X-ray Absorption under Operating Conditions for Solid-Oxide Fuel Cells Electrocatalysts: The Case of LSCF/YSZ
Abstract
1. Introduction
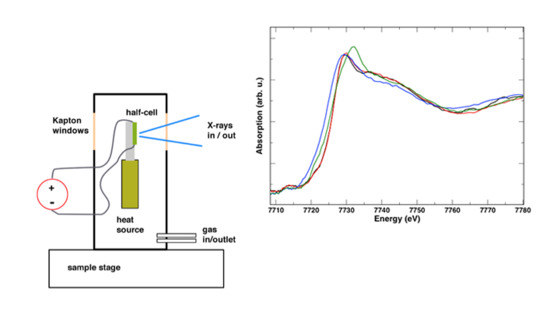
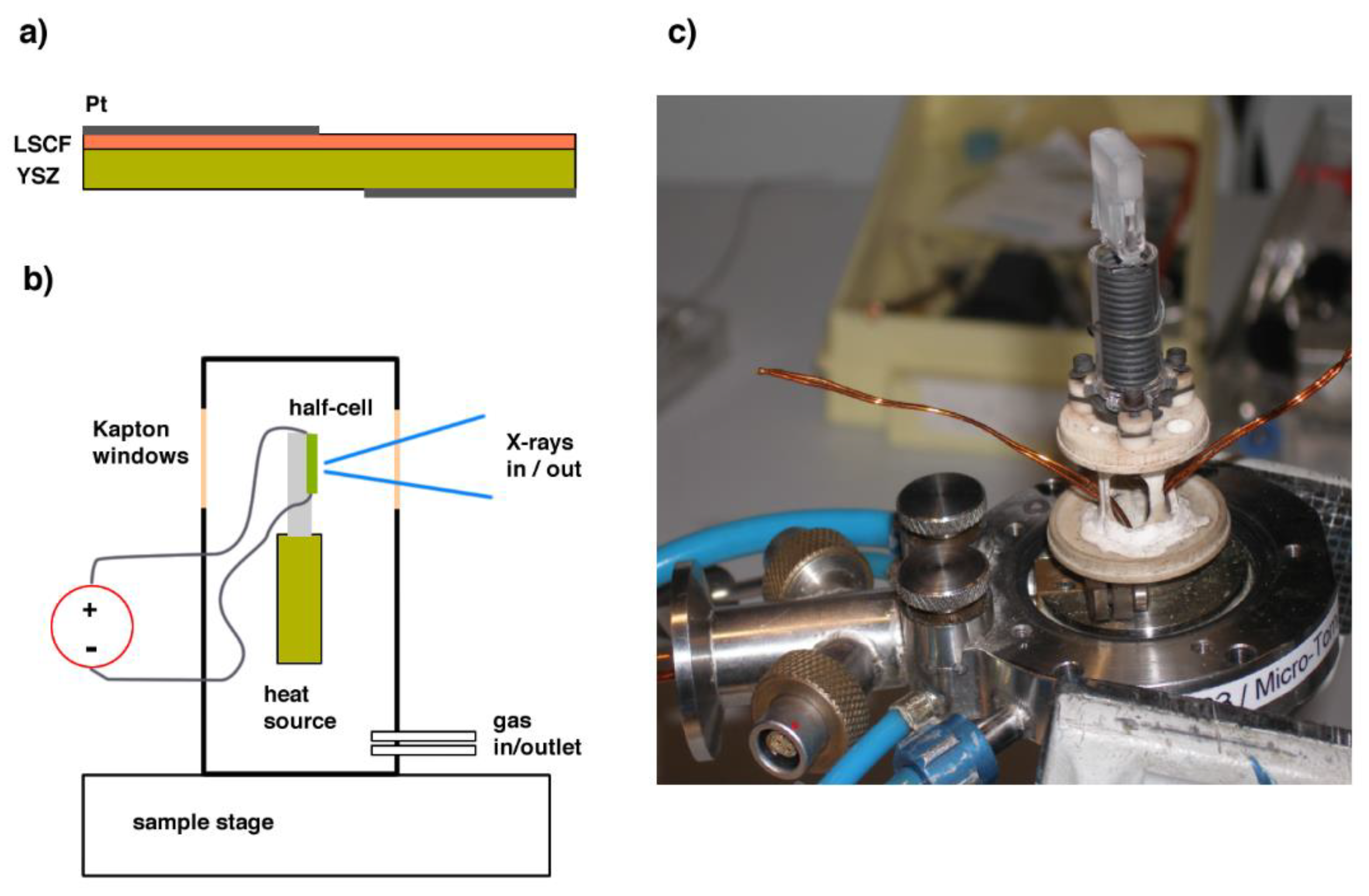
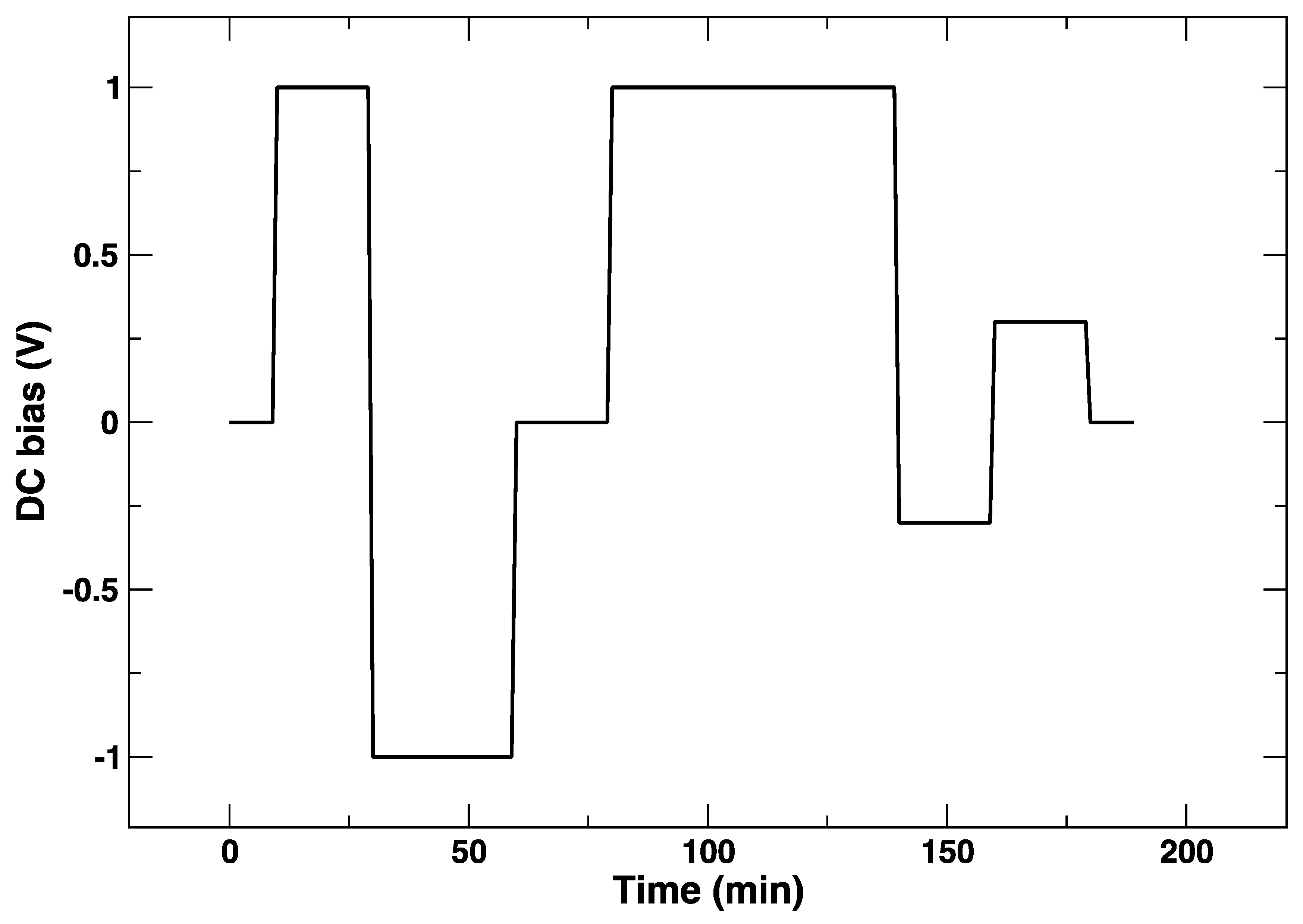
2. Materials and Methods
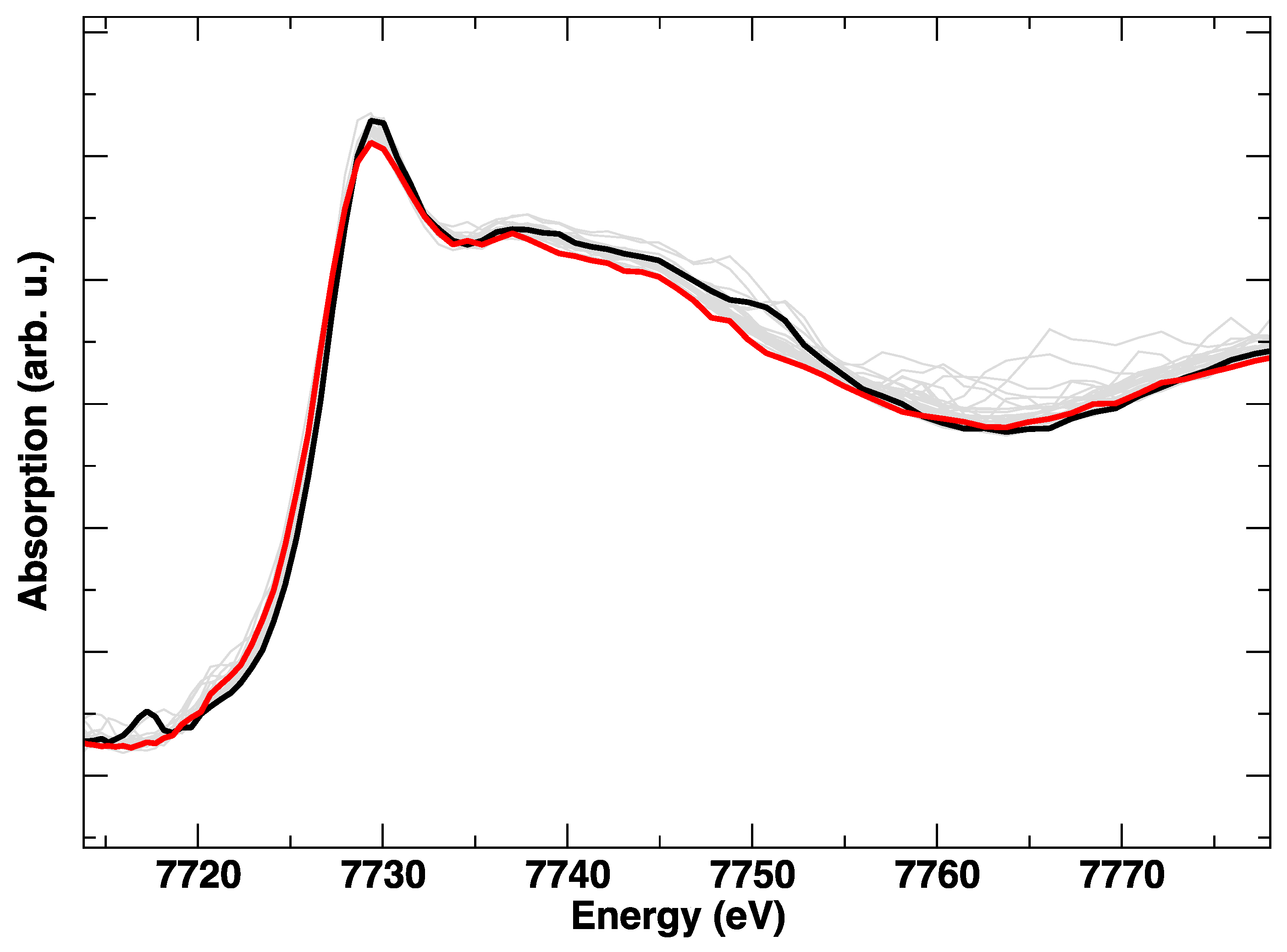
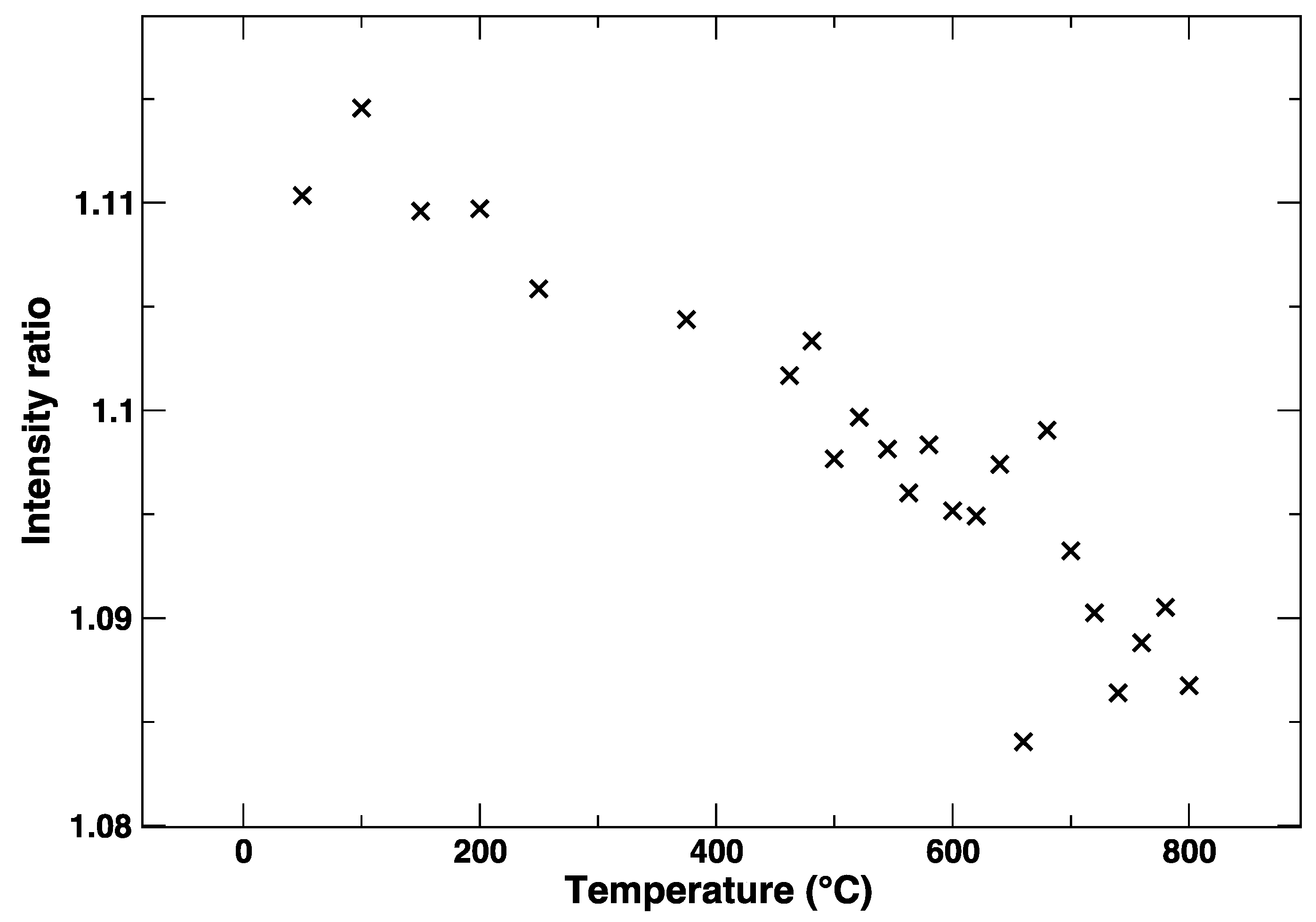
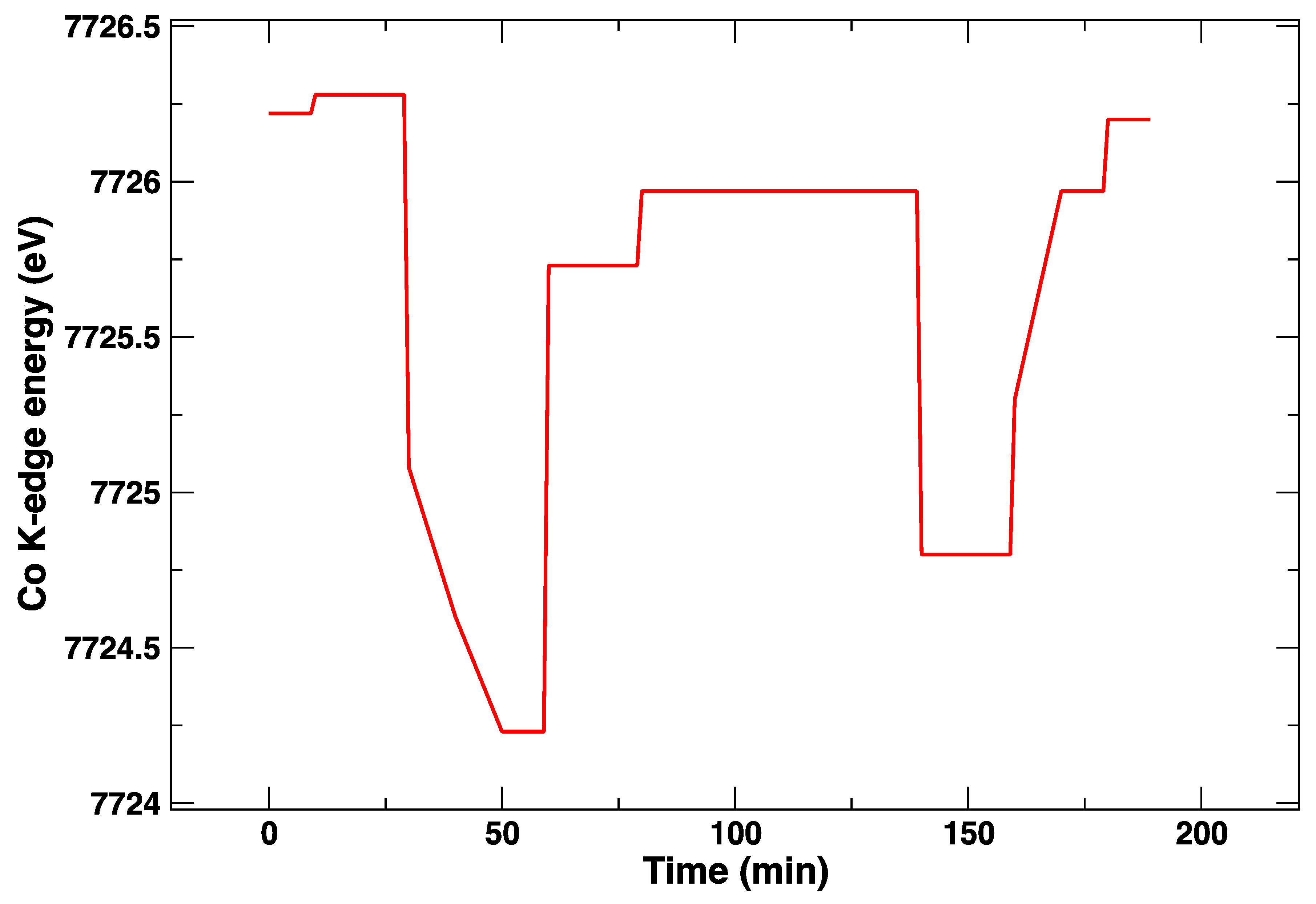
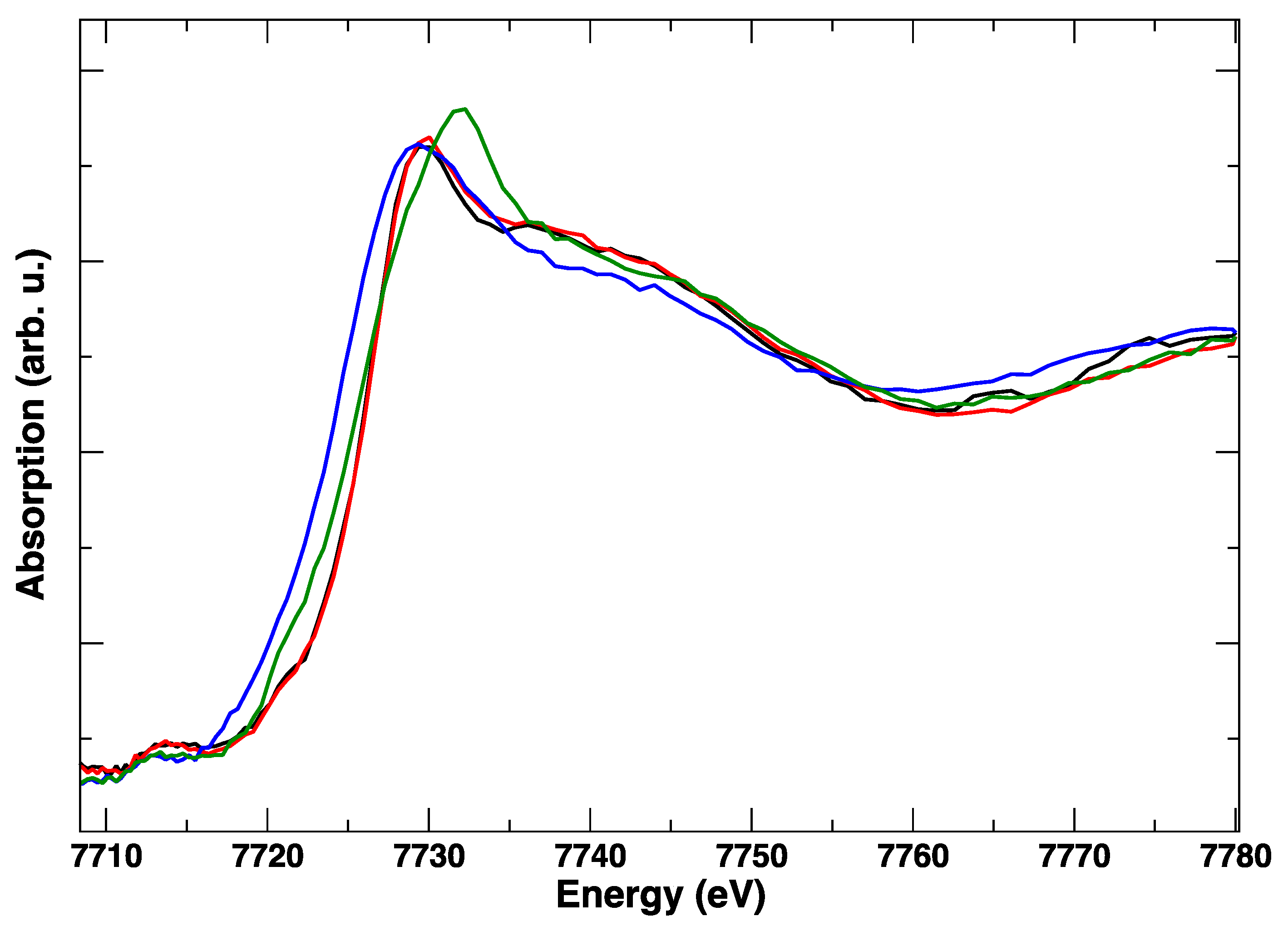
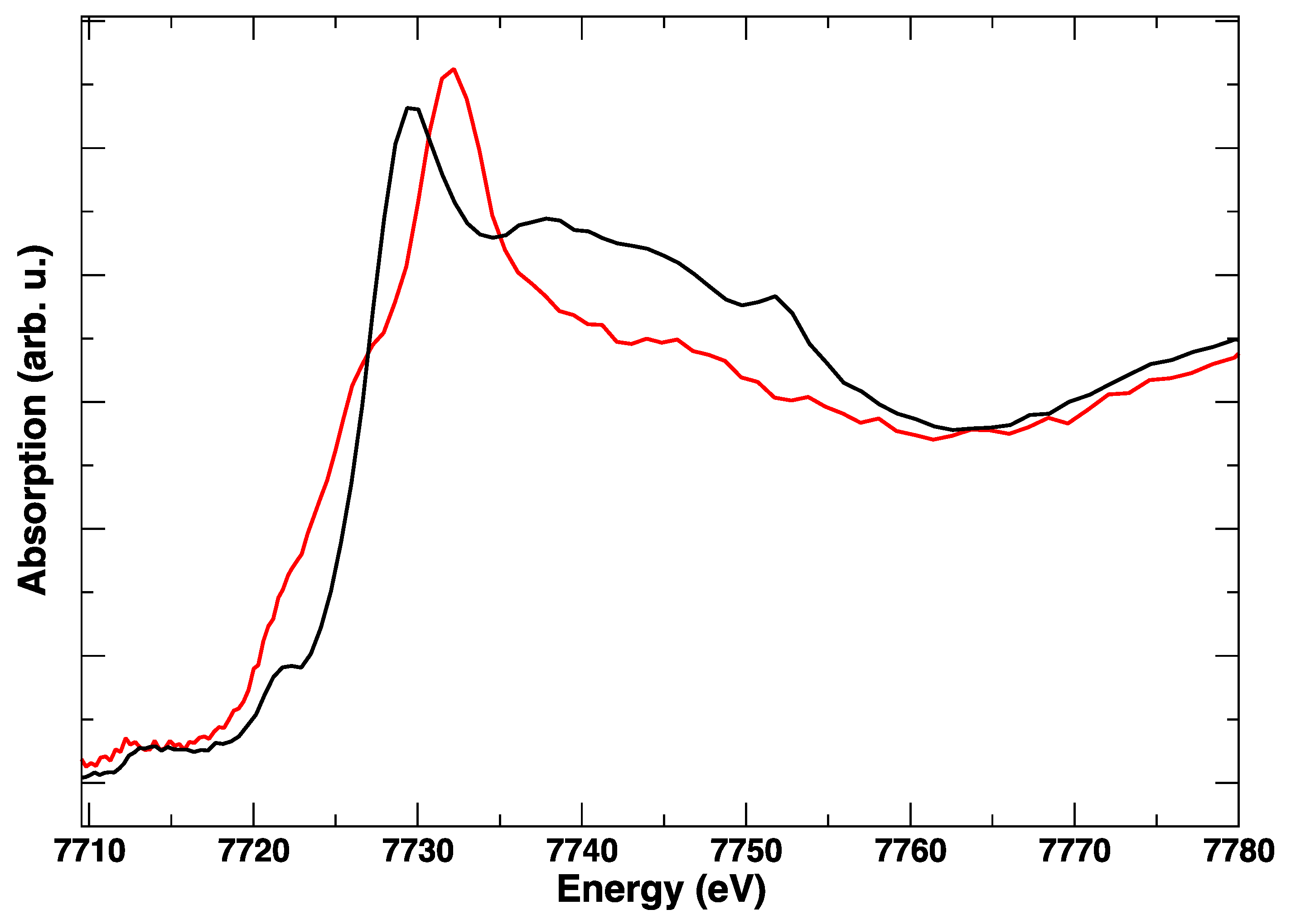
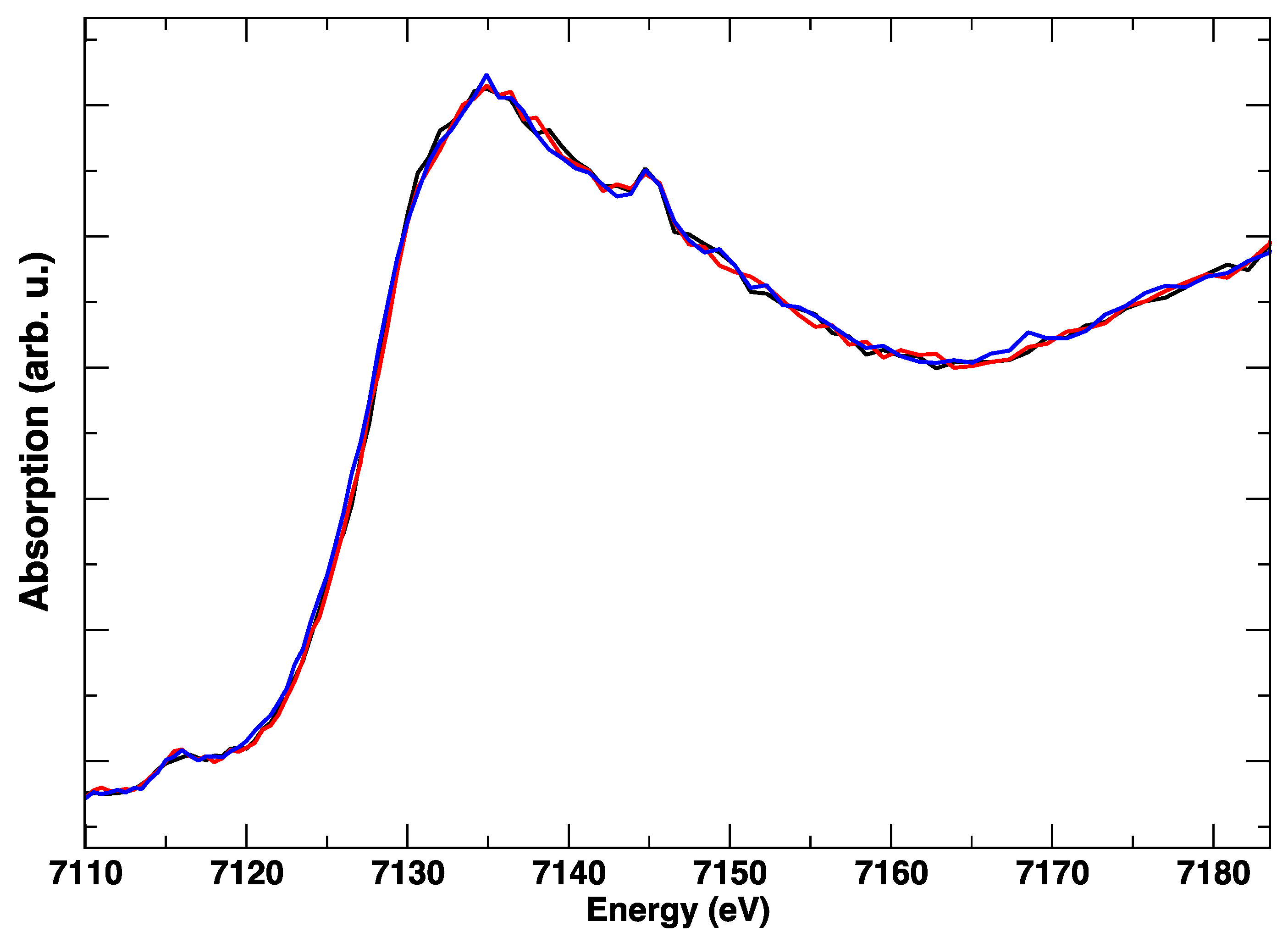
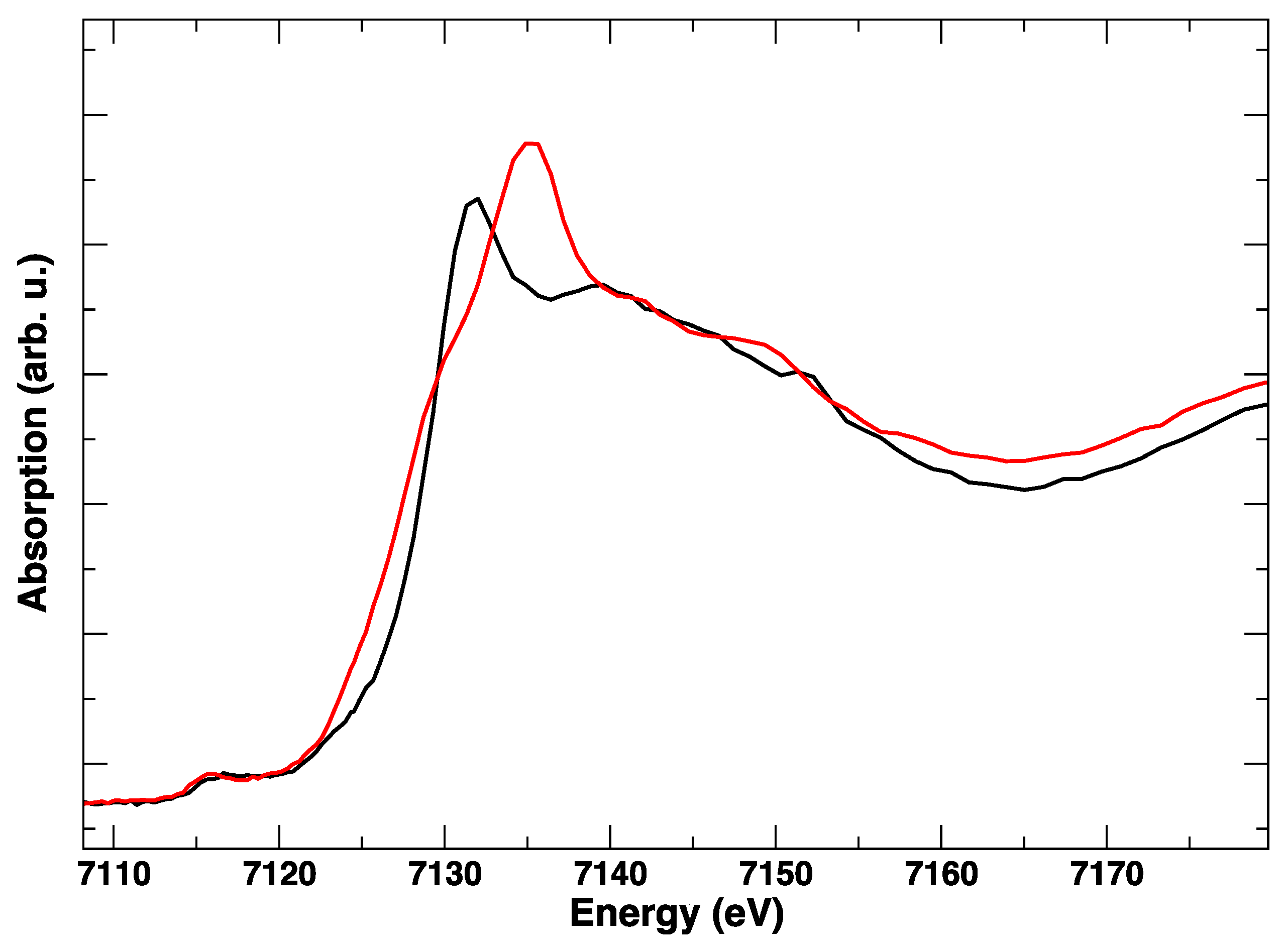
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawada, T.; Oh, M.Y.; Watanabe, H.; Kimura, Y.; Fujimaki, Y.; Masumitsu, T.; Watanabe, S.; Hashimoto, S.; Amezawa, K. Compositional and Mechanical Stabilities of a (La,Sr)(Co,Fe)O3−δ Cathode under SOFC Operation. ECS Trans. 2012, 45, 307–312. [Google Scholar] [CrossRef]
