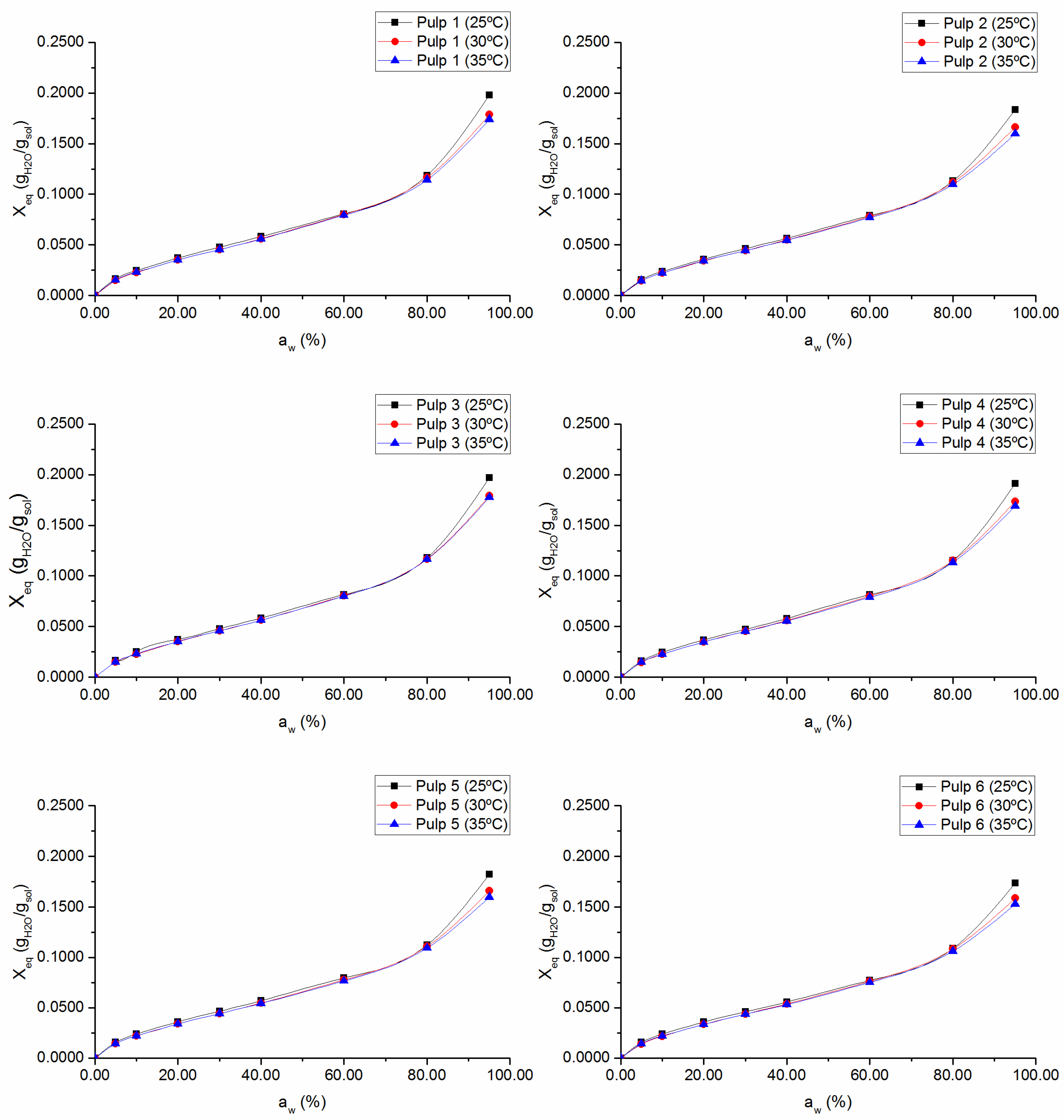
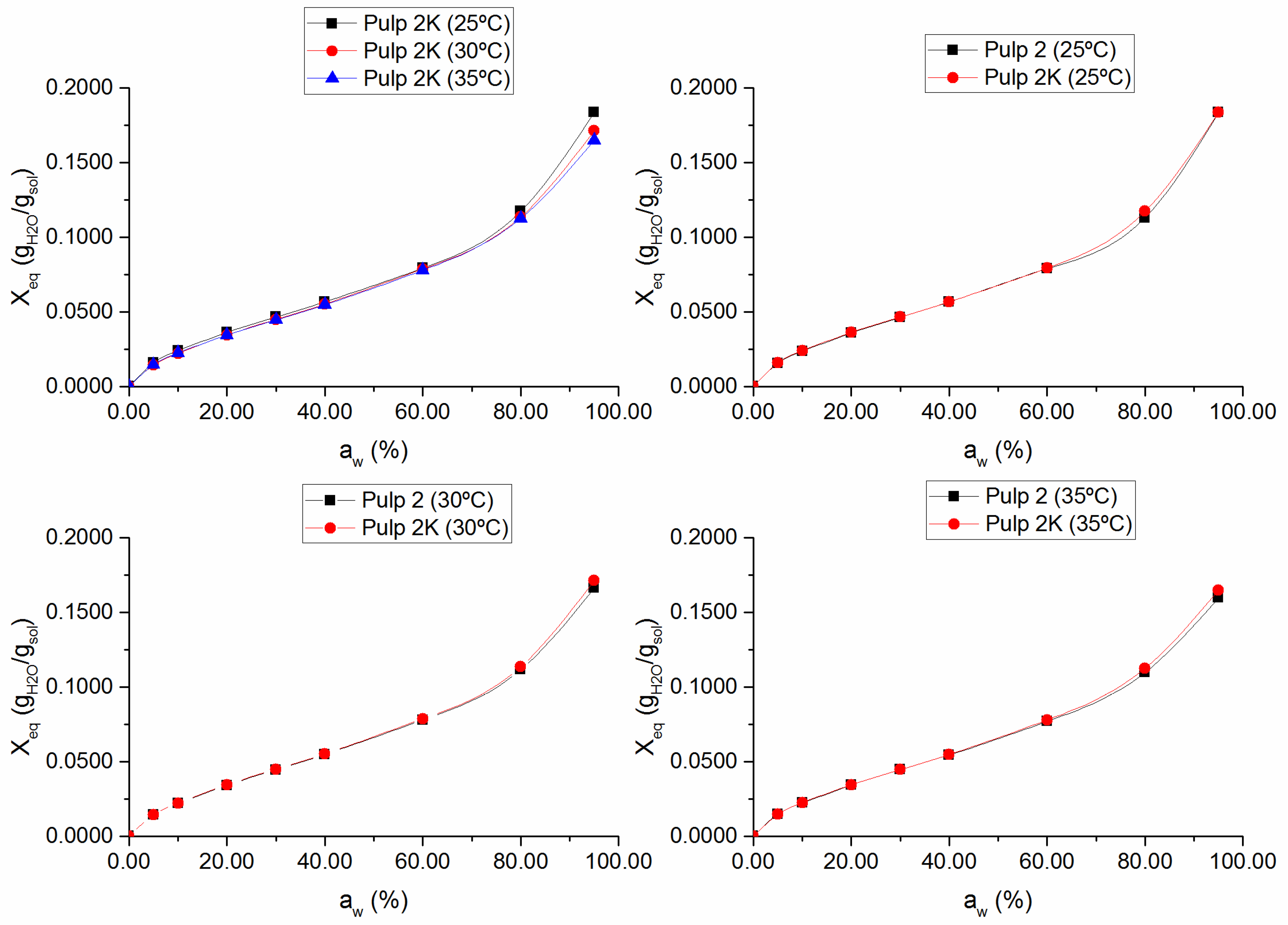
3.1. Sorption Isotherms and Sorption Enthalpies
Sorption isotherms for
1–
6 pulps and the
2K pulp, at 25 °C, 30 °C, and 35 °C are presented in
Figure 1 and
Figure 2, respectively. The obtained isotherms are consistent with type II isotherms, typical of hydrophilic polymers, such as cellulose, possessing free hydroxyl groups, which are largely responsible for water sorption [
14,
27]. An increase in temperature induces a reduction in the equilibrium moisture content at a specific water activity, being more significant at higher
values, as should be expected for fibrous cellulosic materials [
15,
28]. The increase in the temperature increases the state of excitation of the water molecules and, consequently, decreases the attractive forces between them, due to an increase between their mutual distances. This decrease in binding energy between water molecules and the surface of the absorbent material results in the breakdown of these bonds, and, therefore, in a decrease of the equilibrium moisture content for a given water activity value.
Figure 2 also shows that knots content did not significantly affect the equilibrium moisture content. This means that fibers in bundles maintained the accessibility comparable with that of well-separated fibers. In practice, inaccessible surfaces of aggregated fibers in cellulosic materials become accessible via the cleavage of intermolecular hydrogen bonds by water, thus promoting fiber swelling and increasing the effective surface area [
14,
19]. Hence, the agglomeration of fibers is not a problem for their accessibility inside the knots.
