Hybrid Cathodes Composed of K3V2(PO4)3 and Carbon Materials with Boosted Charge Transfer for K-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of KVP-Based Composites
2.2. Materials Characterization
2.3. Electrochemical Measurements
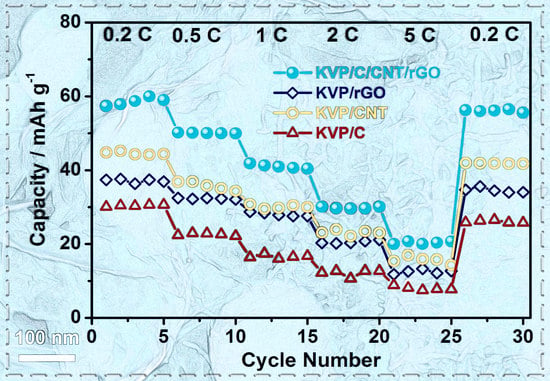
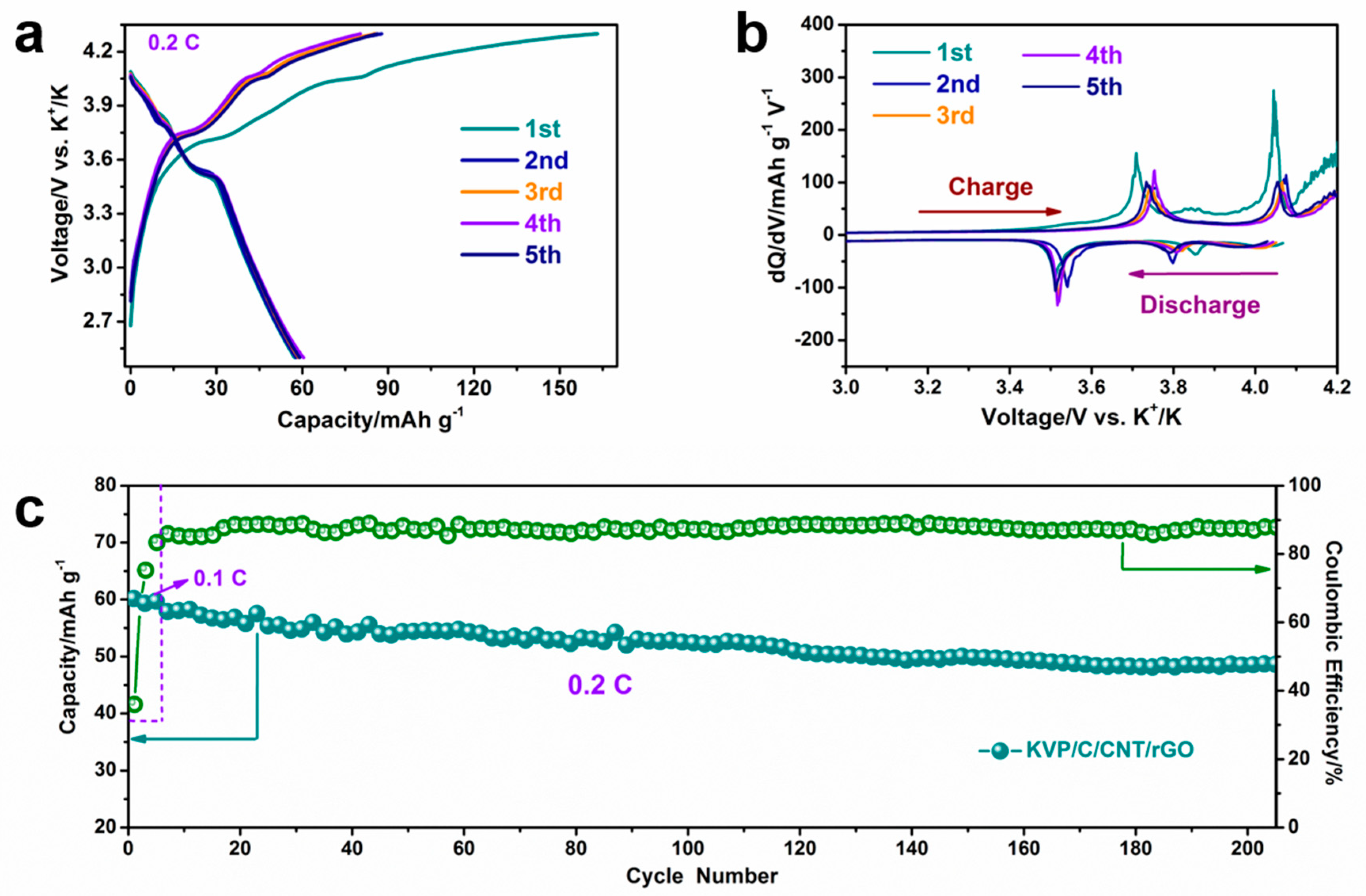
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, B.; Qian, D.; Wang, Z.; Meng, Y.S. Recent progress in cathode materials research for advanced lithium ion batteries. Mater. Sci. Eng. R Rep. 2013, 44, 51–65. [Google Scholar] [CrossRef]
- Gao, M.R.; Xu, Y.F.; Jiang, J.; Yu, S.H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3017. [Google Scholar] [CrossRef]
- Chen, D.; Tan, H.T.; Rui, X.H.; Zhang, Q.; Feng, Y.Z.; Geng, H.B.; Li, C.C.; Huang, S.M.; Yu, Y. Oxyvanite V3O5: A New Intercalation-Type Anode for Lithium-Ion Battery. InfoMat 2019, 1, 251–259. [Google Scholar]
- Rui, X.; Yan, Q.; Skyllas-Kazacos, M.; Lim, T.M. Li3V2(PO4)3 cathode materials for lithium-ion batteries: A review. J. Power Sources 2014, 258, 19–38. [Google Scholar] [CrossRef]
- Chen, S.; Wu, C.; Shen, L.; Zhu, C.; Huang, Y.; Xi, K.; Maier, J.; Yu, Y. Challenges and Perspectives for NASICON-Type Electrode Materials for Advanced Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1700431. [Google Scholar] [CrossRef]
- Tan, H.; Chen, D.; Rui, X.; Yu, Y. Peering into Alloy Anodes for Sodium-Ion Batteries: Current Trends, Challenges, and Opportunities. Adv. Funct. Mater. 2019, 29, 1908745. [Google Scholar] [CrossRef]
- Tan, H.; Xu, L.; Geng, H.; Rui, X.; Li, C.; Huang, S. Nanostructured Li3V2(PO4)3 Cathodes. Small 2018, 14, 1800567. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, D.; Liu, Y.; Han, W.; Chu, H.; Rui, X. Integrated Charge Transfer in Li3V2(PO4)3/C for High-Power Li-Ion Batteries. Int. J. Electrochem. Sci. 2017, 12, 9925–9932. [Google Scholar] [CrossRef]
- Chen, D.; Rui, X.; Zhang, Q.; Geng, H.; Gan, L.; Zhang, W.; Li, C.; Huang, S.; Yu, Y. Persistent zinc-ion storage in mass-produced V2O5 architectures. Nano Energy 2019, 60, 171–178. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Recent Progress in Rechargeable Potassium Batteries. Adv. Funct. Mater. 2018, 28, 1802938. [Google Scholar] [CrossRef]
