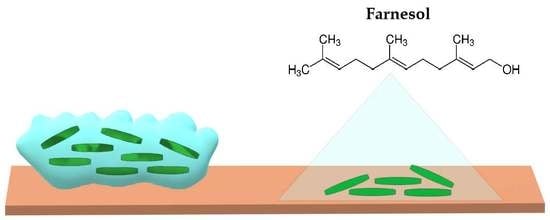
Farnesol-Containing Macromolecular Systems for Antibiofilm Strategies
Abstract
1. Introduction
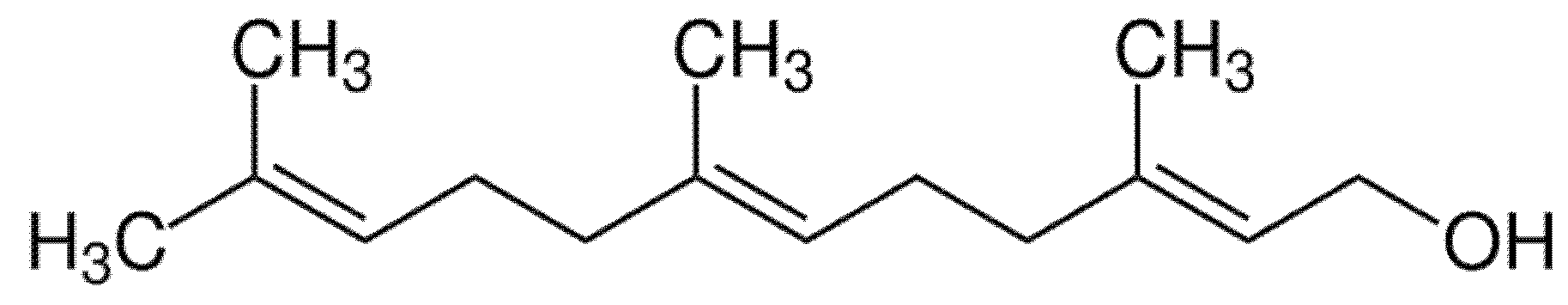
2. Farnesol and Its Antibiofilm Activity
3. Farnesol-Containing Polymer Materials
3.1. Nanoparticles and Liposomes Containing Farnesol
3.1.1. Antibacterial Materials
3.1.2. Antifungal Materials
3.2. Skin Reparative Therapies and Transdermal Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Polke, M.; Jacobsen, I.D. Quorum sensing by farnesol revisited. Curr. Genet. 2017, 63, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Zawrotniak, M.; Wojtalik, K.; Rapala-Kozik, M. Farnesol, a Quorum-sensing molecule of Candida albicans triggers the release of neutrophil extracellular traps. Cells 2019, 8, 1611. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.K.S.; Rudden, M.; Smyth, T.J.; Dooley, J.S.G.; Marchant, R.; Banat, I.M. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl. Microbiol. Biotechnol. 2019, 103, 3521–3535. [Google Scholar] [CrossRef] [PubMed]
- Dancewicz, K.; Gliszczynska, A.; Halarewicz, A.; Wawrzenczyk, C.; Gabrys, B. Effect of farnesol and its synthetic derivatives on the settling behaviour of the peach potato aphid Myzus persicae (Sulz.). Pestycydy 2010, 1–4, 51–57. [Google Scholar]
- Jamalian, A.; Shams-Ghahfarokhi, M.; Jaimand, K.; Pashootan, N.; Amani, A.; Razzaghi-Abyaneh, M.J. Chemical composition and antifungal activity of Matricaria recutita flower essential oil against medically important dermatophytes and soil-borne pathogens. Mycol. Med. 2012, 22, 308–315. [Google Scholar] [CrossRef]
- Wroblewska-Kurdyk, A.; Dancewicz, K.; Gliszczynska, A.; Gabrys, B. New insight into the behaviour modifying activity of two natural sesquiterpenoids farnesol and nerolidol towards Myzus persicae (Sulzer) (Homoptera: Aphididae). Bull. Entomol. Res. 2020, 110, 249–258. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, M.; Arora, N.; Pruthi, V.; Poluri, K.M. Chemistry and Biology of Farnesol and its derivatives: Quorum sensing molecules with immense therapeutic potential. Curr. Top. Med. Chem. 2018, 18, 1937–1954. [Google Scholar] [CrossRef]
- De Matos, S.P.; Teixeira, H.F.; de Lima, Á.A.N.; Veiga-Junior, V.F.; Koester, L.S. Essential oils and isolated terpenes in nanosystems designed for topical administration: A Review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef]
- Vitiello, G.; Silvestri, B.; Luciani, G. Learning from nature: Bioinspired strategies towards antimicrobial nanostructured systems. Curr. Top. Med. Chem. 2018, 18, 22–41. [Google Scholar] [CrossRef]
