Recent Advancements in the Understanding of the Surface Chemistry in TiO2 Photocatalysis
Abstract
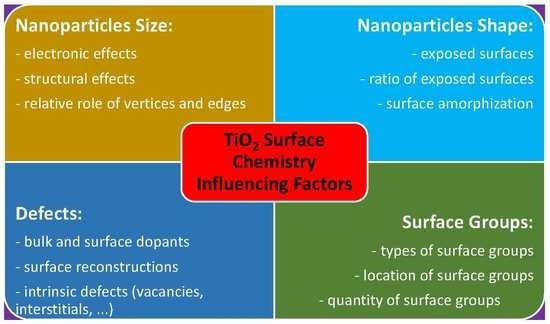
1. Introduction
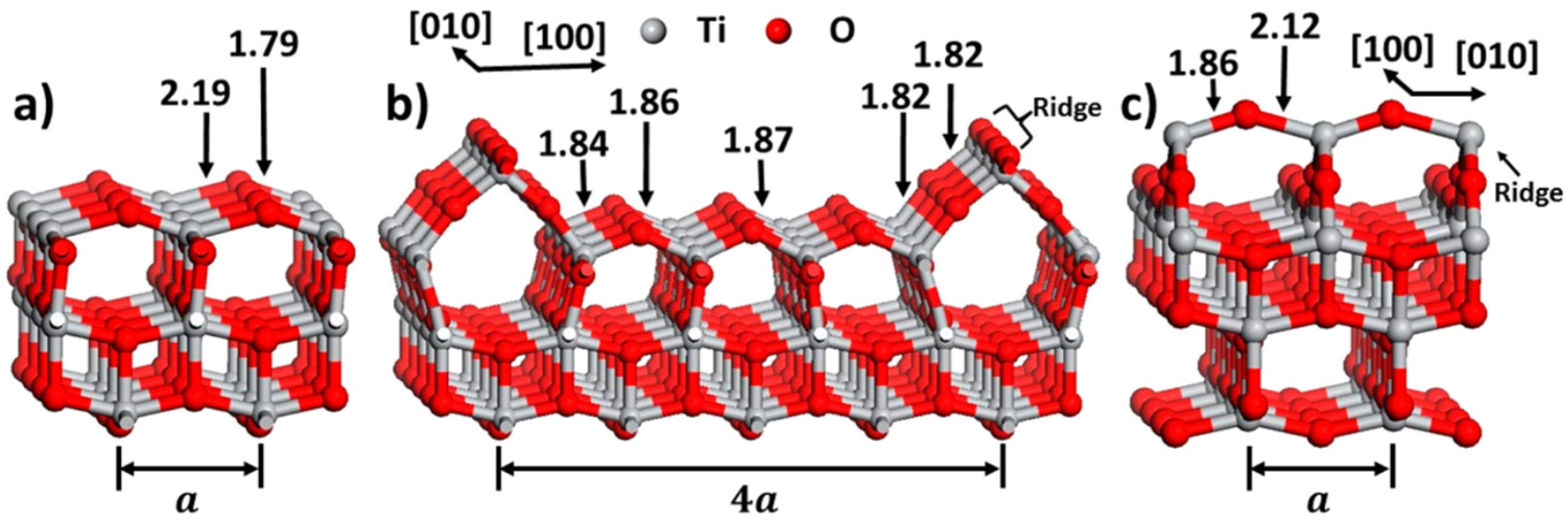
2. The Surface Structure of Bulk TiO2
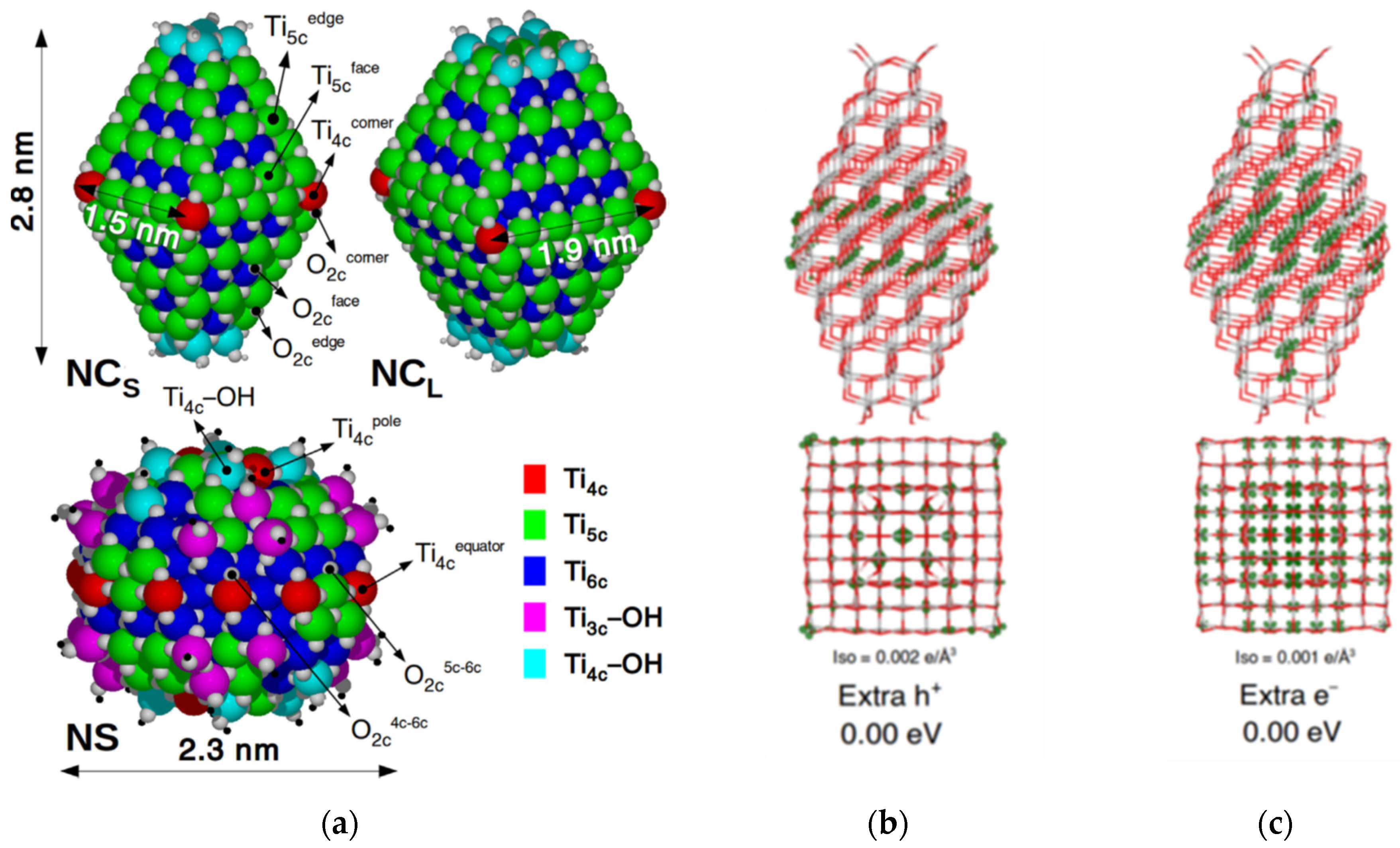
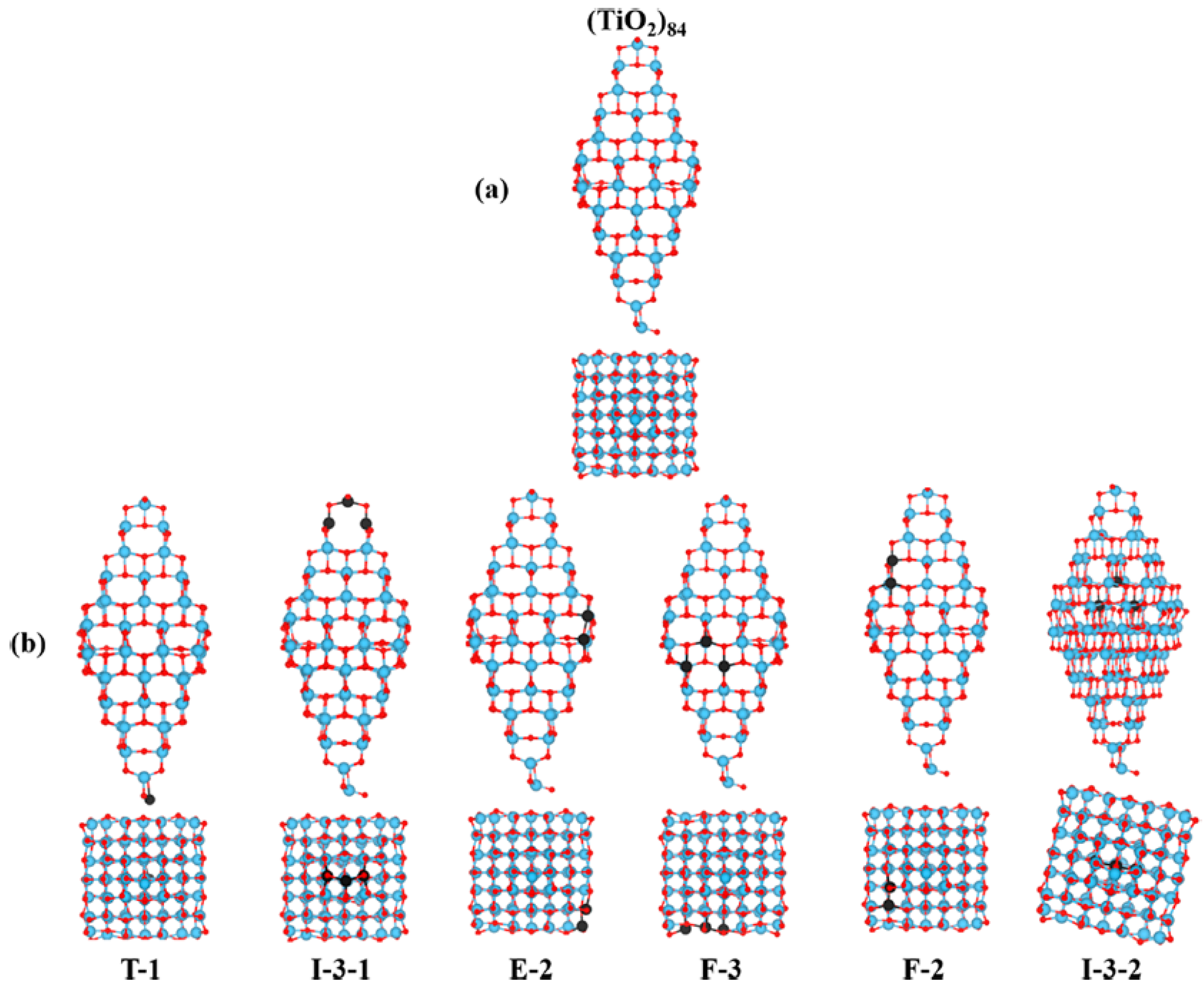
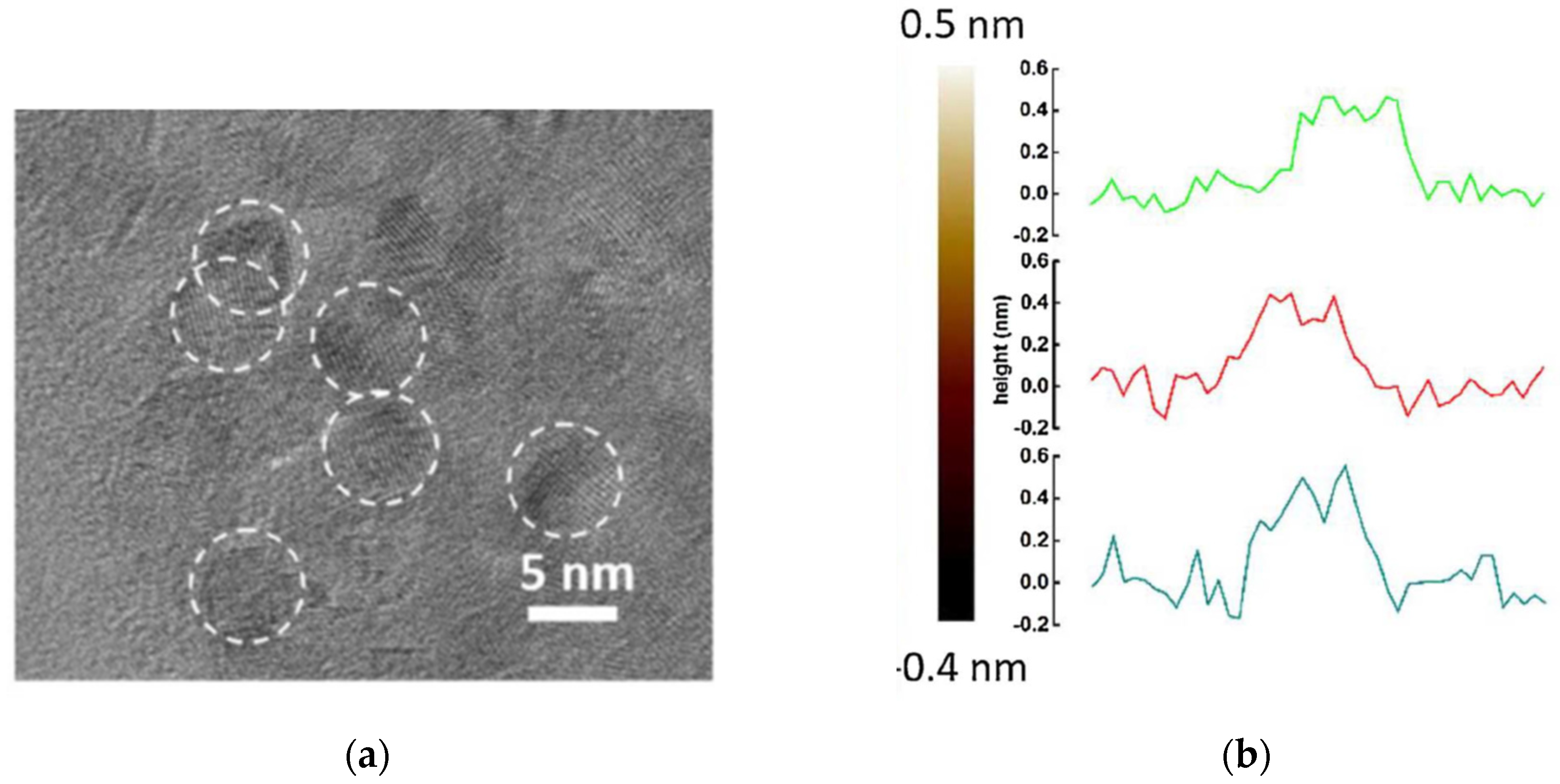
3. The Structure of the Surfaces of TiO2 Nanoparticles
4. The Surface Structure of Two-Dimensional (2D) TiO2
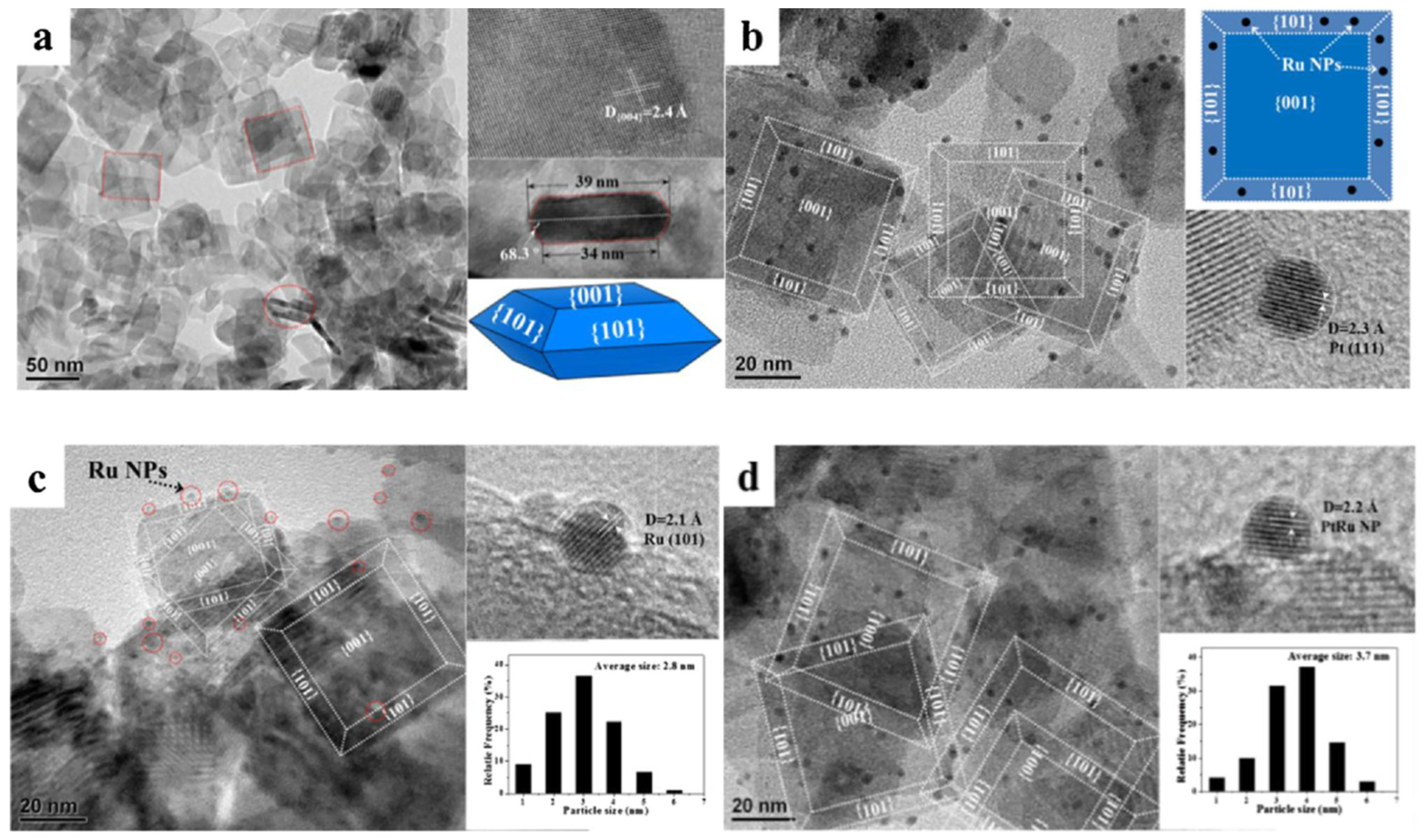
5. Metal-Loaded TiO2 Surfaces
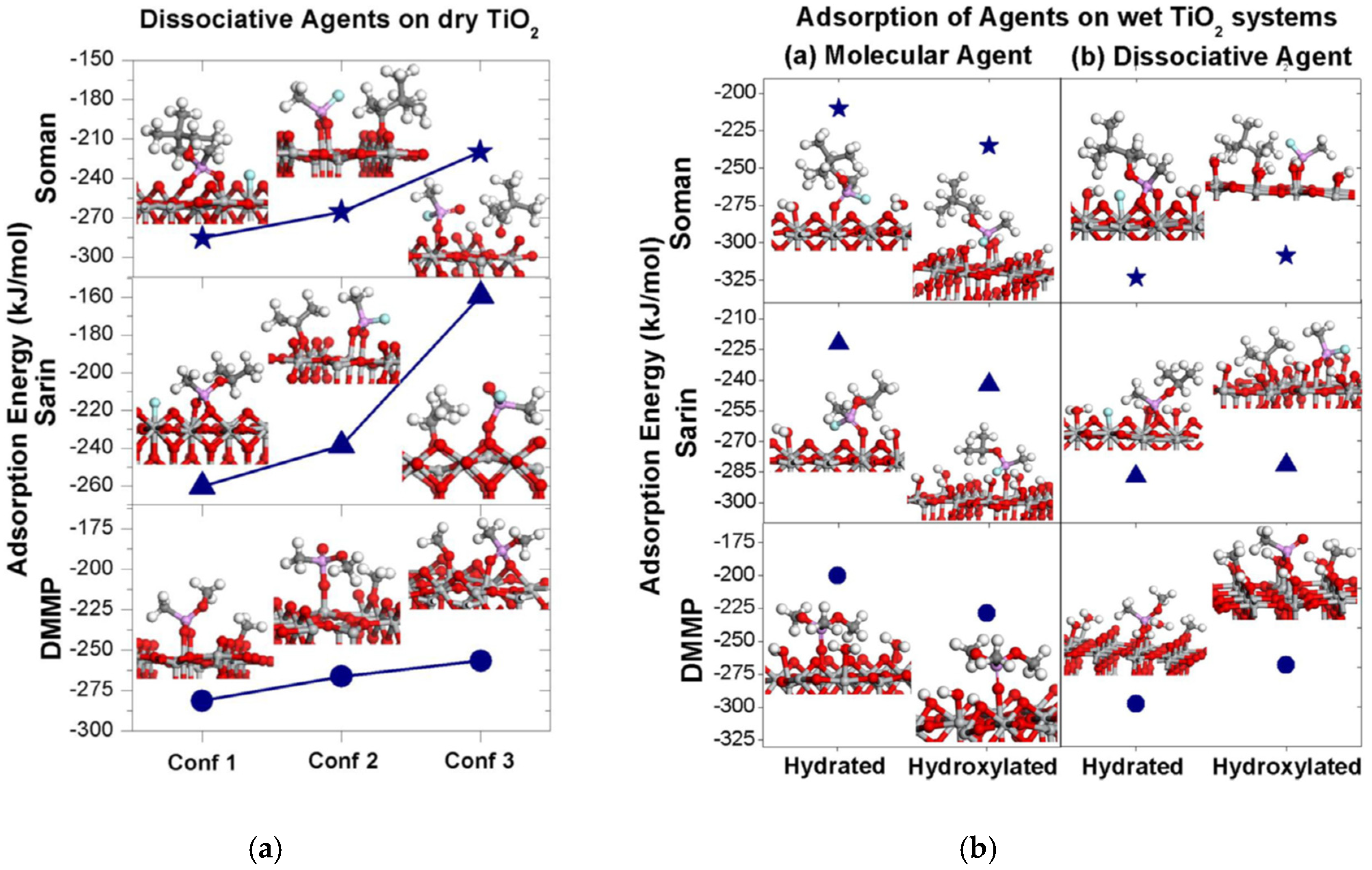
6. Adsorption over TiO2 Surfaces
7. Doping of TiO2 and Its Effect on Adsorption
8. Dark Reactions over TiO2 Surfaces
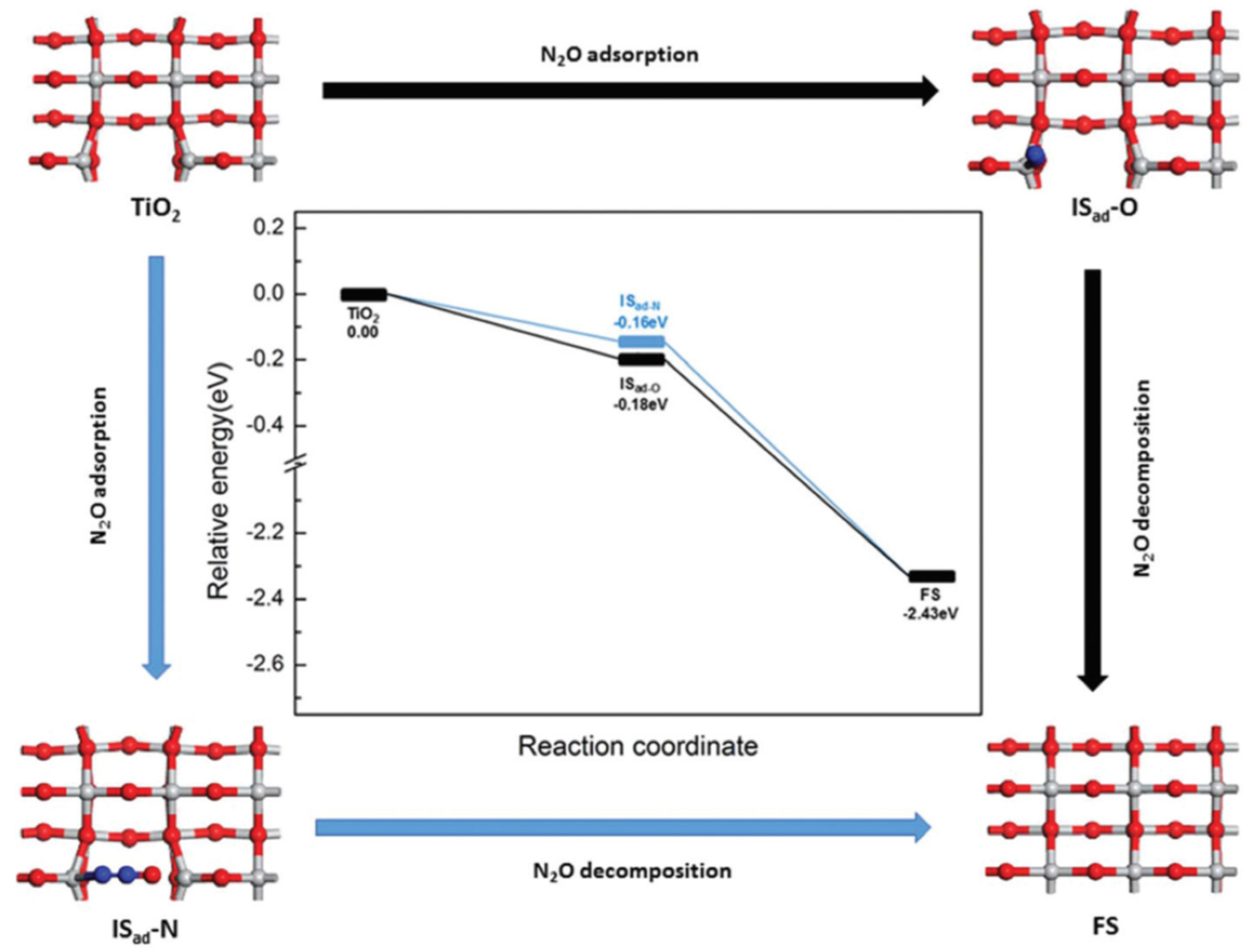
9. Surface Structure Effects in Photoreactions over TiO2
9.1. Clean Anatase TiO2
9.2. Oxygen Vacancies
10. Transient Phenomena in TiO2 as a Tool for Understanding Its Photocatalytic Properties
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C.; Lu, H.; Zhang, J.; Yang, Z.; Zhu, G.; Yin, F.; Gao, J.; Chen, C.; Xin, X. Abnormal p-type sensing response of TiO2 nanosheets with exposed {001} facets. J. Alloys Compd. 2017, 705, 112–117. [Google Scholar] [CrossRef]
- Pližingrová, E.; Klementová, M.; Bezdička, P.; Boháček, J.; Barbieriková, Z.; Dvoranová, D.; Mazúr, M.; Krýsa, J.; Šubrt, J.; Brezová, V. 2D-Titanium dioxide nanosheets modified with Nd, Ag and Au: Preparation, characterization and photocatalytic activity. Catal. Today 2017, 281, 165–180. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Besov, A.S.; Vorontsov, A.V. Fast elimination of organic airborne compounds by adsorption and catalytic oxidation over aerosol TiO2. Catal. Commun. 2008, 9, 2598–2600. [Google Scholar] [CrossRef]
- Tsydenov, D.E.; Shutilov, A.A.; Zenkovets, G.A.; Vorontsov, A.V. Hydrous TiO2 materials and their application for sorption of inorganic ions. Chem. Eng. J. 2014, 251, 131–137. [Google Scholar] [CrossRef]
- Li, C.; Koenigsmann, C.; Ding, W.; Rudshteyn, B.; Yang, K.R.; Regan, K.P.; Konezny, S.J.; Batista, V.S.; Brudvig, G.W.; Schmuttenmaer, C.A.; et al. Facet-Dependent Photoelectrochemical Performance of TiO2 Nanostructures: An Experimental and Computational Study. J. Am. Chem. Soc. 2015, 137, 1520–1529. [Google Scholar] [CrossRef]
- Peng, Y.-K.; Tsang, S.C.E. Facet-dependent photocatalysis of nanosize semiconductive metal oxides and progress of their characterization. Nano Today 2018, 18, 15–34. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, H.; Saidi, W.A.; Nguyen, M.C.; Wang, C.Z.; Ho, K.; Yang, J.; Zhao, J. Role of Surface Stress on the Reactivity of Anatase TiO2 (001). J. Phys. Chem. Lett. 2017, 8, 1764–1771. [Google Scholar] [CrossRef]
- Yin, W.-J.; Wen, B.; Zhou, C.; Selloni, A.; Liu, L.-M. Excess electrons in reduced rutile and anatase TiO2. Surf. Sci. Rep. 2018, 73, 58–82. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Yu, J.; Parkin, I.P.; Fujishima, A.; Nakata, K. Intrinsic intermediate gap states of TiO2 materials and their roles in charge carrier kinetics. J. Photochem. Photobiol. C Photochem. Rev. 2019, 39, 1–57. [Google Scholar] [CrossRef]
- Vorontsov, A.V. Cluster models of photocatalytic anatase TiO2 nanoparticles and their computational characterization. Catal. Today 2015, 252, 168–176. [Google Scholar] [CrossRef]
- Fazio, G.; Ferrighi, L.; Di Valentin, C. Photoexcited carriers recombination and trapping in spherical vs. faceted TiO2 nanoparticles. Nano Energy 2016, 27, 673–689. [Google Scholar] [CrossRef]
