Nitrogen-Doped Ordered Mesoporous Carbons Supported Co3O4 Composite as a Bifunctional Oxygen Electrode Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Nitrogen-Doped Ordered Mesoporous Carbon (N-HNMK-3)
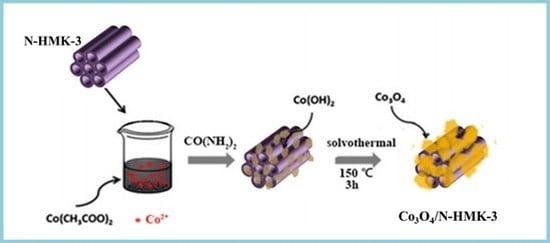
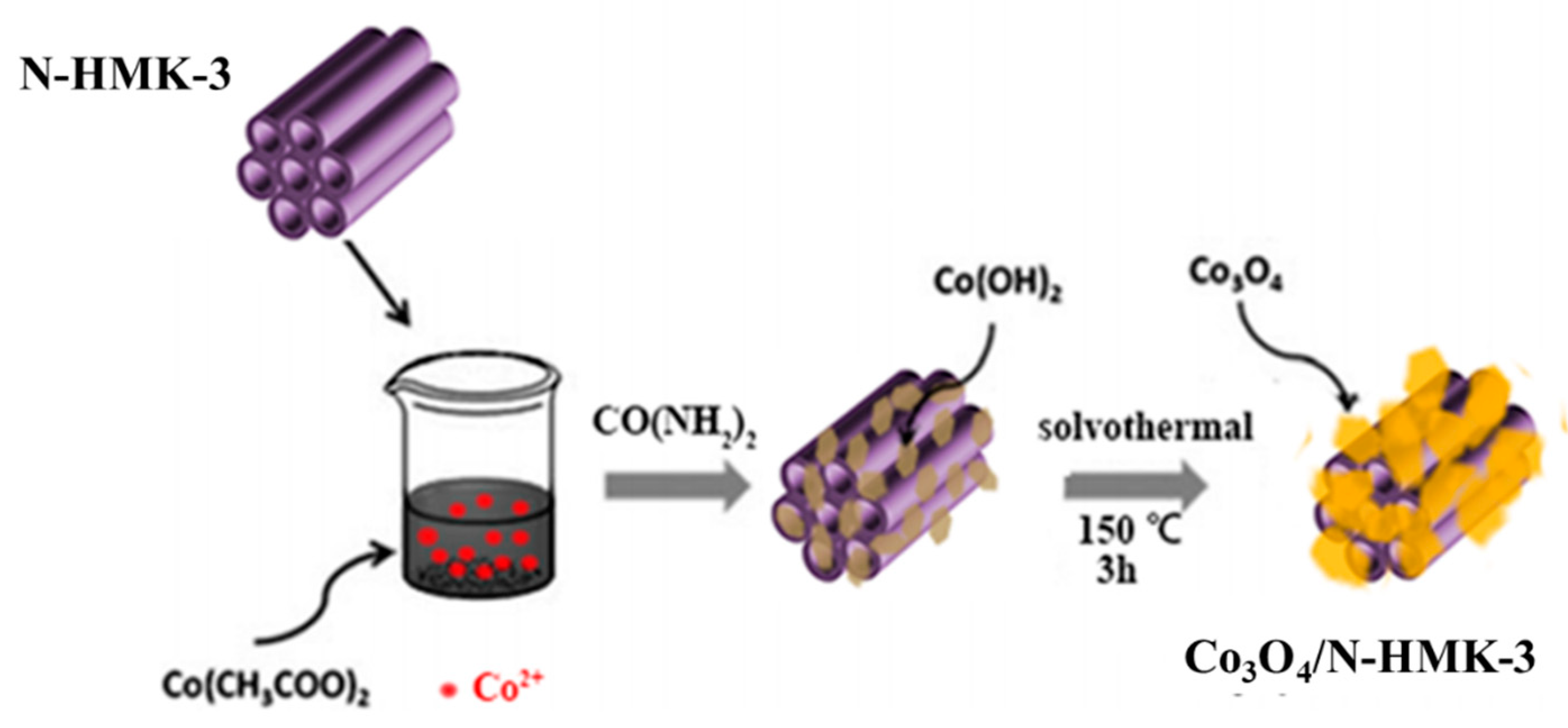
2.3. Synthesis of Co3O4/N-HNMK-3 Electrocatalyst
2.4. Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
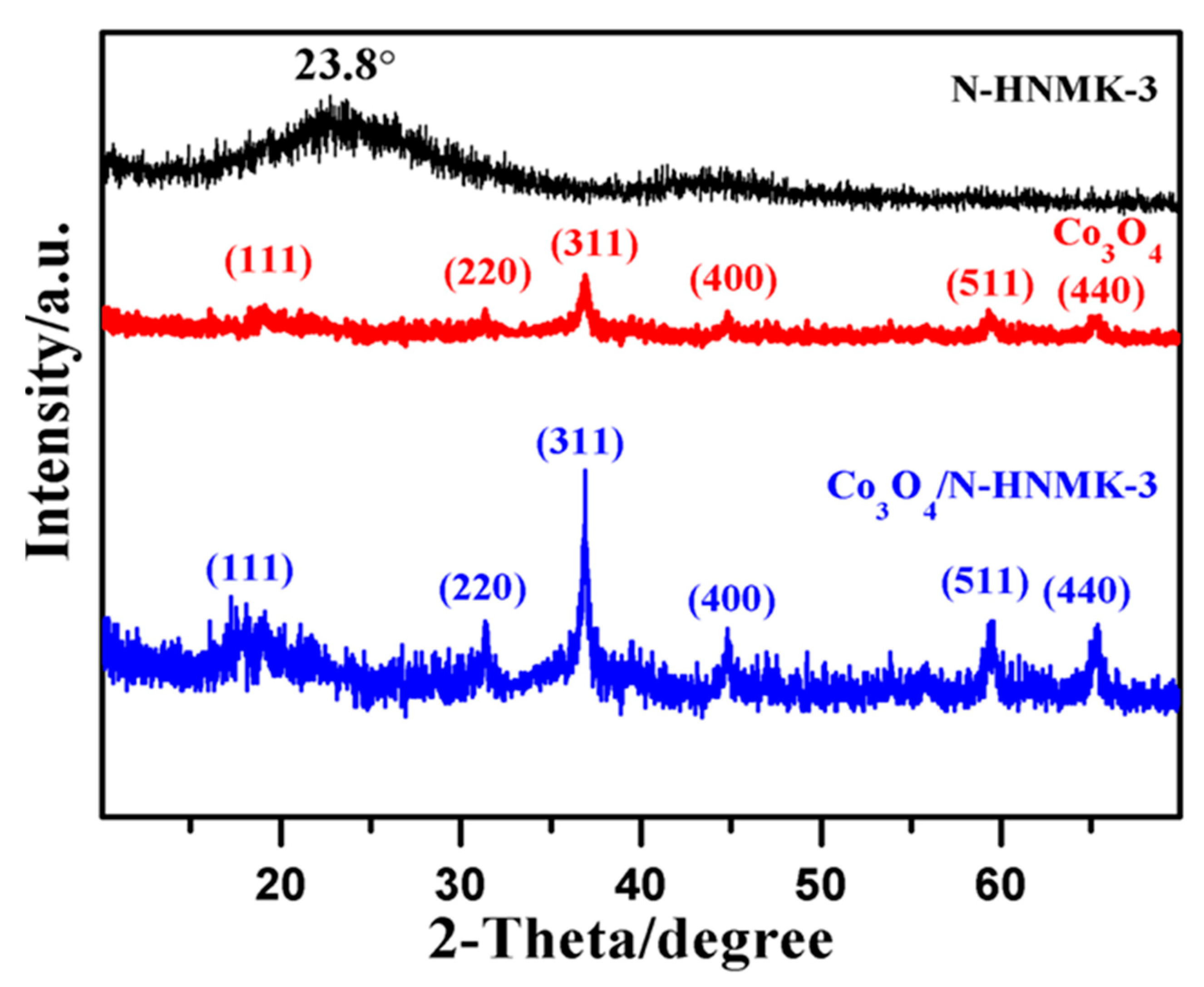
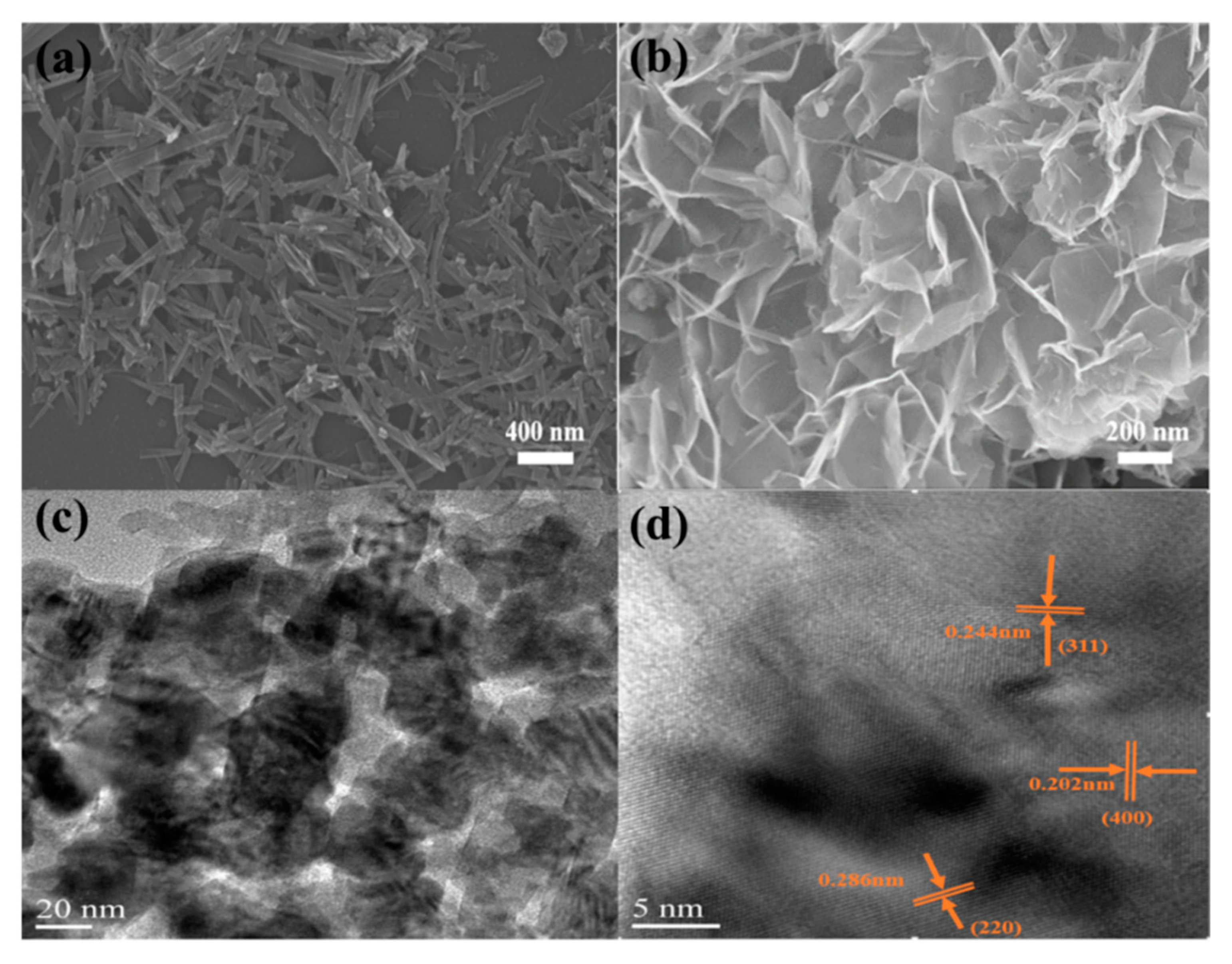
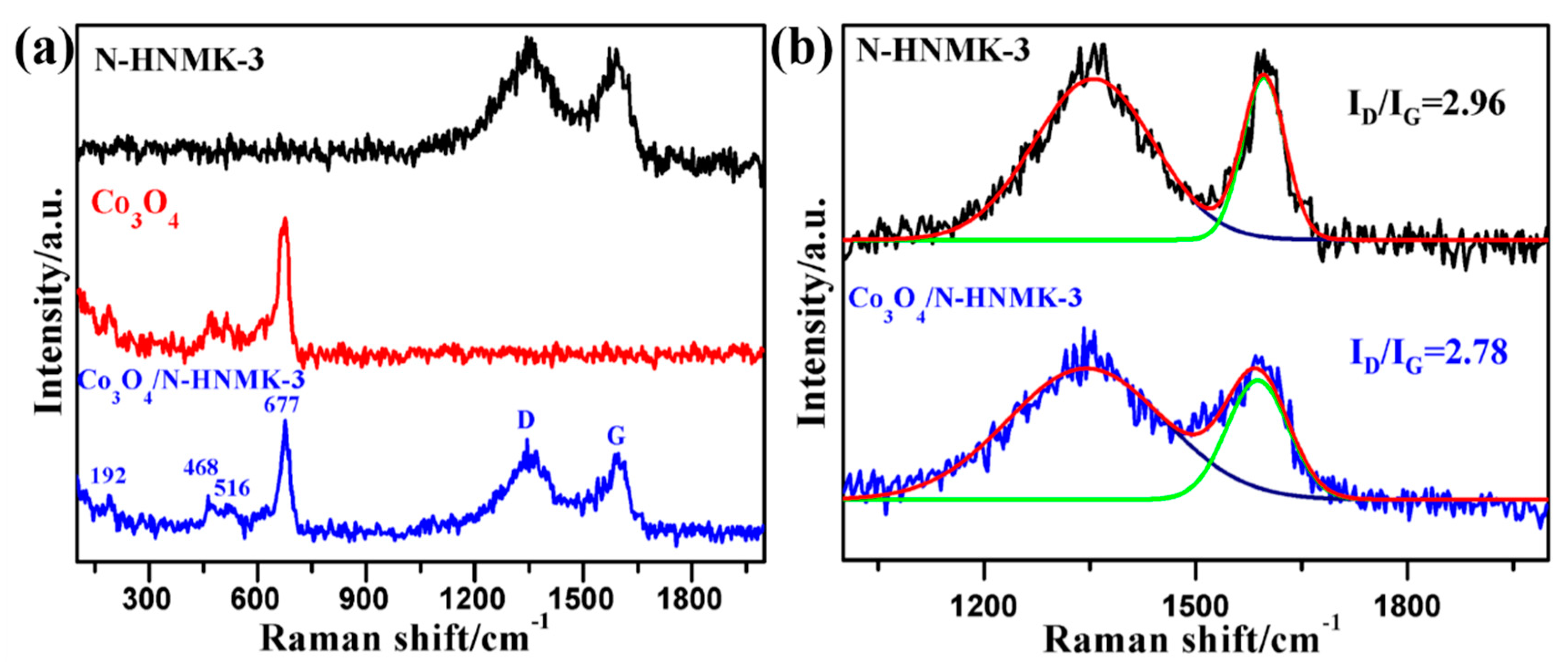
3.1. Structure, Morphology, and Chemical Composition
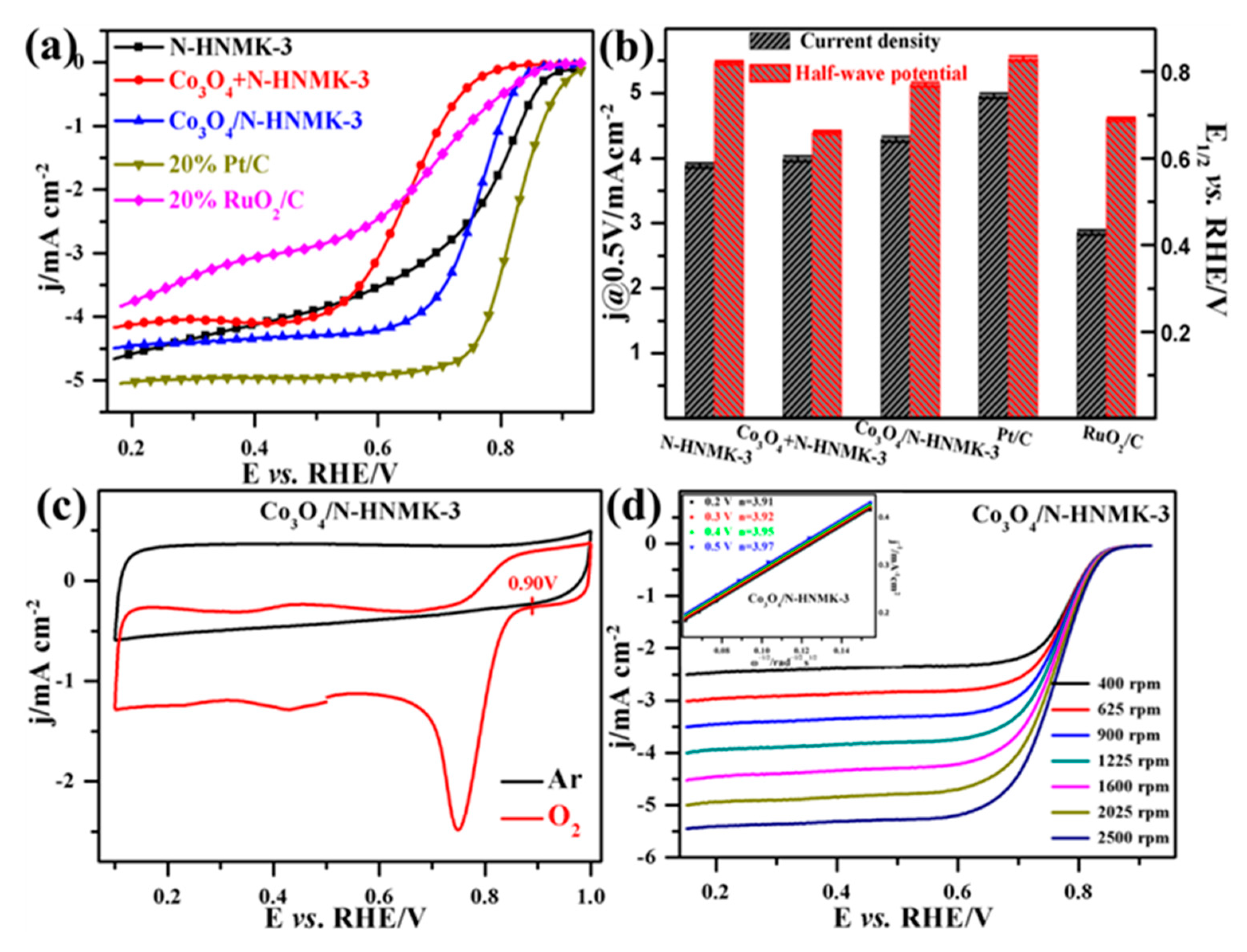
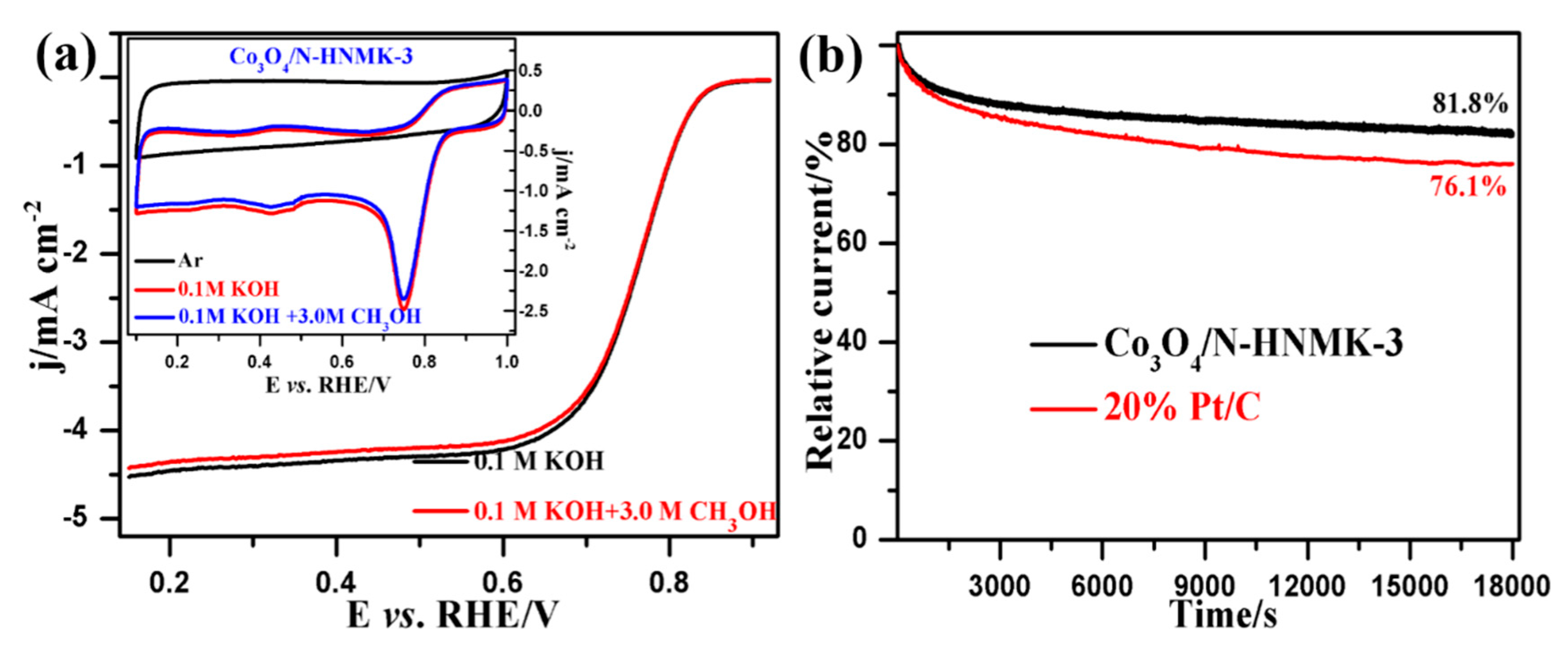
3.2. Electrocatalytic ORR Performance
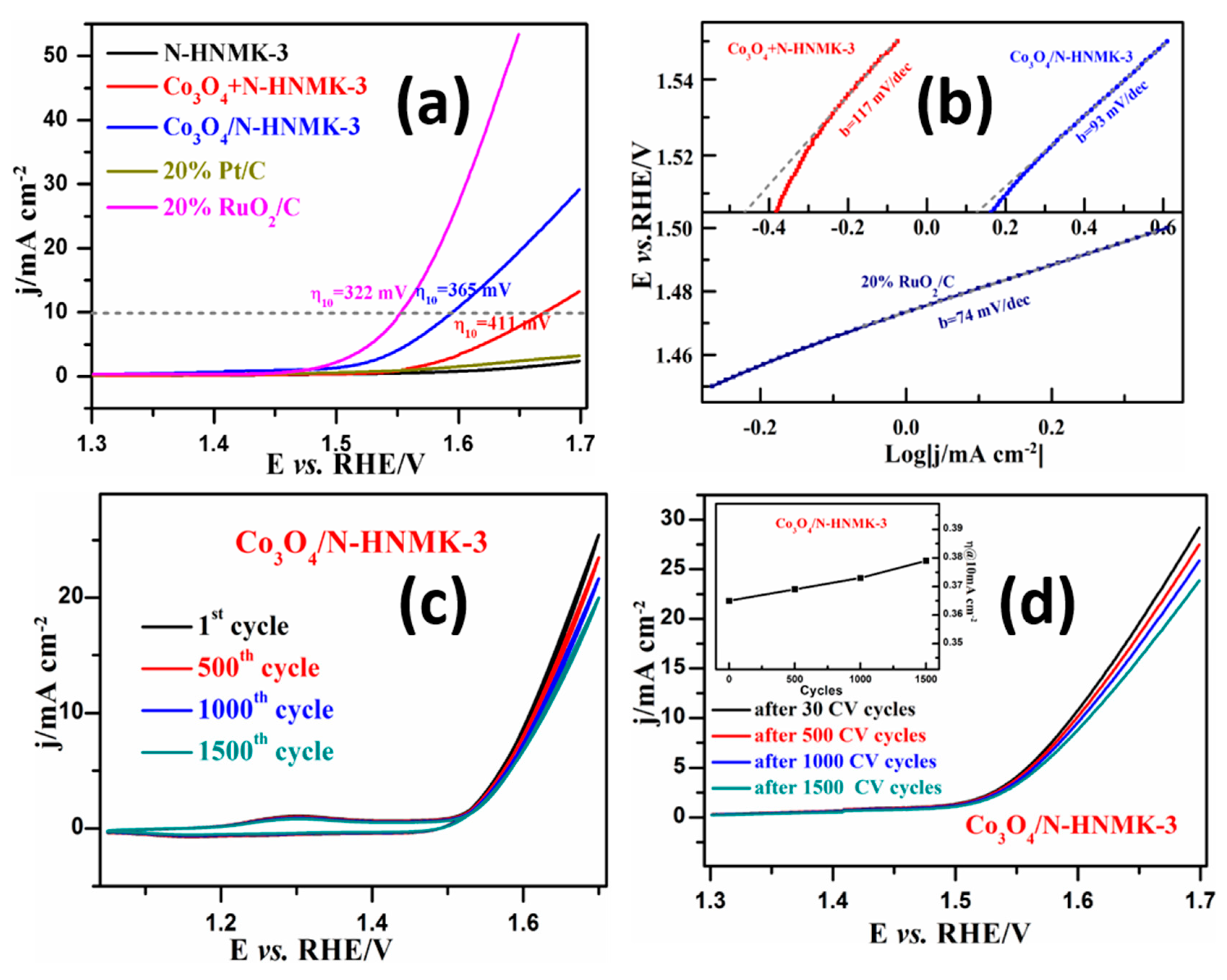
3.3. Electrocatalytic OER Performance
3.4. The Bifunctional ORR/OER Performance
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Postole, G.; Auroux, A. The poisoning level of Pt/C catalysts used in PEM fuel cells by the hydrogen feed gas impurities: The bonding strength. Int. J. Hydrogen 2011, 36, 6817–6825. [Google Scholar] [CrossRef]
- Zhang, X.; Chan, S.H.; Ho, H.K.; Tan, S.-C.; Li, M.; Li, G.; Li, J.; Feng, Z. Towards a smart energy network: The roles of fuel/electrolysis cells and technological perspectives. Int. J. Hydrogen 2015, 40, 6866–6919. [Google Scholar] [CrossRef]