- Baumann, F.S.; Fleig, J.; Konuma, M.; Starke, U.; Habermeier, H.-U.; Maier, J. Strong Performance Improvement of La0.6Sr0.4Co0.8Fe0.2O3−δ SOFC Cathodes by Electrochemical Activation. J. Electrochem. Soc. 2005, 152, A2074–A2079. [Google Scholar] [CrossRef]
- Backhaus-Ricoult, M.; Adib, K.; St. Clair, T.; Luerssen, B.; Gregoratti, L.; Barinov, A. In-situ study of operating SOFC LSM/YSZ cathodes under polarization by photoelectron microscopy. Solid State Ion. 2008, 179, 891–895. [Google Scholar] [CrossRef]
- Nenning, A.; Opitz, A.K.; Rameshan, C.; Rameshan, R.; Blume, R.; Havecker, M.; Knop-Gericke, A.; Rupprechter, G.; Klötzer, B.; Fleig, J. Ambient Pressure XPS Study of Mixed Conducting Perovskite-Type SOFC Cathode and Anode Materials under Well-Defined Electrochemical Polarization. J. Phys. Chem. C 2016, 120, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Orikasa, Y.; Ina, T.; Fukutsuka, T.; Amezawa, K.; Kawada, T.; Uchimoto, Y. X-ray Absorption Spectroscopic Studies on Electronic Structure in La0.6Sr0.4Co0.8Fe0.2O3−δ Perovskite-Type Oxides. ECS Trans. 2008, 13, 201–205. [Google Scholar]
- Giannici, F.; Longo, A.; Balerna, A.; Martorana, A. Dopant−Host Oxide Interaction and Proton Mobility in Gd:BaCeO3. Chem. Mater. 2009, 21, 597–603. [Google Scholar] [CrossRef]
- Lupetin, P.; Giannici, F.; Gregori, G.; Martorana, A.; Maier, J. Effects of grain boundary decoration on the electrical conduction of nanocrystalline CeO2. J. Electrochem. Soc. 2012, 159, B417–B425. [Google Scholar] [CrossRef]
