Effective Surface Structure Changes and Characteristics of Activated Carbon with the Simple Introduction of Oxygen Functional Groups by Using Radiation Energy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
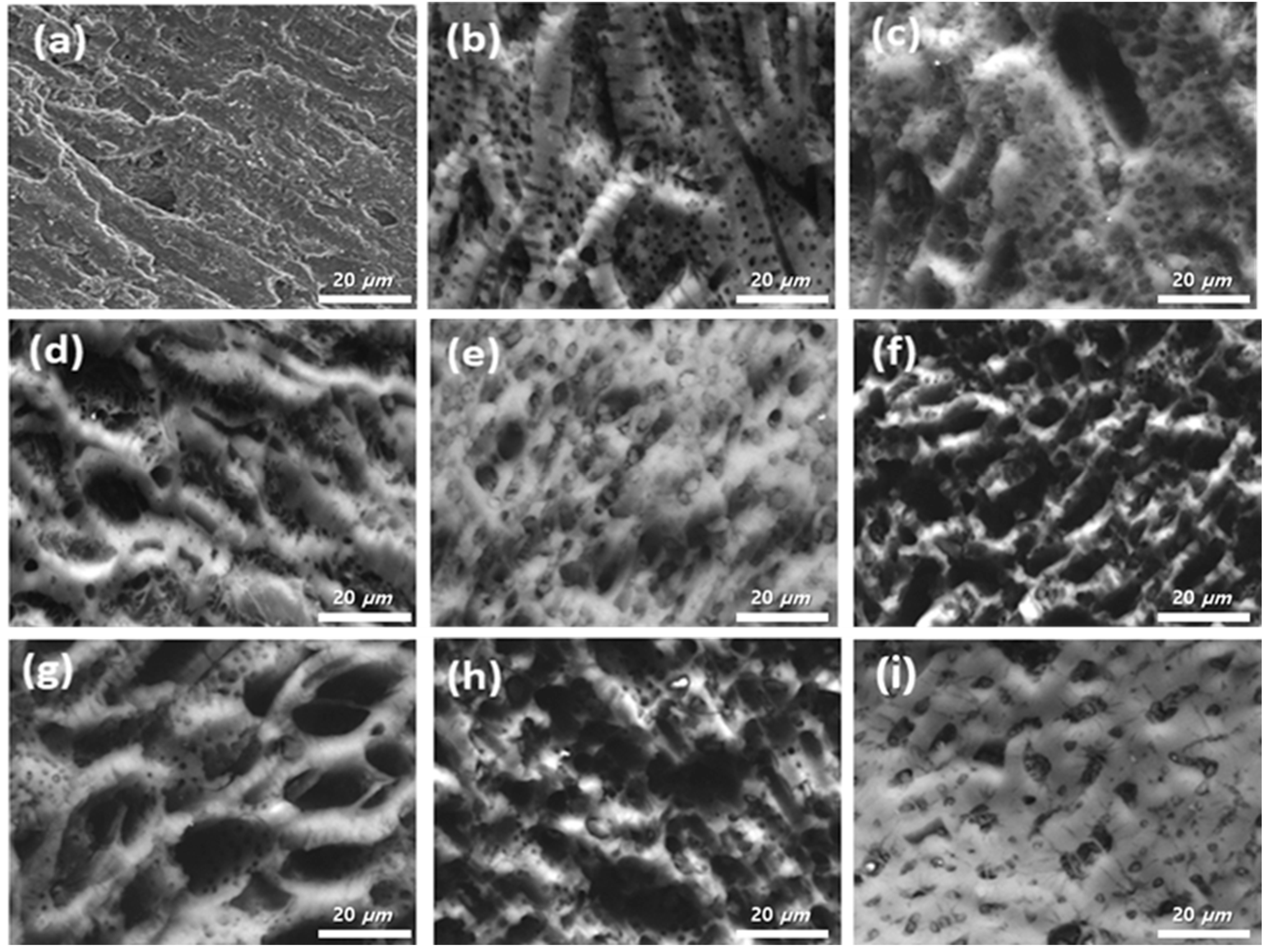
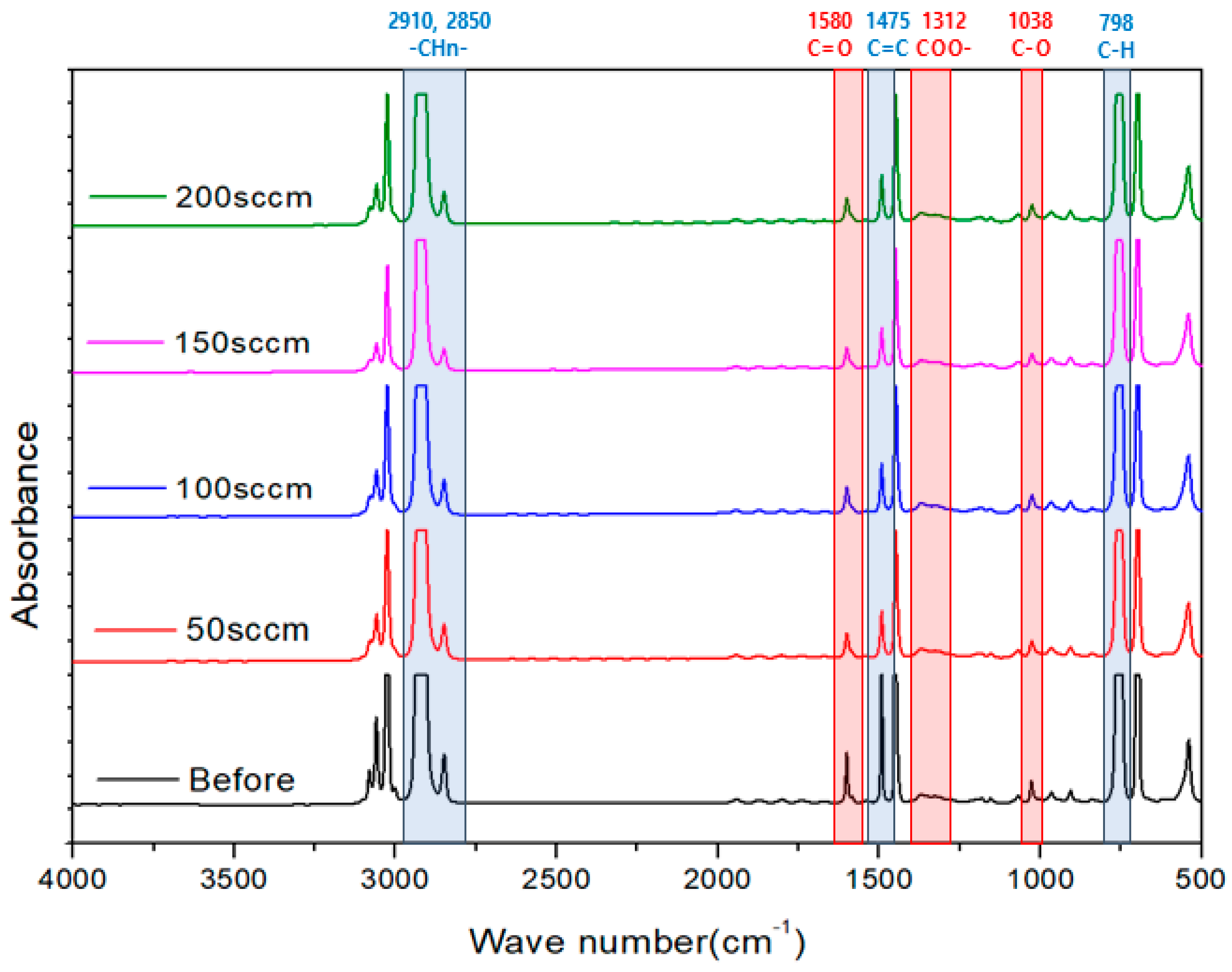
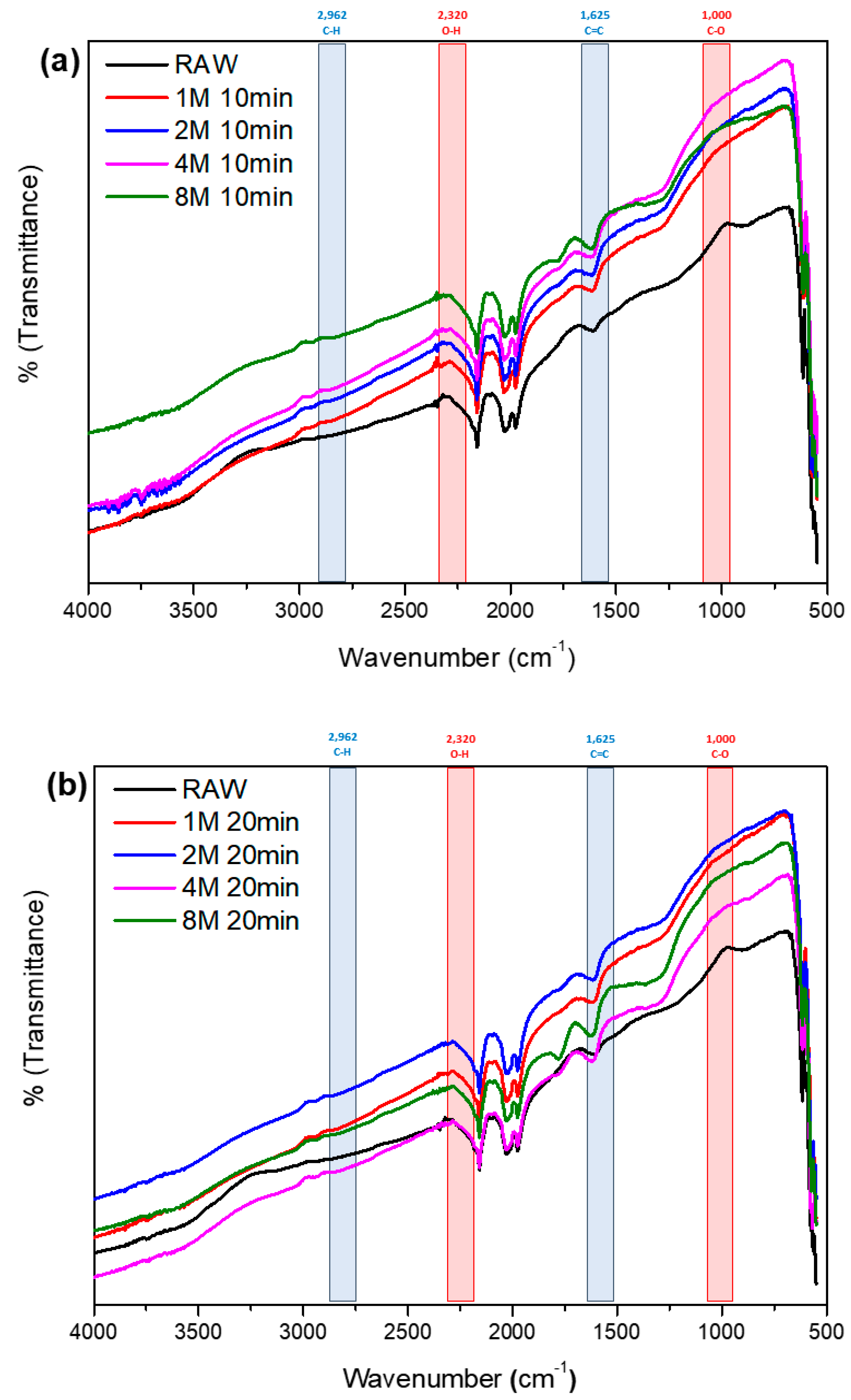
3.1. Surface Characteristic Analysis
3.2. Specific Surface Area Analysis According to Plasma and Microwave Radiation Surface Modification Method
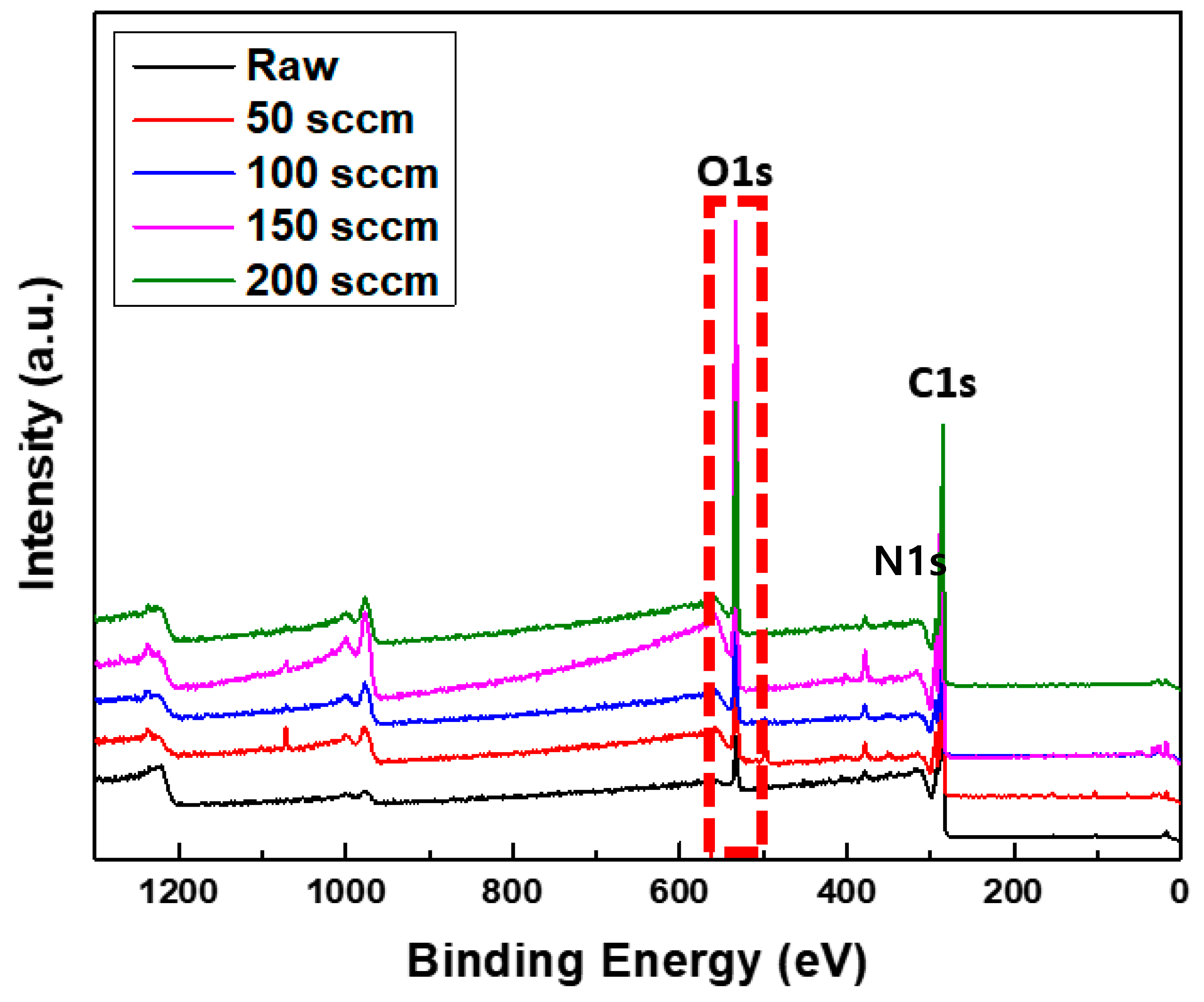
3.3. Surface Element Analysis of Activated Carbon
3.4. Surface Element Analysis of Activated Carbon
4. Conclusions
- 1.
- The plasma surface treatment method demonstrated the effective introduction of oxygen functional groups onto the activated carbon surface, depending on the variation in oxygen flow rates. However, contrary to expectations, when the oxygen flow rate was increased, an irregular introduction of oxygen functional groups onto the activated carbon surface was observed. These results are presumed to be a consequence of the repetitive influx and detachment of ionized oxygen atoms on the activated carbon surface induced by strong plasma energy.
- 2.
- The microwave treatment method shows a decrease in specific surface area and total pore volume of activated carbon with prolonged surface chemical reaction time or higher concentrations of nitric acid. These results are attributed to the etching