- Crick, D.C.; Andres, D.A.; Waechter, C.J. Farnesol is utilized for protein isoprenylation and the biosynthesis of cholesterol in mammalian cells. Biochem. Biophys. Res. Commun. 1995, 211, 590–599. [Google Scholar] [CrossRef]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Van Dijck, P.; Jabra-Rizk, M.A. Modulation of Staphylococcus aureus response to antimicrobials by the Candida albicans quorum sensing molecule farnesol. Antimicrob. Agents Chemother. 2017, 61, e01573-17. [Google Scholar] [CrossRef] [PubMed]
- Shchepin, R.; Hornby, J.M.; Burger, E.; Niessen, T.; Dussault, P.; Nickerson, K.W. Quorum sensing in Candida albicans: Probing farnesol’s mode of action with 40 natural and synthetic farnesol analogs. Chem. Biol. 2003, 10, 743–750. [Google Scholar] [CrossRef]
- Rodriques, C.F.; Černáková, L. Farnesol and Tyrosol: Secondary metabolites with a crucial quorum-sensing role in Candida biofilm development. Genes 2020, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Watanabe, T.; Mikami, T.; Matsumoto, T. Farnesol, a morphogenetic autoregulatory substance in the dimorphic fungus Candida albicans, inhibits hyphae growth through suppression of a mitogen-activated protein kinase cascade. Biol. Pharm. Bull. 2004, 27, 751–752. [Google Scholar] [CrossRef]
- Davis-Hanna, A.; Piispanen, A.E.; Stateva, L.I.; Hogan, D.A. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol. Microbiol. 2008, 67, 47–62. [Google Scholar] [CrossRef]
- Zakikhany, K.; Naglik, J.R.; Schmidt-Westhausen, A.; Holland, G.; Schaller, M.; Hube, B. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol. 2007, 9, 2938–2954. [Google Scholar] [CrossRef]
- Martin, R.; Moran, G.P.; Jacobsen, I.D.; Heyken, A.; Domey, J.; Sullivan, D.J.; Kurzai, O.; Hube, B. The Candida albicans-specific gene EED1encodes a key regulator of hyphal extension. PLoS ONE 2011, 6, e18394. [Google Scholar] [CrossRef]
- Nickerson, K.W.; Atkin, A.L. Deciphering fungal dimorphism: Farnesol’s unanswered questions. Mol. Microbiol. 2017, 103, 567–575. [Google Scholar] [CrossRef]
- Nickerson, K.W.; Atkin, A.L.; Hornby, J.M. Quorum sensing in dimorphic fungi: Farnesol and beyond. Appl. Environ. Microbiol. 2006, 72, 3805–3813. [Google Scholar] [CrossRef]
