Surface Investigation on Electrochemically Deposited Lead on Gold
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Core-Level Spectroscopy
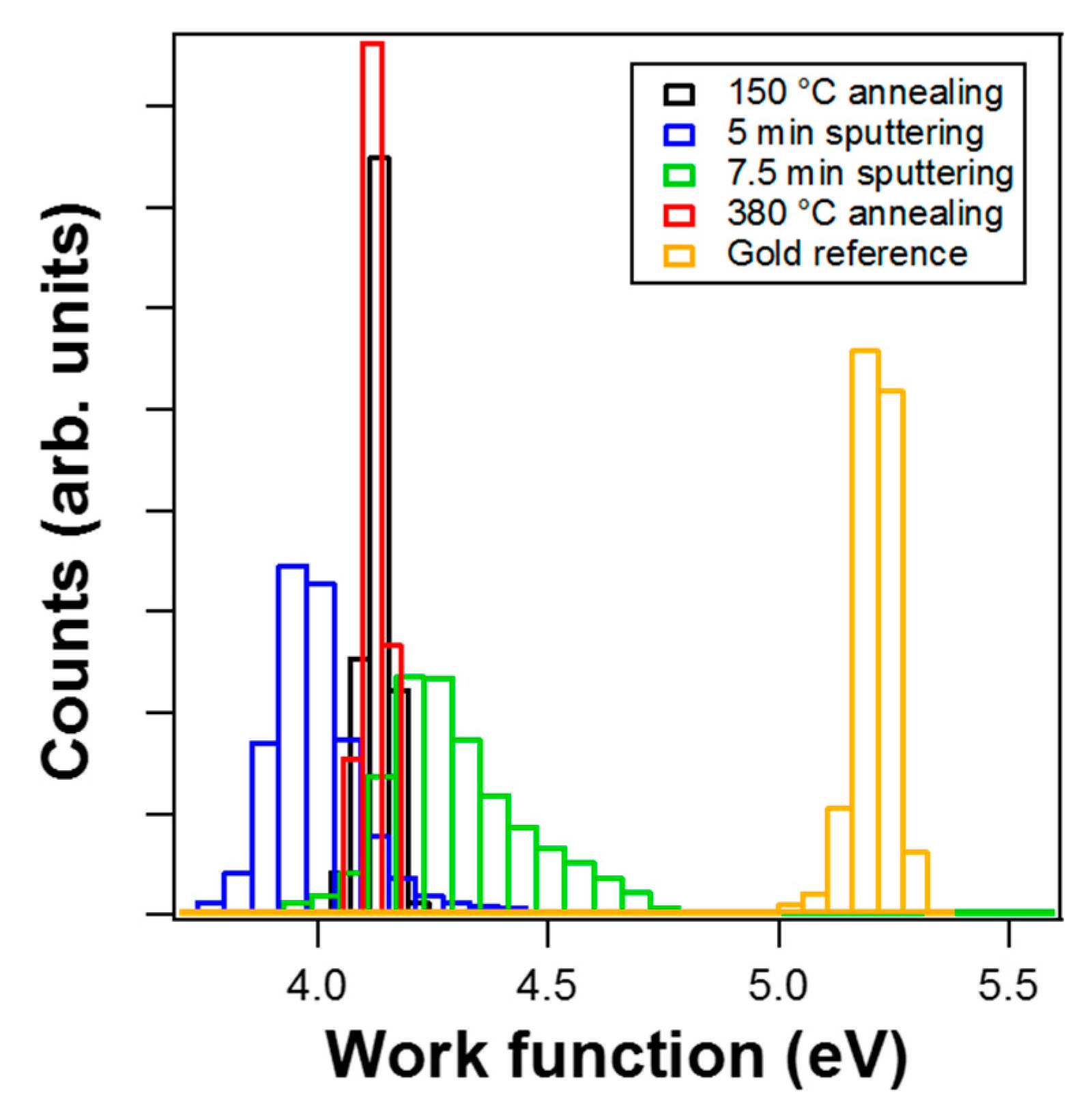
3.2. Valence Band Photoemission and Photoemission Microscopy
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Obretenov, W.; Schmidt, U.; Lorenz, W.J.; Staikov, G.; Budevski, E.; Carnal, D.; Müller, U.; Siegenthaler, H.; Schmidt, E. Underpotential Deposition and Electrocrystallization of Metals An Atomic View by Scanning Tunneling Microscopy. J. Electrochem. Soc. 1993, 140, 692–703. [Google Scholar] [CrossRef]
- Hamelin, A.; Lipkowski, J. Underpotential deposition of lead on gold single crystal faces: Part II. General discussion. J. Electroanal. Chem. Interfacial Electrochem. 1984, 171, 317–330. [Google Scholar] [CrossRef]
- Wu, G.Y.; Schwarzacher, W. The effect of halide additives on the electrodeposition of Pb on polycrystalline Au. J. Electroanal. Chem. 2009, 629, 164–168. [Google Scholar] [CrossRef]
- Adzic, R.; Yeager, E.; Cahan, B. Optical and Electrochemical Studies of Underpotential Deposition of Lead on Gold Evaporated and Single-Crystal Electrodes. J. Electrochem. Soc. 1974, 121, 474–484. [Google Scholar] [CrossRef]
- Alvarez-Rizatti, M.; Jüttner, K. Electrocatalysis of oxygen reduction by UPD of lead on gold single-crystal surfaces. J. Electroanal. Chem. Interfacial Electrochem. 1983, 144, 351–363. [Google Scholar] [CrossRef]
- Green, M.P.; Hanson, K.J.; Carr, R.; Lindau, I. STM Observations of the Underpotential Deposition and Stripping of Pb on Au(111) under Potential Sweep Conditions. J. Electrochem. Soc. 1990, 137, 3493–3498. [Google Scholar] [CrossRef]
- Green, M.P.; Hanson, K.J. Alloy formation in an electrodeposited monolayer. Surf. Sci. 1991, 259, L743–L749. [Google Scholar] [CrossRef]
- Toney, M.F.; Gordon, J.G.; Samant, M.G.; Borges, G.L.; Melroy, O.R. In-Situ Atomic Structure of Underpotentially Deposited Monolayers of Pb and Tl on Au(111) and Ag(111): A Surface X-ray Scattering Study. J. Phys. Chem. 1995, 99, 4733–4744. [Google Scholar] [CrossRef]
- Lorenz, W.J.; Staikov, G. 2D and 3D thin film formation and growth mechanisms in metal electrocrystallization—An atomistic view by in situ STM. Surf. Sci. 1995, 335, 32–43. [Google Scholar] [CrossRef]
