Electrophoretic Deposition of Hydroxyapatite–Chitosan–Titania on Stainless Steel 316 L
Abstract
1. Introduction
2. Materials and Methods
2.1. Suspensions Preparation
2.2. Electrophoretic Deposition
2.3. Corrosion Resistance
3. Results and Discussion
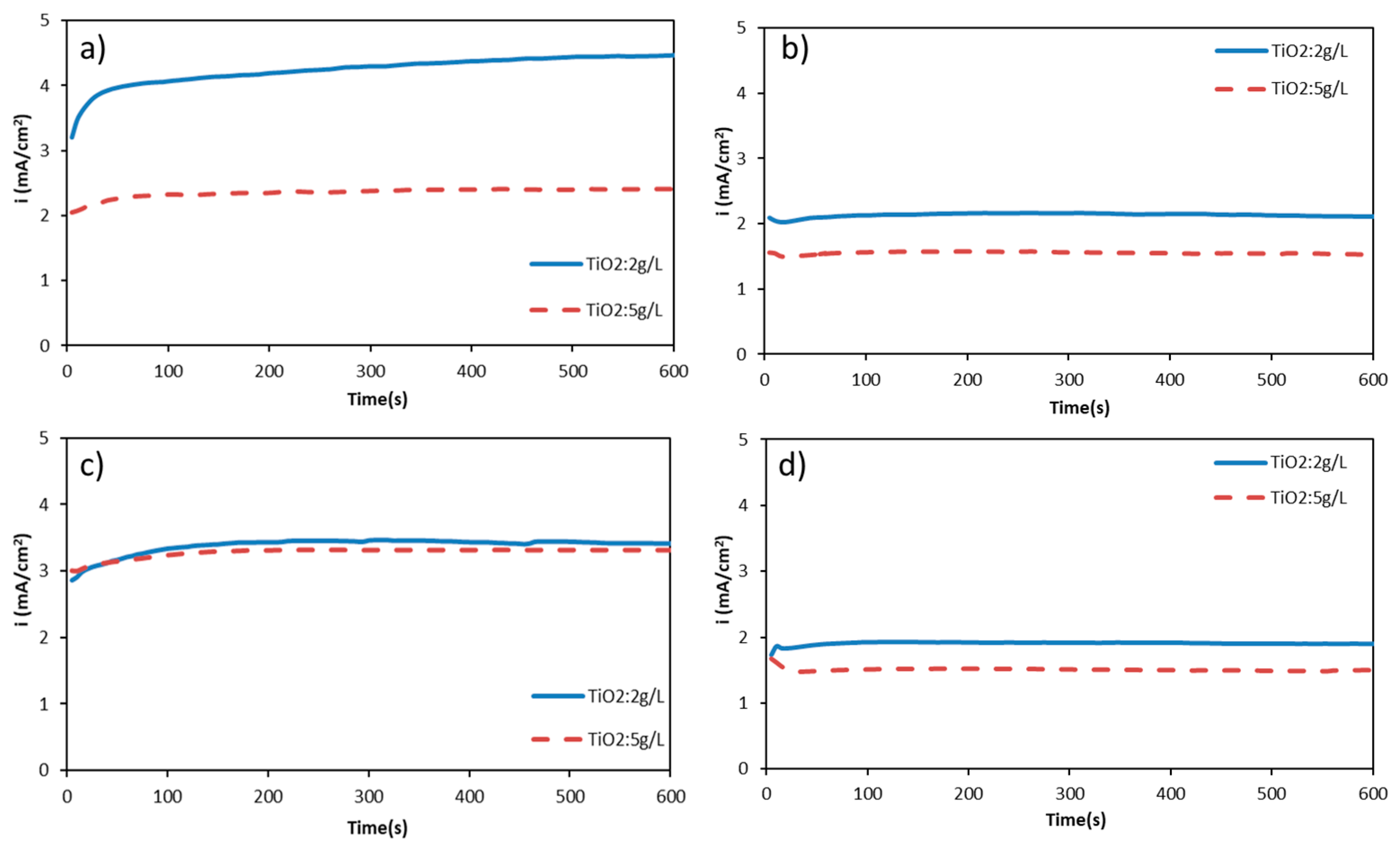
3.1. Current Density
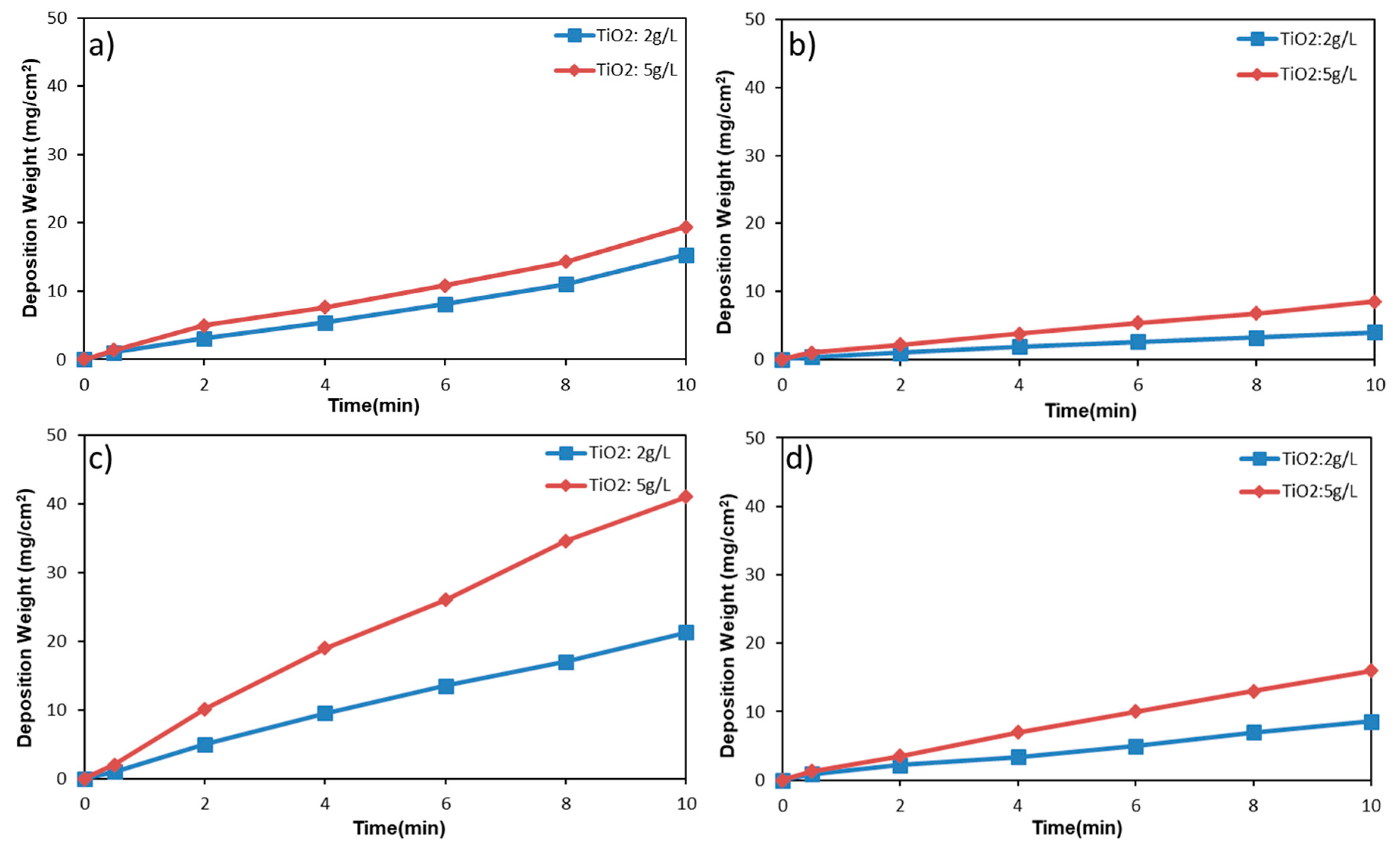
3.2. Kinetics of Deposition
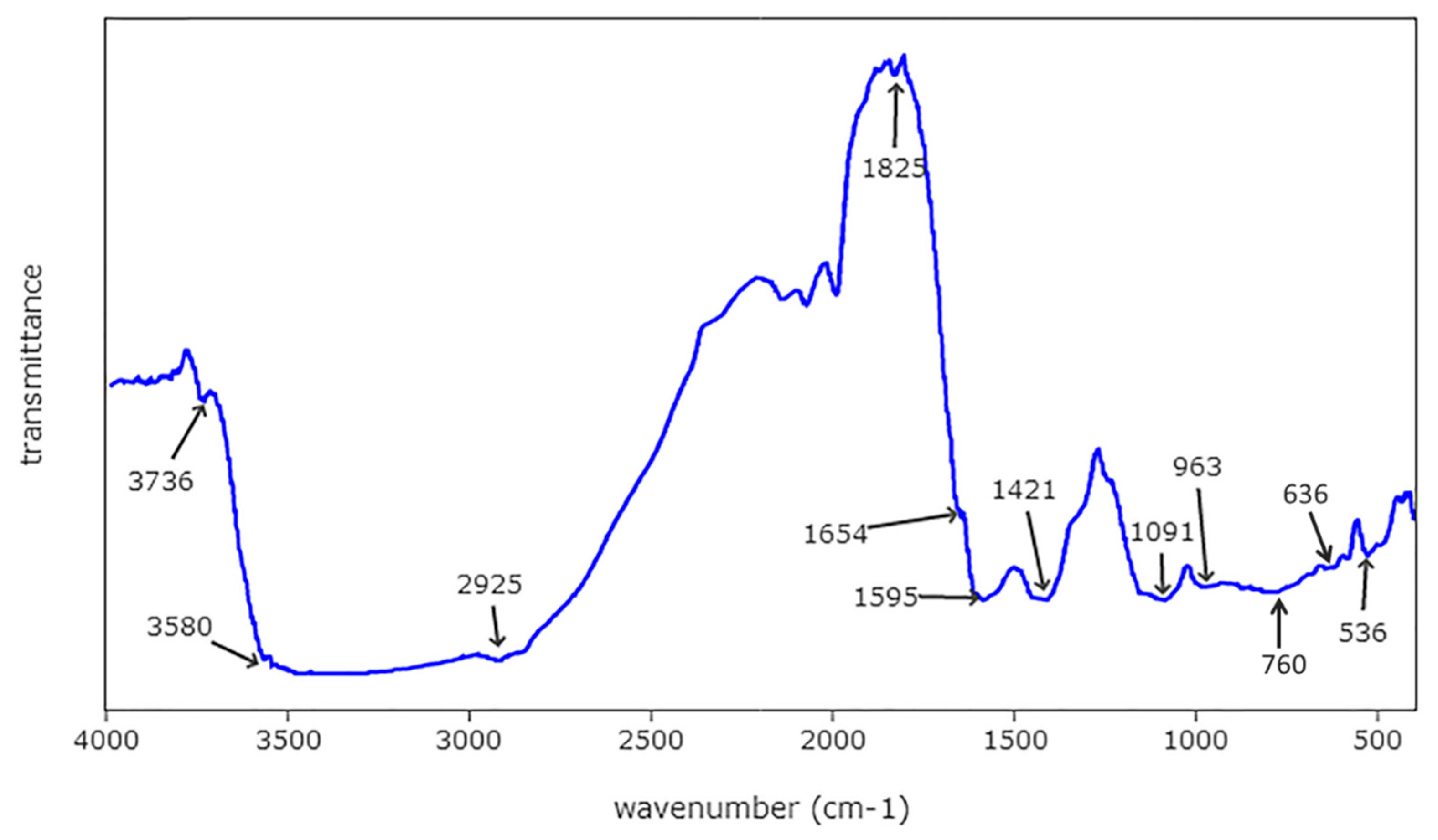
3.3. FTIR Analysis
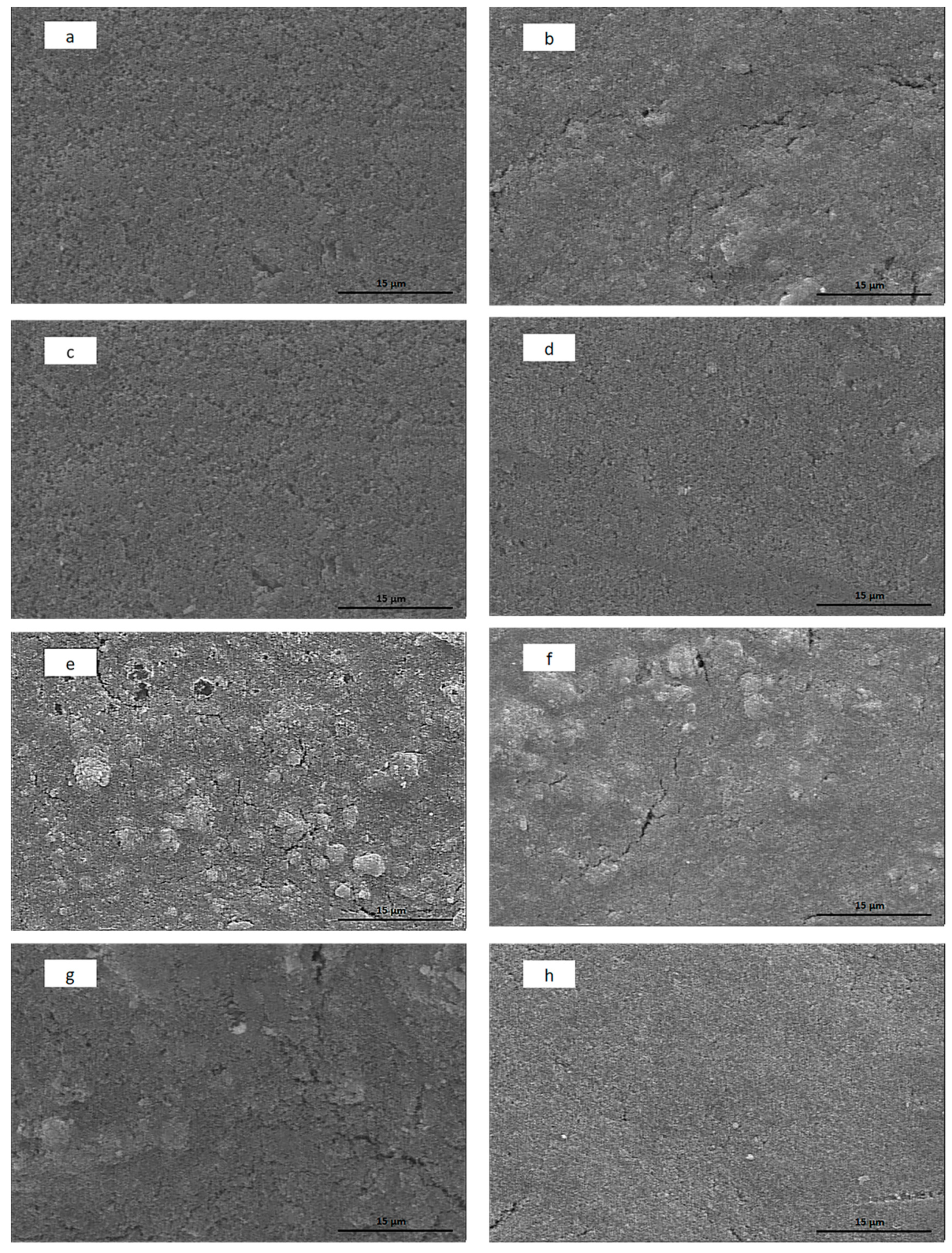
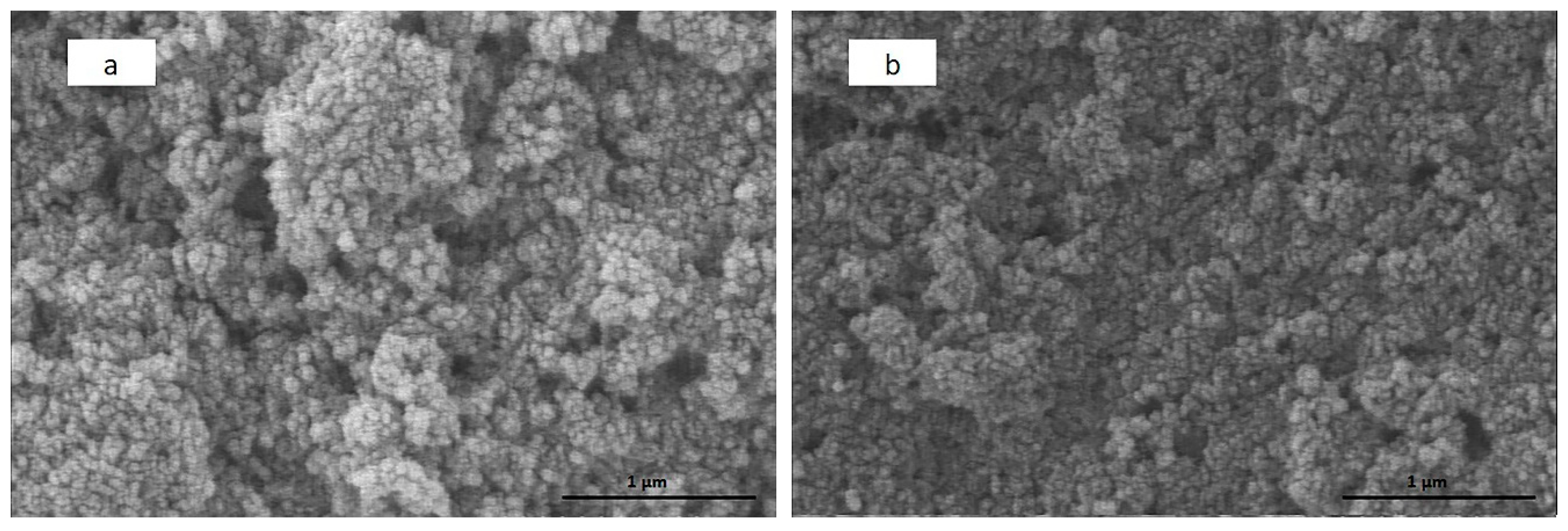
3.4. SEM Analysis
3.5. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite hydroxyapatite-chitosan coatings. Mater. Charact. 2007, 58, 339–348. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Kot, M.; Seuss, S.; Kędzierska, A.; Czyrska-Filemonowicz, A.; Boccaccini, A.R. Electrophoretic deposition and characterization of HA/chitosan nanocomposite coatings on Ti6Al7Nb alloy. Met. Mater. Int. 2015, 21, 96–103. [Google Scholar] [CrossRef]
- Rojaee, R.; Fathi, M.; Raeissi, K. Electrophoretic deposition of nanostructured hydroxyapatite coating on AZ91 magnesium, alloy implants with different surface treatments. Appl. Surf. Sci. 2013, 285, 664–673. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Ardhaoui, M.; Benyounis, K.; Looney, L.; Stokes, J.T. Plasma sprayed hydroxyapatite coatings: Understanding process relationships using design of experiment analysis. Surf. Coat. Techol. 2015, 283, 29–36. [Google Scholar] [CrossRef]
