Hydrogenation of ZnFe2O4 Flat Films: Effects of the Pre-Annealing Temperature on the Photoanodes Efficiency for Water Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Photoelectrodes Preparation
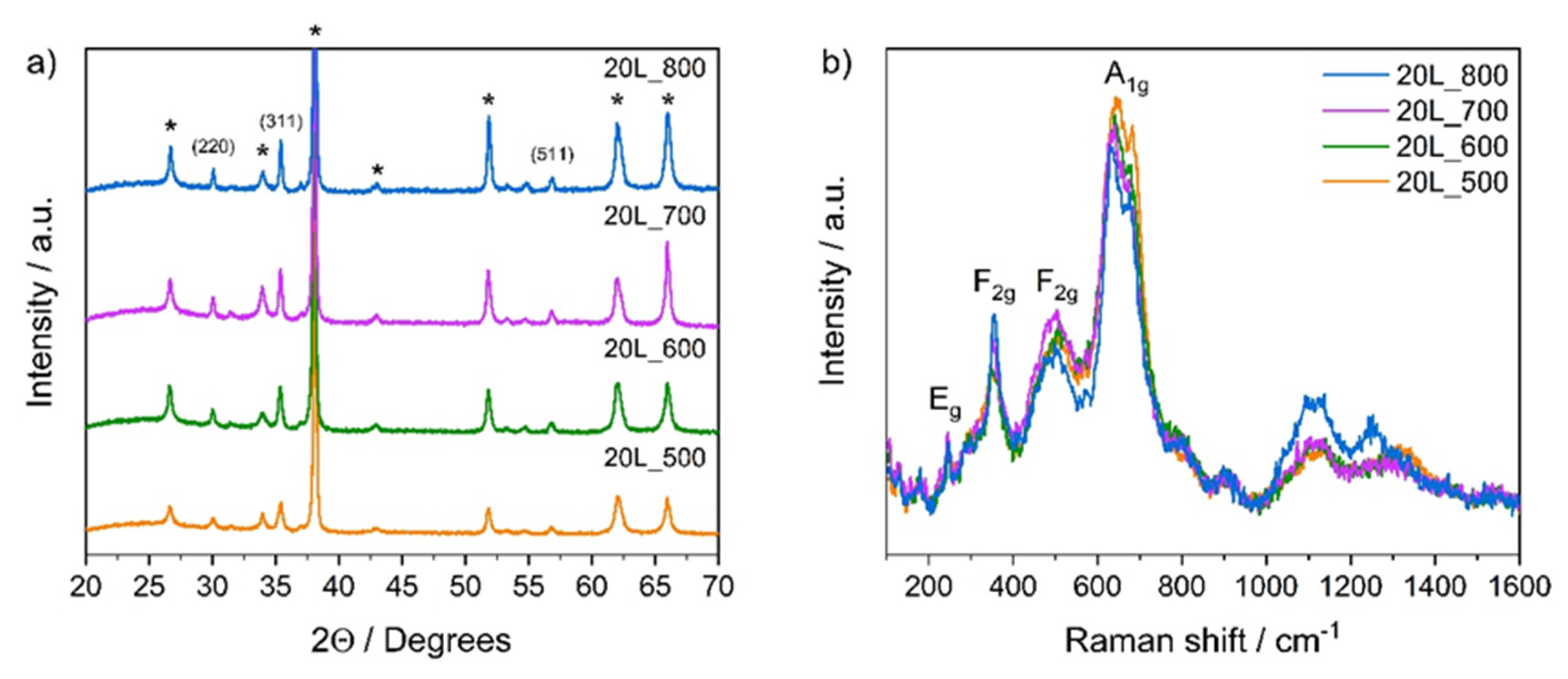
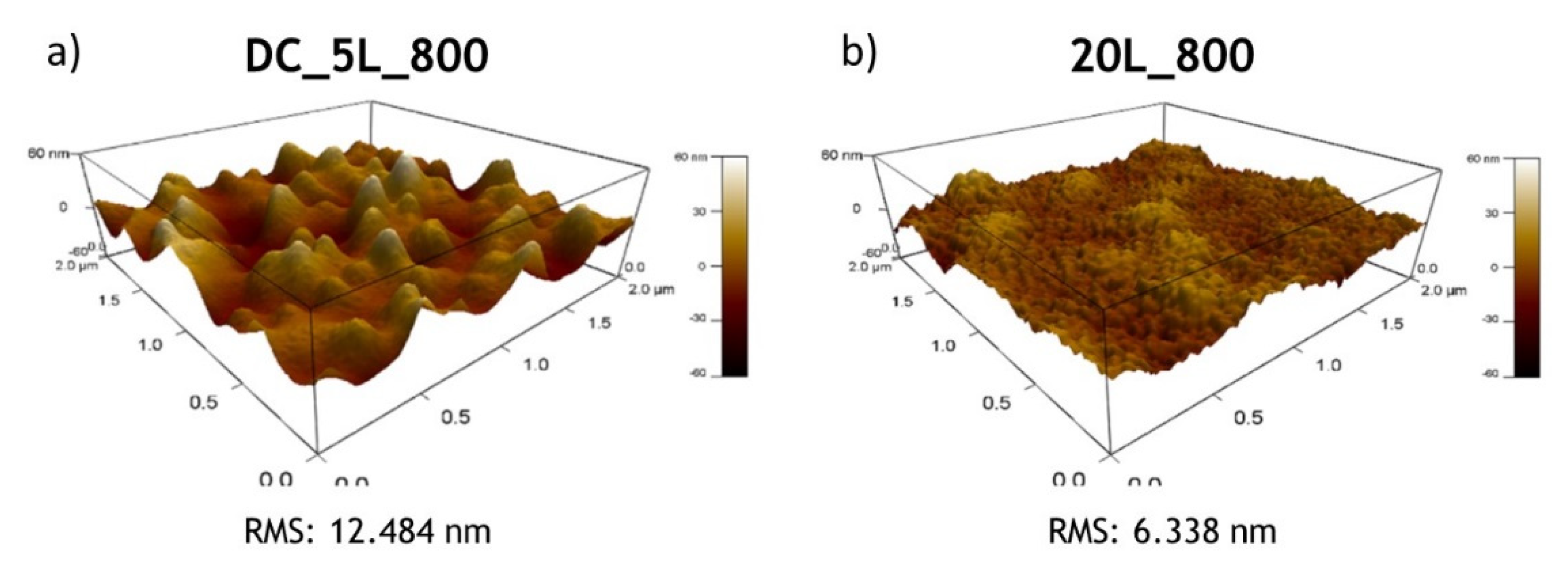
2.3. Structural, Morphological and Optical Characterizations
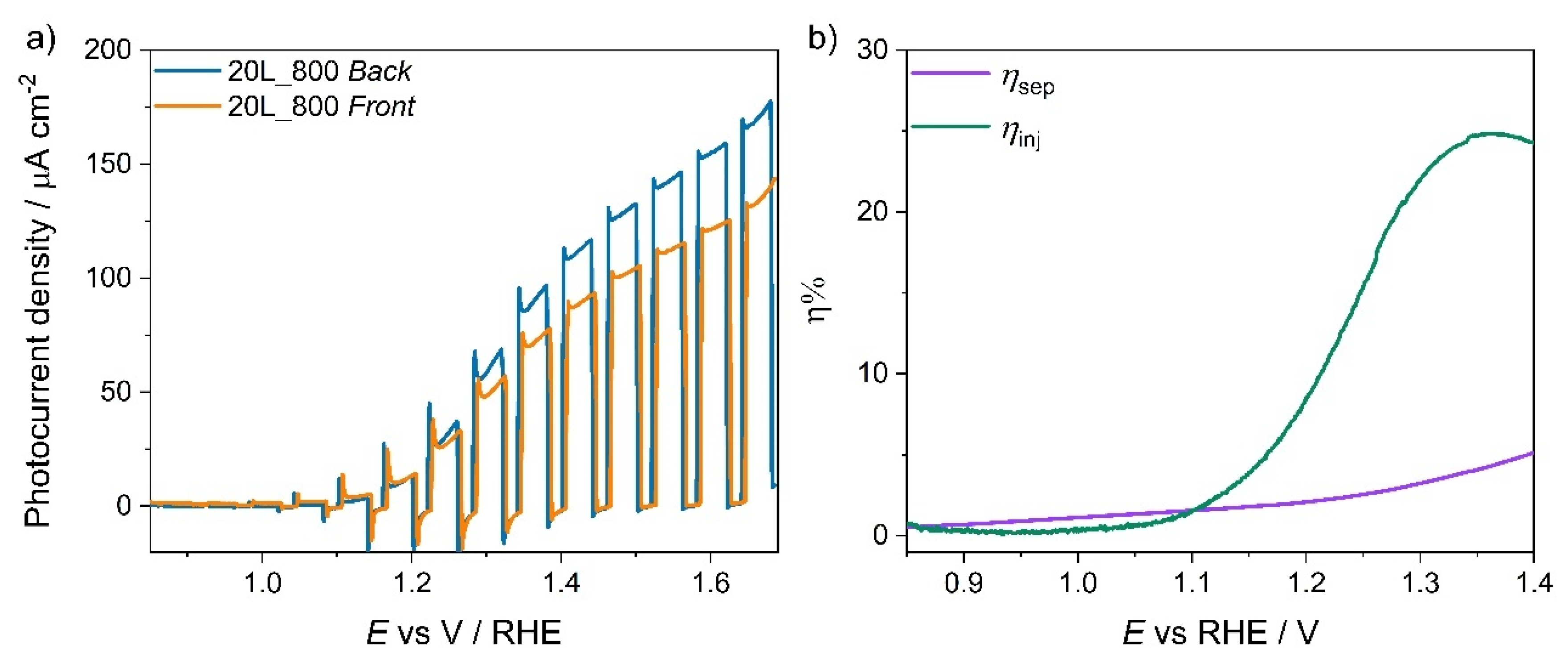
2.4. Photoelectrochemical Tests
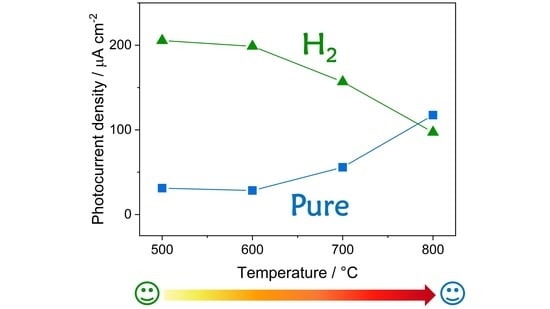
3. Results
3.1. Photoelectrodes Characterization
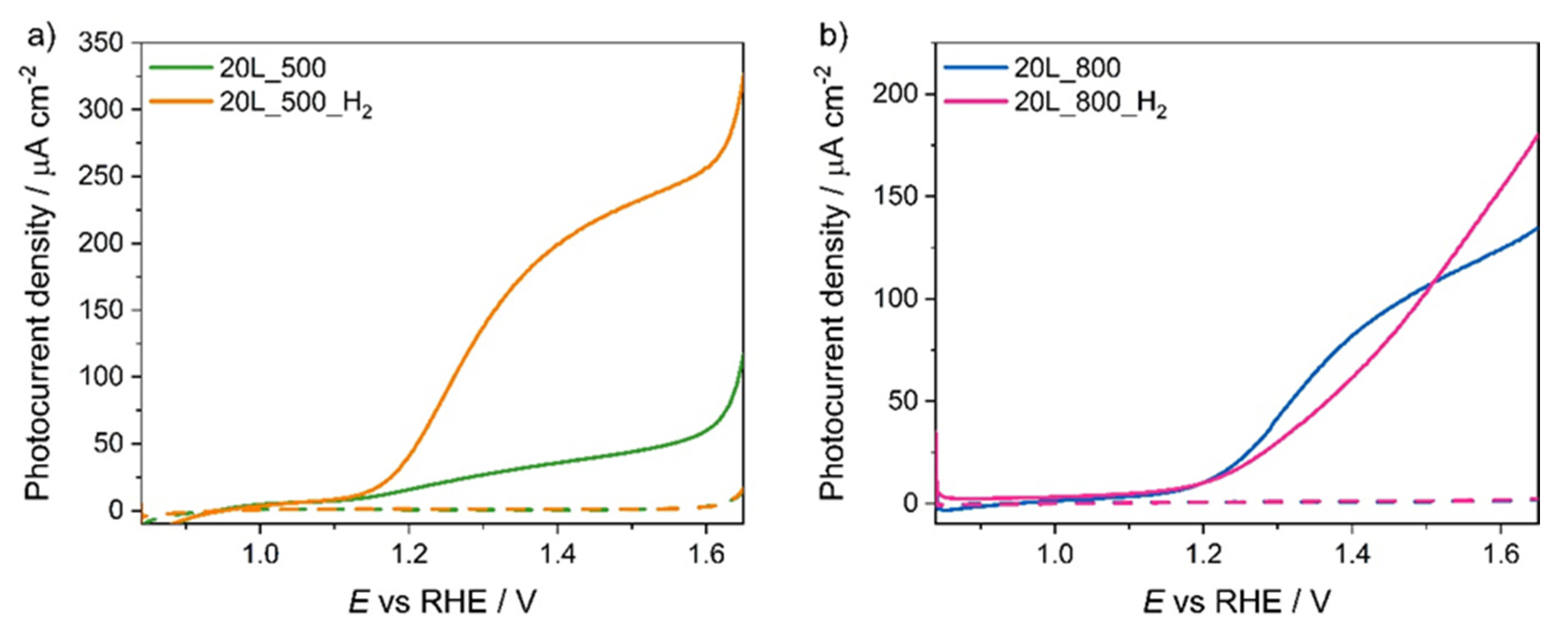
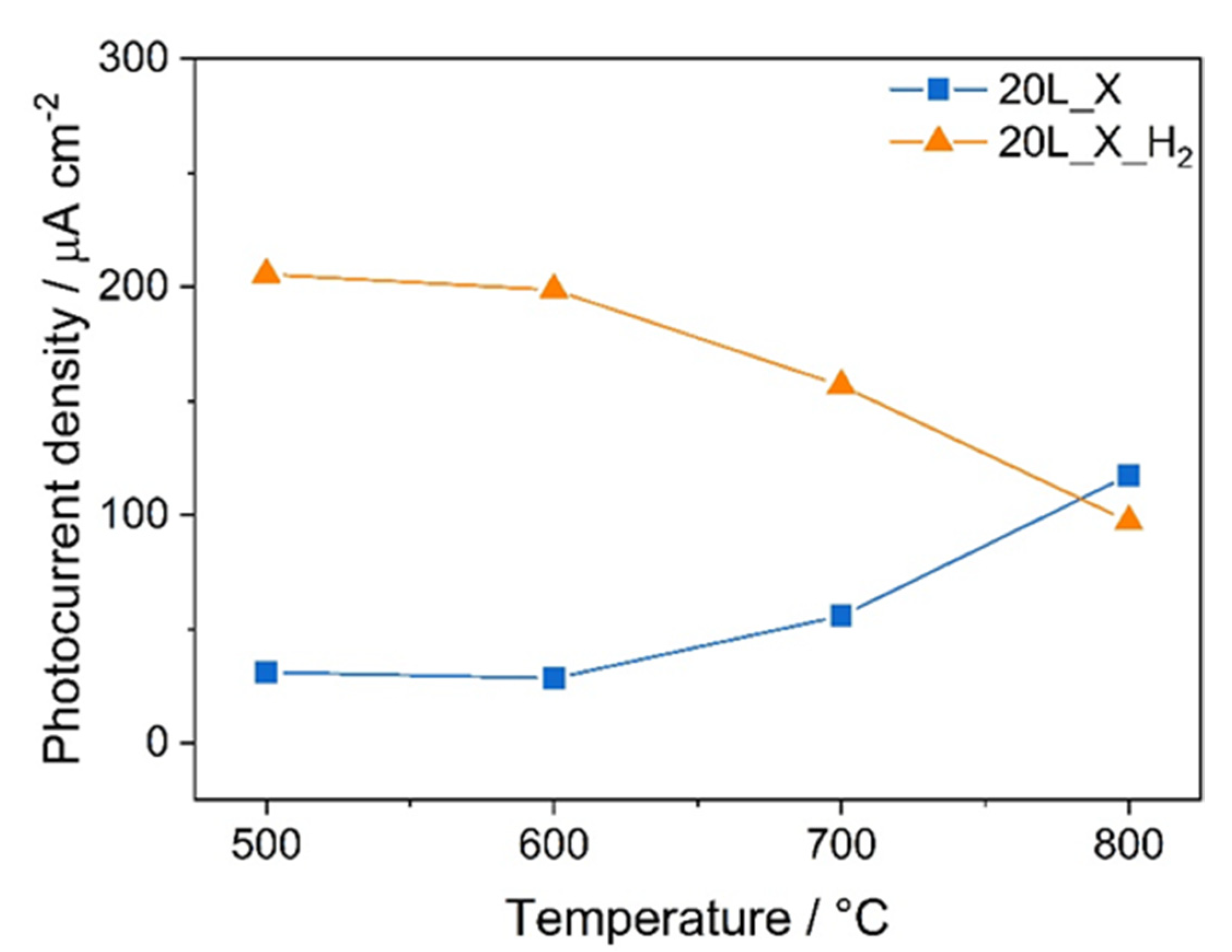
3.2. Photoelectrochemical Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Lewis, N.S. Research opportunities to advance solar energy utilization. Science 2016, 351, 1920. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J. Design of semiconductor photoelectrochemical systems for solar energy conversion. J. Phys. Chem. 1982, 86, 172–177. [Google Scholar] [CrossRef]
- Nielander, A.C.; Shaner, M.R.; Papadantonakis, K.M.; Francis, S.A.; Lewis, N.S. A taxonomy for solar fuels generators. Energy Environ. Sci. 2015, 8, 16–25. [Google Scholar] [CrossRef]
- Prévot, M.S.; Sivula, K. Photoelectrochemical Tandem Cells for Solar Water Splitting. J. Phys. Chem. C 2013, 117, 17879–17893. [Google Scholar] [CrossRef]
