The Use and Deterioration of Intumescent Fire-Retardant Paint on Louise Nevelson’s Erol Beker Chapel of the Good Shepherd
Abstract
1. Introduction
1.1. The Nevelson Chapel—Composition and Condition
1.2. Alkyd Resins, PVAc Paints, and Intumescent Fire-Resistant Coatings
2. Materials and Methods
3. Results
3.1. Optical Microscopy
3.2. SEM-EDX
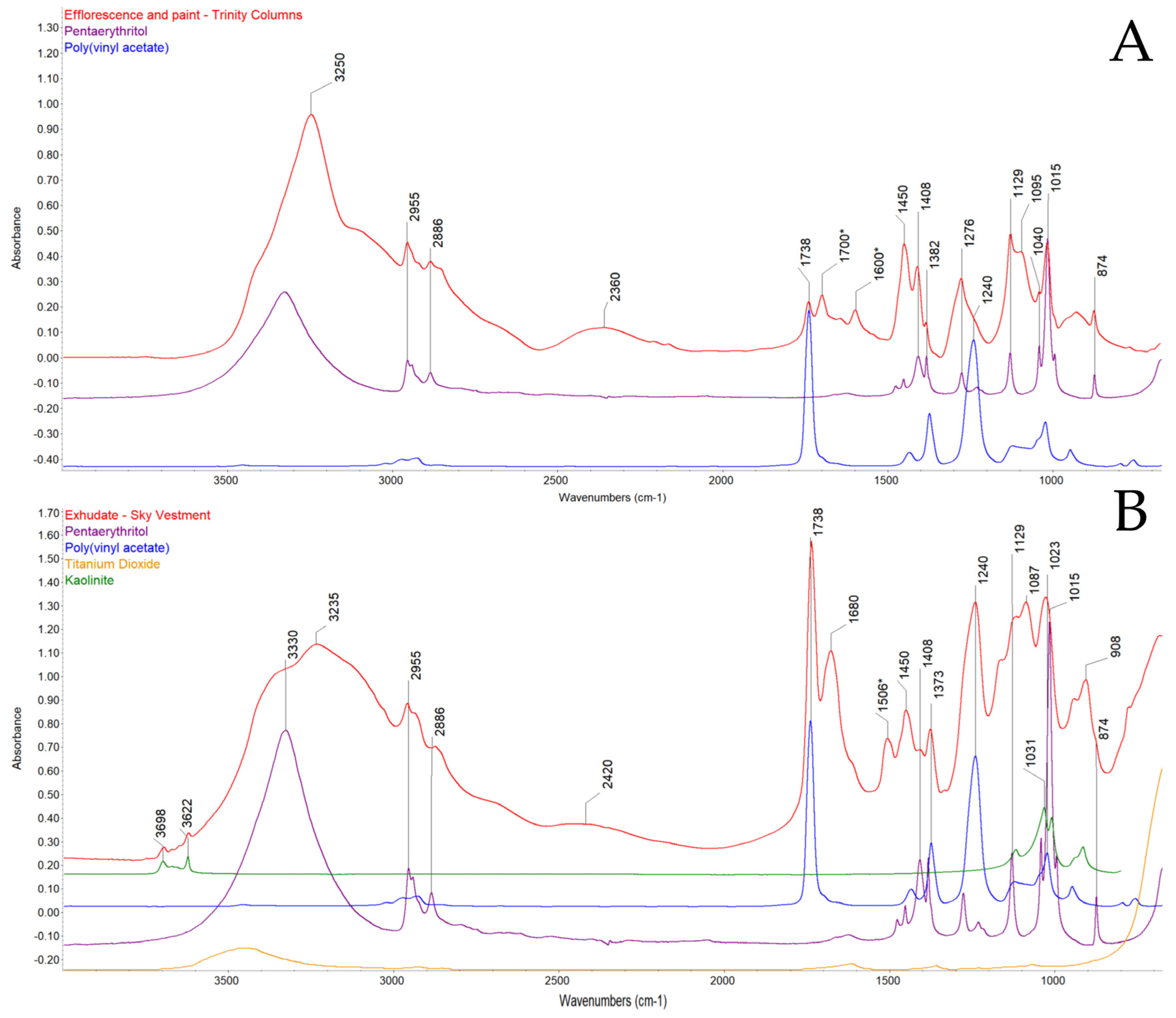
3.3. FTIR Spectroscopy
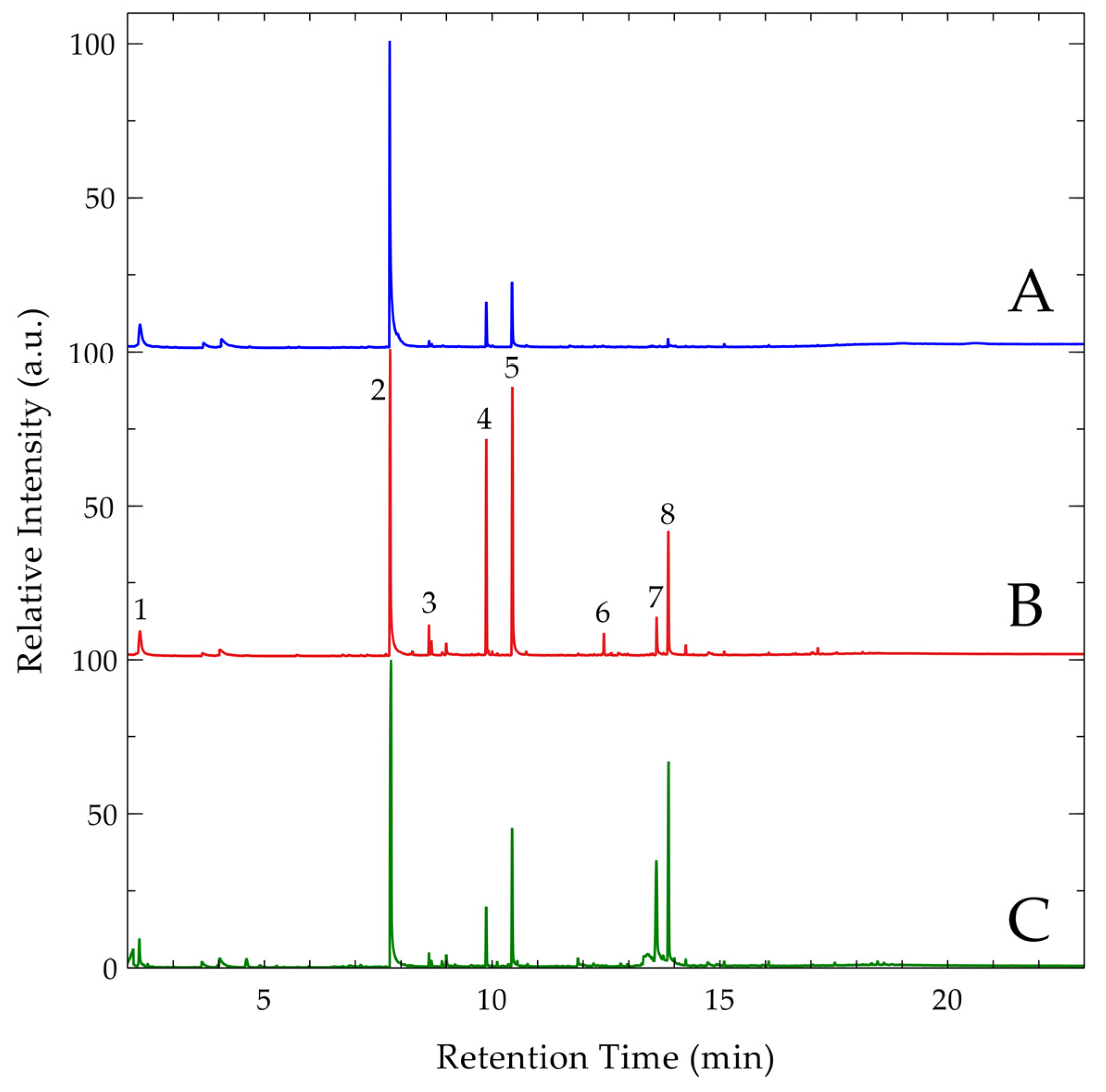
3.4. Pyrolysis GCMS
4. Discussion
4.1. IFR Coatings in the Works of Louise Nevelson
The soft and powdery characteristics of the paint, too, seem to be what [conservator Susan Schussler] reported also in 1983. (…) [t]he sculpture was originally painted with the fireretardant (sic) Everseal products (and deteriorated), and (…) as stated, she repainted portions with the same product and (…) in the early 1990’s it again was painted with the same or similar fire retardant paint (…).[33]
4.2. Environmental Degradation in IFR Coatings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVAc | Poly(vinyl acetate) |
| PER | Pentaerythritol |
| IFR | Intumescent, fire-retardant coating |
| FTIR | Fourier transform infrared spectroscopy |
| SEM-EDX | Scanning electron microscopy with energy-dispersive X-ray spectroscopy |
| GCMS | Gas chromatography/mass spectrometry |
| XRF | X-ray fluorescence spectroscopy |
| NMR | Nuclear magnetic resonance spectroscopy |
| HVAC | Heating, ventilation, and air conditioning |
| MPP | Melamine polyphosphate |
| MEL | Melamine |
| APP | Ammonium polyphosphate |
| DICY | Dicyandiamide |
| SEM-BSE | Scanning electron microscopy backscattered electron |
| SI | Supplementary information |
References
- Nunberg, S.; Alcala, S.; Tomkiewicz, C.; Kehlet, C.; McGlinchy, C.; Dittmer, J. Conservation of a White Louise Nevelson Installation: Gel Systems Explored. In Gels in the Conservation of Art; Angelova, L., Ormsby, B., Townsend, J., Wolbers, R., Eds.; Archetype: London, UK, 2017; pp. 299–305. [Google Scholar]
- Nunberg, S.; Kehlet, C.; Alcala, S.; Tomkiewicz, C.; McGlinchy, C.; Henry, M.C.; Dittmer, J. Conservation of a White Louise Nevelson Installation: Treatment Choices Based on Ethical Discussions and Analytical Studies. In Proceedings of the ICOM Committee for Conservation 18th Triennial Meeting, Copenhagen, Denmark, 4–8 September 2017; Pulido & Nunes; ICOM Committee for Conservation: Neuchâtel, Switzerland, 2017. [Google Scholar]