- Yousefpour, M.; Afshar, A.; Chen, J.; Zhang, X. Electrophoretic deposition of porous hydroxyapatite coatings using polytetrafluoroethylene particles as templates. Mater. Sci. Eng. C 2007, 27, 1482–1486. [Google Scholar] [CrossRef]
- Eliaz, N.S.T.M.; Sridhar, T.M.; Kamachi Mudali, U.; Raj, B. Electrochemical and electrophoretic deposition of hydroxyapatite for orthopaedic applications. Surf. Eng. 2005, 21, 238–242. [Google Scholar] [CrossRef]
- Sidane, D.; Chicot, D.; Yala, S.; Ziani, S.; Khireddine, H.; Iost, A.; Decoopman, X. Study of the mechanical behavior and corrosion resistance of hydroxyapatite sol–gel thin coatings on 316 L stainless steel pre-coated with titania film. Thin Solid Films 2015, 593, 71–80. [Google Scholar] [CrossRef]
- Demnati, I.; Parco, M.; Grossin, D.; Fagoaga, I.; Drouet, C.; Barykin, G.; Combes, C.; Braceras, I.; Gonçalvès, S.; Rey, C. Hydroxyapatite coating on titanium by a low energy plasma spraying mini-gun. Surf. Coat. Technol. 2012, 206, 2346–2353. [Google Scholar] [CrossRef]
