1. Introduction
Heritage institutions face challenges with sustainability. There are pressing twin issues: climate control energy use, increasing global warming (leading to the current and increasing climate emergency), and economic sustainability due to recent energy price increases. This work considers the sustainability aspects of preventive conservation and one conservation treatment for glass artefacts. There is a focus on global warming potential as this is driving recent climate changes [
1].
It has been recognised for some time that certain glass objects are much more environmentally sensitive or ‘unstable’. The term ‘unstable glass’ is not one type of glass but rather encompasses a range of glass compositions and states. Some are chemically unstable due to a low proportion of formative and stabilising compounds like silica and lime in their structure [
2,
3]. Others could be more durable but have undergone severe corrosion and, therefore, have a fragile, altered surface. In all cases, such glass actively deteriorates in normal museum conditions and, therefore, requires specialist intervention and strictly controlled environments.
Recent research [
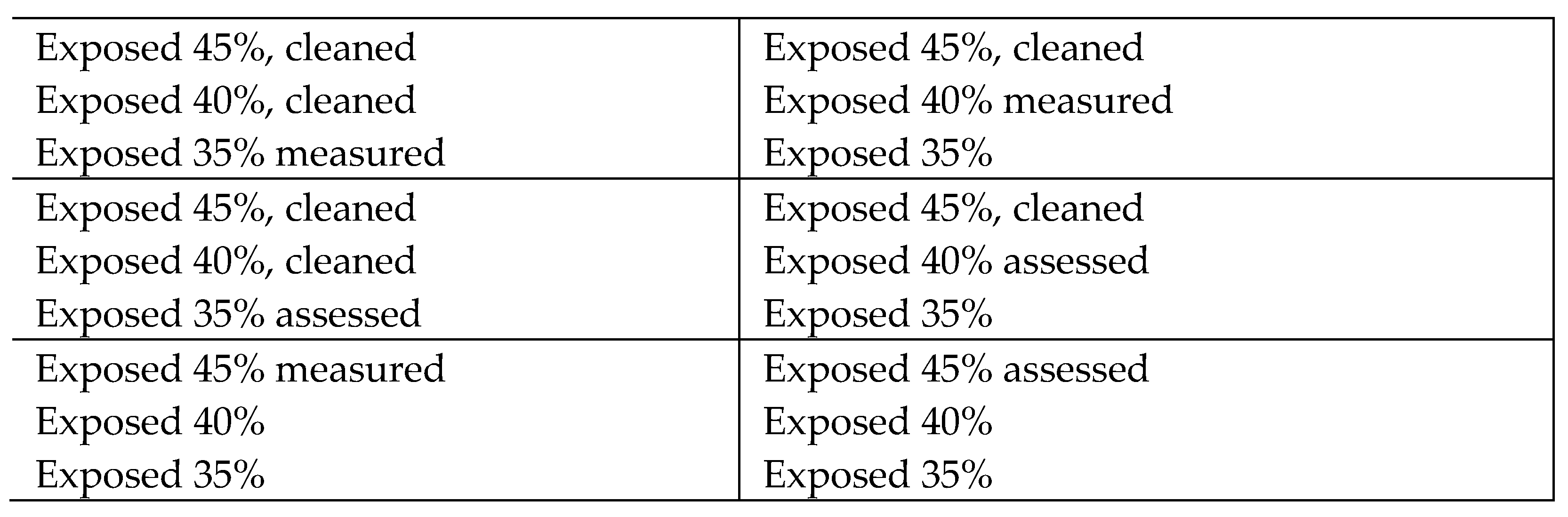
4] has recommended RH conditions of 40–42% RH for unstable glasses. This is difficult to achieve and even difficult to validate. Most heritage institutions use RH probes with accuracies of ±2 or 3%. Whilst more accurate probes are available (Rotronic Hygroclip II probes have stated accuracy + 0.8%), their cost limits their use. Even with this accuracy level, validating a 2% RH band is challenging. A wider band (40–50% RH) is recommended for other glass objects, although the exact recommended ranges vary between authors (
Table 1). Maintaining this tight RH range for unstable glass is energy- or carbon-intensive, but only a small portion of glass objects require it. Moving air was also recommended to slow deterioration rates [
4].
