refroming of methane. Top. Catal. 2011, 54, 170–178. [Google Scholar] [CrossRef]
- Joo, S.; Seong, A.; Kwon, O.; Kim, K.; Lee, J.H.; Gorte, R.J.; Vohs, J.M.; Han, J.W.; Kim, G. Highly active dry methane reforming catalysts with boosted in situ grown Ni-Fe nanoparticles on perovskite via atomic layer deposition. Sci. Adv. 2020, 6, 2375–2548. [Google Scholar] [CrossRef] [PubMed]
- Touahra, F.; Rabahi, A.; Chebout, R.; Boudjama, A.; Lerari, D.; Sehailia, M.; Halliche, D.; Bachari, K. Enhanced catalytic behaviour of surfae dispersed nickel on LaCuO3 perovskite in the production of syngas: An expedient approach to carbon resistance during CO2 reforming of methane. Int. J. Hydrogen Energy 2016, 41, 2477–2486. [Google Scholar] [CrossRef]
- Osuzawa, O.; Setiabudi, H.; Rasid, R.; Cheng, C. Syngas production via methane dry reforming: A novel application of SmCoO3 perovskite catalyst. J. Nat. Gas Sci. Eng. 2017, 27, 435–448. [Google Scholar] [CrossRef]
- Qi, R.; Jin, R.; An, L.; Bai, X.; Wang, Z. A Ni/perovskite catalyst with low metal content for CO2 reforming of methane. Catal. Commun. 2022, 163, 106419. [Google Scholar] [CrossRef]
- Sagar, T.; Padmakar, D.; Lingaiah, N.; Rao, K.S.R.; Reddy, I.; Prasad, P. Syngas production by CO2 reforming of methane on LaNixAl-xO3 perovskite catalysts: Influence of method of preparation. J. Chem. Sci. 2017, 129, 1787–1794. [Google Scholar] [CrossRef]
- Ma, Y.; Su, P.; Ge, Y.; Wang, F.; Xue, R.; Wang, Z.; Li, Y. A novel LaAlO3 perovskite with large surface area supported Ni-based catalyst for methane dry reforming. Catal. Lett. 2022, 152, 2993–3003. [Google Scholar] [CrossRef]
- Dama, S.; Godhke, S.; Bobade, R.; Gurav, H.R.; Chilukuri, S. Active and durable alkaline earth metal substituted perovskite catalysts for dry reforming of methane. Appl. Catal. B 2018, 224, 146–158. [Google Scholar] [CrossRef]
- Wei, T.; Chia, L.; Zheng, H.; Chi, B.; Pu, J.; Li, J. LaMnO3-based perovskite with in situ exsolved Ni nanoparticles: A highly active, performance stable and coking resistant catalyst for CO2 dry reforming of CH4. Appl. Catal. A 2018, 564, 199–207. [Google Scholar] [CrossRef]
- Yao, X.; Cheng, Q.; Attada, Y.; Ould-Chikh, S.; Ramírez, A.; Bai, X.; Mohamed, H.O.; Li, G.; Shterk, G.; Zheng, L.; et al. Atypical stability of exsolved Ni-Fe alloy nanoparticles on double layered perovskite for CO2 dry reforming og methane. Appl. Catal. B 2023, 328, 122479. [Google Scholar] [CrossRef]
- Wei, T.; Jia, L.; Luo, J.; Chi, B.; Pu, J.; Li, J. CO2 dry reforming of methane with Sr and Ni co-doped LaCrO3 perovskite. Appl. Surf. Sci. 2020, 506, 144699. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Uphade, B.S.; Belhekar, A.A. Oxidative coupling of methane to syngas over LaNiO3 perovskite with and without simultaneous steam and CO2 reforming. J. Catal. 1996, 163, 312–318. [Google Scholar] [CrossRef]
- Provendier, H.; Petit, C.; Estournes, C.; Libs, S.; Kiennemann, A. Stabilisation of active nickel catalysts in partial oxidation of methane to synthesis gas by iron addition. Appl. Catal. A 1999, 180, 163–173. [Google Scholar] [CrossRef]
- Nguyen, T.; Lamacz, A.; Beaunier, P.; Czajkowska, S.; Domanski, M.; Krzton, A.; Le, T.; Djega-Mariadassou, G. Partial oxidation of methane over bifunctional catalyst I. In situ formation of Ni0/La2O3 during temperature-programmed POM reaction over LaNiO3 perovskite. Appl. Catal. B 2014, 152, 360. [Google Scholar] [CrossRef]
- Onn, T.; Monai, M.; Dai, S.; Fonda, E.; Montini, T.; Pan, X.; Graham, G.; Fornasiero, P.; Gorte, R. Smart Pd catalyst with improved thermal stability supported on high-surface area LaFeO3 prepared by atomic layer deposition. J. Am. Chem. Soc. 2018, 140, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Basahel, S.; Medkhali, A.; Mokhtar, M.; Narasimharao, K. Noble metal (Pd, Pt and Rh) incorporated LaFeO3 perovskite oxides for catalytic oxidative cracking of n-propane. Catal. Today 2022, 397, 81–93. [Google Scholar] [CrossRef]
- Kousi, K.; Neagu, D.; Bekris, L.; Cali, E.; Kerherve, G.; Papaioannou, E.; Payne, D.; Metcalfe, I. Low-temperature methane conversion with perovskite-supported exo/endo-particles. J. Mater. Chem. A 2020, 8, 12406–12417. [Google Scholar] [CrossRef]
- Lindenthal, L.; Popovic, J.; Rameshan, R.; Huber, J.; Schrenk, F.; Ruh, T.; Nenning, A.; Löffler, S.; Opitz, A.K.; Rameshan, C. Novel perovskite catalysts for CO2 utilization—Exsolution enhanced reverse water-gas shift activity. Appl. Catal. B 2021, 292, 120183. [Google Scholar] [CrossRef]
- Lindenthal, L.; Huber, J.; Drexler, H.; Ruh, T.; Rameshan, R.; Schrenk, F.; Löffler, S.; Rameshan, C. In situ growth of exsolved nanoparticles under varying rWGS reaction conditions—A catalysis and near ambient pressure-XPS study. Catalysts 2021, 11, 1484. [Google Scholar] [CrossRef]
- Huang, R.; Lim, C.; Jang, M.; Hwang, J.; Han, J. Exsolved metal-boosted active perovskite catalyst for water-gas shift reaction. J. Catal. 2021, 400, 148–159. [Google Scholar] [CrossRef]
- Maneerung, T.; Hidajat, K.; Kawi, S. K-doped LaNiO3 perovskite for high.temperature water-gas shift of reformate gas: Role of potassium on suppressing methanation. Int. J. Hydrogen Energy 2017, 42, 9840–9857. [Google Scholar] [CrossRef]
- Maluf, S.S.; Tanabe, E.Y.; Nascente, P.A.P.; Assaf, E.M. Study of water-gas shift reaction over La1-ySryNixCo1-xO3. Top. Catal. 2011, 54, 210–218. [Google Scholar] [CrossRef]