- He, D.H.; Wang, P.; Liu, P.; Liu, X.K.; Ma, F.C.; Zhao, J. HA coating fabricated by electrochemical deposition on modified Ti6Al4V alloy. Surf. Coat. Technol. 2016, 301, 6–12. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Farnoush, H.; Aldıç, G.; Çimenoğlu, H. Functionally graded HA–TiO2 nanostructured composite coating on Ti–6Al–4V substrate via electrophoretic deposition. Surf. Coat. Technol. 2015, 265, 7–15. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Molaei, A.; Yousefpour, M. Electrophoretic deposition of chitosan–bioglass®–hydroxyapatite–halloysite nanotube composite coating. Rare Met. 2018, 1–8. [Google Scholar] [CrossRef]
- Mishyn, V.; Aspermair, P.; Leroux, Y.; Happy, H.; Knoll, W.; Boukherroub, R.; Szunerits, S. “Click” Chemistry on Gold Electrodes Modified with Reduced Graphene Oxide by Electrophoretic Deposition. Surfaces 2019, 2, 193–204. [Google Scholar] [CrossRef]
- Hamaker, H.C. Formation of a deposit by electrophoresis. Trans. Faraday Soc. 1940, 35, 279–287. [Google Scholar] [CrossRef]
- Javidi, M.; Javadpour, S.; Bahrololoom, M.E.; Ma, J. Electrophoretic deposition of natural hydroxyapatite on medical grade 316L stainless steel. Mater. Sci. Eng. C 2008, 28, 1509–1515. [Google Scholar] [CrossRef]