The sorption isotherms allow for evaluating the capacity of the monolayer (
Xm) in the fluff pulp, while adjusting the GAB model to the experimental data and infer the effective parameters (Equations (3) and (4)). Only “free” hydroxyl groups not involved in strong intramolecular and intermolecular hydrogen bonding bind water molecules, thus forming a monolayer at low water activities (a
w ≤ 0.40) [
27,
28]. This monolayer is discontinuous, because of randomly distributed free hydroxyl groups and their differences in accessibility. Bleached
Eucalyptus kraft pulps contain notable amounts of glucuronoxylan, which is located essentially in the outer layers of the fibers [
29]. Accordingly, the content of xylan and its chemical structure (mainly the quantity of uronic moieties) contribute to the absorption of water by the fluff pulps and must be considered in the interpretation of the results of the isothermal sorption.
Table 3 presents the obtained monolayer capacity values. This parameter measures the availability of active sites on the material for water sorption to occur [
14,
15,
16] and it was used to estimate the specific surface area of the absorbent material,
as Equation (11):
where
is the water molecular weight,
the Avogadro number, and
the effective cross-sectional area of the water molecule (0.125 nm
2 for water at 298 K) [
21].
values and, consequently, S values decrease with temperature (
Table 4). This result is in accordance with considerations of other studies that attributed this issue to a reduction in the total number of active sites for water binding as a result of physical or chemical changes on the fiber surface that is induced by temperature [
14,
15,
16,
17,
18,
19,
20,
21,
28].
