Scientific Art in Glass: Archaeometric Analysis and Conservation of Blaschka Models
Abstract
1. Introduction
2. Materials
3. Methods
3.1. Visible Fluorescence Analysis (UVF)
3.2. X-Ray Computed Tomography (CT)
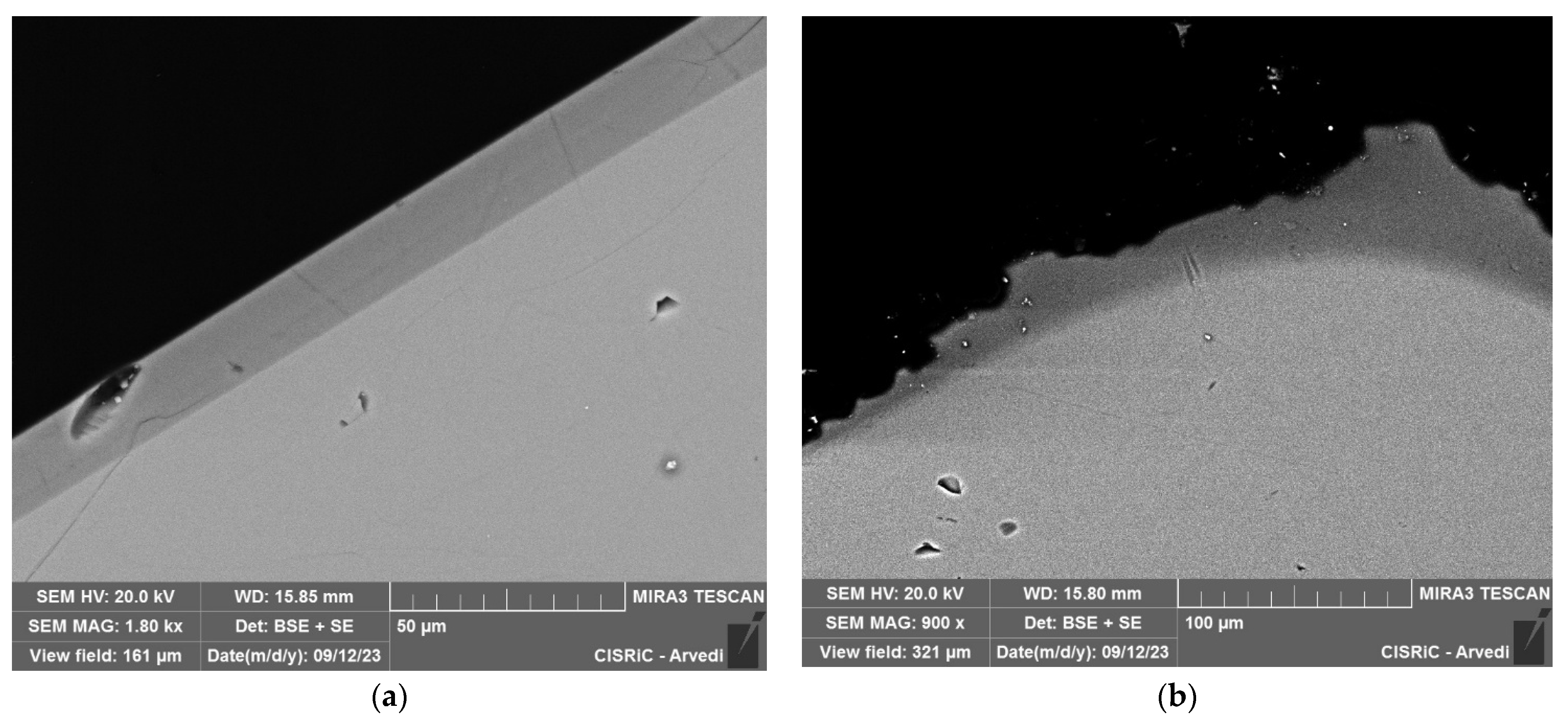
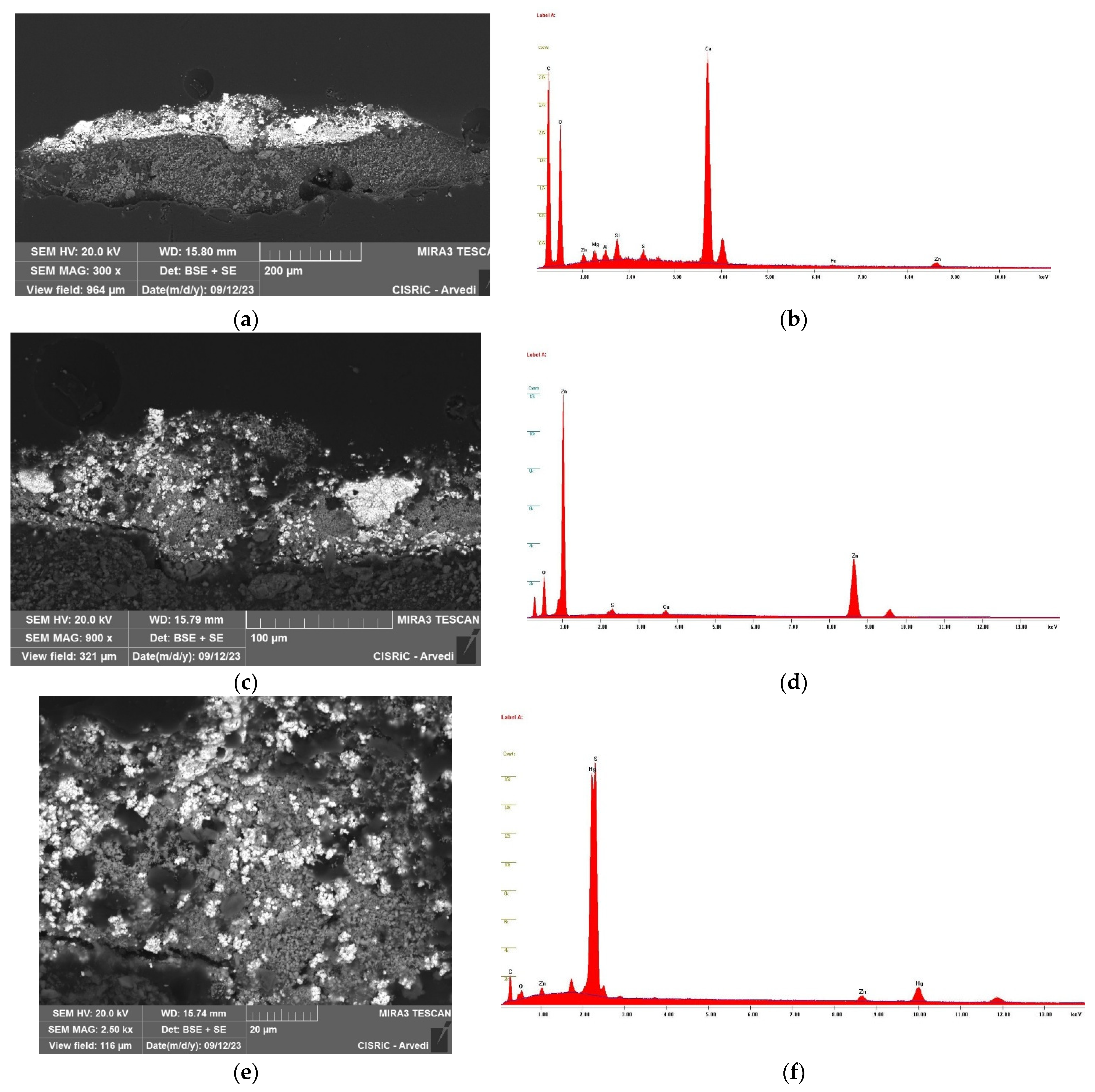
3.3. Scanning Electron Microscope (SEM) with Energy Dispersive X-Ray Spectrometer (EDS)
3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
4. Results and Discussion
4.1. Investigation of Glass Models’ Structure and Semi-Quantitative Composition (CT and SEM-EDS)
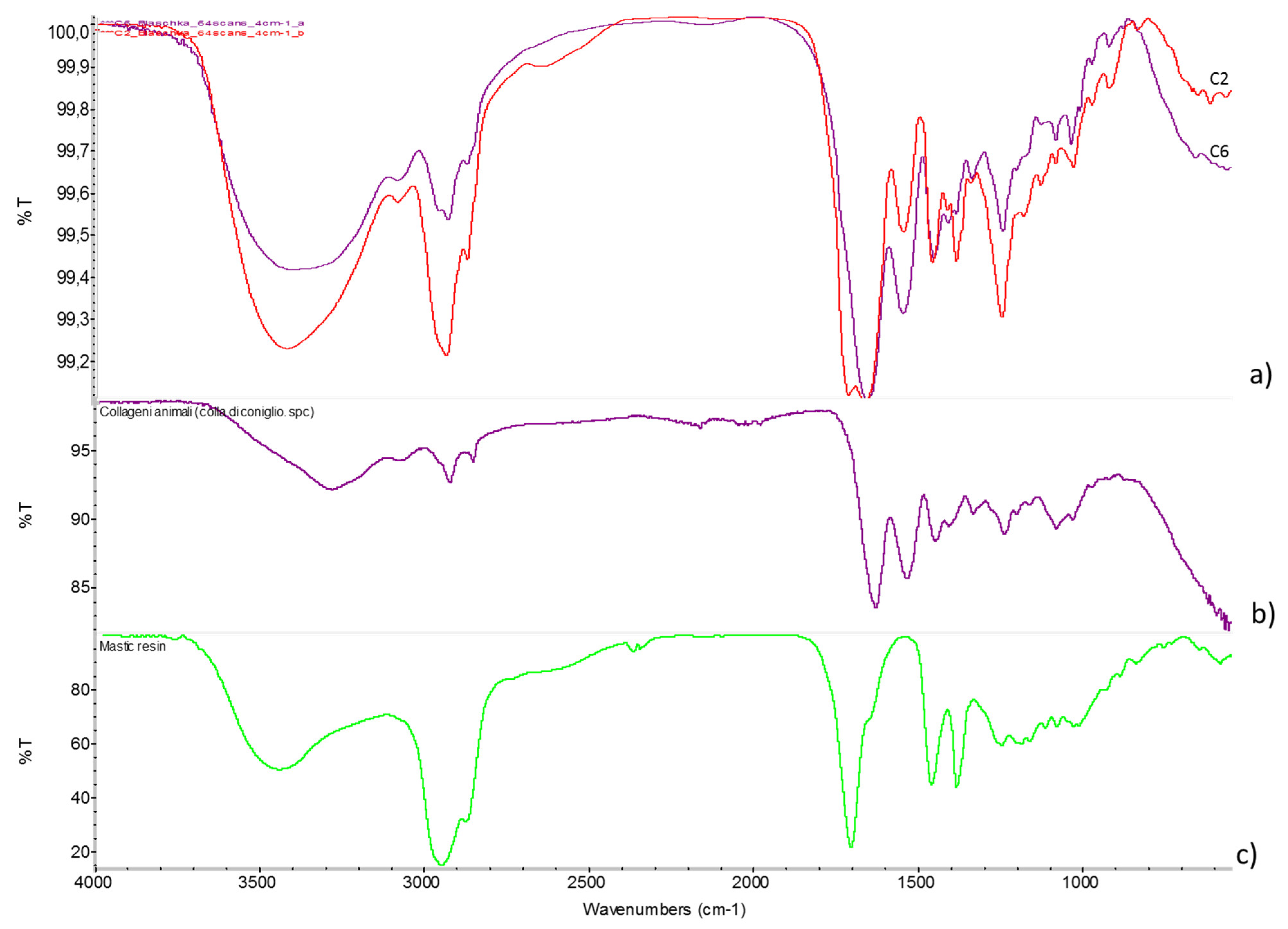
4.2. Analysis of the Adhesive (UVF and FTIR)
4.3. Conservation Measures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, J.; Meechan, C. Nature in glass. The models of Leopold and Rudolf Blaschka. In Things Fall Apart. Museum Conservation in Practice; Buttler, C., Davis, M., Eds.; National Museum of Wales Books: Cardiff, UK, 2006; pp. 164–171. [Google Scholar]
- Reiling, H. The Blaschkas’ glass animal models: Origins of design. J. Glass Stud. 1998, 40, 105–126. [Google Scholar]
- Ruggiero, A.; Larson, K.A. The Blaschka legacy in worldwide collections: A new resource. J. Glass Stud. 2017, 59, 419–428. [Google Scholar]
- van Giffen, N.A.R. The Case of the Hydrating Hydra: Examination and Treatment of a Blaschka Glass Invertebrate Model. In Objects Specialty Group Postprints; Hamilton, E., Dodson, K., Eds.; American Institute for Conservation of Historic and Artistic Works: Washington, DC, USA, 2017; pp. 351–371. [Google Scholar]
- Rossi-Wilcox, S.M.; Reiling, P.; Bisaga, P. The Blaschkas’ Lampworking Tables. J. Glass Stud. 2003, 45, 167–176. [Google Scholar]
- van Giffen, N.A.R.; Eremin, K.; Drier, T.O. Imitating Nature: The Materials and Preservation Needs of the Blaschka Glass Models. J. Glass Stud. 2015, 27, 213–224. [Google Scholar]
- Koob, S.P.; Fulton, S.E.; Rossi-Wilcox, S.M. Botanical wonders: The conservation and exhibition of the Harvard glass flowers. Stud. Conserv. 2008, 53 (Suppl. S1), 44–49. [Google Scholar] [CrossRef]
- Smith McNally, R.; Buschini, N. The Harvard Glass Flowers: Materials and Techniques. J. Am. Inst. Conserv. 1993, 32, 231–240. [Google Scholar] [CrossRef]
- Rossi-Wilcox, S.M.; Whitehouse, D. Drawing upon Nature: Studies for the Blaschkas’ Glass Models; The Corning Museum of Glass: Corning, NY, USA, 2007. [Google Scholar]
- Rossi-Wilcox, S.M. A Brief History of Harvard’s Glass Flowers Collection and Its Development. J. Glass Stud. 2015, 57, 197–211. [Google Scholar]
- Bertini, M.; Verveniotou, E.; Lowe, M.; Miller, C.G. Laser ablation inductively coupled plasma mass spectrometry investigation of late 19th Century Blaschka marine invertebrate glass models. J. Archaeol. Sci. Rep. 2016, 6, 506–517. [Google Scholar] [CrossRef]
- van Giffen, N.A.R.; Eremin, K.; Kirk, S.; Tate, J. Deterioration and Preservation of Blaschka Glass. In Proceedings of the Interim Meeting of the ICOM-CC Working Group, Corning NY, USA, 3–6 October 2010; Roemich, H., Ed.; The Corning Museum of Glass: New York, NY, USA, 2010; pp. 53–62. [Google Scholar]
