Abstract
We describe a novel electrochemical cell for X-ray absorption spectroscopy (XAS) experiments during electrical polarization suitable for high-temperature materials such as those used in solid oxide fuel cells. A half-cell LSCF/YSZ was then investigated under cathodic and anodic conditions (850 °C and applied electrical bias ranging from +1 V to −1 V in air). The in situ XAS measurements allowed us to follow the LSCF degradation into simple oxides. The rapid deterioration of LSCF is ascribed to the formation of excess of oxygen vacancies leading to the collapse of the mixed perovskite structure.
1. Introduction
Solid-oxide fuel cells (SOFC) represent a forefront theme in materials research, since they allow the production of electrical energy from hydrogen or hydrocarbon fuels with very high thermodynamic efficiency. SOFC electrodes act as catalysts (for oxidation of the fuel at the anode, and for reduction of oxygen at the cathode), and as mixed electron/ion conductors. Although a great deal of performance testing is routinely carried out on a wide variety of electrocatalysts to optimize their composition and microstructure, advanced structural characterization studies have been relatively uncommon so far. In this paper, we studied, under operating conditions (at high temperature and under an external electrical potential), the atomic and electronic structure of La0.6Sr0.4Co0.8Fe0.2O3 (LSCF), which is a technologically relevant electrode material for high-temperature and intermediate-temperature SOFC. This material has been the subject of a large number of studies in the last several decades.
Kawada et al. [1] reported on the chemical stability of LSCF, ultimately concluding that it is stable at high temperatures and under different atmospheres. However, cation segregation on one surface was observed when an oxygen partial pressure gradient was applied to LSCF, which resulted in Co-rich and Sr-rich precipitates on the surface exposed to higher P(O2).
In general, few examples of characterization in operating conditions have been reported in the literature to date, regarding both in-house and large-scale facilities techniques. Among the few studies in the literature on this topic, Baumann et al. [2] observed a strong activation for the oxygen reduction reaction in an LSCF/YSZ model system, associated with a steep decrease of the resistivity of LSCF, due to an applied electrical potential (either cathodic or anodic) of the order of several volts. This effect was found to last for several hours after the polarization, which suggests a more profound transformation rather than a simple change of oxidation state. X-ray photoelectron spectroscopy (XPS) performed on films subject to different treatments were found to be enriched with Sr and Co on the surface.
An ever-increasing interest is developing at synchrotrons around the world to improve in situ/operando experimental setups in order to control the chemical/physical environment and the electrical loading applied to the sample under the beam. This allows for accurately monitoring the chemical changes in technologically relevant materials under service conditions. This approach was used, for instance, to study several SOFC cathode-electrolyte-air triple boundary surfaces using photoelectron microscopy under polarization [3]. More recently, Nenning et al. [4] examined the electrochemistry and XPS spectra of lanthanum strontium ferrite (LSF) and lanthanum strontium cobaltite (LSC) thin film electrodes over YSZ, during polarization (around 0.5 V either cathodic or anodic) and in different atmospheres (H2 or O2). Given the surface-sensitive conditions of such an XPS investigation, a pronounced difference was found in the behavior of cobalt compared to all other elements, which was attributed by the authors to the higher reducibility of the Co ions. In fact, in an earlier soft X-ray absorption spectroscopy study of LSCF under different oxygen partial pressures at 800 °C, it was already found that the Co absorption edge is modified by the oxygen non-stoichiometry, while the Fe edge is not [5].
Among synchrotron techniques, X-ray absorption spectroscopy allows one to obtain information about the atomic and electronic structure of the samples, so that it is particularly suited for SOFC electrode materials, often constituted by variable valence elements that can modify their oxidation state and the local structure in dependence of reaction environment and applied potential [6,7,8,9]. Regarding this concern, the development of reliable experimental protocols [10] is essential to obtain significant information on SOFC materials [11,12,13,14]. Among the issues addressed by the above cited authors, Lai et al. investigated catalyst poisoning, arguing that deactivation of an LSCF cathode by CO2 is enhanced under a cathodic bias. Skinner et al. observed that cathodes constituted by Ruddlesden-Popper phases did not undergo significative redox phenomena under polarization. Irvine et al. studied the processes occurring at the electrode-electrolyte interface, and, in particular, assessed the reversible exsolution phenomena occurring during the operation.
We have recently developed an electrochemical cell for the X-ray structural characterization of SOFC electrocatalysts under operating conditions: high temperature (up to 800 °C), controlled atmosphere, and electrical loading. To this aim, we modified a thermochemical cell for in-situ X-ray spectroscopy with an electrically insulating quartz sample holder, and current collectors in order to impose a voltage bias on the sample.
In this paper, we present our first results with this setup on a LSCF/YSZ cathode/electrolyte half-cell. By applying an external voltage bias, ranging from −1 V to +1 V, and employing X-ray absorption spectroscopy at a high temperature, we were able to investigate the different role of iron and cobalt concerning the oxidation state and structural rearrangement, as a function of time, temperature, and electrical polarization in operating conditions.
2. Materials and Methods
A dense La0.6Sr0.4Co0.8Fe0.2O3 (LSCF) film of 200 nm thickness was deposited by pulsed laser deposition on yttria-stabilized zirconia (100) oriented crystal (YSZ, 9.5 mol% Y2O3, 5 mm × 5 mm × 0.5 mm, CrysTec GmbH, Berlin, Germany) from a commercial LSCF target (HITEC, Karlsruhe, Germany). The deposition was carried out with a laser pulse energy of 1.6 J/cm2 and a pulse frequency of 5 Hz, which keeps the substrate temperature at 770 °C under an oxygen partial pressure of 0.4 mbar. After deposition, the films were annealed at 640 °C for 30 min in oxygen.