- Herrero, E.; Buller, L.J.; Abruña, H.D. Underpotential Deposition at Single Crystal Surfaces of Au, Pt, Ag and Other Materials. Chem. Rev. 2001, 101, 1897–1930. [Google Scholar] [CrossRef]
- Hsieh, S.-J.; Gewirth, A.A. Poisoning the catalytic reduction of peroxide on Pb underpotential deposition modified Au surfaces with iodine. Surf. Sci. 2002, 498, 147–160. [Google Scholar] [CrossRef]
- Ganon, J.P.; Clavilier, J. Electrochemical adsorption of lead and bismuth at gold single crystal surfaces with vicinal (111) orientations. I. Surf. Sci. 1984, 145, 487–518. [Google Scholar] [CrossRef]
- Samant, M.G.; Toney, M.F.; Borges, G.L.; Blum, L.; Melroy, O.R. Grazing incidence x-ray diffraction of lead monolayers at a silver (111) and gold (111) electrode/electrolyte interface. J. Phys. Chem. 1988, 92, 220–225. [Google Scholar] [CrossRef]
- Tao, N.J.; Pan, J.; Li, Y.; Oden, P.I.; DeRose, J.A.; Lindsay, S.M. Initial stage of underpotential deposition of Pb on reconstructed and unreconstructed Au(111). Surf. Sci. 1992, 271, L338–L344. [Google Scholar] [CrossRef]
- Chen, C.H.; Washburn, N.; Gewirth, A.A. In situ atomic force microscope study of lead underpotential deposition on gold (111): Structural properties of the catalytically active phase. J. Phys. Chem. 1993, 97, 9754–9760. [Google Scholar] [CrossRef]
- Crepaldi, A.; Pons, S.; Frantzeskakis, E.; Calleja, F.; Etzkorn, M.; Seitsonen, A.P.; Kern, K.; Brune, H.; Grioni, M. Combined ARPES and STM study of Pb/Au(111) Moiré structure: One overlayer, two symmetries. Phys. Rev. B 2013, 87. [Google Scholar] [CrossRef]
- Hernández, J.; Solla-Gullón, J.; Herrero, E.; Aldaz, A.; Feliu, J.M. Characterization of the Surface Structure of Gold Nanoparticles and Nanorods Using Structure Sensitive Reactions. J. Phys. Chem. B 2005, 109, 12651–12654. [Google Scholar] [CrossRef]
- Jeyabharathi, C.; Zander, M.; Scholz, F. Underpotential deposition of lead on quasi-spherical and faceted gold nanoparticles. J. Electroanal. Chem. 2017. [Google Scholar] [CrossRef]
- Liu, Y.; Bliznakov, S.; Dimitrov, N. Comprehensive Study of the Application of a Pb Underpotential Deposition-Assisted Method for Surface Area Measurement of Metallic Nanoporous Materials. J. Phys. Chem. C 2009, 113, 12362–12372. [Google Scholar] [CrossRef]