- Tang, S.; Tian, B.; Guo, Y.J.; Zhu, Z.A.; Guo, Y.P. Chitosan/carbonated hydroxyapatite composite coatings: Fabrication, structure and biocompatibility. Surf. Coat. Technol. 2014, 251, 210–216. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic deposition of biomaterials. J. R. Soc. Interface 2010, 7, S581–S613. [Google Scholar] [CrossRef]
- Ma, R.; Zhitomirsky, I. Electrophoretic deposition of chitosan–albumin and alginate–albumin films. Surf. Eng. 2011, 27, 51–56. [Google Scholar] [CrossRef]
- Pishbin, F.; Simchi, A.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition of chitosan/45S5 Bioglass® composite coatings for orthopaedic applications. Surf. Coat. Technol. 2011, 205, 5260–5268. [Google Scholar] [CrossRef]
- Đošić, M.; Eraković, S.; Janković, A.; Vukašinović-Sekulić, M.; Matić, I.Z.; Stojanović, J.; Rhee, K.Y.; Mišković-Stanković, V.; Park, S.J. In vitro investigation of electrophoretically deposited bioactive hydroxyapatite/chitosan coatings reinforced by graphene. J. Ind. Eng. Chem. 2017, 47, 336–347. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I. Electrodeposition of composite hydroxyapatite–chitosan films. Mater. Chem. Phys. 2005, 94, 245–251. [Google Scholar] [CrossRef]
- Sorkhi, L.; Farrokhi-Rad, M.; Shahrabi, T. Electrophoretic deposition of chitosan in different alcohols. J. Coat. Technol. Res. 2014, 11, 739–746. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Iizuka, S.; Osaka, A.; Monma, H.; Tanaka, J. The effect of citric acid addition on chitosan/hydroxyapatite composites. Colloids Surf. A 2003, 214, 111–118. [Google Scholar] [CrossRef]
