Porous Carbon for CO2 Capture Technology: Unveiling Fundamentals and Innovations
Abstract
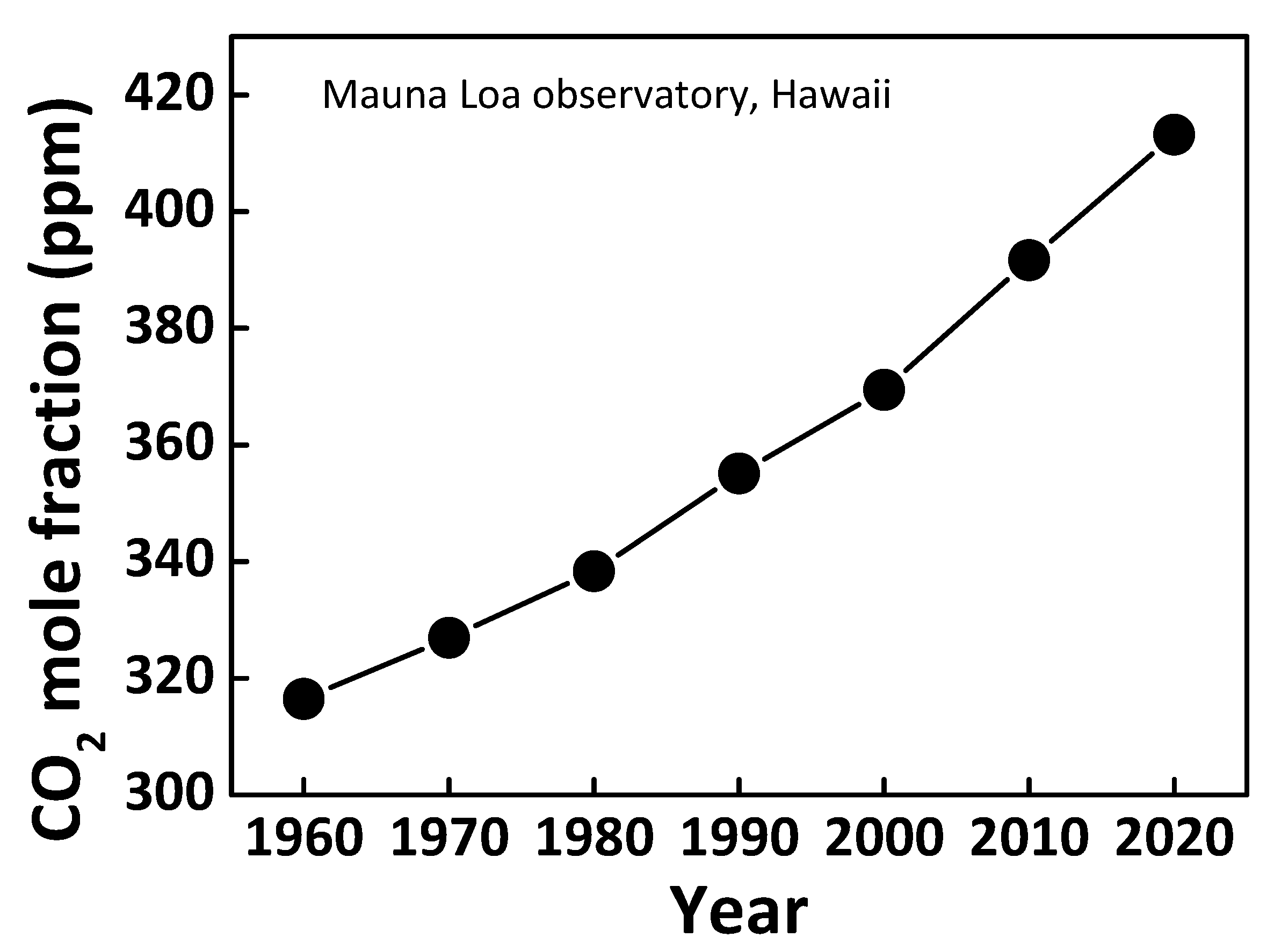
:1. Introduction
2. Carbon as a Promising Candidate for CO2 Capture Technology
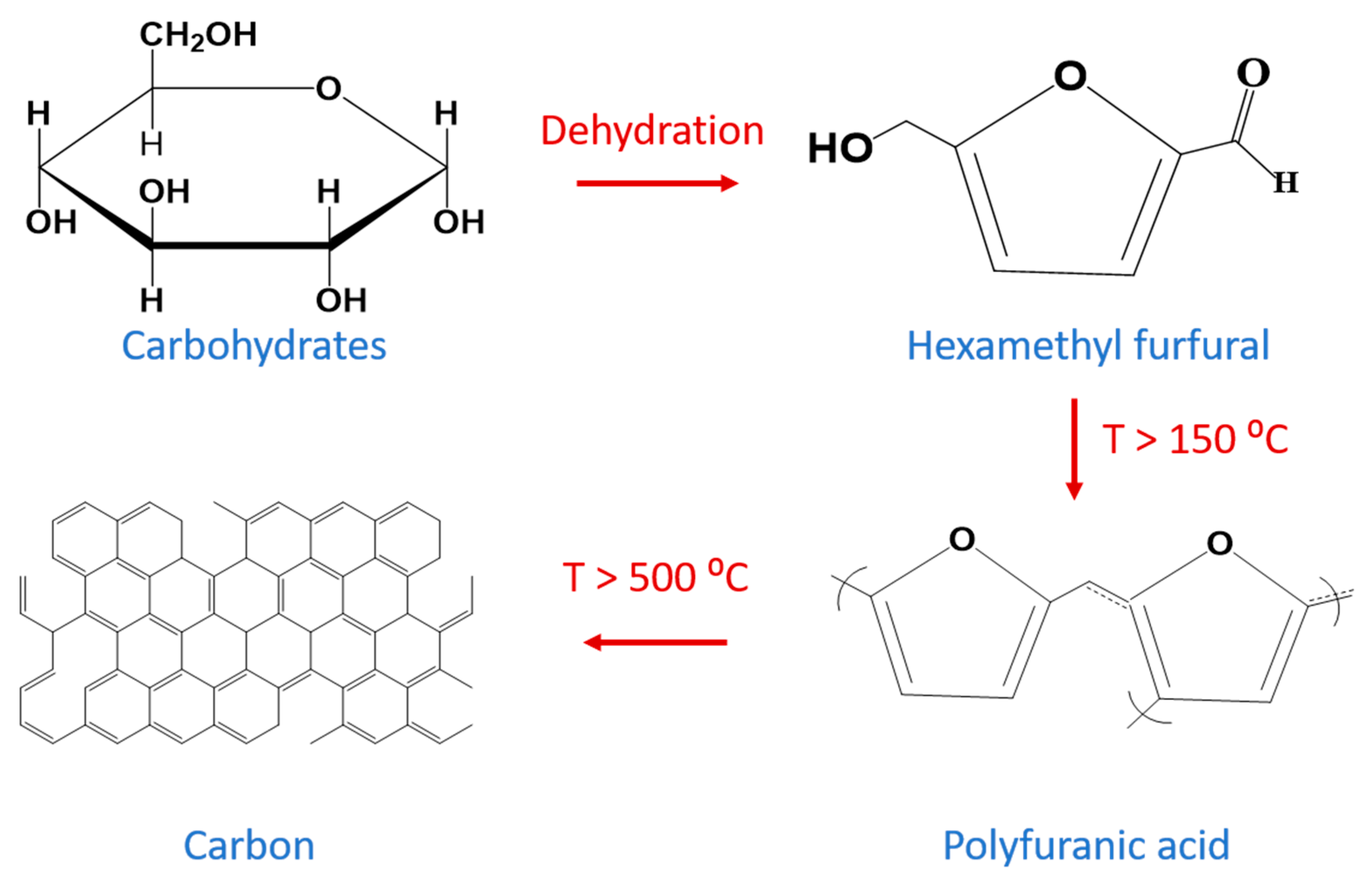
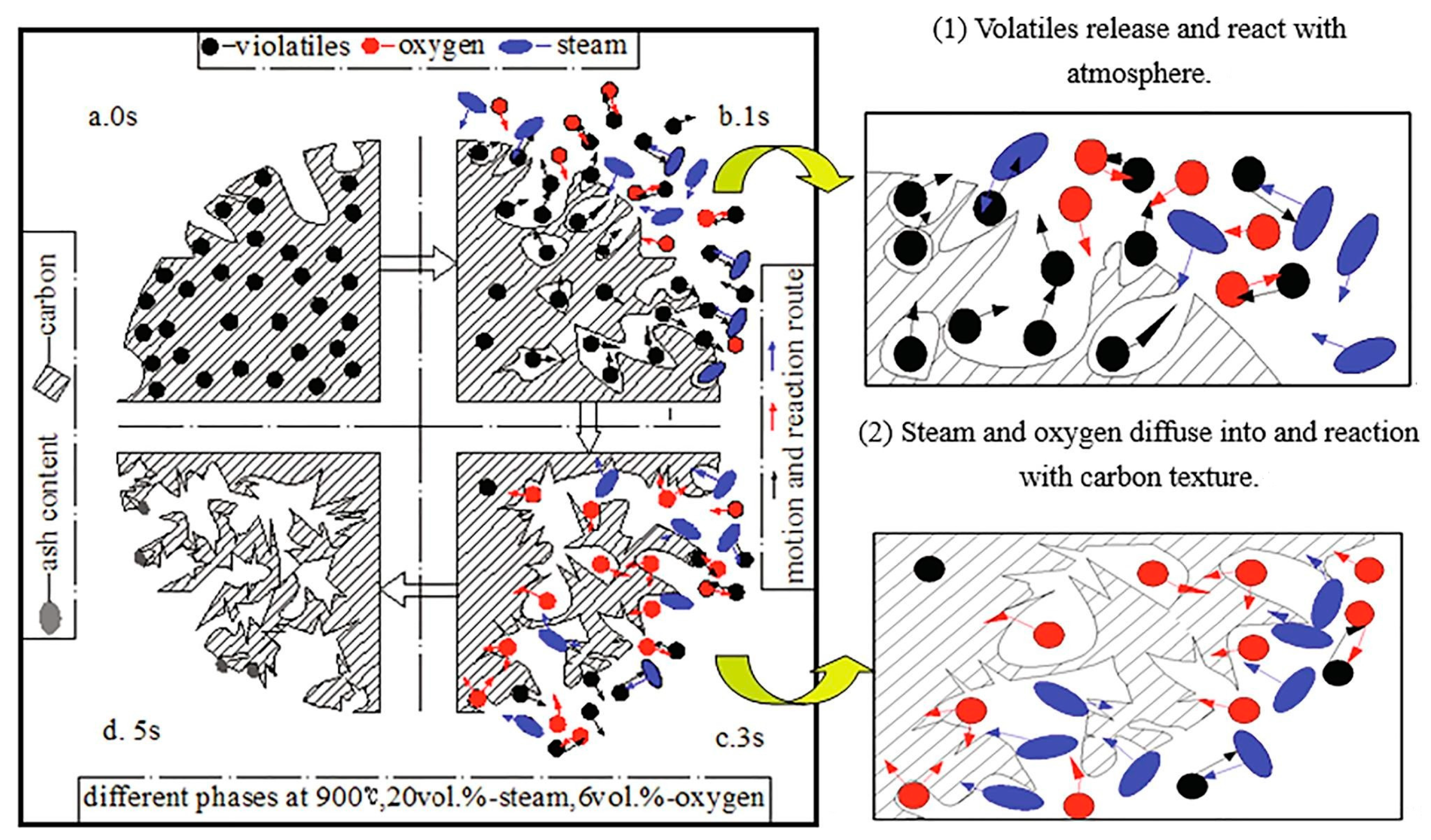
2.1. Carbon Products Preparation Mechanism
2.2. Factors Influencing CO2 Adsorption on the Carbon Surface
3. Strategies for Modifying Carbon Structures
3.1. Chemical Activation
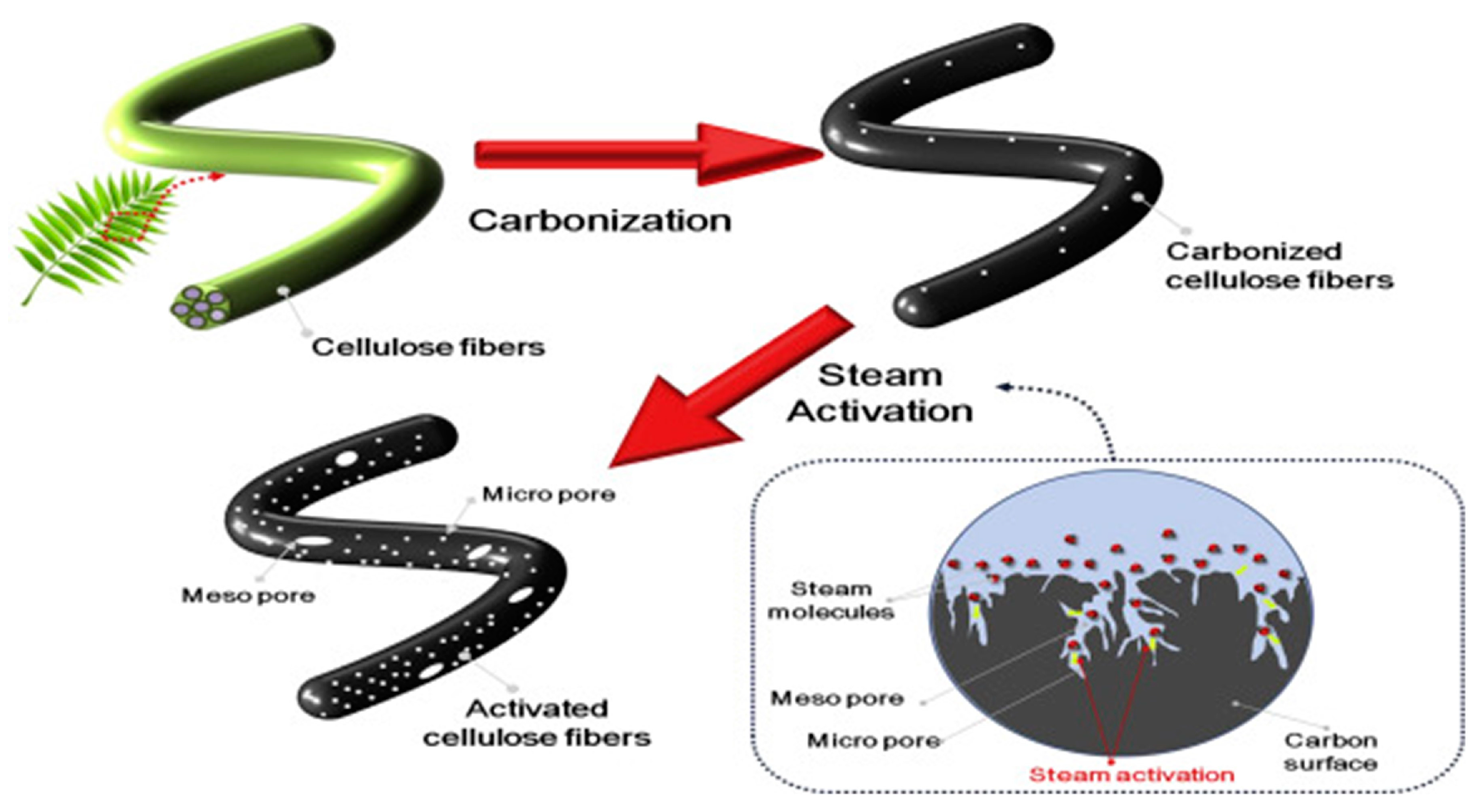
3.2. Physical Activation
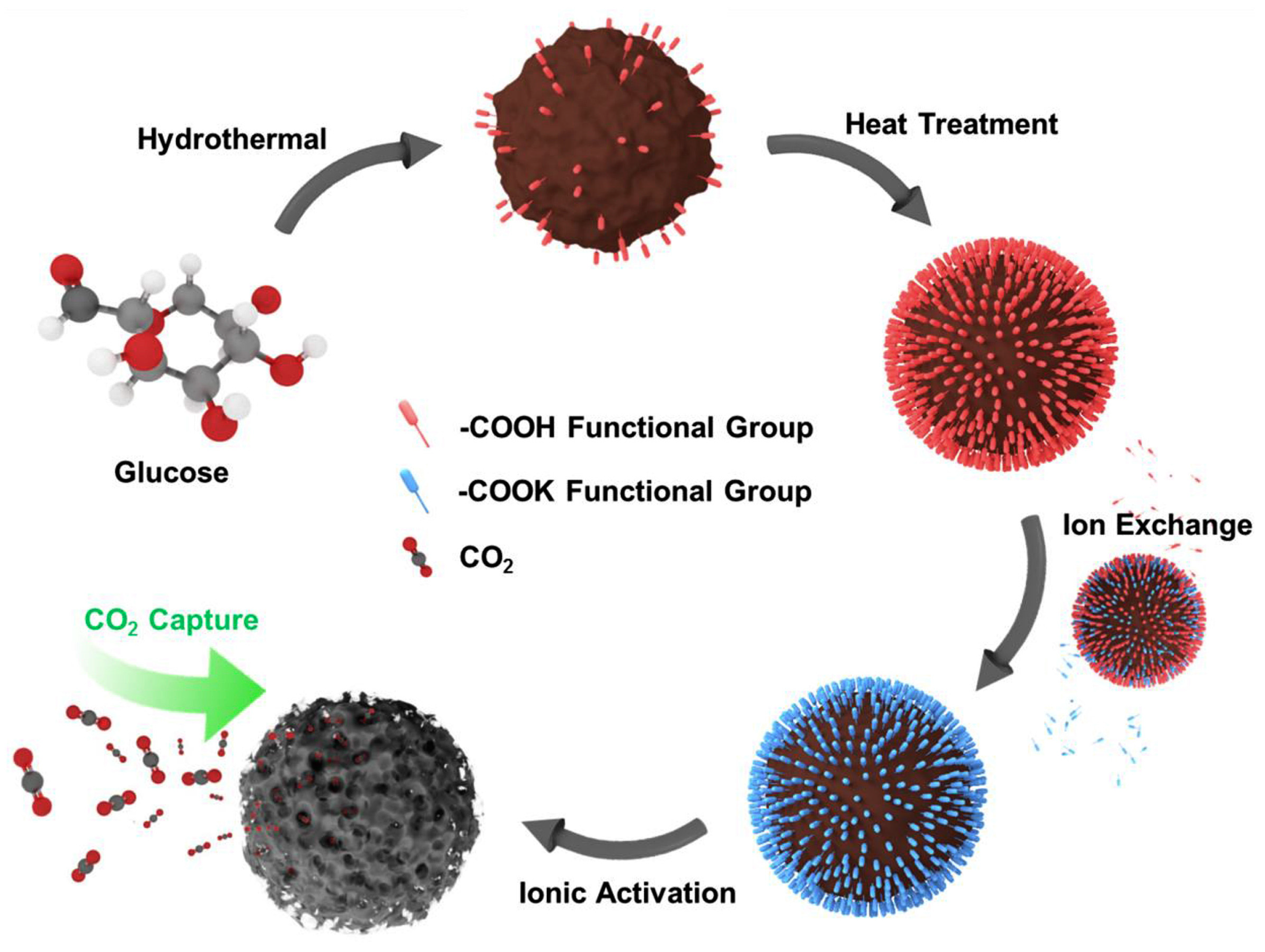
3.3. Metal Ion Activation
3.4. Hard Template Activation
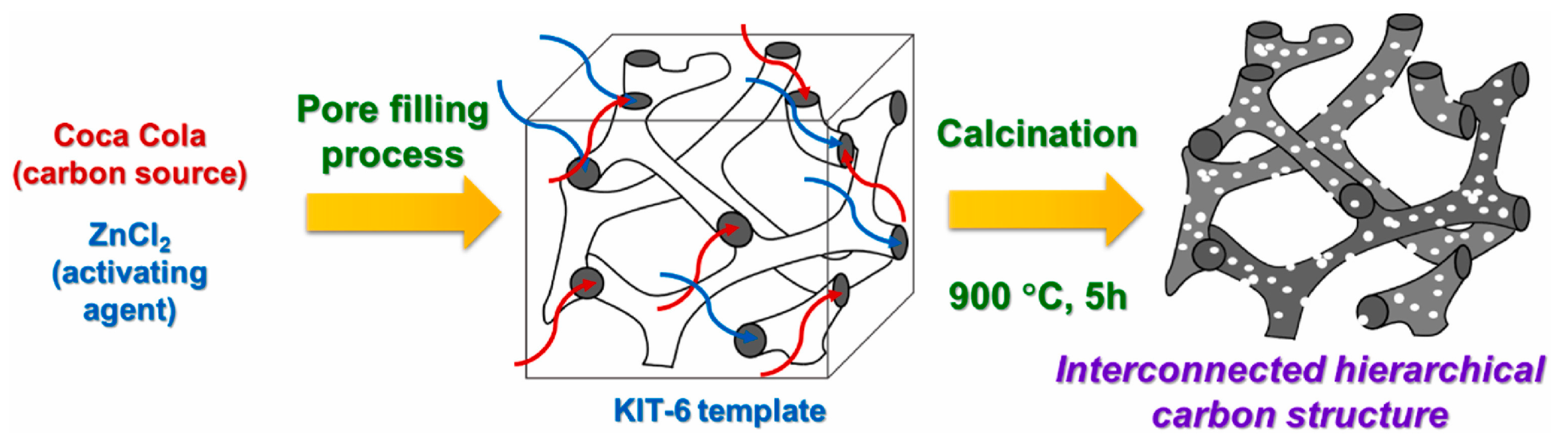
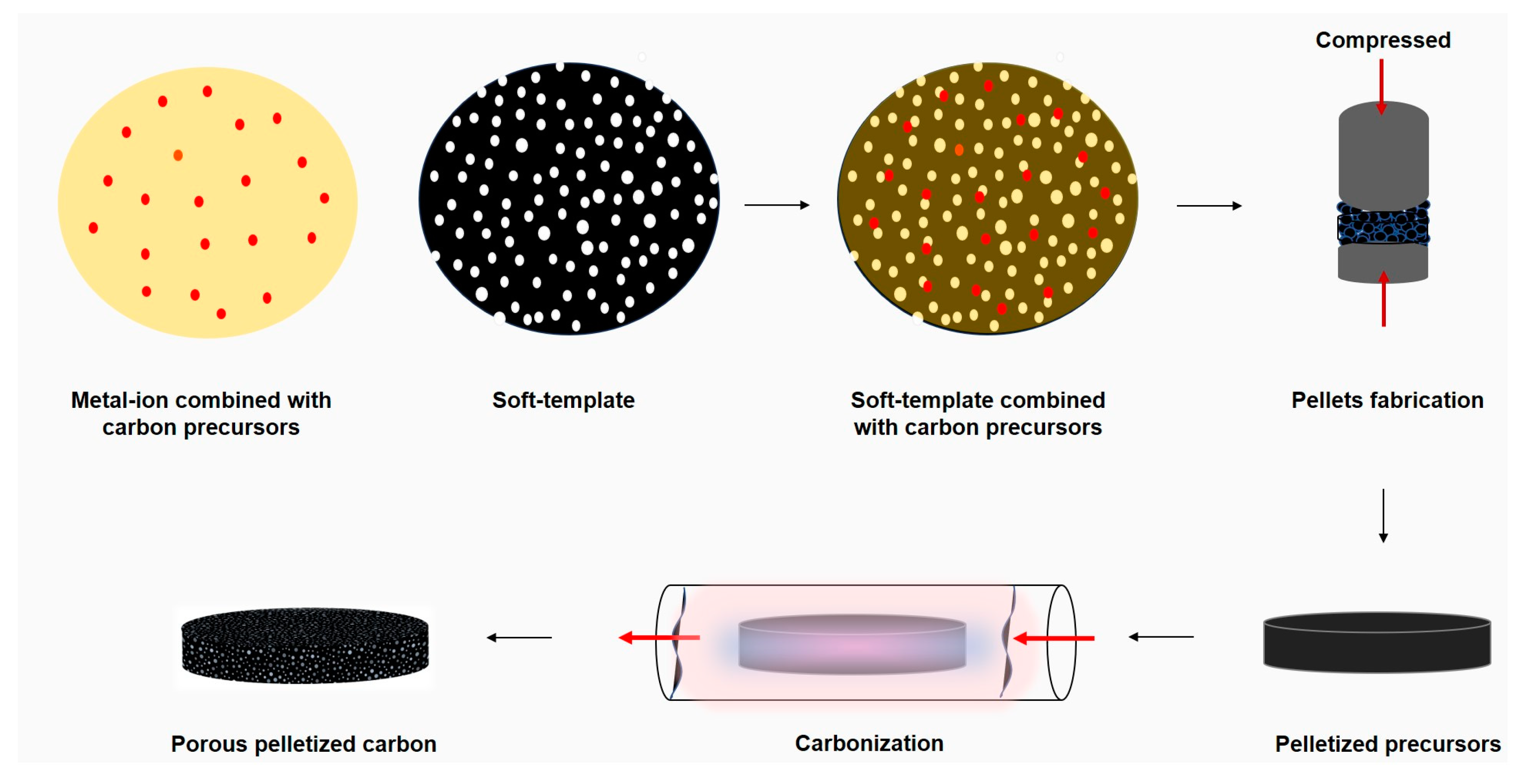
3.5. Soft Template Activation
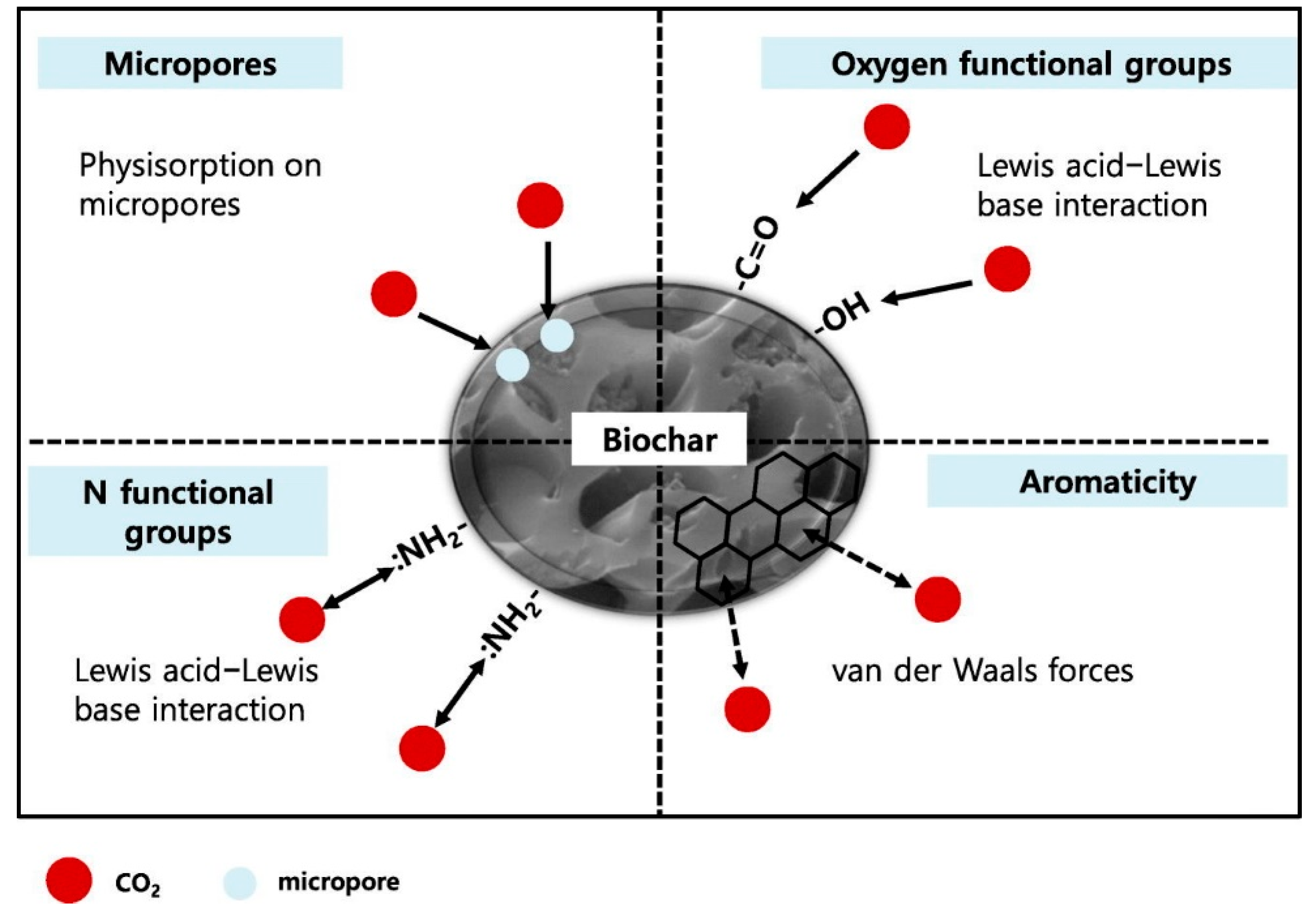
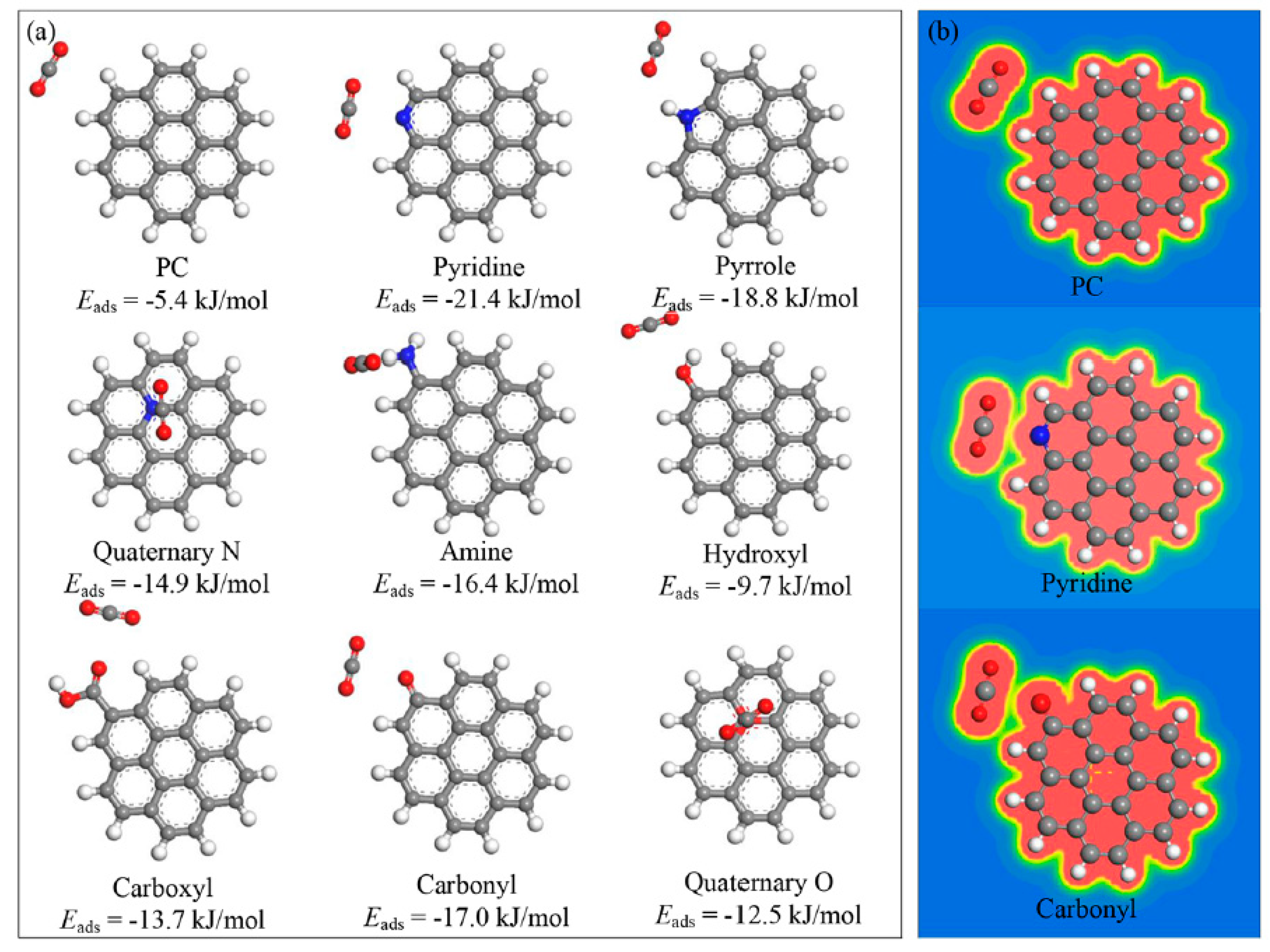
4. Significant Role of Surface Functional Groups in Porous Carbon
5. Envisioning Future Research Prospects and Forging a Focused Strategy
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bari, G.A.R.; Kang, H.J.; Lee, T.G.; Hwang, H.J.; An, B.H.; Seo, H.W.; Ko, C.H.; Hong, W.H.; Jun, Y.S. Dual-templating-derived porous carbons for low-pressure CO2 capture. Carbon Lett. 2023, 33, 811–822. [Google Scholar] [CrossRef]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging trends in porous materials for CO2capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef]
- Ahmad, H.H.; Saleem, F.; Arif, H. Evaluation of Catastrophic Global Warming due to Coal Combustion, Paradigm of South Asia. Int. J. Innov. Sci. Technol. 2021, 3, 198–207. [Google Scholar] [CrossRef]
- Pedraza, J.; Zimmermann, A.; Tobon, J.; Schomäcker, R.; Rojas, N. On the road to net zero-emission cement: Integrated assessment of mineral carbonation of cement kiln dust. Chem. Eng. J. 2021, 408, 127346. [Google Scholar] [CrossRef]
- Janakiram, S.; Santinelli, F.; Costi, R.; Lindbråthen, A.; Nardelli, G.M.; Milkowski, K.; Ansaloni, L.; Deng, L. Field trial of hollow fiber modules of hybrid facilitated transport membranes for flue gas CO2 capture in cement industry. Chem. Eng. J. 2021, 413, 127405. [Google Scholar] [CrossRef]
- Fujikawa, S.; Selyanchyn, R.; Kunitake, T. A new strategy for membrane-based direct air capture. Polym. J. 2021, 53, 111–119. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, Y.; Wupardrasta, Y.; Desai, K. Selection of amine combination for CO2 capture in a packed bed scrubber. Resour. -Effic. Technol. 2016, 2, S165–S170. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Chen, C. Experimental study of energy requirement of CO2 desorption from rich solvent. Energy Procedia 2013, 37, 1836–1843. [Google Scholar] [CrossRef]
- Patel, H.A.; Byun, J.; Yavuz, C.T. Carbon Dioxide Capture Adsorbents: Chemistry and Methods. ChemSusChem 2017, 10, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef]
- Wang, M.; Joel, A.S.; Ramshaw, C.; Eimer, D.; Musa, N.M. Process intensification for post-combustion CO2 capture with chemical absorption: A critical review. Appl. Energy 2015, 158, 275–291. [Google Scholar] [CrossRef]
- Zhang, S.; Du, M.; Shao, P.; Wang, L.; Ye, J.; Chen, J.; Chen, J. Carbonic Anhydrase Enzyme-MOFs Composite with a Superior Catalytic Performance to Promote CO 2 Absorption into Tertiary Amine Solution. Environ. Sci. Technol. 2018, 52, 12708–12716. [Google Scholar] [CrossRef]
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Sabatino, F.; Grimm, A.; Gallucci, F.; van Sint Annaland, M.; Kramer, G.J.; Gazzani, M. A comparative energy and costs assessment and optimization for direct air capture technologies. Joule 2021, 5, 2047–2076. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. A Comparative Study of the CO2 Absorption in Some Solvent-Free Alkanolamines and in Aqueous Monoethanolamine (MEA). Environ. Sci. Technol. 2016, 50, 7239–7246. [Google Scholar] [CrossRef]
- Mazari, S.A.; Ali, B.S.; Jan, B.M.; Saeed, I.M. Degradation study of piperazine, its blends and structural analogs for CO2 capture: A review. Int. J. Greenh. Gas Control 2014, 31, 214–228. [Google Scholar] [CrossRef]
- Chang, B.; Shi, W.; Yin, H.; Zhang, S.; Yang, B. Poplar catkin-derived self-templated synthesis of N-doped hierarchical porous carbon microtubes for effective CO2 capture. Chem. Eng. J. 2019, 358, 1507–1518. [Google Scholar] [CrossRef]
- Oschatz, M.; Antonietti, M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018, 11, 57–70. [Google Scholar] [CrossRef]
- Wang, X.; He, T.; Hu, J.; Liu, M. The progress of nanomaterials for carbon dioxide captureviathe adsorption process. Environ. Sci. Nano. 2021, 8, 890–912. [Google Scholar] [CrossRef]
- Zhang, Z.; Cano, Z.P.; Luo, D.; Dou, H.; Yu, A.; Chen, Z. Rational design of tailored porous carbon-based materials for CO2 capture. J. Mater. Chem. A Mater. 2019, 7, 20985–21003. [Google Scholar] [CrossRef]