- Kehlet, C.; Nunberg, S.; Alcala, S.; Dittmer, J. Nuclear Magnetic Resonance Analysis for Treatment Decisions: The Case of a White Sculptural Environment by Louise Nevelson. Microchem. J. 2018, 137, 480–484. [Google Scholar] [CrossRef]
- Angelova, L.V.; Berrie, B.H.; de Ghetaldi, K.; Kerr, A.; Weiss, R.G. Partially Hydrolyzed Poly(Vinyl Acetate)-Borax-Based Gel-like Materials for Conservation of Art: Characterization and Applications. Stud. Conserv. 2014, 60, 227–244. [Google Scholar] [CrossRef]
- Angelova, L.V.; Terech, P.; Natali, I.; Dei, L.; Carretti, E.; Weiss, R.G. Cosolvent Gel-like Materials from Partially Hydrolyzed Poly(Vinyl Acetate)S and Borax. Langmuir 2011, 27, 11671–11682. [Google Scholar] [CrossRef]
- Learner, T. Modern Paints: Uncovering the Choices. In Modern Paints Uncovered: Proceedings from the Modern Paints Uncovered Symposium; Getty Conservation Institute: Los Angeles, CA, USA, 2007; pp. 3–16. [Google Scholar]
- Ploeger, R.; Scalarone, D.; Chiantore, O. The Characterization of Commercial Artists’ Alkyd Paints. J. Cult. Herit. 2008, 9, 412–419. [Google Scholar] [CrossRef]
- La Nasa, J.; Degano, I.; Modugno, F.; Colombini, M.P. Industrial Alkyd Resins: Characterization of Pentaerythritol and Phthalic Acid Esters Using Integrated Mass Spectrometry. Rapid Commun. Mass Spectrom. 2014, 29, 225–237. [Google Scholar] [CrossRef]
- Novak, M.; Ormsby, B. Poly(Vinyl Acetate) Paints: A Literature Review of Material Properties, Ageing Characteristics, and Conservation Challenges. Polymers 2023, 15, 4348. [Google Scholar] [CrossRef]
- Lake, S.; Ordonez, E.; Schilling, M. A Technical Investigation of Paints Used by Jackson Pollock in His Drip or Poured Paintings. Stud. Conserv. 2004, 49 (Suppl. S2), 137–141. [Google Scholar] [CrossRef]
- Dooley, K.A.; Coddington, J.; Krueger, J.; Conover, D.M.; Loew, M.; Delaney, J.K. Standoff Chemical Imaging Finds Evidence for Jackson Pollock’s Selective Use of Alkyd and Oil Binding Media in a Famous “Drip” Painting. Anal. Methods 2017, 9, 28–37. [Google Scholar] [CrossRef]