- Adžić, R.R.; Tripkovic, A.V.; Markovic, N.M. Oxygen reduction on electrode surfaces modified by foreign metal ad-atoms: Lead ad-atoms on gold. J. Electroanal. Chem. 1980, 114, 37–51. [Google Scholar] [CrossRef]
- Wang, Y.; Laborda, E.; Plowman, B.J.; Tschulik, K.; Ward, K.R.; Palgrave, R.G.; Damm, C.; Compton, R.G. The strong catalytic effect of Pb(II) on the oxygen reduction reaction on 5 nm gold nanoparticles. Phys. Chem. Chem. Phys. 2014, 16, 3200–3208. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.; Solla-Gullón, J.; Herrero, E.; Aldaz, A.; Feliu, J.M. Methanol oxidation on gold nanoparticles in alkaline media: Unusual electrocatalytic activity. Electrochim. Acta 2006, 52, 1662–1669. [Google Scholar] [CrossRef]
- Chen, Y.; Milenkovic, S.; Hassel, A.W. {110}-Terminated Square-Shaped Gold Nanoplates and Their Electrochemical Surface Reactivity. ChemElectroChem 2017, 4, 557–564. [Google Scholar] [CrossRef]
- Yu, L.; Akolkar, R. Lead underpotential deposition for the surface characterization of silver ad-atom modified gold electrocatalysts for glucose oxidation. J. Electroanal. Chem. 2017, 792, 61–65. [Google Scholar] [CrossRef]
- Brankovic, S.R.; Dimitrov, N.; Sieradzki, K. Surfactant Mediated Electrochemical Deposition of Ag on Au(111). Electrochem. Solid State Lett. 1999, 2, 443–445. [Google Scholar] [CrossRef]
- Wu, D.; Solanki, D.J.; Joi, A.; Dordi, Y.; Dole, N.; Litvnov, D.; Brankovic, S.R. Pb Monolayer Mediated Thin Film Growth of Cu and Co: Exploring Different Concepts. J. Electrochem. Soc. 2019, 1666, D3013–D3021. [Google Scholar] [CrossRef]
- Sieradzki, K.; Dimitrov, N. Electrochemical Defect-Mediated Thin-Film Growth. Science 1999, 284, 138. [Google Scholar] [CrossRef]
- Hwang, S.; Oh, I.; Kwak, J. Electrodeposition of Epitaxial Cu(111) Thin Films on Au(111) Using Defect-Mediated Growth. J. Am. Chem. Soc. 2001, 123, 7176–7177. [Google Scholar] [CrossRef]
- Viyannalage, L.T.; Vasilic, R.; Dimitrov, N. Epitaxial Growth of Cu on Au(111) and Ag(111) by Surface Limited Redox ReplacementAn Electrochemical and STM Study. J. Phys. Chem. C 2007, 111, 4036–4041. [Google Scholar] [CrossRef]
- Fayette, M.; Liu, Y.; Bertrand, D.; Nutariya, J.; Vasiljevic, N.; Dimitrov, N. From Au to Pt via Surface Limited Redox Replacement of Pb UPD in One-Cell Configuration. Langmuir 2011, 27, 5650–5658. [Google Scholar] [CrossRef]