- Sukor, N.R.; Shamsuddin, A.H.; Mahlia, T.M.I.; Isa, M.F.M. Techno-economic analysis of CO2 capture technologies in offshore natural gas field: Implications to carbon capture and storage in Malaysia. Processes 2020, 8, 350. [Google Scholar] [CrossRef]
- Micari, M.; Dakhchoune, M.; Agrawal, K.V. Techno-economic assessment of postcombustion carbon capture using high-performance nanoporous single-layer graphene membranes. J. Memb. Sci. 2021, 624, 119103. [Google Scholar] [CrossRef]
- John, J.M.; Alwi, S.R.W.; Omoregbe, D.I. Techno-economic analysis of carbon dioxide capture and utilisation analysis for an industrial site with fuel cell integration. J. Clean. Prod. 2021, 281, 124920. [Google Scholar] [CrossRef]
- Hao, G.P.; Li, W.C.; Qian, D.; Wang, G.H.; Zhang, W.P.; Zhang, T.; Wang, A.Q.; Schüth, F.; Bongard, H.J.; Lu, A.H. Structurally designed synthesis of mechanically stable poly(benzoxazine-co- resol)-based porous carbon monoliths and their application as high-performance CO2 capture sorbents. J. Am. Chem. Soc. 2011, 133, 11378–11388. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Churipard, S.R.; Peter, S.C. An overview of the materials and methodologies for CO2capture under humid conditions. J. Mater. Chem. A Mater. 2021, 9, 26498–26527. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, K.X.; Teng, B.; Zhang, T.; Han, Y. A perfluorinated covalent triazine-based framework for highly selective and water-tolerant CO2 capture. Energy Environ. Sci. 2013, 6, 3684–3692. [Google Scholar] [CrossRef]
- Masala, A.; Vitillo, J.G.; Mondino, G.; Grande, C.A.; Blom, R.; Manzoli, M.; Marshall, M.; Bordiga, S. CO2 capture in dry and wet conditions in UTSA-16 metal-organic framework. ACS Appl. Mater. Interfaces 2017, 9, 455–463. [Google Scholar] [CrossRef]
- Yang, J.; Yue, L.; Hu, X.; Wang, L.; Zhao, Y.; Lin, Y.; Sun, Y.; DaCosta, H.; Guo, L. Efficient CO2 Capture by Porous Carbons Derived from Coconut Shell. Energy Fuels 2017, 31, 4287–4293. [Google Scholar] [CrossRef]
- Xing, W.; Liu, C.; Zhou, Z.; Zhang, L.; Zhou, J.; Zhuo, S.; Yan, Z.; Gao, H.; Wang, G.; Qiao, S.Z. Superior CO2 uptake of N-doped activated carbon through hydrogen-bonding interaction. Energy Environ. Sci. 2012, 5, 7323–7327. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- To, J.W.; He, J.; Mei, J.; Haghpanah, R.; Chen, Z.; Kurosawa, T.; Chen, S.; Bae, W.G.; Pan, L.; Tok, J.B.H.; et al. Hierarchical N-Doped Carbon as CO2 Adsorbent with High CO2 Selectivity from Rationally Designed Polypyrrole Precursor. J. Am. Chem. Soc. 2016, 138, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Creamer, A.E.; Gao, B. Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef]
- Yang, I.; Jung, M.; Kim, M.S.; Choi, D.; Jung, J.C. Physical and chemical activation mechanisms of carbon materials based on the microdomain model. J. Mater. Chem. A Mater. 2021, 9, 9815–9825. [Google Scholar] [CrossRef]
- Quan, C.; Zhou, Y.; Wang, J.; Wu, C.; Gao, N. Biomass-based carbon materials for CO2capture: A review. J. CO2 Util. 2023, 68, 102373. [Google Scholar] [CrossRef]
- Malini, K.; Selvakumar, D.; Kumar, N.S. Activated carbon from biomass: Preparation, factors improving basicity and surface properties for enhanced CO2capture capacity—A review. J. CO2 Util. 2023, 67, 102318. [Google Scholar] [CrossRef]
- Xing, W.; Liu, C.; Zhou, Z.; Zhou, J.; Wang, G.; Zhuo, S.; Xue, Q.; Song, L.; Yan, Z. Oxygen-containing functional group-facilitated CO2 capture by carbide-derived carbons. Nanoscale Res. Lett. 2014, 9, 189. [Google Scholar] [CrossRef]
- Khosrowshahi, M.S.; Abdol, M.A.; Mashhadimoslem, H.; Khakpour, E.; Emrooz, H.B.M.; Sadeghzadeh, S.; Ghaemi, A. The role of surface chemistry on CO2 adsorption in biomass-derived porous carbons by experimental results and molecular dynamics simulations. Sci. Rep. 2022, 12, 8917. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Hou, C.; Liu, J. Quaternary functionalized mesoporous adsorbents for ultra-high kinetics of CO2 capture from air. Sci. Rep. 2020, 10, 21429. [Google Scholar] [CrossRef]
- Yang, Y.B.; Hao, Q.; Müller-Plathe, F.; Böhm, M.C. Monte Carlo Simulations of SO2, H2S, and CO2 Adsorption in Charged Single-Walled Carbon Nanotube Arrays. J. Phys. Chem. C 2020, 124, 5838–5852. [Google Scholar] [CrossRef]
- Wang, R.; Xi, S.C.; Wang, D.Y.; Dou, M.; Dong, B. Defluorinated Porous Carbon Nanomaterials for CO2 Capture. ACS Appl. Nano Mater. 2021, 4, 10148–10154. [Google Scholar] [CrossRef]
- Hu, M.; He, J.; Zhao, Z.; Strobel, T.A.; Hu, W.; Yu, D.; Sun, H.; Liu, L.; Li, Z.; Ma, M.; et al. Compressed glassy carbon: An ultrastrong and elastic interpenetrating graphene network. Sci. Adv. 2017, 3, 3213. [Google Scholar] [CrossRef]
- Mao, H.; Tang, J.; Chen, J.; Wan, J.; Hou, K.; Peng, Y.; Halat, D.M.; Xiao, L.; Zhang, R.; Lv, X.; et al. Designing hierarchical nanoporous membranes for highly efficient gas adsorption and storage. Sci. Adv. 2020, 6, eabb0694. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, H.J.; Kang, H.J.; Bari, G.A.R.; Lee, T.G.; An, B.H.; Cho, S.Y.; Jun, Y.S. Hierarchical porous, N-containing carbon supports for high loading sulfur cathodes. Nanomaterials 2021, 11, 408. [Google Scholar] [CrossRef]