- Khaselev, O.; Turner, J.A. A Monolithic Photovoltaic-Photoelectrochemical Device for Hydrogen Production via Water Splitting. Science 1998, 280, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Prévot, M.S.; Li, Y.; Guijarro, N.; Sivula, K. Improving charge collection with delafossite photocathodes: A host–guest CuAlO2/CuFeO2 approach. J. Mater. Chem. A 2016, 4, 3018–3026. [Google Scholar] [CrossRef]
- Lhermitte, C.R.; Polo, A.; Yao, L.; Boudoire, F.A.; Guijarro, N.; Sivula, K. Generalized Synthesis for the Production of Transparent Thin Films of Ternary Metal Oxide Electrodes. Unpublished.
- Abdi, F.F.; Berglund, S.P. Recent developments in complex metal oxide photoelectrodes. J. Phys. D Appl. Phys. 2017, 50, 193002. [Google Scholar] [CrossRef]
- Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Lee, D.K.; Lee, D.; Lumley, M.A.; Choi, K.S. Progress on ternary oxide-based photoanodes for use in photoelectrochemical cells for solar water splitting. Chem. Soc. Rev. 2019, 48, 2126–2157. [Google Scholar] [CrossRef] [PubMed]
- Buriak, J.M.; Toro, C.; Choi, K.S. Chemistry of Materials for Water Splitting Reactions. Chem. Mater. 2018, 30, 7325–7327. [Google Scholar] [CrossRef]
- Lhermitte, C.R.; Sivula, K. Alternative Oxidation Reactions for Solar-Driven Fuel Production. ACS Catal. 2019, 9, 2007–2017. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Doping TiO2 with p-block elements: Effects on photocatalytic activity. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 13–28. [Google Scholar] [CrossRef]