For archaeological glass or objects where sampling is straightforward, cross sections and SEM-EDX or EPMA provide unambiguous analyses to separate unstable from stable glass. Complete objects are much more challenging. Research has indicated that surface ion concentrations and Germanium ATR-FTIR can detect the earliest stages of degradation, indicating unstable compositions in the environment [
16]. Germanium ATR has more sensitivity than the more common diamond ATR technique [
17]. The techniques of reflectance FTIR, Raman, NIR, and OCT were found to be able to detect more degraded glass but lack sensitivity for the early stages of deterioration. The vast majority of glass is assessed by eye by conservators. Some issues were discussed in a previous work [
16] using this approach, and attempts have been made to quantify the comparison with surface ions.
The sustainability of managing glass collections has been investigated. The RH band to be maintained has already been shown to have a large impact on energy use and carbon footprint [
18].
Maintaining a 2% RH control band is difficult. Four methods have previously been shown to be capable of this in some conditions.
Munters MG50 dehumidifiers coupled with lower air exchange rate (AER) showcases and large amounts of silica gel. The dehumidifier works on a humidistat, turning on when the RH rises to its set point. As the capacity is most often much larger than a single showcase, this results in a distinctive saw-tooth pattern. As the dehumidifier turns on, it dries too much; when it turns off, the showcase’s RH slowly increases again. Adding silica gel at the dehumidifier’s inlet reduces the size of these fluctuations. The correct combination of showcase AER, volume, and loading of silica gel (<0.8/day, 2 m3, and 10 kg/m3 Prosorb) and dehumidifier capacity can reduce them to the 2% recommended.
Miniclima units work by adding conditioned air to the showcase when the humidistat registers showcase RHs below 40% or above 42%. The drying occurs via a Peltier cooler. Careful positioning of the inlet/outlet and humidistat is essential for correct conditioning.
Hahn RK2 units condition air and then provide a low airflow to the showcase based on a humidistat. The low airflow can mean that it is some time before the correct RH is established. The unit also works on the Peltier principle to dehumidify. It also allows continuous chemical filtration.
Saturated potassium carbonate solutions have been reported to maintain RH very close to 43% [
19].
Whilst it is possible to maintain RH with silica gels and low air exchange rate showcases, this extremely tight RH band is considered to entail reconditioning of the gel too frequently for most institutions.
Some museums, including the British Museum, Victoria and Albert Museum, and the Corning Museum, use showcases to store glass, but a large amount is stored on shelving units in storerooms. With very low AER storerooms, desiccant dehumidifiers can maintain 40–42% RH, and in most storerooms, 40–50% is achievable. It has been shown that air conditioning units use more energy the tighter the RH band used [
18].
The performance and carbon footprint of several methods for controlling showcases, including sorbents, saturated potassium carbonate, dehumidifiers and sorbents, Miniclima, and RK2, have been measured. The energy used to maintain these conditions has been estimated for storerooms with dehumidifiers. However, as RH is not the only environmental risk to glass, the performance of the conditioning systems in removing formic acid has been assessed, and the suitability of emission tests such as the BEMMA scheme [
20] and ISO16000 series [
21] for showcase materials for glass has been investigated. Formic acid is present in many showcases (9) and has been reported to accelerate glass deterioration at low concentrations (12t).
The approach to conditioning a full room is more complex. The air exchange rate is even more significant for buildings than showcases. There are two common methods to measure building air exchange rates: tracer gas and constant pressurisation. Tracer gas decay gives a direct measure of the air exchange rate, but only for the one to three days during the measurement. The value will change due to atmospheric conditions, particularly solar gain, wind velocity, and pressure changes. The constant pressurisation method, normally expressed as m3/m2/h@50Pa of the envelope, requires the application of an actual pressure difference to obtain an actual air exchange rate. The pressure difference between the room and the external environment will vary hourly and throughout the year.
A method to predict dehumidifier performance and energy usage for showcases has been developed and extensively tested [
22]. The amount of water vapour per time period is calculated from the room temperature and RH (measured at a time period, e.g., 30 min or 60 min), the controlled showcase RH, and its volume and air exchange rate. The run time of the dehumidifier in the time period is estimated from capacity data from the manufacturer (using room temperature and RH).