- Wang, R.; Jia, J.; Jin, Q.; Chen, H.; Liu, H.; Yin, Q.; Zhao, Z. Forming mechanism of coke microparticles from polymerization of aqueous organics during hydrothermal carbonization process of biomass. Carbon 2022, 192, 50–60. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]
- Zhuang, X.; Liu, J.; Zhang, Q.; Wang, C.; Zhan, H.; Ma, L. A review on the utilization of industrial biowaste via hydrothermal carbonization. Renew. Sustain. Energy Rev. 2022, 154, 111877. [Google Scholar] [CrossRef]
- Silva, F.V.E.; Monteggia, L.O. Pyrolysis of algal biomass obtained from high-rate algae ponds applied to wastewater treatment. Front. Energy Res. 2015, 3, 31. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Huang, H.; Xiao, R.; Li, R.; Zhang, Z. Influence of temperature and residence time on characteristics of biochars derived from agricultural residues: A comprehensive evaluation. Process Saf. Environ. Prot. 2020, 139, 218–229. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- Kang, H.J.; Bari, G.A.R.; Lee, T.G.; Khan, T.T.; Park, J.W.; Hwang, H.J.; Cho, S.Y.; Jun, Y.S. Microporous carbon nanoparticles for lithium-sulfur batteries. Nanomaterials 2020, 10, 2012. [Google Scholar] [CrossRef]
- Sekirifa, M.L.; Hadj-Mahammed, M.; Pallier, S.; Baameur, L.; Richard, D.; Al-Dujaili, A.H. Preparation and characterization of an activated carbon from a date stones variety by physical activation with carbon dioxide. J. Anal. Appl. Pyrolysis. 2013, 99, 155–160. [Google Scholar] [CrossRef]
- Ogungbenro, A.E.; Quang, D.V.; Al-Ali, K.A.; Vega, L.F.; Abu-Zahra, M.R.M. Physical synthesis and characterization of activated carbon from date seeds for CO2 capture. J. Environ. Chem. Eng. 2018, 6, 4245–4252. [Google Scholar] [CrossRef]
- Singh, G.; Lakhi, K.S.; Sil, S.; Bhosale, S.V.; Kim, I.; Albahily, K.; Vinu, A. Biomass derived porous carbon for CO2 capture. Carbon 2019, 148, 164–186. [Google Scholar] [CrossRef]
- Kupgan, G.; Liyana-Arachchi, T.P.; Colina, C.M. NLDFT Pore Size Distribution in Amorphous Microporous Materials. Langmuir 2017, 33, 11138–11145. [Google Scholar] [CrossRef]
- Dantas, S.; Struckhoff, K.C.; Thommes, M.; Neimark, A.V. Pore size characterization of micro-mesoporous carbons using CO2 adsorption. Carbon 2021, 173, 842–848. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Mehio, N.; Dai, S.; Jiang, D.E. Quantum mechanical basis for kinetic diameters of small gaseous molecules. J. Phys. Chem. A 2014, 118, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, B.; Gorbounov, M.; Soltani, S.M. Impact of Surface Functional Groups and Their Introduction Methods on the Mechanisms of CO2 Adsorption on Porous Carbonaceous Adsorbents. Carbon Capture Sci. Technol. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Lim, G.; Lee, K.B.; Ham, H.C. Effect of N-Containing Functional Groups on CO2 Adsorption of Carbonaceous Materials: A Density Functional Theory Approach. J. Phys. Chem. C 2016, 120, 8087–8095. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Hao, J.; Ma, R.; Guo, Q.; Gao, H.; Bai, H. Nitrogen and Oxygen Codoped Porous Carbon with Superior CO2 Adsorption Performance: A Combined Experimental and DFT Calculation Study. Ind. Eng. Chem. Res. 2019, 58, 13390–13400. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Biomass derived low-cost microporous adsorbents for efficient CO2 capture. Fuel 2015, 148, 246–254. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Choi, S.W.; Shang, J.; Hanif, A.; Dissanayake, P.D.; Tsang, D.C.; Kwon, J.H.; Lee, K.B.; Ok, Y.S. Carbon dioxide capture in biochar produced from pine sawdust and paper mill sludge: Effect of porous structure and surface chemistry. Sci. Total Environ. 2020, 739, 139845. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Xing, W.; Xue, Q.; Yan, Z.; Zhuo, S.; Qiao, S.Z. Critical role of small micropores in high CO2 uptake. Phys. Chem. Chem. Phys. 2013, 15, 2523–2529. [Google Scholar] [CrossRef]
- Sun, N.; Sun, C.; Liu, J.; Liu, H.; Snape, C.E.; Li, K. Surface-modified spherical activated carbon materials for pre-combustion carbon dioxide capture. RSC Adv. 2015, 5, 33681–33690. [Google Scholar] [CrossRef]
- Gao, A.; Guo, N.; Yan, M.; Li, M.; Wang, F.; Yang, R. Hierarchical porous carbon activated by CaCO3 from pigskin collagen for CO2 and H2 adsorption. Microporous Mesoporous Mater. 2018, 260, 172–179. [Google Scholar] [CrossRef]