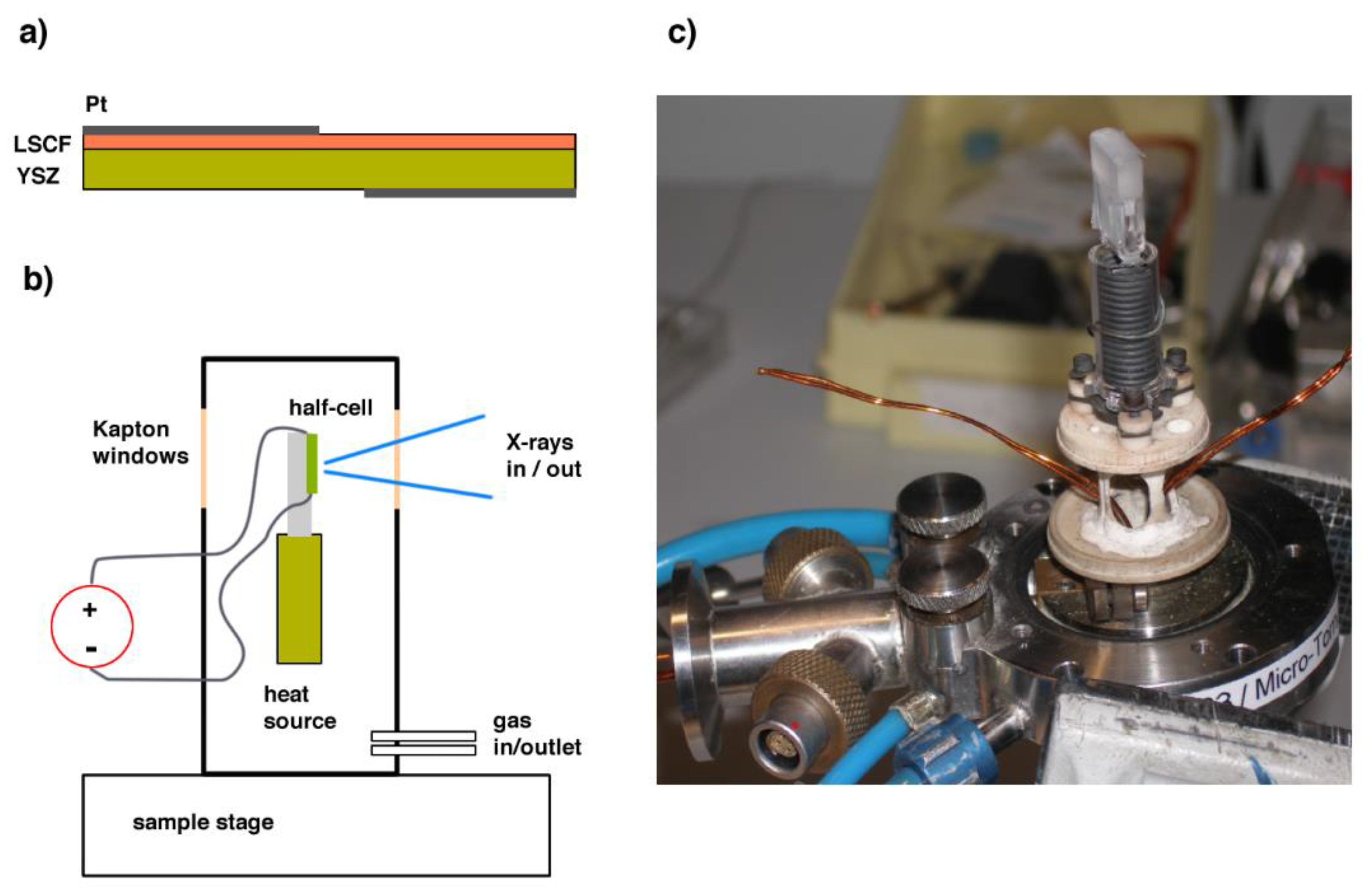
Platinum current collectors were deposited by sputtering on opposite sides of the bilayer, covering half of the surface, and enabling the other half to be studied in the spectroscopy experiment (see Figure 1). The sample was mounted on a fused quartz sample holder inside the “Microtomo” furnace developed at the European Synchrotron Radiation Facility (Grenoble, France) for in situ X-ray absorption spectroscopy. The voltage across the electrode-electrolyte bilayer is applied through an external I-V source, through two platinum wires ending with platelets, which are brought in contact with the current collectors on the sample. For this experiment, a constant electrical bias was applied to the half-cell, which ranged from −1 V (cathodic) to +1 V (anodic). In this convention, “cathodic” refers to a negative polarization of the LSCF side electrode with respect to the counter-electrode on the YSZ side (oxygen ions flowing from LSCF to YSZ).
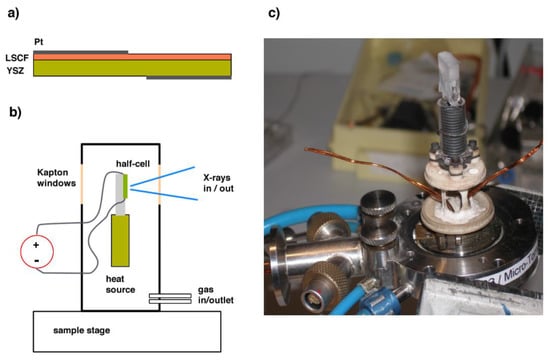
Figure 1.
(a) LSCF/YSZ half-cell. (b) Schematic of the X-ray absorption spectroscopy setup. (c) Inner view of the cell.