Energy parameters
and
of the GAB sorption model are shown in
Table 5 and
Table 6, respectively. Parameter
is essentially related to differences in enthalpy between monolayer (
) and multilayer (
), and it measures the binding energy of the forces between water molecules and the fiber surface [
14,
15,
16,
17,
18,
19]. The
values presented in
Table 5 suggest that the effective binding force between water molecules and the surface of agglomerated and compact fibers (knots) is weaker than between water molecules and non-agglomerated fibers, since the
C value for
2K pulp is lower in comparison to
value for
2 pulp. Parameter
is related to differences between enthalpy in the bulk liquid (
) and multilayer [
14,
15].
varies between 0 and 1; when it approaches one, water molecules that are beyond the monolayer are not structured in a multilayer and there is almost no distinction between multilayer molecules and bulk liquid. The lower the
value, the more sorbed molecules are structured in a multilayer [
14,
15]. The
values are very similar for all samples, varying in the range between 0.7 and 0.8 (
Table 6), which indicate that the multilayer is structured and its properties are distinct from the bulk liquid [
14]. The temperature has negative effect on
and positive effect on
, as it has already been reported for other cellulosic materials [
14,
15,
28]. These results are consistent with the definition of these parameters, i.e., C decreases with increasing temperature due to the decreasing of monolayer binding energy and K increases with increasing temperature, which indicates that multilayer water molecules become more similar to those in bulk liquid, due to the increase of their excitation state.
Enthalpy differences and entropy accommodation factors between monolayer and multilayer (
and
, respectively), and between bulk liquid and the multilayer (
and
, respectively) revealed that
are positive (
Table 7), because the interaction of water with primary sorption sites is exothermic [
15].
. values are smaller, because multilayer molecules interact less than monolayer molecules with the surface of the absorbent material and, consequently, multilayer molecules are less firmly bounded in comparison to the monolayer molecules [
14,
15,
16]. These results show that the enthalpy that is associated with adsorbed water molecules follows the order:
, i.e., the interactions of water molecules in the monolayer are stronger in comparison with the multilayer. The
values are smaller than 1, because, from an entropic point of view, the water molecules have a larger degree of freedom in the multilayer. Similarly, the
values are greater than 1 due to higher molecule’s entropy of the molecules in the bulk liquid.
. values are larger than
values because of a more significant entropic contribution due to the strongly increased number of configurations and mobility of molecules in the bulk liquid compared to molecules in the multilayer.
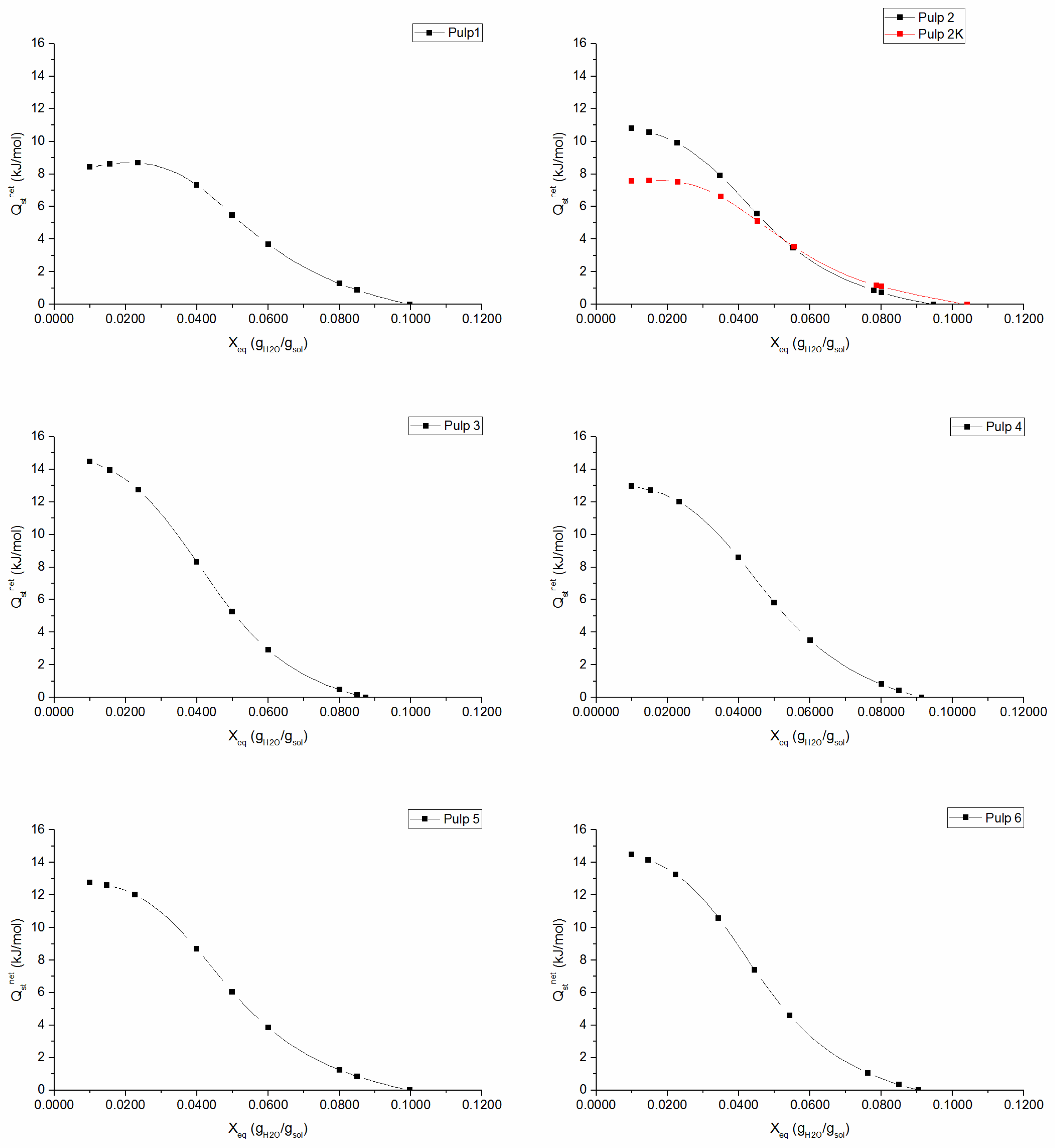