- Vorontsov, A.V. Effect of the Structure of Small Anatase Nanoparticles on the Localization of Photogenerated Charge Carriers. Kinet. Catal. 2017, 58, 688–694. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Tsydenov, D.E. Arrangement of acid sites on the surfaces of anatase titanium dioxide nanoparticles according to cluster models. Kinet. Catal. 2014, 55, 409–415. [Google Scholar] [CrossRef]
- Lamiel-Garcia, O.; Ko, K.C.; Lee, J.Y.; Bromley, S.T.; Illas, F. When Anatase Nanoparticles Become Bulklike: Properties of Realistic TiO2 Nanoparticles in the 1–6 nm Size Range from All Electron Relativistic Density Functional Theory Based Calculations. J. Chem. Theory Comput. 2017, 13, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Selli, D.; Fazio, G.; Di Valentin, C. Modelling realistic TiO2 nanospheres: A benchmark study of SCC-DFTB against hybrid DFT. J. Chem. Phys. 2017, 147, 164701. [Google Scholar] [CrossRef]
- Selli, D.; Fazio, G.; Di Valentin, C. Using Density Functional Theory to Model Realistic TiO2 Nanoparticles, Their Photoactivation and Interaction with Water. Catalysts 2017, 7, 357. [Google Scholar] [CrossRef]
- Valero, R.; Morales-García, Á.; Illas, F. Theoretical Modeling of Electronic Excitations of Gas-Phase and Solvated TiO2 Nanoclusters and Nanoparticles of Interest in Photocatalysis. J. Chem. Theory Comput. 2018, 14, 4391–4404. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Smirniotis, P.G. Size and surface groups effects in decahedral anatase nanoparticles for photocatalytic applications. J. Photochem. Photobiol. Chem. 2018, 363, 51–60. [Google Scholar] [CrossRef]
- Le, N.Q.; Schweigert, I.V. Modeling Electronic Trap States at Interfaces between Anatase Nanoparticles. J. Phys. Chem. C 2017, 121, 14254–14260. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Smirniotis, P.G. Semiempirical computational study of oxygen vacancies in a decahedral anatase nanoparticle. Int. J. Quantum Chem. 2019, 119, e25806. [Google Scholar] [CrossRef]
- Morales-García, Á.; Lamiel-García, O.; Valero, R.; Illas, F. Properties of Single Oxygen Vacancies on a Realistic (TiO2)84 Nanoparticle: A Challenge for Density Functionals. J. Phys. Chem. C 2018, 122, 2413–2421. [Google Scholar] [CrossRef]
- Morita, K.; Yasuoka, K. Density functional theory study of atomic and electronic properties of defects in reduced anatase TiO2 nanocrystals. AIP Adv. 2018, 8, 035119. [Google Scholar] [CrossRef]
- Drozd, V.S.; Zybina, N.A.; Abramova, K.E.; Parfenov, M.Y.; Kumar, U.; Valdés, H.; Smirniotis, P.G.; Vorontsov, A.V. Oxygen vacancies in nano-sized TiO2 anatase nanoparticles. Solid State Ion. 2019, 339, 115009. [Google Scholar] [CrossRef]
- Yin, G.; Huang, X.; Chen, T.; Zhao, W.; Bi, Q.; Xu, J.; Han, Y.; Huang, F. Hydrogenated Blue Titania for Efficient Solar to Chemical Conversions: Preparation, Characterization, and Reaction Mechanism of CO2 Reduction. ACS Catal. 2018, 8, 1009–1017. [Google Scholar] [CrossRef]
- Ko, K.C.; Lee, J.Y.; Illas, F. Modeling realistic titania nanoparticles. In Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2018; Volume 12, pp. 205–238. ISBN 978-0-08-102232-0. [Google Scholar]