effect induced by the high concentration of nitric acid solution and intense microwave energy, leading to the surface modification of activated carbon. Additionally, the introduction of oxygen functional groups contributes to the phenomenon of pore blockage on the activated carbon surface.
- 3.
- The oxygen functional groups introduced onto the activated carbon surface in this manner exhibit a higher electronegativity compared to carbon, resulting in a negative charge. Additionally, carbon atoms in most hydrocarbon compounds, such as VOCs, tend to carry a positive charge when encountering oxygen atoms with high electronegativity. Consequently, oxygen functional groups and VOC molecules are anticipated to form chemical bonds through electrostatic interactions, contributing to the adsorption process.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Separ. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Li, L.; Suain, L.; Junxin, L. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ma, C.; Horlyck, J.; Liu, R.; Yun, J. Development of pharmaceutical VOCs elimination by catalytic processes in China. Catalysts 2020, 10, 668. [Google Scholar] [CrossRef]
- Bai, B.C.; Lee, H.U.; Lee, C.W.; Lee, Y.S.; Im, J.S. N2 plasma treatment on activated carbon fibers for toxic gas removal: Mechanism study by electrochemical investigation. Chem. Engin. J. 2016, 306, 260–268. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C. Indoor air purification by photocatalyst TiO2 immobilized on an activated carbon filter installed in an air cleaner. Chem. Engin. Sci. 2005, 60, 103–109. [Google Scholar] [CrossRef]