The net isosteric heat of sorption is an important thermodynamic parameter, which measures the binding energy of the forces between the water vapor molecules and the absorbent material. It gives useful information regarding the sorption mechanism, since it can help to interpret the type of water binding that is occurring at a given moisture content [
15,
16,
30,
31,
32,
33]. The results presented in
Figure 3 show the existence of three classes of water with a continuous transition from tightly bound water to free water molecules. In the monolayer region, water molecules are tightly bound to the material, corresponding to high interaction energy (high
values). At increasing moisture content, net isosteric heat of sorption decreases, since most active sites become occupied and sorption occurs on the less active sites, which results in lower heats of sorption. Ultimately,
approaches zero, which indicates that the total heat of sorption is equal to the heat of vaporization of pure water, i.e., the region where adsorbed water molecules behave as molecules in the liquid state with properties like those of the bulk liquid. The knots effect is well evidenced in
Figure 3 for
2K pulp, as there is a noticeable decrease in isosteric heat of adsorption in the monolayer region. This result is in accordance with the previous conclusion, i.e., the binding energy between aggregated fibers in knots and water molecules is weaker when compared to well-separated fiber-water binding forces. The maximum net isosteric heat of sorption, which can be theoretically defined as
[
14], can be associated to physical interpretation of GAB parameters:
and
are related to sorption energy in monolayer and
is related to multilayer interactions. In fact, a higher monolayer sorption enthalpy (
) corresponds to a higher value of
(e.g., in the case of
3 and
6 pulps,
Figure 3). It was also found a clear relation between
and
:
3 and
6 pulps have the lowest multilayer sorption enthalpy, i.e., lower
and, consequently,
reaches zero at lower moisture content, demonstrating that these have a less structured multilayer and their water molecules have properties that are similar to bulk liquid.
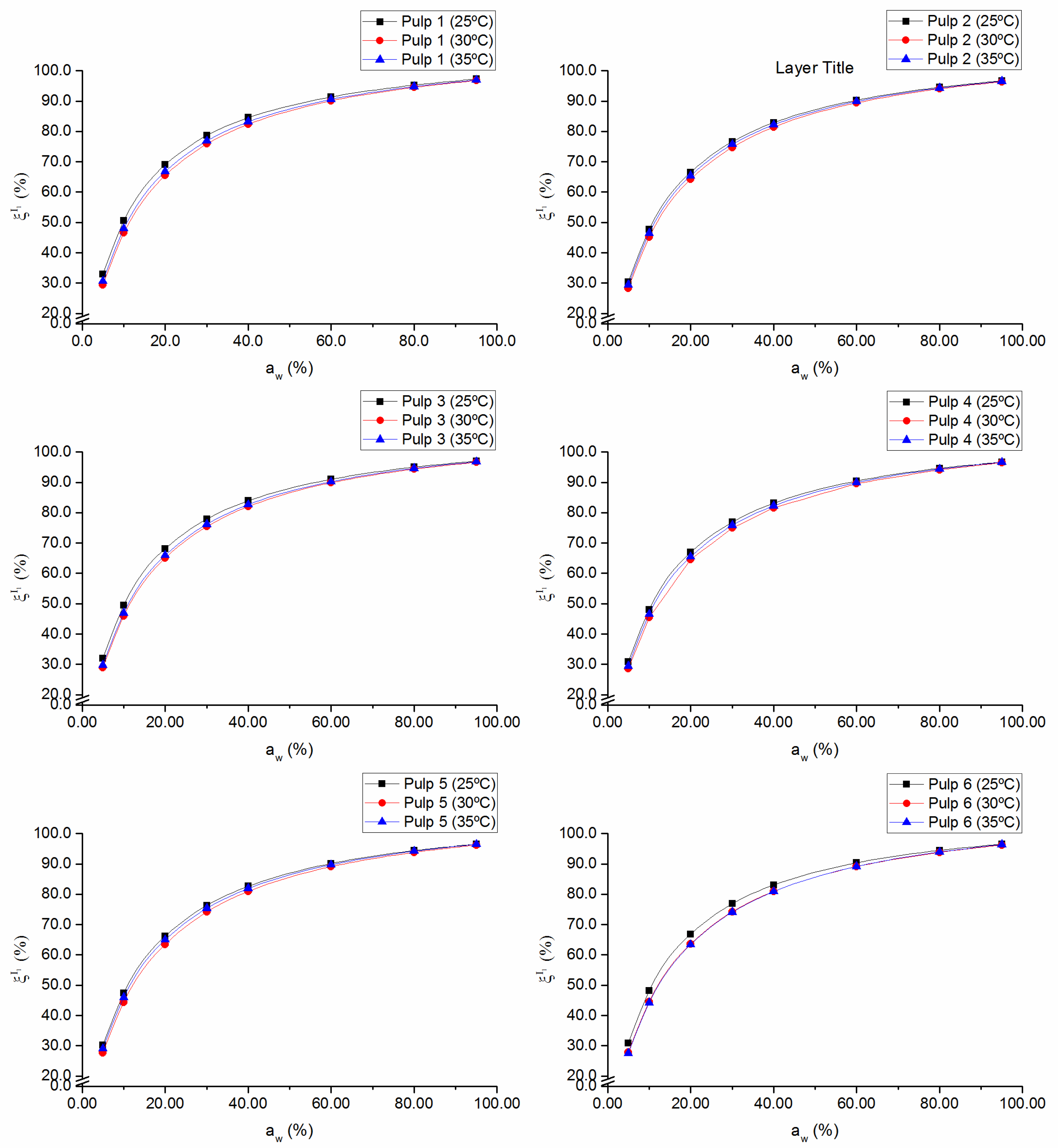
The fraction of occupied sites in the monolayer,
, plotted as a function of water activity, evidences the assumption of incomplete surface coverage of the adsorbent material and the formation of the multilayer (
Figure 4). At low water activity values, the surface has more available sites for the water molecules to form hydrogen bonds with the surface and, therefore, there is a more pronounced growth in
at low relative pressure (up to
= 40%). As the monolayer of water molecules forms, the surface becomes less accessible due to the “jamming” of the adsorbate water molecules, favoring bonds between water molecules instead of bonds between water molecules and the surface [
21]. For each pulp, the maximum fraction of occupied sites in the monolayer is higher than 96%, which indicates good surface affinity of the absorbent material for water molecules. As could be expected, by analogy with
Xm,
values were negatively affected by temperature, especially at relatively low
aw (≤ 40%) (
Figure 4).