- van Giffen, N.A.R.; Eremin, K.; Newman, R. The Harvard Glass Flowers and more: A technical study. In Proceedings of the Annales du 18 Congrès de l’Association Internationale Pour l’Histoire du Verre, Thessaloniki, Greece, 21–25 September 2009; pp. 475–480. [Google Scholar]
- Harvell, D. A Sea of Glass; University of California Press: Oakland, CA, USA, 2016. [Google Scholar]
- Fulton, S.E.; Rossi-Wilcox, S.M. Harvard’s glass flowers: A case study in travelling a fragile collection. J. Am. Inst. Conserv. 2008, 47, 15–26. [Google Scholar] [CrossRef]
- Brill, E.R.; Huber, F.; Brown, D.O. Sea Creatures in Glass: The Blaschka Marine Animals at Harvard; Scala Arts Publishers: New York, NY, USA, 2016. [Google Scholar]
- Doyle, H.; Callaghan, E.; Reynaud, E.G. Blaschka invertebrate models in Irish institutions. J. Hist. Collect. 2017, 29, 439–450. [Google Scholar] [CrossRef]
- Sigwart, J.D. Crystal creatures: Context for the Dublin Blaschka Congress. Hist. Biol. 2008, 20, 1–10. [Google Scholar] [CrossRef]
- Kessler, C.G.; Russell, H.D. Leopold and Rudolf Blaschka’s nudibranch glass models. Nautilus 1978, 92, 167–172. [Google Scholar]
- Swinney, G.N. Enchanted invertebrates: Blaschka models and other simulacra in National Museums Scotland. Hist. Biol. 2008, 20, 39–50. [Google Scholar] [CrossRef]
- Brierley, L. Art forms in nature, examination and conservation of a Blaschka glass, model of the protozoan Aulosphaera elegantissima. Stud. Conserv. 2009, 54, 255–267. [Google Scholar] [CrossRef]
- Reiling, H. The Blaschkas’ glass animal models: Illustrations of 19 century zoology. Sci. Hist. 2000, 26, 131–144. [Google Scholar]
- Callaghan, E.; Egger, B.; Doyle, H.; Reynaud, E.G. Taxonomic revision of Leopold and Rudolf Blaschkas’ Glass Models of Invertebrates 1888 Catalogue, with correction of authorities. J. Nat. Sci. Collect. 2020, 7, 34–43. [Google Scholar]
- Shaw, M.D.; Szczepanski, J.Z.; Murray, S.F.; Hodge, S.; Vink, C.J. Ideas made glass: Blaschka glass models at Canterbury Museum. Rec. Canterb. Mus. 2017, 31, 5–84. [Google Scholar]
- Whitman, J.D.; Viscardi, P.; Reynaud, E.G. Recording of Blaschka glass invertebrate models: A method and workflow for imaging using standardized methods. J. Nat. Sci. Collect. 2022, 10, 115–145. [Google Scholar]
- Dublin Blaschka Congress. Proceedings of the Dublin Blaschka Congress; Taylor & Francis: Abingdon, Oxon, UK, 2008. [Google Scholar]
- Fried, P.; Woodward, J.; Brown, D.; Harvell, D.; Hanken, J. 3D scanning of antique glass by combining photography and computed tomography. Digit. Appl. Archaeol. Cult. Herit. 2020, 18, e00147. [Google Scholar] [CrossRef]
- Jungck, J.R.; Wagner, R.; Van Loo, D.; Grossman, B.; Khiripet, N.; Khiripet, J.; Khantuwan, W.; Hagan, M. Art Forms in Nature: Radiolaria from Haeckel and Blaschka to 3D Nanotomography, Quantitative Image Analysis, Evolution, and Contemporary Art. Theory Biosci. 2019, 138, 159–187. [Google Scholar] [CrossRef]
