Cyclic Voltammetry Characterization of Au, Pd, and AuPd Nanoparticles Supported on Different Carbon Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metal Nanoparticles/CNFs Preparation
2.2. Electrochemical Characterization
2.3. Morphological Characterization
3. Results and Discussion
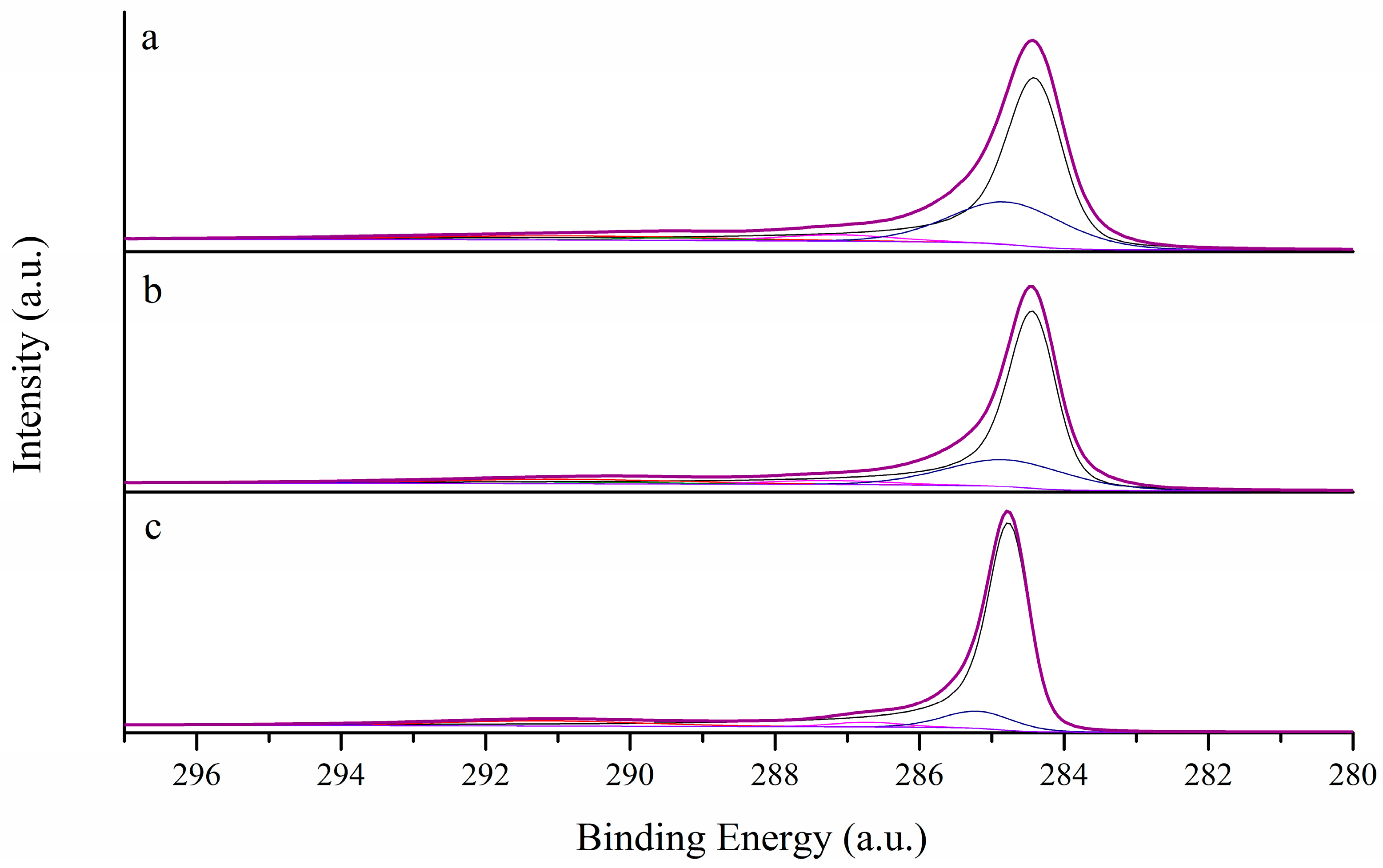
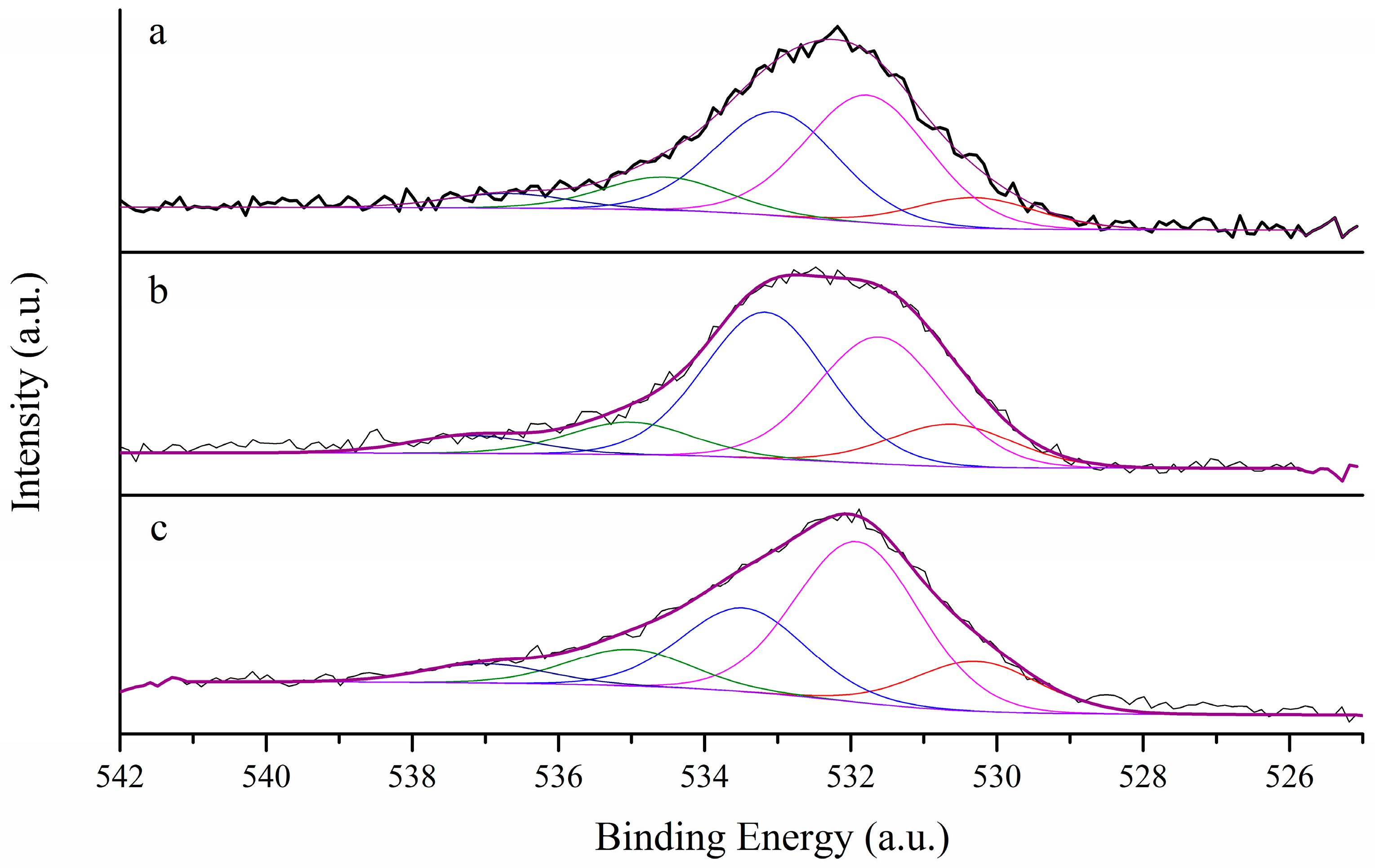
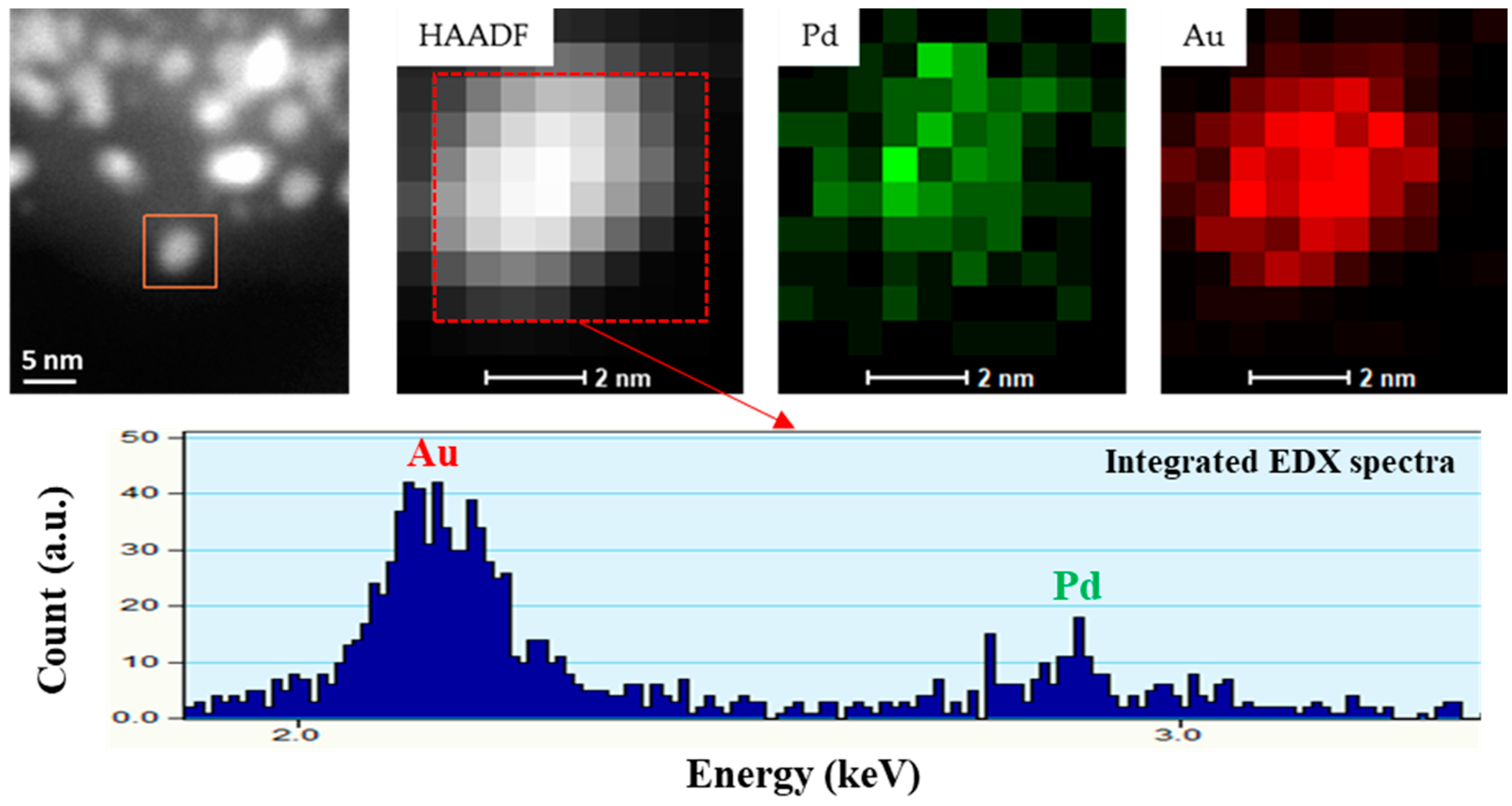
3.1. Morphological Characterization
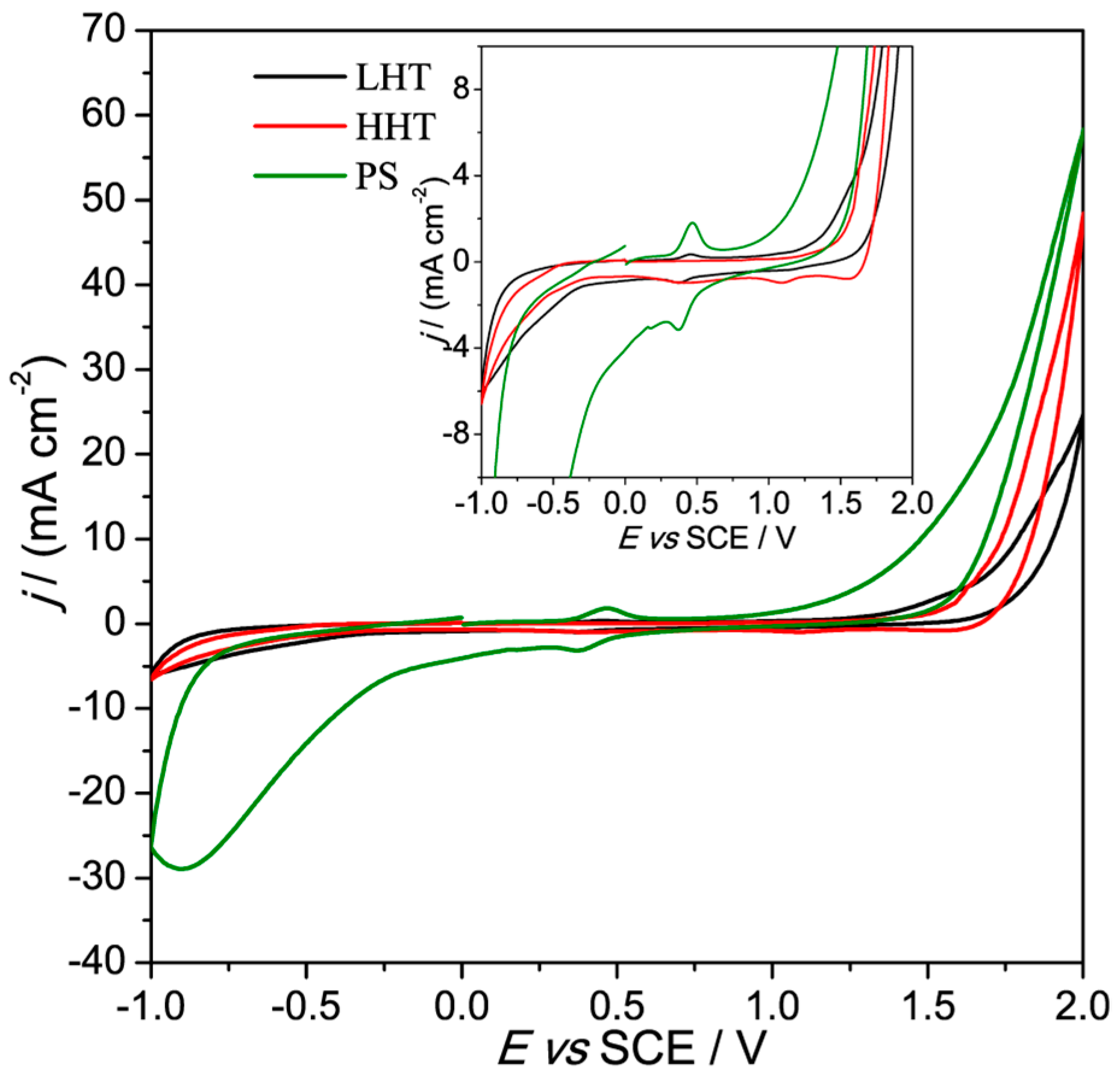
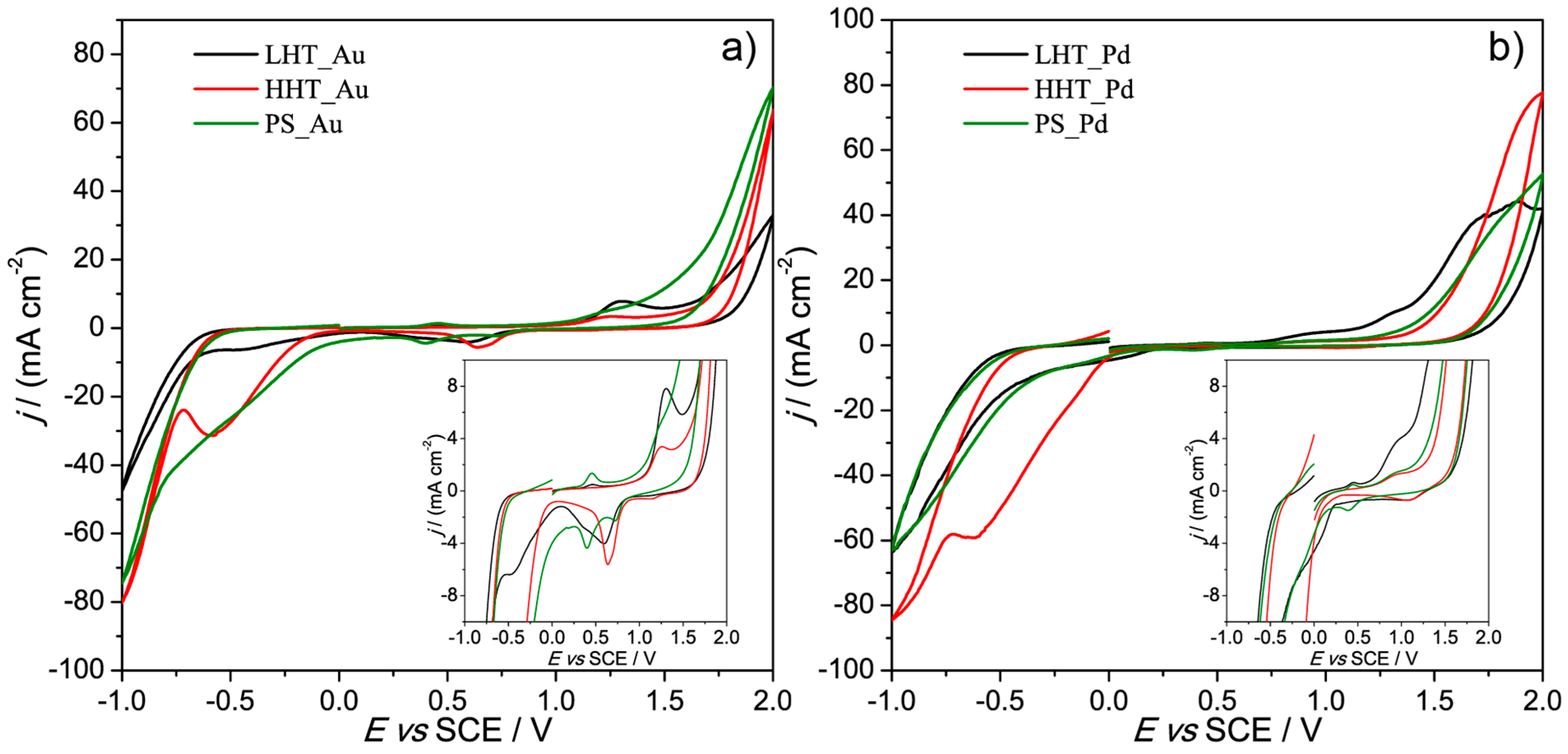
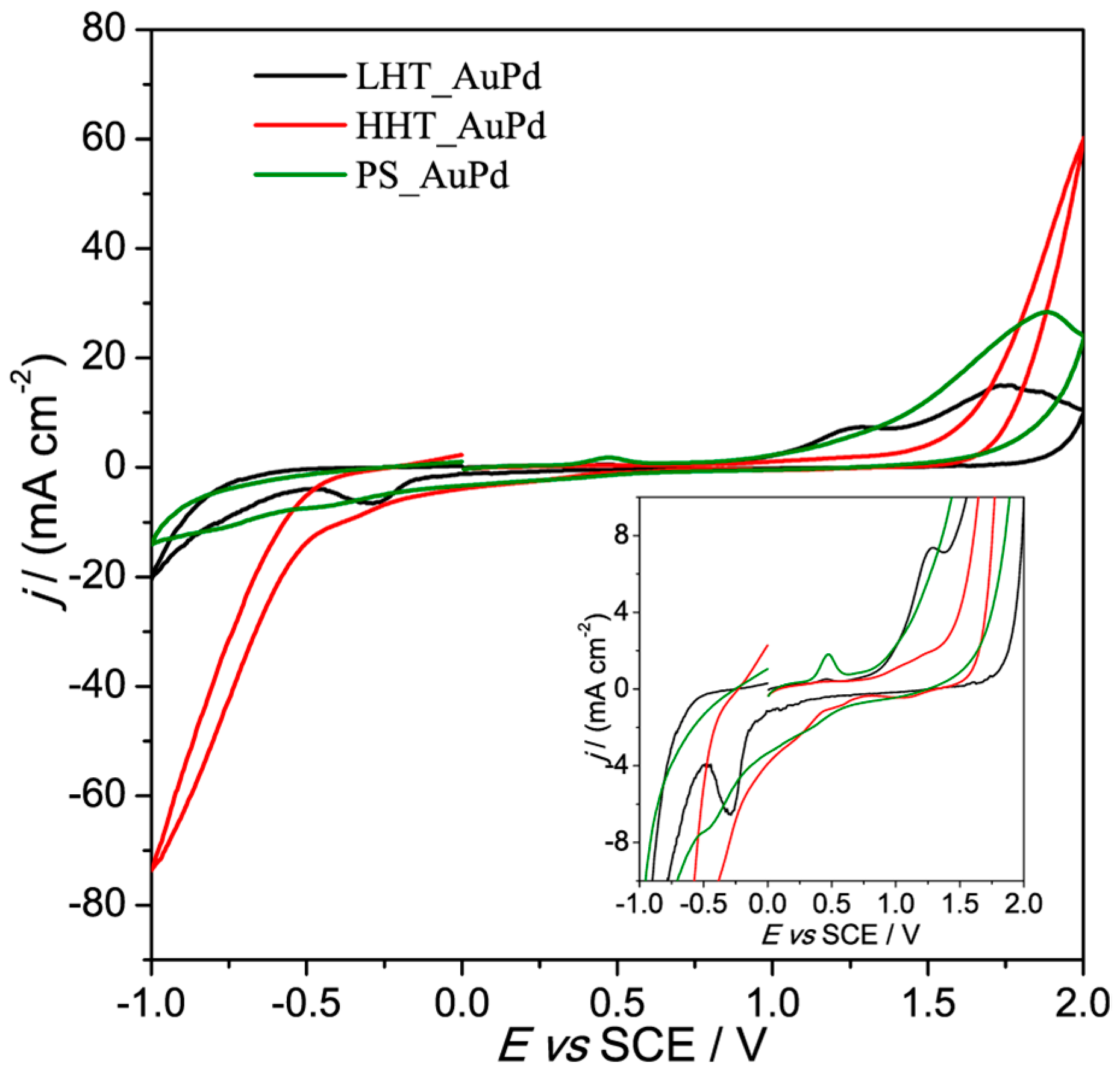
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Campbell, F.W.; Compton, R.G. The use of nanoparticles in electroanalysis: An updated review. Anal. Bioanal. Chem. 2010, 396, 241–259. [Google Scholar] [CrossRef]
- Sardar, R.; Funston, A.M.; Mulvaney, P.; Murray, R.W. Gold Nanoparticles: Past, Present, and Future. Langmuir 2009, 25, 13840–13851. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, A.; Mondini, S.; Marelli, M.; Pifferi, V.; Falciola, L.; Ponti, A.; Ferretti, A.M.; Polito, L. Synthesis of Water Dispersible and Catalytically Active Gold-Decorated Cobalt Ferrite Nanoparticles. Langmuir 2016, 32, 7117–7126. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Goodman, D.W. The nature of the metal-metal bond in bimetallic surfaces. Science 1992, 257, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Toshima, N.; Yonezawa, T. Bimetallic nanoparticles—Novel materials for chemical and physical applications. New J. Chem. 1998, 22, 1179–1201. [Google Scholar] [CrossRef]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Wang, D.; Su, D.S.; Prati, L. New challenges in gold catalysis: Bimetallic systems. Catal. Sci. Technol. 2015, 5, 55–68. [Google Scholar] [CrossRef]