3.2. Contact Angles of Pulps
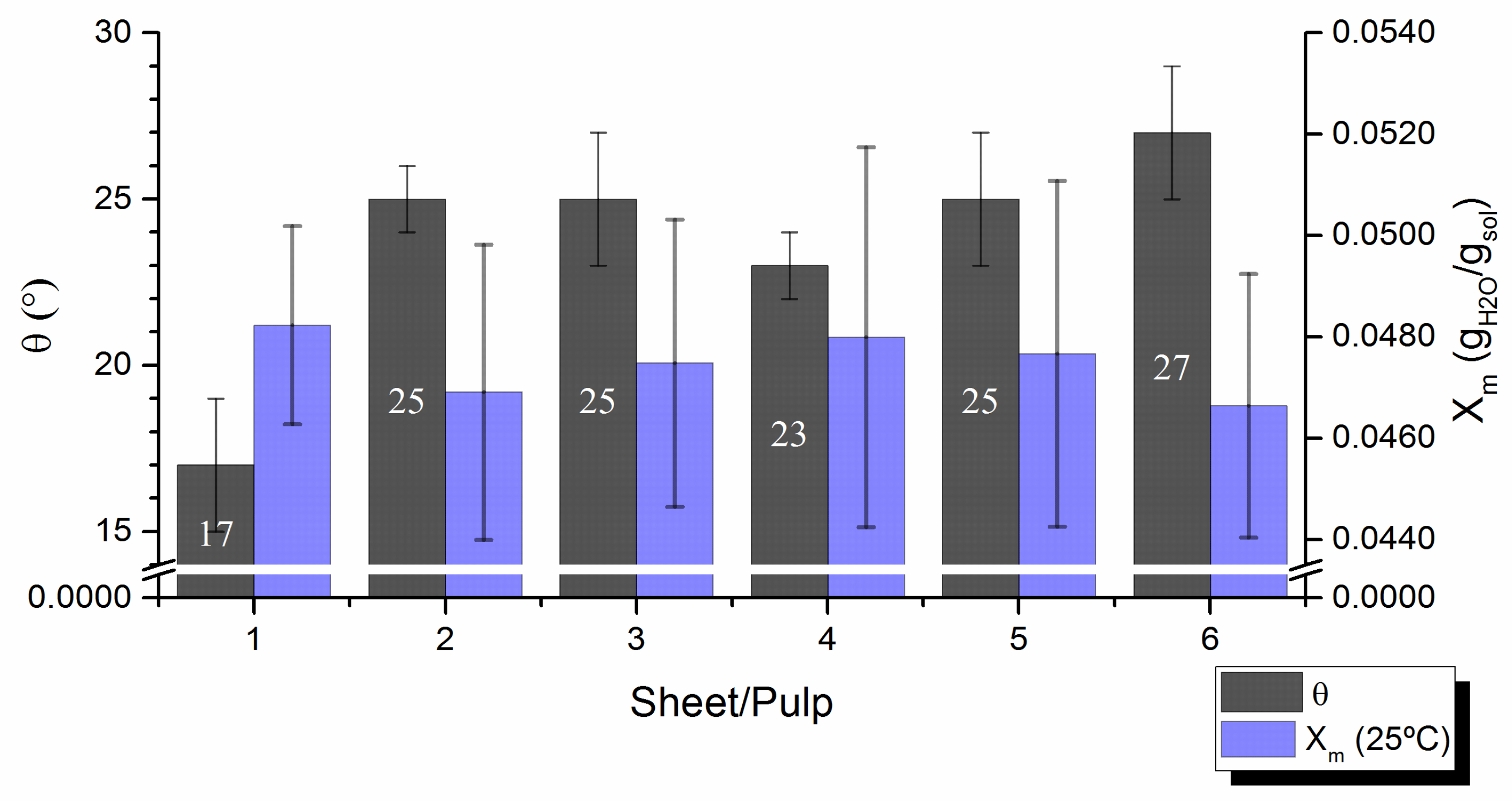
Contact angles,
, were measured on laboratory pulp handsheets with smooth surface in order to minimize the effect of surface roughness. At least similar roughnesses were detected for all samples when evaluated by known Bendtsen test according to ISO 8791/2:2013, making the results of contact angle tests comparable. Contact angles varied between 17° and 27° (
Figure 5), thus demonstrating common strong pulp wettability, as solid-liquid attraction largely prevailed over the liquid-liquid one [
34]. The contact angles, that reflect the wettability of the handsheet surface, must relate to the affinity of the pulp fibers for water (i.e.,
Xm and S).