This fraction is multiplied by the power of the dehumidifier to estimate energy usage. The air from the room surrounds all the joints of the showcases, so it is reasonable to use it as the external condition. Four sets of measurements underneath showcases did not show significant differences in climate from the room conditions, and most showcase designs are well sealed underneath. The method was modified to generate an estimate for storage rooms. This situation is more complex, with uncertainty as to where the air exchanging with the internal room environment comes from. For a room within a room design, the external room temperature and RH can be used. This approach is probably also valid for rooms with external walls, with no entrances or windows and a vapour barrier incorporated into the external wall. For rooms with external windows or permeable external walls, floors, or ceilings, the relative amount of ingress from outside or other adjacent rooms is unknown. These volumes will have different (and often very different) temperatures and RHs. An approach to estimate from uncontrolled T/RH data was investigated. A previous year’s temperature RH data in the uncontrolled room was used along with the control RH (generally 40 or 50%), the room volume, and the air exchange rate. Situations with dehumidifiers to condition whole rooms have been investigated.
Research has shown that the degradation rate of glass reduces as RH reduces, but the lowest RH values investigated were 40% [
12,
23]. Initial experiments have been undertaken to assess the conditions required to minimise the degradation rate with five glass compositions, including two potassium glasses. The glasses were selected from suitable-sized pieces that could reasonably undergo the conditions investigated. The deterioration rate at 35, 40, and 45% RH has been previously measured in still air for a series of sodium glass daguerreotype cover glasses [
24]. Further glass types were tested. Tests at 40% were also run in moving air. Formic acid has been shown to accelerate deterioration at very low concentrations, 30.5 µg/m
3 [
23]. Formaldehyde has also been shown to have a significant effect in some situations [
25]. Tests with the more aggressive formic acid were undertaken.
3. Results and Discussion
3.1. Showcase Performance and Carbon Footprints
The estimated embedded carbon was 93 kg CO
2-eq for the Munters MG50, 47 for the Miniclima EBC 08, and 58 for the RK2 unit. The performance in the laboratory trials for the units and their energy usage is shown in
Table 5.
In the test showcases, the MG50 worked well in all instances. The Miniclima struggled to dehumidify the larger showcase (G) sufficiently at higher RHs and especially higher RHs combined with higher temperatures. Reducing the AER to 0.04 allowed it to perform sufficiently. The RK2 performed better but still struggled in the high-temperature RH region. Energy consumption was fairly similar for the three systems and relatively high, as expected from the very tight RH band. The consumption increased as the dehumidification load increased, with the Munters MG50 being more efficient at higher temperatures and the Miniclima and RK2 less efficient. Widening the control band to 40–50% significantly reduced the energy consumed in all instances tested by 2 to 3 times. The dehumidifier showed the highest energy reduction, as the controller allowed the RH to move within most of the full range. The Miniclima and RK2 still kept tight ±2% RH bands. However, the less tight specification is likely to mean they keep within the band all the time and can be used successfully in larger showcases with higher air exchange rates. With sufficient data, a required energy surface against temperature and RH could be produced to predict the energy usage of Miniclimas and RK2s. This has already been developed using the manufacturer’s data for Munters MG50 dehumidifiers [
22]. The potassium carbonate kept a 2% band around 43%. This is slightly higher than desired. The formic acid concentrations measured were below the threshold determined by Robinet in half of the test situations.
The installed showcases showed similar results (
Table 6), with the dehumidifier working well and the Miniclima performing most of the time but struggling with high RH and temperature conditions. The RK2 performed very well in a moderate environment room but was overwhelmed for a small proportion of the time when the temperature rose above 25 °C and the RH was above 72%. All units successfully kept the 40–50% RH band. The energy use was much higher for the tighter RH band, 3 to 4 times that for the wider band. The room environments were different, indicating the results are, to a degree, generalisable. All showcases were glass and metal construction, but previous measurements with acrylic showcases gave similar results. Controlling with PROSORB would require frequent changes for newly conditioned gel. The period between changes is very variable and depends on the season (and room conditions). These are calculated results, with an accuracy that cannot be achieved with most RH probes.