- Jayaraju, N.; Viravapandian, D.; Kim, G.Y.; Banga, D.; Stickney, J.L. Electrochemical Atomic Layer Deposition (E-ALD) of Pt Nanofilms Using SLRR Cycles. J. Electrochem. Soc. 2012, 159, D616–D622. [Google Scholar] [CrossRef]
- Ambrozik, S.; Rawlings, B.; Vasiljevic, N.; Dimitrov, N. Metal deposition via electroless surface limited redox replacement. Electrochem. Commun. 2014, 44, 19–22. [Google Scholar] [CrossRef]
- Dimitrov, N. Recent Advances in the Growth of Metals, Alloys, and Multilayers by Surface Limited Redox Replacement (SLRR) Based Approaches. Electrochim. Acta 2016, 209, 599–622. [Google Scholar] [CrossRef]
- Al Amri, Z.; Mercer, M.P.; Vasiljevic, N. Surface Limited Redox Replacement Deposition of Platinum Ultrathin Films on Gold: Thickness and Structure Dependent Activity towards the Carbon Monoxide and Formic Acid Oxidation reactions. Electrochim. Acta 2016, 210, 520–529. [Google Scholar] [CrossRef]
- Brankovic, S.R. Fundamentals of Metal Deposition via Surface Limited Redox Replacement of Underpotentially-Deposited Monolayer. Electrochem. Soc. Interface 2018, 27, 57–63. [Google Scholar] [CrossRef]
- Brankovic, S.R.; Wang, J.X.; Adžić, R.R. Metal monolayer deposition by replacement of metal adlayers on electrode surfaces. Surf. Sci. 2001, 474, L173–L179. [Google Scholar] [CrossRef]
- Mercer, M.P.; Plana, D.; Fermiotan, D.J.; Morgan, D.; Vasiljevic, N. Growth of epitaxial Pt1-xPbx alloys by surface limited redox replacement and study of their adsorption properties. Langmuir 2015, 31, 10904–10912. [Google Scholar] [CrossRef]
- Vasiljevic, N. Electrodeposition of Pt-bimetallic Model Systems for Electrocatalysis and Electrochemical Surface Science. Electrochem. Soc. Interface 2018, 27, 71–75. [Google Scholar] [CrossRef]
- Zhang, J.; Vukmirovic, M.B.; Sasaki, K.; Nilekar, A.U.; Mavrikakis, M.; Adzic, R.R. Mixed-Metal Pt Monolayer Electrocatalysts for Enhanced Oxygen Reduction Kinetics. J. Am. Chem Soc. 2005, 127, 12480–12481. [Google Scholar] [CrossRef]
- Bromberg, L.; Fayette, M.; Martens, B.; Luo, Z.P.; Wang, Y.; Xu, D.; Zhang, J.; Fang, J.; Dimitrov, N. Catalytic Performance Comparison of Shape-Dependent Nanocrystals and Oriented Ultrathin Films of Pt4Cu Alloy in the Formic Acid Oxidation Process. Electrocatalysis 2012, 1–13. [Google Scholar] [CrossRef]