- Sebaa, S.; Boucherit-Otmani, Z.; Courtois, P. Effects of tyrosol and farnesol on Candida albicans biofilm. Mol. Med. Rep. 2019, 19, 3201–3209. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Y.; Cao, Y.B.; Xu, Z.; Ying, K.; Li, Y.; Xie, Y.; Zhu, Z.Y.; Chen, W.S.; Jiang, Y.Y. cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob. Agents Chemother. 2005, 49, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xia, J.; Li, C.; Zuo, L.; Wei, X. The possible molecular mechanisms of farnesol on the antifungal resistance of C. albicans biofilms: The regulation of CYR1 and PDE2. BMC Microbiol. 2018, 18, 203. [Google Scholar] [CrossRef] [PubMed]
- Padder, S.A.; Prasad, R.; Shah, A.H. Quorum sensing: A less known mode of communication among fungi. Microbiol. Res. 2018, 210, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A.; Meiller, T.F.; James, C.E.; Shirtliff, M.E. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Agents Chemother. 2006, 50, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Kong, E.F.; Ibrahim, A.; Piepenbrink, K.; Shetty, A.C.; McCracken, C.; Bruno, V.; Jabra-Rizk, M.A. Candida albicans quorum-sensing molecule farnesol modulates staphyloxanthin production and activates the thiol-based oxidative-stress response in Staphylococcus aureus. Virulence 2019, 10, 625–642. [Google Scholar] [CrossRef]
- Jeon, J.G.; Pandit, S.; Xiao, J.; Greqoire, S.; Falsetta, M.L.; Klein, M.I. Influences of trans-trans farnesol, a membrane-targeting sesquiterpenoid, on Streptococcus mutans physiology and survival within mixed-species oral biofilms. J. Oral Sci. 2011, 3, 98–106. [Google Scholar] [CrossRef]
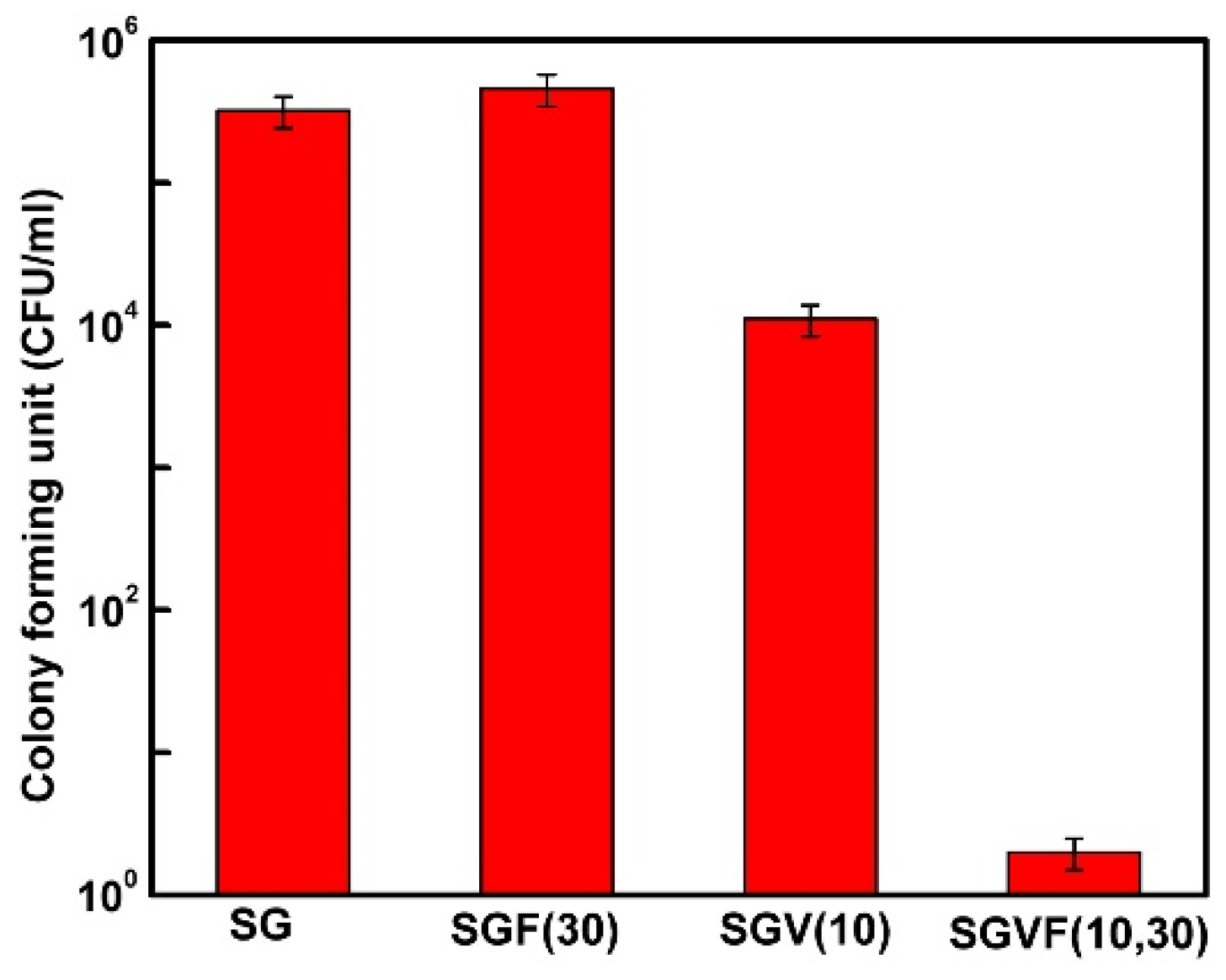
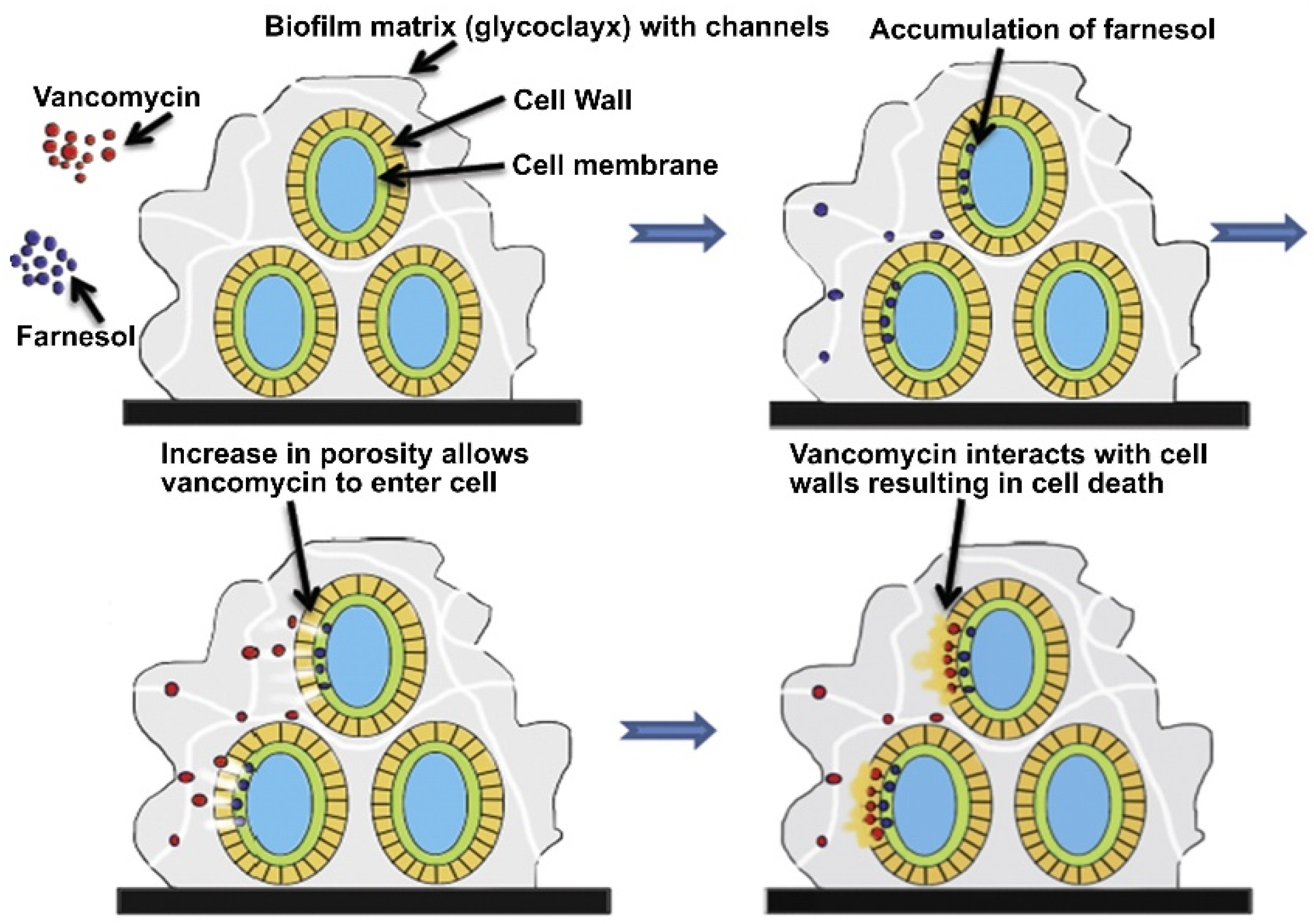
- Pammi, M.; Liang, R.; Hicks, J.M.; Barrish, J.; Versalovic, J. Farnesol decreases biofilms of Staphylococcus epidermidis and exhibits synergy with nafcillin and vancomycin. Pediatr. Res. 2011, 70, 578–583. [Google Scholar] [CrossRef]
- Dixon, E.F.; Hall, R.A. Noisy neighbourhoods: Quorum sensing infungal–polymicrobial infections. Cell. Microbiol. 2015, 17, 1431–1441. [Google Scholar] [CrossRef]
- Cugini, C.; Calfee, M.W.; Farrow, J.M., III; Morales, D.K.; Pesci, E.C.; Hogan, D.A. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol. Microbiol. 2007, 65, 896–906. [Google Scholar] [CrossRef]
- Brehm-Stecher, B.F.; Johnson, E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 2003, 47, 3357–3360. [Google Scholar] [CrossRef]
