Naringenin Release to Biomembrane Models by Incorporation into Nanoparticles. Experimental Evidence Using Differential Scanning Calorimetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SLN and NLC Preparation
2.3. Speed Vacuum Drying
2.4. Characterization of Nanoparticles
2.5. Stability Tests
2.6. Preparation of MLV
2.7. DSC Analysis
2.7.1. SLN, NLC, and MLV Calorimetric Analysis
2.7.2. Calorimetric Analysis of Dry Samples
2.7.3. Naringenin and MLV Contact Kinetic
2.7.4. SLN/MLV and NLC/MLV Contact Kinetic
3. Results and Discussion
3.1. Nanoparticle Characterization
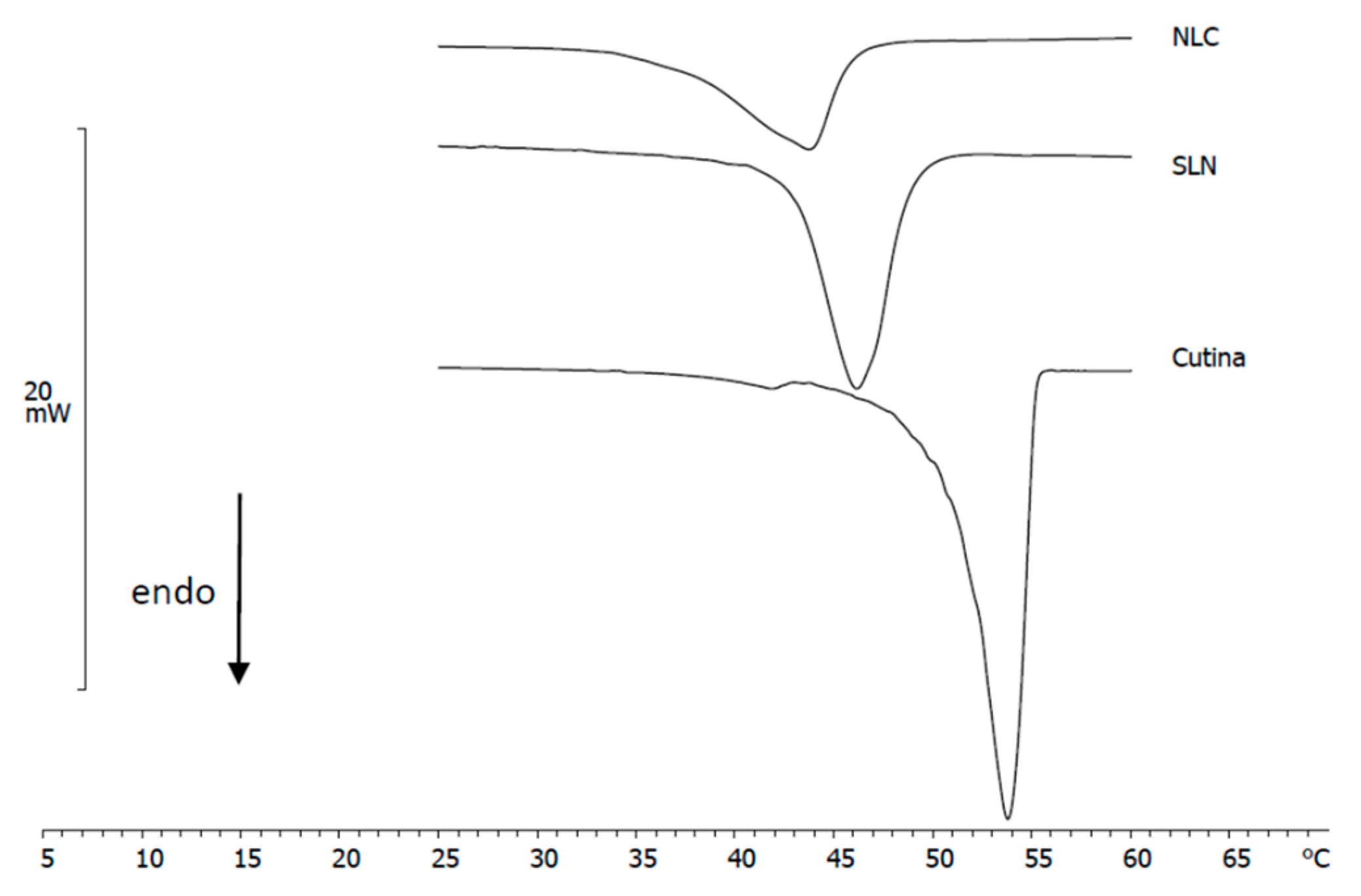
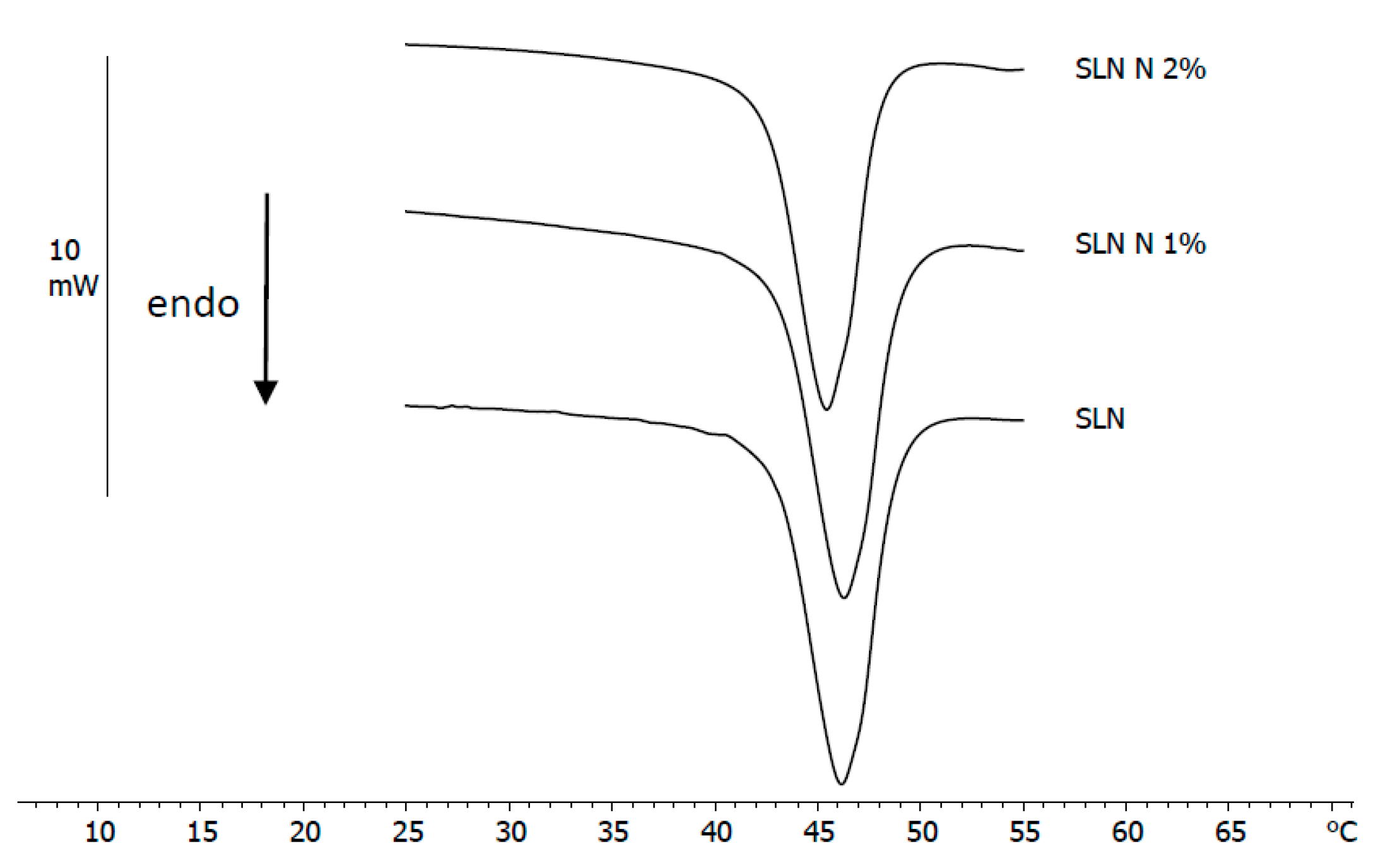
3.2. DSC Analysis of Nanoparticles
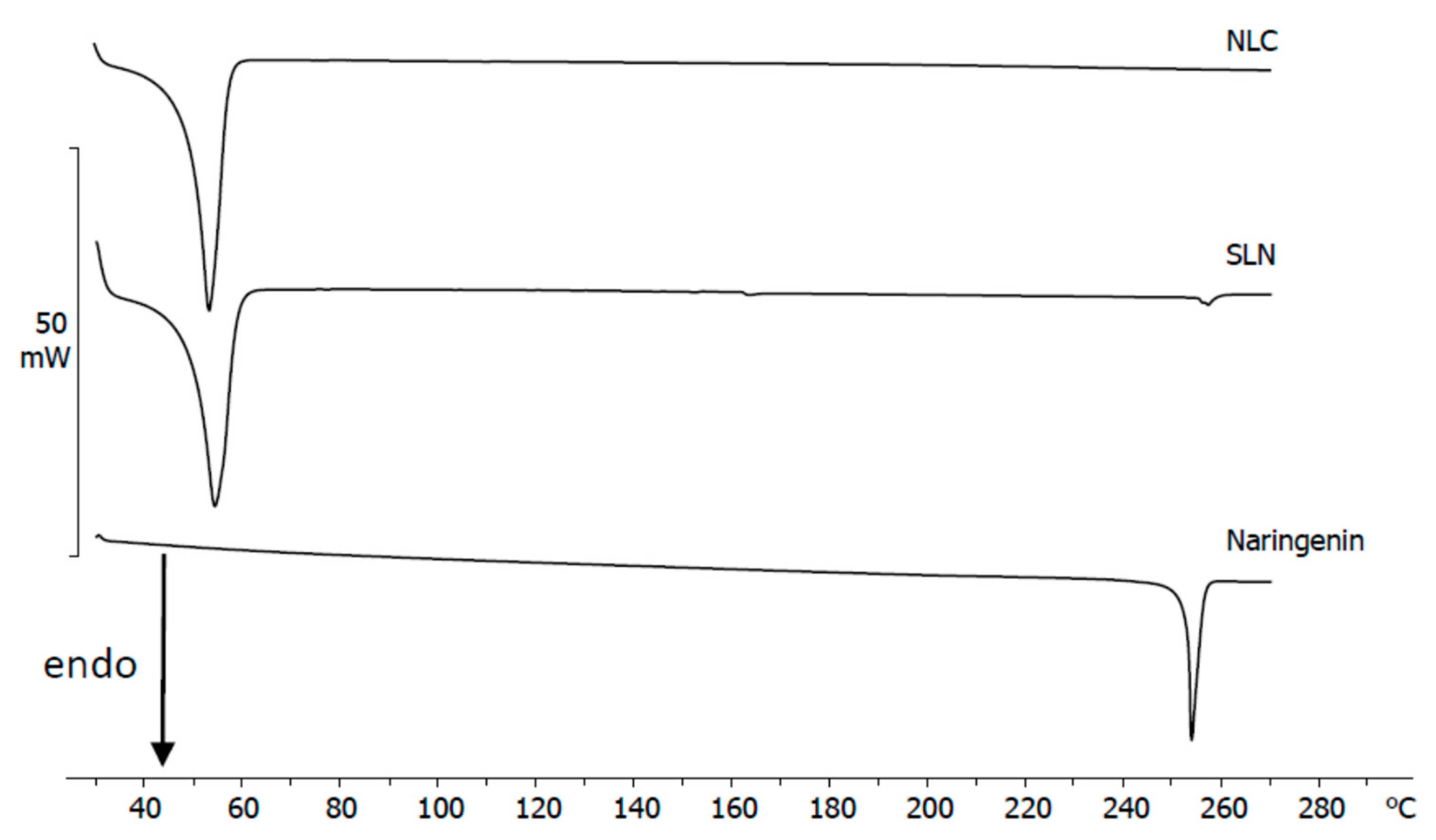
3.3. Calorimetric Analysis of Dry Samples
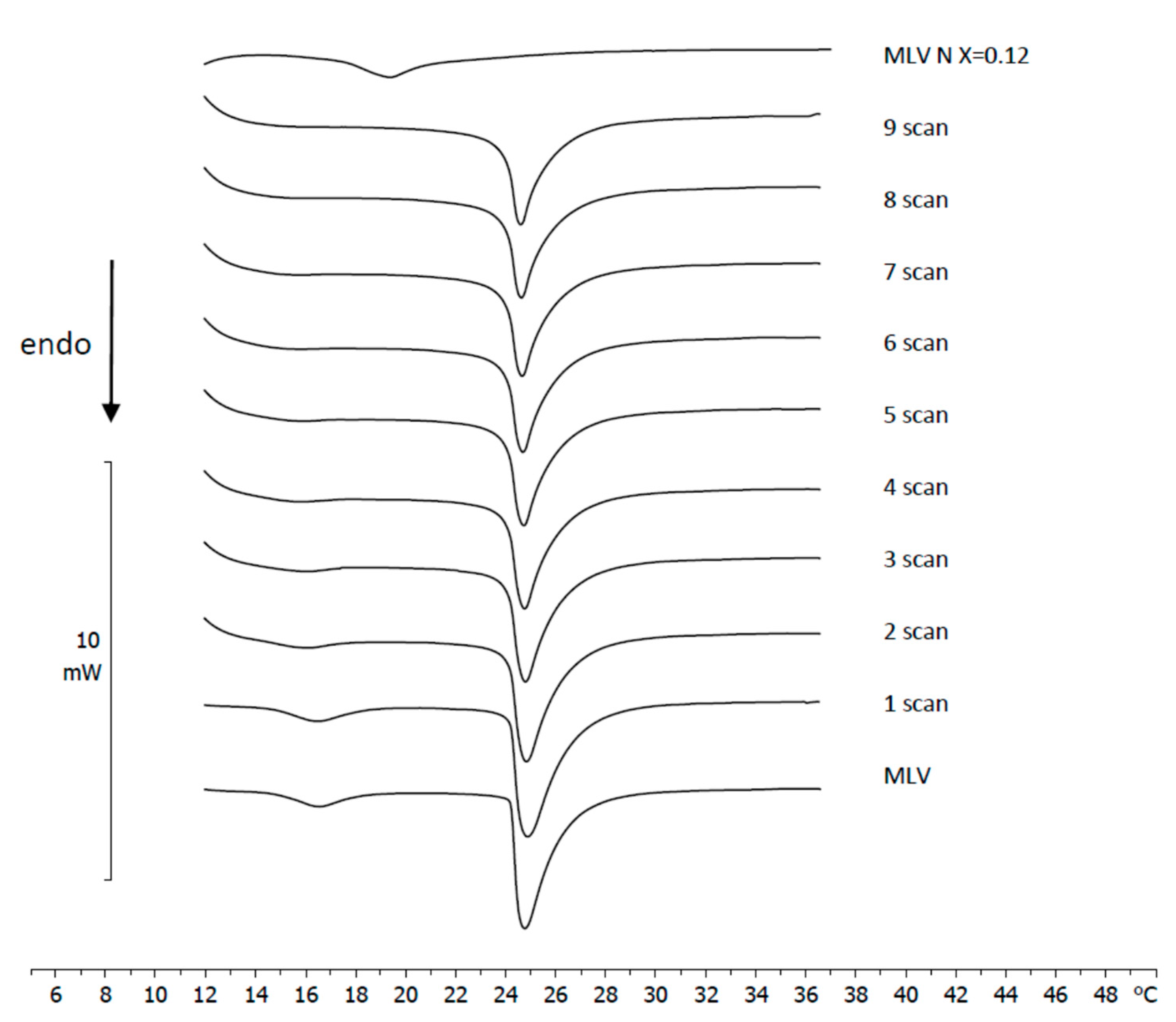
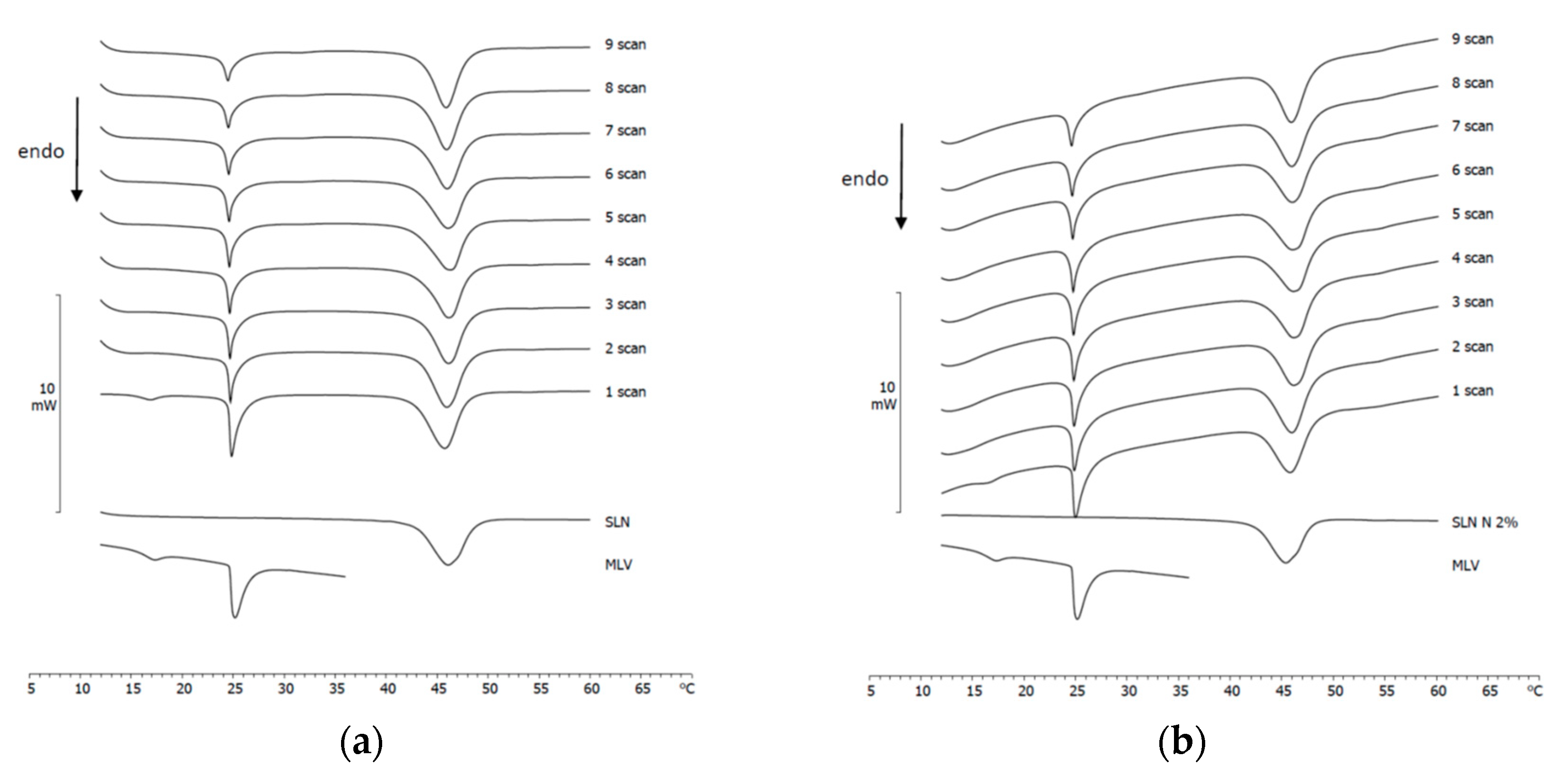
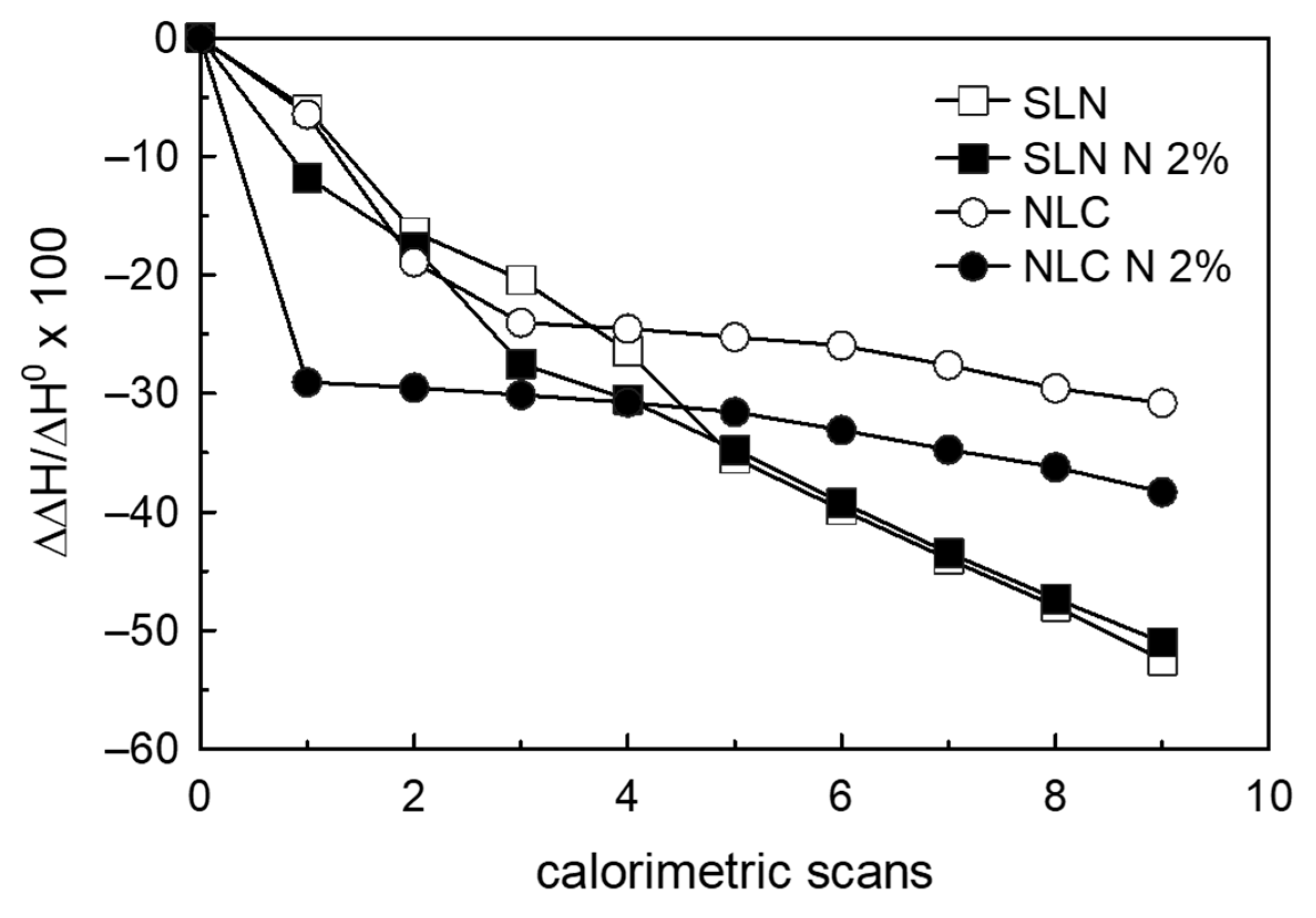
3.4. Naringenin and MLV Contact Kinetic
3.5. SLN/MLV and NLC/MLV Contact Kinetic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Felgines, C.; Texier, O.; Morand, C.; Manach, C.; Scalbert, A.; Régerat, F.; Rémésy, C. Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 27, G1148–G1154. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Kim, H.K.; Kim, H.J.; Do, G.M.; Jeong, T.S.; Park, Y.B.; Choi, M.S. Hypocholesterolemic and antioxidative effects of naringenin and its two metabolites in high-cholesterol fed rats. Transl. Res. 2007, 149, 15–21. [Google Scholar] [CrossRef]
- Ashraful, A.M.; Nusrat, S.; Mahbubur, R.M.; Shaikh, J.U.; Hasan, M.R.; Satyajit, D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar]
- Chen, C.; Wei, Y.Z.; He, X.M.; Li, D.D.; Wang, G.Q.; Li, J.J.; Zhang, F. Naringenin produces neuroprotection against lps-induced dopamine neurotoxicity via the inhibition of microglial NLRP3 inflammasome activation. Front. Immunol. 2019, 10, 936. [Google Scholar] [CrossRef] [Green Version]
- Tayyab, M.; Farheen, S.M.; Khanam, N.; Hossain, M.M.; Shahi, M.H. Antidepressant and neuroprotective effects of naringenin via sonic hedgehog-gli1 cell signaling pathway in a rat model of chronic unpredictable mild stress. Neuromol. Med. 2019, 21, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.; Going, S.; Chow, H.-H.; Patil, B.; Thomson, C. The effect of daily consumption of grapefruit on body weight, lipids, and blood pressure in healthy, overweight adults. Metab. Clin. Exp. 2012, 61, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G.; Malcolm, J.; Arnold, O.; Spence, J.D. Grapefruit juice–drug interactions. Br. J. Clin. Pharmacol. 1998, 46, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Bressler, R. Grapefruit juice and drug interactions. Exploring mechanisms of this interaction and potential toxicity for certain drugs. Geriatrics 2006, 61, 12–18. [Google Scholar]
- Cui, W.; He, Z.; Zhang, Y.; Fan, Q.; Feng, N. Naringenin cocrystals prepared by solution crystallization method for improving bioavailability and anti-hyperlipidemia effects. AAPS PharmSciTech 2019, 20, 115. [Google Scholar] [CrossRef]
- Copmans, D.; Orellana-Paucar, A.M.; Steurs, G.; Zhang, Y.; Ny, A.; Foubert, K.; Exarchou, V.; Siekierska, A.; Kim, Y.; De Borggraeve, W.; et al. Methylated flavonoids as anti-seizure agents: Naringenin 4′, 7-dimethyl ether attenuates epileptic seizures in zebrafish and mouse models. Neurochem. Int. 2018, 112, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, S.; Firempong, C.K.; Zhang, H.; Wang, M.; Zhang, Y.; Zhu, Y.; Yu, J.; Xu, X. Enhanced