- Isinkaralar, K.; Turkyilmaz, A. Simultaneous adsorption of selected VOCs in the gas environment by low-cost adsorbent from Ricinus communis. Carbon Lett. 2022, 32, 1781–1789. [Google Scholar] [CrossRef]
- Sivaranjanee, R.; Kumar, P.S.; Rangasamy, G. A critical review on biochar for environmental applications. Carbon Lett. 2023, 33, 1407–1432. [Google Scholar] [CrossRef]
- Kocaman, S. Evaluation of adsorption characteristics of new-generation CNT-based adsorbents: Characterization, modeling, mechanism, and equilibrium study. Carbon Lett. 2023, 33, 883–897. [Google Scholar] [CrossRef]
- Rajeshkumar, L.; Ramesh, M.; Bhuvaneswari, V.; Balaji, D. Carbon nano-materials (CNMs) derived from biomass for energy storage applications: A review. Carbon Lett. 2023, 33, 661–690. [Google Scholar] [CrossRef]
- Kang, H.J.; Yang, S.Y.; Kim, T.M.; Kim, Y.R. A study on the improvement of hydrophilic properties of activated carbon surface by nitric acid treatment. J. Korean Appl. Sci. Technol. 2021, 38, 1241–1248. [Google Scholar]
- Vold, M.J. The effect of adsorption on the van der waals interaction of spherical colloidal particles. J. Colloid Sci. 1961, 16, 1–12. [Google Scholar] [CrossRef]