- Polo, A.; Grigioni, I.; Dozzi, M.V.; Selli, E. Sensitizing effects of BiVO4 and visible light induced production of highly reductive electrons in the TiO2/BiVO4 heterojunction. Catal. Today 2020, 340, 19–25. [Google Scholar] [CrossRef]
- Lhermitte, C.R.; Garret Verwer, J.; Bartlett, B.M. Improving the stability and selectivity for the oxygen-evolution reaction on semiconducting WO3 photoelectrodes with a solid-state FeOOH catalyst. J. Mater. Chem. A 2016, 4, 2960–2968. [Google Scholar] [CrossRef]
- Grigioni, I.; Abdellah, M.; Corti, A.; Dozzi, M.V.; Hammarström, L.; Selli, E. Photoinduced Charge-Transfer Dynamics in WO3/BiVO4 Photoanodes Probed through Midinfrared Transient Absorption Spectroscopy. J. Am. Chem. Soc. 2018, 140, 14042–14045. [Google Scholar] [CrossRef]
- Dotan, H.; Sivula, K.; Grätzel, M.; Rothschild, A.; Warren, S.C. Probing the photoelectrochemical properties of hematite (α-Fe2O3) electrodes using hydrogen peroxide as a hole scavenger. Energy Environ. Sci. 2011, 4, 958–964. [Google Scholar] [CrossRef]
- Lhermitte, C.R.; Bartlett, B.M. Advancing the Chemistry of CuWO4 for Photoelectrochemical Water Oxidation. Acc. Chem. Res. 2016, 49, 1121–1129. [Google Scholar] [CrossRef]
