The Antibacterial Performance of Implant Coating Made of Vancomycin-Loaded Polymer Material: An In Vitro Study
Abstract
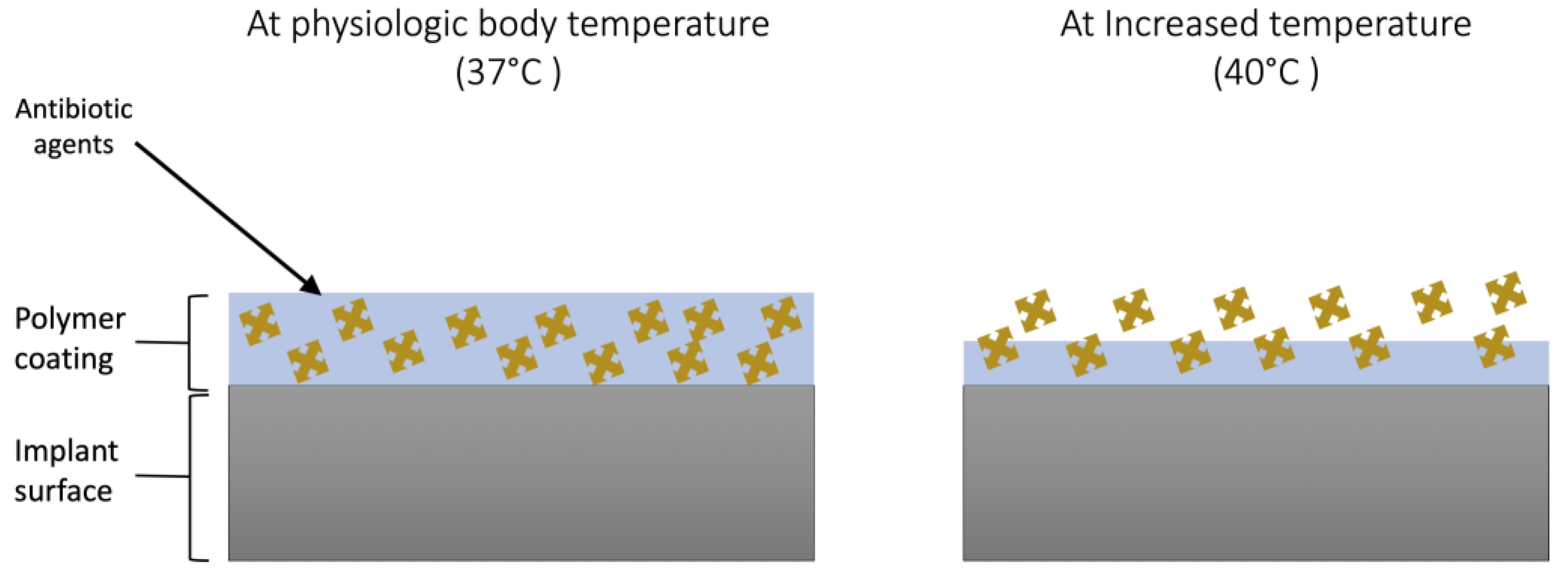
:1. Introduction
2. Materials and Methods
2.1. Polymer Synthesis
2.2. Polymer Coating
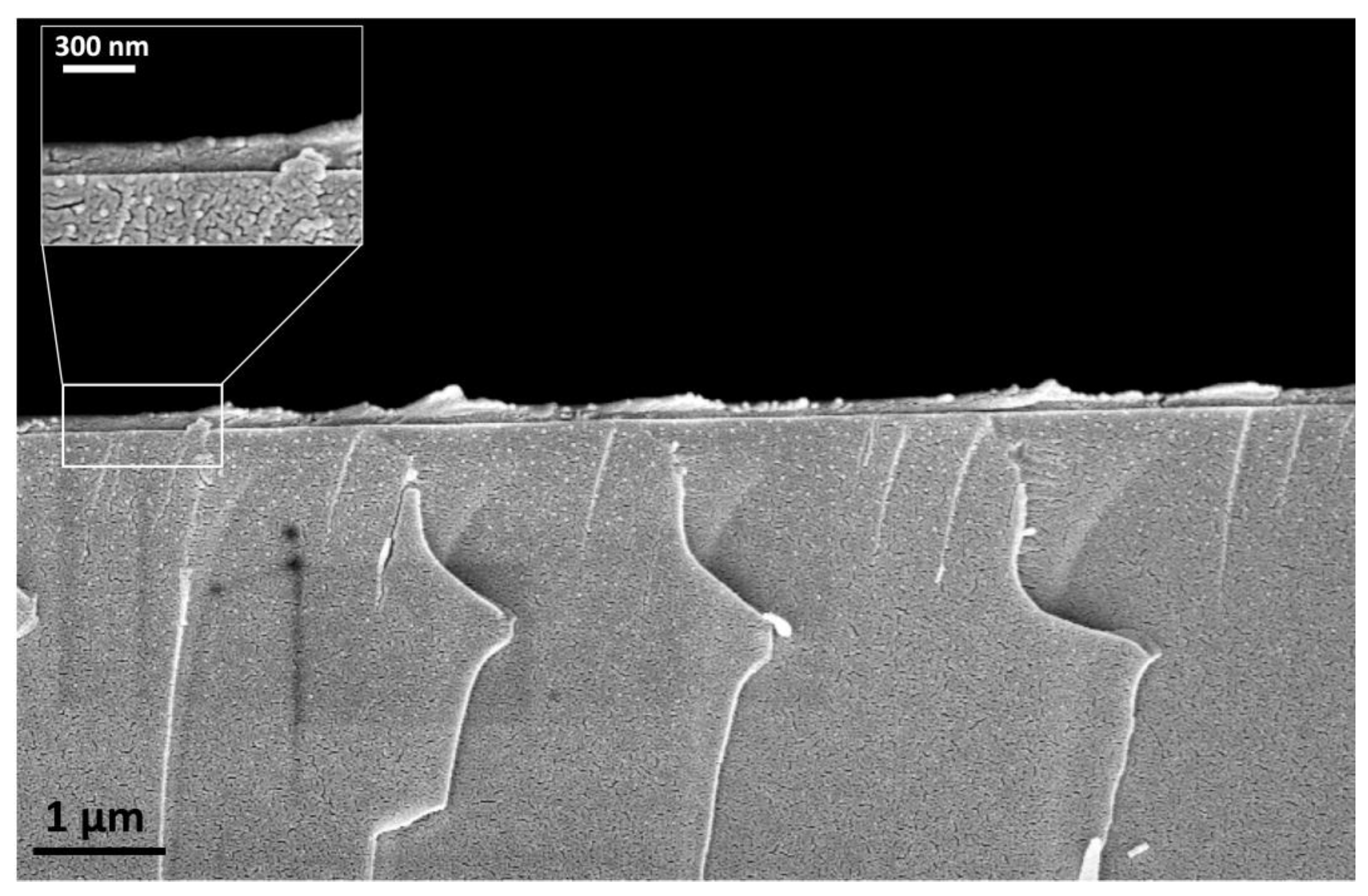
2.3. Scanning Electron Microscopy (SEM)
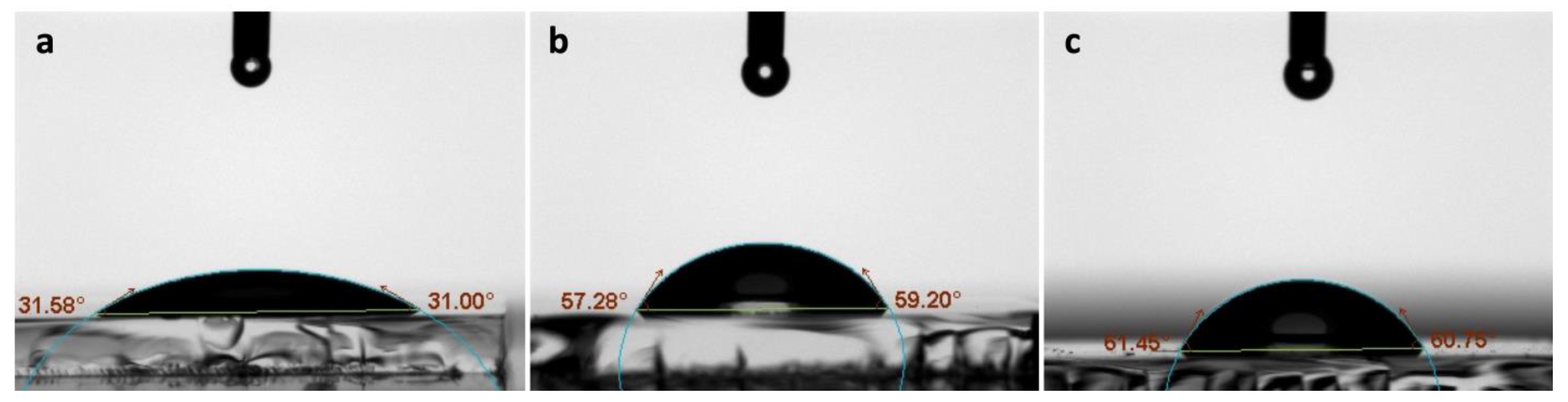
2.4. CA Measurement
2.5. Loading of Vancomycin on the Coated Layers
2.6. Bacterial Live/Dead Analysis
2.7. Analysis
3. Results
3.1. SEM
3.2. CA Measurements
3.3. Live/Dead Bacterial Analysis
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neoh, K.G.; Hu, X.; Zheng, D.; Kang, E.T. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials 2012, 33, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Al-Radha, A.S.D.; Dymock, D.; Younes, C.; O’Sullivan, D. Surface properties of titanium and zirconia dental implant materials and their effect on bacterial adhesion. J. Dent. 2012, 40, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral. Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef]
- Jahoda, D.; Nyc, O.; Pokorný, D.; Landor, I.; Sosna, A. Antibiotic treatment for prevention of infectious complications in joint replacement. Acta Chir. Orthop. Traumatol. Cech. 2006, 73, 108–114. [Google Scholar] [CrossRef]
- Zimmerli, W.; Moser, C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol. Med. Microbiol. 2012, 65, 158–168. [Google Scholar] [CrossRef]
- Niu, S.; Cao, X.; Zhang, Y.; Zhu, Q.; Zhu, J.; Zhen, P. Peri-implant and systemic effects of high-/low-affinity bisphosphonate-hydroxyapatite composite coatings in a rabbit model with peri-implant high bone turnover. BMC Musculoskelet. Disord. 2012, 13, 97. [Google Scholar] [CrossRef]
- Norton, L.W.; Koschwanez, H.E.; Wisniewski, N.A.; Klitzman, B.; Reichert, W.M. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J. Biomed. Mater. Res. Part A 2007, 81, 858–869. [Google Scholar] [CrossRef]
- Decker, J.F.; Lee, J.; Cortella, C.A.; Polimeni, G.; Rohrer, M.D.; Wozney, J.M.; Hall, J.; Susin, C.; Wikesjo, U.M. Evaluation of implants coated with recombinant human bone morphogenetic protein-2 and vacuum-dried using the critical-size supraalveolar peri-implant defect model in dogs. J. Periodontol. 2010, 81, 1839–1849. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhu, S.; Luo, E.; Li, J.; Feng, G.; Liao, Y.; Hu, J. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials 2010, 31, 9006–9014. [Google Scholar] [CrossRef]