- Schrinner, M.; Proch, S.; Mei, Y.; Kempe, R.; Miyajima, N.; Ballauff, M. Stable Bimetallic Gold–Platinum Nanoparticles Immobilized on Spherical Polyelectrolyte Brushes: Synthesis, Characterization, and Application for the Oxidation of Alcohols. Adv. Mater. 2008, 20, 1928–1933. [Google Scholar] [CrossRef]
- Kim, C.; Dionigi, F.; Beermann, V.; Wang, X.; Möller, T.; Strasser, P. Alloy Nanocatalysts for the Electrochemical Oxygen Reduction (ORR) and the Direct Electrochemical Carbon Dioxide Reduction Reaction (CO2 RR). Adv. Mater. 2018, 1805617. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.; Wu, P.; Zhang, H.; Zhou, B.; Cai, C. Bimetallic Pt-Au nanocatalysts electrochemically deposited on graphene and their electrocatalytic characteristics towards oxygen reduction and methanol oxidation. Phys. Chem. Chem. Phys. 2011, 13, 4083–4094. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, A.; Zhang, T.; Mou, C.Y. Catalysis by gold: New insights into the support effect. Nano Today 2013, 8, 403–416. [Google Scholar] [CrossRef]
- Bracey, C.L.; Ellis, P.R.; Hutchings, G.J. Application of copper-gold alloys in catalysis: Current status and future perspectives. Chem. Soc. Rev. 2009, 38, 2231–2243. [Google Scholar] [CrossRef]
- Gliech, M.; Klingenhof, M.; Görlin, M.; Strasser, P. Supported metal oxide nanoparticle electrocatalysts: How immobilization affects catalytic performance. Appl. Catal. A Gen. 2018, 568, 11–15. [Google Scholar] [CrossRef]