3.2. Formic Acid Testing
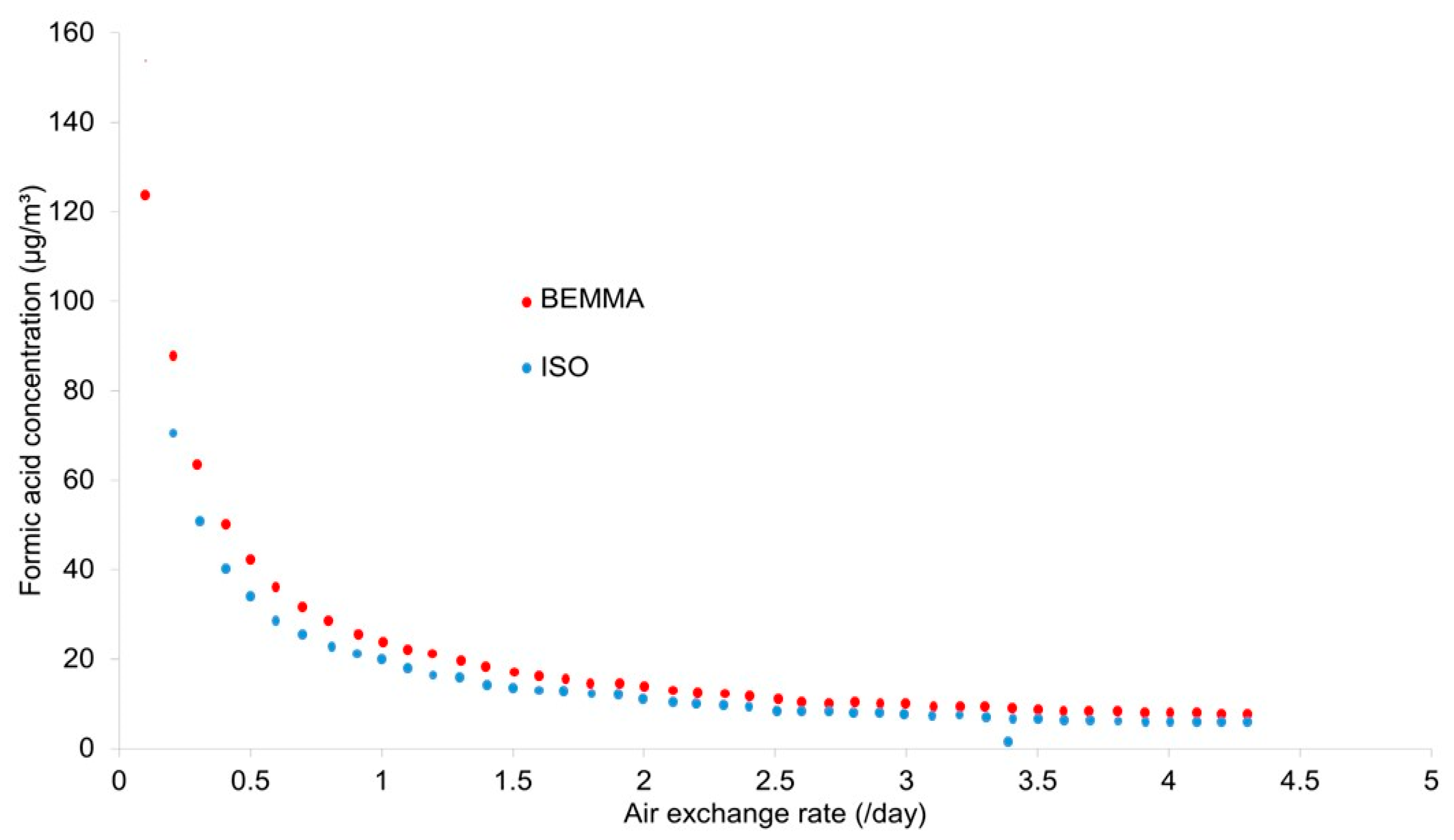
The continuous RK2 system reduced the formic acid concentration below the detection limit in all trials. Both the dehumidifier and Miniclima-controlled cases had formic acid present above the reported threshold (30.5 µg/m3) due to their intermittent operation. Higher room RH levels reduced the formic acid present.
Results of the formic acid calculations are shown in
Figure 2.
With a detection limit of 15 µg/m
3 in the conditions of the BEMMA test, the reported damage level will be reached with the desktop showcase considered at AERs below 0.9/day; a high proportion of such showcases have AERs below this value [
41]. The ISO 16000-based test is lower, 0.5/day, but still well above the commonly used 0.1/day specification for AER for showcases. This indicates that, in some instances, a material can pass both tests and then go on to accelerate the degradation of susceptible glasses.