- Offermanns, V.; Andersen, O.Z.; Falkensammer, G.; Andersen, I.H.; Almtoft, K.P.; Sorensen, S.; Sillassen, M.; Jeppesen, C.S.; Rasse, M.; Foss, M.; et al. Enhanced osseointegration of endosseous implants by predictable sustained release properties of strontium. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.S.; Antoci, V., Jr.; Harrison, G.; Patal, P.; Freeman, T.A.; Shapiro, I.M.; Parvizi, J.; Hickok, N.J.; Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J. Orthop. Res. 2009, 27, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef]
- Li, L.L.; Wang, L.M.; Xu, Y.; Lv, L.X. Preparation of gentamicin-loaded electrospun coating on titanium implants and a study of their properties in vitro. Arch. Orthop. Trauma Surg. 2012, 132, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Aberoumandi, S.M.; Khalilov, R.; Davaran, S.; Nasibova, A.; Abbasi, E.; Saghfi, S.; Akbarzadeh, A. An update on clinical applications of nanoparticles in brain and retinal disease (CNS). Adv. Biol. Earth Sci. 2017, 2, 125–142. [Google Scholar]
- Nasibova, A. Generation of nanoparticles in biological systems and their application prospects. Adv. Biol. Earth Sci. 2023, 8, 140–146. [Google Scholar]
- Hu, Y.; Cai, K.; Luo, Z.; Xu, D.; Xie, D.; Huang, Y.; Yang, W.; Liu, P. TiO2 nanotubes as drug nanoreservoirs for the regulation of mobility and differentiation of mesenchymal stem cells. Acta Biomater. 2012, 8, 439–448. [Google Scholar] [CrossRef]
- Kung, S.; Devlin, H.; Fu, E.; Ho, K.Y.; Liang, S.Y.; Hsieh, Y.D. The osteoinductive effect of chitosan-collagen composites around pure titanium implant surfaces in rats. J. Periodontal Res. 2011, 46, 126–133. [Google Scholar] [CrossRef]
- Gulati, K.; Ramakrishnan, S.; Aw, M.S.; Atkins, G.J.; Findlay, D.M.; Losic, D. Biocompatible polymer coating of titania nanotube arrays for improved drug elution and osteoblast adhesion. Acta Biomater. 2012, 8, 449–456. [Google Scholar] [CrossRef]