solubility and bioavailability of naringenin via liposomal nanoformulation: Preparation and in vitro and in vivo evaluations. AAPS PharmSciTech 2017, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque de Oliveira Mendes, L.; Sousa Ponciano, C.; Depieri Cataneo, A.H.; Fanini Wowk, P.; Bordignon, J.; Silva, H.; Vieira de Almeida, M.; Pereira Avila, E. The anti-Zika virus and anti-tumoral activity of the citrus flavanone lipophilic naringenin-based compounds. Chem.-Biol. Int. 2020, 331, 109218. [Google Scholar] [CrossRef]
- Mehnert, W.; Mader, K. Solid Lipid Nanoparticle; production, characterization and applications. Avd. Drug Deliv. Rev. 2001, 2–3, 165–196. [Google Scholar] [CrossRef]
- Radtke, M. Nanostructured lipid carriers. a novel generation of solid lipid drug carriers. Pharm. Technol. Eur. 2005, 17, 45–50. [Google Scholar]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef]
- Wisniewska-Becker, A.; Gruszecki, W.I. Biomembrane models. In Drug-Biomebrane Interaction Studies; The Application of Calorimetric Techniques; Pignatello, R., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 46–95. [Google Scholar]
- Castelli, F.; Sarpietro, M.G.; Micieli, D.; Ottimo, S.; Pitarresi, G.; Tripodo, G.; Carlisi, B.; Giammona, G. Differential Scanning Calorimetry Study on Drug Release from an Inulin-based Hydrogel and its Interaction with a Biomembrane Model: pH and Loading Effect. Eur. J. Pharm. Sci. 2008, 35, 76–85. [Google Scholar] [CrossRef]
- Sarpietro, M.G.; Torrisi, C.; Di Sotto, A.; Castelli, F. Interaction of limonene, terpineol, and 1,8 cineol with a model of biomembrane: A DSC study. Thermochim. Acta 2021, 700, 178938. [Google Scholar] [CrossRef]
- Pignatello, R.; Musumeci, T.; Basile, L.; Carbone, C.; Puglisi, G. Biomembrane models and drug-biomembrane interaction studies: Involvement in drug design and development. J. Pharm. Bioallied Sci. 2011, 3, 4–14. [Google Scholar] [CrossRef]
- Chiu, M.H.; Prenner, E.J. Differential scanning calorimetry: An invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J. Pharm. Bioallied Sci. 2011, 3, 39–59. [Google Scholar] [PubMed]
- Montenegro, L.; Sarpietro, M.G.; Ottimo, S.; Puglisi, G.; Castelli, F. Differential scanning calorimetry studies on sunscreen loaded solid lipid nanoparticles prepared by the phase inversion temperature method. Int. J. Pharm. 2011, 415, 301–306. [Google Scholar] [CrossRef]