- Zhang, X.; Elsayed, I.; Song, X.; Shmulsky, R.; Hassan, E.B. Microporous carbon nanoflakes derived from biomass cork waste for CO2 capture. Sci. Total Environ. 2020, 748, 142465. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Y.; Yao, F.; Wang, X.; Zheng, H.; Ye, G.; Cheng, H.; Wu, J.; Huang, H.; Ye, D. One-pot synthesis of N-doped petroleum coke-based microporous carbon for high-performance CO2 adsorption and supercapacitors. J. Environ. Sci. 2024, 139, 93–104. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Vendrell, X.; Kiełbasa, K.; Michalkiewicz, B. Biomass waste fern leaves as a material for a sustainable method of activated carbon production for CO2 capture. Biomass Bioenergy 2023, 175, 106880. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, D.; Lui, G.; Li, G.; Jiang, G.; Cano, Z.P.; Deng, Y.P.; Du, X.; Yin, S.; Chen, Y.; et al. In-situ ion-activated carbon nanospheres with tunable ultramicroporosity for superior CO2 capture. Carbon 2019, 143, 531–541. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Alvim-Ferraz, M.C.M.; Gaspar, C.M.T.B. Micropore size distribution of activated carbons impregnated after carbonization. J. Porous Mater. 2003, 10, 47–55. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Sapag, K.; Rodríguez-Reinoso, F. Tailoring biomass-based activated carbon for CH4 storage by combining chemical activation with H3PO4 or ZnCl2 and physical activation with CO2. Carbon 2016, 110, 138–147. [Google Scholar] [CrossRef]
- Kim, H.S.; Kang, M.S.; Yoo, W.C. Highly Enhanced Gas Sorption Capacities of N-Doped Porous Carbon Spheres by Hot NH3 and CO2 Treatments. J. Phys. Chem. C 2015, 119, 28512–28522. [Google Scholar] [CrossRef]
- Khuong, D.A.; Nguyen, H.N.; Tsubota, T. Activated carbon produced from bamboo and solid residue by CO2 activation utilized as CO2 adsorbents. Biomass Bioenergy 2021, 148, 106039. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Lee, S.S.; Seo, D.C.; Tsang, D.C.W.; Ok, Y.S. Steam activation of biochars facilitates kinetics and pH-resilience of sulfamethazine sorption. J. Soils Sediments 2016, 16, 889–895. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, J.; Jin, C.; Wang, Z.; Wang, T.; Cheng, X.; Ma, C. Effects of temperature, oxygen and steam on pore structure characteristics of coconut husk activated carbon powders prepared by one-step rapid pyrolysis activation process. Bioresour Technol 2020, 310, 123413. [Google Scholar] [CrossRef]
- Muthmann, J.; Bläker, C.; Pasel, C.; Luckas, M.; Schledorn, C.; Bathen, D. Characterization of structural and chemical modifications during the steam activation of activated carbons. Microporous Mesoporous Mater. 2020, 309, 110549. [Google Scholar] [CrossRef]
- Heo, Y.J.; Park, S.J. A role of steam activation on CO2 capture and separation of narrow microporous carbons produced from cellulose fibers. Energy 2015, 91, 142–150. [Google Scholar] [CrossRef]
- Bardestani, R.; Kaliaguine, S. Steam activation and mild air oxidation of vacuum pyrolysis biochar. Biomass Bioenergy 2018, 108, 101–112. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.M.; Dallmeyer, I.; Garcia-Perez, M. Modification of biochar surface by air oxidation: Role of pyrolysis temperature. Biomass Bioenergy 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Xiao, F.; Bedane, A.H.; Mallula, S.; Sasi, P.C.; Alinezhad, A.; Soli, D.; Hagen, Z.M.; Mann, M.D. Production of granular activated carbon by thermal air oxidation of biomass charcoal/biochar for water treatment in rural communities: A mechanistic investigation. Chem. Eng. J. Adv. 2020, 4, 100035. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Soltani, S.M. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Zaker, A.; Hammouda, S.B.; Sun, J.; Wang, X.; Li, X.; Chen, Z. Carbon-based materials for CO2 capture: Their production, modification and performance. J. Environ. Chem. Eng. 2023, 11, 109741. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Z.; Xing, W.; Zhu, T.; Shen, H.; Zhuo, S. N-doped microporous carbons derived from direct carbonization of K+ exchanged meta-aminophenol-formaldehyde resin for superior CO2 sorption. Chem. Commun. 2015, 51, 4591–4594. [Google Scholar] [CrossRef]
- Zhou, Z.Q.J.; Li, Z.; Xing, W.; Shen, H.; Bi, X.; Zhu, T.; Zhuo, S. A New Approach to Tuning Carbon Ultramicropore Size at Sub-Angstrom Level for Maximizing Specific Capacitance and CO2 Uptake. Adv. Funct. Mater. 2016, 26, 7955–7964. [Google Scholar] [CrossRef]
- Itoi, H.; Nishihara, H.; Kogure, T.; Kyotani, T. Three-dimensionally arrayed and mutually connected 1.2-nm nanopores for high-performance electric double layer capacitor. J. Am. Chem. Soc. 2011, 133, 1165–1167. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, N.; Li, J.; Deng, X.; Sha, J.; He, C. Hard-template synthesis of three-dimensional interconnected carbon networks: Rational design, hybridization and energy-related applications. Nano Today 2019, 29, 100796. [Google Scholar] [CrossRef]
- Joseph, S.; Singh, G.; Lee, J.M.; Yu, X.; Breese, M.B.; Ruban, S.M.; Bhargava, S.K.; Yi, J.; Vinu, A. Hierarchical carbon structures from soft drink for multi-functional energy applications of Li-ion battery, Na-ion battery and CO2 capture. Carbon 2023, 210, 118085. [Google Scholar] [CrossRef]