- Tan, H.; Feng, Y.; Rui, X.; Yu, Y.; Huang, S. Metal Chalcogenides: Paving the Way for High-Performance Sodium/Potassium-Ion Batteries. Small Methods 2019, 10, 1900563. [Google Scholar] [CrossRef]
- Yang, D.; Liu, C.; Rui, X.; Yan, Q. Embracing high performance potassium-ion batteries with phosphorus-based electrodes: A review. Nanoscale 2019, 11, 15402–15417. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Y.; Yang, B.; Liu, L.; He, D. A Dual Carbon-based Potassium Dual Ion Battery with Robust Comprehensive Performance. Small 2018, 14, 1801836. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, X.; Rui, X.; Chen, D.; Huang, S. Na3V2(PO4)3: An advanced cathode for sodium-ion batteries. Nanoscale 2019, 11, 2556–2576. [Google Scholar]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef]
- Liao, J.; Hu, Q.; Che, B.; Ding, X.; Chen, F.; Chen, C. Competing with other polyanionic cathode materials for potassium-ion batteries via fine structure design: new layered KVOPO4 with a tailored particle morphology. J. Mater. Chem. A 2019, 7, 15244–15251. [Google Scholar] [CrossRef]
- Komaba, S.; Hasegawa, T.; Dahbi, M.; Kubota, K. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem. Commun. 2015, 60, 172–175. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yang, D.; Rui, X.; Yu, Y.; Huang, S. Advanced Cathodes for Potassium-Ion Battery. Curr. Opin. Electrochem. 2019, 18, 24–30. [Google Scholar] [CrossRef]
- Jian, Z.; Luo, W.; Ji, X. Carbon Electrodes for K-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 11566. [Google Scholar] [CrossRef]
- Luo, W.; Wan, J.; Ozdemir, B.; Bao, W.; Chen, Y.; Dai, J.; Lin, H.; Xu, Y.; Gu, F.; Barone, V. Potassium Ion Batteries with Graphitic Materials. Nano Lett. 2015, 15, 7671–7677. [Google Scholar] [CrossRef]
- Han, J.; Xu, M.; Niu, Y.; Li, G.N.; Wang, M.; Zhang, Y.; Jia, M.; Li, C.M. Exploration of K2Ti8O17 as an anode material for potassium-ion batteries. Chem. Commun. 2016, 52, 11274–11276. [Google Scholar] [CrossRef] [PubMed]
- Sultana, I.; Ramireddy, T.; Rahman, M.M.; Chen, Y.; Glushenkov, A.M. Tin-based composite anodes for potassium-ion batteries. Chem. Commun. 2016, 52, 9279–9282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Mao, J.; Li, S.; Chen, Z.; Guo, Z. Phosphorus-Based Alloy Materials for Advanced Potassium-Ion Battery Anode. J. Am. Chem. Soc. 2017, 139, 3316–3319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, I.; Rahman, M.M.; Ramireddy, T.; Ying, C.; Glushenkov, A.M. High capacity potassium-ion battery anodes based on black phosphorus. J. Mater. Chem. A 2017, 5, 23506–23512. [Google Scholar] [CrossRef]