- Zhang, J.W.; Sun, K.K.; Li, D.D.; Deng, T.; Lu, G.P.; Cai, C. Pd-Ni bimetallic nanoparticles supported on active carbon as an efficient catalyst for hydrodeoxygenation of aldehydes. Appl. Catal. A Gen. 2019, 569, 190–195. [Google Scholar] [CrossRef]
- Peneau, V.; He, Q.; Shaw, G.; Kondrat, S.A.; Davies, T.E.; Miedziak, P.; Forde, M.; Dimitratos, N.; Kiely, C.J.; Hutchings, G.J. Selective catalytic oxidation using supported gold-platinum and palladium-platinum nanoalloys prepared by sol-immobilisation. Phys. Chem. Chem. Phys. 2013, 15, 10636–10644. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.C.; Moncada, A.B.; Acevedo, D.F.; Planes, G.A.; Miras, M.C.; Barbero, C.A. Electroanalysis using modified hierarchical nanoporous carbon materials. Faraday Discuss. 2013, 164, 147. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Schiavoni, M.; Prati, L. Material science for the support design: A powerful challenge for catalysis. Catal. Sci. Technol. 2012, 2. [Google Scholar] [CrossRef]
- Romero Hernández, A.; Manríquez, M.E.; Ezeta Mejia, A.; Arce Estrada, E.M. Shape Effect of AuPd Core-Shell Nanostructures on the Electrocatalytical Activity for Oxygen Reduction Reaction in Acid Medium. Electrocatalysis 2018, 9, 752–761. [Google Scholar] [CrossRef]
- Yang, J.; Deng, S.; Lei, J.; Ju, H.; Gunasekaran, S. Electrochemical synthesis of reduced graphene sheet-AuPd alloy nanoparticle composites for enzymatic biosensing. Biosens. Bioelectron. 2011, 29, 159–166. [Google Scholar] [CrossRef]
- Jin, C.; Wan, C.; Dong, R. Electrocatalytic Activity Enhancement of Pd Nanoparticles Supported on Reduced Graphene Oxide by Surface Modification with Au. J. Electrochem. Soc. 2017, 164, H696–H700. [Google Scholar] [CrossRef]