3.3. Room Carbon Footprints
Table 7 shows the room details, the measured air exchange rates, and the estimated and measured energy use of Munters MG50 and MCS 300 dehumidifiers.
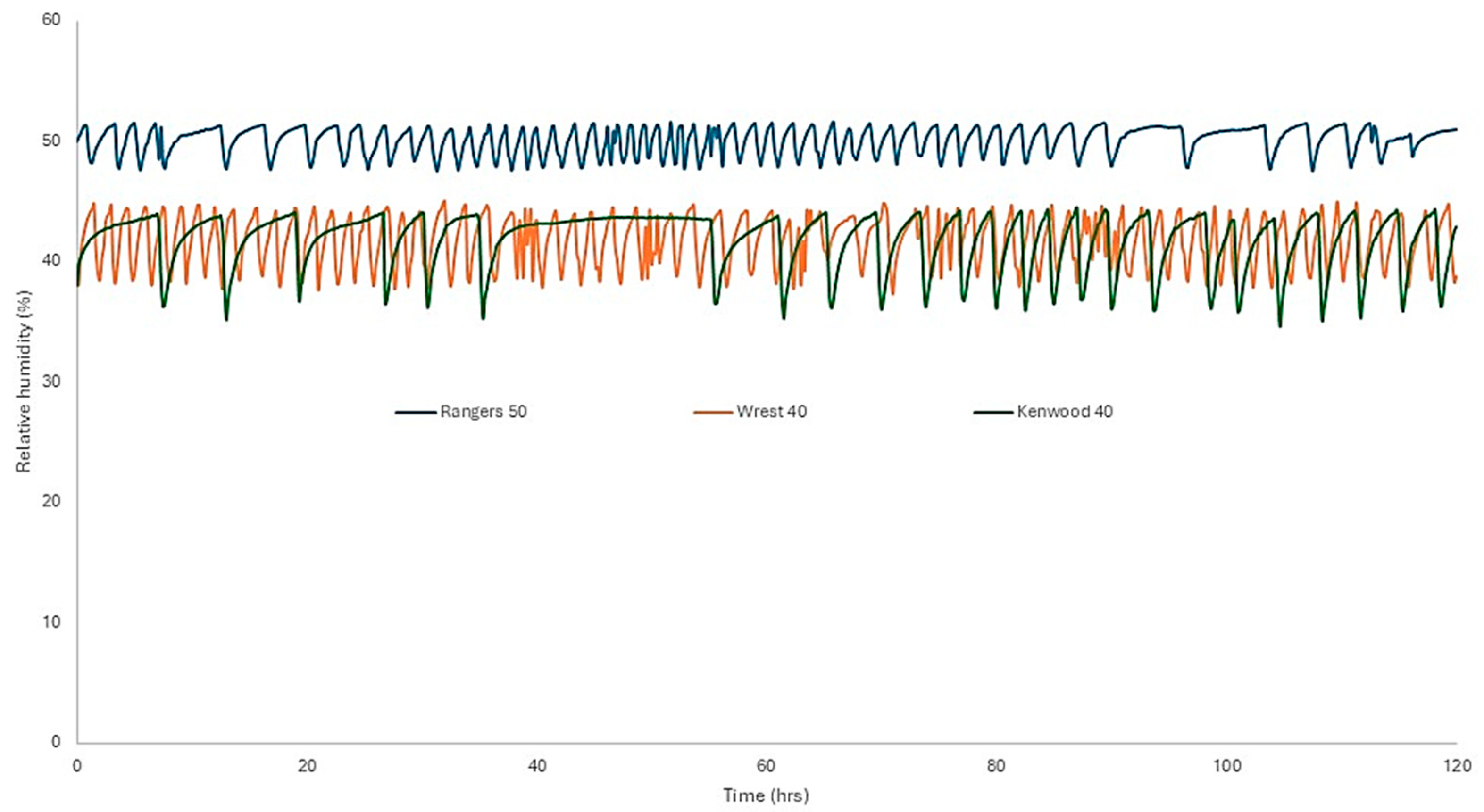
With the levels of air exchange in most of the store buildings, Munters dehumidifiers can, at best, maintain a 4% RH band. With the lowest air exchange rate, achieved by sealing the windows at Ranger’s House, a 2% band was achieved at 50% RH.