- van Giffen, N.A.R.; Koob, S.P. Cleaning and Repairing Blaschka Glass Models: Challenges in the Preservation of Exquisite Reproductions of Nature. In Recent Advances in Glass and Ceramics Conservation 2016, Proceedings of the ICOM-CC Working Group Glass and Ceramics Interim Meeting, The Eugeniusz Geppert Academy of Art and Design, Wrocław, Poland, 25–29 May 2016; International Council of Museums—Committee for Conservation (ICOM-CC): Paris, France, 2016; pp. 205–214. [Google Scholar]
- Weyl, W.A. Coloured Glasses; Society of Glass: Sheffield, UK, 1951. [Google Scholar]
- Janssens, K. Electron Microscopy. In Modern Methods for Analysisng Archaeological and Historical Glass; Janssens, K., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 129–154. [Google Scholar]
- Davidson, S. Conservation and Restoration of Glass, 2nd ed.; Butterworth-Heinemann: London, UK, 2003. [Google Scholar]
- Pellegrini, D.; Duce, C.; Bonaduce, I.; Biagi, S.; Ghezzi, L.; Colombini, M.P.; Tinè, M.R.; Bramanti, E. Fourier transform infrared spectroscopic study of rabbit glue/inorganic pigments mixtures in fresh and aged reference paint reconstructions. Microchem. J. 2016, 124, 31–35. [Google Scholar] [CrossRef]
- Vahur, S.; Teearu, A.; Peets, P.; Joosu, L.; Leito, I. ATR-FT-IR spectral collection of conservation materials in the extended region of 4000–80 cm−1. Anal. Bioanal. Chem. 2016, 408, 3373–3379. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Yeo, S.Y. Characterization of Animal Protein-Based Binders in Ancient Chinese Wall Paintings Using Atomic Force Microscopy and Fourier Transform Infrared Spectroscopy. Appl. Spectrosc. 2022, 76, 1191–1205. [Google Scholar] [CrossRef]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Infrared Spectroscopy in Conservation Science; The Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Bruni, S.; Guglielmi, V. Identification of archaeological triterpenic resins by the non-separative techniques FTIR and 13C NMR: The case of Pistacia resin (mastic) in comparison with frankincense. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2014, 121, 613–622. [Google Scholar] [CrossRef]
- Noake, E.; Lau, D.; Nel, P. Identification of cellulose nitrate based adhesive repairs in archaeological pottery of the University of Melbourne’s Middle Eastern archaeological pottery collection using portable FTIR-ATR spectroscopy and PCA. Herit. Sci. 2017, 5, 3. [Google Scholar] [CrossRef]
- Berthumeyrie, S.; Collin, S.; Bussiere, P.O.; Therias, S. Photooxidation of cellulose nitrate: New insights into degradation mechanisms. J. Hazard. Mater. 2014, 272, 137–147. [Google Scholar] [CrossRef]
- Mitchell, G.; France, F.; Nordon, A.; Tang, P.L.; Gibson, L.T. Assessment of historical polymers using attenuated total reflectance-Fourier transform infra-red spectroscopy with principal component analysis. Herit. Sci. 2013, 1, 1. [Google Scholar] [CrossRef]
- NORMA UNI 10829:1999; Works of Art of Historical Importance—Ambient Conditions or the Conservation—Measurement and Analysis. UNI: Milan/Rome, Italy, 1999.