- Shang, L.; Zhao, F.; Zeng, B. Sensitive voltammetric determination of vanillin with an AuPd nanoparticles-graphene composite modified electrode. Food Chem. 2014, 151, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.E.; Krtil, P.; Kibler, L.A.; Jacob, T. Bimetallic alloys in action: Dynamic atomistic motifs for electrochemistry and catalysis. Phys. Chem. Chem. Phys. 2014, 16, 15029–15042. [Google Scholar] [CrossRef] [PubMed]
- Pluntke, Y.; Kibler, L.A.; Kolb, D.M. Unique activity of Pd monomers: Hydrogen evolution at AuPd(111) surface alloys. Phys. Chem. Chem. Phys. 2008, 10, 3684–3688. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.R.; Amonette, J.E.; Engelhard, M.H.; Gaspar, D.J.; Karakoti, A.S.; Kuchibhatla, S.; Nachimuthu, P.; Nurmi, J.T.; Qiang, Y.; Sarathy, V.; et al. Characterization challenges for nanomaterials. In Surface and Interface Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; Volume 40, pp. 529–537. [Google Scholar]
- Villa, A.; Dimitratos, N.; Chan-Thaw, C.E.; Hammond, C.; Veith, G.M.; Wang, D.; Manzoli, M.; Prati, L.; Hutchings, G.J. Characterisation of gold catalysts. Chem. Soc. Rev. 2016, 45. [Google Scholar] [CrossRef] [PubMed]
- Holt, L.R.; Plowman, B.J.; Young, N.P.; Tschulik, K.; Compton, R.G. The Electrochemical Characterization of Single Core-Shell Nanoparticles. Angew. Chem. Int. Ed. 2016, 55, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Plowman, B.J.; Sidhureddy, B.; Sokolov, S.V.; Young, N.P.; Chen, A.; Compton, R.G. Electrochemical Behavior of Gold–Silver Alloy Nanoparticles. ChemElectroChem 2016, 3, 1039–1043. [Google Scholar] [CrossRef]