- Giannici, F.; Gregori, G.; Aliotta, C.; Longo, A.; Maier, J.; Martorana, A. Structure and oxide ion conductivity: Local order, defect interactions and grain boundary effects in acceptor-doped ceria. Chem. Mater. 2014, 26, 5994–6006. [Google Scholar] [CrossRef]
- Giannici, F.; Canu, G.; Gambino, M.; Longo, A.; Salomé, M.; Viviani, M.; Martorana, A. Electrode–Electrolyte Compatibility in Solid-Oxide Fuel Cells: Investigation of the LSM–LNC Interface with X-ray Microspectroscopy. Chem. Mater. 2015, 27, 2763–2766. [Google Scholar] [CrossRef]
- Hagen, A.; Traulsen, M.L.; Kiebach, W.R.; Johansen, B.S. Spectroelectrochemical cell for in situ studies of solid oxide fuel cells. J. Synchrotron Rad. 2012, 19, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Woolley, R.J.; Ryan, M.P.; Skinner, S.J. In Situ Measurements on Solid Oxide Fuel Cell Cathodes—Simultaneous X-ray Absorption and AC Impedance Spectroscopy on Symmetrical Cells. Fuel Cells 2013, 13, 1080–1087. [Google Scholar] [CrossRef]
- Longo, A.; Liotta, L.F.; Banerjee, D.; La Parola, V.; Puleo, F.; Cavallari, C.; Sahle, C.J.; Moretti Sala, M.; Martorana, A. The Effect of Ni Doping on the Performance and Electronic Structure of LSCF Cathodes Used for IT-SOFCs. J. Phys. Chem. C 2018, 122, 1003–1013. [Google Scholar] [CrossRef]
- Lai, S.Y.; Ding, D.; Liu, M.; Liu, M.; Alamgir, F.M. Operando and In Situ X-ray Spectroscopies of Degradation in La0.6Sr0.4Co0.2Fe0.8O3−δ Thin Film Cathodes in Fuel Cells. ChemSusChem 2014, 7, 3078–3087. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.T.S.; Neagu, D.; Verbraeken, M.C.; Christodoulos, C.; Graves, C.; Mogensen, M.B. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 2016, 1, 15014. [Google Scholar] [CrossRef]