- Qiao, S.Z.; Jiao, Y.; Zheng, Y.; Jaroniec, M. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.-J. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; He, T.; Alonso-Vante, N. In situ free-surfactant synthesis and ORR-electrochemistry of carbon-supported Co3S4 and CoSe2 nanoparticles. Chem. Mater. 2007, 20, 26–28. [Google Scholar] [CrossRef]
- Xu, X.; Liang, H.; Ming, F.; Qi, Z.; Xie, Y.; Wang, Z. Prussian Blue Analogues Derived Penroseite (Ni,Co)Se2 Nanocages Anchored on 3D Graphene Aerogel for Efficient Water Splitting. ACS Catal. 2017, 7, 6394–6399. [Google Scholar] [CrossRef]
- Zhong, H.; Campos-Roldán, C.A.; Zhao, Y.; Zhang, S.; Feng, Y.; Alonso-Vante, N. Recent Advances of Cobalt-Based Electrocatalysts for Oxygen Electrode Reactions and Hydrogen Evolution Reaction. Catalysts 2018, 8, 559. [Google Scholar] [CrossRef]
- Jia, X.; Gao, S.; Liu, T.; Li, D.; Tang, P.; Feng, Y. Controllable Synthesis and Bi-functional Electrocatalytic Performance towards Oxygen Electrode Reactions of Co3O4/N-RGO Composites. Electrochim. Acta 2017, 226, 104–112. [Google Scholar] [CrossRef]