A steady air flux flowed inside the cell during the measurement. In this cell, the sample holder can be mounted at either 45 degrees, or parallel to the incoming beam, which allows X-ray absorption spectroscopy in a transmission or a fluorescence mode. In particular, the X-ray absorption near edge structure (XANES) data presented in this paper were acquired at the Fe K-edge (7112 eV) and at the Co K-edge (7709 eV) in a fluorescence mode using a multi-element solid state Ge detector.
3. Results
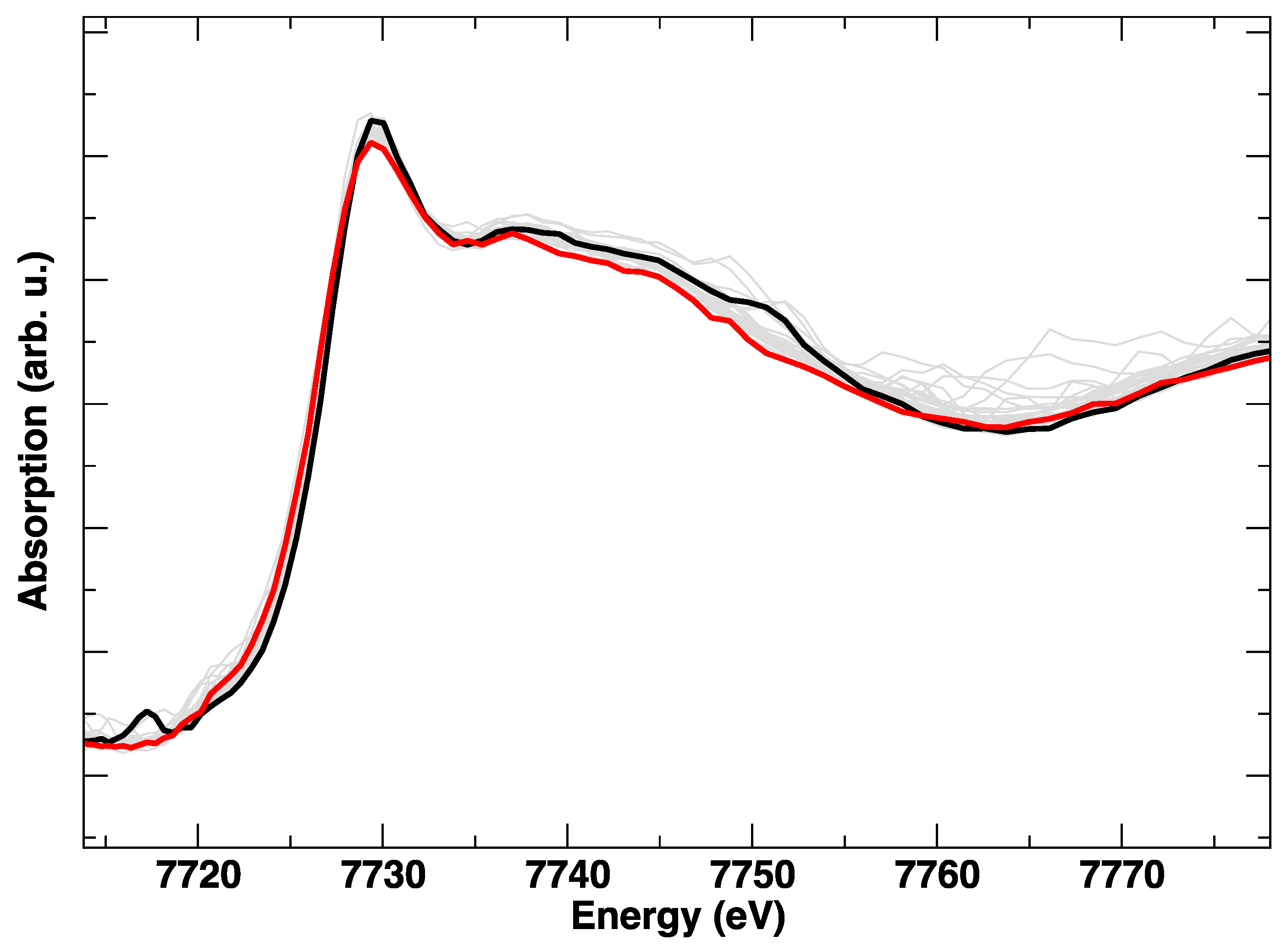
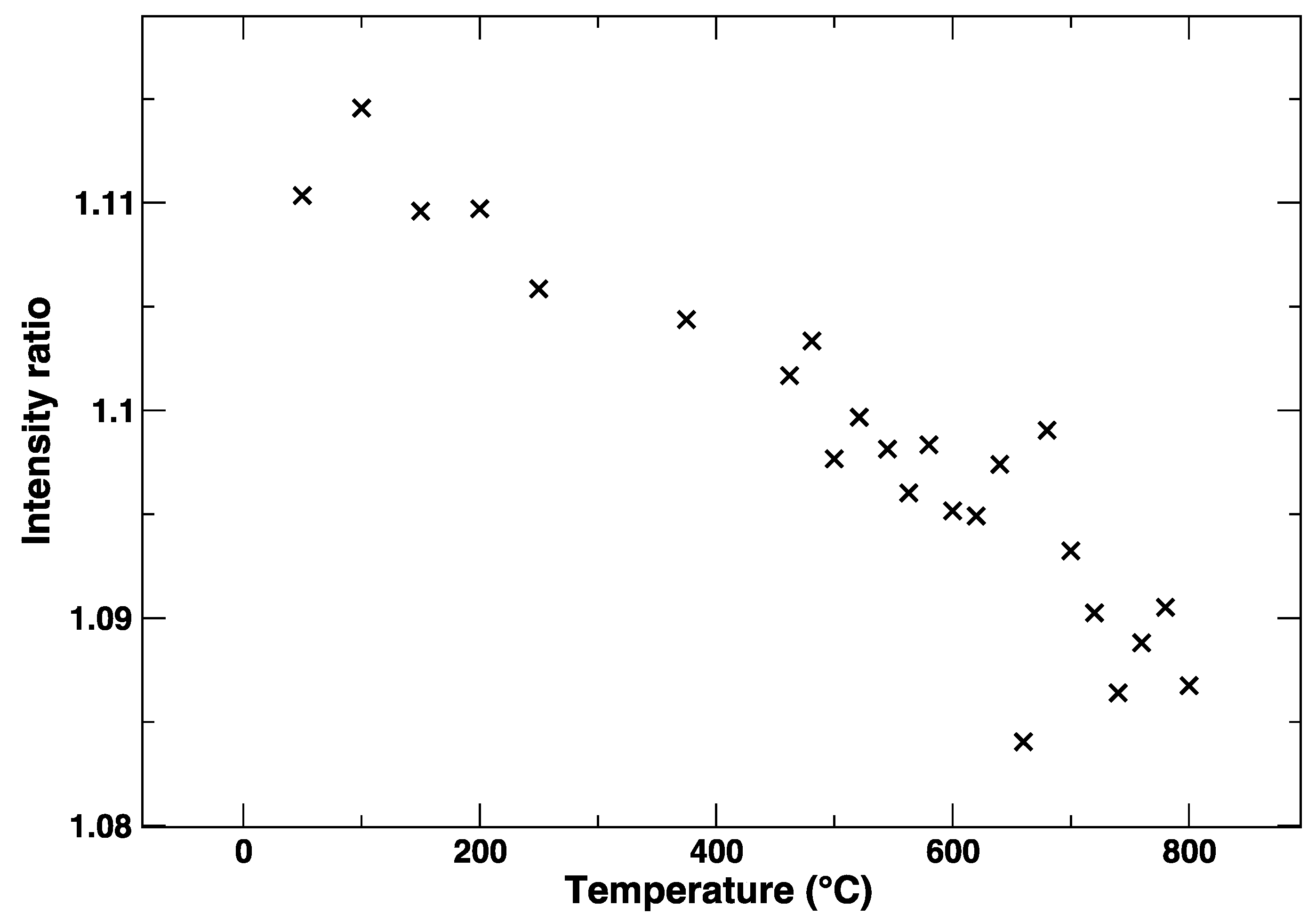
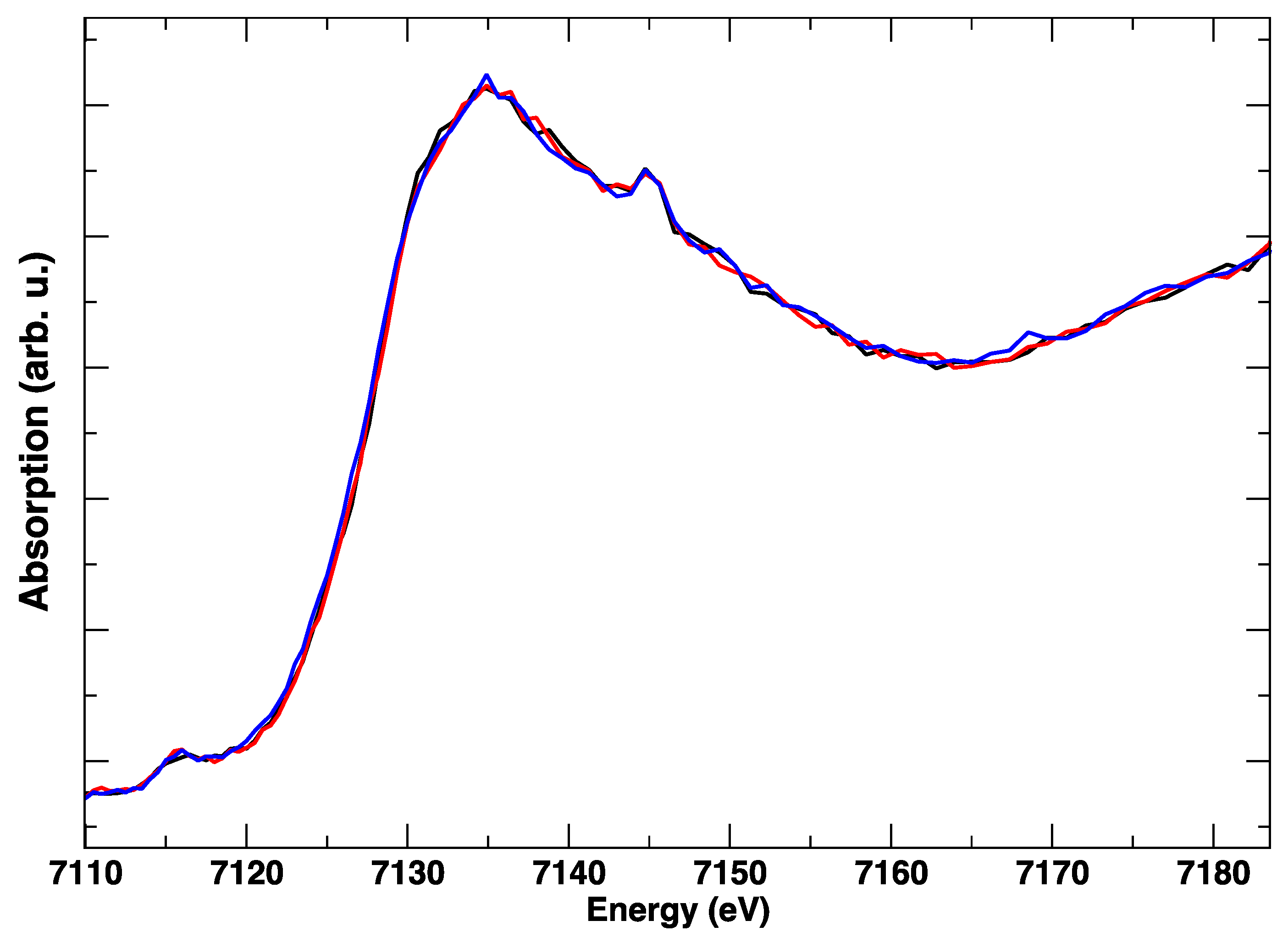
During the heating of LSCF from RT to 800 °C, the Co K-edge shows a gradual variation, which is evident from Figure 2 and Figure 3. In particular, from Figure 3, where the intensity ratio between the features at 7729 eV and 7734 eV is plotted, we get an impression how continuous the evolution of the XANES spectrum is with the temperature, without evident discontinuities due to the known rhombohedral to a cubic structural transition below 500 °C [15]. It is likely that the progressive changes in the edge are linked to the structural rearrangement, but also to the expected increase of oxygen vacancies concentration with the temperature.