- de Haart, L.G.J.; Blasse, G. Photoelectrochemical Properties of Ferrites with the Spinel Structure. J. Electrochem. Soc. 1985, 132, 2933–2938. [Google Scholar] [CrossRef]
- Zhu, X.; Guijarro, N.; Liu, Y.; Schouwink, P.; Wells, R.A.; Le Formal, F.; Sun, S.; Gao, C.; Sivula, K. Spinel structural disorder influences solar-water-splitting performance of ZnFe2O4 nanorod photoanodes. Adv. Mater. 2018, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, N.; Bornoz, P.; Prévot, M.; Yu, X.; Zhu, X.; Johnson, M.; Jeanbourquin, X.; Le Formal, F.; Sivula, K. Evaluating spinel ferrites MFe2O4 (M = Cu, Mg, Zn) as photoanodes for solar water oxidation: Prospects and limitations. Sustain. Energy Fuels 2018, 2, 103–117. [Google Scholar] [CrossRef]
- Dillert, R.; Taffa, D.H.; Wark, M.; Bredow, T.; Bahnemann, D.W. Research Update: Photoelectrochemical water splitting and photocatalytic hydrogen production using ferrites (MFe2O4) under visible light irradiation. APL Mater. 2015, 3, 104001. [Google Scholar] [CrossRef]
- Prévot, M.S.; Jeanbourquin, X.A.; Bourée, W.S.; Abdi, F.; Friedrich, D.; van de Krol, R.; Guijarro, N.; Le Formal, F.; Sivula, K. Evaluating Charge Carrier Transport and Surface States in CuFeO2 Photocathodes. Chem. Mater. 2017, 29, 4952–4962. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.J.; Kim, J.H.; Jang, J.W.; Choi, S.H.; Lee, J.S. Defective ZnFe2O4 nanorods with oxygen vacancy for photoelectrochemical water splitting. Nanoscale 2015, 7, 19144–19151. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Chen, S.; Huang, Y.; Sum, T.C.; Chen, Z. New insight into the roles of oxygen vacancies in hematite for solar water splitting. Phys. Chem. Chem. Phys. 2017, 19, 1074–1082. [Google Scholar] [CrossRef]
- Rioult, M.; Stanescu, D.; Fonda, E.; Barbier, A.; Magnan, H. Oxygen Vacancies Engineering of Iron Oxides Films for Solar Water Splitting. J. Phys. Chem. C 2016, 120, 7482–7490. [Google Scholar] [CrossRef]
- Liu, Y.; Le Formal, F.; Boudoire, F.; Yao, L.; Sivula, K.; Guijarro, N. Insights into the interfacial carrier behaviour of copper ferrite (CuFe2O4) photoanodes for solar water oxidation. J. Mater. Chem. A 2019, 7, 1669–1677. [Google Scholar] [CrossRef]
- Prévot, M.S.; Guijarro, N.; Sivula, K. Enhancing the Performance of a Robust Sol–Gel-Processed p-Type Delafossite CuFeO2 Photocathode for Solar Water Reduction. ChemSusChem 2015, 8, 1359–1367. [Google Scholar] [CrossRef]
- Wang, Z.; Schiferl, D.; Zhao, Y.; O’Neill, H.S.C. High pressure Raman spectroscopy of spinel-type ferrite ZnFe2O4. J. Phys. Chem. Solids 2003, 64, 2517–2523. [Google Scholar] [CrossRef]