- Rui, X.; Sim, D.; Wong, K.; Zhu, J.; Liu, W.; Xu, C.; Tan, H.; Xiao, N.; Hng, H.H.; Lim, T.M. Li3V2(PO4)3 nanocrystals embedded in a nanoporous carbon matrix supported on reduced graphene oxide sheets: Binder-free and high rate cathode material for lithium-ion batteries. J. Power Sources 2012, 214, 171–177. [Google Scholar] [CrossRef]
- Rui, X.; Li, C.; Chen, C. Synthesis and characterization of carbon-coated LiV(PO) cathode materials with different carbon sources. Electrochim. Acta 2009, 54, 3374–3380. [Google Scholar] [CrossRef]
- Rui, X.; Li, C.; Liu, J.; Cheng, T.; Chen, C. The Li3V2(PO4)3/C composites with high-rate capability prepared by a maltose-based sol–gel route. Electrochim. Acta 2010, 55, 6761–6767. [Google Scholar] [CrossRef]
- Rui, X.; Sun, W.; Wu, C.; Yu, Y.; Yan, Q. An Advanced Sodium-Ion Battery Composed of Carbon Coated Na3V2(PO4)3 in a Porous Graphene Network. Adv. Mater. 2015, 27, 6670–6676. [Google Scholar] [CrossRef]
- Hong, S.; Kim, Y.; Park, Y.; Choi, A.; Choi, N.S.; Lee, K.T. Charge Carriers in Rechargeable Batteries: Na Ions vs. Li Ions. Energy Environ. Sci. 2014, 45, 2067–2081. [Google Scholar] [CrossRef]
- Han, J.; Li, G.; Liu, F.; Wang, M.Q.; Xu, M.W. Investigation of K3V2(PO4)3/C nanocomposite as high-potential cathode materials for potassium-ion batteries. Chem. Commun. 2017, 53, 1805–1808. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, K.; Gao, Y.; Luo, H.; Li, L. Recent Progress in the Applications of Vanadium-Based Oxides on Energy Storage: From Low-Dimensional Nanomaterials Synthesis to 3D Micro/Nano-Structures and Free-Standing Electrodes Fabrication. Adv. Energy Mater. 2017, 7, 1700547. [Google Scholar]
- Liu, T.; Bo, W.; Gu, X.; Lei, W.; Min, L.; Gao, L.; Wang, D.; Zhang, S. All-climate sodium ion batteries based on the NASICON electrode materials. Nano Energy 2016, 30, 756–761. [Google Scholar] [CrossRef]