- Vilian, A.E.; Song, J.Y.; Lee, Y.S.; Hwang, S.K.; Kim, H.J.; Jun, Y.S.; Huh, Y.S.; Han, Y.K. Salt-templated three-dimensional porous carbon for electrochemical determination of gallic acid. Biosens. Bioelectron. 2018, 117, 597–604. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, B.; Zhu, L.; Shao, Z. Molten salt synthesis of nitrogen-doped carbon with hierarchical pore structures for use as high-performance electrodes in supercapacitors. Carbon 2015, 93, 48–58. [Google Scholar] [CrossRef]
- Liu, X.; Antonietti, M. Moderating black powder chemistry for the synthesis of doped and highly porous graphene nanoplatelets and their use in electrocatalysis. Adv. Mater. 2013, 25, 6284–6290. [Google Scholar] [CrossRef]
- Liu, X.; Giordano, C.; Antonietti, M. A facile molten-salt route to graphene synthesis. Smal 2014, 10, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Fechler, N.; Fellinger, T.P.; Antonietti, M. ‘salt templating’: A simple and sustainable pathway toward highly porous functional carbons from ionic liquids. Adv. Mater. 2013, 25, 75–79. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, Y.; Sun, X.; Mokaya, R. Preparation and hydrogen storage properties of zeolite-templated carbon materials nanocast via chemical vapor deposition: Effect of the zeolite template and nitrogen doping. J. Phys. Chem. B 2006, 110, 18424–18431. [Google Scholar] [CrossRef] [PubMed]
- Asasian-Kolur, N.; Sharifian, S.; Haddadi, B.; Jordan, C.; Harasek, M. Ordered Porous Carbon Preparation by Hard Templating Approach for Hydrogen Adsorption Application; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Hu, H.; Yang, Q.; Cai, J. From metal-organic frameworks to porous carbon materials: Recent progress and prospects from energy and environmental perspectives. Nanoscale 2020, 12, 4238–4268. [Google Scholar] [CrossRef]
- Xu, X.; Xu, C.; Liu, J.; Jin, R.; Luo, X.; Shu, C.; Chen, H.; Guo, C.; Xu, L.; Si, Y. The synergistic effect of ‘soft-hard template’ to in situ regulate mass transfer and defective sites of doped-carbon nanostructures for catalysis of oxygen reduction. J. Alloys Compd. 2023, 939, 168782. [Google Scholar] [CrossRef]
- Lee, J.; Han, S.; Hyeon, T. Synthesis of new nanoporous carbon materials using nanostructured silica materials as templates. J. Mater. Chem. 2004, 14, 478–486. [Google Scholar] [CrossRef]
- Rehman, A.; Heo, Y.J.; Nazir, G.; Park, S.J. Solvent-free, one-pot synthesis of nitrogen-tailored alkali-activated microporous carbons with an efficient CO2 adsorption. Carbon 2021, 172, 71–82. [Google Scholar] [CrossRef]
- Shi, J.; Xu, J.; Cui, H.; Yan, N.; Zou, J.; Liu, Y.; You, S. Synthesis of highly porous N-doped hollow carbon nanospheres with a combined soft template-chemical activation method for CO2 capture. Ind. Crops Prod. 2023, 280, 115952. [Google Scholar] [CrossRef]
- Chuenchom, L.; Kraehnert, R.; Smarsly, B.M. Recent progress in soft-templating of porous carbon materials. Soft Matter 2012, 8, 10801–10812. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Z.; Chi, J.; Lei, E.; Liu, Y.; Yin, Y.; Yang, Z.; Ma, C.; Li, W.; Luo, S.; et al. Soft-template hydrothermal synthesis of N and B co-doped walnut-shaped porous carbon spheres with hydrophilic surfaces for supercapacitors. Appl. Surf. Sci. 2023, 638, 158016. [Google Scholar] [CrossRef]
- Kang, H.J.; Huh, Y.S.; Im, W.B.; Jun, Y.S. Molecular cooperative assembly-mediated synthesis of ultra-high-performance hard carbon anodes for dual-carbon sodium hybrid capacitors. ACS Nano 2019, 13, 11935–11946. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Hu, H.; Teng, X.L.; Zhu, Y.F.; Zhang, Y.L.; Chao, H.X.; Yang, H.; Wang, X.S.; Wu, M.B. Templating synthesis of porous carbons for energy-related applications: A review. New Carbon Mater. 2022, 37, 25–45. [Google Scholar] [CrossRef]
- Jun, Y.S.; Lee, E.Z.; Wang, X.; Hong, W.H.; Stucky, G.D.; Thomas, A. From melamine-cyanuric acid supramolecular aggregates to carbon nitride hollow spheres. Adv. Funct. Mater. 2013, 23, 3661–3667. [Google Scholar] [CrossRef]
- Nicolae, S.A.; Szilágyi, Á.; Titirici, M.M. Soft templating production of porous carbon adsorbents for CO2 and H2S capture. Carbon 2020, 169, 193–204. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Wang, P.; Wang, Z.; Zuo, C.; Chen, W.; Ao, T. Facile fabrication of N-doped hierarchical porous carbons derived from soft-templated ZIF-8 for enhanced adsorptive removal of tetracycline hydrochloride from water. J. Hazard Mater. 2022, 423, 127103. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, Y.; Zhang, D.; Lin, Z.; Lin, J.; Li, S.; Guo, S. Construction of 3D hierarchical honeycomb macro/meso/micro-porous carbon with soft and hard templates for high-performance sodium-ion batteries. Mater. Lett. 2023, 334, 133737. [Google Scholar] [CrossRef]
- Bin, A.A.; Binti, J.H. Waste Recycling Technologies for Nanomaterials Manufacturing: Manufacturing of Nanoalumina by Recycling of Aluminium Cans Waste Aiman; Springer: Cham, Switzerland, 2021; Available online: https://link.springer.com/book/10.1007/978-3-030-68031-2 (accessed on 8 July 2023).