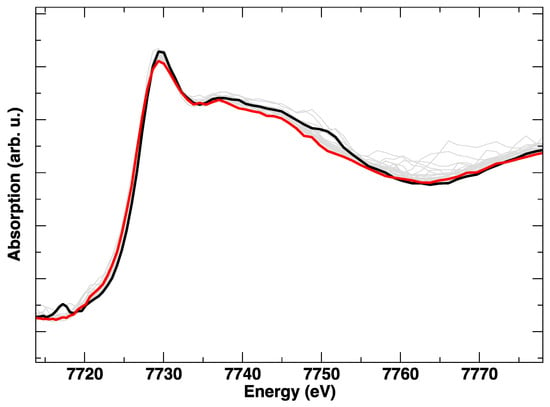
Figure 2.
Co K-edge XANES spectra during heating, from room temperature (black line) to 800 °C (red line). Intermediate spectra are plotted in grey.
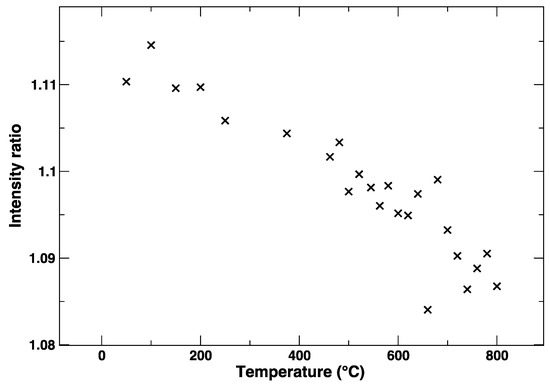
Figure 3.
Intensity ratio between the Co K-edge XANES maximum at 7729 eV and the local minimum at 7734 eV.
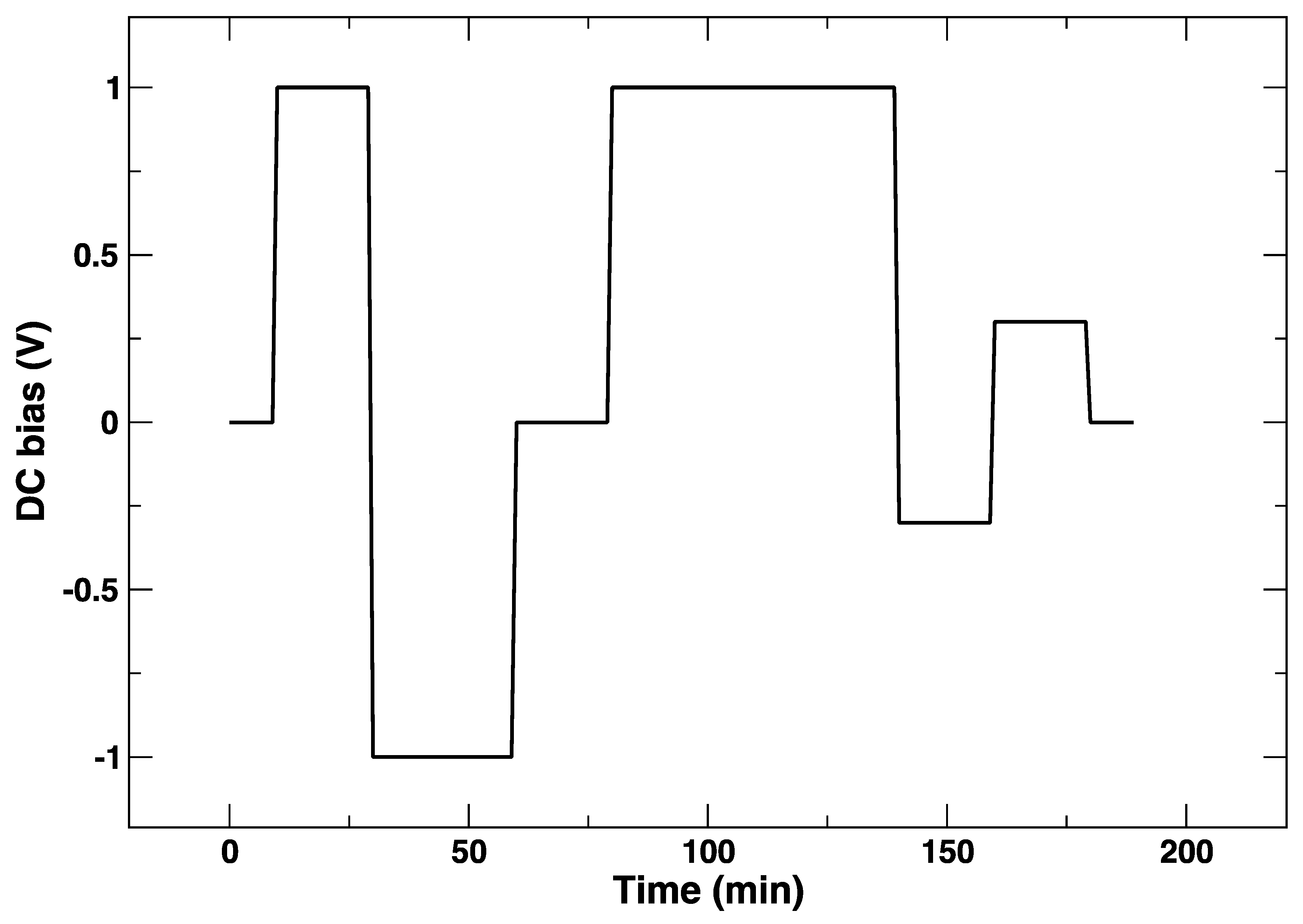
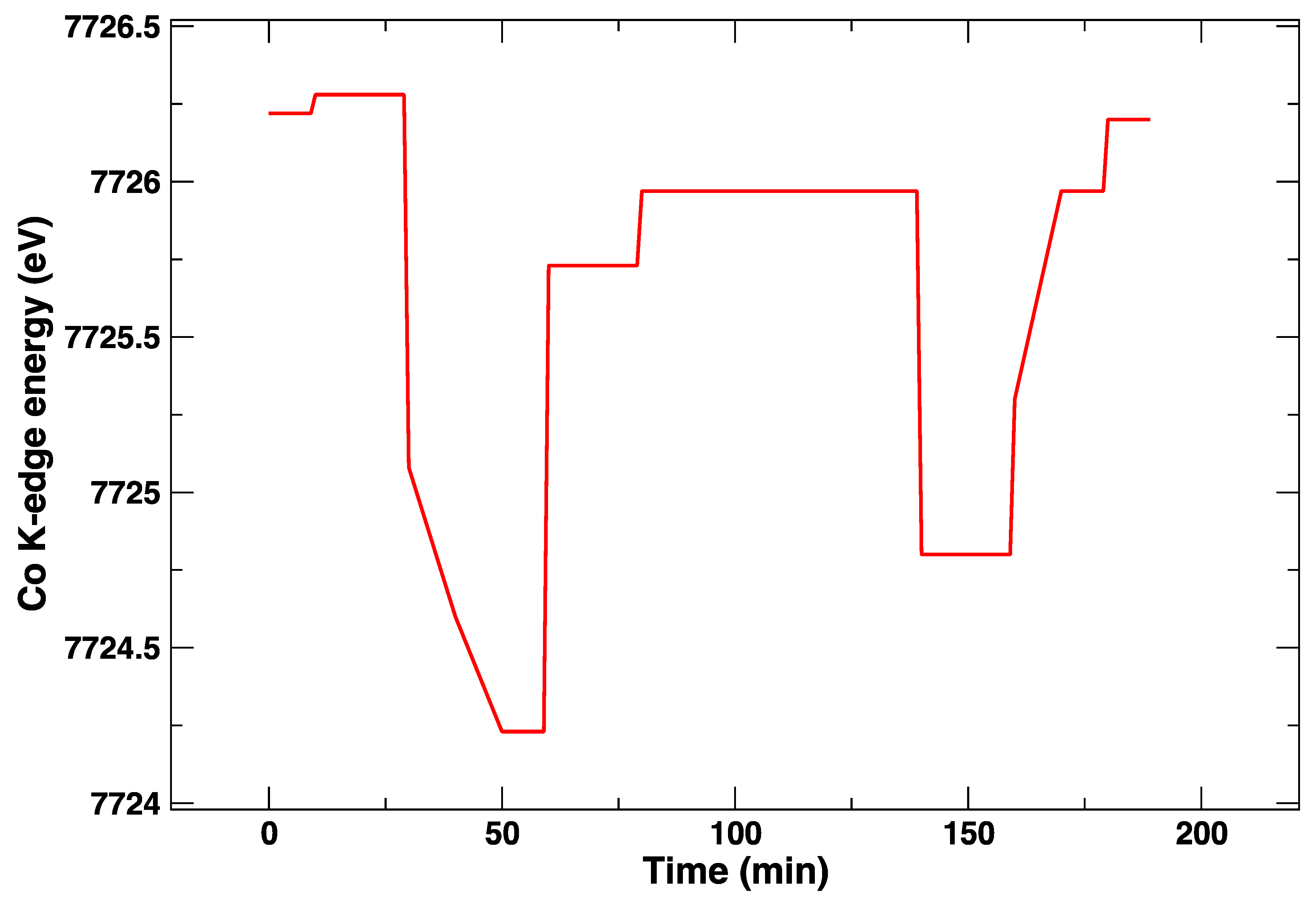
The applied electrical bias as a function of time is plotted in Figure 4. The position of the absorption K-edge of cobalt (i.e., its oxidation state) is plotted in the same way, and it follows almost instantaneously the bias changes (Figure 5). On the other hand, it can be observed in the plot that, within the first cycle of cathodic/anodic polarization, there is a continuous transformation of the edge that takes about 30 min, which suggests a diffusive process and/or a chemical transformation different from a simple change of oxidation state. One