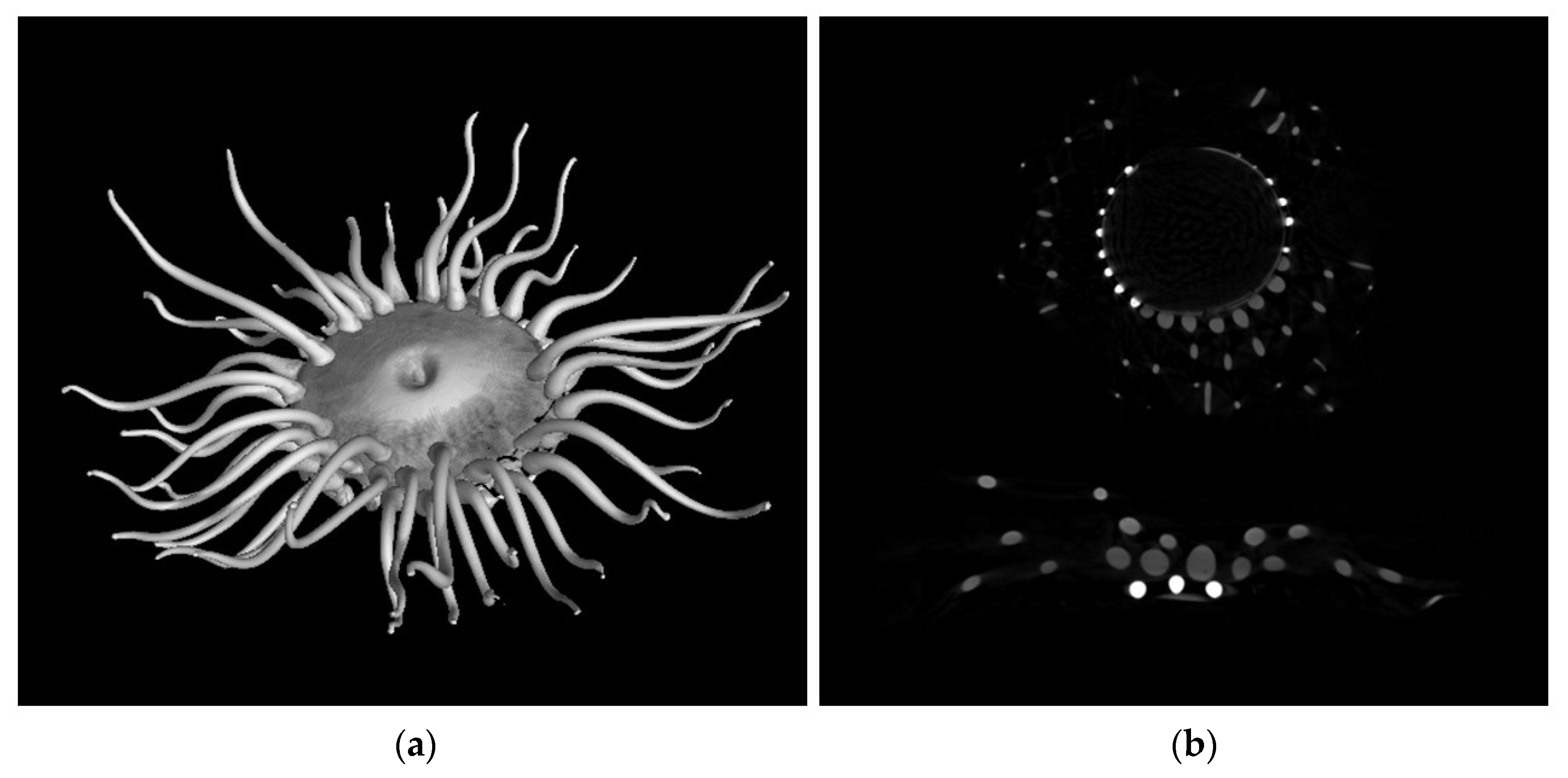
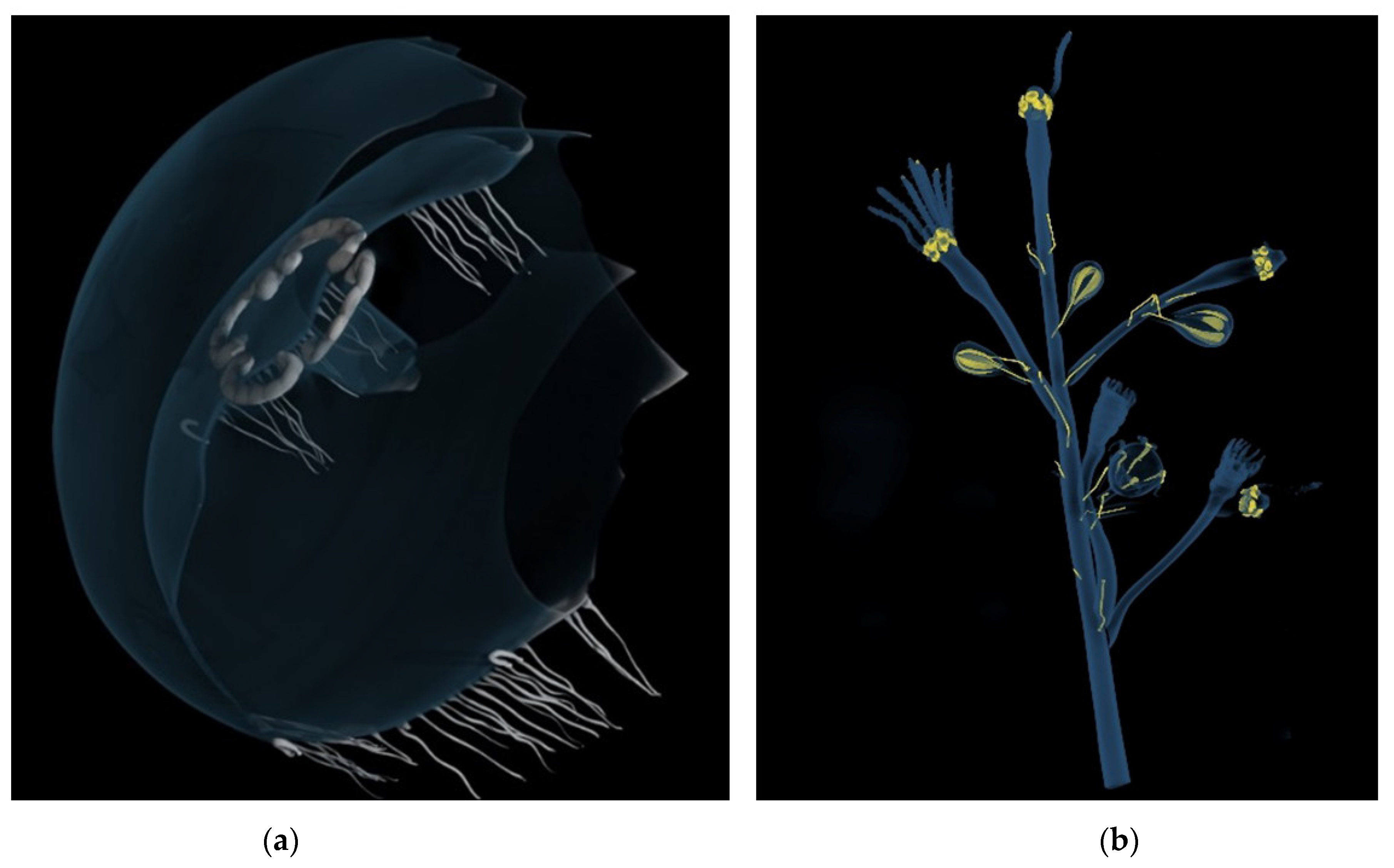
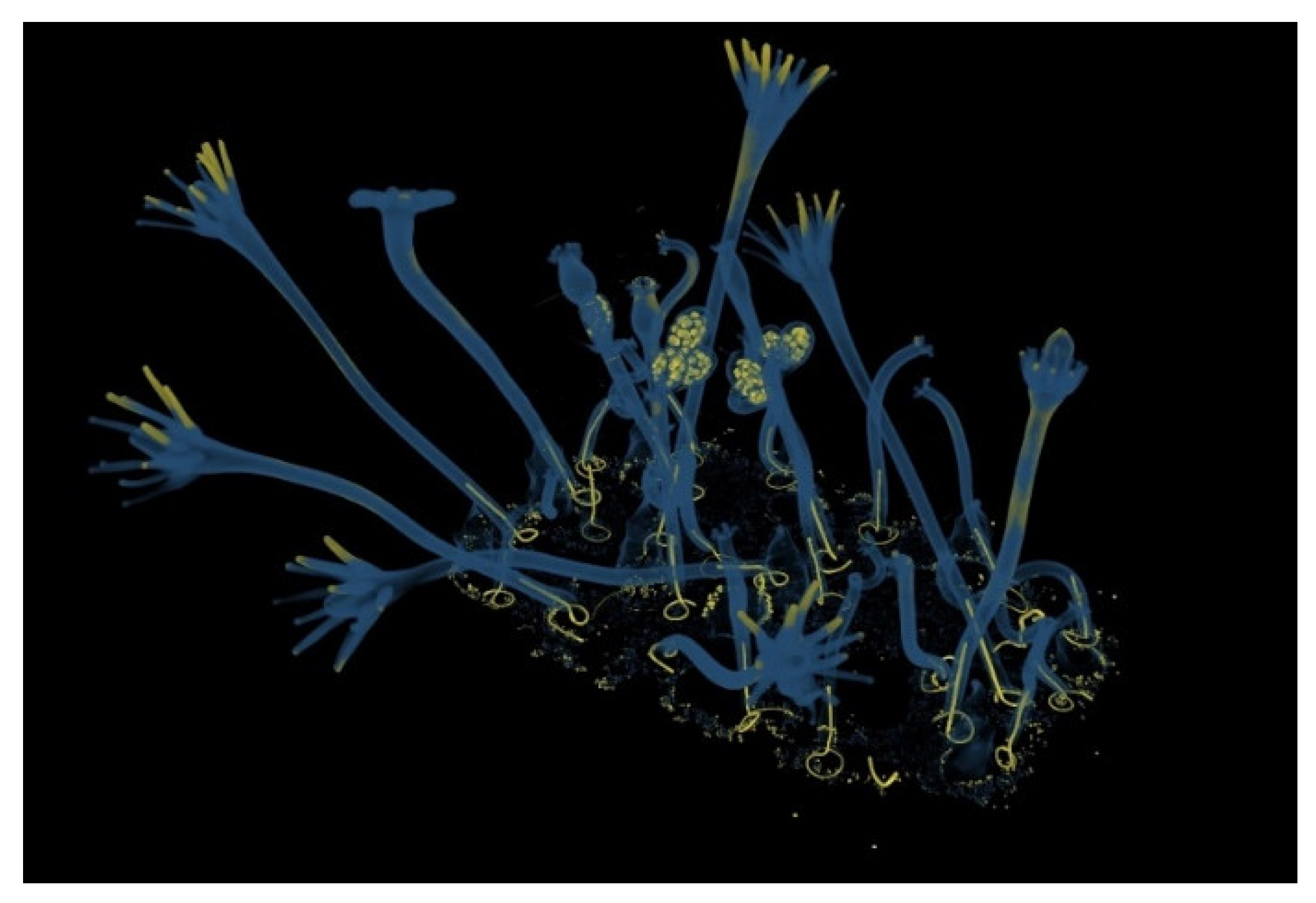
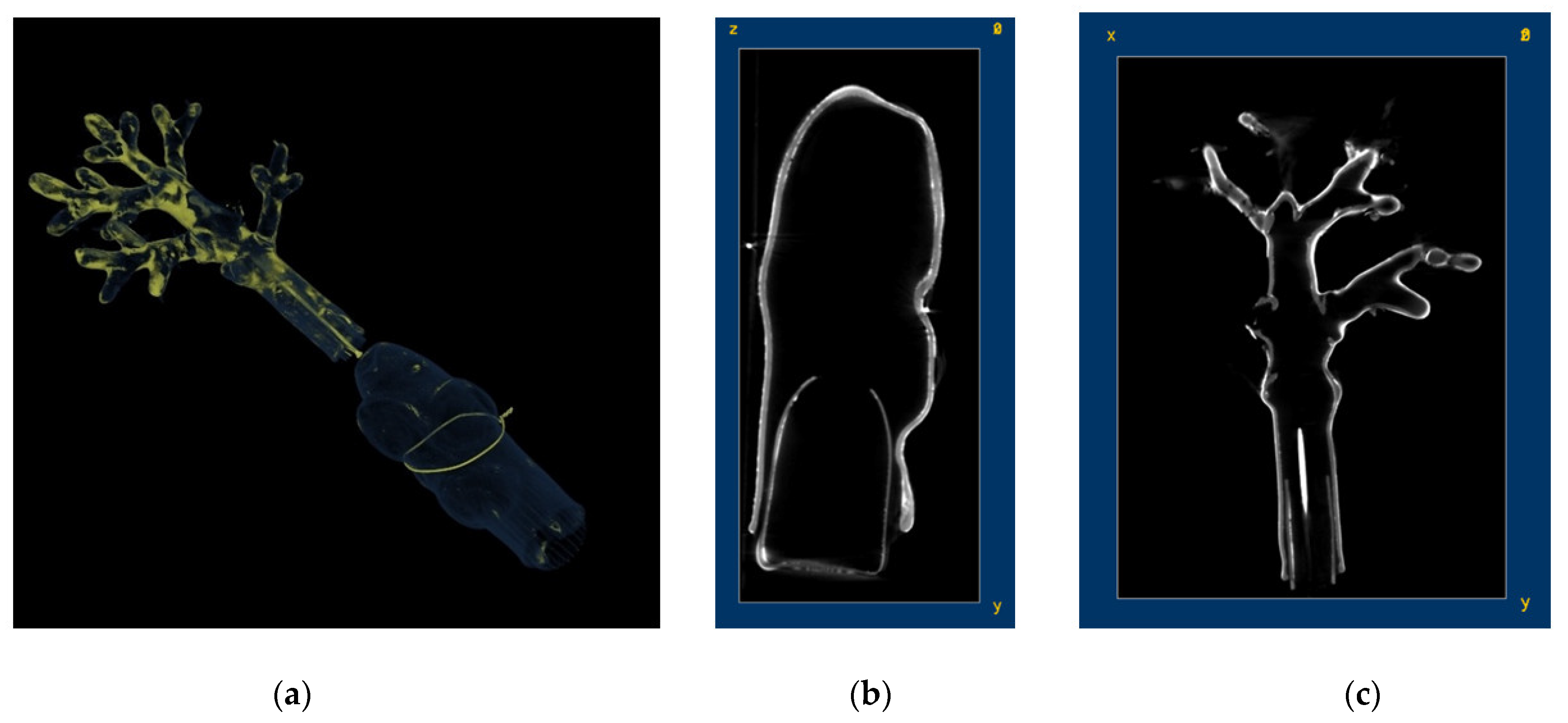
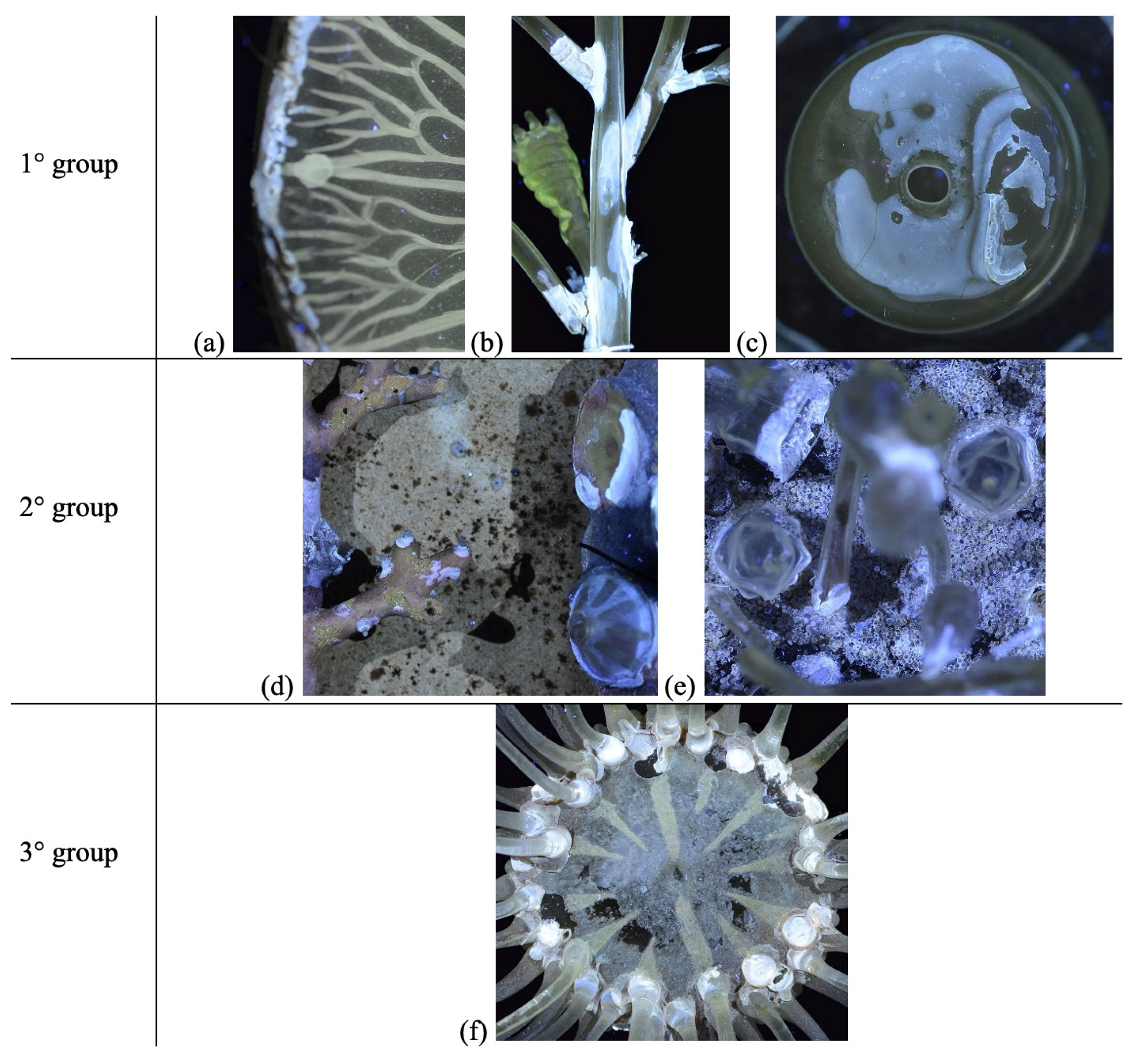
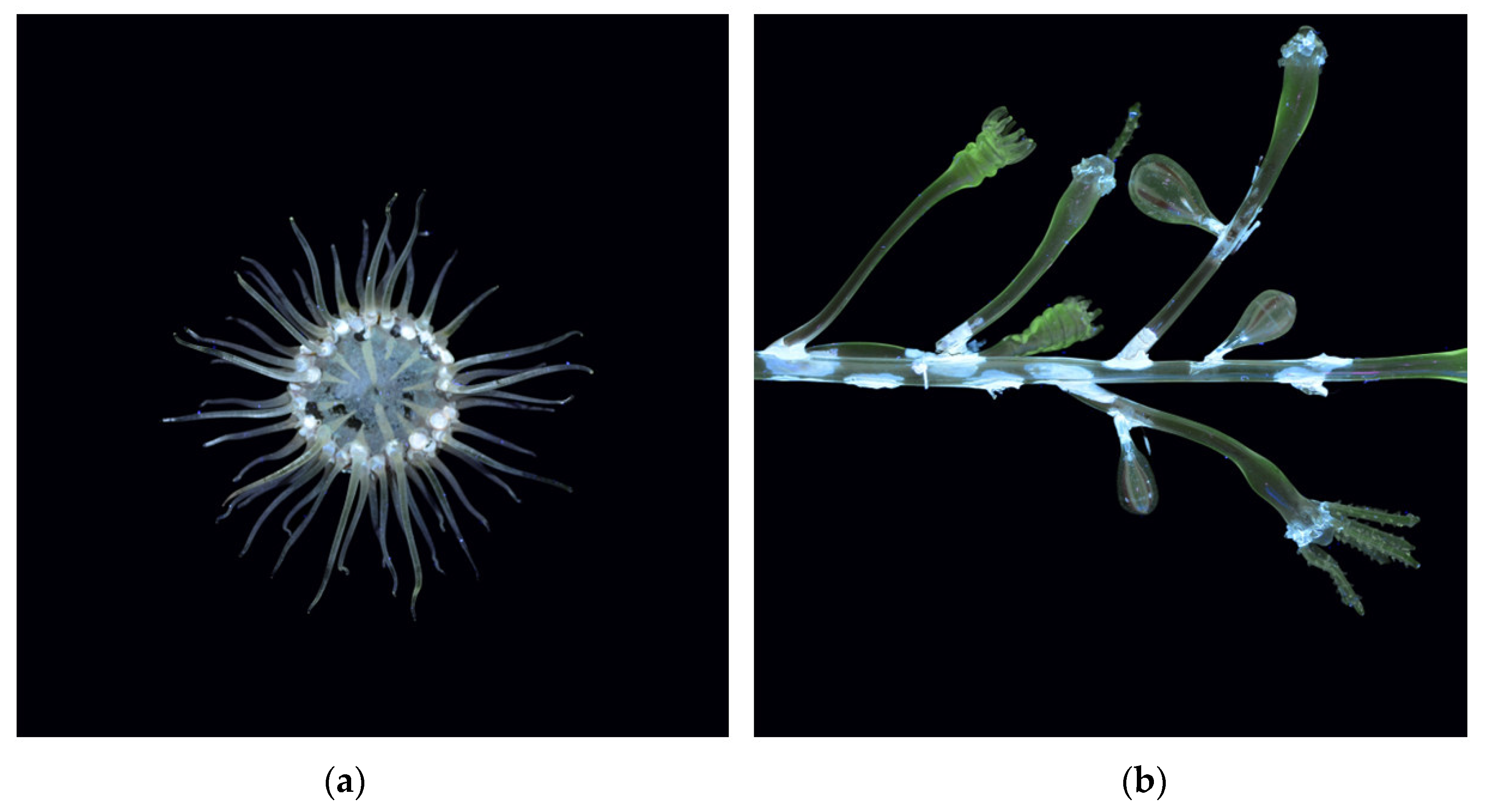
| Model | Name | Collection |
|---|---|---|
 | Anthea cereus sea anemone (A: oral disc and tentacles; B: body and base) | Liceo Ugo Foscolo, Pavia |
 | Aurelia aurita jellyfish | Liceo Ugo Foscolo, Pavia |
 | Bougainvillia fruticosa | Liceo Giovanni Prati, Trento |
 | Corallium rubrum Red coral (A: Corallium rubrum; B: enlargement of Corallium rubrum terminal branch) | Liceo Ugo Foscolo, Pavia |
 | Hydractinia echianta | Liceo Giovanni Prati, Trento |
| Model | Samples | Sample Material | Position of Sampling | CT Indication for Sampling |
|---|---|---|---|---|
| Aurelia aurita | AA1 (bell) AA2 (tentacle)  | Glass |  | Bell and tentacles have a different radiopacity. No sampling was allowed of the pink glass |
| Anthea cereus | AC1 (hemispheres—highly radiopaque area) AC2 (tentacle)  | Glass |  | The tentacles and the corolla are less radiopaque than the small hemispheres attached to the edge of the central part |