- Huang, C.C.; Li, H.S.; Chen, C.H. Effect of surface acidic oxides of activated carbon on adsorption of ammonia. J. Hazard. Mater. 2008, 159, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jia, Z.; Xu, X.; Chen, Y.; Peng, W.; Zhang, J.; Wang, H.; Li, S.; Wen, J. Preparation of antimicrobial activated carbon fiber by loading with silver nanoparticles. Colloids Surf. A 2022, 633, 127868. [Google Scholar] [CrossRef]
- Valdés, H.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Zaror, C.A. Effect of ozone treatment on surface properties of activated carbon. Langmuir 2002, 18, 2111–2116. [Google Scholar] [CrossRef]
- Rodrigues, C.C.; Moraes Jr, D.; Nóbrega, S.W.; Barboza, M.G. Ammonia adsorption in a fixed bed of activated carbon. Bioresour. Technol. 2007, 98, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, J.H.; Hwang, J. Inactivation and filtration of bioaerosols using carbon fiber ionizer assisted activated carbon fiber filter. Part. Aerosol Res. 2010, 6, 185–192. [Google Scholar]
- Kim, M.J.; Jung, M.J.; Kim, M.I.; Choi, S.S.; Lee, Y.S. Adsorption characteristics of toluene gas using fluorinated phenol-based activated carbons. Appl. Chem. Eng. 2015, 26, 587–592. [Google Scholar] [CrossRef][Green Version]
- Khasri, A.; Bello, O.S.; Ahmad, M.A. Mesoporous activated carbon from pentace species sawdust via microwave-induced KOH activation: Optimization and methylene blue adsorption. Res. Chem. Intermed. 2018, 44, 5737–5757. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, B.J. Influence of oxygen plasma treatment on hydrogen chloride removal of activated carbon fibers. J. Colloid Interface Sci. 2004, 275, 590–595. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.G.; Yang, H.C.; Kim, J.H.; Lee, J.K. Adsorption characteristics of sulfur dioxide on pellet type zeolites. Korean Chem. Eng. Res. 2003, 41, 129–133. [Google Scholar]
- Guo, J.; Lua, A.C. Preparation of activated carbons from oil-palm-stone chars by microwave-induced carbon dioxide activation. Carbon 2000, 38, 1985–1993. [Google Scholar] [CrossRef]
- Liu, Q.S.; Zheng, T.; Li, N.; Wang, P.; Abulikemu, G. Modification of bamboo-based activated carbon using microwave radiation and its effects on the adsorption of methylene blue. Appl. Surf. Sci. 2010, 256, 3309–3315. [Google Scholar] [CrossRef]
- Lee, D.; Hong, S.H.; Paek, K.H.; Ju, W.T. Adsorbability enhancement of activated carbon by dielectric barrier discharge plasma treatment. Surf. Coat. Technol. 2005, 200, 2277–2282. [Google Scholar] [CrossRef]
- Ortiz Ortega, E.; Hosseini, S.; Martinez-Chapa, O.S.; Madou, M.J. Aging of plasma-activated carbon surfaces: Challenges and opportunities. Appl. Surf. Sci. 2021, 565, 150362. [Google Scholar] [CrossRef]