- Chua, P.-H.; Neoh, K.-G.; Kang, E.-T.; Wang, W. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials 2008, 29, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Jou, C.-H.; Yuan, L.; Lin, S.-M.; Hwang, M.-C.; Chou, W.-L.; Yu, D.-G.; Yang, M.-C. Biocompatibility and antibacterial activity of chitosan and hyaluronic acid immobilized polyester fibers. J. Appl. Polym. Sci. 2007, 104, 220–225. [Google Scholar] [CrossRef]
- Kavanagh, C.A.; Rochev, Y.A.; Gallagher, W.M.; Dawson, K.A.; Keenan, A.K. Local drug delivery in restenosis injury: Thermoresponsive co-polymers as potential drug delivery systems. Pharmacol. Ther. 2004, 102, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215. [Google Scholar] [CrossRef]
- Ramanan, R.M.; Chellamuthu, P.; Tang, L.; Nguyen, K.T. Development of a temperature-sensitive composite hydrogel for drug delivery applications. Biotechnol. Prog. 2006, 22, 118–125. [Google Scholar] [CrossRef]
- Pişskin, E.; Dinçer, S.; Türk, M. Gene delivery: Intelligent but just at the beginning. J. Biomater. Sci. Polym. Ed. 2004, 15, 1181–1202. [Google Scholar] [CrossRef]
- Stile, R.A.; Burghardt, W.R.; Healy, K.E. Synthesis and Characterization of Injectable Poly(N-isopropylacrylamide)-Based Hydrogels That Support Tissue Formation in Vitro. Macromolecules 1999, 32, 7370–7379. [Google Scholar] [CrossRef]
- Shahi, S.; Özcan, M.; Maleki Dizaj, S.; Sharifi, S.; Al-Haj Husain, N.; Eftekhari, A.; Ahmadian, E. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol. Mech. Methods 2019, 29, 368–377. [Google Scholar] [CrossRef]
- Schwarz, F.; Wieland, M.; Schwartz, Z.; Zhao, G.; Rupp, F.; Geis-Gerstorfer, J.; Schedle, A.; Broggini, N.; Bornstein, M.M.; Buser, D.; et al. Potential of chemically modified hydrophilic surface characteristics to support tissue integration of titanium dental implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 544–557. [Google Scholar] [CrossRef]
- Tugulu, S.; Löwe, K.; Scharnweber, D.; Schlottig, F. Preparation of superhydrophilic microrough titanium implant surfaces by alkali treatment. J. Mater. Sci. Mater. Med. 2010, 21, 2751–2763. [Google Scholar] [CrossRef]
- Ventre, M.; Causa, F.; Netti, P.A. Determinants of cell-material crosstalk at the interface: Towards engineering of cell instructive materials. J. R. Soc. Interface 2012, 9, 2017–2032. [Google Scholar] [CrossRef] [PubMed]