- Tan, H.; Li, Y.; Jiang, X.; Tang, J.; Wang, Z.; Qian, H.; Mei, P.; Malgras, V.; Bando, Y.; Yamauchi, Y. Perfectly ordered mesoporous iron-nitrogen doped carbon as highly efficient catalyst for oxygen reduction reaction in both alkaline and acidic electrolytes. Nano Energy 2017, 36, 286–294. [Google Scholar] [CrossRef]
- Shen, H.; Gracia-Espino, E.; Ma, J.; Tang, H.; Mamat, X.; Wagberg, T.; Hu, G.; Guo, S. Atomically FeN2 moieties dispersed on mesoporous carbon: A new atomic catalyst for efficient oxygen reduction catalysis. Nano Energy 2017, 35, 9–16. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Y.; Wang, K.; Liang, Y.; Wu, D.; Tsiakaras, P.; Song, S. A novel sulfur-nitrogen dual doped ordered mesoporous carbon electrocatalyst for efficient oxygen reduction reaction. Appl. Catal. B: Environ. 2016, 189, 1–11. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Fuertes, A.B.; Sevilla, M.; Titirici, M.-M.; Solis, M.S. Efficient metal-free N-doped mesoporous carbon catalysts for ORR by a template-free approach. Carbon 2016, 106, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef]
- Mengxia, S.; Changping, R.; Yan, C. Covalent entrapment of cobalt-iron sulfides in N-doped mesoporous carbon: Extraordinary bifunctional electrocatalysts for oxygen reduction and evolution reactions. ACS Appl. Mater. Interfaces 2015, 7, 1207. [Google Scholar]