- Wang, T.; Liu, L.; Ge, G.; Liu, M.; Zhou, W.; Chang, K.; Yang, F.; Wang, D.; Ye, J. Two-dimensional titanium oxide nanosheets rich in titanium vacancies as an efficient cocatalyst for photocatalytic water oxidation. J. Catal. 2018, 367, 296–305. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wang, M.; Pei, C.; Liu, B.; Yuan, Y.; Liu, S.; Yang, H. Enhancing the Sensing Properties of TiO2 Nanosheets with Exposed {001} Facets by a Hydrogenation and Sensing Mechanism. Inorg. Chem. 2017, 56, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Sun, Z.; Dou, S.X. Theoretically Manipulating Quantum Dots on Two-Dimensional TiO2 Monolayer for Effective Visible Light Absorption. ACS Appl. Mater. Interfaces 2017, 9, 8255–8262. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, H.; Xiong, J.; Guo, B.; Liang, S.; Wu, L. Photocatalytic hydrogen evolution over monolayer H1.07Ti1.73O4·H2O nanosheets: Roles of metal defects and greatly enhanced performances. Appl. Catal. B Environ. 2018, 221, 473–481. [Google Scholar] [CrossRef]
- Wang, H.; Song, Y.; Xiong, J.; Bi, J.; Li, L.; Yu, Y.; Liang, S.; Wu, L. Highly selective oxidation of furfuryl alcohol over monolayer titanate nanosheet under visible light irradiation. Appl. Catal. B Environ. 2018, 224, 394–403. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, W.; Wu, P. The electronic structure and optical properties of donor-acceptor codoped TiO2 nanosheets from hybrid functional calculations. Mater. Chem. Phys. 2017, 186, 333–340. [Google Scholar] [CrossRef]
- Gao, H.; Hu, G.; Sui, J.; Mu, C.; Shangguan, W.; Kong, M.; Shentu, W. Scalable preparation of defect-rich free-standing TiO2 sheets with visible-light photocatalytic activity. Appl. Catal. B Environ. 2018, 226, 337–345. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Smirniotis, P.G. Structure, electronic and optical properties of bilayer anatase nanoribbons. Comput. Mater. Sci. 2018, 155, 266–281. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Valdés, H. Quantum size effect and visible light activity of anatase nanosheet quantum dots. J. Photochem. Photobiol. Chem. 2019, 379, 39–46. [Google Scholar] [CrossRef]
- Wang, L.; Wei, D.; Kang, S.; Xie, X.; Shi, Y.; Liu, S. Two-Dimensional Titania: Structures and Properties Predicted by First Principle Calculation. J. Phys. Chem. C 2018, 122, 22911–22919. [Google Scholar] [CrossRef]
- Haick, H.; Paz, Y. Long-Range Effects of Noble Metals on the Photocatalytic Properties of Titanium Dioxide. J. Phys. Chem. B 2003, 107, 2319–2326. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Lu, D.; Wu, W.; Ding, J.; Zhao, X.; Xiong, R.; Yang, M.; Wu, P.; Chen, F.; et al. Visible-light-driven water splitting from dyeing wastewater using Pt surface-dispersed TiO2 -based nanosheets. J. Alloys Compd. 2017, 699, 183–192. [Google Scholar] [CrossRef]
- Uner, D. The Effect of Addition of Pt on the Gas Phase Photocatalysis over TiO2. In Environmentally Benign Photocatalysts; Anpo, M., Kamat, P.V., Eds.; Springer: New York, NY, USA, 2010; pp. 479–501. ISBN 978-0-387-48441-9. [Google Scholar]
- Falconer, J.L.; Magrini-Bair, K.A. Photocatalytic and Thermal Catalytic Oxidation of Acetaldehyde on Pt/TiO2. J. Catal. 1998, 179, 171–178. [Google Scholar] [CrossRef]