Figure 5 shows that the wettability of the handsheet’s surface is clearly related to fiber’s affinity for water: the sheet with lower contact angle has been produced from the pulp that has better ability to retain water molecules on their surface (higher value of
Xm). Hence, a surface with fibers that are more accessible to form hydrogen bonds with water provides higher hydrophilicity to the pulp, which, in turn, correspond to lower contact angles measured on handsheets formed from it. The experimental results unambiguously confirm that the wettability of the pulp handsheets and the affinity of the fiber surface with water are inextricably related. Pulps with extreme contact angles (e.g.,
1 pulp with Ө = 17° and
6 pulp with Ө = 27°,
Figure 5) did not correlate univocally with their chemical composition (amount of hemicelluloses or carboxylic moieties,
Table 1), or with their basic fiber morphology (average fiber length/width or coarseness,
Table 2). On the other hand, a tendency of pulp´s contact angle to increase with fiber´s deformation (curl and kink,
Table 2) was clearly traceable (r
2 = 0.7–0.8, figure not shown).
The deformations of the pulp fibers, such as curl and kink, are mostly irreversible being caused by distortion and compression of the fibril layers [
35,
36,
37]. The misalignment of the fibril lamellas lead to wavy layers (
Figure S1, Supplementary Materials), where some adjacent surfaces in the creases approach each other, forming intrafiber cross-links by hydrogen bonding of free hydroxyl groups [
37]. As a consequence, the accessible area of the damaged fibers can be slightly reduced, which caused the lower monolayer capacity and the wettability of the fiber mesh, resulting in Ө increase. On the other hand, the surfaces of deformed fibers, which contain partially disordered cellulose fibrils, are more accessible when compared to surfaces of non-deformed fibers [
36]. This fact can explain the larger ΔHc (
Table 7) and
(
Figure 3) of more curled and kinked pulp fibers (
Table 2) observed experimentally. These accessible surfaces of deformed fibers can also readily form the intensive hydrogen bonds between the fibers in the handsheets, thus contributing to the reduction in paper surface wettability (increases in Ө). Therefore, the contact angle of the fiber web reflects the apparent wettability of the surface and not necessarily just the intrinsic contribution of the constituent fibers [
38]. Nevertheless, the contact angle is one of the most common and reliable analyses for assessing the wettability of cellulosic materials.
3.3. Capillarity Absorption
Porosity (
), specific volume (
), absorption capacity (
), and absorption time (
) of
1–
6 pads without knots and
1K–
6K pads with knots revealed notable differences, depending on the fiber network structure in pads (
Table 8). These differences influenced the absorption properties. Thus, the knots turn the fiber network much more compact (less bulky), negatively affecting the porosity of air-laid pads. However, this fact did not lead to the expected drop in C
abs of pads formed from knotted pulp compared to knotless one (
Table 8), although within a series of
1–
6 or
1K–
6K pads some tendency to decrease of C
abs was observed with the decrease in the specific volume of the pad. Furthermore, predicted by Equation (2) the maximal absorption capacity (C
am) of pads was more than five times greater than those obtained experimentally. This apparent discrepancy can be explained by the collapse of pad pores upon wetting. The contraction of the volume of the pad occurs essentially under the gravity of the absorbed liquid, due to the low mechanical resistance of the wet mesh [
12]. Hence, the C
abs depended significantly on the strength of network under wetting. In this context, a certain positive effect of knots on the C
abs can be explained by the inclusion of rigid fiber bundles in the network that preserve it from collapse under moistening. As already pointed out, the accessibility of fibers within the knots is not much worse than that of disintegrated fibers (
Figure 2). However, the monolayer sorption enthalpy (
) and the net isosteric heat of sorption (
) of fibers in knots are lower than those of well-separated fibers (
Figure 3 and
Table 7), due to the more intense interfiber interactions in the formers. This, together with lower pore size inside than outside of knots, must lead to the lower penetration rate of water into the pad (Equation (1)). Such behavior was experimentally confirmed for most of the evaluated pulps (
Table 8).
According to common knowledge, the strength of the network becomes better when using longer, coarser, and curvier fibers [
10,
12,
39]. Among six examined pulps, which were obtained from the same wood specie and by similar processing mode, the fiber length/width and coarseness varied little (
Table 2). However, the fiber deformation parameters of pulps (curl and kink) varied quite substantially. The minimal fiber deformations were registered for
1 pulp and the maximal for the
6 pulp. These pulps have the opposite surface affinity for water in terms of
Xm,
,
and contact angles (
Table 3,
Table 7 and
Table 8 and
Figure 3 and
Figure 5) and demonstrated the opposite absorption capacity and the absorption rate (
Table 8). Contrary to expectations, more hydrophilic
1 pulp showed less absorption capacity and absorption rate than less hydrophilic
6 pulp. The only explanation is the structure of the fiber network of
6 pad
, comprised of fibers that are more curled, kinked, and coarser than those of
1 pad, which promoted a more bulky/porous and robust structure than that formed in the
1 pad. Therefore, the structure of the fiber network is a more important factor affecting absorptivity than the intrinsic hydrophilicity of the constituent fibers, at least in the range of contact angles of ca. 15–30°.