- Nurmi, K. Material Analysis and Conservation Treatment of Louise Nevelson’s Sculpture Dawn’s Image, Night. 2022. Available online: https://digitalcommons.buffalostate.edu/art_con_projects/22 (accessed on 23 September 2024).
- Mohd Sabee, M.M.S.; Itam, Z.; Beddu, S.; Zahari, N.M.; Mohd Kamal, N.L.; Mohamad, D.; Zulkepli, N.A.; Shafiq, M.D.; Abdul Hamid, Z.A. Flame Retardant Coatings: Additives, Binders, and Fillers. Polymers 2022, 14, 2911. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, G.; Su, Q. Influence of Hydrothermal Aging Process on Components and Properties of Waterborne Fire-Resistive Coatings. J. Coat. Technol. Res. 2014, 11, 207–216. [Google Scholar] [CrossRef]
- Hansen-Bruhn, I.; Poulsen, A.V.; Abildgaard, U.; Ravnsbæk, J.B.; Hinge, M. Effect of Titania, Barite, and Kaolinite Fillers on Char Layer Formation in Water-Based Intumescent Fire-Retardant Coatings. J. Coat. Technol. Res. 2022, 19, 1067–1075. [Google Scholar] [CrossRef]
- Mariappan, T. Recent Developments of Intumescent Fire Protection Coatings for Structural Steel: A Review. J. Fire Sci. 2016, 34, 120–163. [Google Scholar] [CrossRef]
- Vandersall, H.L. Intumescent Coating Systems, Their Development and Chemistry. J. Fire Flammabl. 1971, 2, 97–140. [Google Scholar]
- Soares, I.; Ferreira, J.L.; Silva, H.; Rodrigues, M.P. Fire-Retardant and Fire-Resistant Coatings: From Industry to the Potential Use on Cultural Heritage. J. Cult. Herit. 2024, 68, 316–327. [Google Scholar] [CrossRef]
- Challinor, J.M. A Pyrolysis-Derivatisation-Gas Chromatography Technique for the Structural Elucidation of Some Synthetic Polymers. J. Anal. Appl. Pyrolysis 1989, 16, 323–333. [Google Scholar] [CrossRef]
- Renuka Devi, K.B.; Santhi, K.B.; Mathivanan, R. Spectra and Normal Coordinate Analysis of Poly Vinyl Acetate. Optoelectron. Adv. Mater.-Rapid Commun. 2012, 6, 205–210. [Google Scholar]
- Ramamoorthy, P.; Krishnamurthy, N. Vibration Spectrum of Pentaerythritol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1997, 53, 655–663. [Google Scholar] [CrossRef]
- Müller, U.; Philipp, M.; Gaukler, J.C.; Possart, W.; Sanctuary, R.; Krüger, J.K. Dissolution, Transport and Reaction at a DICY/DGEBA Interface. J. Adhes. 2012, 88, 253–276. [Google Scholar] [CrossRef]