- Shinde, Y.; Wadhai, S.; Ponkshe, A.; Kapoor, S.; Thakur, P. Decoration of Pt on the metal free RGO-TiO2 composite photocatalyst for the enhanced photocatalytic hydrogen evolution and photocatalytic degradation of pharmaceutical pollutant β blocker. Int. J. Hydrog. Energy 2018, 43, 4015–4027. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, X.; Zhao, Y.; Wang, L.; Zhao, Z.; Huang, X.; Liu, J.; Li, J. Efficient photocatalysts of TiO2 nanocrystals-supported PtRu alloy nanoparticles for CO2 reduction with H2O: Synergistic effect of Pt-Ru. Appl. Catal. B Environ. 2018, 236, 445–457. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Y.; Wu, X.; Zheng, H.; Zhao, Z.; Liu, J.; Li, J. Graphene-wrapped Pt/TiO2 photocatalysts with enhanced photogenerated charges separation and reactant adsorption for high selective photoreduction of CO2 to CH4. Appl. Catal. B Environ. 2018, 226, 360–372. [Google Scholar] [CrossRef]
- Yang, L.; Wang, W.; Jiang, H.; Zhang, Q.; Shan, H.; Zhang, M.; Zhu, K.; Lv, J.; He, G.; Sun, Z. Improved SERS performance of single-crystalline TiO2 nanosheet arrays with coexposed {001} and {101} facets decorated with Ag nanoparticles. Sens. Actuators B Chem. 2017, 242, 932–939. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Jia, L.; Xing, X. Reduction of HCHO with OH− on Pt loading anatase TiO2 (001) surface: A DFT calculation. Catal. Commun. 2017, 92, 23–26. [Google Scholar] [CrossRef]
- Schlexer, P.; Chen, H.-Y.T.; Pacchioni, G. CO2 Activation and Hydrogenation: A Comparative DFT Study of Ru10/TiO2 and Cu10/TiO2 Model Catalysts. Catal. Lett. 2017, 147, 1871–1881. [Google Scholar] [CrossRef]
- Schvval, A.B.; Juan, A.; Cabeza, G.F. Theoretical study of the role of the interface of Ag4 nanoclusters deposited on TiO2(110) and TiO2(101). Appl. Surf. Sci. 2019, 490, 343–351. [Google Scholar] [CrossRef]
- Singhal, N.; Ali, A.; Vorontsov, A.; Pendem, C.; Kumar, U. Efficient approach for simultaneous CO and H2 production via photoreduction of CO2 with water over copper nanoparticles loaded TiO2. Appl. Catal. Gen. 2016, 523, 107–117. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Li, W.; Li, X.; Tian, H.; Wei, X.; Ren, Z.; Han, G. Ultrathin Anatase TiO2 Nanosheets for High-Performance Photocatalytic Hydrogen Production. Small 2017, 13, 1604115. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Zhuo, H.; Wei, Z.; Zhuang, G.; Zhong, X.; Deng, S.; Li, X.; Wang, J. Oxygen vacancies on TiO2 promoted the activity and stability of supported Pd nanoparticles for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 2264–2272. [Google Scholar] [CrossRef]
- Barbieriková, Z.; Dvoranová, D.; Brezová, V.; Džunuzović, E.; Sredojević, D.N.; Lazić, V.; Nedeljković, J.M. Visible-light-responsive surface-modified TiO2 powder with 4-chlorophenol: A combined experimental and DFT study. Opt. Mater. 2019, 89, 237–242. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Smirniotis, P.G. Benchmarking semiempirical and DFT methods for the interaction of thiophene and diethyl sulfide molecules with a Ti(OH)4(H2O) cluster. J. Mol. Model. 2017, 23, 223. [Google Scholar] [CrossRef]
- Geldof, D.; Tassi, M.; Carleer, R.; Adriaensens, P.; Roevens, A.; Meynen, V.; Blockhuys, F. Binding modes of phosphonic acid derivatives adsorbed on TiO2 surfaces: Assignments of experimental IR and NMR spectra based on DFT/PBC calculations. Surf. Sci. 2017, 655, 31–38. [Google Scholar] [CrossRef]
- Quintero, Y.C.; Nagarajan, R. Molecular and dissociative adsorption of DMMP, Sarin and Soman on dry and wet TiO2(110) using density functional theory. Surf. Sci. 2018, 675, 26–35. [Google Scholar] [CrossRef]
- Trubitsyn, D.A.; Vorontsov, A.V. Molecular and reactive adsorption of dimethyl methylphosphonate over (001) and (100) anatase clusters. Comput. Theor. Chem. 2013, 1020, 63–71. [Google Scholar] [CrossRef]