- Lei, W.; Deng, Y.-P.; Li, G.; Cano, Z.P.; Wang, X.; Luo, D.; Liu, Y.; Wang, D.; Chen, Z. Two-Dimensional Phosphorus-Doped Carbon Nanosheets with Tunable Porosity for Oxygen Reactions in Zinc-Air Batteries. ACS Catal. 2018, 8, 2464–2472. [Google Scholar] [CrossRef]
- Chai, G.-L.; Qiu, K.; Qiao, M.; Titirici, M.-M.; Shang, C.; Guo, Z.X. Active sites engineering leads to exceptional ORR and OER bifunctionality in P,N Co-doped graphene frameworks. Environ. Sci. 2017, 10, 1186–1195. [Google Scholar] [CrossRef] [Green Version]
- Patra, S.; Choudhary, R.; Roy, E.; Madhuri, R.; Sharma, P.K.; Sharma, D.P.K. Heteroatom-doped graphene ‘Idli’: A green and foody approach towards development of metal free bifunctional catalyst for rechargeable zinc-air battery. Nano Energy 2016, 30, 118–129. [Google Scholar] [CrossRef]
- Han, J.-S.; Chung, D.Y.; Ha, D.-G.; Kim, J.-H.; Choi, K.; Sung, Y.-E.; Kang, S.-H. Nitrogen and boron co-doped hollow carbon catalyst for the oxygen reduction reaction. Carbon 2016, 105, 1–7. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, S.; Jiang, J.; Feng, Y. Improved Electrocatalytic Performance of Tailored Metal-Free Nitrogen-Doped Ordered Mesoporous Carbons for the Oxygen Reduction Reaction. ChemElectroChem 2018, 5, 1899–1904. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, A.; Yan, J.; Sun, G.; Sun, L.; Zhang, T. Synthesis and Electrochemical Performance of Heteroatom-Incorporated Ordered Mesoporous Carbons. Chem. Mater. 2010, 22, 5463–5473. [Google Scholar] [CrossRef]
- Granozzi, G.; Balbuena, P.B.; Ma, J.; Habrioux, A.; Luo, Y.; Ramos-Sanchez, G.; Calvillo, L.; Alonso-Vante, N. Electronic interaction between platinum nanoparticles and nitrogen-doped reduced graphene oxide: Effect on the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 11891–11904. [Google Scholar]
- Wang, C.; Shi, P.; Yao, W. Synergistic effect of Co3O4 nanoparticles and graphene as catalysts for peroxymonosulfate-based orange II degradation with high oxidant utilization efficiency. J. Phys. Chem. C 2016, 120, 336–344. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H.-M. Graphene Anchored with Co3O4 Nanoparticles as Anode of Lithium Ion Batteries with Enhanced Reversible Capacity and Cyclic Performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Gorlin, Y.; Jaramillo, T.F. A Bifunctional Nonprecious Metal Catalyst for Oxygen Reduction and Water Oxidation. J. Am. Chem. Soc. 2010, 132, 13612–13614. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, X.; Evans, D.G.; Yang, W. Well-dispersed Co3O4/Co2MnO4 nanocomposites as a synergistic bifunctional catalyst for oxygen reduction and oxygen evolution reactions. Nanoscale 2013, 5, 5312. [Google Scholar] [CrossRef]
- Ge, X.; Liu, Y.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Xiao, P.; Zhang, Z.; Lim, S.H.; Li, B.; Wang, X.; et al. Dual-Phase Spinel MnCo2O4 and Spinel MnCo2O4/Nanocarbon Hybrids for Electrocatalytic Oxygen Reduction and Evolution. ACS Appl. Mater. Interfaces 2014, 6, 12684–12691. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Kim, B.J.; Chen, Z. One-pot synthesis of a mesoporous NiCo2O4 nanoplatelet and graphene hybrid and its oxygen reduction and evolution activities as an efficient bi-functional electrocatalyst. J. Mater. Chem. A 2013, 1, 4754. [Google Scholar] [CrossRef]
- Liu, S.; Bian, W.; Yang, Z.; Tian, J.; Jin, C.; Shen, M.; Zhou, Z.; Yang, R. A facile synthesis of CoFe2O4/biocarbon nanocomposites as efficient bi-functional electrocatalysts for the oxygen reduction and oxygen evolution reaction. J. Mater. Chem. A 2014, 2, 18012–18017. [Google Scholar] [CrossRef]