- Berrio Escobar, J.F.; Pastrana Restrepo, M.H.; Márquez Fernández, D.M.; Martínez Martínez, A.; Giordani, C.; Castelli, F.; Sarpietro, M.G. Synthesis and interaction of sterol-uridine conjugate with DMPC liposomes studied by differential scanning calorimetry. Colloids Surf. B Biointerfaces 2018, 166, 203–209. [Google Scholar] [CrossRef]
- Librando, V.; Accolla, M.L.; Minniti, Z.; Pappalardo, M.; Castelli, F.; Cascio, O.; Sarpietro, M.G. Calorimetric evidence of interaction of brominated flame retardants with membrane model. Environ. Toxicol. Pharm. 2015, 39, 1154–1160. [Google Scholar] [CrossRef]
- Montenegro, L.; Ottimo, S.; Puglisi, G.; Castelli, F.; Sarpietro, M.G. Idebenone loaded solid lipid nanoparticles interact with biomembrane models: Calorimetric evidence. Mol. Pharm. 2012, 9, 2534–2541. [Google Scholar] [CrossRef] [PubMed]
- Siekmann, B.; Westesen, K. Thermoanalysis of the recrystallization process of melt-homogenized glyceride nanoparticles. Colloids Surf. B Biointerfaces 1994, 3, 159–175. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Walde, P. Preparation of vesicles (liposomes). In ASP Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2004; Volume 9, pp. 43–79. [Google Scholar]