On the other hand, the better absorption capacity and the absorption rate of
2 and
6 pulps in relation to
3 and
5 pulps does not match well to the proposition that the structure of the network is a unique dominant factor, because ε, υ, and the main deformation parameters of the fibers in pulps (curl and kink) did not vary significantly as to justify this assumption (
Table 2 and
Table 8). At the same time,
2 and
6 pulps had one of the highest contents of carboxylic groups, which mainly belong to the glucuronoxylan that is present in the pulps (
Table 1). The latter suggests that the chemical composition of the fibers also has an important contribution to absorption, because it is known that hemicelluloses and other components containing carboxylic moieties contribute largely to the swelling of the pulp [
10,
40]. The swelling of the polymeric network is one of the basic mechanisms in the absorption by porous materials [
10,
41].
Interestingly, the
4 pulp containing the high amount of the hemicelluloses and carboxyl groups (
Table 1) and having the coarsest and most deformed fibers among the examined pulps (
Table 2), allowing for porous and bulky pads, showed the worst absorption capacity, and the lowest absorption rate (
Table 8). At least partially, these findings can be explained by the smaller number of knots in
4 pulp (
Table 8) and one of the shorter lengths of fiber (
Table 2).
The relationships between the surface properties of the pulp fibers, their chemical composition, and morphology with respect to the absorption capacity of the resulting air-produced formulations are not straightforward and multi-dependent, as can be seen from the above reasoning. To better trace these relationships, statistical analyzes of the absorption capacity in relation to the pulp properties were performed.
3.4. Multivariate Analysis
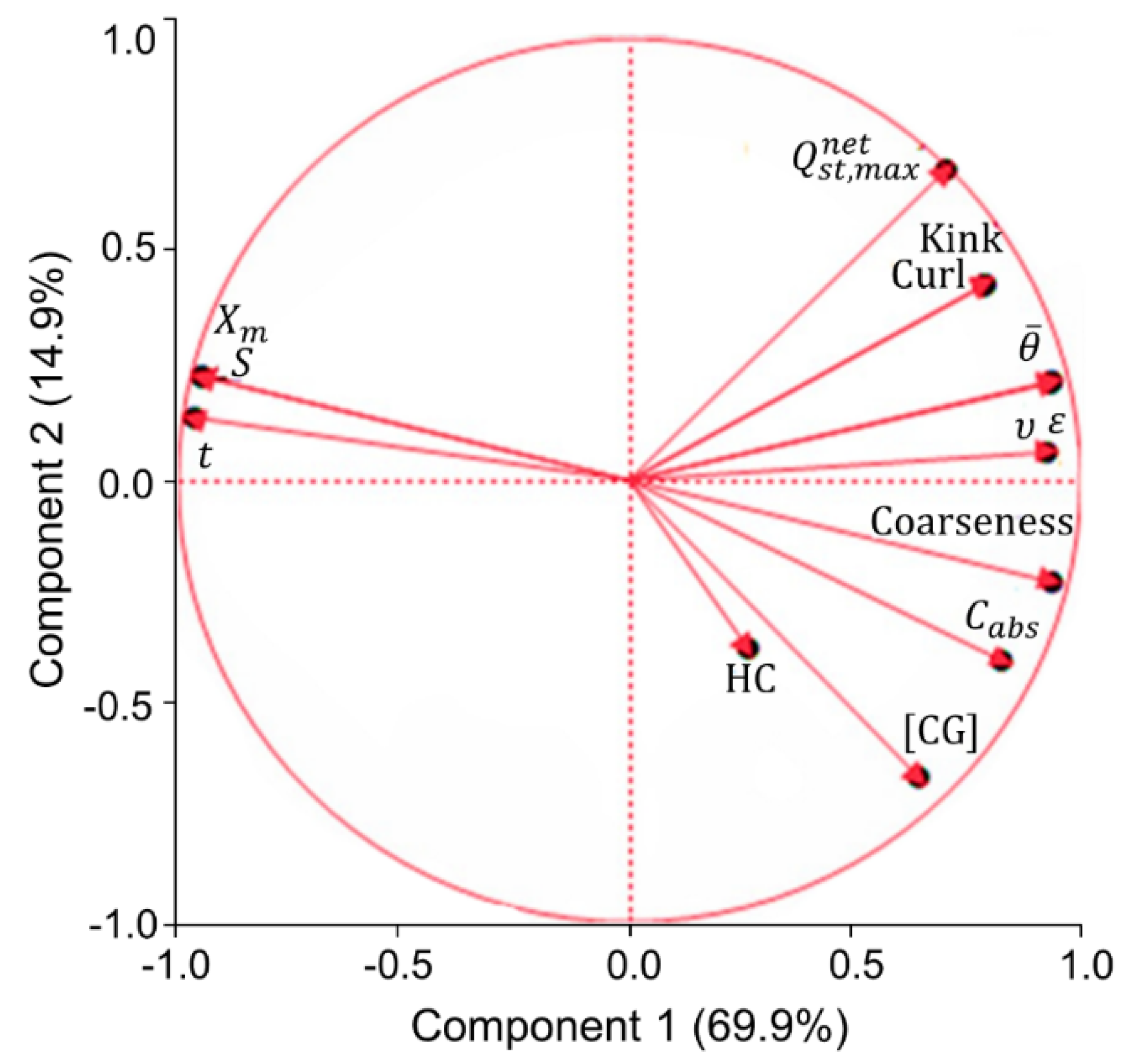
Principal component analysis (PCA) was used to monitor the main relationships between the absorption capacity of the studied pulps and their surface properties, chemical composition, and fiber morphology (
Figure 6). The PCA reflects the correlation between the variables, expressing their contribution to the two principal components (PC), in terms of relative variation. Thus, two variables that are located at the same quadrant indicate a positive correlation, since them positively or negatively contribute to the two PCs, and this correlation is as stronger as closer they are. When located in opposite quadrants, two variables present have a negative correlation with each other, because they translate into opposite effects for the PCs. When located in parallel quadrants, two variables apparently have the same contribution to one PC and the opposite contribution to the other. However, it is necessary to consider the total variation that is explained by each of the PCs. In this case, component 1 (PC1) explains 69.9%, while the component 2 (PC2) explains only 14.9% of variations (
Figure 6). Therefore, the correlations between the different variables will be analyzed, especially in relation to their contribution to PC1 describing mainly the fiber network properties.