- Yin, C.Y.; Aroua, M.K.; Daud, W.M.A.W. Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Separ. Purif. Technol. 2007, 52, 403–415. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis. 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Lim, C.; Kwak, C.H.; Jeong, S.G.; Kim, D.; Lee, Y.S. Enhanced CO2 adsorption of activated carbon with simultaneous surface etching and functionalization by nitrogen plasma treatment. Carbon Lett. 2023, 33, 139–145. [Google Scholar] [CrossRef]
- Wu, J.; Xu, F.; Li, S.; Ma, P.; Zhang, X.; Liu, Q.; Fu, R.; Wu, D. Porous polymers as multifunctional material platforms toward task-specific applications. Adv. Mater. 2019, 31, 1802922. [Google Scholar] [CrossRef] [PubMed]
- Hesas, R.H.; Wan Daud, W.M.A.; Sahu, J.N.; Arami-Niya, A. The effects of a microwave heating method on the production of activated carbon from agricultural waste: A review. J. Anal. Appl. Pyrolysis 2013, 100, 1–11. [Google Scholar] [CrossRef]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Świątkowski, A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Jung, M.J.; Yu, H.R.; Lee, D.; Lee, Y.S. Effect of boric acid treatment on the electrochemical properties of the phenol-based Activated carbon. Appl. Chem. Engin. 2013, 24, 201–207. [Google Scholar]
- Yu, H.R.; Cho, S.; Bai, B.C.; Yi, K.B.; Lee, Y.S. Effects of fluorination on carbon molecular sieves for CH4/CO2 gas separation behavior. Int. J. Greenh. Gas Control. 2012, 10, 278–284. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, X.; Wang, Y.; Cui, Z.; Tang, J. SF6 abatement in a packed bed plasma reactor: Role of zirconia size and optimization using RSM. J. Ind. Eng. Chem. 2021, 94, 205–216. [Google Scholar] [CrossRef]