- Jørgensen, K.; Ipsen, J.H.; Mouritsen, O.G.; Bennett, D.; Zuckermann, M.J. A general model for the interactionof foreign molecules with lipid membranes: Drugs andanaesthetics. Biochim. Biophys. Acta 1991, 1062, 227–238. [Google Scholar] [CrossRef]
- Jørgensen, K.; Ipsen, J.H.; Mouritsen, O.G.; Bennett, D.; Zuckermann, M.J. The effects of density fluctuationson the partitioning of foreign molecules into lipid bilayers:application to anaesthetics and insecticides. Biochim. Biophys. Acta 1991, 1067, 241–253. [Google Scholar] [CrossRef]


| Sample | Cetil Palmitate (g) | Tegin O (g) | Oleth-20 (g) | Isopropyl Myristate (g) | Naringenin (g) | H2O (g) |
|---|---|---|---|---|---|---|
| SLN | 1.40 | 0.880 | 1.720 | - | - | 16.000 |
| SLN N 1% | 1.40 | 0.880 | 1.720 | - | 0.014 | 15.986 |
| SLN N 2% | 1.40 | 0.880 | 1.720 | - | 0.028 | 15.930 |
| NLC | 1.22 | 0.772 | 1.520 | 0.500 | - | 15.980 |
| NLC N 1% | 1.22 | 0.772 | 1.520 | 0.500 | 0.012 | 15.975 |
| NLC N 2% | 1.22 | 0.772 | 1.520 | 0.500 | 0.024 | 15.927 |
| Sample | Size ± SD (nm) | P.I. ± SD |