- Heidari, F.; Bahrololoom, M.E.; Vashaee, D.; Tayebi, L. In situ preparation of iron oxide nanoparticles in natural hydroxyapatite/chitosan matrix for bone tissue engineering application. Ceram. Int. 2015, 41, 3094–3100. [Google Scholar] [CrossRef]
- Song, L.; Gan, L.; Xiao, Y.F.; Wu, Y.; Wu, F.; Gu, Z.W. Antibacterial hydroxyapatite/chitosan complex coatings with superior osteoblastic cell response. Mater. Lett. 2011, 65, 974–977. [Google Scholar] [CrossRef]
- Hahn, B.D.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Choi, J.H.; Kim, H.E.; Kim, S.G. Aerosol deposition of hydroxyapatite–chitosan composite coatings on biodegradable magnesium alloy. Surf. Coat. Technol. 2011, 205, 3112–3118. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Sorkhi, L.; Farrokhi-Rad, M.; Shahrabi, T. Electrophoretic deposition of hydroxyapatite–chitosan nanocomposite coatings in different alcohols. Surf. Coat. Technol. 2013, 216, 106–114. [Google Scholar] [CrossRef]
- Sarkar, A.; Kannan, S. In situ synthesis, fabrication and Rietveld refinement of the hydroxyapatite/titania composite coatings on 316 L SS. Ceram. Int. 2014, 40, 6453–6463. [Google Scholar] [CrossRef]