- McCurry, D.; Kamundi, M.; Fayette, M.; Wafula, F.; Dimitrov, N. All Electrochemical Fabrication of a Platinized Nanoporous Au Thin-Film Catalyst. ACS Appl. Mater. Interfaces 2011, 3, 4459–4468. [Google Scholar] [CrossRef] [PubMed]
- Adzic, R.R.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Shao, M.; Wang, J.X.; Nilekar, A.U.; Mavrikakis, M.; Valerio, J.A.; Uribe, F. Platinum Monolayer Fuel Cell Electrocatalysts. Top. Catal. 2007, 46, 249–262. [Google Scholar] [CrossRef]
- Fayette, M.; Nutariya, J.; Vasiljevic, N.; Dimitrov, N. A Study of Pt Dissolution during Formic Acid Oxidation. ACS Catalysis 2013, 3, 1709–1718. [Google Scholar] [CrossRef]
- Wu, D.; Solanki, D.J.; Ramirez, J.L.; Yang, W.; Joi, A.; Dordi, Y.; Dole, N.; Brankovic, S.R. Electroless Deposition of Pb Monolayer: A New Process and Application to Surface Selective Atomic Layer Deposition. Langmuir 2018, 34, 11384–11394. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Hanson, K.; Scherson, D.; Xing, X.; Richter, M.; Ross, P.; Carr, R.; Lindau, I. In situ scanning tunneling microscopy studies of the underpotential deposition of lead on gold (111). J. Phys. Chem. 1989, 93, 2181–2184. [Google Scholar] [CrossRef]
- Stafford, G.R.; Bertocci, U. In Situ Stress and Nanogravimetric Measurements During Underpotential Deposition of Pb on (111)-Textured Au. J. Phys. Chem. C 2007, 111, 17580–17586. [Google Scholar] [CrossRef]
- Shin, J.W.; Bertocci, U.; Stafford, G.R. Stress Response to Surface Alloying and Dealloying during Underpotential Deposition of Pb on (111)-Textured Au. J. Phys. Chem. C 2010, 114, 7926–7932. [Google Scholar] [CrossRef]
- Nutariya, J.; Velleuer, J.; Schwarzacher, W.; Vasiljevic, N. Surface alloying/dealloying in Pb/Au (111) system. ECS Trans. 2010, 28, 15–25. [Google Scholar] [CrossRef]
- Biberian, J.P.; Rhead, G.E. Spontaneous alloying of a gold substrate with lead monolayers. J. Phys. F Met. Phys. 1973, 3, 675–682. [Google Scholar] [CrossRef]
- Perdereau, J.; Biberian, J.P.; Rhead, G.E. Adsorption and surface alloying of lead monolayers on (111) and (110) faces of gold. J. Phys. F Met. Phys. 1974, 4, 798. [Google Scholar] [CrossRef]