can appreciate that, after an initial polarization at +1 V leading to a slight shift towards a larger Co K-edge value and an abrupt change to −1 V yielding to a reduction of Co (lower values of the Co K-edge), the return to +1 V does not bring the Co K-edge to the initial energy values. Although the energy difference is rather small, this might be indicative of irreversible processes occurring in the LSCF layer after only 60 min. This is supported by the subsequent voltage step. In this case, during the application of −1 V, the edge energy does not reach the initial values, which suggests that the LSCF film already underwent some permanent structural modifications.
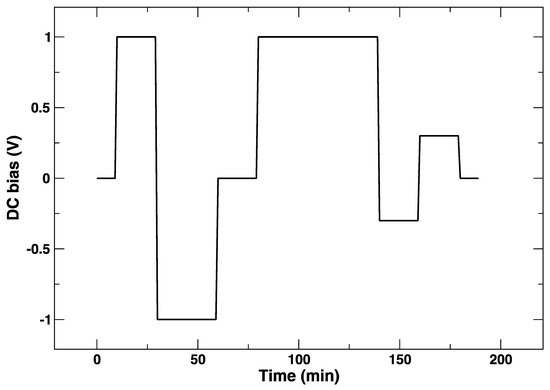
Figure 4.
DC bias applied during the in situ Co K-edge XANES experiment at 850 °C.
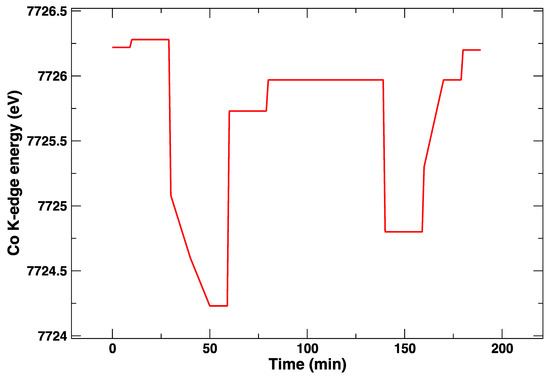
Figure 5.
Energy of the Co K-edge, measured as the maximum of the first derivative of the XANES spectrum, as a function of time at 850 °C.