- Rudshteyn, B.; Negre, C.F.A.; Oliboni, R.S.; Monti, A.; Chen, J.; Crabtree, R.H.; Rego, L.G.C.; Batista, V.S. Inferring Protonation States of Hydroxamate Adsorbates on TiO2 Surfaces. J. Phys. Chem. C 2017, 121, 11985–11990. [Google Scholar] [CrossRef]
- Setvin, M.; Hulva, J.; Wang, H.; Simschitz, T.; Schmid, M.; Parkinson, G.S.; Di Valentin, C.; Selloni, A.; Diebold, U. Formaldehyde Adsorption on the Anatase TiO2 (101) Surface: Experimental and Theoretical Investigation. J. Phys. Chem. C 2017, 121, 8914–8922. [Google Scholar] [CrossRef]
- Vorontsov, A.V. Structural and electronic effects in acetone adsorption over TiO2 anatase clusters as the first stage of photocatalytic oxidation. J. Nanoparticle Res. 2017, 19, 326. [Google Scholar] [CrossRef]
- Li, F.; Huang, W.-H.; Gong, X.-Q. Unique adsorption behaviors of NO and O2 at hydrogenated anatase TiO2 (101). Chin. Chem. Lett. 2018, 29, 765–768. [Google Scholar] [CrossRef]
- Lang, X.; Liang, Y.; Sun, L.; Zhou, S.; Lau, W.-M. Interplay between Methanol and Anatase TiO2 (101) Surface: The Effect of Subsurface Oxygen Vacancy. J. Phys. Chem. C 2017, 121, 6072–6080. [Google Scholar] [CrossRef]
- Nadeem, I.M.; Harrison, G.T.; Wilson, A.; Pang, C.L.; Zegenhagen, J.; Thornton, G. Bridging Hydroxyls on Anatase TiO2 (101) by Water Dissociation in Oxygen Vacancies. J. Phys. Chem. B 2018, 122, 834–839. [Google Scholar] [CrossRef]
- Chen, K.; Chen, C.; Ren, X.; Alsaedi, A.; Hayat, T. Interaction mechanism between different facet TiO2 and U(VI): Experimental and density-functional theory investigation. Chem. Eng. J. 2019, 359, 944–954. [Google Scholar] [CrossRef]
- Zhang, J.; Liao, J.; Yang, F.; Xu, M.; Lin, S. Regulation of the Electroanalytical Performance of Ultrathin Titanium Dioxide Nanosheets toward Lead Ions by Non-Metal Doping. Nanomaterials 2017, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Yang, F.; Wang, C.-Z.; Lin, S. The crystal facet-dependent electrochemical performance of TiO2 nanocrystals for heavy metal detection: Theoretical prediction and experimental proof. Sens. Actuators B Chem. 2018, 271, 195–202. [Google Scholar] [CrossRef]
- Gao, Y.; Lockart, M.; Kispert, L.D.; Bowman, M.K. Photoinduced Charge Separation in Retinoic Acid on TiO2: Comparison of Three Anchoring Modes. J. Phys. Chem. C 2019, 123, 24634–24642. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Zhou, H.; Zou, C.; Xu, K.; Luo, X.; Yu, T.; Zhang, W.; Liu, Y.; Yuan, C. A systematic study on the crystal facets-dependent gas sensing properties of anatase TiO2 with designed {010}, {101} and {001} facets. Ceram. Int. 2019, 45, 6282–6290. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, Y.; Ding, Z.; Zuo, M.; Jing, C. Modulating High-Index Facets on Anatase TiO2: Modulating High-Index Facets on Anatase TiO2. Eur. J. Inorg. Chem. 2018, 2018, 683–693. [Google Scholar] [CrossRef]
- Di Liberto, G.; Tosoni, S.; Pacchioni, G. Nitrogen doping in coexposed (001)–(101) anatase TiO2 surfaces: A DFT study. Phys. Chem. Chem. Phys. 2019, 21, 21497–21505. [Google Scholar] [CrossRef]
- Sasani, A.; Baktash, A.; Mirabbaszadeh, K.; Khoshnevisan, B. Structural and electronic properties of Mg and Mg-Nb co-doped TiO2 (101) anatase surface. Appl. Surf. Sci. 2016, 384, 298–303. [Google Scholar] [CrossRef]
- Hiremath, V.; Shavi, R.; Seo, J.G. Controlled oxidation state of Ti in MgO-TiO2 composite for CO2 capture. Chem. Eng. J. 2017, 308, 177–183. [Google Scholar] [CrossRef]