- Semighini, C.P.; Hornby, J.M.; Dumitru, R.; Nickerson, K.W.; Harris, S.D. Farnesol-induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi. Mol. Microbiol. 2006, 59, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Zhou, Y.; Wei, X. Farnesol induces apoptosis-like cell death in the pathogenic fungus Aspergillus flavus. Mycologia 2014, 106, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, I.; Spielberg, S.; Michael Weber, M.; Albrecht-Eckardt, D.; Bläss, M.; Claus, R.; Barz, C.; Scherlach, K.; Hertweck, C.; Löffler, J.; et al. The fungal quorum-sensing molecule farnesol activates innate immune cells but suppresses cellular adaptive immunity. mBio 2015, 6, e00143-15. [Google Scholar] [CrossRef] [PubMed]
- Mosel, D.D.; Dumitru, R.; Hornby, J.M.; Atkin, A.L.; Nickerson, K.W. Farnesol concentrations required to block germ tube formation in Candida albicans in the presence and absence of serum. Appl. Environ. Microbiol. 2005, 71, 4938–4940. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, J. Polymers against Microorganisms. In On the Race to Efficient Antimicrobial Materials, 1st ed.; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar]
- Nowacka, M.; Rygała, A.; Kręgiel, D.; Kowalewska, A. Poly(silsesquioxanes) and poly(siloxanes) grafted with N-acetylcysteine for eradicating mature bacterial biofilms in water environment. Coll. Surf. B Biointerf. 2018, 172, 627–634. [Google Scholar] [CrossRef]
- Kręgiel, D.; Rygała, A.; Kolesińska, B.; Nowacka, M.; Herc, A.S.; Kowalewska, A. Antimicrobial and antibiofilm N-acetyl-L-cysteine grafted siloxane polymers with potential for use in water systems. Int. J. Mol. Sci. 2019, 20, 2011. [Google Scholar] [CrossRef]
- Santos, M.R.E.; Fonseca, A.C.; Mendonça, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Recent developments in antimicrobial polymers: A review. Materials 2016, 9, 599. [Google Scholar] [CrossRef]
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chen, C.-Y.; Lu, Y.-Y.; Lin, Y.-S. Recent advances in antimicrobial polymers: A mini-review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef]
- Liu, N.-Y.; Zhu, J.-Y.; Zhang, T.; Dong, S.-L. Characterization of two odorant binding proteins in Spodoptera exigua reveals functional conservation and difference. Comp. Biochem. Phys. A 2017, 213, 20–27. [Google Scholar] [CrossRef]
- Kim, C.; Kim, H. Synthesis of Farnesyl-Terminated Carbosilane Dendrimer. Synthesis 2005, 3, 381–386. [Google Scholar] [CrossRef]
- Parker, D.J.; Jones, H.A.; Petcher, S.; Cervini, L.; Griffin, J.M.; Akhtarb, R.; Hasell, T. Low cost and renewable sulfur-polymers by inverse vulcanisation, and their potential for mercury capture. J. Mater. Chem. A 2017, 5, 11682–11692. [Google Scholar] [CrossRef]