Figure 3 shows a week’s data for RH monitoring in selected rooms.
The Kenwood 40 trace shows much longer-term oscillations than the Rangers 50 or Wrest 40. This is probably due to the larger surface area of sorbent material present in the print store. This reduces the rate of RH increase after the dehumidifier has shut off. Tighter control could be achieved by sealing doors and windows (where present), as evidenced by the reduction in air exchange rates at both Dover Castle and Ranger’s House when windows were sealed. The stores all have conventional door wooden fittings.
Reducing the setpoint from 50% to 40% increased energy use by 54, 80, and 84% in the three storerooms where this was tested. The energy calculations were reasonably accurate—they were within 30% of the measured energy usage in all instances using tracer gas air exchange rates. The energy usage estimates using air exchange rates from pressurisation tests were significantly higher (as were the air exchange rate estimates). Improving the sealing of windows made the largest improvement in the air exchange rate. All the windows present were of the sash type; guidance has been published on reducing leakage from such windows [
42,
43]. The ASHRAE handbook chapter states that to achieve their AA rating (±5% RH fluctuations), moisture vapour barriers are required [
44]. None of the buildings tested had this feature.
3.4. Impact of Environmental Condition Tests
The calculated durability (NBO/T) for the glasses studied is shown in
Figure 4.
The glass compositions covered a wide range of durability, from just above 0.5 to slightly over 0.9. There was no systematic trend with the main network modifier.
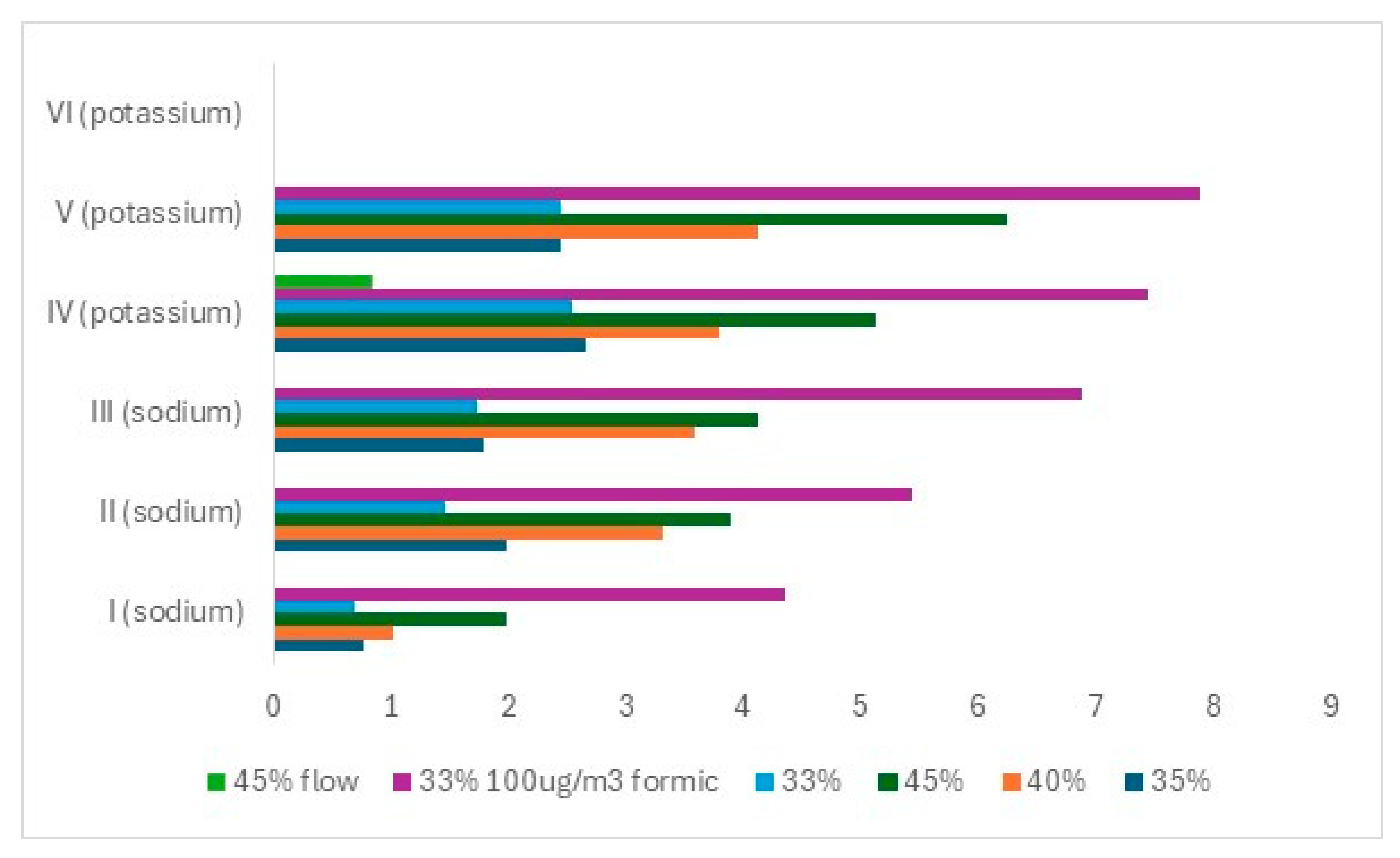
The ion concentrations on the glass surfaces after ageing are shown in
Figure 5.