- Tschulik, K.; Ngamchuea, K.; Ziegler, C.; Beier, M.G.; Damm, C.; Eychmueller, A.; Compton, R.G. Core-Shell Nanoparticles: Characterizing Multifunctional Materials beyond Imaging—Distinguishing and Quantifying Perfect and Broken Shells. Adv. Funct. Mater. 2015, 25, 5149–5158. [Google Scholar] [CrossRef]
- Ranđelović, M.; Momčilović, M.; Matović, B.; Babić, B.; Barek, J. Cyclic voltammetry as a tool for model testing of catalytic Pt- and Ag-doped carbon microspheres. J. Electroanal. Chem. 2015, 757, 176–182. [Google Scholar] [CrossRef]
- Tessonnier, J.P.; Rosenthal, D.; Hansen, T.W.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfänder, N.; Timpe, O.; Su, D.S.; et al. Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon N. Y. 2009, 47, 1779–1798. [Google Scholar] [CrossRef]
- Campisi, S.; Sanchez Trujillo, F.; Motta, D.; Davies, T.; Dimitratos, N.; Villa, A. Controlling the Incorporation of Phosphorus Functionalities on Carbon Nanofibers: Effects on the Catalytic Performance of Fructose Dehydration. C 2018, 4, 9. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Xie, F.Y.; Xie, W.G.; Gong, L.; Zhang, W.H.; Chen, S.H.; Zhang, Q.Z.; Chen, J. Surface characterization on graphitization of nanodiamond powder annealed in nitrogen ambient. Surf. Interface Anal. 2010, 42, 1514–1518. [Google Scholar] [CrossRef]
- Martínez, M.T.; Callejas, M.A.; Benito, A.M.; Cochet, M.; Seeger, T.; Ansón, A.; Schreiber, J.; Gordon, C.; Marhic, C.; Chauvet, O.; et al. Sensitivity of single wall carbon nanotubes to oxidative processing: Structural modification, intercalation and functionalisation. Carbon N. Y. 2003, 41, 2247–2256. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon N. Y. 