- Jeremy Jones, W.; Orville-Thomas, W.J. The Infra-Red Spectrum and Structure of Dicyandiamide. Trans. Faraday Soc. 1959, 55, 193. [Google Scholar] [CrossRef]
- Yang, S.; Chen, Z. The Study on Aging and Degradation Mechanism of Ammonium Polyphosphate in Artificial Accelerated Aging. Procedia Eng. 2018, 211, 906–910. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, W.; Duan, W.; Li, Y.; Wang, Q. In Situ Synthesized and Dispersed Melamine Polyphosphate Flame Retardant Epoxy Resin Composites. J. Appl. Polym. Sci. 2018, 136, 47194. [Google Scholar] [CrossRef]
- Zapata, J.C.; Syme, A.-M.; Rowell, K.; Burns, B.P.; Clark, E.S.; Gorman, M.N.; Jacob, L.; Kapodistrias, P.; Kedziora, D.J.; Lempriere, F.A.R.; et al. Computational Infrared Spectroscopy of 958 Phosphorus-Bearing Molecules. Front. Astron. Space Sci. 2021, 8, 639068. [Google Scholar] [CrossRef]
- Byrd, A.; Stockton, M.; Nunberg, S.; Dittmer, J.; Kehlet, C. Multi-Analytical Study of Synthetic Paint Binders Toward Conservation of a Sculptural Environment from the Seventies; Poster Presentation; Pratt Institute: Brooklyn, NY, USA, 2014. [Google Scholar]
- Soares, A.; Pestana, M. Polyterpene Resisns: Part I—A Brief Historical Review. Silva Lusit. 2020, 28, 181–195. [Google Scholar] [CrossRef]
- Learner, T. The Analysis of Synthetic Paints by Pyrolysis–Gas Chromatography–Mass Spectrometry (PyGCMS). Stud. Conserv. 2001, 46, 225–241. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Bitossi, G.; Osete-Cortina, L.; de la Cruz-Cañizares, J.; Yusá-Marco, D.J. Characterization of Polyvinyl Resins Used as Binding Media in Paintings by Pyrolysis–Silylation–Gas Chromatography–Mass Spectrometry. Anal. Bioanal. Chem. 2007, 391, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Weil, E.D. Fire-Protective and Flame-Retardant Coatings—A State-of-The-Art Review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Deogon, M.S. A Study of Intumescent Coatings. Ph.D. Thesis, Brunel University, Uxbridge, UK, 1989; p. 90. [Google Scholar]
- General Services Administration: Fine Arts Program. In Conservation Report: Louise Nevelson—Bicentennial Dawn, 1976; ID: AA123; 2007.
- General Services Administration: Public Building Service; Schussler, S. In Final Conservation Record: Louise Nevelson—Bicentennial Dawn, 1976; 1983.