- Shakir, S.; Abd-ur-Rehman, H.M.; Yunus, K.; Iwamoto, M.; Periasamy, V. Fabrication of un-doped and magnesium doped TiO2 films by aerosol assisted chemical vapor deposition for dye sensitized solar cells. J. Alloys Compd. 2018, 737, 740–747. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Kumar, M.; Singhal, N.; Jain, S.L.; Babalola, J.O.; Vorontsov, A.V.; Kumar, U. Engineering and modeling the effect of Mg doping in TiO2 for enhanced photocatalytic reduction of CO2 to fuels. Catal. Sci. Technol. 2018, 8, 3686–3694. [Google Scholar] [CrossRef]
- Xing, Z.; Li, Z.; Wu, X.; Wang, G.; Zhou, W. In-situ S-doped porous anatase TiO2 nanopillars for high-efficient visible-light photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2016, 41, 1535–1541. [Google Scholar] [CrossRef]
- Xiong, Y.; He, D.; Jaber, R.; Cameron, P.J.; Edler, K.J. Sulfur-Doped Cubic Mesostructured Titania Films for Use as a Solar Photocatalyst. J. Phys. Chem. C 2017, 121, 9929–9937. [Google Scholar] [CrossRef]
- Yan, X.; Yuan, K.; Lu, N.; Xu, H.; Zhang, S.; Takeuchi, N.; Kobayashi, H.; Li, R. The interplay of sulfur doping and surface hydroxyl in band gap engineering: Mesoporous sulfur-doped TiO2 coupled with magnetite as a recyclable, efficient, visible light active photocatalyst for water purification. Appl. Catal. B Environ. 2017, 218, 20–31. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Valdés, H. Insights into the visible light photocatalytic activity of S-doped hydrated TiO2. Int. J. Hydrog. Energy 2019, 44, 17963–17973. [Google Scholar] [CrossRef]
- Vorontsov, A.V. Molecular and dissociative adsorption of a diethylsulfide molecule on (010) and (001) faces of a TiO2 anatase nanoparticle. J. Struct. Chem. 2015, 56, 813–822. [Google Scholar] [CrossRef]
- Zhou, W.-Y.; Liu, J.-Y.; Song, J.-Y.; Li, J.-J.; Liu, J.-H.; Huang, X.-J. Surface-Electronic-State-Modulated, Single-Crystalline (001) TiO2 Nanosheets for Sensitive Electrochemical Sensing of Heavy-Metal Ions. Anal. Chem. 2017, 89, 3386–3394. [Google Scholar] [CrossRef]
- Xiong, F.; Yin, L.-L.; Wang, Z.; Jin, Y.; Sun, G.; Gong, X.-Q.; Huang, W. Surface Reconstruction-Induced Site-Specific Charge Separation and Photocatalytic Reaction on Anatase TiO2 (001) Surface. J. Phys. Chem. C 2017, 121, 9991–9999. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Q.; Li, C.; Li, J.; Yang, J. Surface heterojunction between (001) and (101) facets of ultrafine anatase TiO2 nanocrystals for highly efficient photoreduction CO2 to CH4. Appl. Catal. B Environ. 2016, 198, 378–388. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, X. Reversing the Photocatalytic Activity Orders of Anatase TiO2 Facets by Surface Treatment. ChemistrySelect 2016, 1, 5838–5841. [Google Scholar] [CrossRef]
- Chen, M.; Ma, J.; Zhang, B.; He, G.; Li, Y.; Zhang, C.; He, H. Remarkable synergistic effect between {001} facets and surface F ions promoting hole migration on anatase TiO2. Appl. Catal. B Environ. 2017, 207, 397–403. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, L.; Yang, D.; Zhang, J.; Li, Y.-J.; Zou, K.; Deng, W.-Q. Niobium-Doped (001)-Dominated Anatase TiO2 Nanosheets as Photoelectrode for Efficient Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 9576–9583. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Song, W.; Liu, B.; Zhong, W.; Deng, J.; Zheng, H.; Liu, J.; Gong, X.-Q.; Zhao, Z. Facet-dependent photocatalytic performance of TiO2: A DFT study. Appl. Catal. B Environ. 2016, 198, 1–8. [Google Scholar] [CrossRef]