- Sugiyama, H.; Hattori, Y. Selective and enhanced CO2 adsorption on fluorinated activated carbon fibers. Chem. Phys. Lett. 2020, 758, 137909. [Google Scholar] [CrossRef]

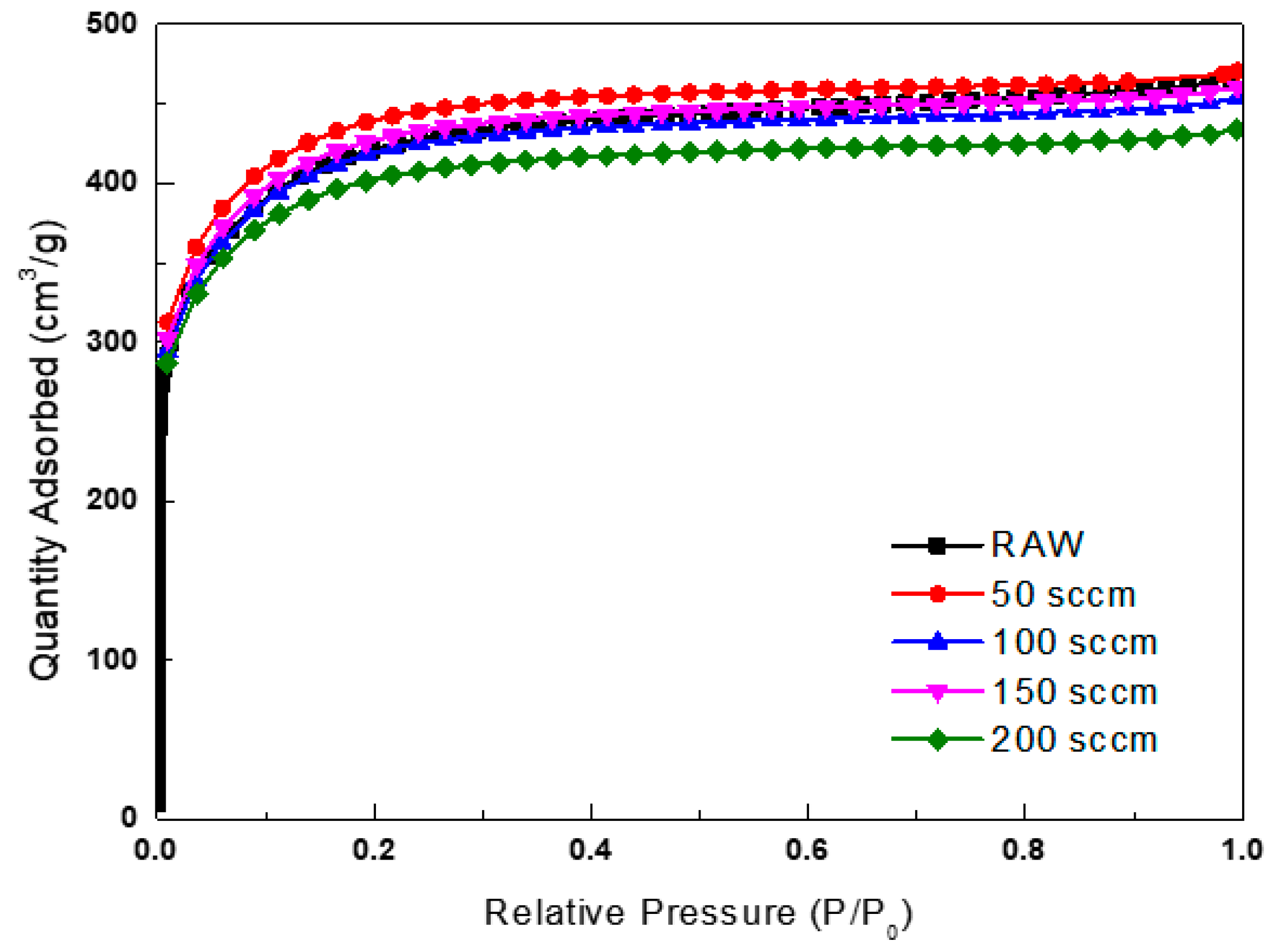
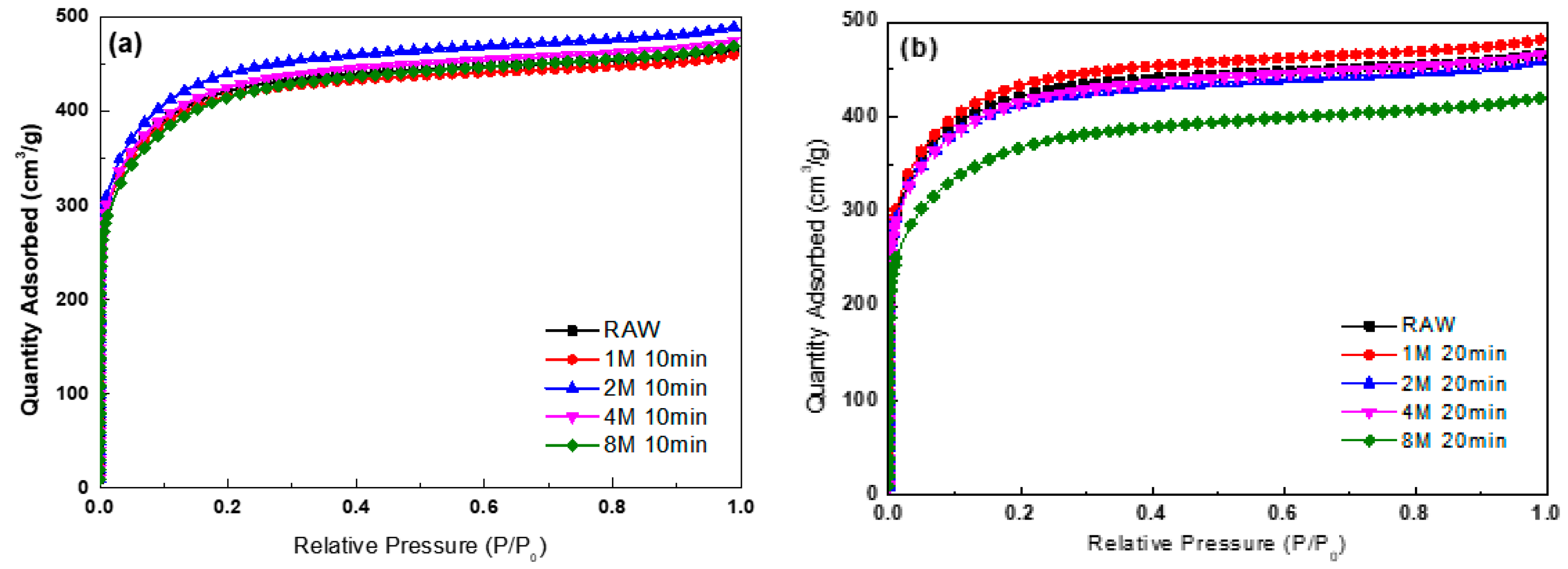
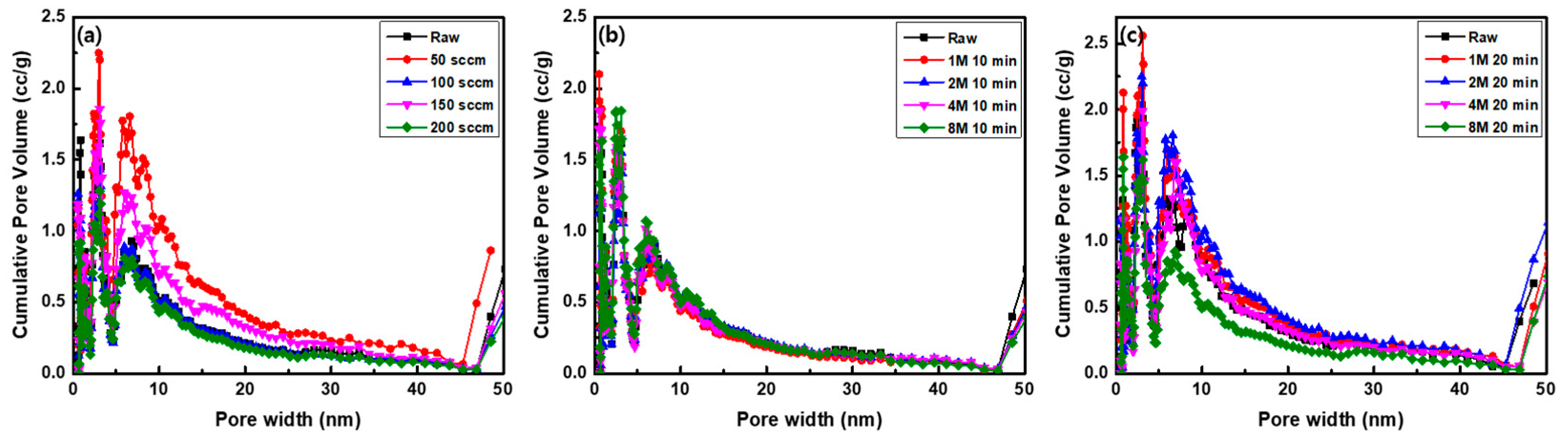

| Sample | SBET (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| Raw | 1562 | 0.89 | 0.729 | 1.85 |
| 50 cc/min | 1639 | 0.86 | 0.70 | 1.78 |
| 100 cc/min | 1546 | 0.81 | 0.67 | 1.81 |
| 150 cc/min | 1590 | 0.83 | 0.71 | 1.80 |
| 200 cc/min | 1503 | 0.79 | 0.64 | 1.79 |
| Sample | Sbet (m2/g) | Total Pore Volume (cm2/g) | Micropore Volume (cm2/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| RAW | 1562 | 0.89 | 0.68 | 1.85 |
| 1 M 10 min | 1542 | 0.82 | 0.67 | 1.85 |
| 2 M 10 min | 1628 | 0.84 | 0.72 | 1.86 |
| 4 M 10 min | 1573 | 0.83 | 0.69 | 1.87 |
| 8 M 10 min | 1527 | 0.83 | 0.68 | 1.91 |
| 1 M 20 min | 1608 | 0.87 | 0.71 | 1.86 |
| 2 M 20 min | 1527 | 0.82 | 0.67 | 1.86 |
| 4 M 20 min | 1531 | 0.81 | 0.68 | 1.89 |
| 8 M 20 min | 1347 | 0.77 | 0.61 | 1.93 |
| Sample Name | XPS Peaks | ||||
|---|---|---|---|---|---|
| C1s | O1s | N1s | |||
| Raw | 94.82 | 4.76 | 0.41 | ||
| Microwave | 1 M | 10 min | 92.51 | 7.49 | - |
| 20 min | 90.46 | 9.54 | - | ||
| 2 M | 10 min | 89.89 | 10.41 | - | |
| 20 min | 90.48 | 9.53 | - | ||
| 4 M | 10 min | 90.27 | 9.73 | - | |