- García, A.J. Interfaces to Control Cell-Biomaterial Adhesive Interactions. In Polymers for Regenerative Medicine; Werner, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 171–190. [Google Scholar] [CrossRef]
- Xu, L.-C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [PubMed]
- Milleret, V.; Tugulu, S.; Schlottig, F.; Hall, H. Alkali treatment of microrough titanium surfaces affects macrophage/monocyte adhesion, platelet activation and architecture of blood clot formation. Eur. Cell Mater. 2011, 21, 430–444; discussion 444. [Google Scholar] [CrossRef]
- Wall, I.; Donos, N.; Carlqvist, K.; Jones, F.; Brett, P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone 2009, 45, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, A.; Omar, O.M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titanium oral implants: Surface characteristics, interface biology and clinical outcome. J. R. Soc. Interface 2010, 7 (Suppl. S5), S515–S527. [Google Scholar] [CrossRef] [PubMed]
- Wiącek, A.E.; Terpiłowski, K.; Jurak, M.; Worzakowska, M. Low-temperature air plasma modification of chitosan-coated PEEK biomaterials. Polym. Test. 2016, 50, 325–334. [Google Scholar] [CrossRef]
- Wiącek, A.E.; Terpiłowski, K.; Jurak, M.; Worzakowska, M. Effect of low-temperature plasma on chitosan-coated PEEK polymer characteristics. Eur. Polym. J. 2016, 78, 1–13. [Google Scholar] [CrossRef]
- Terpiłowski, K.; Wiącek, A.E.; Jurak, M. Influence of nitrogen plasma treatment on the wettability of polyetheretherketone and deposited chitosan layers. Adv. Polym. Technol. 2018, 37, 1557–1569. [Google Scholar] [CrossRef]
- Alenezi, A.; Hulander, M.; Atefyekta, S.; Andersson, M. Development of a photon induced drug-delivery implant coating. Mater. Sci. Eng. C 2019, 98, 619–627. [Google Scholar] [CrossRef]
- Chen, S.; Darby, I. Dental implants: Maintenance, care and treatment of peri-implant infection. Aust. Dent. J. 2003, 48, 212–220; quiz 263. [Google Scholar] [CrossRef]
- Ahmadov, I.; Bandaliyeva, A.; Nasibova, A.; Hasanova, F.; Khalilov, R. The synthesis of the silver nanodrugs in the medicinal plant baikal skullcap (scutellaria baicalensis georgi) and their antioxidant, antibacterial activity. Adv. Biol. Earth Sci. 2020, 5, 103–118. [Google Scholar]