All glasses showed the most deterioration at 45%, followed by 40%, then 35%, and finally, 33%. The sodium glass I appeared to be the most resistant, with II and III similar. The potassium-containing glasses were less stable. The effect of RH exposure does not follow the calculated durabilities. This highlights the possible limitations of the NBO/T equation to accurately evaluate the role of components like sodium and potassium or calcium and magnesium oxides, which are chemically equivalent but impart different degrees of durability to the glass. Furthermore, the high silica content of all tested samples meant that they had a good base level of stability, meaning that the differences in their preservation may need many decades of exposure to be better highlighted.
The presence of 80 µg/m3 of formic acid dramatically accelerated the deterioration rate of all the glasses. The flowing air reduced deterioration dramatically by over 80% for the single glass tested. The crystals on glass I in clean air at 33% were identified as sodium carbonate. Those formed when exposed to a formic acid environment were sodium formate.
Two images of the exposed glass are shown in
Figure 6 and
Figure 7. The images were taken with the red channel of the RGB colour space. This has been found to compare better to standards on glass surfaces [
45].
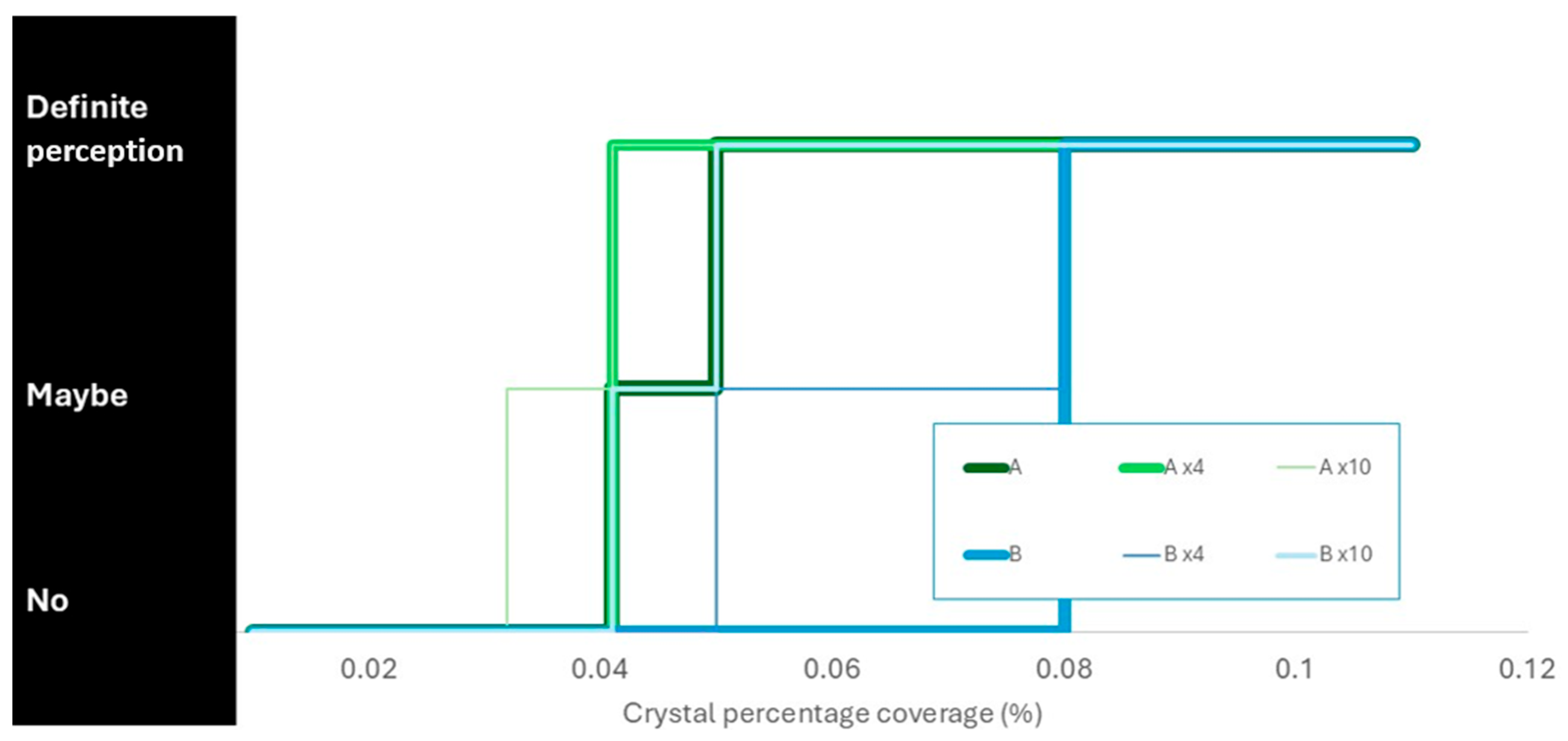
The two conservators’ perceptions of the glass showing signs of deterioration are summarised in
Figure 8.
Conservator A perceived crystals at lower percentage coverages than Conservator B in all instances. The magnification helped, but Conservator A only rated 0.03% coverage as maybe and did not perceive 0.01% coverage at all, even with ×10 magnification. The ion chromatography easily detected both these levels and had an estimated detection limit 10x smaller than the lowest level tested (giving a coverage of 0.01%).
4. Conclusions
Experiments and site measurements evaluated three active control systems and two passive approaches. Dehumidifiers controlled RH well in all instances tested, whilst Miniclima and RK2 units performed most of the time. The Peltier dehumidification in these units struggled with very high room RH and higher temperatures. The