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Ketteler, G.; Ashby, P.; Mun, B.S.; Ratera, I.; Bluhm, H.; Kasemo, B.; Salmeron, M. In situ photoelectron spectroscopy study of water adsorption on model biomaterial surfaces. J. Phys. Condens. Matter 2008, 20, 184024. [Google Scholar] [CrossRef]
- Wang, D.; Villa, A.; Su, D.; Prati, L.; Schlögl, R. Carbon-supported gold nanocatalysts: Shape effect in the selective glycerol oxidation. ChemCatChem 2013, 5, 2717–2723. [Google Scholar] [CrossRef]
- Sanchez, F.; Alotaibi, M.H.; Motta, D.; Chan-Thaw, C.E.; Rakotomahevitra, A.; Tabanelli, T.; Roldan, A.; Hammond, C.; He, Q.; Davies, T.; et al. Hydrogen production from formic acid decomposition in the liquid phase using Pd nanoparticles supported on CNFs with different surface properties. Sustain. Energy Fuels 2018, 2, 2705–2716. [Google Scholar] [CrossRef]
- Franklin, R.E. Homogeneous and heterogeneous graphitization of carbon. Nature 1956, 177, 239. [Google Scholar] [CrossRef]
- Chen, X.; Deng, X.; Kim, N.Y.; Wang, Y.; Huang, Y.; Peng, L.; Huang, M.; Zhang, X.; Chen, X.; Luo, D.; et al. Graphitization of graphene oxide films under pressure. Carbon N. Y. 2018, 132, 294–303. [Google Scholar] [CrossRef]
- Wilgosz, K.; Chen, X.; Kierzek, K.; Machnikowski, J.; Kalenczuk, R.J.; Mijowska, E. Template method synthesis of mesoporous carbon spheres and its applications as supercapacitors. Nanoscale Res. Lett. 2012, 7, 269. [Google Scholar] [CrossRef]