- Xu, S.; Niu, M.; Zhao, G.; Ming, S.; Li, X.; Zhu, Q.; Ding, L.X.; Kim, M.; Alothman, A.A.; Mushab, M.S.S.; et al. Size control and electronic manipulation of Ru catalyst over B, N co-doped carbon network for high-performance hydrogen evolution reaction. Nano Res. 2023, 16, 6212–6219. [Google Scholar] [CrossRef]
- Jin, X.; Wang, X.; Liu, Y.; Kim, M.; Cao, M.; Xie, H.; Liu, S.; Wang, X.; Huang, W.; Nanjundan, A.K.; et al. Nitrogen and Sulfur Co-Doped Hierarchically Porous Carbon Nanotubes for Fast Potassium Ion Storage. Small 2022, 18, 42. [Google Scholar] [CrossRef]
- Emran, M.Y.; Shenashen, M.A.; El-Safty, S.A.; Selim, M.M. Design of porous S-doped carbon nanostructured electrode sensor for sensitive and selective detection of guanine from DNA samples. Microporous Mesoporous Mater. 2021, 320, 111097. [Google Scholar] [CrossRef]
- Khosropour, H.; Rezaei, B.; Alinajafi, H.A.; Ensafi, A.A. Electrochemical sensor based on glassy carbon electrode modified by polymelamine formaldehyde/graphene oxide nanocomposite for ultrasensitive detection of oxycodone. Microchimica Acta 2021, 188, 1. [Google Scholar] [CrossRef]
- Emran, M.Y.; Shenashen, M.A.; Elmarakbi, A.; Selim, M.M.; El-Safty, S.A. Nitrogen-doped carbon hollow trunk-like structure as a portable electrochemical sensor for noradrenaline detection in neuronal cells. Anal. Chim. Acta 2022, 1192, 339380. [Google Scholar] [CrossRef]
- Wu, D.; Yang, Y.; Liu, J.; Zheng, Y. Plasma-Modified N/O-Doped Porous Carbon for CO2Capture: An Experimental and Theoretical Study. Energy Fuels 2020, 34, 6077–6084. [Google Scholar] [CrossRef]
- Wu, D.; Liu, J.; Yang, Y.; Zheng, Y. Nitrogen/Oxygen Co-Doped Porous Carbon Derived from Biomass for Low-Pressure CO2Capture. Ind. Eng. Chem. Res. 2020, 59, 14055–14063. [Google Scholar] [CrossRef]
- Ashourirad, B.; Arab, P.; Islamoglu, T.; Cychosz, K.A.; Thommes, M.; El-Kaderi, H.M. A cost-effective synthesis of heteroatom-doped porous carbons as efficient CO2 sorbents. J. Mater. Chem. A Mater. 2016, 4, 14693–14702. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Klepel, O.; Hunger, B. Temperature-programmed desorption (TPD) of carbon dioxide on alkali-metal cation-exchanged faujasite type zeolites. J. Therm. Anal. Calorim. 2005, 80, 201–206. [Google Scholar] [CrossRef]
- Jagiello, J.; Chojnacka, A.; Pourhosseini, S.E.M.; Wang, Z.; Beguin, F. A dual shape pore model to analyze the gas adsorption data of hierarchical micro-mesoporous carbons. Carbon 2021, 178, 113–124. [Google Scholar] [CrossRef]
- Hotová, G.; Slovák, V.; Soares, O.S.G.P.; Figueiredo, J.L.; Pereira, M.F.R. Oxygen surface groups analysis of carbonaceous samples pyrolysed at low temperature. Carbon 2018, 134, 255–263. [Google Scholar] [CrossRef]
- Wu, R.; Ye, Q.; Wu, K.; Wang, L.; Dai, H. Highly efficient CO2 adsorption of corn kernel-derived porous carbon with abundant oxygen functional groups. J. CO2 Util. 2021, 51, 101620. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Y.; Zhang, T.C.; Ouyang, L.; Yuan, S. Phytic acid-induced self-assembled chitosan gel-derived N, P—Co-doped porous carbon for high-performance CO 2 capture and supercapacitor. J. Power Sources 2022, 517, 230727. [Google Scholar] [CrossRef]
- Maklavany, D.M.; Rouzitalab, Z.; Amini, A.M.; Askarieh, M.; Silvestrelli, P.L.; Seif, A.; Orooji, Y.; Rashidi, A. One-step approach to Quaternary (B, N, P, S)-Doped hierarchical porous carbon derived from Quercus Brantii for highly selective and efficient CO2 Capture: A combined experimental and extensive DFT study. Chem. Eng. J. 2023, 453, 139950. [Google Scholar] [CrossRef]
- Ma, X.; Xu, W.; Su, R.; Shao, L.; Zeng, Z.; Li, L.; Wang, H. Insights into CO2 capture in porous carbons from machine learning, experiments and molecular simulation. Sep. Purif. Technol. 2023, 306, 122521. [Google Scholar] [CrossRef]
- Khodabakhshi, S.; Kiani, S.; Niu, Y.; White, A.O.; Suwaileh, W.; Palmer, R.E.; Barron, A.R.; Andreoli, E. Facile and environmentally friendly synthesis of ultramicroporous carbon spheres: A significant improvement in CVD method. Carbon 2021, 171, 426–436. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Bao, A. Design of hierarchically structured porous boron/nitrogen codoped carbon materials with excellent performance for CO2capture. Ind. Eng. Chem. Res. 2021, 60, 2710–2718. [Google Scholar] [CrossRef]
- Song, C.; Ye, W.; Liu, Y.; Huang, H.; Zhang, H.; Lin, H.; Lu, R.; Zhang, S. Facile preparation of porous carbon derived from industrial biomass waste as an efficient CO2adsorbent. ACS Omega 2020, 5, 28255–28263. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.F.; Zhong, J.J.; Qian, X.; Song, S.L.; Zhang, Y.G.; Li, D.H. Nitrogen-enriched porous polyacrylonitrile-based carbon fibers for CO2 Capture. Ind. Eng. Chem. Res. 2018, 57, 11608–11616. [Google Scholar] [CrossRef]
- Song, C.; Liu, M.; Ye, W.; Liu, Y.; Zhang, H.; Lu, R.; Zhang, S. Nitrogen-Containing Porous Carbon for Highly Selective and Efficient CO2 Capture. Energy Fuels 2019, 33, 12601–12609. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, X.F.; Li, L.; Jin, L.; Zhang, Y.G.; Song, S.L.; Liu, R.P. Nitrogen-Enriched Porous Carbon Fiber as a CO2 Adsorbent with Superior CO2 Selectivity by Air Activation. Energy Fuels 2019, 33, 12558–12567. [Google Scholar] [CrossRef]