- Manteghi, A.; Ahmadi, S.; Arabi, H. Polyolefin elastomer grafted unsaturated hindered phenol esters: Synthesis and antioxidant behavior. Des. Monomers Polym. 2016, 19, 569–576. [Google Scholar] [CrossRef][Green Version]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Zhang, Y.; Li, Q.; Ma, Z.; Dong, J.Y.; Hu, Y. Synthesis and properties of polyethylene-bound antioxidants. Macromol. Chem. Phys. 2014, 215, 763–775. [Google Scholar] [CrossRef]
- Laschke, M.W.; Strohe, A.; Scheuer, C.; Eglin, D.; Verrier, S.; Alini, M.; Pohlemann, T.; Menger, M.D. In vivo biocompatibility and vascularization of biodegradable porous polyurethane scaffolds for tissue engineering. Acta Biomaterialia 2009, 5, 1991–2001. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Eglin, D.; Grad, S.; Gogolewski, S.; Alini, M. Farsenol-modified biodegradable polyurethanes for cartilage tissue engineering. J. Biomed. Mater. Res. A. 2010, 92, 393–408. [Google Scholar] [CrossRef]
- Hawser, S.P.; Douglas, L.J. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 1994, 62, 915–921. [Google Scholar] [CrossRef]
- Deveau, A.; Hogan, D.A. Linking quorum sensing regulation and biofilm formation by Candida albicans. Methods Mol. Biol. 2011, 692, 219–233. [Google Scholar]
- Bhattacharyya, S.; Agrawal, A.; Knabe, C.; Ducheyne, P. Sol gel silica controlled release thin films for the inhibition of methicillin-resistant Staphylococcus aureus. Biomaterials 2014, 35, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.E. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Schilling, A.; Neuner, E.; Rehm, S.J. Vancomycin: A 50-something-year-old antibiotic we still don’t understand. Cleve. Clin. J. Med. 2011, 78, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Leite, B.; Teixeira, P.; Cerca, N.; Azeredo, J.; Oliveira, S. Farnesol as antibiotics adjuvant in Staphylococcus epidermidis control in vitro. Am. J. Med. Sci. 2011, 341, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Bhadani, A.; Rane, J.; Veresmortean, C.; Banerjee, S.; John, G. Bio-inspired surfactants capable of generating plant volatiles. Soft Matter. 2015, 11, 3076–3082. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Blankschtein, D.; Shefer, A.; Shefer, S. Thermodynamic prediction of active ingredient loading in polymeric microparticles. J. Controll. Release 1999, 60, 77–100. [Google Scholar] [CrossRef]
- Sousa, F.L.; Horta, S.; Santos, M.; Rocha, S.M.; Trindade, T. Release behavior of trans,trans-farnesol entrapped in amorphous silica capsules. Results Pharma Sci. 2012, 2, 52–56. [Google Scholar] [CrossRef]