- Coletta, V.C.; Gonçalves, R.V.; Bernardi, M.I.B.; Hanaor, D.A.H.; Assadi, M.H.N.; Marcos, F.C.F.; Nogueira, F.G.E.; Assaf, E.M.; Mastelaro, V.R. Cu-modified SrTiO3 perovskites toward enhanced water-gas shift catalysis: A combined experimental and computational study. ACS Appl. Energy Mater. 2021, 4, 452–461. [Google Scholar] [CrossRef]
- Watanabe, R.; Sekine, Y.; Takamatsu, H.; Samaoto, Y.; Aramaki, S.; Matsukata, M.; Kikuchi, E. Pt and/or Pd-supported/incorporated catalyst on perovskite-type oxide for water gas shift reaction. Top. Catal. 2010, 53, 621–628. [Google Scholar] [CrossRef]
- Sekine, Y.; Takamatsu, H.; Aramaki, S.; Ichishima, K.; Takada, M.; Matsukata, M.; Kikuchi, E. Synergistic effect of Pt or Pd and perovskite oxide for water gas shift reaction. Appl. Catal. A 2009, 352, 214–222. [Google Scholar] [CrossRef]
- Daza, Y.; Kent, R.; Yung, M.; Kuhn, J. Carbon dioxide conversion by reverse water-gas shift chemical looping on perovskite-type oxides. Ind. Eng. Chem. Res. 2014, 53, 5828–5837. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, F.; Wang, N.; Li, X. Cu-Y2O3 catalyst derived from Cu2Y2O5 perovskite for water gas shift reaction: The effect of reduction temperature. Catalysts 2022, 12, 481. [Google Scholar] [CrossRef]
- Orsini, F.; Ferrero, D.; Cannone, S.F.; Santarelli, M.; Felli, A.; Boaro, M.; de Leitenburg, C.; Trovarelli, A.; Llorca, J.; Dimitrakopoulos, G.; et al. Exsolution-enhanced reverse water-gas shift chemical looping activity of Sr2FeMo0.6Ni0.4O6-d. Chem. Eng. J. 2023, 475, 146083. [Google Scholar] [CrossRef]
- Ploner, K.; Götsch, T.; Kogler, G.; Thalinger, R.; Bernardi, J.; Zhao, Q.; Zhuo, C.; Klötzer, B.; Penner, S. Structural and catalytic properties of Ag- and Co3O4-impregnated strontium titanium ferrite SrTi0.7Fe0.3O3-d in methanol steam reforming. Ind. Eng. Chem. Res. 2017, 56, 13654–13662. [Google Scholar] [CrossRef]
- Provendier, H.; Petit, C.; Kiennemann, A. Steam reforming of methane on LaNixFe1-xO3 (0 ≤ x ≤ 1) perovkites. Reactivity and characterisation after test. Comptes Rendus L’académie Des Sci. Ser. IIC-Chem. 2001, 4, 57–66. [Google Scholar]
- Zhang, X.; Su, Y.; Pei, C.; Zhao, Z.; Liu, R.; Gong, J. Chemical Looping steam reforming of methane over Ce-doped perovskites. Chem. Eng. J. 2020, 223, 115707. [Google Scholar]
- Ou, Z.; Zhang, Z.; Qin, C.; Xia, H.; Deng, T.; Niu, J.; Ran, J.; Wu, C. Highly active and stable Ni/perovskite catalysts in steam methane reforming for hydrogen production. Sustain. Energy Fuels 2021, 5, 1845. [Google Scholar] [CrossRef]
- Natile, M.; Poletto, F.; Galenda, A.; Glisenti, A.; Montini, T.; De Rogatis, L.; Fornasiero, P. La0.6Sr0.4CoyFe1-yO3 perovskites: Influence of the Co/Fe atomic ratio on properties and catalytic activity toward alcohol steam reforming. Chem. Mater. 2008, 20, 2314–2327. [Google Scholar] [CrossRef]
- Dama, S.; Ghudke, S.; Bobade, R.; Gurav, H.; Chilukuri, S. Tuning the dimensionality of layered Srn+1Tin-xNixO3n+1 perovskite structures for improved activity in syngas generation. J. Catal. 2018, 360, 27–39. [Google Scholar] [CrossRef]
- Yang, E.; Kim, N.; Noh, Y.; Lim, S.; Jung, J.; Lee, J.; Hong, G.; Moon, D. Steam reforming of methane over La1-xCexNiO3 perovskite catalysts. Int. J. Hydrogen Energy 2015, 40, 11831–11839. [Google Scholar] [CrossRef]
- Aguero, F.; Morales, M.; Larregola, S.; Izurieta, E.; Lopez, E.L.; Cadus, E. La1-xCaxAl1-yNiyO3 perovskites used as catalyst precursors of nickel-based catalysts for ethanaol steam reforming. Int. J. Hydrogen Energy 2015, 40, 15510–15520. [Google Scholar] [CrossRef]
- Aguero, F.; Alonso, J.; Fernandez-Diaz, M.; Cadus, L. Ni-based catalysts obtained from perovskite oxides for ethaol steam reforming. J. Fuel Chem. Technol. 2018, 46, 1332–1341. [Google Scholar] [CrossRef]
- da Lima, S.; da Silva, A.; da Costa, L.; Assaf, J.; Mattos, L.; Sarkari, R.; Venugopal, A.; Noronha, R. Hydrogen production through oxidative steam reforming of ethanol over Ni-based catalysts derived from La1-xCexNiO3. Appl. Catal. B 2012, 121, 1–9. [Google Scholar] [CrossRef]
- Lin, K.; Wang, C.; Chien, S. Catalytic performance of steam reforming of ethanol at low temperature over LaNiO3 perovskite. Int. J. Hydrogen Energy 2013, 38, 3226–3232. [Google Scholar] [CrossRef]
- Zhao, L.; Han, T.; Wang, H.; Zhang, L.; Liu, Y. Ni-Co alloy catalysts from La1-xNixCoO3 perovskite supported on zirconia for steam reforming of ethanol. Appl. Catal. B 2016, 187, 19–29. [Google Scholar] [CrossRef]
- Ramesh, S.; Yang, E.; Jung, J.; Moon, D. Copper decorated perovskite an efficient catalyst for low temperature hydroen production by steam reforming of glycerol. Int. J. Hydrogen Energy 2015, 40, 11428–11435. [Google Scholar] [CrossRef]
- Wu, G.; Li, S.; Zhang, C.; Wang, T.; Gong, J. Glycerol steam reforming over perovskite-derived nickel-based catalysts. Appl. Catal. B 2014, 144, 277–285. [Google Scholar] [CrossRef]
- Aman, D.; Radwan, D.; Ebaid, M.; Mikhail, S.; van Steen, E. Comparing nickel and cobalt perovskites for steam reforming of glycerol. Mol. Catal. 2018, 452, 60–67. [Google Scholar] [CrossRef]
- Oemar, U.; Ang, P.S.; Hidajat, K.; Kawi, S. Promotional effect of Fe on perovskite LaNixFe1-xO3 catalyst for hydrogen production via steam reforming of toluene. Int. J. Hydrogen Energy 2013, 38, 5525–5534. [Google Scholar] [CrossRef]