- Häßler, D.; Mund, M.; Daus, L.-H.; Hothan, S.; Schaumann, P.; Schartel, B. Durability of Intumescent Coatings and Recommendations for Test Concepts for a Working Life of More than 10 Years. Fire Saf. J. 2024, 146, 104173. [Google Scholar] [CrossRef]
- Jimenez, M.; Bellayer, S.; Revel, B.; Duquesne, S.; Bourbigot, S. Omprehensive Study of the Influence of Different Aging Scenarios on the Fire Protective Behavior of an Epoxy Based Intumescent Coating. Ind. Eng. Chem. Res. 2013, 52, 729–743. [Google Scholar] [CrossRef]
- Jimenez, M.; Bellayer, S.; Naik, A.; Bachelet, P.; Duquesne, S.; Bourbigot, S. Topcoats versus Durability of an Intumescent Coating. Ind. Eng. Chem. Res. 2016, 55, 9625–9632. [Google Scholar] [CrossRef]
- Daus, L.-H.; Schartel, B.; Wachtendorf, V.; Mangelsdorf, R.; Korzen, M. A Chain Is No Stronger than Its Weakest Link: Weathering Resistance of Water-Based Intumescent Coatings for Steel Applications. J. Fire Sci. 2020, 39, 72–102. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J. Influences of Glass Flakes on Fire Protection and Water Resistance of Waterborne Intumescent Fire Resistive Coating for Steel Structure. Prog. Org. Coat. 2011, 70, 150–156. [Google Scholar] [CrossRef]
- Anees, S.M.; Dasari, A. A Review on the Environmental Durability of Intumescent Coatings for Steels. J. Mater. Sci. 2017, 53, 124–145. [Google Scholar] [CrossRef]