- Sousa, F.L.; Santos, M.; Rocha, S.M.; Trindade, T. Encapsulation of essential oils in SiO2 microcapsules and release behaviour of volatile compounds. J. Microencapsul. 2014, 7, 627–635. [Google Scholar] [CrossRef]
- Singh, R.; Smitha, M.S.; Singh, S.P. The role of nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotechnol. 2014, 14, 1–12. [Google Scholar] [CrossRef]
- Qayyum, S.; Khan, A.U. Nanoparticles vs. biofilms: A battle against another paradigm of antibiotic resistance. Med. Chem. Commun. 2016, 7, 1479–1498. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Su, L.; van der Mei, H.C.; Jutte, P.C.; Ren, Y.; Busscher, H.J. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev. 2019, 48, 428–446. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.-M.; Rice, K.C.; Li, X.; Yu, F.; Reinhardt, R.A.; Bayles, K.W.; Wang, D. Tooth-binding micelles for dental caries prevention. Antimicrob. Agents Chemother. 2009, 53, 4898–4902. [Google Scholar] [CrossRef]
- Mogen, A.B.; Chen, F.; Ahn, S.-J.; Burne, R.A.; Wang, D.; Rice, K.C. Pluronics-formulated farnesol promotes efficient killing and demonstrates novel interactions with Streptococcus mutans Biofilms. PLoS ONE 2015, 10, e0133886. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Giuntini, F.; Della Pepa, C.; Dosio, F.; Serpe, L. Recent developments in antibacterial therapy: Focus on stimuli-responsive drug-delivery systems and therapeutic nanoparticles. Molecules 2019, 24, 1991. [Google Scholar] [CrossRef]
- Abraham, T.; Mao, M.; Tan, C. Engineering approaches of smart, bio-inspired vesicles for biomedical applications. Phys. Biol. 2018, 15, 061001. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Garcia-Gonzalez, C.A.; Bucio, E.; Concheiro, A. Stimuli-responsive polymers for antimicrobial therapy: Drug targeting, contact killing surfaces and competitive release. Expert Opin. Drug Deliv. 2016, 13, 1109–1119. [Google Scholar] [CrossRef]
- Benoit, D.S.W.; Sims, K.R., Jr.; Fraser, D. Nanoparticles for oral biofilm treatments. ACS Nano 2019, 13, 4869–4875. [Google Scholar] [CrossRef]
- Hu, C.; Wang, L.-L.; Lin, Y.-Q.; Liang, H.-M.; Zhou, S.-Y.; Zheng, F.; Feng, X.-L.; Rui, Y.-Y.; Shao, L.-Q. Nanoparticles for the treatment of oral biofilms: Current state, mechanisms, influencing factors, and prospects. Adv. Healthcare Mater. 2019, 8, 1901301. [Google Scholar] [CrossRef]
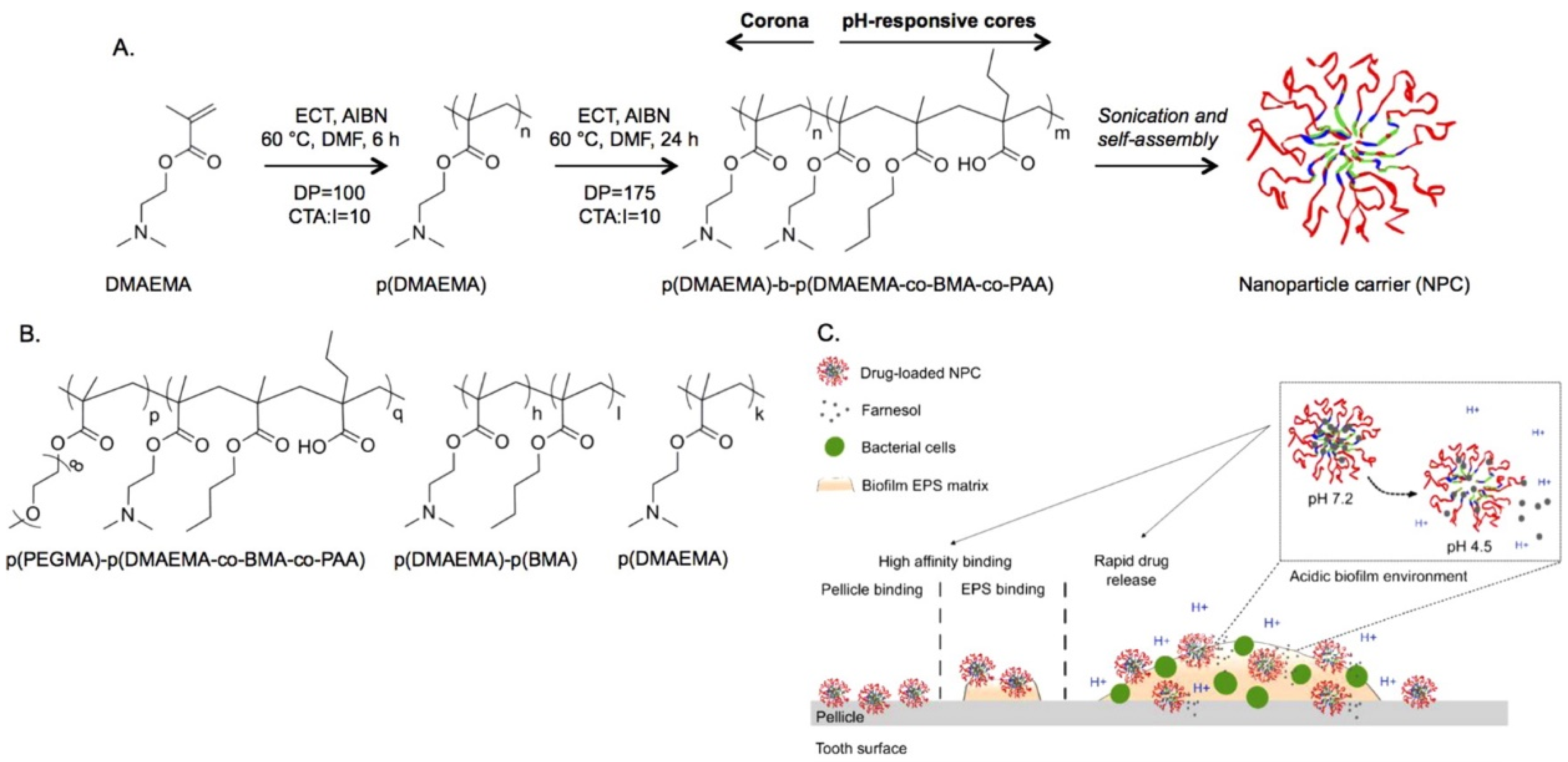
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S.W. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef]