- Setvin, M.; Shi, X.; Hulva, J.; Simschitz, T.; Parkinson, G.S.; Schmid, M.; Di Valentin, C.; Selloni, A.; Diebold, U. Methanol on Anatase TiO2 (101): Mechanistic Insights into Photocatalysis. ACS Catal. 2017, 7, 7081–7091. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Guan, Z.; Li, Q.; He, C.; Yang, J. Synergistic effect of surface and bulk single-electron-trapped oxygen vacancy of TiO2 in the photocatalytic reduction of CO2. Appl. Catal. B Environ. 2017, 206, 300–307. [Google Scholar] [CrossRef]
- Wang, L.; Song, W.; Deng, J.; Zheng, H.; Liu, J.; Zhao, Z.; Gao, M.; Wei, Y. Facet-dependent photocatalytic decomposition of N2O on the anatase TiO2: A DFT study. Nanoscale 2018, 10, 6024–6038. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Luo, Y. New Mechanism for Photocatalytic Reduction of CO2 on the Anatase TiO2 (101) Surface: The Essential Role of Oxygen Vacancy. J. Am. Chem. Soc. 2016, 138, 15896–15902. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, Š.; Zbořil, R.; Schmuki, P. Photocatalysis with Reduced TiO2: From Black TiO2 to Cocatalyst-Free Hydrogen Production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef]
- Dong, G.; Wang, X.; Chen, Z.; Lu, Z. Enhanced Photocatalytic Activity of Vacuum-activated TiO2 Induced by Oxygen Vacancies. Photochem. Photobiol. 2018, 94, 472–483. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, M.; Guan, Z.; Li, Q.; Yang, J. Effect of annealing ambience on the formation of surface/bulk oxygen vacancies in TiO2 for photocatalytic hydrogen evolution. Appl. Surf. Sci. 2018, 428, 640–647. [Google Scholar] [CrossRef]
- Wan, P.; Hood, Z.D.; Adhikari, S.P.; Xu, Y.; Yang, S.; Wu, S. Enhancing the photoresponse and photocatalytic properties of TiO2 by controllably tuning defects across {101} facets. Appl. Surf. Sci. 2018, 434, 711–716. [Google Scholar] [CrossRef]
- Li, J.-J.; Cai, S.-C.; Yu, E.-Q.; Weng, B.; Chen, X.; Chen, J.; Jia, H.-P.; Xu, Y.-J. Efficient infrared light promoted degradation of volatile organic compounds over photo-thermal responsive Pt-rGO-TiO2 composites. Appl. Catal. B Environ. 2018, 233, 260–271. [Google Scholar] [CrossRef]
- Li, X.; Shen, R.; Ma, S.; Chen, X.; Xie, J. Graphene-based heterojunction photocatalysts. Appl. Surf. Sci. 2018, 430, 53–107. [Google Scholar] [CrossRef]
- Martins, P.M.; Ferreira, C.G.; Silva, A.R.; Magalhães, B.; Alves, M.M.; Pereira, L.; Marques, P.A.A.P.; Melle-Franco, M.; Lanceros-Méndez, S. TiO2/graphene and TiO2/graphene oxide nanocomposites for photocatalytic applications: A computer modeling and experimental study. Compos. Part B Eng. 2018, 145, 39–46. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Kumar, M.; Singh, B.; Oninla, V.O.; Babalola, J.O.; Valdés, H.; Vorontsov, A.V.; Kumar, U. Self-assembled reduced graphene oxide-TiO2 nanocomposites: Synthesis, DFTB+ calculations, and enhanced photocatalytic reduction of CO2 to methanol. Carbon 2019, 147, 385–397. [Google Scholar] [CrossRef]
- Piskorz, W. Attaching titania clusters of various size to reduced graphene oxide and its impact on the conceivable photocatalytic behavior of the junctions—A DFT/D + U and TD DFTB modeling. J. Phys. 2019, 31, 404001. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J. Enhanced photocatalytic H2 -production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B Environ. 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Berger, T.; Sterrer, M.; Diwald, O.; Knözinger, E.; Panayotov, D.; Thompson, T.L.; Yates, J.T. Light-Induced Charge Separation in Anatase TiO2 Particles. J. Phys. Chem. B 2005, 109, 6061–6068. [Google Scholar] [CrossRef]