- Andreas, H.A.; Conway, B.E. Examination of the double-layer capacitance of an high specific-area C-cloth electrode as titrated from acidic to alkaline pHs. Electrochim. Acta 2006, 51, 6510–6520. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of Carbon Nanotubes Chemistry of Carbon Nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Bonanni, A.; Pumera, M.; Miyahara, Y. Influence of gold nanoparticle size (2-50 nm) upon its electrochemical behavior: An electrochemical impedance spectroscopic and voltammetric study. Phys. Chem. Chem. Phys. 2011, 13, 4980–4986. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, V.; Chan-Thaw, C.; Campisi, S.; Testolin, A.; Villa, A.; Falciola, L.; Prati, L.; Pifferi, V.; Chan-Thaw, C.E.; Campisi, S.; et al. Au-Based Catalysts: Electrochemical Characterization for Structural Insights. Molecules 2016, 21, 261. [Google Scholar] [CrossRef] [PubMed]
- Grdeń, M.; Łukaszewski, M.; Jerkiewicz, G.; Czerwiński, A. Electrochemical behaviour of palladium electrode: Oxidation, electrodissolution and ionic adsorption. Electrochim. Acta 2008, 53, 7583–7598. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Scott, K. Performance of carbon nanofiber supported Pd–Ni catalysts for electro-oxidation of ethanol in alkaline medium. J. Power Sources 2010, 195, 5246–5251. [Google Scholar] [CrossRef]
| Catalyst | C sp2 (%) | C sp3 (%) | sp2/sp3 | ID/IG |
|---|---|---|---|---|
| PS | 75.8 | 18.6 | 4.1 | 0.75 |
| LHT | 83.8 | 12.0 | 7.0 | 0.60 |
| HHT | 90.8 | 3.4 | 16.8 | 0.11 |
| Sample | O1S | C1s | Atomic Ratio % C:O | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C = O | C-O-H | C-O-C | COOH | H2O | sp2 | sp3 | C = O | C = C | |||
| PS | BE | 530.2 | 531.8 | 533.0 | 534.6 | 536.7 | 284.4 | 284.9 | 287.2 | 290.0 | 88.3:11.7 |