- Zhou, J.; Horev, B.; Hwang, G.; Klein, M.I.; Koo, H.; Benoit, D.S.W. Characterization and optimization of pH responsive polymer nanoparticles for drug delivery to oral biofilms. J. Mater. Chem. B 2016, 4, 3075–3085. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, L.; Chen, L.; Lin, Y.; Luo, Z.; Chen, Z.; Li, T.; Wu, J.; Zhong, Z. Farnesal-loaded pH-sensitive polymeric micelles provided effective prevention and treatment on dental caries. J. Nanobiotechnol. 2020. [Google Scholar] [CrossRef]
- Dora, C.P.; Singh, S.K.; Kumar, S.; Datusalia, A.K.; Deep, A. Development and characterization of nanoparticles of glibenclamide by solvent displacement method. Acta Pol. Pharm. 2010, 67, 283–290. [Google Scholar] [PubMed]
- Sims, K.R., Jr.; Liu, Y.; Hwang, G.; Jung, H.I.; Koo, H.; Benoit, D.S.W. Enhanced design and formulation of nanoparticles for anti-biofilm drug delivery. Nanoscale 2019, 11, 219–236. [Google Scholar] [CrossRef]
- Barot, T.; Rawtani, D.; Kulkarni, P.; Hussain, C.M.; Akkireddy, S. Physicochemical and biological assessment of flowable resin composites incorporated with farnesol loaded halloysite nanotubes for dental applications. J. Mech. Behav. Biomed. Mater. 2020, 104, 103675. [Google Scholar] [CrossRef]
- Cavallaro, G.; Chiappisi, L.; Pasbakhsh, P.; Gradzielski, M.; Lazzara, G. A structural comparison of halloysite nanotubes of different origin by Small-Angle Neutron Scattering (SANS) and Electric Birefringence. Appl. Clay Sci. 2018, 160, 71–80. [Google Scholar] [CrossRef]
- Pandey, G.; Munguambe, D.M.; Tharmavaram, M.; Rawtani, D.; Agrawal, Y.K. Halloysite nanotubes-An efficient ‘nano-support’ for the immobilization of α-amylase. Appl. Clay Sci. 2017, 136, 184–191. [Google Scholar] [CrossRef]
- De Castilho, A.R.F.; Rosalen, P.L.; de Souza Arau´jo, I.J.; Kitagawa, I.L.; de Araujo Costa, C.A.; Janal, M.N.; Alves, M.C.; Duarte, S.; Filho, P.N.L.; Stipp, R.N.; et al. Trans,trans-farnesol, an antimicrobial natural compound, improves glass ionomer cement properties. PLoS ONE 2019, 14, e0220718. [Google Scholar] [CrossRef]
- Chávez-Andrade, G.-M.; Tanomaru-Filho, M.; Basso Bernardi, M.I.; de Toledo Leonardo, R.; Faria, G.; Guerreiro-Tanomaru, J.M. Antimicrobial and biofilm anti-adhesion activities of silver nanoparticles and farnesol against endodontic microorganisms for possible application in root canal treatment. Arch. Oral Biol. 2019, 107, 104481. [Google Scholar] [CrossRef]
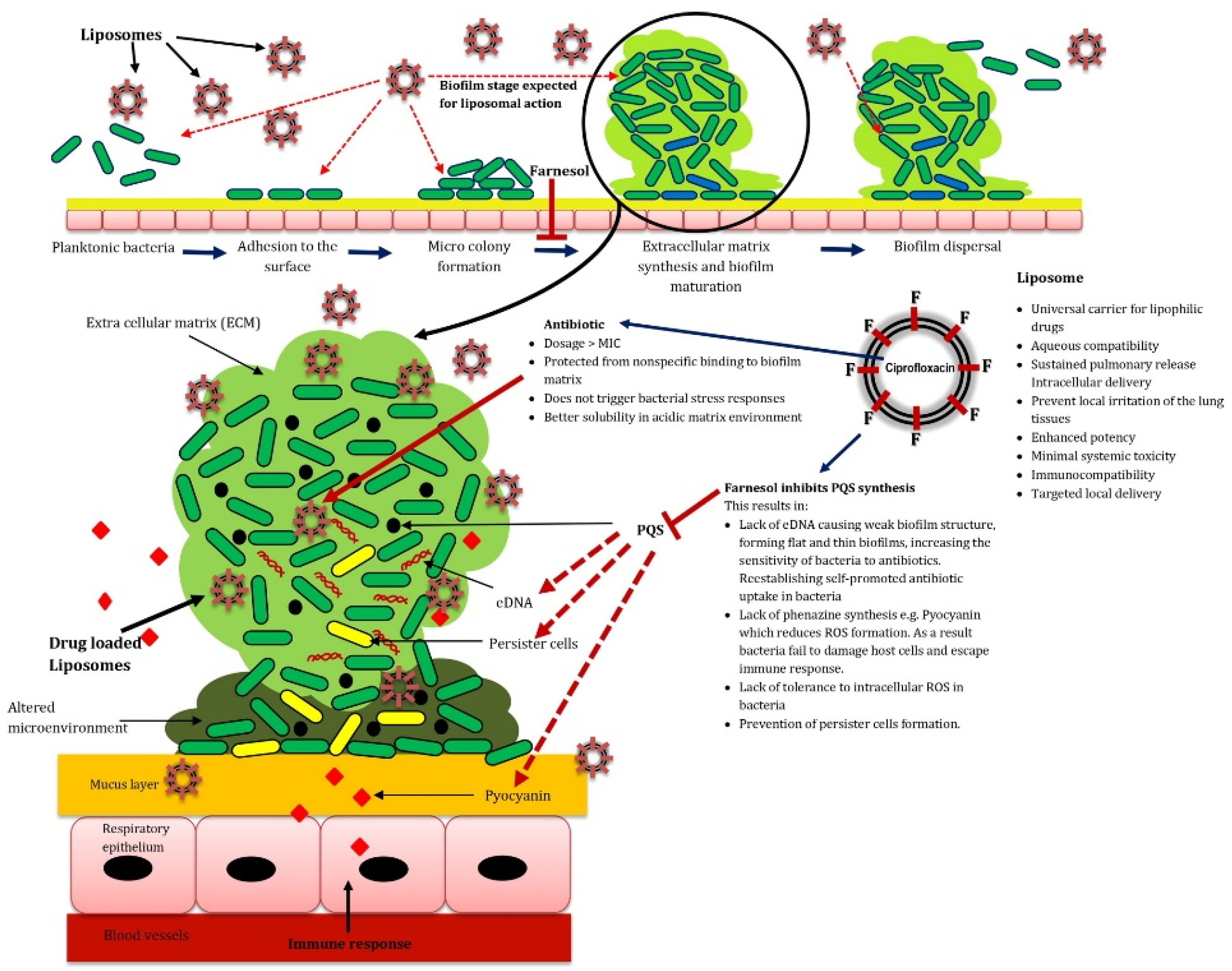
- Bandara, H.M.H.N.; Herpin, M.J.; Kolacny, D., Jr.; Harb, A.; Romanovicz, D.; Smyth, H.D.C. Incorporation of farnesol significantly increases the efficacy of liposomal Ciprofloxacin against Pseudomonas aeruginosa biofilms in vitro. Mol. Pharm. 2016, 13, 2760–2770. [Google Scholar] [CrossRef]