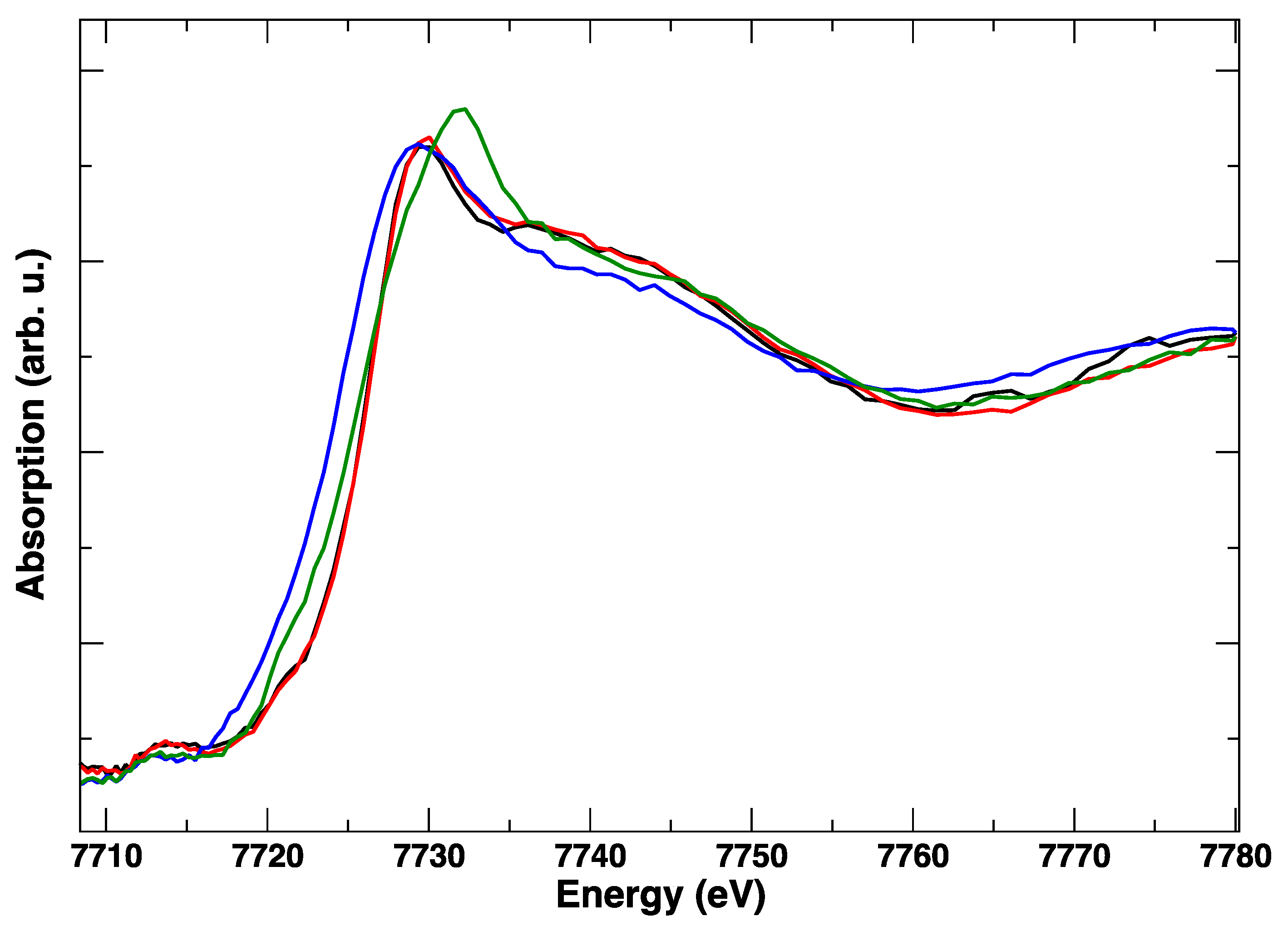
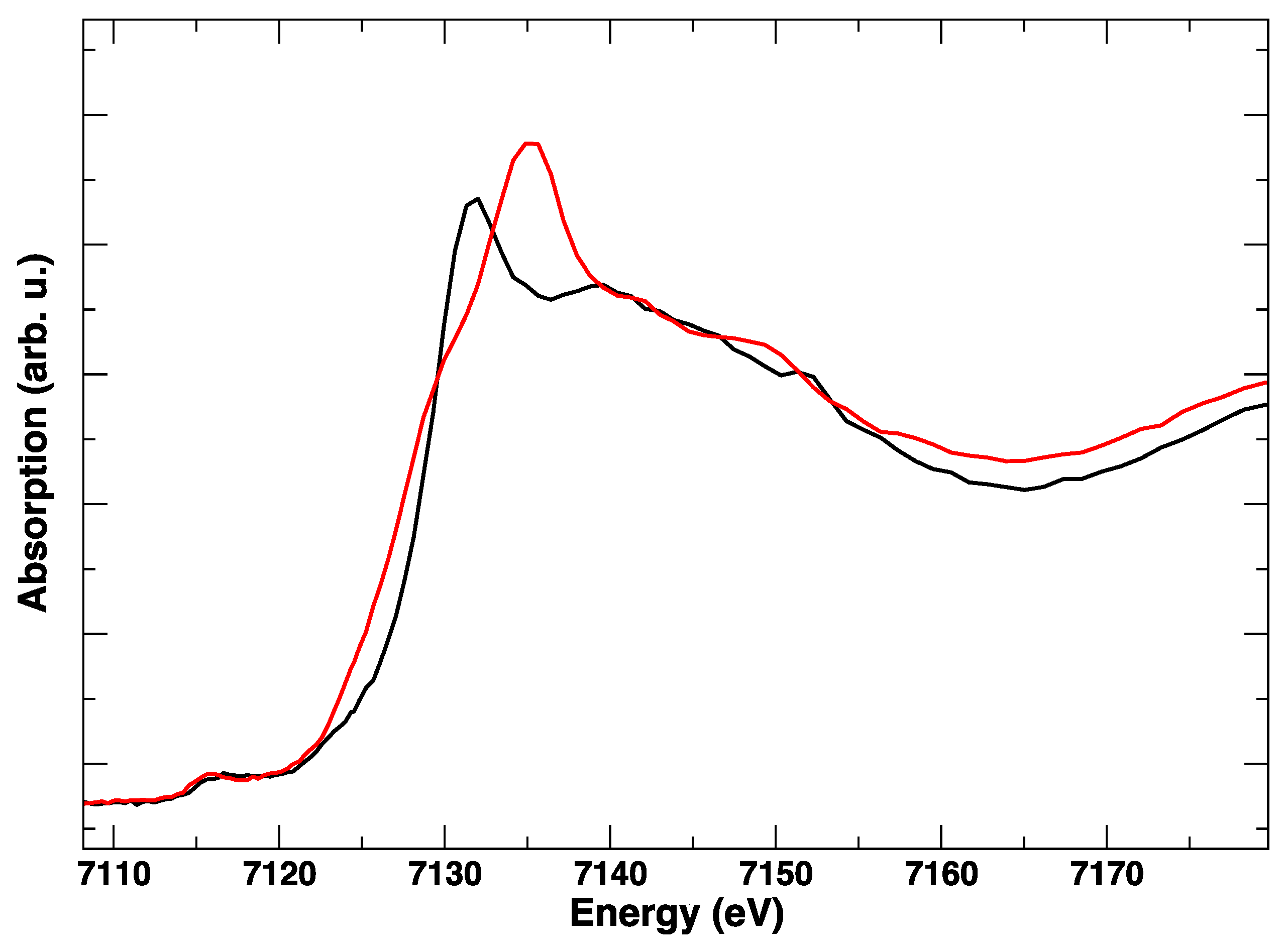
These edge transformations show a clear irreversibility, which is evident when comparing the red spectrum in Figure 6 and corresponds to the initial anodic bias and the final (green) spectrum, taken in anodic polarization, but after the cathodic step. The postmortem Co K-edge resembles that of the spinel Co3O4 [15]: it is completely different from the starting LSCF and this modification is maintained irrespective of the applied potential. The room temperature spectra, taken before and after polarization cycles, in Figure 7, confirm with higher resolution the final transformation of LSCF.
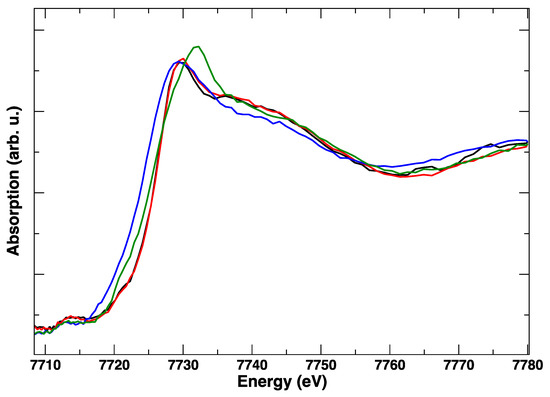
Figure 6.
Representative Co K-edge XANES spectra at 850 °C during two cycles of the electrical polarization experiment. No applied bias (black), +1 V (red), −1 V (blue), +1 V (green).