- Wang, F.-X.; Pan, G.-B.; Liu, Y.-D.; Xiao, Y. Pb deposition onto Au(111) from acidic chloroaluminate ionic liquid. Chem. Phys. Lett. 2010, 488, 112–115. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, J.S.; Han, J.Y.; Seo, J.M.; Lee, C.K.; Hong, S.C. Surface alloying of a Co film on the Cu(001) surface. Surf. Sci. 2000, 453, 47–58. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M. Effect of Surface Composition on Electronic Structure, Stability, and Electrocatalytic Properties of Pt-Transition Metal Alloys: Pt-Skin versus Pt-Skeleton Surfaces. J. Am. Chem. Soc. 2006, 128, 8813–8819. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zhang, H.; Meng, Y.; He, B.; Yu, L. Dissolution Engineering of Platinum Alloy Counter Electrodes in Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 11448–11452. [Google Scholar] [CrossRef] [PubMed]
- Ali-Löytty, H.; Hannula, M.; Honkanen, M.; Östman, K.; Lahtonen, K.; Valden, M. Grain orientation dependent Nb–Ti microalloying mediated surface segregation on ferritic stainless steel. Corros. Sci. 2016, 112, 204–213. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- Mun, B.S.; Watanabe, M.; Rossi, M.; Stamenkovic, V.R.; Markovic, N.M.; Ross, P.N. A study of electronic structures of Pt3M (M=Ti,V,Cr,Fe,Co,Ni) polycrystalline alloys with valence-band photoemission spectroscopy. J. Chem. Phys. 2005, 123, 204717. [Google Scholar] [CrossRef]
- Renault, O.; Brochier, R.; Roule, A.; Haumesser, P.H.; Krömker, B.; Funnemann, D. Work-function imaging of oriented copper grains by photoemission. Surf. Interface Anal. 2006, 38, 375–377. [Google Scholar] [CrossRef]
- Tiwari, D.; Cattelan, M.; Harniman, R.L.; Sarua, A.; Abbas, A.; Bowers, J.W.; Fox, N.A.; Fermin, D.J. Mapping Shunting Paths at the Surface of Cu2ZnSn(S,Se)4 Films via Energy-Filtered Photoemission Microscopy. iScience 2018, 9, 36–46. [Google Scholar] [CrossRef]
- Vasilic, R.; Vasiljevic, N.; Dimitrov, N. Open Circuit Stability of Underpotentially Deposited Pb Monolayer on Cu(111). J. Elecatroanal. Chem. 2005, 580, 203–212. [Google Scholar] [CrossRef]
- Tiwari, D.; Cattelan, M.; Harniman, R.L.; Sarua, A.; Fox, N.; Koehler, T.; Klenk, R.; Fermin, D.J. Impact of Sb and Na Doping on the Surface Electronic Landscape of Cu2ZnSnS4 Thin Films. ACS Energy Lett. 2018, 2977–2982. [Google Scholar] [CrossRef]
- Kim, K.S.; O’Leary, T.J.; Winograd, N. X-ray photoelectron spectra of lead oxides. Anal. Chem. 1973, 45, 2214–2218. [Google Scholar] [CrossRef]