- Ren, Z.; Wang, Y.; Ma, S.; Duan, S.; Yang, X.; Gao, P.; Zhang, X.; Cai, Q. Effective bone regeneration using thermosensitive poly (N-isopropylacrylamide) grafted gelatin as injectable carrier for bone mesenchymal stem cells. ACS Appl. Mater. Interfaces 2015, 7, 19006–19015. [Google Scholar] [CrossRef] [PubMed]
- Meenach, S.A.; Anderson, A.A.; Suthar, M.; Anderson, K.W.; Hilt, J.Z. Biocompatibility analysis of magnetic hydrogel nanocomposites based on poly (N-isopropylacrylamide) and iron oxide. J. Biomed. Mater. Res. Part. A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 91, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Kurt, S.; Thor, A. A hydrophilic dental implant surface exhibits thrombogenic properties in vitro. Clin. Implant. Dent. Relat. Res. 2013, 15, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Hamlet, S.; Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Bosshardt, D.D.; Ivanovski, S. Gene expression profile of osseointegration of a hydrophilic compared with a hydrophobic microrough implant surface. Clin. Oral. Implant. Res. 2011, 22, 365–372. [Google Scholar] [CrossRef]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Wieland, M.; Dard, M.; Becker, J. Chemisch modifizierte, ultra-hydrophile Titanimplantatoberflächen. Mund Kiefer Gesichtschirurgie 2007, 11, 11–17. [Google Scholar] [CrossRef]
- Von Eiff, C.; Peters, G.; Heilmann, C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect. Dis. 2002, 2, 677–685. [Google Scholar] [CrossRef]
- Vuong, C.; Otto, M. Staphylococcus epidermidis infections. Microbes Infect. 2002, 4, 481–489. [Google Scholar] [CrossRef]
- Loll, P.J.; Axelsen, P.H. The structural biology of molecular recognition by vancomycin. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 265–289. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Li, S.; Fei, J. Clindamycin-loaded titanium prevents implant-related infection through blocking biofilm formation. J. Biomater. Appl. 2022, 36, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.; Von Holy, A. Bacterial biofilms within the clinical setting: What healthcare professionals should know. J. Hosp. Infect. 2006, 64, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Chen, Z.; Yang, X.; Cen, L.; Zhang, X.; Gao, P. Layer-by-layer self-assembly of minocycline-loaded chitosan/alginate multilayer on titanium substrates to inhibit biofilm formation. J. Dent. 2014, 42, 1464–1472. [Google Scholar] [CrossRef]