- Qian, L.; Lu, Z.; Xu, T.; Wu, X.; Tian, Y.; Li, Y.; Huo, Z.; Sun, X.; Duan, X. Trinary Layered Double Hydroxides as High-Performance Bifunctional Materials for Oxygen Electrocatalysis. Adv. Mater. 2015, 5, 1500245. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, W.; Chen, S.; Nie, Y.; Xiong, K.; Wei, Z. Cobalt carbonate hydroxide/C: An efficient dual electrocatalyst for oxygen reduction/evolution reactions. Chem. Commun. 2014, 50, 15529–15532. [Google Scholar] [CrossRef] [PubMed]
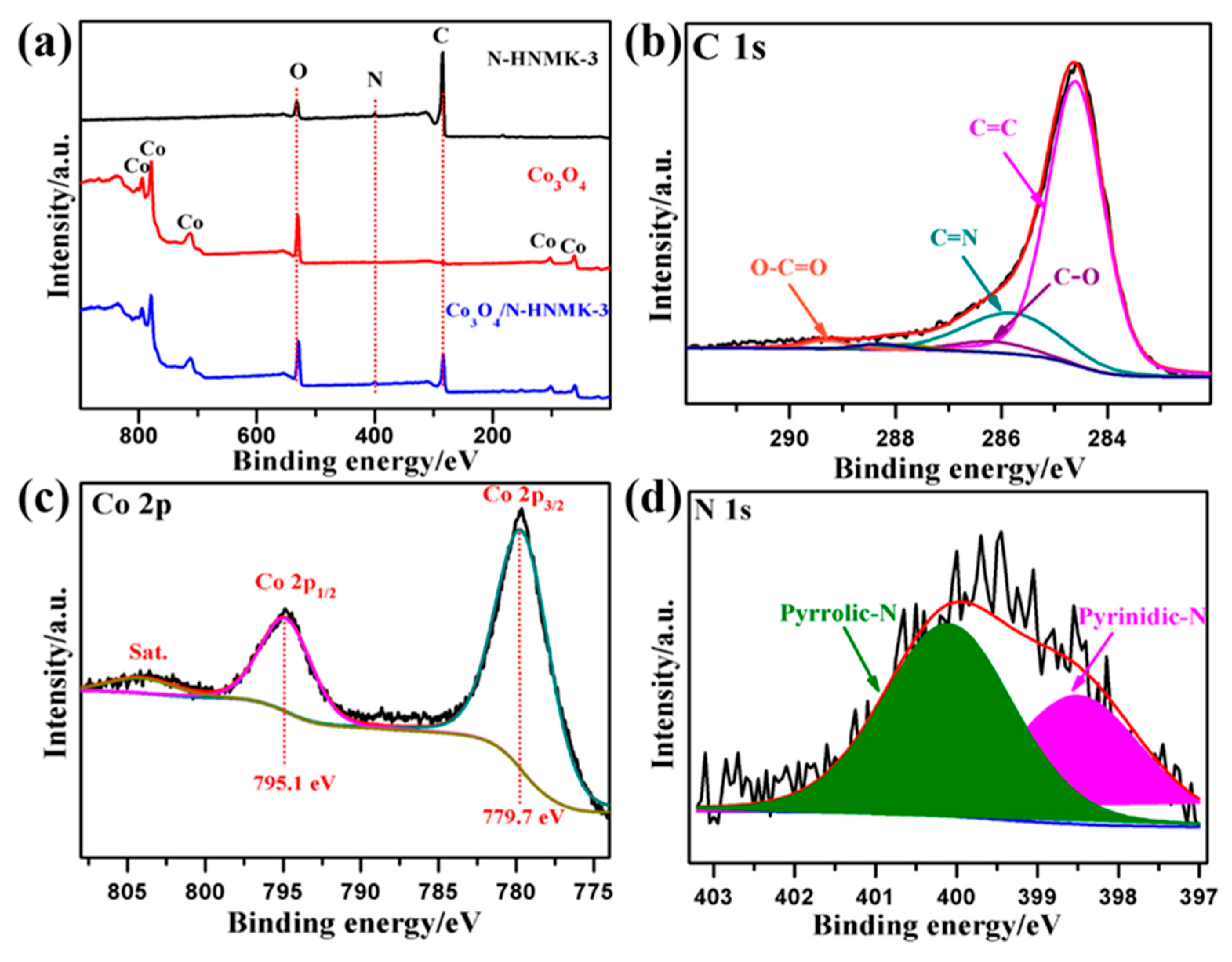
| Samples | C | N | Co | O | Pyridinic-N | Pyrrolic-N |
|---|---|---|---|---|---|---|
| N-HNMK-3 | 88.8 | 2.6 | 8.6 | 31.2 | 68.8 | |
| Co3O4 | 42.8 | 57.2 | ||||
| Co3O4/N-HNMK-3 | 60.6 | 2.2 | 9.4 | 27.8 | 29.8 | 70.2 |
| Catalysts | E vs. RHE at j = −3 mA cm−2 | E vs. RHE at j = 10 mA cm−2 | ΔE (EOER@10 mA cm−2)-(EORR@−3 mA cm−2) | Refs. |
|---|---|---|---|---|
| N-HNMK-3 | 0.69 | 2.13 | 1.44 | This work |
| Co3O4+N-HNMK-3 | 0.56 | 1.69 | 1.13 | This work |
| Co3O4/N-HNMK-3 | 0.73 | 1.59 | 0.86 | This work |
| 20% Pt/C | 0.81 | 2.07 | 1.26 | This work |
| 20% RuO2/C | 0.44 | 1.55 | 1.11 | This work |
| Co3O4/Co2MnO4 | 0.68 | 1.77 | 1.09 | 27 |
| MnCo2O4/Nanocarbon | 0.79 | 1.75 | 0.96 | 28 |
| NiCo2O4/Graphene | 0.60 | 1.68 | 1.08 | 29 |