- Wang, S.; Katsuki, M.; Dokiya, M.; Hashimoto, T. High temperature properties of La0.6Sr0.4Co0.8Fe0.2O3−δ phase structure and electrical conductivity. Solid State Ion. 2003, 159, 71–78. [Google Scholar] [CrossRef]
- Fröba, M.; Köhn, R.; Bouffaud, G.; Richard, O.; van Tendeloo, G. Fe2O3 Nanoparticles within Mesoporous MCM-48 Silica: In Situ Formation and Characterization. Chem. Mater. 1999, 11, 2858–2865. [Google Scholar] [CrossRef]
- Kuzmin, A.; Chaboy, J. EXAFS and XANES analysis of oxides at the nanoscale. IUCrJ 2014, 1, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Kim-Lohsoontorn, P.; Bae, J. Electrochemical performance of solid oxide electrolysis cell electrodes under high-temperature coelectrolysis of steam and carbon dioxide. J. Power Sources 2011, 196, 7161–7168. [Google Scholar] [CrossRef]
- Soldati, A.L.; Baqué, L.; Napolitano, F.; Serquis, A. Cobalt–iron red–ox behavior in nanostructured La0.4Sr0.6Co0.8Fe0.2O3−δ cathodes. J. Solid State Chem. 2013, 198, 253–261. [Google Scholar] [CrossRef]
- Baumann, F.S.; Fleig, J.; Habermeier, H.-U.; Maier, J. Impedance spectroscopic study on well-defined (La,Sr)(Co,Fe)O3−δ model electrodes. Solid State Ion. 2006, 177, 1071–1081. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannici, F.; Gregori, G.; Longo, A.; Chiara, A.; Maier, J.; Martorana, A. X-ray Absorption under Operating Conditions for Solid-Oxide Fuel Cells Electrocatalysts: The Case of LSCF/YSZ. Surfaces 2019, 2, 32-40. https://doi.org/10.3390/surfaces2010003
Giannici F, Gregori G, Longo A, Chiara A, Maier J, Martorana A. X-ray Absorption under Operating Conditions for Solid-Oxide Fuel Cells Electrocatalysts: The Case of LSCF/YSZ. Surfaces. 2019; 2(1):32-40. https://doi.org/10.3390/surfaces2010003
Chicago/Turabian StyleGiannici, Francesco, Giuliano Gregori, Alessandro Longo, Alessandro Chiara, Joachim Maier, and Antonino Martorana. 2019. "X-ray Absorption under Operating Conditions for Solid-Oxide Fuel Cells Electrocatalysts: The Case of LSCF/YSZ" Surfaces 2, no. 1: 32-40. https://doi.org/10.3390/surfaces2010003
APA StyleGiannici, F., Gregori, G., Longo, A., Chiara, A., Maier, J., & Martorana, A. (2019). X-ray Absorption under Operating Conditions for Solid-Oxide Fuel Cells Electrocatalysts: The Case of LSCF/YSZ. Surfaces, 2(1), 32-40. https://doi.org/10.3390/surfaces2010003