- Vahabi, H.; Sonnier, R.; Ferry, L. Effects of Ageing on the Fire Behaviour of Flame-Retarded Polymers: A Review. Polym. Int. 2015, 64, 313–328. [Google Scholar] [CrossRef]
- Bruckner, T.M.; Singewald, T.D.; Gruber, R.; Hader-Kregl, L.; Klotz, M.; Müller, M.; Luckeneder, G.; Rosner, M.; Kern, C.; Hafner, M.; et al. Water Absorption and Leaching of a 1K Structural Model Epoxy Adhesive for the Automotive Industry. Polym. Test. 2022, 117, 107870. [Google Scholar] [CrossRef]
- Yang, X.F.; Tallman, D.E.; Bierwagen, G.P.; Croll, S.G.; Rohlik, S. Blistering and Degradation of Polyurethane Coatings under Different Accelerated Weathering Tests. Polym. Degrad. Stab. 2002, 77, 103–109. [Google Scholar] [CrossRef]
- Angelova, L.V.; Ormsby, B.; Richardson, E. Diffusion of Water from a Range of Conservation Treatment Gels into Paint Films Studied by Unilateral NMR. Microchem. J. 2016, 124, 311–320. [Google Scholar] [CrossRef]


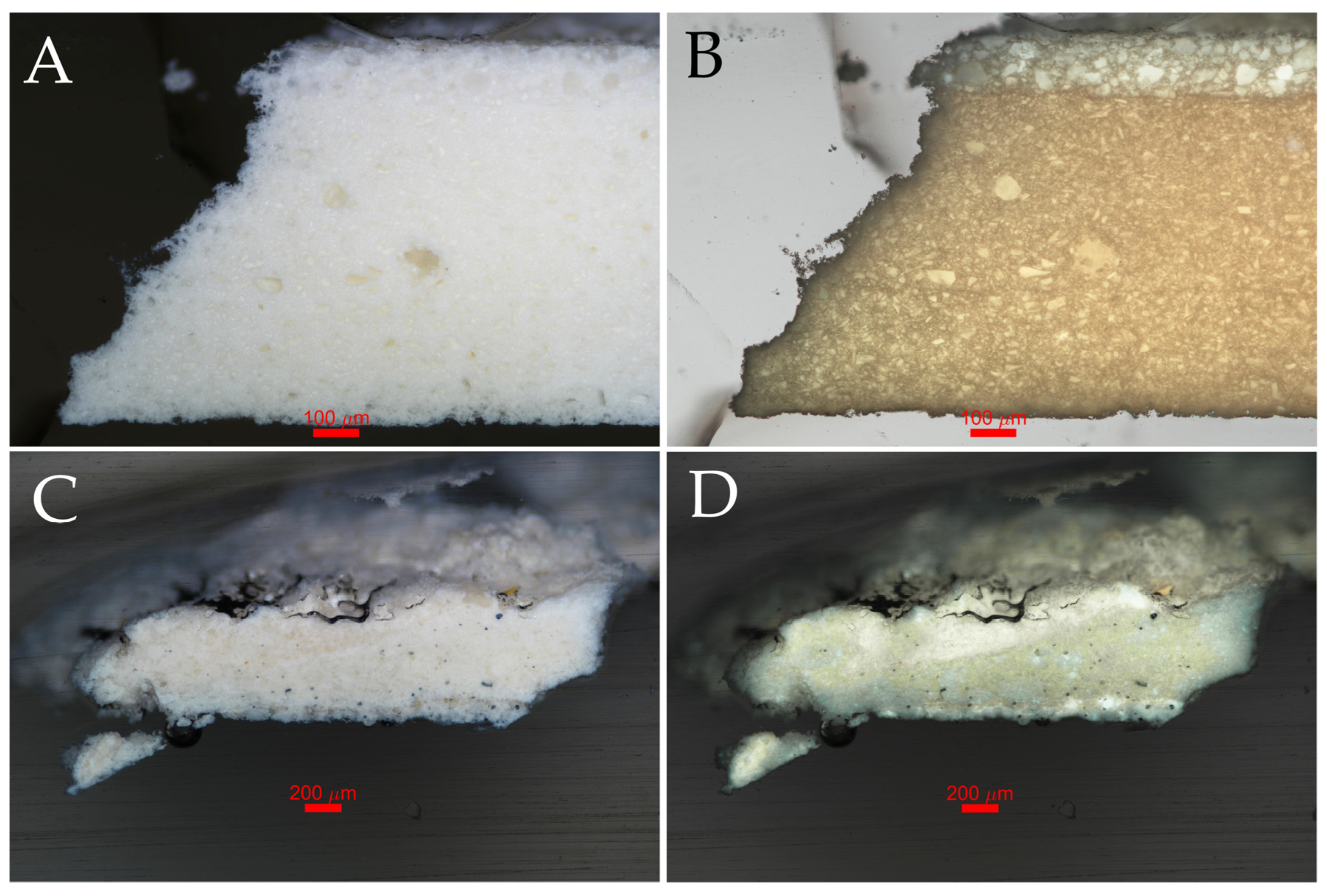

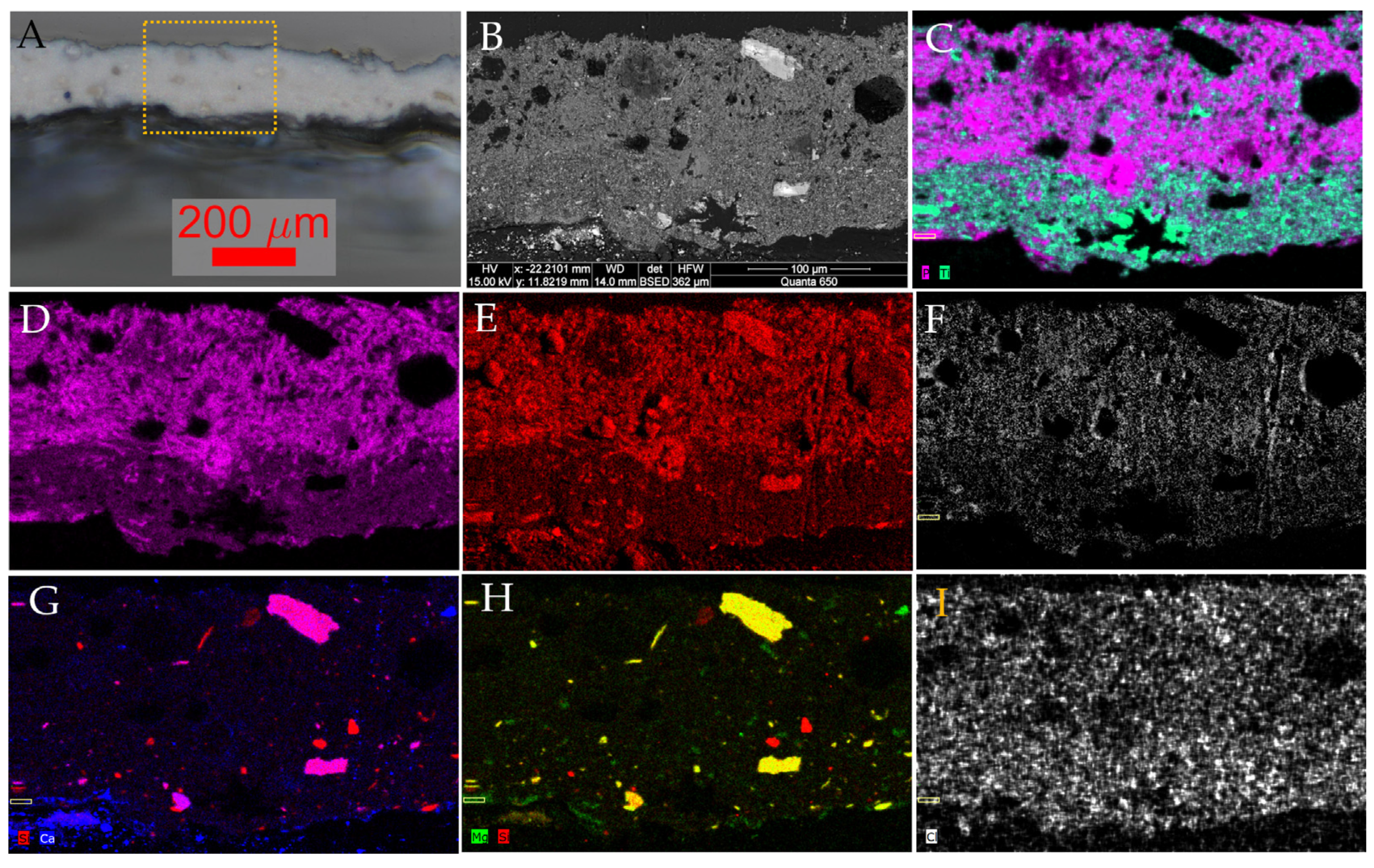
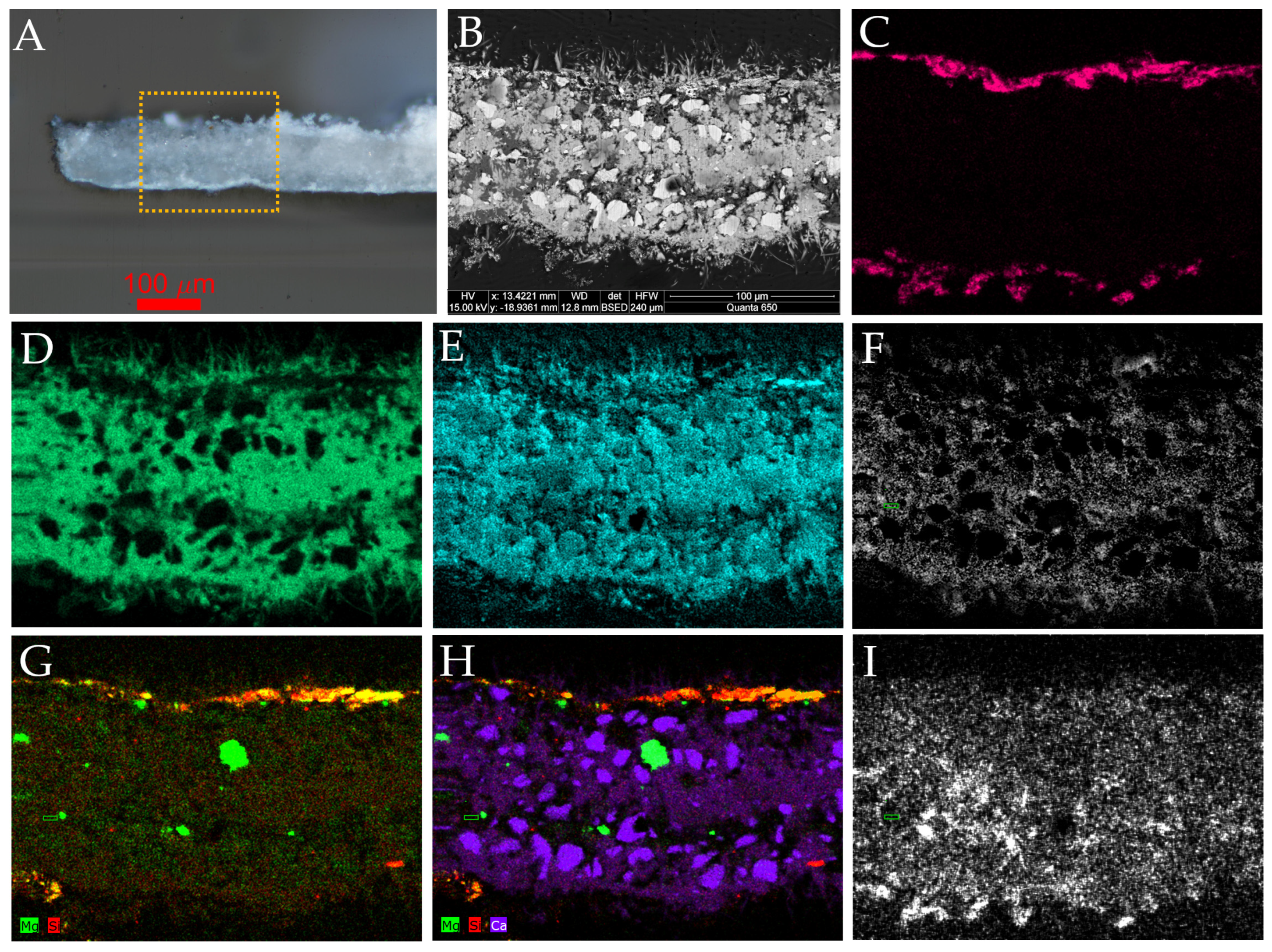
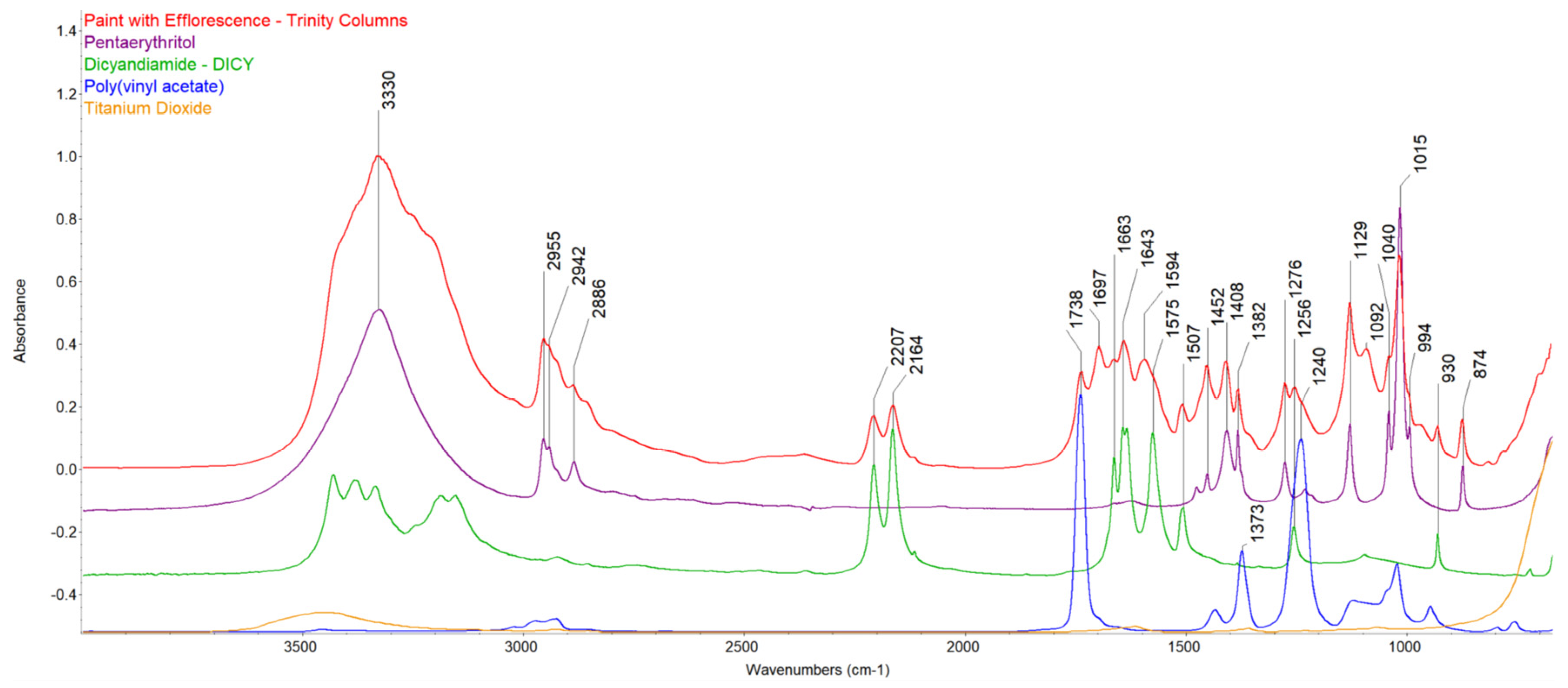
| Sculpture | Description of Samples Analysed | Methods of Analysis |
|---|---|---|