| Corallium rubrum | CR1 (polyp with paint layer) | Glass + paint layer |  | Higher radiopacity of the paint layer |
| Hydractinia echinata | HE1 (tentacle—low-radiopacity area) HE2 (spheres)  | Glass |  | Highly radiopaque small spheres are glued to the base and the ends of some tentacles are also quite radiopaque. No sampling was allowed of the yellow glass |
| wt% | AA1 | AA2 | AC1 | AC2 | AC2_sup * | CR1 | CR1_sup * | HE1 | HE2 |
|---|---|---|---|---|---|---|---|---|---|
| Na2O | 13.9 | 14.36 | 4.12 | 14.85 | 1.49 | 14.74 | 3.14 | 14.33 | 7.58 |
| MgO | 0.21 | 0.17 | 0.53 | n.d. | n.d. | 0.14 | 0.18 | 0.19 | 0.89 |
| Al2O3 | 2.13 | 1.9 | 0.14 | 1.24 | 1.89 | 3.04 | 3.42 | 1.88 | 0.72 |
| SiO2 | 72.1 | 71.54 | 47.49 | 69.47 | 83.84 | 69.99 | 79.49 | 71.35 | 61.08 |
| SO3 | 0.33 | 0.74 | 2.2 | n.d. | n.d. | 0.79 | 0.68 | 0.78 | n.d. |
| PbO2 | 0.32 | n.d. | 31.46 | n.d. | n.d. | n.d. | n.d. | n.d. | 15.29 |
| Cl2O | 0.23 | 0.21 | 0.98 | 1.22 | 1.41 | 0.30 | 0.18 | 0.22 | 0.28 |