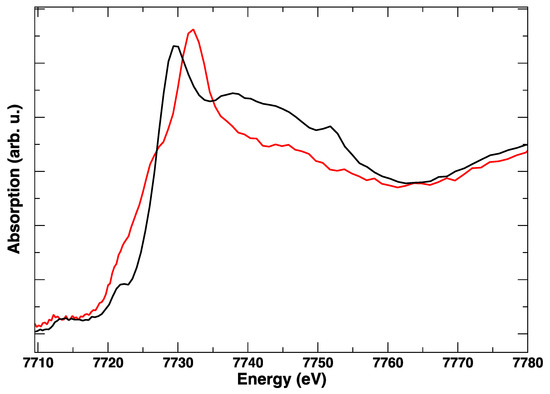
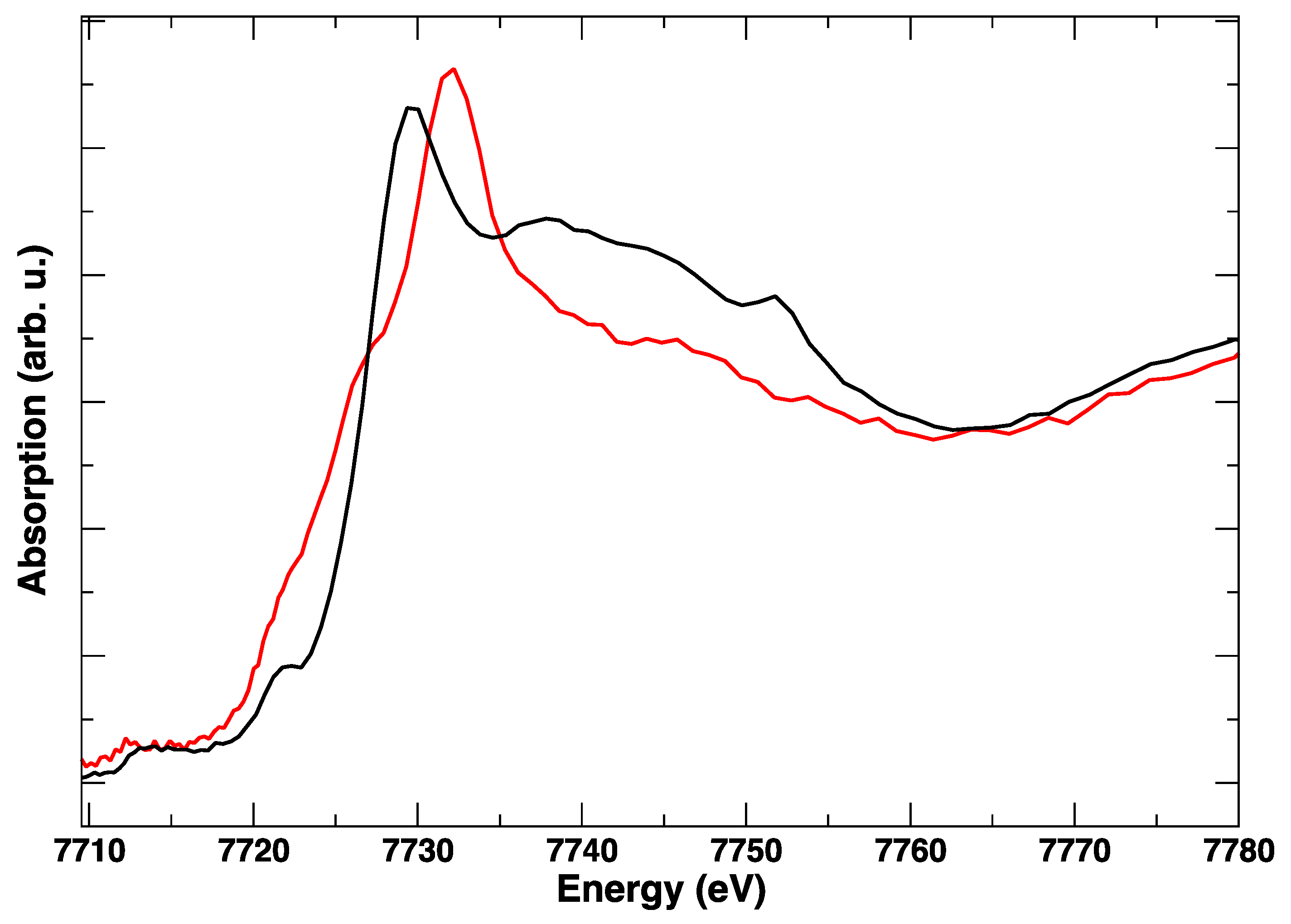
Figure 7.
Room temperature Co K-edge XANES spectra of LSCF before treatment (black line) and after the in situ electrical polarization experiment (red line).
A subsequent polarization cycle (Figure 8) with simultaneous acquisition of the Fe K-edge spectra highlights that: (1) the Fe edge is almost unchanged in response to the electric bias, (2) the spectrum is different from LSCF, and resembles Fe2O3 nanoparticles [16,17].
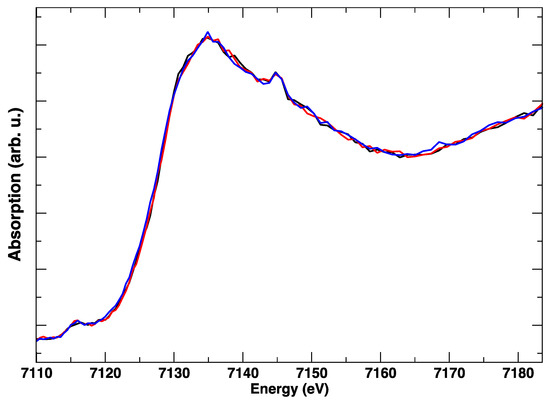
Figure 8.
Fe K-edge XANES spectra measured at 850 °C with an applied electrical bias: +1 V (black), −1 V (red), 0 V (blue), over the course of 40 minutes.
The evidence points toward the complete and irreversible transformation of the material. The post mortem XANES spectrum on the iron K-edge shows a deep modification with respect to the fresh sample (Figure 9), with the treated sample resembling Fe2O3 [16,17]. It appears that the polarization cycling has produced a total breakdown of the LSCF structure, with de-mixing of the starting material in iron oxides, cobalt oxides, and other compounds. We propose that such a rapid structural breakdown is induced both by the high cathodic voltage (−1 V) and by the abrupt cycling between cathodic and anodic (+1 V) conditions. It has been noted in the past that the stability of the LSCF phase is tested and optimized for SOFC operation (that is, cathodic polarization), and not for the inverse operation that is characteristic of an electrolyzer. In fact, the current density curves show a pronounced asymmetry between cathodic and anodic polarization for LSCF cathodes compared to other materials such as lanthanum strontium manganites [18].
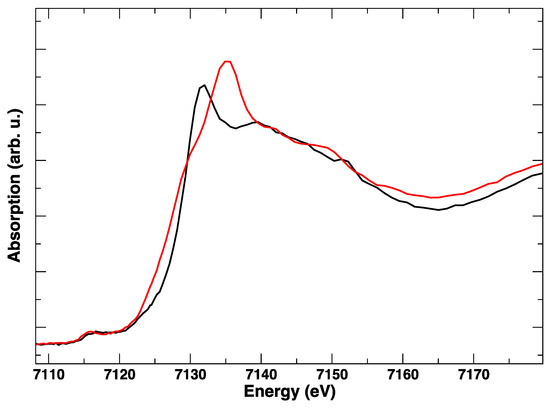
Figure 9.
Room temperature Fe K-edge XANES spectra of LSCF before treatment (black line) and after the in situ electrical polarization experiment (red line).
4. Discussion
This paper presents one of the few structural studies of SOFC electrodes during operation. In particular, we investigated a LSCF/YSZ half-cell under electrical polarization. On the basis of the post mortem XANES spectra of iron and cobalt, we can state that, as a consequence of the high temperature polarization cycling, the LSCF structure was disrupted, producing the de-mixing of the spinel Co3O4, with a Co oxidation state intermediate between +2 and +3, and of hematite Fe2O3 with Fe oxidation state +3. This means that both cationic species are reduced compared to the LSCF mixed oxide [19]. It has been reported before that an applied cathodic bias increases the concentration of oxygen vacancies [20]. It appears then that the increased concentration of oxygen vacancies above a certain level induces the collapse of the perovskite mixed oxide, which is also favored by the high temperature. This eventually leads to a rearrangement of the mixed oxide toward the simple oxides retaining an oxidation state lower than that of LSCF.