In general, the PCA supports previous conclusions that the affinity of the fiber surface with water, expressed by
Xm and
S and the surface wettability (measured by the contact angle,
Ө), are not necessarily related to the improved capillarity absorption properties (
Cabs and
t). In fact, statistically,
Xm and
S have positive correlation with absorption time (
) and negative correlation with absorption capacity (
Cabs). The opposite effect is verified in relation to the correlations between the average contact angle (
) and these absorption properties. The PCA also shows that higher coarseness correlated with the lower accessible surface area (
S) and the monolayer capacity (
Xm) and the increased fiber deformations (kink and curl) to the lesser wettability of handsheet’s surface, i.e., larger contact angles (
Figure 6). The reasons for such correlations have been previously discussed when analyzing the affinity of the pulp surface with water (
Section 3.2) and the relationship between the absorption capacity and the structure of the fiber network in air-laid pads (
Section 3.3).
The expectable positive correlation was found regarding the presence of highly hydrophilic components in pulps (hemicelluloses and other polysaccharides containing carboxylic groups) and their absorption capacity (
Figure 6). The absorptivity of pulps also positively correlated with fiber coarseness and, to a lesser extent, with curled and kinked fibers. In fact, the network with a higher coarseness of fibers usually exhibits better absorption properties, because these bulky fibers possess less interfiber bonding areas, resulting in a network with high specific volume, high porosity, and, therefore, improved absorbency. The positive contribution of deformed fibers to improve the specific volume (
ν) and the porosity (
ε) of air-laid pads has also been discussed previously and favored the pulp’s absorption capacity. It is worth mentioning that the deformations of the fibers provided the greatest contribution to
ν and
ε of the pads. These deformations occurred during the production of kraft pulp, due to the variable cooking and bleaching processing conditions. These conditions also affected the chemical composition of pulp due to the variable degradation of hemicelluloses [
42]. Dry defibration is another cause of fiber deformation, which depends on the manufacturing history, e.g., pulping and bleaching conditions.
Although the content of hemicelluloses (HC) in pulps is expectedly related to the carboxyl groups content (CG) (
Figure 6), a much weaker relationship exists between the HC content and the absorption capacity (C
abs). HC usually contributes negatively to the air-laid fiber network strength due to the weakening of wet mesh, thus decreasing the network flexibility and leading to less curled fibers [
8]. Consequently, the effect of HC on
Cabs is not straightforward. On the one hand, HC favors the network swelling and water retention and, on the other hand, HC deteriorate the air-laid fiber network structure and strength.
HC contributed negatively to the wettability of the fibers in pads (negative contribution to PC2), according to the PCA data. Thus, the
Xm and HC located, even in the opposite quadrants, indicating a negative correlation with each other (
Figure 6). This feature is in line with the known fact that HC can negatively affect the wettability of the fiber web, resulting in an increase in
Ө with the extent of HC present in the pulp [
38,
43,
44]. The plausible explanation deals with intensive formation of hydrogen bonds between free hydroxyl groups on the surface of cellulose fibrils and amorphous hemicelluloses. As a result, the amount of free hydroxyl groups in the cellulose fibrils is decreasing and the interfibrillar space is reduced being filled with HC, thus diminishing the monolayer capacity of fibrils. The removal of moderate amounts of hemicelluloses can lead to better wettability of fibers, because the lower the hemicellulose content, the looser the cellulose fibrils network in fiber, and, consequently, fiber mesh will have higher specific volume and higher porosity. However, with a very low HC content, when the fibers are dried intensively, their surface is collapsed due to the intensive aggregation of fibrils within cell wall and the wettability of the fibers decreases [
38,
40]. Therefore, hemicelluloses can have both positive and negative effects on fiber wettability.
It is noteworthy that, among the studied pulps, the variations in HC content did not exceed 20% and the eventual direct relationship between the decrease in HC content (
Table 1) and the increase in
Ө of pulp fibers (
Table 8) was not clearly detectable. It was recently reported that the capacity of pulp fiber to swell depends not only on the amount of hemicelluloses in eucalypt kraft pulp, but also on their location within the cell wall [
45]. The last feature depended on the pulping severity, mainly the alkali load and the cooking temperature. Hence, it is reasonable to propose that the wettability and absorption capacity of kraft pulps in study also depended not only on the HC content, but also on the HC distribution in the cell wall of fibers.