- Lee, C.K. Fabrication, characterization and wear corrosion testing of bioactive hydroxyapatite/nano-TiO2 composite coatings on anodic Ti–6Al–4V substrate for biomedical applications. Mater. Sci. Eng. B 2012, 177, 810–818. [Google Scholar] [CrossRef]
- Rath, P.C.; Besra, L.; Singh, B.P.; Bhattacharjee, S. Titania/hydroxyapatite bi-layer coating on Ti metal by electrophoretic deposition: Characterization and corrosion studies. Ceram. Int. 2012, 38, 3209–3216. [Google Scholar] [CrossRef]
- Albayrak, O.; El-Atwani, O.; Altintas, S. Hydroxyapatite coating on titanium substrate by electrophoretic deposition method: Effects of titanium dioxide inner layer on adhesion strength and hydroxyapatite decomposition. Surf. Coat. Technol. 2008, 202, 2482–2487. [Google Scholar] [CrossRef]
- Farnoush, H.; Mohandesi, J.A.; Çimenoğlu, H. Micro-scratch and corrosion behavior of functionally graded HA-TiO2 nanostructured composite coatings fabricated by electrophoretic deposition. J. Mech. Behav. Biomed. Mater. 2015, 46, 31–40. [Google Scholar] [CrossRef]
- Farnoush, H.; Mohandesi, J.A.; Fatmehsari, D.H.; Moztarzadeh, F. A kinetic study on the electrophoretic deposition of hydroxyapatite–titania nanocomposite based on a statistical approach. Ceram. Int. 2012, 38, 6753–6767. [Google Scholar] [CrossRef]