- Mahurin, S.M.; Fulvio, F.; Hillesheim, C.; Nelson, K.M.; Veith, G.M.; Dai, S. Directed synthesis of nanoporous carbons from task-specific ionic liquid precursors for the adsorption of CO2. ChemSusChem 2014, 7, 3284–3289. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Xiong, L.; Zhong, J.J.; Jin, L.; Yan, J.L.; Mu, B.; Zhang, Y.G.; Song, S.L. Nitrogen-Containing Porous Carbon Fibers Prepared from Polyimide Fibers for CO2Capture. Ind. Eng. Chem. Res. 2020, 59, 18106–18114. [Google Scholar] [CrossRef]
- Pevida, C.; Drage, T.C.; Snape, C.E. Silica-templated melamine-formaldehyde resin derived adsorbents for CO2 capture. Carbon 2008, 46, 1464–1474. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Zheng, M.; Zhao, H.; Zhao, Y.; Sun, Z. Hierarchically Structured Porous Nitrogen-Doped Carbon for Highly Selective CO2 Capture. ACS Sustain. Chem. Eng. 2016, 4, 298–304. [Google Scholar] [CrossRef]
- Estevez, L.; Barpaga, D.; Zheng, J.; Sabale, S.; Patel, R.L.; Zhang, J.G.; McGrail, B.P.; Motkuri, R.K. Hierarchically Porous Carbon Materials for CO2 Capture: The Role of Pore Structure. Ind. Eng. Chem. Res. 2018, 57, 1262–1268. [Google Scholar] [CrossRef]
- Hong, L.; Ju, S.; Liu, X.; Zhuang, Q.; Zhan, G.; Yu, X. Highly Selective CO2 Uptake in Novel Fishnet-like Polybenzoxazine-Based Porous Carbon. Energy Fuels 2019, 33, 11454–11464. [Google Scholar] [CrossRef]
- Wahab, M.A.; Na, J.; Masud, M.K.; Hossain, M.S.A.; Alothman, A.A.; Abdala, A. Nanoporous carbon nitride with a high content of inbuilt N site for the CO2 capture. J. Hazard Mater. 2021, 408, 124843. [Google Scholar] [CrossRef]
- Zeeshan, M.; Yalcin, K.; Oztuna, F.E.S.; Unal, U.; Keskin, S.; Uzun, A. A new class of porous materials for efficient CO2 separation: Ionic liquid/graphene aerogel composites. Carbon 2021, 171, 79–87. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, C.; Li, Z.; Li, X.; Wang, H.; Feng, N.; Wan, H.; Guan, G. Controllable construction of N-enriched hierarchically porous carbon nanosheets with enhanced performance for CO2 capture. Chem. Eng. J. 2019, 371, 414–423. [Google Scholar] [CrossRef]
- Srinivas, G.; Krungleviciute, V.; Guo, Z.X.; Yildirim, T. Exceptional CO2 capture in a hierarchically porous carbon with simultaneous high surface area and pore volume. Energy Environ. Sci. 2014, 7, 335–342. [Google Scholar] [CrossRef]
- Kumbhar, D.; Palliyarayil, A.; Reghu, D.; Shrungar, D.; Umapathy, S.; Sil, S. Rapid discrimination of porous bio-carbon derived from nitrogen rich biomass using Raman spectroscopy and artificial intelligence methods. Carbon 2021, 178, 792–802. [Google Scholar] [CrossRef]
- Ma, C.; Bai, J.; Hu, X.; Jiang, Z.; Wang, L. Nitrogen-doped porous carbons from polyacrylonitrile fiber as effective CO2 adsorbents. J. Environ. Sci. 2023, 125, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Alloush, A.M.; Abdulghani, H.; Amasha, H.A.; Saleh, T.A.; Al Hamouz, O.C.S. Microwave-assisted synthesis of novel porous organic polymers for effective selective capture of CO2. J. Ind. Eng. Chem. 2022, 113, 215–225. [Google Scholar] [CrossRef]
- Gou, J.; Liu, C.; Lin, J.; Yu, C.; Fang, Y.; Liu, Z.; Guo, Z.; Tang, C.; Huang, Y. Densification and pelletization of porous boron nitride fibers for effective CO2 adsorption. Ceram. Int. 2022, 48, 11636–11643. [Google Scholar] [CrossRef]
- Dhoke, C.; Zaabout, A.; Cloete, S.; Amini, S. Review on reactor configurations for adsorption-based CO2 capture. Ind. Eng. Chem. Res. 2021, 60, 3779–3798. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Z.; Zeng, L.; Wang, F. Pressure drop in honeycomb adsorption filters filled with granular activated carbon. Powder Technol. 2021, 393, 550–558. [Google Scholar] [CrossRef]
- Rezaei, F.; Grahn, M. Thermal management of structured adsorbents in CO 2 capture processes. Ind. Eng. Chem. Res. 2012, 51, 4025–4034. [Google Scholar] [CrossRef]
- Rezaei, F.; Webley, P. Structured adsorbents in gas separation processes. Sep. Purif. Technol. 2010, 70, 243–256. [Google Scholar] [CrossRef]
- Rezaei, F.; Webley, P. Optimum structured adsorbents for gas separation processes. Chem. Eng. Sci. 2009, 64, 5182–5191. [Google Scholar] [CrossRef]
- Akhtar, F.; Andersson, L.; Ogunwumi, S.; Hedin, N.; Bergström, L. Structuring adsorbents and catalysts by processing of porous powders. J. Eur. Ceram. Soc. 2014, 34, 1643–1666. [Google Scholar] [CrossRef]
- Borchardt, L.; Michels, N.L.; Nowak, T.; Mitchell, S.; Pérez-Ramírez, J. Structuring zeolite bodies for enhanced heat-transfer properties. Microporous Mesoporous Mater. 2015, 208, 196–202. [Google Scholar] [CrossRef]
- Tang, S.H.; Zaini, M.A.A. Development of activated carbon pellets using a facile low-cost binder for effective malachite green dye removal. J. Clean. Prod. 2020, 253, 119970. [Google Scholar] [CrossRef]
- Cousin-Saint-Remi, J.; Van der Perre, S.; Segato, T.; Delplancke, M.P.; Goderis, S.; Terryn, H.; Baron, G.; Denayer, J. Highly Robust MOF Polymeric Beads with a Controllable Size for Molecular Separations. ACS Appl Mater Interfaces 2019, 11, 13694–13703. [Google Scholar] [CrossRef]
- Valizadeh, B.; Nguyen, T.N.; Smit, B.; Stylianou, K.C. Porous Metal–Organic Framework@Polymer Beads for Iodine Capture and Recovery Using a Gas-Sparged Column. Adv. Funct. Mater. 2018, 28, 1801596. [Google Scholar] [CrossRef]