| K2O | 3.91 | 3.94 | 9.87 | 7.43 | 4.31 | 3.58 | 4.04 | 3.94 | 10.79 |
| CaO | 6.32 | 6.55 | 1.75 | 5.28 | 6.31 | 6.79 | 8.17 | 6.63 | 2.65 |
| MnO | 0.31 | 0.35 | 0.18 | 0.25 | 0.37 | 0.31 | 0.48 | 0.40 | 0.33 |
| Fe2O3 | 0.26 | 0.24 | 0.47 | 0.27 | 0.38 | 0.30 | 0.23 | 0.27 | 0.38 |
| Model | Sample | Zone of Provenance | Type of Glass | ||
|---|---|---|---|---|---|
| Aurelia aurita | AA1 | Bell | Mixed alkali | ||
| AA2 | Tentacle | Mixed alkali | |||
| Anthea cereus | AC1 | Lower edge of the A element | Lead glass | ||
| AC2 | Tentacle | Mixed alkali | |||
| Bougainvillia fruticosa | - | - | - | ||
| Corallium rubrum | CR1 | Glass | Polyp of the A element | Mixed alkali | |
| Colour | Exterior layer | Polyp of the A element | Adhesive | ||
| Interior layer | Polyp of the A element | Zinc oxide and cinnabar | |||
| Hydractinia echinata | HE1 | Tentacle | Mixed alkali | ||
| HE2 | Sphere on the base | Lead glass | |||
| Models | Sample | Sampling Area | Description | FT-IR | Composition |
|---|---|---|---|---|---|