- Mohan, L.; Durgalakshmi, D.; Geetha, M.; Narayanan, T.S.; Asokamani, R. Electrophoretic deposition of nanocomposite (HAp+ TiO2) on titanium alloy for biomedical applications. Ceram. Int. 2012, 38, 3435–3443. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Mangalaraj, D.; Ponpandian, N. Controlled growth and investigations on the morphology and mechanical properties of hydroxyapatite/titania nanocomposite thin films. Compos. Sci. Technol. 2010, 70, 1645–1651. [Google Scholar] [CrossRef]
- Kavitha, K.; Sutha, S.; Prabhu, M.; Rajendran, V.; Jayakumar, T. In situ synthesized novel biocompatible titania–chitosan nanocomposites with high surface area and antibacterial activity. Carbohydr. Polym. 2013, 93, 731–739. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
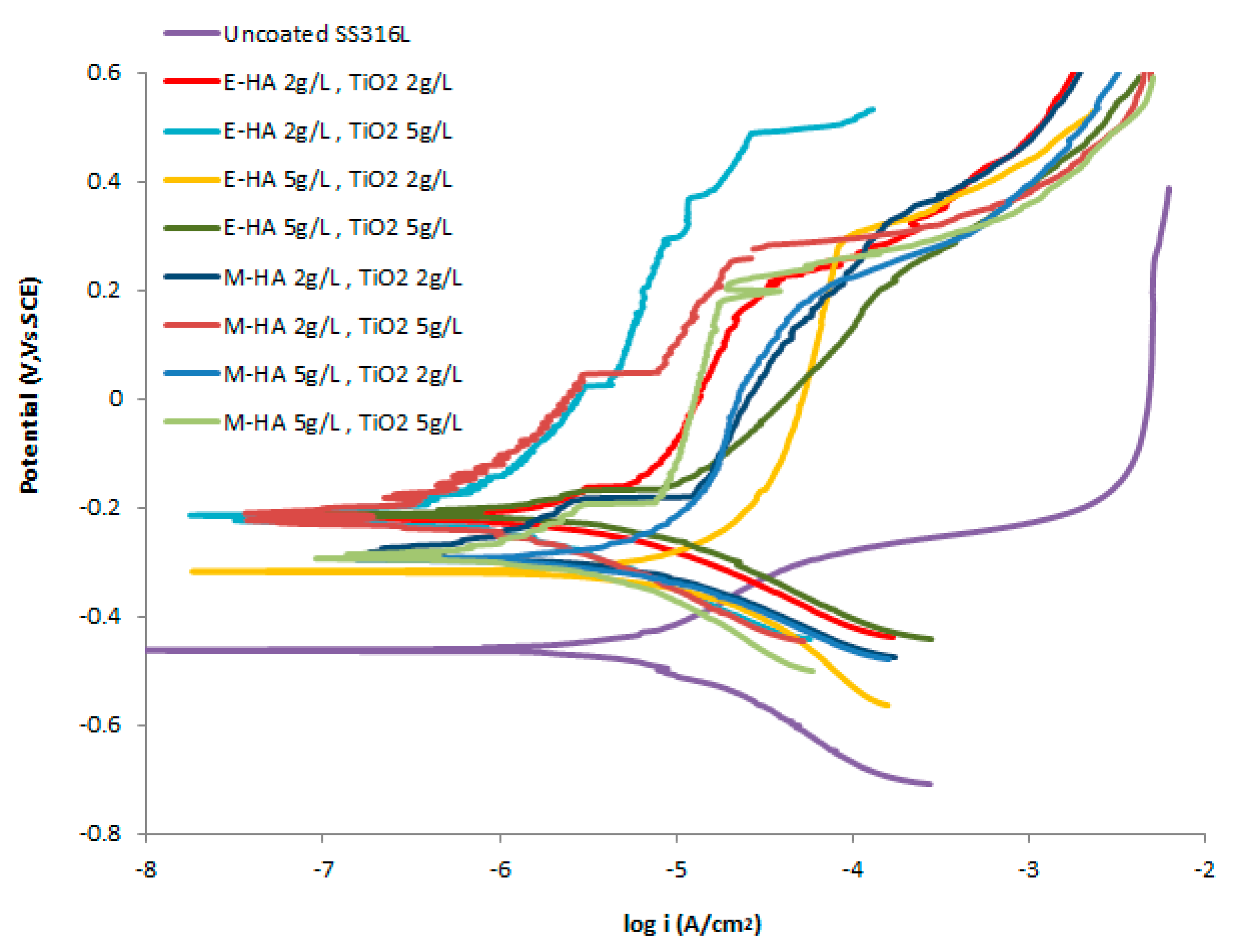
| Suspension for Coating | Icorr (A/cm2) | Ecorr vs. SCE (V) |
|---|---|---|
| Uncoated Stainless steel 316 L | 1.86 × 10−6 | −0.46 |
| Ethanol—0.5 g/L Chitosan, HA 2 g/L and Titania 2 g/L | 2.51 × 10−7 | −0.23 |
| Ethanol—0.5 g/L Chitosan, HA 2 g/L and Titania 5 g/L | 9.33 × 10−8 | −0.22 |
| Ethanol—0.5 g/L Chitosan, HA 5 g/L and Titania 2 g/L | 1.41 × 10−6 | −0.32 |