|---|---|---|
| SLN | 39.62 ± 0.42 | 0.287 ± 0.060 |
| SLN N 1% | 31.28 ± 1.32 | 0.350 ± 0.062 |
| SLN N 2% | 34.75 ± 0.54 | 0.304 ± 0.046 |
| NLC | 31.94 ± 0.82 | 0.304 ± 0.034 |
| NLC N 1% | 29.10 ± 0.24 | 0.277 ± 0.054 |
| NLC N 2% | 29.93 ± 0.73 | 0.335 ± 0.043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrisi, C.; Di Guardia, M.; Castelli, F.; Sarpietro, M.G. Naringenin Release to Biomembrane Models by Incorporation into Nanoparticles. Experimental Evidence Using Differential Scanning Calorimetry. Surfaces 2021, 4, 295-305. https://doi.org/10.3390/surfaces4040025
Torrisi C, Di Guardia M, Castelli F, Sarpietro MG. Naringenin Release to Biomembrane Models by Incorporation into Nanoparticles. Experimental Evidence Using Differential Scanning Calorimetry. Surfaces. 2021; 4(4):295-305. https://doi.org/10.3390/surfaces4040025
Chicago/Turabian StyleTorrisi, Cristina, Marco Di Guardia, Francesco Castelli, and Maria Grazia Sarpietro. 2021. "Naringenin Release to Biomembrane Models by Incorporation into Nanoparticles. Experimental Evidence Using Differential Scanning Calorimetry" Surfaces 4, no. 4: 295-305. https://doi.org/10.3390/surfaces4040025