- Bourée, W.S.; Prévot, M.S.; Jeanbourquin, X.A.; Guijarro, N.; Johnson, M.; Formal, F.L.; Sivula, K. Robust Hierarchically Structured Biphasic Ambipolar Oxide Photoelectrodes for Light-Driven Chemical Regulation and Switchable Logic Applications. Adv. Mater. 2016, 28, 9308–9312. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.S.; Warule, S.S.; Muduli, S.; Kale, B.B.; Jouen, S.; Lefez, B.; Hannoyer, B.; Ogale, S.B. Maghemite (hematite) core (shell) nanorods via thermolysis of a molecular solid of Fe-complex. Dalt. Trans. 2011, 40, 8003–8011. [Google Scholar] [CrossRef] [PubMed]
- Murugappan, K.; Silvester, D.S.; Chaudhary, D.; Arrigan, D.W.M. Electrochemical Characterization of an Oleyl-coated Magnetite Nanoparticle-Modified Electrode. ChemElectroChem 2014, 1, 1211–1218. [Google Scholar] [CrossRef]
- Bott, A.W. Electrochemistry of Semiconductors. Curr. Separ. 1998, 17, 87–91. [Google Scholar] [CrossRef]
- Gao, Y.; Hamann, T.W. Quantitative hole collection for photoelectrochemical water oxidation with CuWO4. Chem. Commun. 2017, 53, 1285–1288. [Google Scholar] [CrossRef]
- Bassi, P.S.; Antony, R.P.; Boix, P.P.; Fang, Y.; Barber, J.; Wong, L.H. Crystalline Fe2O3/Fe2TiO5 heterojunction nanorods with efficient charge separation and hole injection as photoanode for solar water oxidation. Nano Energy 2016, 22, 310–318. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Jang, J.W.; Kim, J.Y.; Choi, S.H.; Magesh, G.; Lee, J.; Lee, J.S. Awakening solar water-splitting activity of ZnFe2O4 nanorods by hybrid microwave annealing. Adv. Energy Mater. 2015, 5, 1–9. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polo, A.; Lhermitte, C.R.; Dozzi, M.V.; Selli, E.; Sivula, K. Hydrogenation of ZnFe2O4 Flat Films: Effects of the Pre-Annealing Temperature on the Photoanodes Efficiency for Water Oxidation. Surfaces 2020, 3, 93-104. https://doi.org/10.3390/surfaces3010009
Polo A, Lhermitte CR, Dozzi MV, Selli E, Sivula K. Hydrogenation of ZnFe2O4 Flat Films: Effects of the Pre-Annealing Temperature on the Photoanodes Efficiency for Water Oxidation. Surfaces. 2020; 3(1):93-104. https://doi.org/10.3390/surfaces3010009
Chicago/Turabian StylePolo, Annalisa, Charles R. Lhermitte, Maria Vittoria Dozzi, Elena Selli, and Kevin Sivula. 2020. "Hydrogenation of ZnFe2O4 Flat Films: Effects of the Pre-Annealing Temperature on the Photoanodes Efficiency for Water Oxidation" Surfaces 3, no. 1: 93-104. https://doi.org/10.3390/surfaces3010009
APA StylePolo, A., Lhermitte, C. R., Dozzi, M. V., Selli, E., & Sivula, K. (2020). Hydrogenation of ZnFe2O4 Flat Films: Effects of the Pre-Annealing Temperature on the Photoanodes Efficiency for Water Oxidation. Surfaces, 3(1), 93-104. https://doi.org/10.3390/surfaces3010009