Author Contributions
Supervision, J.M. and A.M.; Writing—original draft, F.G., G.G., A.L. and A.C.
Funding
We acknowledge funding through MIUR projects Futuro in Ricerca “INnovative Ceramic and hybrid materials for proton-conducting fuel cells at Intermediate Temperature: design, characterization, and device assembly (INCYPIT)”, PON R&C 2007-1013 “Tecnologie ad alta Efficienza per la Sostenibilità Energetica ed ambientale On-board (TESEO)”, PRIN2010 “Celle a combustibile ad ossido solido operanti a temperatura intermedia alimentate con biocombustibili (BIOITSOFC)”.
Acknowledgments
We acknowledge the ESRF for provision of beamtime. We thank H. Vitoux (ESRF) for assistance in the cell design and during the experiment, B. Stuhlhofer (MPI) for thin film deposition, R. Merkle (MPI) for helpful discussion, and the Glass workshop of MPI for the sample holder fabrication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kawada, T.; Oh, M.Y.; Watanabe, H.; Kimura, Y.; Fujimaki, Y.; Masumitsu, T.; Watanabe, S.; Hashimoto, S.; Amezawa, K. Compositional and Mechanical Stabilities of a (La,Sr)(Co,Fe)O3−δ Cathode under SOFC Operation. ECS Trans. 2012, 45, 307–312. [Google Scholar] [CrossRef]
- Baumann, F.S.; Fleig, J.; Konuma, M.; Starke, U.; Habermeier, H.-U.; Maier, J. Strong Performance Improvement of La0.6Sr0.4Co0.8Fe0.2O3−δ SOFC Cathodes by Electrochemical Activation. J. Electrochem. Soc. 2005, 152, A2074–A2079. [Google Scholar] [CrossRef]
- Backhaus-Ricoult, M.; Adib, K.; St. Clair, T.; Luerssen, B.; Gregoratti, L.; Barinov, A. In-situ study of operating SOFC LSM/YSZ cathodes under polarization by photoelectron microscopy. Solid State Ion. 2008, 179, 891–895. [Google Scholar] [CrossRef]
- Nenning, A.; Opitz, A.K.; Rameshan, C.; Rameshan, R.; Blume, R.; Havecker, M.; Knop-Gericke, A.; Rupprechter, G.; Klötzer, B.; Fleig, J. Ambient Pressure XPS Study of Mixed Conducting Perovskite-Type SOFC Cathode and Anode Materials under Well-Defined Electrochemical Polarization. J. Phys. Chem. C 2016, 120, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Orikasa, Y.; Ina, T.; Fukutsuka, T.; Amezawa, K.; Kawada, T.; Uchimoto, Y. X-ray Absorption Spectroscopic Studies on Electronic Structure in La0.6Sr0.4Co0.8Fe0.2O3−δ Perovskite-Type Oxides. ECS Trans. 2008, 13, 201–205. [Google Scholar]
- Giannici, F.; Longo, A.; Balerna, A.; Martorana, A. Dopant−Host Oxide Interaction and Proton Mobility in Gd:BaCeO3. Chem. Mater. 2009, 21, 597–603. [Google Scholar] [CrossRef]
- Lupetin, P.; Giannici, F.; Gregori, G.; Martorana, A.; Maier, J. Effects of grain boundary decoration on the electrical conduction of nanocrystalline CeO2. J. Electrochem. Soc. 2012, 159, B417–B425. [Google Scholar] [CrossRef]
- Giannici, F.; Gregori, G.; Aliotta, C.; Longo, A.; Maier, J.; Martorana, A. Structure and oxide ion conductivity: Local order, defect interactions and grain boundary effects in acceptor-doped ceria. Chem. Mater. 2014, 26, 5994–6006. [Google Scholar] [CrossRef]
- Giannici, F.; Canu, G.; Gambino, M.; Longo, A.; Salomé, M.; Viviani, M.; Martorana, A. Electrode–Electrolyte Compatibility in Solid-Oxide Fuel Cells: Investigation of the LSM–LNC Interface with X-ray Microspectroscopy. Chem. Mater. 2015, 27, 2763–2766. [Google Scholar] [CrossRef]
- Hagen, A.; Traulsen, M.L.; Kiebach, W.R.; Johansen, B.S. Spectroelectrochemical cell for in situ studies of solid oxide fuel cells. J. Synchrotron Rad. 2012, 19, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Woolley, R.J.; Ryan, M.P.; Skinner, S.J. In Situ Measurements on Solid Oxide Fuel Cell Cathodes—Simultaneous X-ray Absorption and AC Impedance Spectroscopy on Symmetrical Cells. Fuel Cells 2013, 13, 1080–1087. [Google Scholar] [CrossRef]
- Longo, A.; Liotta, L.F.; Banerjee, D.; La Parola, V.; Puleo, F.; Cavallari, C.; Sahle, C.J.; Moretti Sala, M.; Martorana, A. The Effect of Ni Doping on the Performance and Electronic Structure of LSCF Cathodes Used for IT-SOFCs. J. Phys. Chem. C 2018, 122, 1003–1013. [Google Scholar] [CrossRef]
- Lai, S.Y.; Ding, D.; Liu, M.; Liu, M.; Alamgir, F.M. Operando and In Situ X-ray Spectroscopies of Degradation in La0.6Sr0.4Co0.2Fe0.8O3−δ Thin Film Cathodes in Fuel Cells. ChemSusChem 2014, 7, 3078–3087. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.T.S.; Neagu, D.; Verbraeken, M.C.; Christodoulos, C.; Graves, C.; Mogensen, M.B. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 2016, 1, 15014. [Google Scholar] [CrossRef]
- Wang, S.; Katsuki, M.; Dokiya, M.; Hashimoto, T. High temperature properties of La0.6Sr0.4Co0.8Fe0.2O3−δ phase structure and electrical conductivity. Solid State Ion. 2003, 159, 71–78. [Google Scholar] [CrossRef]
- Fröba, M.; Köhn, R.; Bouffaud, G.; Richard, O.; van Tendeloo, G. Fe2O3 Nanoparticles within Mesoporous MCM-48 Silica: In Situ Formation and Characterization. Chem. Mater. 1999, 11, 2858–2865. [Google Scholar] [CrossRef]
- Kuzmin, A.; Chaboy, J. EXAFS and XANES analysis of oxides at the nanoscale. IUCrJ 2014, 1, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Kim-Lohsoontorn, P.; Bae, J. Electrochemical performance of solid oxide electrolysis cell electrodes under high-temperature coelectrolysis of steam and carbon dioxide. J. Power Sources 2011, 196, 7161–7168. [Google Scholar] [CrossRef]
- Soldati, A.L.; Baqué, L.; Napolitano, F.; Serquis, A. Cobalt–iron red–ox behavior in nanostructured La0.4Sr0.6Co0.8Fe0.2O3−δ cathodes. J. Solid State Chem. 2013, 198, 253–261. [Google Scholar] [CrossRef]
- Baumann, F.S.; Fleig, J.; Habermeier, H.-U.; Maier, J. Impedance spectroscopic study on well-defined (La,Sr)(Co,Fe)O3−δ model electrodes. Solid State Ion. 2006, 177, 1071–1081. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).