- de Almeida Moreira, B.R.; Cruz, V.H.; Pérez, J.F.; da Silva Viana, R. Production of pellets for combustion and physisorption of CO2 from hydrothermal carbonization of food waste—Part I: High-performance solid biofuels. J. Clean. Prod. 2021, 319, 128695. [Google Scholar] [CrossRef]
- Kim, Y.J.S. Adsorption of Carbon Dioxide using Pelletized AC with Amine impregnation. J. Korean Oil Chem. Soc. 2013, 30, 88–89. [Google Scholar] [CrossRef]
- Manohara, G.V.; Maroto-Valer, M.M.; Garcia, S. Binder free novel synthesis of structured hybrid mixed metal oxides (MMOs) for high temperature CO2 capture. Chem. Eng. J. 2021, 415, 128881. [Google Scholar] [CrossRef]

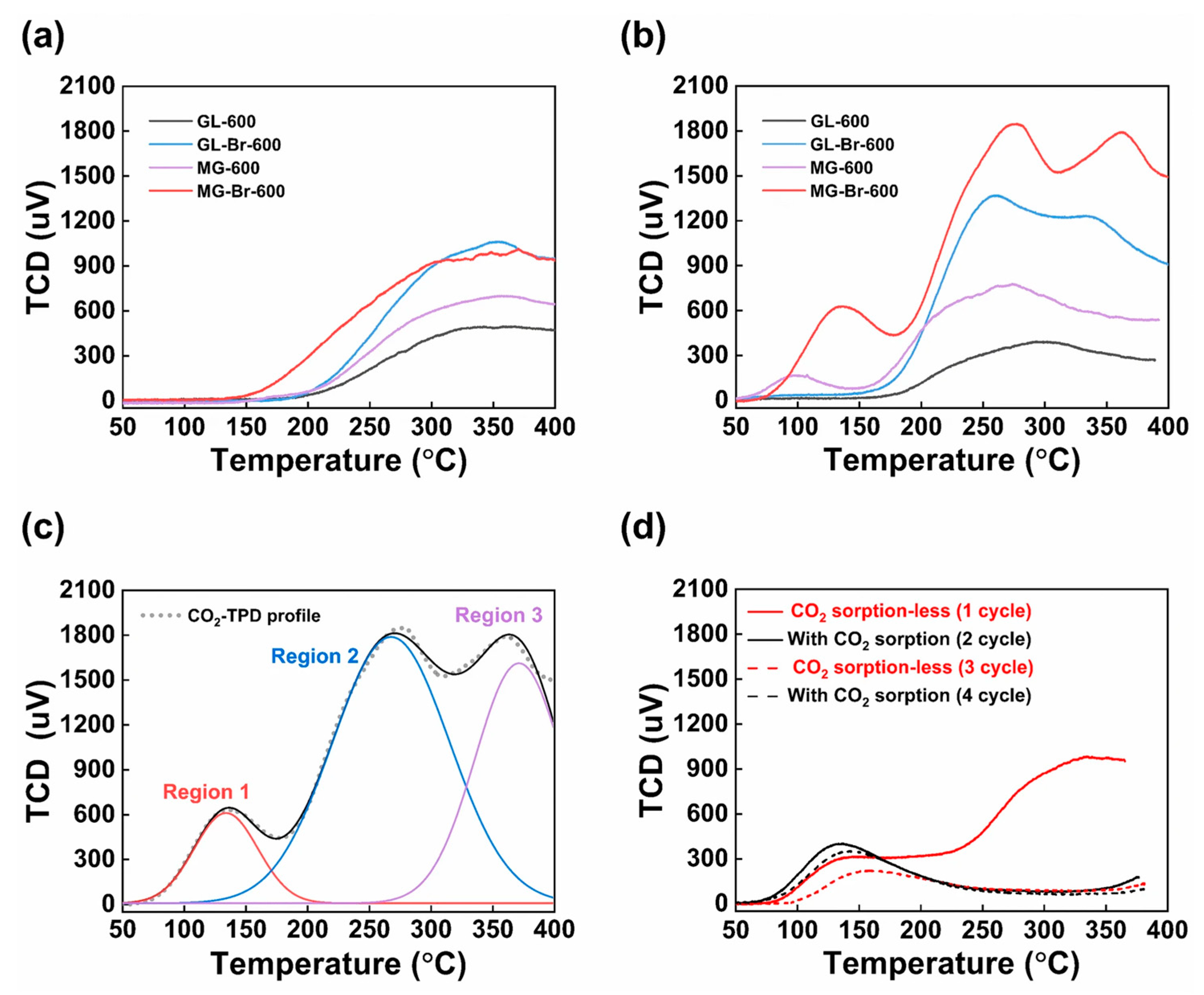
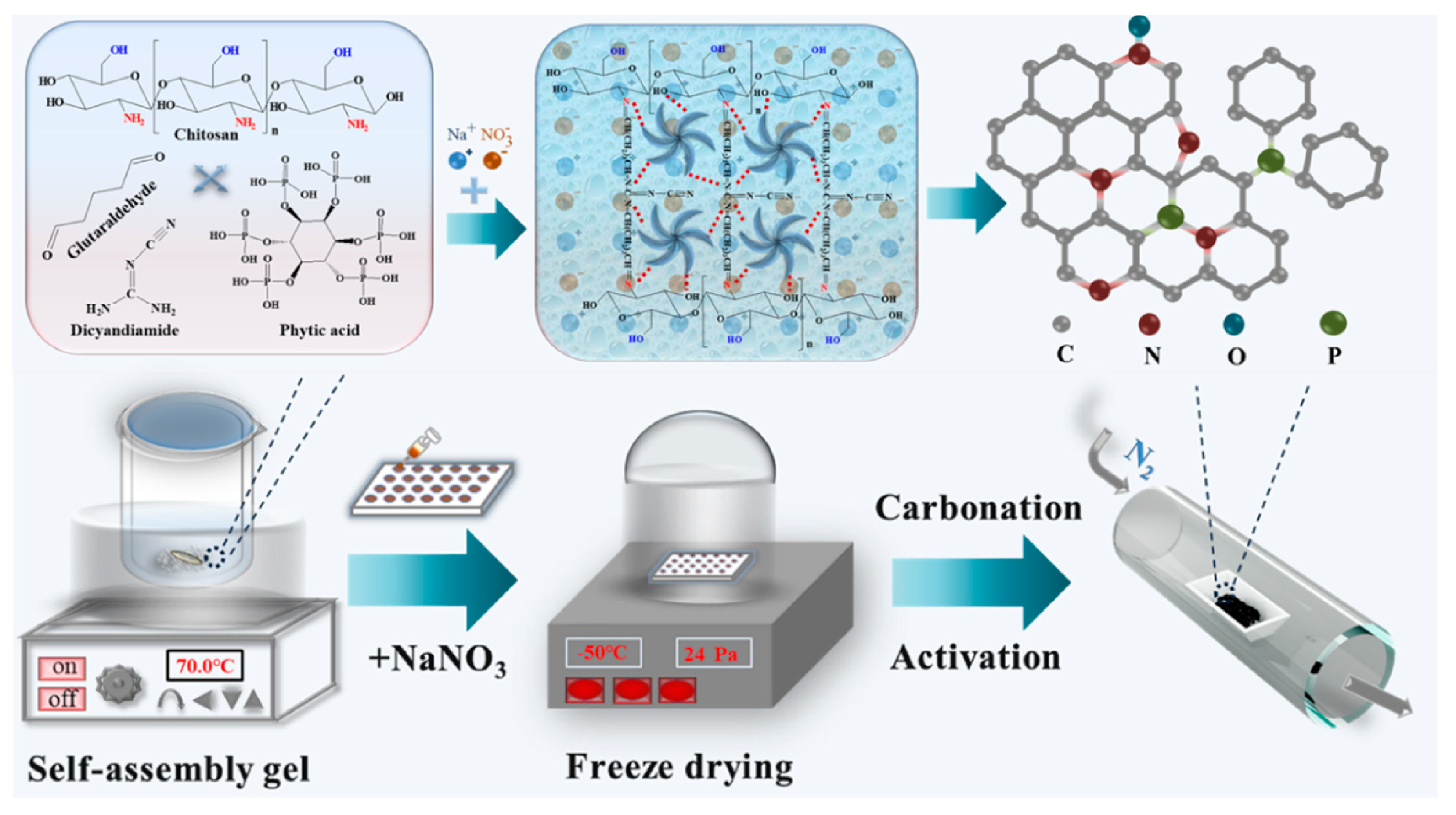
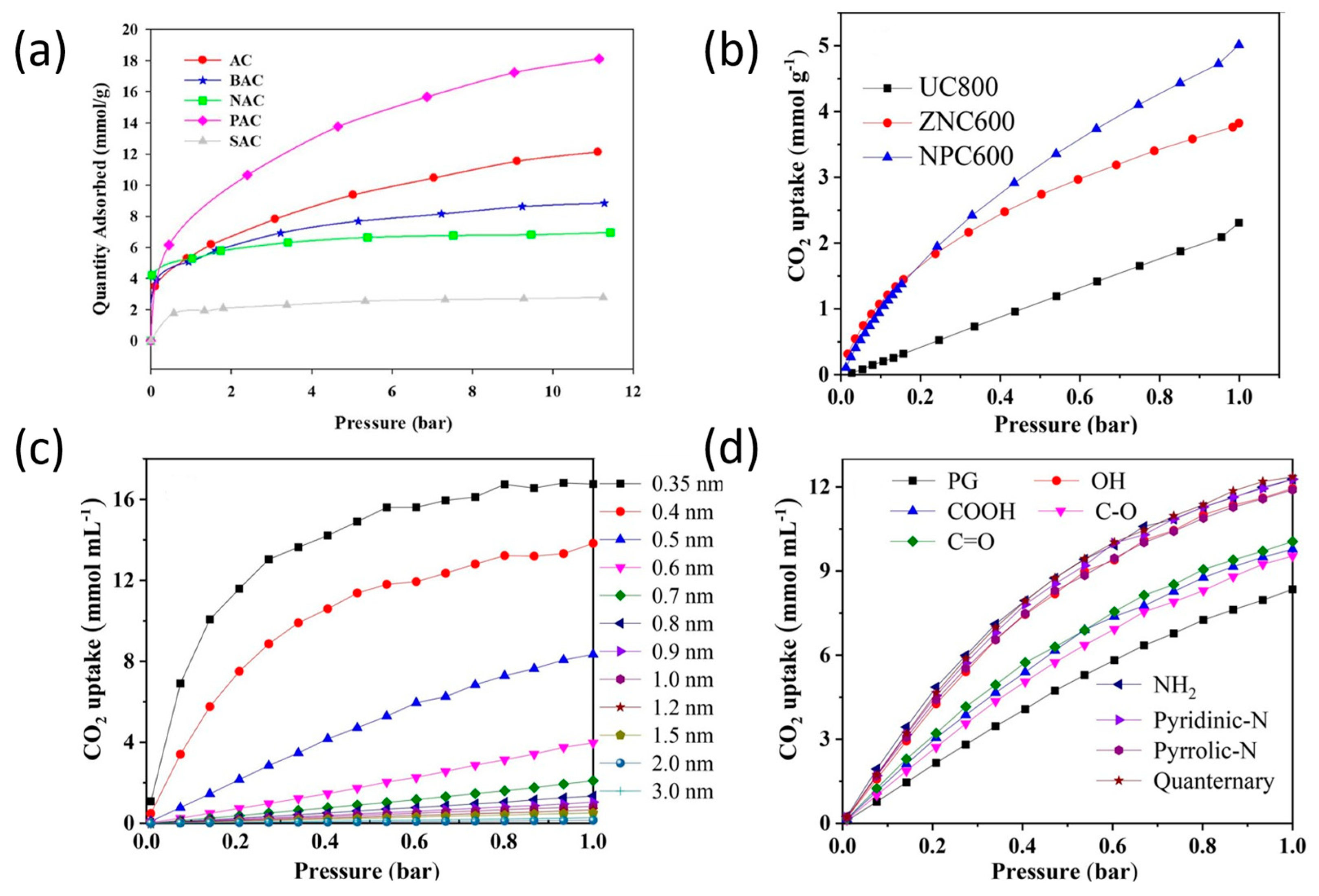
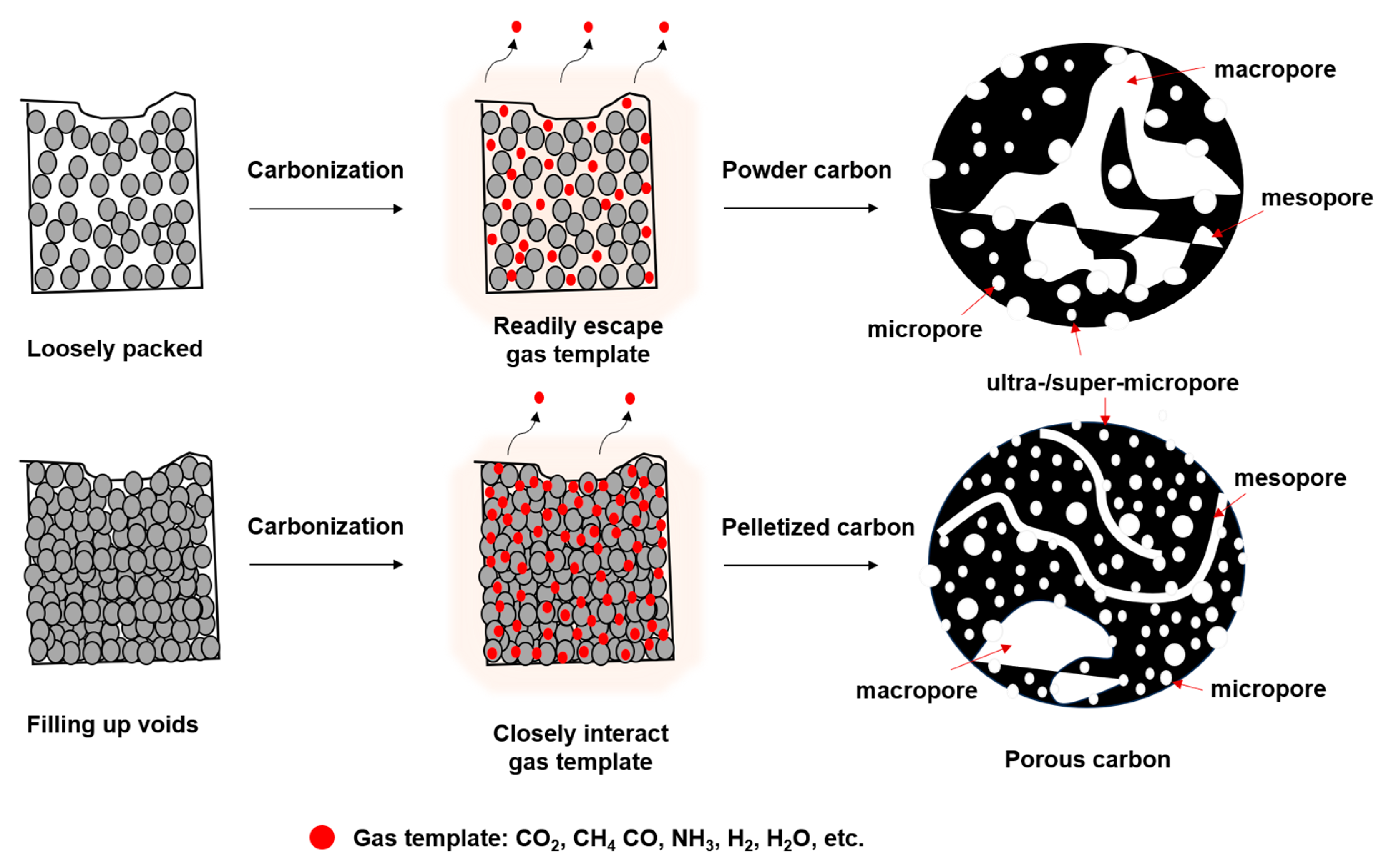
| Activation Process | Carbon Materials | Uptake (1 bar) [mmol g−1] | Kinetics/Heat of Adsorption (Qst) [kJ mol−1] | Selectivity CO2/N2 (15:85) | Ref. |
|---|---|---|---|---|---|
| MS/organic template | N-/O-rich multi-hierarchical porous C | 3.8 (0 °C), 2.9 (25 °C) | Kinetics (Initial kpi, 0.2 mmol g−1 min−0.5) 26 | 43 (25 °C), 31 (0 °C) | [1] |
| Activation free | C (spherical) | 2.9 (25 °C), 4 (0 °C) | 27.5–29.3 | CO2/N2 (15/85) 30 (25 °C) | [128] |
| Template free | B-/N-co-doped C | 2.1 (30 °C) | 28 | – | [129] |
| Chemical activation | C from industrial biomass | 4.2 (25 °C), 6.6 (0 °C), 1.3 (0.15 bar) | 34–18 | 27 | [130] |
| Chemical activation | Defluorinated porous C | 5.0 (25 °C), 8.8 (0 °C) | 27.3 (zero coverage) | 23 (25 °C), 22 (0 °C) | [42] |
| Template free | N-containing C (PAN) | 2.4 (25 °C) | – | Simulated flue gas CO2/N2 (10/90) 0.8 mmol g−1 (25 °C) | [131] |
| Chemical activation | N-containing porous C | 3.7 (25 °C), 6.2 (0 °C), 3.2 (0 °C) | 15–36 | 18 kJ mol–1 (25 °C) 129 kJ mol–1 (25 °C) | [132] |
| Air activation | N-containing porous C fiber | 2.2 (25 °C) | 26.6–30.8 | 183 | [133] |
| Template free | Nanoporous C | 2.7 (25 °C), 4.0 (0 °C) | 31.5 | 30 | [134] |
| Chemical activation | N-containing porous C fiber | 5.0 (25 °C), 1.5 (0.15 bar) | 24.6–25.5 | 24 | [135] |
| Silica template | Porous C | 2.2 (25 °C) | 31.8 | – | [136] |
| Chemical activation | Porous N-containing C | 2.7 (25 °C), 3.8 (25 °C) | ~36 | CO2/N2 (10/90) 134 | [137] |
| Freeze drying/plasma treatment | N-/O-containing porous C | 0.9 (30 °C) | – | 15 | [118] |
| Ice/silica/CO2 templating | N-/O-containing porous C | 3.7 (25 °C) | – | – | [138] |
| Chemical activation | Polybenzooxazine-based porous C | 8.4 (25 °C) | 32–35 | CO2/N2 (10/90) 25 (25 °C), 34 (0 °C) | [139] |
| Hard template | N-containing carbon nitride | 2.0 (25 °C), 2.5 (0 °C) | – | - | [140] |
| Template free | Ionic liquid/graphene aerogel | 0.2 (0 °C) | – | CO2/CH4 (ideal selectivity) 120 (25 °C), 1 mbar | [141] |
| Metal ion activation | N-containing porous C nanosheet | 2.5 (25 °C) | – | 17.5 (25 °C) | [142] |
| Hard template | Porous carbon from MOF | ~2.5 (27 °C) | – | – | [143] |
| Salt template | N-containing C | 3.3 (25 °C) | –– | - | [144] |
| Template free | N-containing C monolith | 3.3 (25 °C), 5 (0 °C) | ~20 | CO2/N2 (14/86) 16 min breakthrough time (25 °C) | [26] |
| Chemical activation | N-containing porous C | 4.0 (25 °C), 6.0 (0 °C) | ~25 | CO2/N2 (10/90) 19 (25 °C) | [145] |
| Hard template | N-based porous polymer | 1.0 (25 °C), 1.6 (0 °C) | – | 63 (25 °C, 1 bar) | [146] |
| Carbon Structure Modification | Advantages | Disadvantages |
|---|---|---|
| Chemical activation | Provides ultra pores, micropores, lower activation temperature | Extra steps of washing to remove corrosive chemicals |
| Physical activation | Provides micro-/meso-/macropores, readily utilized on carbonization | Cannot provide shaped carbon, higher activation temperature |
| Metal ion activation | Ultra-to-super-micropores | Limited carbon source, extra process of washing to remove metal |
| Hard template | Mechanical and structural stability, micro-/mesopores | Extra process to remove template, etching, time-consuming, can damage carbon structure due to vigorous acid or base treatment to remove template |
| Soft template | Self-removal with carbonization, provides multi-shaped carbon | Only meso-/macroporous carbon synthesis, template needs to remain stable during thermal process, template preparation process requires time, meso-/macropores only |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bari, G.A.K.M.R.; Jeong, J.-H. Porous Carbon for CO2 Capture Technology: Unveiling Fundamentals and Innovations. Surfaces 2023, 6, 316-340. https://doi.org/10.3390/surfaces6030023
Bari GAKMR, Jeong J-H. Porous Carbon for CO2 Capture Technology: Unveiling Fundamentals and Innovations. Surfaces. 2023; 6(3):316-340. https://doi.org/10.3390/surfaces6030023
Chicago/Turabian StyleBari, Gazi A. K. M. Rafiqul, and Jae-Ho Jeong. 2023. "Porous Carbon for CO2 Capture Technology: Unveiling Fundamentals and Innovations" Surfaces 6, no. 3: 316-340. https://doi.org/10.3390/surfaces6030023
APA StyleBari, G. A. K. M. R., & Jeong, J.-H. (2023). Porous Carbon for CO2 Capture Technology: Unveiling Fundamentals and Innovations. Surfaces, 6(3), 316-340. https://doi.org/10.3390/surfaces6030023