| CoFe2O4/Biocarbon | 0.69 | 1.67 | 0.98 | 30 |
| O-NiCoFe-LDH | 0.62 | 1.67 | 1.05 | 31 |
| Co(OH)2CO3/C | 0.81 | 1.73 | 0.92 | 32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, S.; Zhong, H.; Alonso-Vante, N.; Li, D.; Tang, P.; Feng, Y. Nitrogen-Doped Ordered Mesoporous Carbons Supported Co3O4 Composite as a Bifunctional Oxygen Electrode Catalyst. Surfaces 2019, 2, 229-240. https://doi.org/10.3390/surfaces2020018
Wang J, Zhang S, Zhong H, Alonso-Vante N, Li D, Tang P, Feng Y. Nitrogen-Doped Ordered Mesoporous Carbons Supported Co3O4 Composite as a Bifunctional Oxygen Electrode Catalyst. Surfaces. 2019; 2(2):229-240. https://doi.org/10.3390/surfaces2020018
Chicago/Turabian StyleWang, Jing, Shuwei Zhang, Haihong Zhong, Nicolas Alonso-Vante, Dianqing Li, Pinggui Tang, and Yongjun Feng. 2019. "Nitrogen-Doped Ordered Mesoporous Carbons Supported Co3O4 Composite as a Bifunctional Oxygen Electrode Catalyst" Surfaces 2, no. 2: 229-240. https://doi.org/10.3390/surfaces2020018
APA StyleWang, J., Zhang, S., Zhong, H., Alonso-Vante, N., Li, D., Tang, P., & Feng, Y. (2019). Nitrogen-Doped Ordered Mesoporous Carbons Supported Co3O4 Composite as a Bifunctional Oxygen Electrode Catalyst. Surfaces, 2(2), 229-240. https://doi.org/10.3390/surfaces2020018