- Costa, A.F.; Araujo, D.E.; Cabral, M.S.; Brito, I.T.; De Menezes Leite, L.B.; Pereira, M.; Amaral, A.C. Development, characterization, and in vitro–in vivo evaluation of polymeric nanoparticles containing miconazole and farnesol for treatment of vulvovaginal candidiasis. Med. Mycol. 2019, 57, 52–62. [Google Scholar] [CrossRef]
- Nikoomanesh, F.; Roudbarmohammadi, S.; Khoobi, M.; Haghighi, F.; Roudbary, M. Design and synthesis of mucoadhesive nanogel containing farnesol: Investigation of the effect on HWP1, SAP6 and Rim101 genes expression of Candida albicans in vitro. Artif. Cell. Nanomed. Biotechnol. 2019, 47, 64–72. [Google Scholar] [CrossRef]
- Wu, G.X.; Huang, H.H.; Chang, H.R.; Kuo, S.M. Evaluation of the UVB-screening capacity and restorative effects exerted by farnesol gel on UVB-caused sunburn. Environ. Toxicol. 2018, 33, 488–507. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Huang, H.H.; Wu, G.X.; Chang, H.R.; Wu, K.L.; Kuo, S.M. Antiaging and smoothness-improving properties of farnesol-based facial masks on rat skin exposed to ultraviolet B. J. Cosmet. Dermatol. 2020, 19, 540–552. [Google Scholar] [CrossRef]
- Wu, Y.C.; Wu, G.X.; Huang, H.H.; Shyh Ming Kuo, S.M. Liposome-encapsulated farnesol accelerated tissue repair in third-degree burns on a rat model. Burns 2019, 45, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; He, S.; Yang, Y.; Jian, D.; Chen, X.; Ding, J. Formulation, characterization and clinical evaluation of propranolol hydrochloride gel for transdermal treatment of superficial infantile hemangioma. Drug Dev. Ind. Pharm. 2015, 41, 1109–1119. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, M.; Kowalewska, A.; Kręgiel, D. Farnesol-Containing Macromolecular Systems for Antibiofilm Strategies. Surfaces 2020, 3, 197-210. https://doi.org/10.3390/surfaces3020015
Nowacka M, Kowalewska A, Kręgiel D. Farnesol-Containing Macromolecular Systems for Antibiofilm Strategies. Surfaces. 2020; 3(2):197-210. https://doi.org/10.3390/surfaces3020015
Chicago/Turabian StyleNowacka, Maria, Anna Kowalewska, and Dorota Kręgiel. 2020. "Farnesol-Containing Macromolecular Systems for Antibiofilm Strategies" Surfaces 3, no. 2: 197-210. https://doi.org/10.3390/surfaces3020015
APA StyleNowacka, M., Kowalewska, A., & Kręgiel, D. (2020). Farnesol-Containing Macromolecular Systems for Antibiofilm Strategies. Surfaces, 3(2), 197-210. https://doi.org/10.3390/surfaces3020015