- Sekine, Y.; Mukai, D.; Murai, Y.; Tochiya, S.; Izutsu, Y.; Sekiguchi, K.; Hosomura, N.; Arai, H.; Kikuchi, E.; Sugiura, Y. Steam reforming of toluene over perovskite-supported Ni catalysts. Appl. Catal. A 2013, 451, 160–167. [Google Scholar] [CrossRef]
- Urasaki, K.; Tokunaga, K.; Sekine, Y.; Matsukata, M.; Kikuchi, E. Production of hydrogen by steam reforming of ethanol over cobalt and nickel catalysts supported on perovskite.type catalysts. Catal. Commun. 2008, 9, 600–604. [Google Scholar] [CrossRef]
- Morales, M.; Segarra, M. Steam reforming and oxidate steam reforming of ethanol over La0.6Sr0.4CoO3-d perovskite as catalyst precursor for hydrogen production. Appl. Catal. A 2015, 502, 305–311. [Google Scholar] [CrossRef]
- Thalinger, R.; Gocyla, M.; Heggen, M.; Dunin-Borkowski, R.; Grünbacher, M.; Stöger-Pollach, M.; Schmidmair, D.; Klötzer, B.; Penner, S. Ni-perovskite interaction and ist structural and catalytic consequences in methane steam reforming and methanation reactions. J. Catal. 2016, 337, 26–35. [Google Scholar] [CrossRef]
- Thalinger, R.; Götsch, T.; Zhuo, C.; Hetaba, W.; Wallisch, W.; Stöger-Pollach, M.; Schmidmair, D.; Klötzer, B.; Penner, S. Rhodium-catalyzed methanation and methane steam reforming reactions on Rhodium-perovskite systems: Metal-support interaction. ChemCatChem 2016, 8, 2057–2067. [Google Scholar] [CrossRef]
- Wang, H.; Fang, Y.; Liu, Y.; Bai, X. Perovskite LaFeO3 supported bi.metal catalyst for syngas methanation. J. Nat. Gas Chem. 2012, 21, 745–752. [Google Scholar] [CrossRef]
- Dokuchits, E.V.; Larina, T.V.; Gerasimov, E.Y.; Pochtar, A.A.; Minyukova, T.P. Syngas conversion over perovskite-like LaCuxTi1-xO3/KIT-6 catalysts. Appl. Catal. A 2020, 608, 117834. [Google Scholar] [CrossRef]
- Yan, B.; Wu, Q.; Cen, J.; Timoshenko, J.; Frenkel, A.; Su, D.; Chen, X.; Parise, J.; Stach, A.; Orlev, A.; et al. Highly active subnanmoeter Rh clusters derived from Rh-doped SrTiO3 for CO2 redcution. Appl. Catal. B 2018, 237, 1003–1011. [Google Scholar] [CrossRef]
- Aryandiyan, H.; Wang, Y.; Scott, J.; Mesgari, S.; Dai, H.; Amal, R. In situ exsolution of bimetallic Rh-Ni nanoalloys: A highly efficient catalyst for CO2 methanation. ACS Appl. Mater. Interfaces 2018, 10, 16352–16357. [Google Scholar] [CrossRef] [PubMed]
- Fierro, J.L.G. Hydrogenation of carbon oxides over perovskite-type oxides. Catal. Rev. 1992, 34, 321–336. [Google Scholar] [CrossRef]
- Gisling, H.J.; Monnier, J.R.; Apai, G. Synthesis, characterization and catalytic activity of LaRhO3. J. Catal. 1987, 103, 407. [Google Scholar] [CrossRef]
- Broussard, J.A.; Wade, L.E. Catalytic conversion of syngas with perovskites. Am. Chem. Soc. Div. Fuel Chem. 1986, 31, 75. [Google Scholar]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, H. Self-generation of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Taniguchi, M.; Uenishi, M.; Kajita, N.; Tan, I.; Nishihata, Y.; Mizuki, J.; Narita, K.; Kimura, M.; Kaneko, K. Self-generating Rh- and Pt-based perovskite catalysts for automotive emissions control. Angew. Chem. Int. Ed. 2006, 45, 5998–6002. [Google Scholar] [CrossRef]
- Tanaka, H.; Tan, I.; Uenishi, M.; Taniguchi, M.; Kimura, M.; Nishihata, Y.; Mizuki, J. LaFePdO3 perovskite automotive catalyst having a self-regenerative function. J. Alloys Compd. 2006, 408, 1071–1077. [Google Scholar] [CrossRef]
- Katz, M.; Graham, G.; Duan, Y.; Liu, H.; Adamo, C.; Schlom, D.; Pan, X. Self-regeration of Pd-LaFeO3 catalysts: New insights from atomic-resolution electron microscopy. J. Am. Chem. Soc. 2011, 133, 18090–18093. [Google Scholar] [CrossRef]
- Kothari, M.; Jeon, Y.; Miller, D.; Pascui, A.; Kilmartin, J.; Wails, D.; Ramos, S.; Chadwick, A.; Irvine, J.T.S. Platinum incorporation into titanate perovskites to deliver emergent active and stable platinum nanparticles. Nat. Chem. 2021, 13, 677–682. [Google Scholar] [CrossRef]
- Glisenti, A.; Pacella, M.; Guiotto, M.; Natile, M.M.; Canu, P. Largely Cu-doped LaCo1-xCux perovskites for TWC: Toward new PGM-free catalysts. Appl. Catal. B 2016, 180, 94–105. [Google Scholar] [CrossRef]
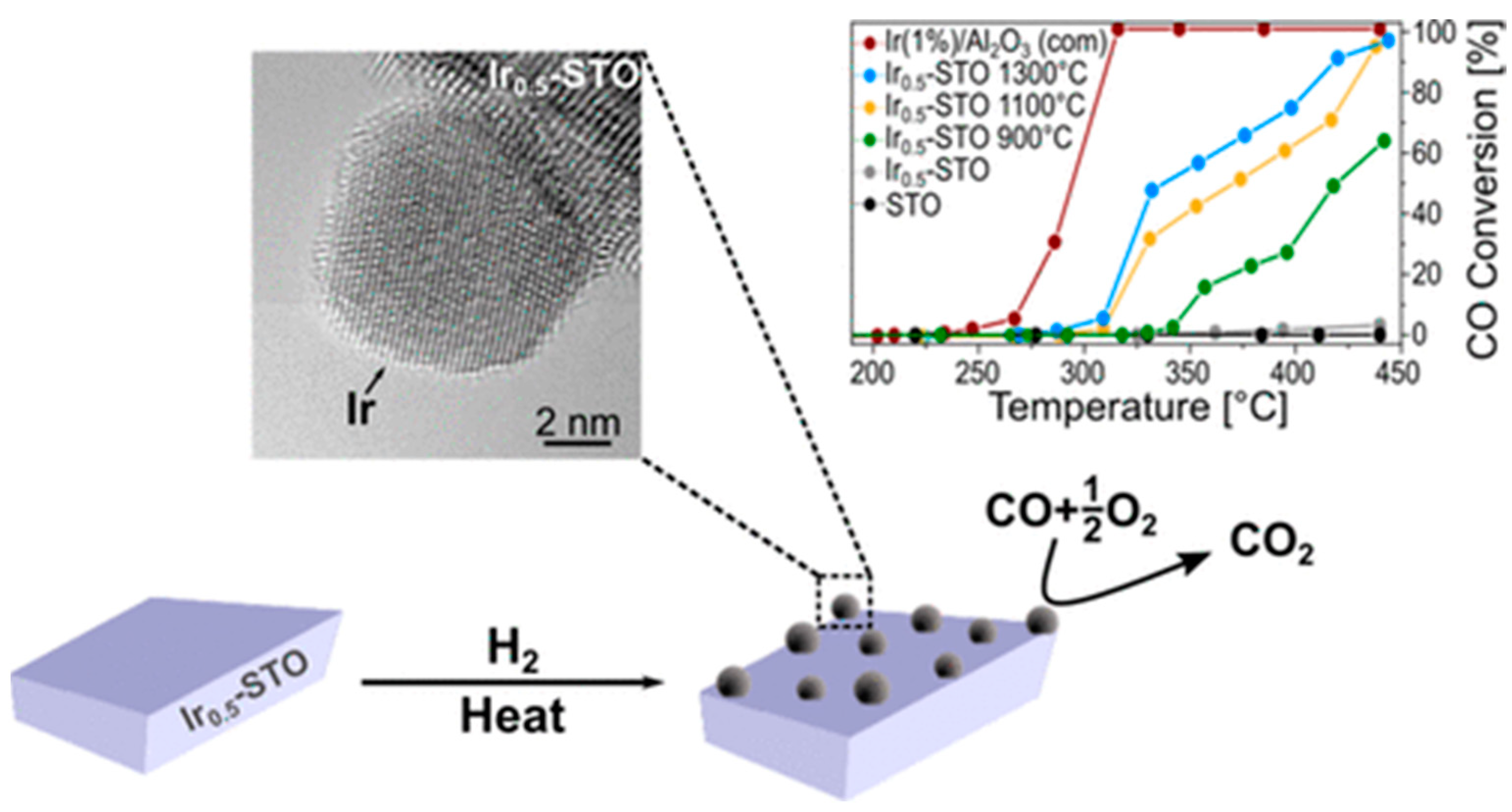
- Calì, E.; Kerherve, G.; Naufal, F.; Kousi, K.; Neagu, D.; Papaioannou, E.I.; Thomas, M.P.; Guiton, B.S.; Metcalfe, I.S.; Irvine, J.T.S.; et al. Exsolution of catalytically active iridium nanparticles from strontium titanate. ACS Appl. Energy Mater. 2020, 12, 37444–37453. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Wang, C.; Shen, K.; Vohs, J.; Gorte, R. Investigation into support effects for Pt and Pd on LaMnO3. Appl. Catal. A 2022, 646, 1188773. [Google Scholar] [CrossRef]
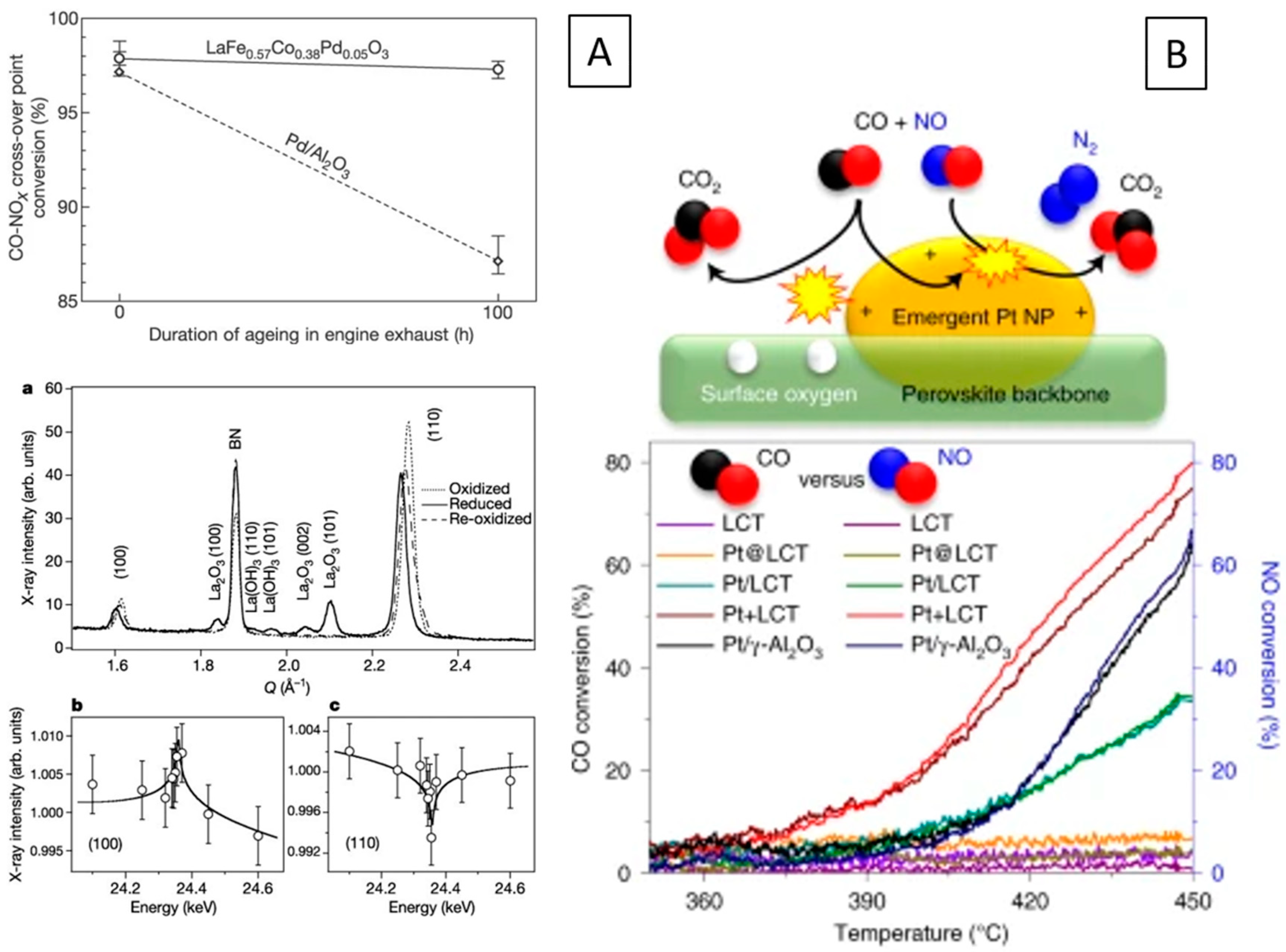
- Thurner, C.W.; Bonmassar, N.; Winkler, D.; Haug, L.; Ploner, K.; Nezhad, P.D.K.; Drexler, X.; Mohammadi, A.; van Aken, P.A.; Kunze-Liebhäuser, J.; et al. Who does the job? How copper can replace noble metals in sustainable catalysis by the formation of copper-mixed oxide interfaces. ACS Catal. 2022, 12, 7696–7708. [Google Scholar] [CrossRef] [PubMed]
- Thurner, C.; Drexler, X.; Haug, L.; Winkler, D.; Kunze-Liebhäuser, J.; Bernardi, J.; Klötzer, B.; Penner, S. When copper is not enough: Advantages and drawbacks of using copper in deNOx reactions over lanthanum manganite perovskite structures. Appl. Catal. B 2023, 331, 122693. [Google Scholar] [CrossRef]