- Wilke, K.; Breuer, H.D. The influence of transition metal doping on the physical and photocatalytic properties of titania. J. Photochem. Photobiol. Chem. 1999, 121, 49–53. [Google Scholar] [CrossRef]
- Paz, Y. Transient IR spectroscopy as a tool for studying photocatalytic materials. J. Phys. Condens. Matter 2019, 31, 503004. [Google Scholar] [CrossRef]
- Serpone, N.; Lawless, D.; Khairutdinov, R.; Pelizzetti, E. Subnanosecond Relaxation Dynamics in TiO2 Colloidal Sols (Particle Sizes Rp = 1.0 − 13.4 nm). Relevance to Heterogeneous Photocatalysis. J. Phys. Chem. 1995, 99, 16655–16661. [Google Scholar] [CrossRef]
- Bahnemann, D.W.; Hilgendorff, M.; Memming, R. Charge Carrier Dynamics at TiO 2 Particles: Reactivity of Free and Trapped Holes. J. Phys. Chem. B 1997, 101, 4265–4275. [Google Scholar] [CrossRef]
- Lawless, D.; Serpone, N.; Meisel, D. Role of hydroxyl radicals and trapped holes in photocatalysis. A pulse radiolysis study. J. Phys. Chem. 1991, 95, 5166–5170. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Choudhury, A. Investigation of the optical property and photocatalytic activity of mixed phase nanocrystalline titania. Appl. Nanosci. 2014, 4, 839–847. [Google Scholar] [CrossRef]
- Bahnemann, D.; Henglein, A.; Lilie, J.; Spanhel, L. Flash photolysis observation of the absorption spectra of trapped positive holes and electrons in colloidal titanium dioxide. J. Phys. Chem. 1984, 88, 709–711. [Google Scholar] [CrossRef]
- Yamakata, A.; Ishibashi, T.; Onishi, H. Time-resolved infrared absorption spectroscopy of photogenerated electrons in platinized TiO2 particles. Chem. Phys. Lett. 2001, 333, 271–277. [Google Scholar] [CrossRef]
- Yamakata, A.; Ishibashi, T.; Onishi, H. Water- and Oxygen-Induced Decay Kinetics of Photogenerated Electrons in TiO2 and Pt/TiO2: A Time-Resolved Infrared Absorption Study. J. Phys. Chem. B 2001, 105, 7258–7262. [Google Scholar] [CrossRef]
- De Sario, P.A.; Pietron, J.J.; Taffa, D.H.; Compton, R.; Schünemann, S.; Marschall, R.; Brintlinger, T.H.; Stroud, R.M.; Wark, M.; Owrutsky, J.C.; et al. Correlating Changes in Electron Lifetime and Mobility on Photocatalytic Activity at Network-Modified TiO2 Aerogels. J. Phys. Chem. C 2015, 119, 17529–17538. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorontsov, A.V.; Valdés, H.; Smirniotis, P.G.; Paz, Y. Recent Advancements in the Understanding of the Surface Chemistry in TiO2 Photocatalysis. Surfaces 2020, 3, 72-92. https://doi.org/10.3390/surfaces3010008
Vorontsov AV, Valdés H, Smirniotis PG, Paz Y. Recent Advancements in the Understanding of the Surface Chemistry in TiO2 Photocatalysis. Surfaces. 2020; 3(1):72-92. https://doi.org/10.3390/surfaces3010008
Chicago/Turabian StyleVorontsov, Alexander V., Héctor Valdés, Panagiotis G. Smirniotis, and Yaron Paz. 2020. "Recent Advancements in the Understanding of the Surface Chemistry in TiO2 Photocatalysis" Surfaces 3, no. 1: 72-92. https://doi.org/10.3390/surfaces3010008
APA StyleVorontsov, A. V., Valdés, H., Smirniotis, P. G., & Paz, Y. (2020). Recent Advancements in the Understanding of the Surface Chemistry in TiO2 Photocatalysis. Surfaces, 3(1), 72-92. https://doi.org/10.3390/surfaces3010008