| Ethanol—0.5 g/L Chitosan, HA 5 g/L and Titania 5 g/L | 5.01 × 10−7 | −0.23 |
| Methanol—0.5 g/L Chitosan, HA 2 g/L and Titania 2 g/L | 3.16 × 10−7 | −0.27 |
| Methanol—0.5 g/L Chitosan, HA 2 g/L and Titania 5 g/L | 6.31 × 10−8 | −0.22 |
| Methanol—0.5 g/L Chitosan, HA 5 g/L and Titania 2 g/L | 1.10 × 10−6 | −0.29 |
| Methanol—0.5 g/L Chitosan, HA 5 g/L and Titania 5 g/L | 2.51 × 10−7 | −0.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorkhi, L.; Farrokhi-Rad, M.; Shahrabi, T. Electrophoretic Deposition of Hydroxyapatite–Chitosan–Titania on Stainless Steel 316 L. Surfaces 2019, 2, 458-467. https://doi.org/10.3390/surfaces2030034
Sorkhi L, Farrokhi-Rad M, Shahrabi T. Electrophoretic Deposition of Hydroxyapatite–Chitosan–Titania on Stainless Steel 316 L. Surfaces. 2019; 2(3):458-467. https://doi.org/10.3390/surfaces2030034
Chicago/Turabian StyleSorkhi, Leila, Morteza Farrokhi-Rad, and Taghi Shahrabi. 2019. "Electrophoretic Deposition of Hydroxyapatite–Chitosan–Titania on Stainless Steel 316 L" Surfaces 2, no. 3: 458-467. https://doi.org/10.3390/surfaces2030034
APA StyleSorkhi, L., Farrokhi-Rad, M., & Shahrabi, T. (2019). Electrophoretic Deposition of Hydroxyapatite–Chitosan–Titania on Stainless Steel 316 L. Surfaces, 2(3), 458-467. https://doi.org/10.3390/surfaces2030034