| 20 min | 88.79 | 11.21 | - | ||
| 8 M | 10 min | 86.47 | 13.53 | - | |
| 20 min | 85.89 | 14.11 | - | ||
| Plasma | 50 cc/min | 64.08 | 34.14 | 1.78 | |
| 100 cc/min | 71.51 | 26.97 | 1.51 | ||
| 150 cc/min | 60.64 | 35.64 | 3.22 | ||
| 200 cc/min | 77.06 | 21.24 | 1.69 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.Y.; Bai, B.C.; Kim, Y.R. Effective Surface Structure Changes and Characteristics of Activated Carbon with the Simple Introduction of Oxygen Functional Groups by Using Radiation Energy. Surfaces 2024, 7, 12-25. https://doi.org/10.3390/surfaces7010002
Yang SY, Bai BC, Kim YR. Effective Surface Structure Changes and Characteristics of Activated Carbon with the Simple Introduction of Oxygen Functional Groups by Using Radiation Energy. Surfaces. 2024; 7(1):12-25. https://doi.org/10.3390/surfaces7010002
Chicago/Turabian StyleYang, So Yeong, Byong Chol Bai, and Yong Ryeol Kim. 2024. "Effective Surface Structure Changes and Characteristics of Activated Carbon with the Simple Introduction of Oxygen Functional Groups by Using Radiation Energy" Surfaces 7, no. 1: 12-25. https://doi.org/10.3390/surfaces7010002
APA StyleYang, S. Y., Bai, B. C., & Kim, Y. R. (2024). Effective Surface Structure Changes and Characteristics of Activated Carbon with the Simple Introduction of Oxygen Functional Groups by Using Radiation Energy. Surfaces, 7(1), 12-25. https://doi.org/10.3390/surfaces7010002