- Mohammadi, A.; Farzi, A.; Thurner, C.; Klötzer, B.; Schwarz, S.; Bernardi, J.; Niaei, A.; Penner, S. Tailoring the metal-perovskite interface for promotional steering of the catalytic NO reduction by CO in the presence of H2O on Pd-lanthanum iron manganite composites. Appl. Catal. B 2022, 307, 121160. [Google Scholar] [CrossRef]
- Simonot, L.; Garin, F.; Maire, G. A comparative study of LaCoO3, Co3O4 and a mix of LaCoO3-Co3O4 II. Catalytic properties for the CO+NO reaction. Appl. Catal. B 1997, 11, 181–191. [Google Scholar] [CrossRef]
- Higo, T.; Omori, Y.; Shigemoto, A.; Ueno, K.; Ogo, S.; Sekine, Y. Promotive effect of H2O on low-temperature NO reduction by CO over Pd/La0.9Ba0.9AlO3-δ. Catal. Today 2020, 352, 192–197. [Google Scholar] [CrossRef]
- Khristova, M.; Petrovic, S.; Terlecki-Baricevic, A.; Mehandjiev, D. Catalytic reduction of NO by CO over Pd–doped Perovskite-type catalysts. Cent. Eur. J. Chem. 2009, 7, 857–863. [Google Scholar]
- Munoz, H.; Korili, S.; Gil, A. Progress and recent strategies in the synthesis and catalytic applications of perovskites based on lanthanum and aluminum. Materials 2002, 15, 3288. [Google Scholar] [CrossRef] [PubMed]
- Kapokova, L.; Pavlova, S.; Bunina, R.; Alikina, G.; Krieger, T.; Ishchenko, A.; Rogov, V.; Sadykov, V. Dry reforming of methane over LnFe0.7Ni0.3O3-δ perovskites: Influence of Ln nature. Catal. Today 2011, 164, 227–233. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Chi, B.; Pu, J.; Li, J. High performance Ni exsolved and Cu added La0.8Ce0.2Mn0.6Ni0.4O3-based perovskites for ethanol steam reforming. Int. J. Hydrogen Energy 2020, 45, 16458–16468. [Google Scholar] [CrossRef]
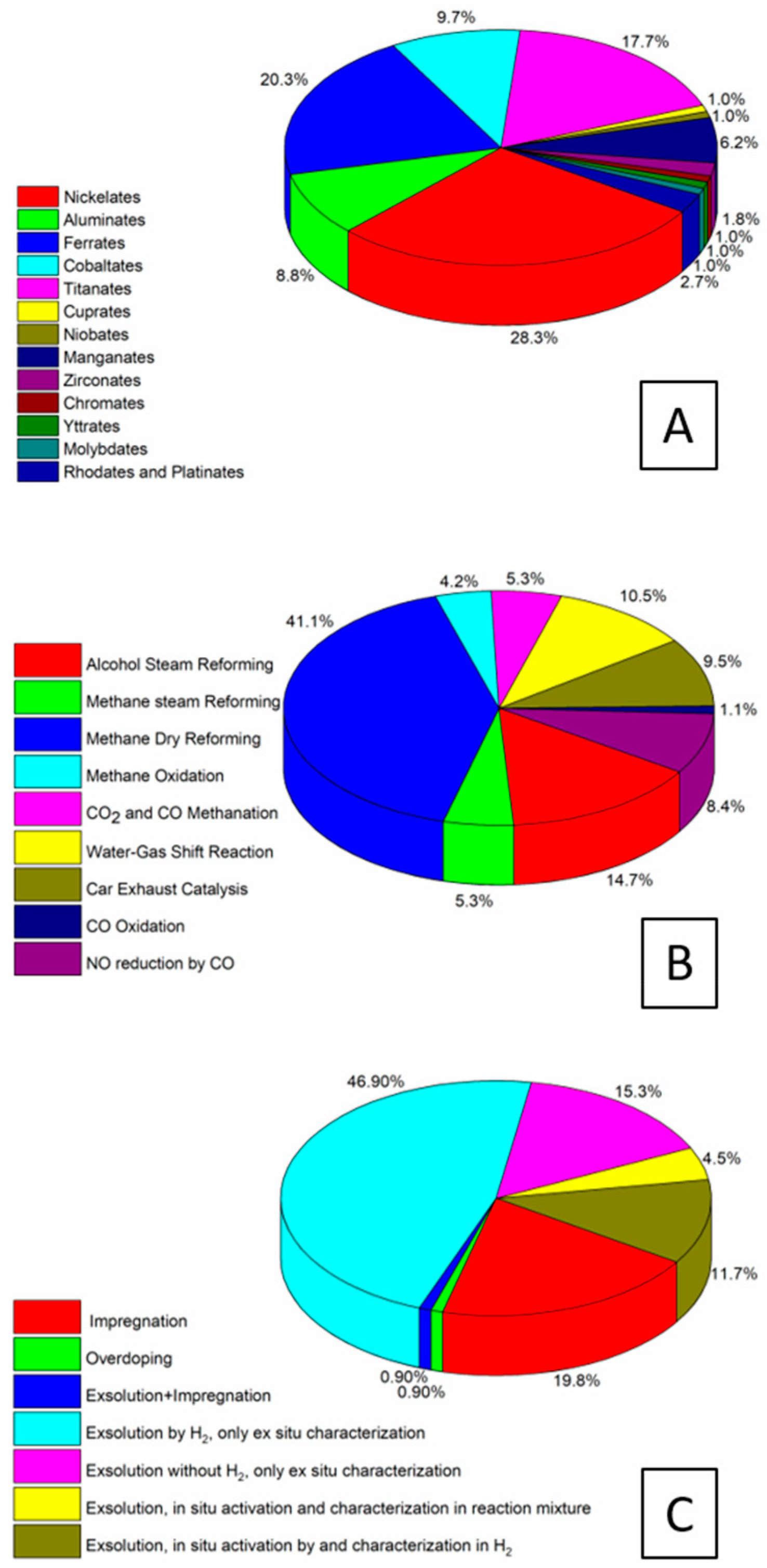
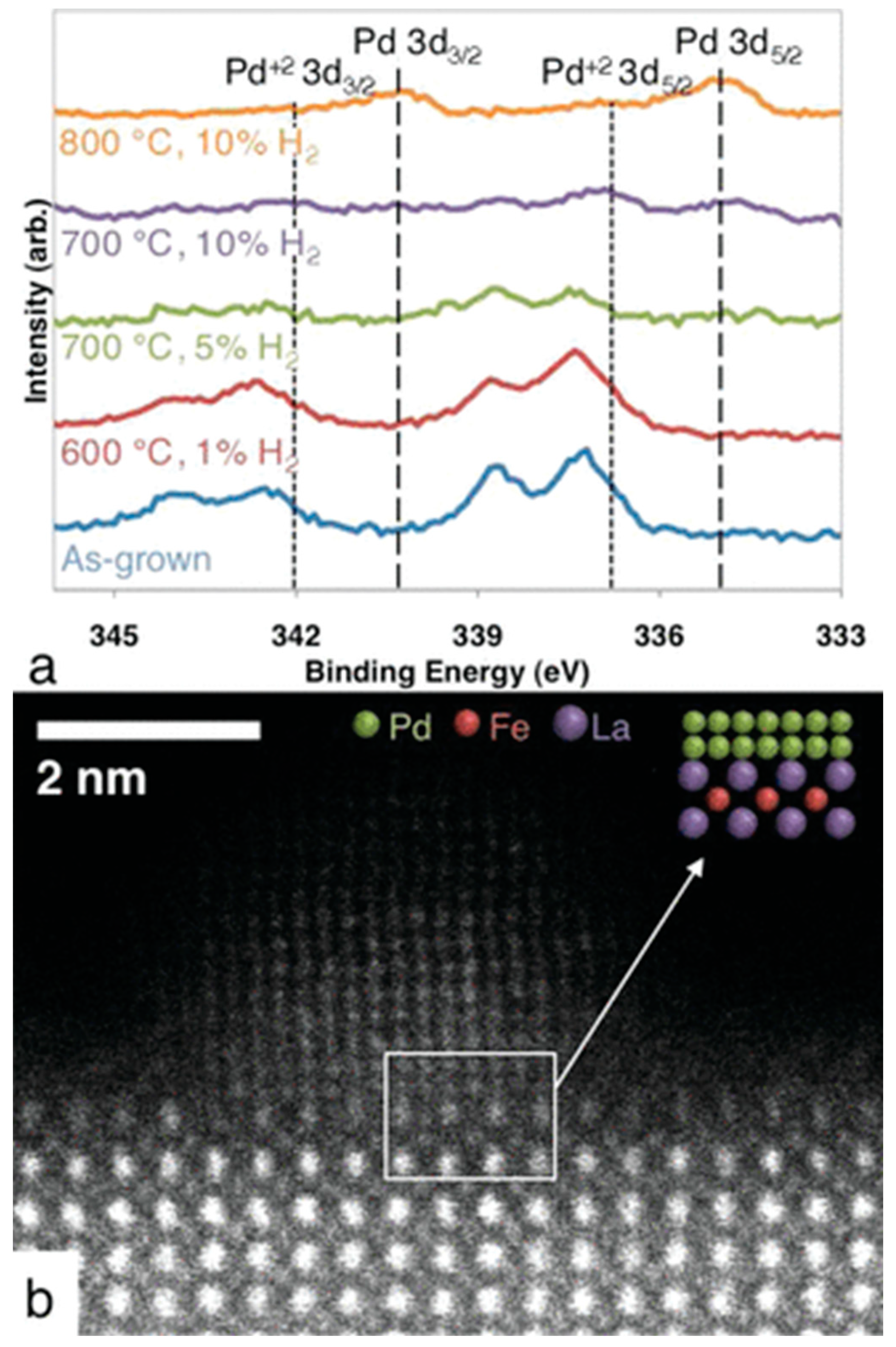
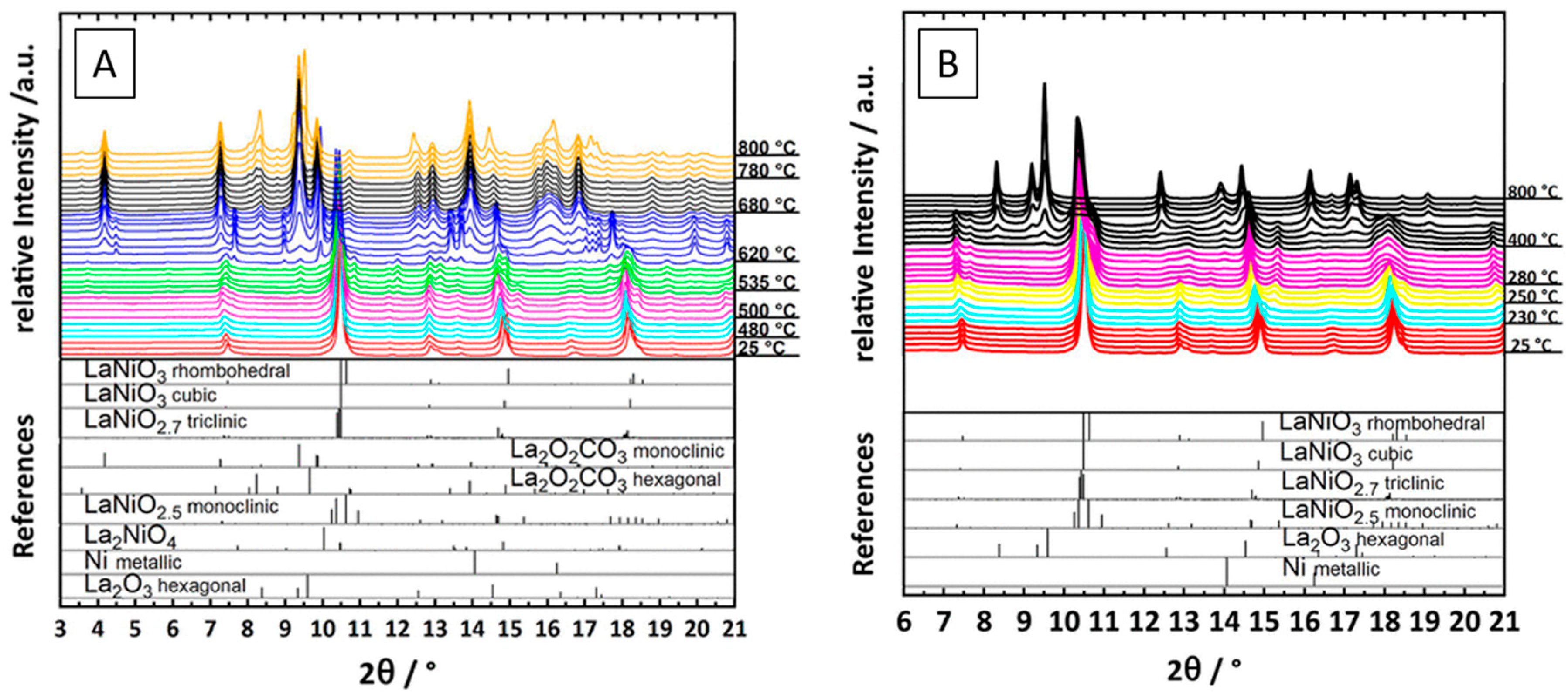
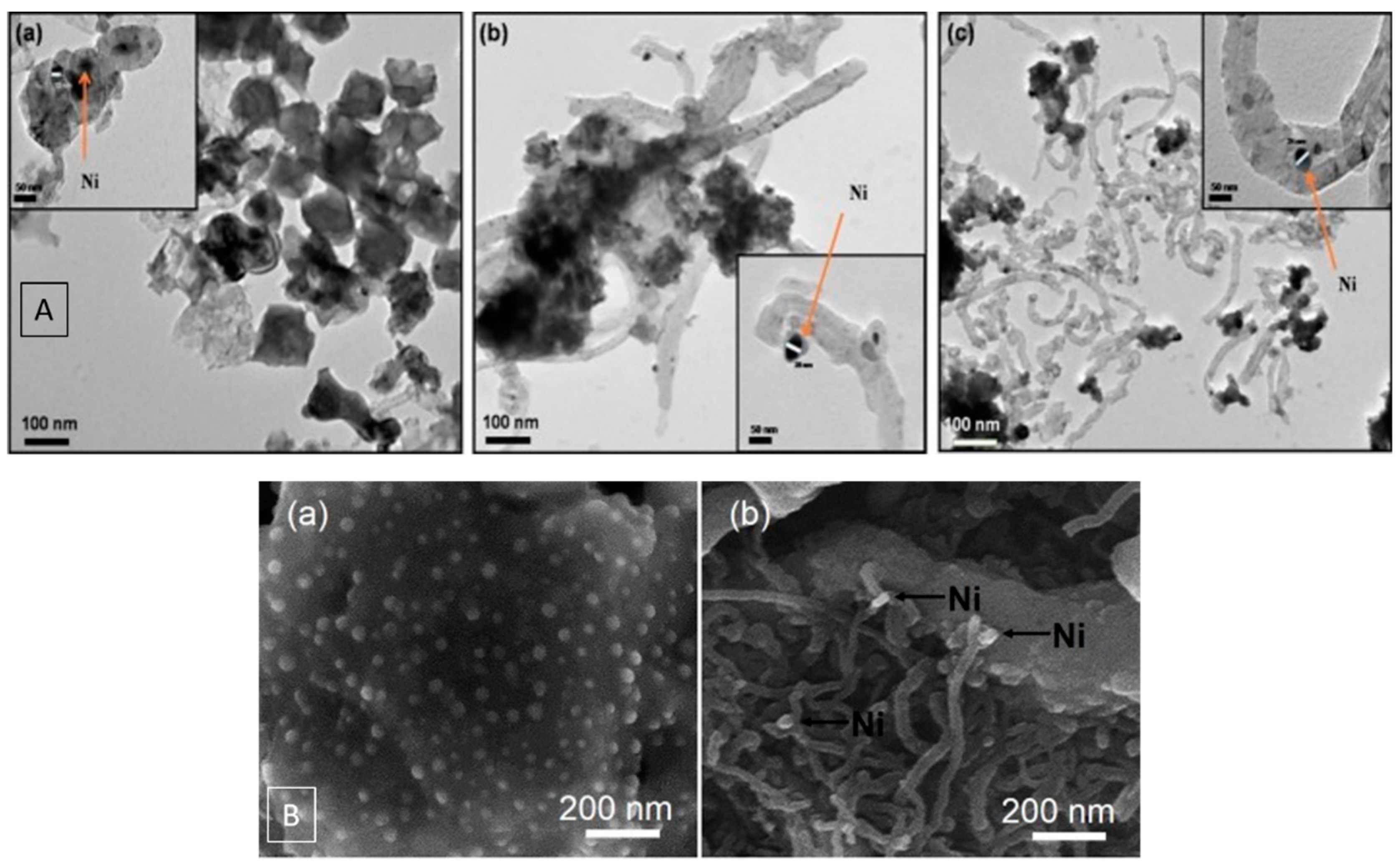
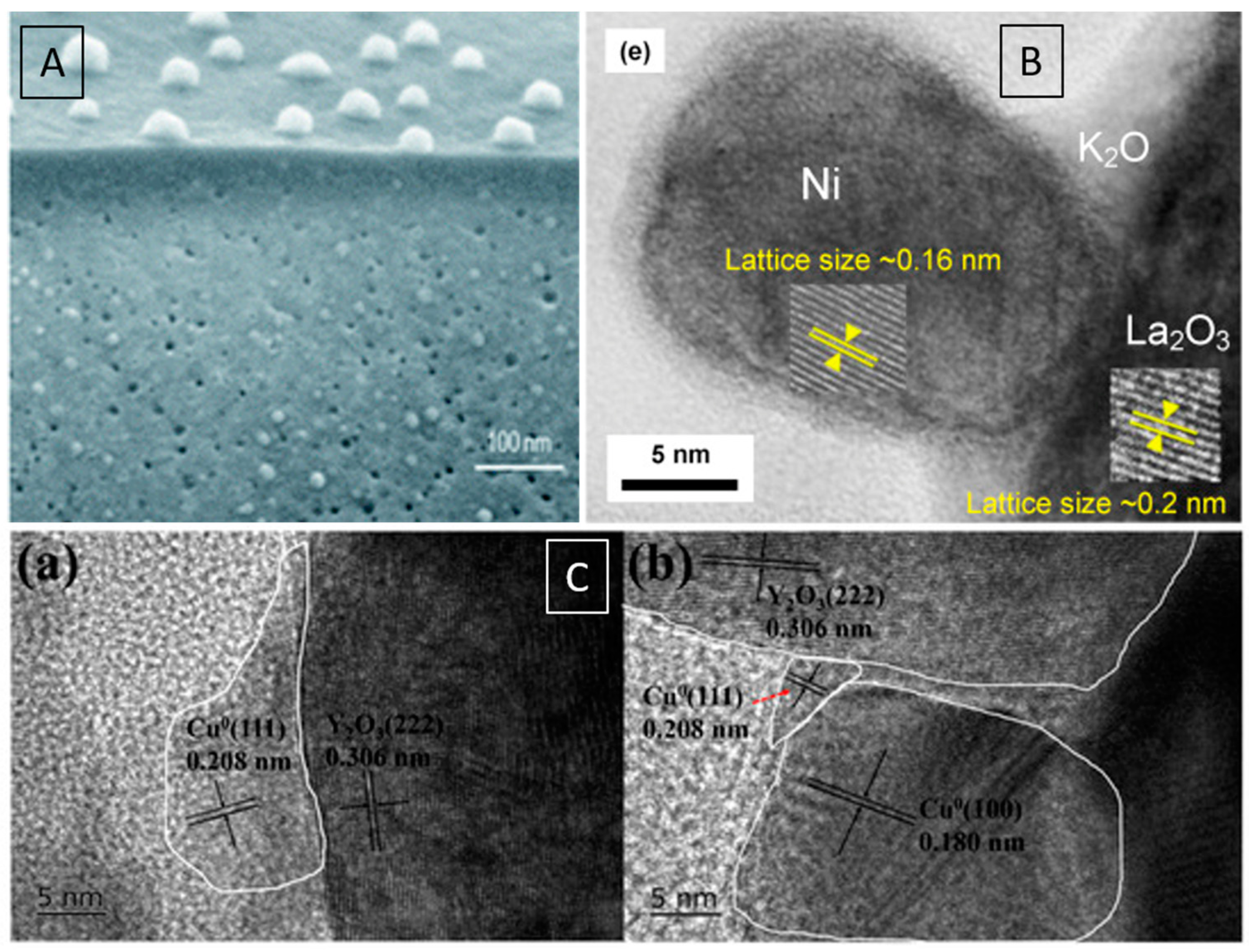
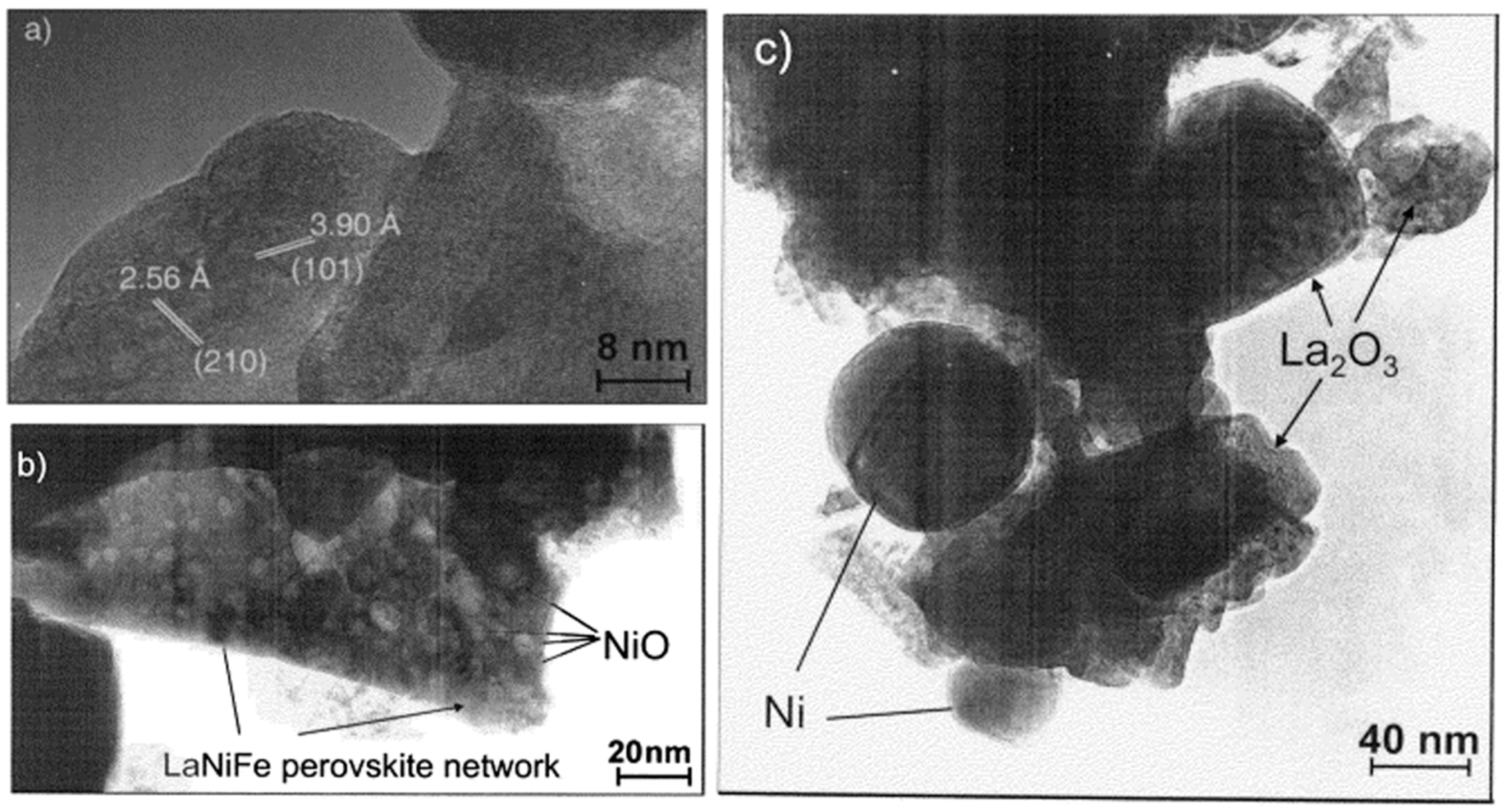
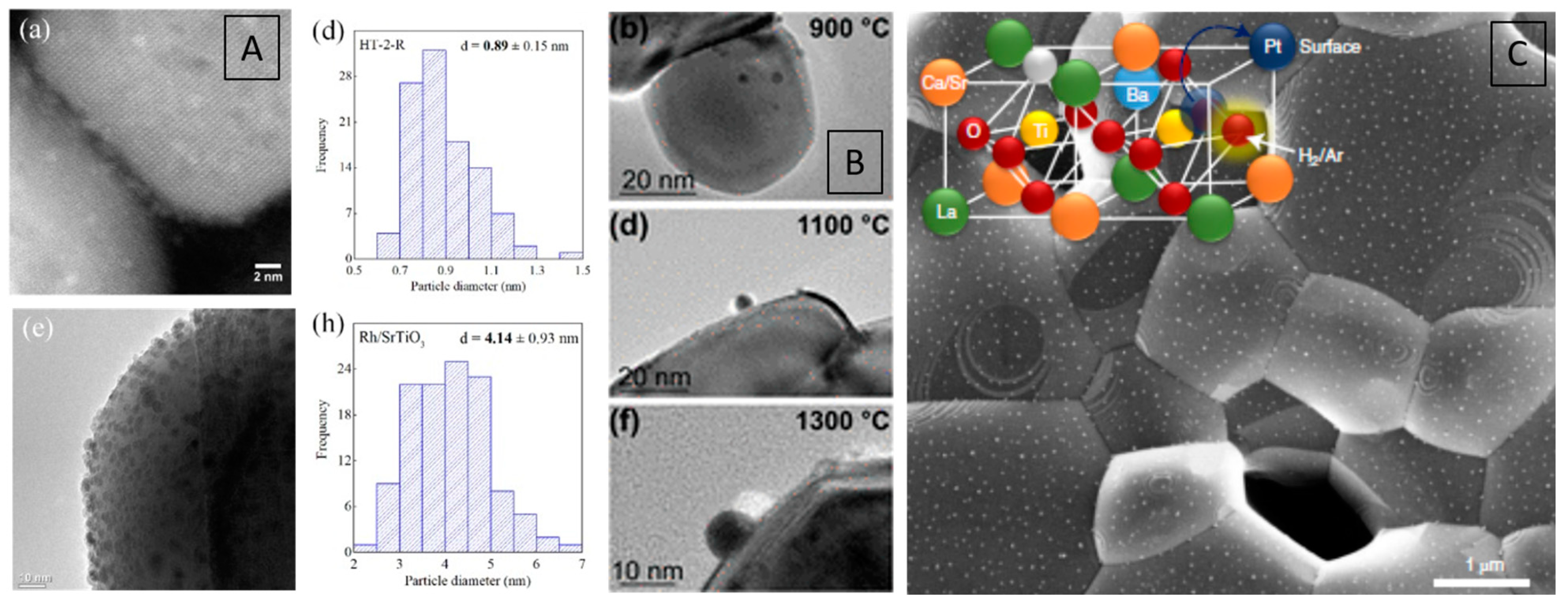
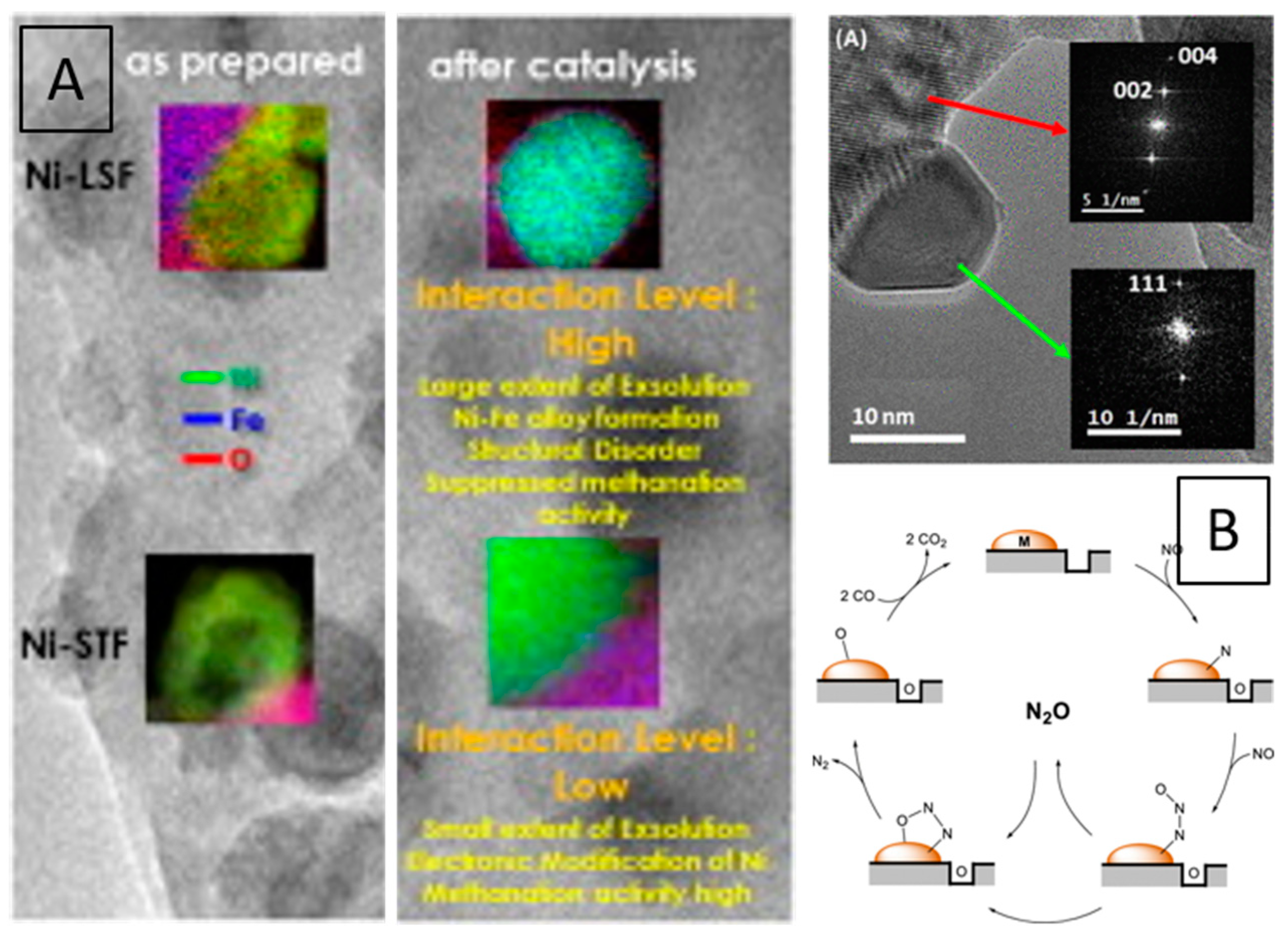
| Dry Reforming | ||||||||
|---|---|---|---|---|---|---|---|---|
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In-Situ: Reduction | In-Situ: Reaction | e/i * | Ref. | |
| Nickelates | ||||||||
| Ni/ | LaNiO3 LaNiO3 (Ru-doped 2.5%, 5.0%) | Dry reforming of CH4 CO2:CH4 (24%:19%), 950 °C | Ni/La2O3 Ni/La2O3 NiRu/La2O3 | Ni/La2O3 Ni/La2O3 NiRu/La2O3 | Ni/La2O3 Ni/La2O3 NiRu/La2O3 | e, i | [35] | |
| Ni/ | LaNiO3 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/La2O3 | Ni/La2O3, La2O2CO3 | Ni/La2O3 | Ni/La2O3, La2O2CO3, La2NiO4 | e | [11] |
| Ni/ | La2NiO4 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/La2O3, La2NiO4, La2O2CO3 | Ni/La2O3, La2NiO4, La2O2CO3 | Ni/La2O3, La2NiO4, La2O2CO3 | e | [13] | |
| Ni/ | Sm1.5Sr0.5NiO4 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/Sm2O3, SrCO3 | Ni/Sm2O3, SrCO3 | Ni/Sm2O3, SrCO3 | Ni/Sm2O3, SrCO3 | e | [34] |
| Ni/ | LaNiO3, La1-xCexNiO3 | Dry reforming of CH4 CO2:CH4 (1:1), 600–800 °C | Ni/La2O3, CeO2 | Ni/La2O3, La2O2CO3, CeO2 | e | [30] | ||
| Ni/ | LaNi1-xRuxO3 | Dry reforming of CH4 CO2:CH4 (1:1), 750–800 °C | Ni/La2O3, RuO2 | Ni/La2O3, La2O2CO3, RuO2 | e | [27] | ||
| Ni-M/ | La0.8Sr0.2Ni0.8M0.2O3 (M = Bi, Co, Cr, Cu, Fe) | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni-M/La2O3, SrO | Ni-M/La2O3, La2O2CO3, SrO | e | [26] | ||
| Ni/ | LaNi0.34Co0.33Mn0.33O3 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/La2O3 | Ni/La2O3, La2O2CO3 | e | [25] | ||
| Ni-Fe/ | La2NiO4, LaNiO3 (Fe doped, LaNixFe1-xO3) | Dry reforming of CH4 CO2:CH4 (1:1), 750 °C | Ni-Fe/La2O3 | Ni-Fe/La2O3 | e, i | [24] | ||
| Ni-M/ | LaNi0.8M0.2O3 (M = Co, Fe) | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni-M/La2O3 | Ni-M/La2O3, La2O2CO3 | Ni-M/La2O3, La2O2CO3 | e | [23] | |
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Ni/ | LaNiO3 | Dry reforming of CH4 CO2:CH4 (1:1), 700–800 °C | Ni/La2O3, La2NiO4 | Ni/La2O3, La2NiO4 | e | [20] | ||
| Ni/ | La2NiO4 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/La2O3 | Ni/La2O3, La2O2CO3 | i | [17] | ||
| Ni/ | La1-xSrxNiO3 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/La2O3, SrCO3 | Ni/La2O3, La2O2CO3, SrCO3 | e | [16] | ||
| Ni/ | LaNiO3, CeSiO2 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/La2O3, CeO2 | Ni/La2O3, La2O2CO3, CeO2 | Ni/La2O3, CeO2 | Ni/La2O3, La2O2CO3, CeO2 | e | [32] |
| Ni-Fe/ | PrBaMn1.6Ni0.3Fe0.1O5+δ | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/Ni3Fe/Ni4Fe/PrBaMnO5+δ | e, i | [48] | |||
| Ferrites | ||||||||
| Ni-Fe/ | LaNi(1-x)FexO3 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/LaFeO3, La2O3 | Ni/LaFeO3, La2O3 | e | [28] | ||
| Ni-Fe/ | La0.9Sr0.1Ni0.5Fe0.5O3 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni-Fe/La2O3 | Ni-Fe/La2O3 | e, i | [18] | ||
| Ni-Fe/ | LaNixFe1-xO3 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni-Fe/La2O3, LaFeO3 | Ni-Fe/La2O3, LaFeO3 | [29] | |||
| Fe-Ni/ | LaFe0.9Ni0.1O3 | Dry reforming of C2H6 CO2:C2H6 (2:1), 600 °C | Ni-Fe/La2O3, LaFeO3 | Ni-Fe/La2O3, La2O2CO3, LaFeO3 | e, i | [38] | ||
| Ni-Fe/ | LaxCe1-xNi0.5Fe0.5O3 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni-Fe/La2O3, LaFeO3, CeO2, NiFe2O4 | Ni-Fe/La2O3, La2O2CO3, LaFeO3, CeO2, NiFe2O4 | e | [33] | ||