- Caykara, T.; Kiper, S.; Demirel, G. Thermosensitive poly(N-isopropylacrylamide-co-acrylamide) hydrogels: Synthesis, swelling and interaction with ionic surfactants. Eur. Polym. J. 2006, 42, 348–355. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef]
- Lewis, G.; Coughlan, D.; Lane, M.; Corrigan, O. Preparation and release of model drugs from thermally sensitive poly (N-isopropylacrylamide) based macrospheres. J. Microencapsul. 2006, 23, 677–685. [Google Scholar] [CrossRef]
- Gollwitzer, H.; Ibrahim, K.; Meyer, H.; Mittelmeier, W.; Busch, R.; Stemberger, A. Antibacterial poly(D,L-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J. Antimicrob. Chemother. 2003, 51, 585–591. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Stemberger, A.; Haas, N.P.; Raschke, M. Biodegradable poly(D,L-lactide) coating of implants for continuous release of growth factors. J. Biomed. Mater. Res. 2001, 58, 449–455. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Lucke, M.; Wildemann, B.; Haas, N.P.; Raschke, M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: A review. Injury 2006, 37 (Suppl. S2), S105–S112. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alenezi, A. The Antibacterial Performance of Implant Coating Made of Vancomycin-Loaded Polymer Material: An In Vitro Study. Surfaces 2023, 6, 304-315. https://doi.org/10.3390/surfaces6030022
Alenezi A. The Antibacterial Performance of Implant Coating Made of Vancomycin-Loaded Polymer Material: An In Vitro Study. Surfaces. 2023; 6(3):304-315. https://doi.org/10.3390/surfaces6030022
Chicago/Turabian StyleAlenezi, Ali. 2023. "The Antibacterial Performance of Implant Coating Made of Vancomycin-Loaded Polymer Material: An In Vitro Study" Surfaces 6, no. 3: 304-315. https://doi.org/10.3390/surfaces6030022
APA StyleAlenezi, A. (2023). The Antibacterial Performance of Implant Coating Made of Vancomycin-Loaded Polymer Material: An In Vitro Study. Surfaces, 6(3), 304-315. https://doi.org/10.3390/surfaces6030022