energy usage and embedded carbon are in the same order of magnitude for the three active systems. The tight RH band recommended for unstable glasses was about 3–4 times more carbon-intensive to maintain. Silica gel (Prosorb or probably Silicagel E) would need changing quite frequently and at different periods over the year. Saturated potassium carbonate worked well at around 43% RH, but this is higher than the recommended range. Both passive systems have much lower carbon footprints.
Formic acid was effectively cleaned with the continuous RK2 unit, but the dehumidifier, Miniclima, and potassium carbonate left potentially damaging concentrations in the showcase in some situations. The single potassium carbonate test would need to be repeated to confirm this result. Some materials will pass the BEMMA and ISO 16000-based emission analyses and potentially reach damaging concentrations in some showcase designs.
Using dehumidifiers in storerooms was found to work, but only very low air exchange rate rooms can maintain the tight RH range. Most can maintain RH below 50%. The windows, doors, and building fabric are important for air exchange. Measurements with tracer gas decay gave much better estimates of dehumidifier energy use than those from pressurisation (fan) tests.
Exposure tests with six glass compositions indicated that reducing RH below 40% will slow deterioration. These results indicate the weakness of the saturated potassium carbonate approach to control, as only 43% can be achieved. Formic acid was confirmed as a very aggressive pollutant. Flowing air was shown to reduce the deterioration rate at 33% RH.
The newly started GoGreen project, Green Strategies to Conserve the Past and Preserve the Future of Cultural Heritage, will investigate the wider aspects of sustainability and the conservation profession’s transition to green practices. The research will include analysis to characterise the stability of glass objects and tailor their environments and preventive conservation strategies.