| Sky Vestment | Restoration paint, soft exudate, original, and overpaints | FTIR, SEM-EDX, Py-GCMS, optical microscopy |
| Cross of the Good Shepherd | Powdery exudate, blister of underbound powdery overpaint, intact overpaint | FTIR, Py-GCMS, optical microscopy |
| Frieze of the Apostles | Blister of overpaint, stained area of paint and wood, intact overpaint | FTIR, optical microscopy |
| Trinity Columns | Paint with staining, powdery efflorescence, intact overpaint | FTIR, optical microscopy |
| Grapes and Wheat Lintel | Surface paint, efflorescence | FTIR, SEM-EDX, Py-GCMS, optical microscopy |
| Cross of the Resurrection | Powdery efflorescence | FTIR |
| Sculpture | Components |
|---|---|
| Sky Vestment-Trinity | Paint: poly(vinyl acetate), pentaerythritol, titanium dioxide, polyphosphate salt 1, kaolinite clay |
| Cross of the Good Shepherd | Paint: poly(vinyl acetate), pentaerythritol, dicyandiamide, polyphosphate salt |
| Wood: cellulose, synthetic wood glue (a formaldehyde-based resin), an epoxy curing agent with similar signals to dicyandiamide | |
| Frieze of the Apostles | Paint: poly(vinyl acetate), pentaerythritol, titanium dioxide, polyphosphate salt |
| Wood: cellulose, natural resin varnish (shellac), wood glue (formaldehyde-based resin), an epoxy curing agent with similar signals to dicyandiamide | |