| Bougainvillia fruticosa | C1 | Central stem | Yellow; hard and brittle | ~3370, 2935, 2974, 1714, 1655, 1543, 1453, 1384, 1245 | Protein adhesive (i.e., animal glue) + natural resin (i.e., mastic) |
| Aurelia aurita | C2 | Manubrium | Yellow/orange; plastic consistency | ~3440, 2937, 2875, ~2645, 1712, 1659, 1544, 1457, 1383, 1246 | Protein adhesive (i.e., animal glue) + natural resin (i.e., mastic) |
| C3 | Tentacle attachment | Yellow/orange | ~3370, 2935, 2872, ~2650, 1712, 1653, 1542, 1460, 1384, 1247 | Protein adhesive (i.e., animal glue) + natural resin (i.e., mastic) | |
| Anthea cereus | C4 | Element B | Yellow; hard and brittle | No valuable results (*) | - |
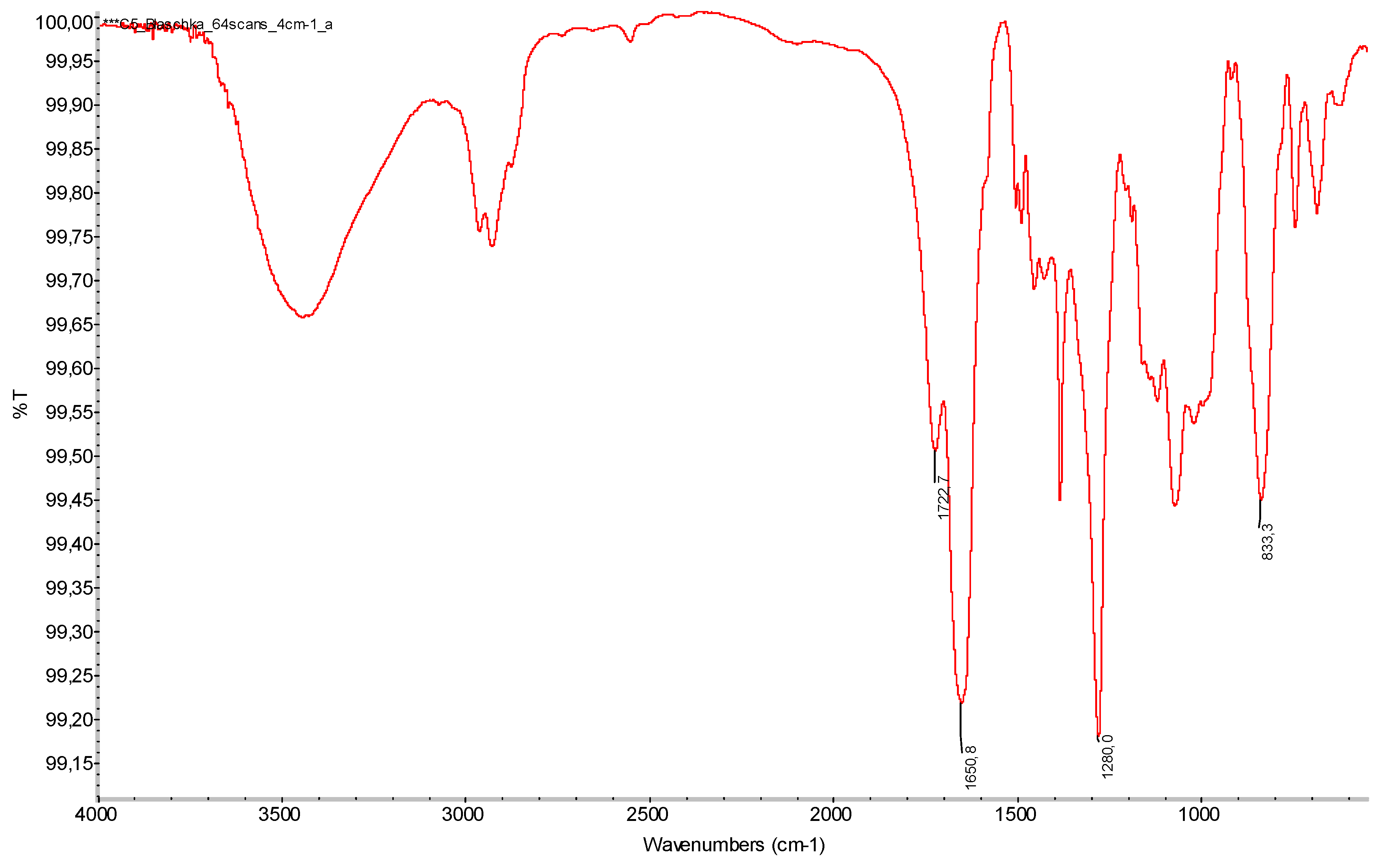
| Corallium rubrum | C5 | Cylindrical element | Yellow/orange; hard and brittle | 2962, 2923, 2872, 1722, 1650, 1280, 1066, 833, 742, 687 | Cellulose nitrate |
| Hydractinia echinata | C6 | Base | Yellow; hard and brittle | ~3370, 2959, 2935, 2974, 1720, 1655, 1543, 1453, 1250 | Protein adhesive (i.e., animal glue) + natural resin (i.e., mastic) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giani, G.; Ferucci, S.; Matteucci, C.; Apicella, S.A.; Tarantola, G.; Morigi, M.P.; Bettuzzi, M.; Riccardi, M.P.; Vandini, M. Scientific