- Desimoni, E.; Casella, G.I.; Morone, A.; Salvi, A.M. XPS determination of oxygen-containing functional groups on carbon-fibre surfaces and the cleaning of these surfaces. Surf. Interface Anal. 1990, 15, 627–634. [Google Scholar] [CrossRef]
- Fadley, C.S. Angle-resolved x-ray photoelectron spectroscopy. Prog. Surf. Sci. 1984, 16, 275–388. [Google Scholar] [CrossRef]
- Light, T.B.; Eldridge, J.M.; Matthews, J.W.; Greiner, J.H. Structure of thin lead oxide layers as determined by x−ray diffraction. J. Appl. Phys. 1975, 46, 1489–1492. [Google Scholar] [CrossRef]
- Brunt, T.A.; Rayment, T.; O’Shea, S.J.; Welland, M.E. Measuring the Surface Stresses in an Electrochemically Deposited Metal Monolayer: Pb on Au(111). Langmuir 1996, 12, 5942–5946. [Google Scholar] [CrossRef]
- Vurens, G.H.; Salmeron, M.; Somorjai, G.A. The preparation of thin ordered transition metal oxide films on metal single crystals for surface science studies. Prog. Surf. Sci. 1989, 32, 333–360. [Google Scholar] [CrossRef]
- Seah, M.P.; Dench, W.A. Quantitative electron spectroscopy of surfaces: A standard data base for electron inelastic mean free paths in solids. Surf. Interface Anal. 1979, 1, 2–11. [Google Scholar] [CrossRef]
- Nyholm, R.; Berndtsson, A.; Martensson, N. Core level binding energies for the elements Hf to Bi (Z=72-83). J. Phys. C 1980, 13, L1091. [Google Scholar] [CrossRef]
- Nicholson, J.A.; Riley, J.D.; Leckey, R.C.G.; Jenkin, J.G.; Liesegang, J.; Azoulay, J. Ultraviolet photoelectron spectroscopy of the valence bands of some Au alloys. Phys. Rev. B 1978, 18, 2561–2567. [Google Scholar] [CrossRef]
- Eastman, D.E.; Grobman, W.D. Photoemission Energy Distributions for Au from 10 to 40 eV Using Synchrotron Radiation. Phys. Rev. Lett. 1972, 28, 1327–1330. [Google Scholar] [CrossRef]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef]
- Evans, S.; Thomas, J.M. Electronic structure of the oxides of lead. Part 1.—A study using X-ray and ultraviolet photoelectron spectroscopy of the oxidation of polycrystalline lead. J. Chem. Soc. Faraday Trans. 1975, 71, 313–328. [Google Scholar] [CrossRef]
- Uda, M.; Nakamura, A.; Yamamoto, T.; Fujimoto, Y. Work function of polycrystalline Ag, Au and Al. J. Electron. Spectros. Relat. Phenom. 1998, 88, 643–648. [Google Scholar] [CrossRef]
- Hoster, H. Surface Alloys; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2014; Volume 3, pp. 329–367. [Google Scholar]