- Islam, S.; Alfaruqi, M.H.; Putro, D.Y.; Mathew, V.; Kim, J. Pyrosynthesis of Na3V2(PO4)3@C Cathodes for Safe and Low-Cost Aqueous Hybrid Batteries. Chemsuschem 2018, 11, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, Y.; Wang, L.; Yu, F.; Ma, J. Na3V2(PO4)3@C as Faradaic Electrodes in Capacitive Deionization for High-Performance Desalination. Nano Lett. 2019, 19, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, B.; Wang, C.; Dou, Y.; Zhang, Q.; Liu, Y.; Gao, H.; Al-Mamun, M.; Pang, W.K.; Guo, Z.; et al. Constructing the best symmetric full K-ion battery with the NASICON-type K3V2(PO4)3. Nano Energy 2019, 60, 432–439. [Google Scholar] [CrossRef]
- Wang, X.; Niu, C.; Meng, J.; Ping, H.; Xu, X.; Wei, X.; Liang, Z.; Zhao, K.; Wen, L.; Yan, M. Novel K3V2(PO4)3/C Bundled Nanowires as Superior Sodium-Ion Battery Electrode with Ultrahigh Cycling Stability. Adv. Energy Mater. 2015, 5, 1500716. [Google Scholar]
- Rajagopalan, R.; Lei, Z.; Shi, X.D.; Hua, K.L. Tuned In Situ Growth of Nanolayered rGO on 3D Na3V2(PO4)3 Matrices: A Step toward Long Lasting, High Power Na-Ion Batteries. Adv. Mater. Interfaces 2016, 3, 1600007. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhou, X.; Li, D.; Cheng, X.; Liu, F.; Yan, Y. Highly Reversible Na Storage in Na3V2(PO4)3 by Optimizing Nanostructure and Rational Surface Engineering. Adv. Energy Mater. 2018, 8, 1800068. [Google Scholar]
- Macfarlane, D.; Chinnareddy, M.V.; Mitra, S.; Forsyth, M.; Kar, M.; Raj, A.K. Stability enhancing ionic liquid hybrid electrolyte for NVP@C cathode based sodium batteries. Sustain. Energy. Fuels 2018, 2, 566–576. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Kuang, X.; Zhu, H.; Xiao, N.; Zhang, Q.; Rui, X.; Yu, Y.; Huang, S. Hybrid Cathodes Composed of K3V2(PO4)3 and Carbon Materials with Boosted Charge Transfer for K-Ion Batteries. Surfaces 2020, 3, 1-10. https://doi.org/10.3390/surfaces3010001
Zhang X, Kuang X, Zhu H, Xiao N, Zhang Q, Rui X, Yu Y, Huang S. Hybrid Cathodes Composed of K3V2(PO4)3 and Carbon Materials with Boosted Charge Transfer for K-Ion Batteries. Surfaces. 2020; 3(1):1-10. https://doi.org/10.3390/surfaces3010001
Chicago/Turabian StyleZhang, Xianghua, Xinyi Kuang, Hanwen Zhu, Ni Xiao, Qi Zhang, Xianhong Rui, Yan Yu, and Shaoming Huang. 2020. "Hybrid Cathodes Composed of K3V2(PO4)3 and Carbon Materials with Boosted Charge Transfer for K-Ion Batteries" Surfaces 3, no. 1: 1-10. https://doi.org/10.3390/surfaces3010001