Art in Glass: Archaeometric Analysis and Conservation of Blaschka Models. Heritage 2025, 8, 376. https://doi.org/10.3390/heritage8090376
Giani G, Ferucci S, Matteucci C, Apicella SA, Tarantola G, Morigi MP, Bettuzzi M, Riccardi MP, Vandini M. Scientific Art in Glass: Archaeometric Analysis and Conservation of Blaschka Models. Heritage. 2025; 8(9):376. https://doi.org/10.3390/heritage8090376
Chicago/Turabian StyleGiani, Gemma, Silvia Ferucci, Chiara Matteucci, Salvatore Andrea Apicella, Gaia Tarantola, Maria Pia Morigi, Matteo Bettuzzi, Maria Pia Riccardi, and Mariangela Vandini. 2025. "Scientific Art in Glass: Archaeometric Analysis and Conservation of Blaschka Models" Heritage 8, no. 9: 376. https://doi.org/10.3390/heritage8090376
APA StyleGiani, G., Ferucci, S., Matteucci, C., Apicella, S. A., Tarantola, G., Morigi, M. P., Bettuzzi, M., Riccardi, M. P., & Vandini, M. (2025). Scientific Art in Glass: Archaeometric Analysis and Conservation of Blaschka Models. Heritage, 8(9), 376. https://doi.org/10.3390/heritage8090376