- Pantelides, S.T.; Mickish, D.J.; Kunz, A.B. Correlation effects in energy-band theory. Phys. Rev. B 1974, 10, 2602–2613. [Google Scholar] [CrossRef]
- Shevchik, N.J. Evolution of energy bands from the Au 5d level in Cd-Au alloys. J. Phys. F 1975, 5, 1860. [Google Scholar] [CrossRef]
- Moruzzi, V.L.; Williams, A.R.; Janak, J.F. Band narrowing and charge transfer in six intermetallic compounds. Phys. Rev. B 1974, 10, 4856–4862. [Google Scholar] [CrossRef]
- Tersoff, J. Surface-Confined Alloy Formation in Immiscible Systems. Phys. Rev. Lett. 1984, 74, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Besenbacher, F.; Nielsen, L.P.; Sprunger, P.T. Chapter 6 Surface alloying in heteroepitaxial metal-on-metal growth. In The Chemical Physics of Solid Surfaces; King, D.A., Woodruff, D.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 8, pp. 207–257. [Google Scholar]
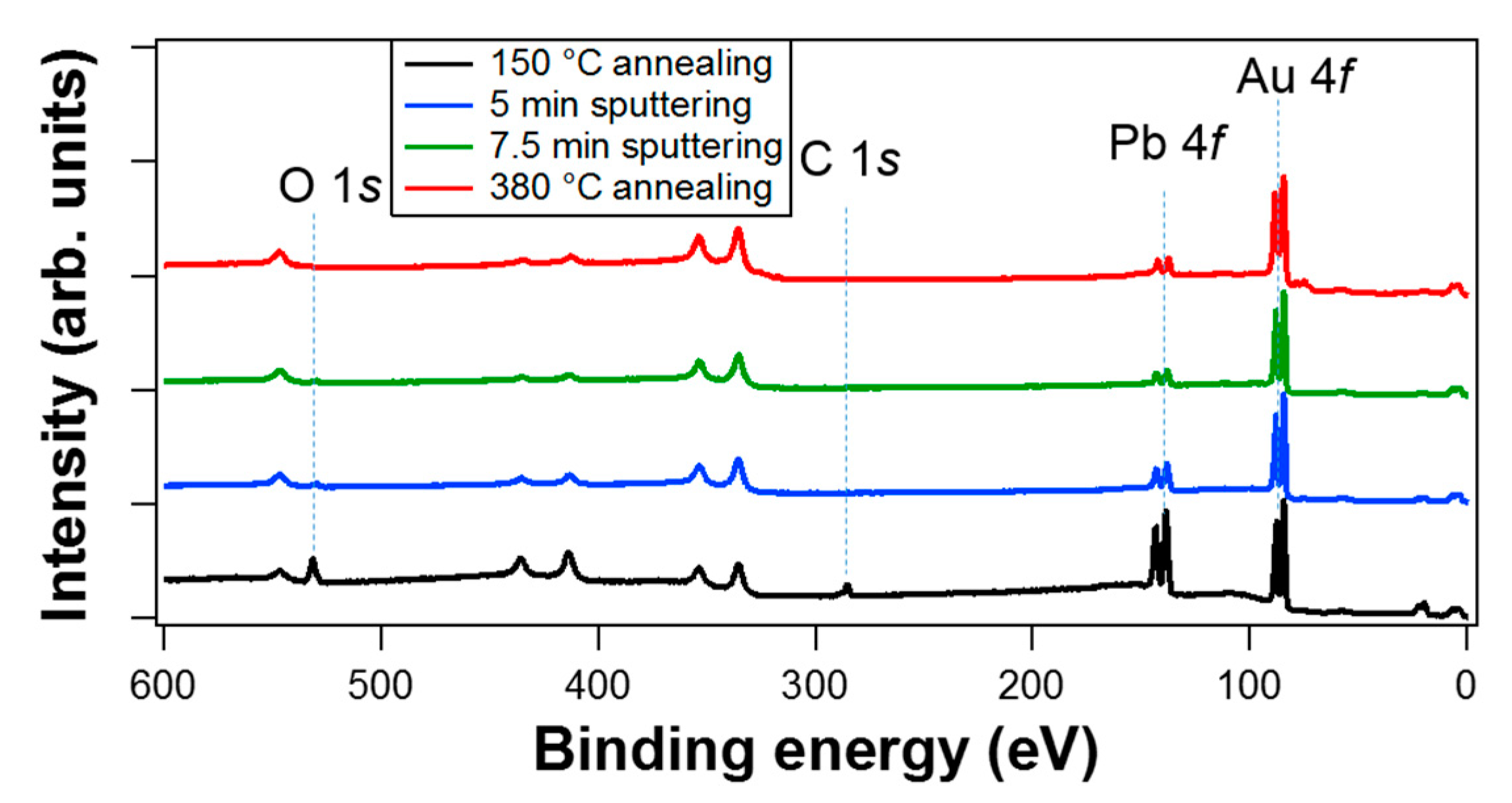
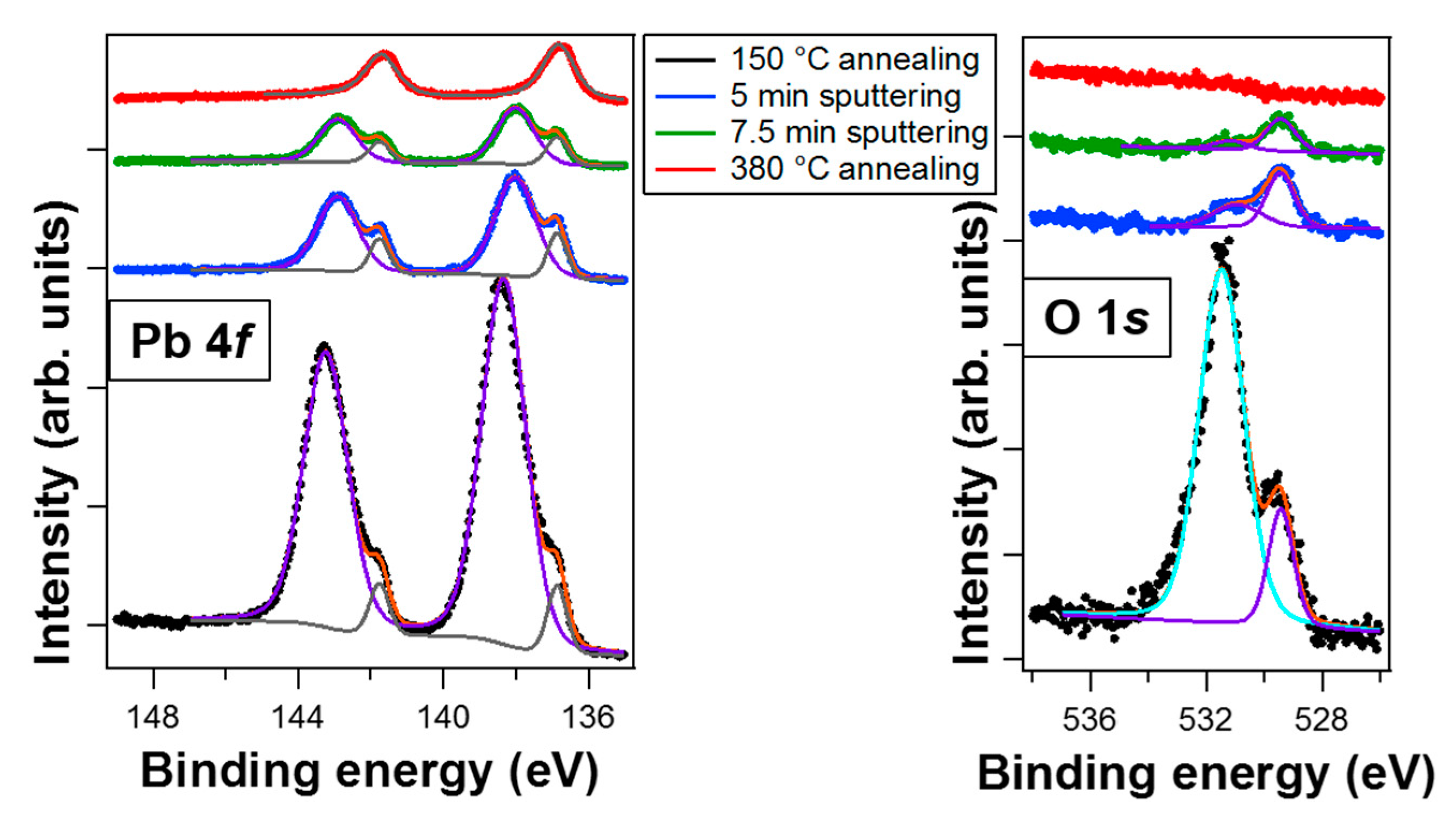
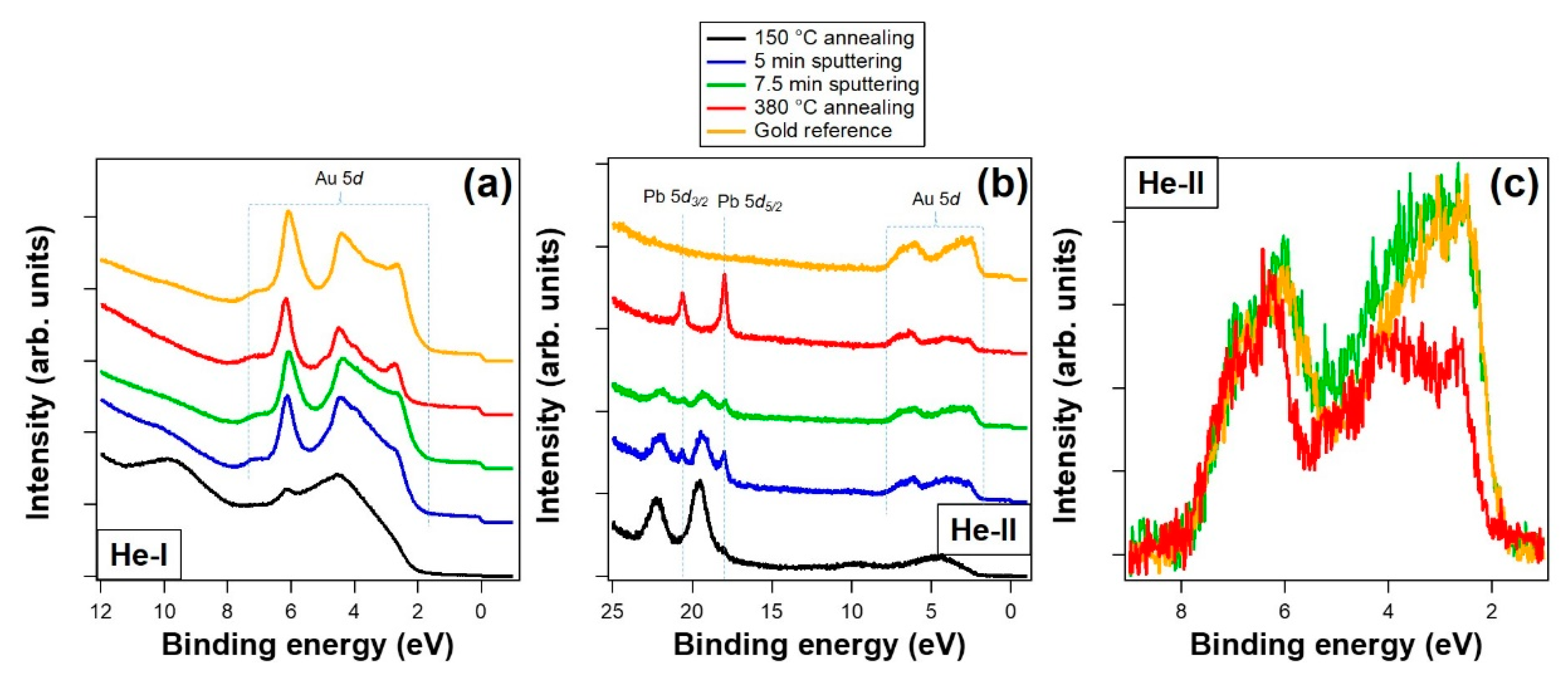
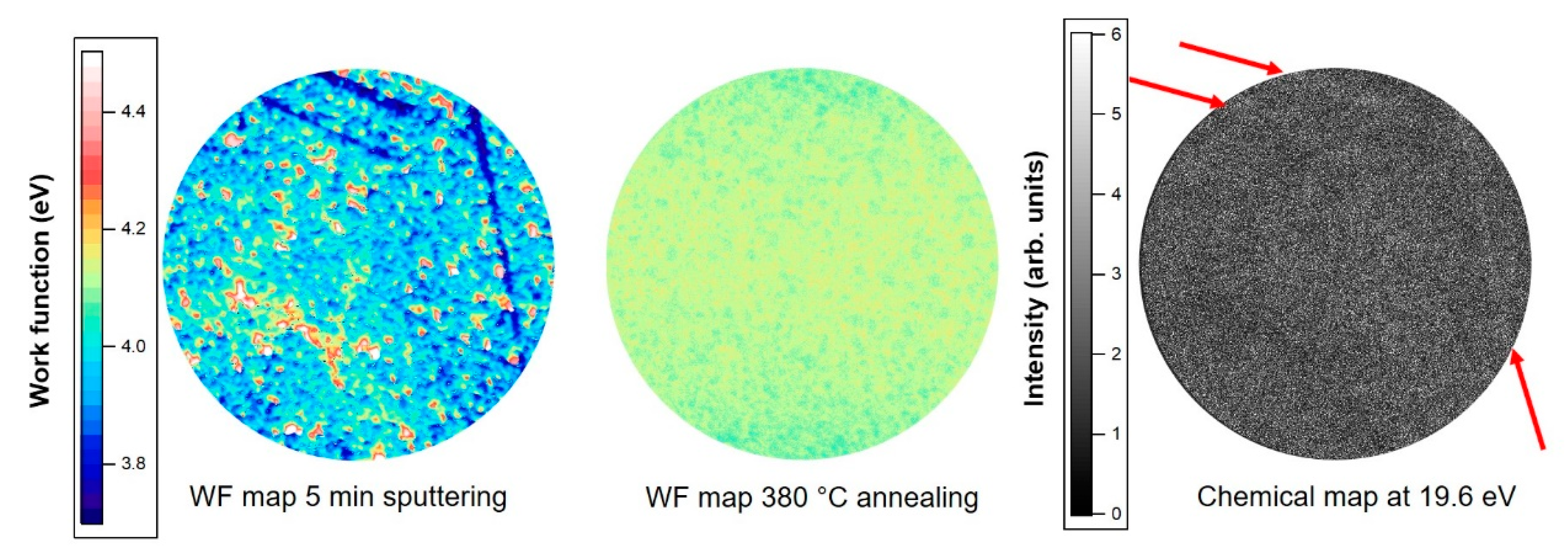
| Treatment | Pb 4f7/2 metal BE | Pb 4f7/2 Oxide BE | Area Pb Oxide/Area Pb Metal | O/Pb (at. %) | Overlayer Coverage |
|---|---|---|---|---|---|
| 150 °C anneal. | 136.9 eV | 138.3 eV | 12.0 | 1.7 | 11.4 Å (3.7 ML) |
| 5 min sputter. | 136.9 eV | 138.1 eV | 4.5 | 0.9 | 5.5 Å (1.8 ML) |
| 7.5 min sputter. | 136.9 eV | 138.0 eV | 3.5 | 0.8 | 3.0 Å (1.0 ML) |
| 380 °C anneal. | 136.8 eV | / | / | / | 2.5 Å (0.9 ML) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepanska, A.; Wan, G.; Cattelan, M.; Fox, N.A.; Vasiljevic, N. Surface Investigation on Electrochemically Deposited Lead on Gold. Surfaces 2019, 2, 56-68. https://doi.org/10.3390/surfaces2010006
Szczepanska A, Wan G, Cattelan M, Fox NA, Vasiljevic N. Surface Investigation on Electrochemically Deposited Lead on Gold. Surfaces. 2019; 2(1):56-68. https://doi.org/10.3390/surfaces2010006
Chicago/Turabian StyleSzczepanska, Alicja, Gary Wan, Mattia Cattelan, Neil A. Fox, and Natasa Vasiljevic. 2019. "Surface Investigation on Electrochemically Deposited Lead on Gold" Surfaces 2, no. 1: 56-68. https://doi.org/10.3390/surfaces2010006
APA StyleSzczepanska, A., Wan, G., Cattelan, M., Fox, N. A., & Vasiljevic, N. (2019). Surface Investigation on Electrochemically Deposited Lead on Gold. Surfaces, 2(1), 56-68. https://doi.org/10.3390/surfaces2010006