| Ni-Fe/ | LnFe0.7Ni0.3O3-δ (Ln = La, Pr, Sm) | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni-Fe/La2O3, La(OH)3, Pr2O3 | Ni-Fe/La2O3, La(OH)3, Pr2O3, PrnO2n+2 | i | [110] | ||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Niobates | ||||||||
| Ni-Nb/ | La2Ni0.8Nb0.2O4 La0.8Sr0.2Ni0.8Nb0.2O4 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/La2O3, LaNbO4, NbOx + SrCO3 | Ni/La2O3, La2O2CO3, LaNbO4, NbOx + SrCO3 | i | [39] | ||
| Titanates | ||||||||
| Ni/ | BaTiO3 (10 wt.-% Ni doping) Ca0.8Sr0.2TiO3 (10 wt.-% Ni doping) | Dry reforming of CH4 CO2:CH4 (1:1), 850 °C | Ni/BaTiO3 Ni/CaTiO3, SrTiO3 | Ni/BaTiO3 Ni/CaTiO3, SrTiO3 | i | [21] | ||
| Cuprates | ||||||||
| Ni/ | La2NixCuyO4 La2BaxNiyCuzO4 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/La2O3 Ni/La2O3, BaCO3 | Ni/La2O3, La2O2CO3 Ni/La2O3, La2O2CO3, BaCO3 | Ni/La2O3, La2O2CO3 Ni/La2O3, La2O2CO3, BaCO3 | e | [12] | |
| Ni-Cu/ | LaNixCu1-xO3 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni-Cu/La2O3, La2CuO4, CuO | Ni-Cu/La2O3, La2CuO4, CuO | e | [31] | ||
| Ni/ | Ni-LaCuO3 | Dry reforming of CH4 CO2:CH4 (1:1), 700 °C | NiCu/La2O3, | NiCu/La2O3, LaCuO3, La2O2CO3 | i | [41] | ||
| Ni-Cu/ | La2(NiCu)O4 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni-Cu/La2O3 | Ni-Cu/La2O3, La2O2CO3 | i | [19] | ||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Cobaltites | ||||||||
| Ni-Co/ | La(CoxNi1-x)Fe0.5O3 | Dry reforming of CH4 CO2:CH4 (1:1), 750 °C | Ni-Co/La2O3, LaFeO3 | Ni-Co/La2O3, La2O2CO3, LaFeO3 | e | [37] | ||
| Sm-Co/ | SmCoO3 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Co/Sm2O3 | Co/Sm2O3 | e | [42] | ||
| Aluminates | ||||||||
| Ni/ | La0.9Ca0.1AlO3 (2.5–10 wt.-% Ni doping) | Dry reforming of CH4 CO2:CH4 (1:1), 700 °C | Ni/LaAlO3 | Ni/LaAlO3 | e | [43] | ||
| Ni/ | LaAlO3, (10 wt.-% Ni doping) LaAlO3, (10 wt.-% Ni doping), CaO support | Dry reforming of CH4 CO2:CH4 (1:1), 750 °C | Ni/LaAlO3 Ni-CaO/LaAlO3 | Ni/LaAlO3 Ni-CaO/LaAlO3 | i | [45] | ||
| Ni/ | LaNixAl1-xO3 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | - | - | e | [44] | ||
| Zirconates | ||||||||
| Ni-Zr/ | MZr1-xNixO3-δ (M = Ca, Sr, Ba) | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/MZr1−xNixO3−δ, MCO3 | Ni/MZr1−xNixO3−δ, MCO3 | i | [46] | ||
| Ni/ | LaNi1-xZnxO3 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/La2O3, La2NiO4, ZnO | Ni/La2O3, La2NiO4, ZnO | e | [31] | ||
| Manganites | ||||||||
| Ni/ | La0.9Mn0.8Ni0.2O3 | Dry reforming of CH4 CO2:CH4 (1:1), 700 °C | Ni/La0.9Mn0.8Ni0.2O3 | e, i | [47] | |||
| Chromates | ||||||||
| Ni/ | La0.8-xSrxCr0.85Ni0.15O3 | Dry reforming of CH4 CO2:CH4 (1:1), 800 °C | Ni/La1−xSrxCrO3 | Ni/La1−xSrxCrO3 | e | [49] | ||
| Methane (Partial) Oxidation | ||||||||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Nickelates | ||||||||
| Ni-Co/ | LaNiO3, LaNi1-xCoxO3, LaCoO3, La0.8(Ca or Sr)0.2NiO3 | Oxidative conversion of CH4 into syngas O2:CH4 (1:1.8), 800 °C | Ni/LaNiO3, La(OH)3 | e, i | [50] | |||
| Ni/ | LaNiO3 | Partial oxidation of CH4 O2:CH4 (6 vol%:1 vol%), 900 °C | Ni/La2O3, La(OH)3 | Ni/La2O3, LaNiO3, LaNiO4,La(OH)3, NiO | e | [52] | ||
| Ferrites | ||||||||
| Ni-Fe/ | LaNixFe1-xO3 | Oxidative conversion of CH4 into syngas O2:CH4 (1:2), 800 °C | Ni-Fe/La2O3, LaNixFe1−xO3, La2Ni2O5 | Ni-Fe/La2O3, LaNixFe1−xO3 | e | [51] | ||
| Pd/ Pt/ Rh/ | LaFeO3 | Catalytic oxidative cracking of n-propane 600 °C | LaFePd/LaFeO3, La2O3 LaFePt/LaFeO3, La2O3 LaFeRh/LaFeO3, La2O3 | e, i | [54] | |||
| Pd/ | LaFeO3, MgAl2O4 (1 wt.-% Pd) | CH4 oxidation O2:CH4 (10:1), 800 °C | Pd/La2O3, Fe2O3, MgAl2O4 | Pd/La2O3, Fe2O3, MgAl2O4 | e | [53] | ||
| Titanates | ||||||||
| CoNi/ Ni/ | La0.7Ce0.1Co0.3Ni0.1Ti0.6O3-δ La0.8Ce0.1Ni0.4Ti0.6O3-δ | Methane oxidation Reduction: 5% CH4 in He, 750 °C Oxidation: 5% O2 in He, 750 °C | CoNi/La0.7Ce0.1Co0.3Ni0.1Ti0.6O3−δ Ni/La0.8Ce0.1Ni0.4Ti0.6O3−δ | CoNi/La0.7Ce0.1Co0.3Ni0.1Ti0.6O3−δ Ni/La0.8Ce0.1Ni0.4Ti0.6O3−δ | e | [55] | ||
| (Reverse) Water Gas Shift Reaction | ||||||||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Ferrites | ||||||||
| FeCo/ FeNi/ | Nd0.6Ca0.4Fe0.9Co0.1O3-δ Nd0.6Ca0.4Fe0.97Co0.03O3-δ Nd0.6Ca0.4Fe0.97Ni0.03O3-δ | Reverse water gas shift reaction CO2:H2 (1:1–15:1), 300–700 °C | Fe/Co/Ni, CaCO3 | Fe/Co/Ni, CaCO3 | Fe/Co/Ni, CaCO3 | Fe/Co/Ni, CaCO3 | e | [57] |
| Pt/ PtPd/ Pd/ | LaCoO3, LaFeO3, LaCrO3, LaNiO3, LaMnO3, SrTiO3, (1 wt.-% doping) | Water gas shift reaction CO:H2O:H2 (1:5:7), 300 °C | Only for LaCoO3 analysed: Pt-Co0/LaCoO3, La2O3 Pd-Co0/LaCoO3 | Only for LaCoO3 analysed: Pt-Co0/LaCoO3, La2O3 Pd-Co0/LaCoO3 | i | [63] | ||
| Pt/ PtPd/ Pd/ | LaCoO3, LaFeO3, LaAlO3, LaFe0.5Co0.5O3, LaAl0.5Co0.5O3, (1 wt.-% doping) | Water gas shift reaction CO:H2O:H2 (1:5:7), 300 °C | LaFe0.5Co0.5O3, LaAl0.5Co0.5O3 | LaFe0.5Co0.5O3, LaAl0.5Co0.5O3 | i | [62] | ||
| Ni-Fe/ | La0.9Fe0.95Ni0.05O3 | Water gas shift reaction CO + H2O (1 vol%:23 vol%), 600 °C | Ni/LaFeO3 | Ni/LaFeO3 | i | [58] | ||
| Ni-Fe/ | Sr2FeMo0.6Ni0.4O6-δ | Reverse water gas shift reaction H2:CO2 (2.7%:20%), 950 °C | Ni-Fe/SrCO3, SrMoO4, Sr3MoO6, Sr3FeMoO7−δ, Sr2FeMoO6 | Ni-Fe/SrCO3, SrMoO4, Sr3MoO6, Sr3FeMoO7−δ, Sr2FeMoO6 | Ni-Fe/SrCO3, SrMoO4, Sr3MoO6, Sr3FeMoO7−δ, Sr2FeMoO6 | Ni-Fe/SrCO3, SrMoO4, Sr3MoO6, Sr3FeMoO7−δ, Sr2FeMoO6 | e | [66] |
| Ni-Co/ | (Nd/La)x(Ca/Sr)1-xFe1-y(Ni/Co)yO3-δ | Reverse water gas shift reaction CO2:H2 (1:1), 600 °C | Fe, Co, Ni/Fe3O4, FeO, CaCO3, (Nd/La)x(Ca/Sr)1−xFe1−y(Ni/Co)yO3−δ | Fe, Co, Ni/Fe3O4, FeO, CaCO3, (Nd/La)x(Ca/Sr)1−xFe1−y(Ni/Co)yO3−δ | Fe, Co, Ni/Fe3O4, FeO, CaCO3, (Nd/La)x(Ca/Sr)1−xFe1−y(Ni/Co)yO3−δ | Fe, Co, Ni/Fe3O4, FeO, CaCO3, (Nd/La)x(Ca/Sr)1−xFe1−y(Ni/Co)yO3−δ | e | [56] |