| Atom % | (10.2) | (40.7) | (33.1) | (9.8) | (6.0) | (75.8) | (18.6) | (3.7) | (1.8) | ||
| LHT | BE | 530.5 | 531.6 | 533.1 | 535.0 | 537.1 | 284.5 | 284.9 | 287.4 | 290.1 | 92.2:7.8 |
| Atom % | (11.6) | (34.2) | (40.3) | (8.8) | (5.1) | (83.8) | (12.0) | (2.6) | (1.6) | ||
| HHT | BE | 530.2 | 531.9 | 533.5 | 535.0 | 537.0 | 284.7 | 285.2 | 287.9 | 290.6 | 97.3:2.7 |
| Atom % | (13.9) | (46.2) | (24.0) | (10.1) | (5.8) | (90.8) | (5.4) | (1.6) | (2.2) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testolin, A.; Cattaneo, S.; Wang, W.; Wang, D.; Pifferi, V.; Prati, L.; Falciola, L.; Villa, A. Cyclic Voltammetry Characterization of Au, Pd, and AuPd Nanoparticles Supported on Different Carbon Nanofibers. Surfaces 2019, 2, 205-215. https://doi.org/10.3390/surfaces2010016
Testolin A, Cattaneo S, Wang W, Wang D, Pifferi V, Prati L, Falciola L, Villa A. Cyclic Voltammetry Characterization of Au, Pd, and AuPd Nanoparticles Supported on Different Carbon Nanofibers. Surfaces. 2019; 2(1):205-215. https://doi.org/10.3390/surfaces2010016
Chicago/Turabian StyleTestolin, Anna, Stefano Cattaneo, Wu Wang, Di Wang, Valentina Pifferi, Laura Prati, Luigi Falciola, and Alberto Villa. 2019. "Cyclic Voltammetry Characterization of Au, Pd, and AuPd Nanoparticles Supported on Different Carbon Nanofibers" Surfaces 2, no. 1: 205-215. https://doi.org/10.3390/surfaces2010016
APA StyleTestolin, A., Cattaneo, S., Wang, W., Wang, D., Pifferi, V., Prati, L., Falciola, L., & Villa, A. (2019). Cyclic Voltammetry Characterization of Au, Pd, and AuPd Nanoparticles Supported on Different Carbon Nanofibers. Surfaces, 2(1), 205-215. https://doi.org/10.3390/surfaces2010016