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Nickelates | ||||||||
| Ni/ | LaNiO3 LaNiO3 (K-doped) | Water gas shift reaction CO + H2O (5 mol%:25 mol%), 350–550 °C Reformate gas CO + H2O + CO2 (10 mol%:25 mol%:40 mol%), 350–550 °C | Ni/La2O3 Ni/La2O3, K2O | i | [59] | |||
| Pt/ PtPd/ Pd/ | LaCoO3, LaFeO3, LaCrO3, LaNiO3, LaMnO3, SrTiO3, (1 wt.-% doping) | Water gas shift reaction CO:H2O:H2 (1:5:7), 300 °C | Only for LaCoO3 analysed: Pt-Co0/LaCoO3, La2O3 Pd-Co0/LaCoO3 | Only for LaCoO3 analysed: Pt-Co0/LaCoO3, La2O3 Pd-Co0/LaCoO3 | i | [63] | ||
| Ni-Co/ | La1-ySryNixCo1-xO3 | Water gas shift reaction CO + H2O (1:2.3), 400 °C | [60] | |||||
| Titanates | ||||||||
| Pt/ PtPd/ Pd/ | LaCoO3, LaFeO3, LaCrO3, LaNiO3, LaMnO3, SrTiO3, (1 wt.-% doping) | Water gas shift reaction CO:H2O:H2 (1:5:7), 300 °C | Only for LaCoO3 analysed: Pt-Co0/LaCoO3, La2O3 Pd-Co0/LaCoO3 | Only for LaCoO3 analysed: Pt-Co0/LaCoO3, La2O3 Pd-Co0/LaCoO3 | i | [63] | ||
| Cu/ | SrTi1-xCuxO3 | Water gas shift reaction CO + H2O (1:3), 300 °C | Cu/CuO, SrCO3, SrTiO3, | e, i | [61] | |||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Cobaltites | ||||||||
| Pt/ PtPd/ Pd/ | LaCoO3, LaFeO3, LaCrO3, LaNiO3, LaMnO3, SrTiO3, (1 wt.-% doping) | Water gas shift reaction CO:H2O:H2 (1:5:7), 300 °C | Only for LaCoO3 analysed: Pt-Co0/LaCoO3, La2O3 Pd-Co0/LaCoO3 | Only for LaCoO3 analysed: Pt-Co0/LaCoO3, La2O3 Pd-Co0/LaCoO3 | i | [63] | ||
| Pt/ PtPd/ Pd/ | LaCoO3, LaFeO3, LaAlO3, LaFe0.5Co0.5O3, LaAl0.5Co0.5O3, (1 wt.-% doping) | Water gas shift reaction CO:H2O:H2 (1:5:7), 300 °C | LaFe0.5Co0.5O3, LaAl0.5Co0.5O3 | LaFe0.5Co0.5O3, LaAl0.5Co0.5O3 | i | [62] | ||
| Ni-Co/ | La1-ySryNixCo1-xO3 | Water gas shift reaction CO + H2O (1:2.3), 400 °C | [60] | |||||
| Co/ | La1-xSrxCoO3-δ | Reverse water gas shift reaction H2:CO2 (1:1), 850 °C | Co/La2O3, La0.75Sr0.25CoO3−δ, La2−xSrxCoO4 | Co/La2O3, SrCO3, La2−xSrxCoO4, La2CoO4, CoO | Co/La2O3, La0.75Sr0.25CoO3−δ, La2−xSrxCoO4 | Co/La2O3, SrCO3, La2−xSrxCoO4, La2CoO4, CoO | e | [64] |
| Yttrates | ||||||||
| Cu/ | Cu2Y2O5 | Water gas shift reaction CO + H2O (1:1), 100–250 °C | Cu/Y2O3 | e | [65] | |||
| Molybdates | ||||||||
| Ni-Fe/ | Sr2FeMo0.6Ni0.4O6-δ | Reverse water gas shift reaction H2:CO2 (2.7%:20%), 950 °C | Ni-Fe/SrCO3, SrMoO4, Sr3MoO6, Sr3FeMoO7−δ, Sr2FeMoO6 | Ni-Fe/SrCO3, SrMoO4, Sr3MoO6, Sr3FeMoO7−δ, Sr2FeMoO6 | Ni-Fe/SrCO3, SrMoO4, Sr3MoO6, Sr3FeMoO7−δ, Sr2FeMoO6 | Ni-Fe/SrCO3, SrMoO4, Sr3MoO6, Sr3FeMoO7−δ, Sr2FeMoO6 | e | [66] |
| Alcohol and Hydrocarbon Steam Reforming | ||||||||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Ferrites | ||||||||
| Ni/ | LaNixFe1-xO3 | Methane steam reforming CH4:H2O (1:1, 1:3), 800 °C CH4:H2O:H2 (1:3:2), 800 °C | La2Ni2O5 (420 °C) Ni (550 °C) | LaFeO3, La2O3, LaNixFe1−xO3 | e | [68] | ||
| Ni/ | LaFeO3, La0.4Ba0.6Co0.2Fe0.8O3-δ (10 wt.-% Ni doping) | Steam reforming of CH4 H2O:CH4 (1:1 and 2:1), 800 °C | Ni/reduced Perovskite, that can be reversibly oxidized again Ni/reduced Perovskite that can be reversibly oxidized again | Ni/reduced Perovskite, that can be reversibly oxidized again Ni/reduced Perovskite that can be reversibly oxidized again | i | [22] | ||
| Ag/ Co3O4/ | SrTi0.7Fe0.3O3-δ, | MeOH steam reforming MeOH:H2O (1:2) 600 °C | Ag/SrTi0.5Fe0.5O3, Ag2O SrTi0.5Fe0.5O3, CoO, Co2O3, Co3O4 | Ag/SrTi0.5Fe0.5O3, Ag2O SrTi0.5Fe0.5O3, CoO, Co2O3, Co3O4 | i | [67] | ||
| Ni/ | Ni-La0.6Sr0.4FeO3-δ, Ni-SrTi0.7Fe0.3O3-δ | Steam reforming of CH4 H2O:CH4 (1:1), 600 °C Methanation reaction CO2:H2 (1:4), 600 °C | Ni/NiO, SrTiFeO3, LaSrFeO3 | Ni/NiO, SrTiFeO3, LaSrFeO3 | e, i | [86] | ||
| Rh/ Rh-Fe/ | Rh-La0.6Sr0.4FeO3-δ, Rh-SrTi0.7Fe0.3O3-δ | Steam reforming of CH4 H2O:CH4 (1:1), 600 °C Methanation reaction CO2:H2 (1:4), 600 °C | Rh/Rh2O3, SrTiFeO3, LaSrFeO3 Rh-Fe/Rh2O3, SrTiFeO3, LaSrFeO3 | Rh/Rh2O3, SrTiFeO3, LaSrFeO3 Rh-Fe/Rh2O3, SrTiFeO3, LaSrFeO3 | e, i | [87] | ||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Ni/ | LaBO3 (B = Al, Fe, Mn), La0.7A0.3AlO3-δ (A = Ca, Ba, Ce, Zn, Sr, Mg), La1-xMgxAlO3-δ (10 wt.-% doping) | Steam reforming of CH4 H2O:CH4 (2:1), 800 °C | Ni/all corresponding Perovskites, no other oxides detected | Ni/all corresponding Perovskites, no other oxides detected | i | [70] | ||
| Fe/ | La0.5Ce0.5FeO3 | Steam reforming of CH4 H2O:CH4 (80 vol.%:5 vol.%), 925 °C | Fe/(La0.5Ce0.5)2O3, La0.5Ce0.5O2−x | Fe/(La0.5Ce0.5)2O3, La0.5Ce0.5O2−x, La0.5Ce0.5FeO3 | e | [69] | ||
| Ni/ | La1-xMgxAl1-yNiyO3 | Ethanol steam reforming 600 °C | LaMgNi3, LaMgNi2, LaMgNi, LaAlO3 | i | [75] | |||
| Co/ | La0.6Sr0.4Co1-yFeyO3-δ | Ethanol steam reforming EtOH:H2O (1:5), 700 °C Methanol steam reforming MeOH:H2O (1:4), 700 °C | Co-Fe/SrCO3, SrLaCoO4, Co3O4, SrLaFeO4, Fe2O3, La2CoO4 La0.6Sr0.4Co1−yFeyO3 | e | [71] | |||
| Ni-Fe/ | LaNixFe1-xO3 | Steam reforming of toluene C7H8:H2O (1:3.4), 650 °C | Ni-Fe/LaFeO3, LaNixFe1−xO3, La2O3 | Ni-Fe/ LaNixFe1−xO3, La2O3 | e | [82] | ||
| Titanates | ||||||||
| Ni/ | SrTiO3, BaTiO3 (10 wt.-% Ni doping) | Steam reforming of CH4 H2O:CH4 (1:1 and 2:1), 800 °C | Ni/SrTiO3 Ni/BaTiO3 | Ni/SrTiO3 Ni/BaTiO3 | i | [22] | ||
| Ag/ Co3O4/ | SrTi0.7Fe0.3O3-δ, | MeOH steam reforming MeOH:H2O (1:2) 600 °C | Ag/SrTi0.5Fe0.5O3, Ag2O SrTi0.5Fe0.5O3, CoO, Co2O3, Co3O4 | Ag/SrTi0.5Fe0.5O3, Ag2O SrTi0.5Fe0.5O3, CoO, Co2O3, Co3O4 | i | [67] | ||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Ni/ Co/ | SrTiO3, BaTiO3, LaAlO3, (5 wt.-% doping) | Steam reforming of ethanol C2H5OH:H2O (1:10), 550 °C | Ni/SrTiO3, BaTiO3, LaAlO3 Co/SrTiO3, BaTiO3, LaAlO3 | Ni/SrTiO3, BaTiO3, LaAlO3 Co/SrTiO3, BaTiO3, LaAlO3 | i | [84] | ||
| Ni/ | Ni-La0.6Sr0.4FeO3-δ, Ni-SrTi0.7Fe0.3O3-δ | Steam reforming of CH4 H2O:CH4 (1:1), 600 °C Methanation reaction CO2:H2 (1:4), 600 °C | Ni/NiO, SrTiFeO3, LaSrFeO3 | Ni/NiO, SrTiFeO3, LaSrFeO3 | e, i | [86] | ||
| Rh/ Rh-Fe/ | Rh-La0.6Sr0.4FeO3-δ, Rh-SrTi0.7Fe0.3O3-δ | Steam reforming of CH4 H2O:CH4 (1:1), 600 °C Methanation reaction CO2:H2 (1:4), 600 °C | Rh/Rh2O3, SrTiFeO3, LaSrFeO3 Rh-Fe/Rh2O3, SrTiFeO3, LaSrFeO3 | Rh/Rh2O3, SrTiFeO3, LaSrFeO3 Rh-Fe/Rh2O3, SrTiFeO3, LaSrFeO3 | e, i | [87] | ||
| Ni/ | Srn+1Tin-xNixO3n+1 | Steam reforming of CH4 H2O:CH4 (3:1), 800 °C | Ni/SrCO3, Srn+1Tin−xNixO3n+1 | Ni/SrCO3, Srn+1Tin−xNixO3n+1 | e | [72] | ||
| Nickelates | ||||||||
| Ni/ | LaNixFe1-xO3 | Methane steam reforming CH4:H2O (1:1, 1:3), 800 °C CH4:H2O:H2 (1:3:2), 800 °C | La2Ni2O5 (420 °C) Ni (550 °C) | LaFeO3, La2O3, LaNixFe1−xO3 | e | [68] | ||
| Ni-Co/ | LaNiO3, LaNi1-xCoxO3, LaCoO3, La0.8(Ca or Sr)0.2NiO3 | Methane steam reforming CH4:H2O:O2:CO2 (12:1:6:1), 850 °C | Ni/LaNiO3, La(OH)3 | e | [50] | |||
| Ni/ | La1-xCexNiO3 | Steam CO2 reforming of CH4 CO2:H2O:CH4 (1:1:1), 900 °C | Ni/CeO2, La2O2CO3, La2O3, NiO | e | [73] | |||
| Ni/ | Srn+1Tin-xNixO3n+1 | Steam reforming of CH4 H2O:CH4 (3:1), 800 °C | Ni/SrCO3, Srn+1Tin−xNixO3n+1 | Ni/SrCO3, Srn+1Tin−xNixO3n+1 | e | [72] | ||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Ni/ | La1-xCexNiO3 | Ethanol steam reforming EtOH:H2O:O2 (2.5%:7.5%:1.25%), 800 °C | Ni/La2O3, La(OH)3, CeOx | e | [76] | |||
| Ni/ | LaNiO3 | Ethanol steam reforming EtOH:H2O (3%:37%), 800 °C | Ni/La2O3, La2O2CO3, LaNiO3 | Ni/La2O3, La2O2CO3, LaNiO3 | e | [77] | ||
| Ni-Co/ | LaNi1-xCoxO3 on ZrO2 | Ethanol steam reforming EtOH:H2O (1:3), 750 °C | Ni-Co/La2O3, La2O2CO3, ZrO2 | Ni-Co/La2O3, La2O2CO3, ZrO2 | e, i | [78] | ||
| Ni-Cu/ | LaNi0.9Cu0.1O3 | Steam reforming of glycerol Steam:Carbon (3:1), 700 °C | Ni-Cu/La2O3, La2O2CO3 | e | [79] | |||
| Ni/ | La1-xCaxNiO3 | Steam reforming of glycerol Steam:Carbon (3:1), 700 °C | Ni/La2O3, CaO, NiO | Ni/CaCO3, La2O2CO3 | e, i | [80] | ||
| Ni/ Co/ | LaNiO3, LaCoO3 | Steam reforming of glycerol Steam:Carbon (2:1), 700 °C | Ni/LaNiO3, La2O2CO3 Co/LaCoO3, La2O2CO3 | e | [81] | |||
| Aluminates | ||||||||
| Ni/ | LaAlO3, (10 wt.-% Ni doping) | Steam reforming of CH4 H2O:CH4 (1:1 and 2:1), 800 °C | Ni/LaAlO3 | Ni/LaAlO3 | I | [22] | ||
| Ni/ | LaAlO3, LaxM1-xAlO3-δ (M = Ca, Sr, Ba) (1–15 wt.-% doping) | Steam reforming of toluene C7H8:H2O (1:14), 600 °C | Ni/LaAlO3, SrLaAlO4, La2O3, SrAl2O4, La(OH)3 | Ni/LaAlO3, SrLaAlO4, La2O3, SrAl2O4, La(OH)3 | i | [83] | ||
| Ni/ | LaBO3 (B = Al, Fe, Mn), La0.7A0.3AlO3-δ (A = Ca, Ba, Ce, Zn, Sr, Mg), La1-xMgxAlO3-δ (10 wt.-% doping) | Steam reforming of CH4 H2O:CH4 (2:1), 800 °C | Ni/all corresponding Perovskites, no other oxides detected | Ni/all corresponding Perovskites, no other oxides detected | i | [70] | ||
| Ni/ | La1-xCaxAl1-yNiyO3 | Ethanol steam reforming EtOH:H2O (1:5), 600 °C | - | - | i | [74] | ||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Cobaltites | ||||||||
| Ni/ | La0.4Ba0.6Co0.2Fe0.8O3-δ (10 wt.-% Ni doping) | Steam reforming of CH4 H2O:CH4 (1:1 and 2:1), 800 °C | Ni/reduced Perovskite, that can be reversibly oxidized again | Ni/reduced Perovskite, that can be reversibly oxidized again | i | [22] | ||
| Ag/ Co3O4/ | SrTi0.7Fe0.3O3-δ, | MeOH steam reforming MeOH:H2O (1:2) 600 °C | Ag/SrTi0.5Fe0.5O3, Ag2O SrTi0.5Fe0.5O3, CoO, Co2O3, Co3O4 | Ag/SrTi0.5Fe0.5O3, Ag2O SrTi0.5Fe0.5O3, CoO, Co2O3, Co3O4 | i | [67] | ||
| Ni-Co/ | LaNiO3, LaNi1-xCoxO3, LaCoO3, La0.8(Ca or Sr)0.2NiO3 | Methane steam reforming CH4:H2O:O2:CO2 (12:1:6:1), 850 °C | Ni/LaNiO3, La(OH)3 | [50] | ||||
| Ni-Co/ | LaNi1-xCoxO3 on ZrO2 | Ethanol steam reforming EtOH:H2O (1:3), 750 °C | Ni-Co/La2O3, La2O2CO3, ZrO2 | Ni-Co/La2O3, La2O2CO3, ZrO2 | i | [78] | ||
| Co/ | La0.6Sr0.4CoO3-δ | Ethanol steam reforming EtOH:H2O (1:3), 800 °C | Co/β-Co, La2O3, La2O2CO3, LaOOH, SrCO3 | Co/(La1−xSrx)2CoO4, CoO, La2O3, La2O2CO3, LaOOH, SrCO3 | e | [85] | ||
| Co/ | La0.6Sr0.4Co1-yFeyO3-δ | Ethanol steam reforming EtOH:H2O (1:5), 700 °C Methanol steam reforming MeOH:H2O (1:4), 700 °C | Co-Fe/SrCO3, SrLaCoO4, Co3O4, SrLaFeO4, Fe2O3, La2CoO4 La0.6Sr0.4Co1−yFeyO3 | e | [71] | |||
| Ni/ Co/ | LaNiO3, LaCoO3 | Steam reforming of glycerol Steam:Carbon (2:1), 700 °C | Ni/LaNiO3, La2O2CO3 Co/LaCoO3, La2O2CO3 | e | [81] | |||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Manganates | ||||||||
| Ni/ | LaBO3 (B = Al, Fe, Mn), La0.7A0.3AlO3-δ (A = Ca, Ba, Ce, Zn, Sr, Mg), La1-xMgxAlO3-δ (10 wt.-% doping) | Steam reforming of CH4 H2O:CH4 (2:1), 800 °C | Ni/all corresponding Perovskites, no other oxides detected | Ni/all corresponding Perovskites, no other oxides detected | i | [70] | ||
| Ni-Cu/ | La0.8Ce0.2Mn0.6Ni0.4O3, CuO | Ethanol steam reforming EtOH:H2O (1:3), 700 °C | Ni-Cu/LaMnO3.15, CeO2, La2CO5, La2NiO4 | e | [111] | |||
| Methanation and Hydrogenation Reactions | ||||||||
| Ferrites | ||||||||
| Ni/ Ni-Fe/ | LaFeO3, (10–30 wt.-% doping) | Syngas methanation H2:CO (3:1), 480 °C | Ni/NiO, LaFeO3, LaFe1−xNixO3, FeNi | Ni/NiO, LaFeO3, La2O2CO3, LaFe1−xNixO3, FeNi | i | [88] | ||
| Titanates | ||||||||
| Rh/ | SrTiO3 (2 mol% Rh doped) | CO2 + H2, 573 K CO2 + C2H6, 823 K | Rh/SrTiO3 Rh0.45/SrTiO3 | Rh0.45/SrTiO3 | Rh0.45/SrTiO3 | e, i | [90] | |
| Aluminates | ||||||||
| Rh-Ni/ | Rh/LaAl0.92Ni0.08O3 | Methanation reaction CO2:H2 (1:4), 600 °C | Rh-Ni/LaAlO3, La2O3 | e | [91] | |||
| Rhodates and Platinates | ||||||||
| Rh/ | LaRhO3 | Fischer-Tropsch synthesis H2:CO2 (1:1), 350–400 °C | Rh/La2O3, Rh2O3 | e | [93] | |||
| Nickelates | ||||||||
| Ni/ | LaNiO3 | Methanation of CO2 H2:CO2 (4:1), 300–500 °C | Ni/La2O3 | Ni/La2O3, La2O2CO3 | e | [36] | ||
| Car Exhaust Catalysis | ||||||||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Ferrites | ||||||||
| Rh/ | LaFe0.95Rh0.05O3 | Automotive exhaust gas catalysis, Gasoline powered V-8 engine, 900 °C, Reversible oxidation-reduction-oxidation possible | - | - | e | [96] | ||
| Pd/ | LaFe0.95Pd0.05O3 | Automotive exhaust gas catalysis, Gasoline powered V-8 engine, 900 °C, Reversible oxidation-reduction-oxidation possible | Pd, LaFeO3 | LaFe0.95Pd0.05O3, Pd, LaFeO3 | e, i | [97] | ||
| Titanates and Zirconates | ||||||||
| Pt/ | La0.4Ca0.3925Ba0.0075Pt0.005Ti0.995O3 La0.4Sr0.3925Ba0.0075Pt0.005Ti0.995O3 | diesel oxidation CO:C3H6:NO:CO2:O2:H2O (1450ppm:105ppm:125ppm:4.5%:10%:5%), 330 °C | Pt | - | e | [99] | ||
| Rh/ | CaTi0.95Rh0.05O3 | Automotive exhaust gas catalysis, Gasoline powered V-8 engine, 900 °C, Reversible oxidation-reduction-oxidation possible | - | - | e | [96] | ||
| Cobaltites | ||||||||
| Pd/ | LaFe0.57Co0.38Pd0.05O3 | Automotive exhaust gas catalysis, Gasoline powered V-8 engine, 900 °C, Reversible oxidation-reduction-oxidation possible | Pd, Co, La2O3 | LaFe0.57Co0.38Pd0.05O3 or Pd, Co, La2O3 | e, i | [95] | ||
| CO Oxidation | ||||||||
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Titanates | ||||||||
| Ir/ | SrIr0.005Ti0.995O3 (900 °C, 1100 °C, 1300 °C) | CO oxidation CO (0.6%) + O2 (1%), 450 °C | Ir0.5-SrTiO3 | Ir0.5-SrTiO3 | e | [101] | ||
| Manganites | ||||||||
| Pt/ Pd/ | M-LaMnO3 | CO oxidation CO:O2 (2:1), 800 °C | Pt-Mn/LaMnO3 Pd, PdO/LaMnO3 | e | [102] | |||
| Ferrites, Aluminates | ||||||||
| Pd/ | LaFeO3, MgAl2O4 (1 wt.-% Pd) | CO oxidation O2:CO (1:2), 800 °C | Pd/La2O3, Fe2O3, MgAl2O4 | Pd/La2O3, Fe2O3, MgAl2O4 | e | [53] | ||
| De-NOx Reactions | ||||||||
| Manganites | ||||||||
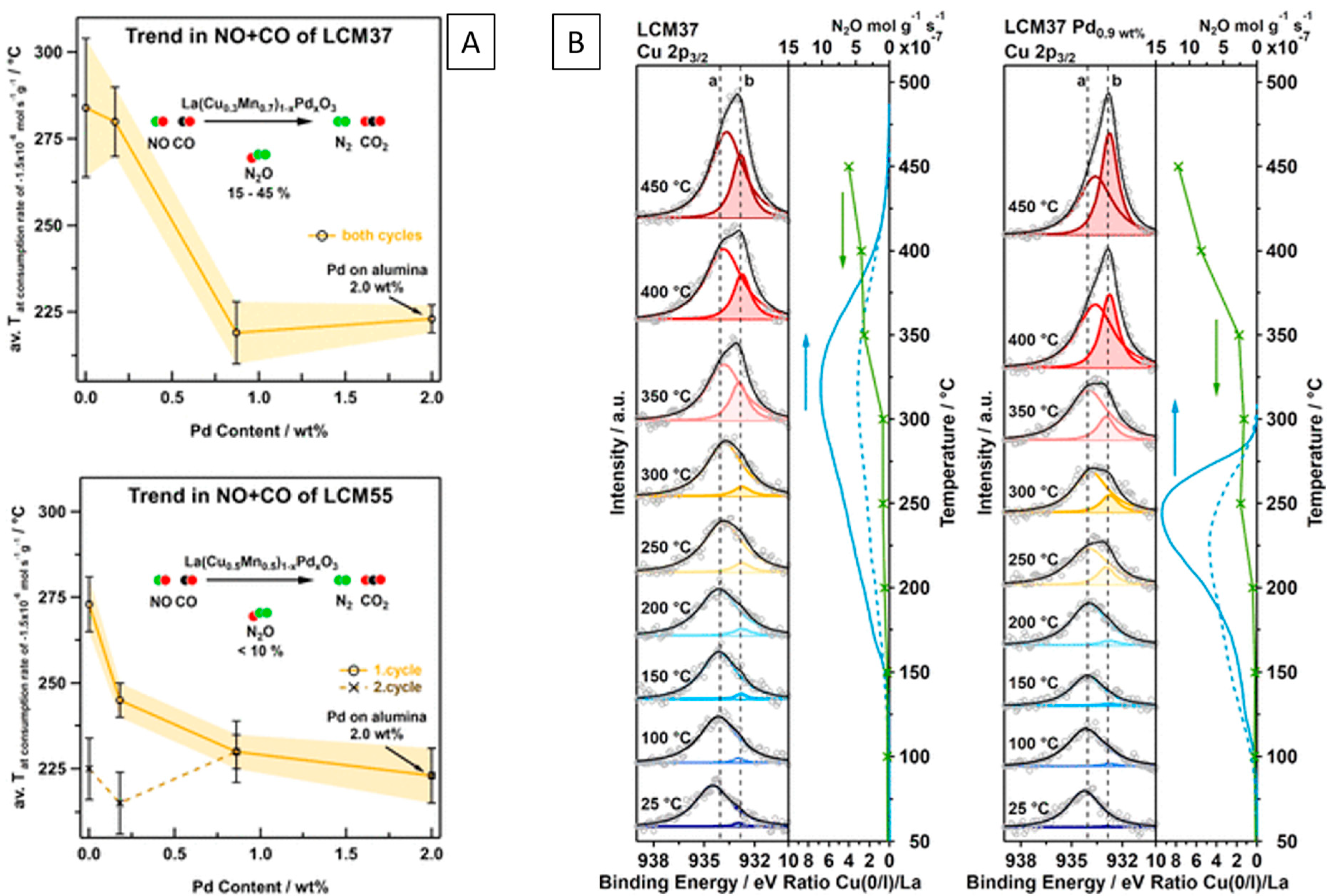
| Cu/ | LaCuxMn1-xO3 LaCuxPdyMn1-x-yO3 | de-NOx reaction NO:CO (1:1), 500 °C | Cu/La2O3, La2CuO4 CuPd/La2O3, La2CuO4 | Cu/La2O3, La2CuO4 CuPd/La2O3, La2CuO4 | Cu/La2O3, La2CuO4 CuPd/La2O3, La2CuO4 | Cu/La2O3, La2CuO4 CuPd/La2O3, La2CuO4 | e, i | [103] |
| Cu/ | LaCu0.5Mn0.5O3-δ LaCuxPdyMn1-x-yO3 | de-NOx reaction NO:CO:H2O (1:1:1), 500 °C NO:CO:H2O:O2 (1:5:4:2), 500 °C | Cu/LaCu0.5Mn0.5O3−δ CuPd/LaCuxPdyMn1−x−yO3 | Cu/LaCu0.5Mn0.5O3−δ CuPd/LaCuxPdyMn1−x−yO3 | Cu/LaCu0.5Mn0.5O3−δ CuPd/LaCuxPdyMn1−x−yO3 | Cu/LaCu0.5Mn0.5O3−δ CuPd/LaCuxPdyMn1−x−yO3 | e, i | [104] |
| Pd/ | La(FexMn1-x)O3 | de-NOx reaction NO:CO:H2O (1:1:1), 650 °C NO:CO:H2O (1:1:5), 650 °C | Pd/La(FexMn1−x)O3 | Pd/La(FexMn1−x)O3 | Pd/La(FexMn1−x)O3 | Pd/La(FexMn1−x)O3 | e, i | [105] |
| System | Reaction Conditions | Phases Obs. after Reduction | Phases Obs. after Catalysis | In Situ: Reduction | In Situ: Reaction | e/i * | Ref. | |
| Cobaltites | ||||||||
| Co/ | LaCoO3, Co3O4 | de-NOx reaction NO:CO (1:1), 500 °C NO:CO:O2:C3H8 (1:2.5:3:0.5), 700 °C | - | - | i | [106] | ||
| Co-Cu/ | LaCo1-xCuxO3 | Three-way catalysis CO:NO (1:1), 400–600 °C CO:O2 (2:1), 400–600 °C | Cu-Co/ LaCo1−xCuxO3, La2O3 | Cu-Co/ LaCo1−xCuxO3, La2O3 | e | [100] | ||
| Aluminates | ||||||||
| Pd/ | LaFeO3, MgAl2O4 (1 wt.-% Pd) | CH4 oxidation O2:CH4 (10:1), 800 °C CO oxidation O2:CO (1:2), 800 °C | Pd/La2O3, Fe2O3, MgAl2O4 | Pd/La2O3, Fe2O3, MgAl2O4 | e | [53] | ||
| Pd/ | Pd-La0.9Ba0.1AlO3-δ | de-NOx reaction NO:CO:O2 (1:4.5:2), 400 °C NO:CO:O2:H2O (1:4.5:2:1), 400 °C | Pd/La0.9Ba0.1AlO3−δ Pd-Ba/LaAlO3 | Pd/La0.9Ba0.1AlO3−δ Pd-Ba/LaAlO3 | i | [107] | ||
| Titanates | ||||||||
| Pt/ | La0.4Ca0.3925Ba0.0075Pt0.005Ti0.995O3 La0.4Sr0.3925Ba0.0075Pt0.005Ti0.995O3 | de-NOx reaction CO + NO (1:1), 330 °C | Pt | e | [99] | |||
| Pd/ | LaTiMgO3 (4.6 wt.-% doping) | de-NOx reaction NO:CO (1:1), 400 °C | - | - | e | [108] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malleier, C.; Penner, S. Metal–Perovskite Interfacial Engineering to Boost Activity in Heterogeneous Catalysis. Surfaces 2024, 7, 296-339. https://doi.org/10.3390/surfaces7020020
Malleier C, Penner S. Metal–Perovskite Interfacial Engineering to Boost Activity in Heterogeneous Catalysis. Surfaces. 2024; 7(2):296-339. https://doi.org/10.3390/surfaces7020020
Chicago/Turabian StyleMalleier, Christoph, and Simon Penner. 2024. "Metal–Perovskite Interfacial Engineering to Boost Activity in Heterogeneous Catalysis" Surfaces 7, no. 2: 296-339. https://doi.org/10.3390/surfaces7020020
APA StyleMalleier, C., & Penner, S. (2024). Metal–Perovskite Interfacial Engineering to Boost Activity in Heterogeneous Catalysis. Surfaces, 7(2), 296-339. https://doi.org/10.3390/surfaces7020020