| Trinity Columns | Poly(vinyl acetate), pentaerythritol, polyphosphate salt, dicyandiamide, natural resin (terpenoid fraction) |
| Cross of the Resurrection | Poly(vinyl acetate), pentaerythritol, dolomite (calcium magnesium carbonate), pyrophyllite (aluminum silicate commonly associated with quartz or kaolinite clay), unidentified melamine salt 2, unidentified inorganic filler 3 |
| Grapes and Wheat Lintel | Poly(vinyl acetate), pentaerythritol, titanium dioxide, dicyandiamide, polyphosphate salt, calcium carbonate (chalk), unidentified melamine salt, unidentified inorganic filler |
| Wood: cellulose, natural resin varnish (shellac), synthetic wood glue (formaldehyde-based resin), an epoxy curing agent with similar signals to dicyandiamide. |
| Peak | Retention Time (min) | Compound |
|---|---|---|
| 1 (SV) 1 | 2.26 | Benzene |
| 1 (GWL 2, CGS 3) | 2.27 | N,N,N′-trimethyl-1,2-ethanediamine |
| 2 | 7.76 | Trimethylphosphate |
| 3 | 8.615 | 1-ethenyl-2-methylbenzene |
| 4 | 9.875 | Pentaerythrotetramethyl ether |
| 5 | 10.445 | 3-methoxy-2,2-bis(methoxymethyl)-1-propanol |
| 6 | 12.455 | 1,2,3,4,5,6-hexa-o-methyl-myo-inositol |
| 7 (CGS) | 13.61 | N,N,N′,N′-tetramethyl-1,3,5-triazine-2,4,6-triamine |
| 7 (SV) | 13.61 | 2-N-diethylmelamine |
| 8 (GWL, CGS) | 13.865 | Altretamine |
| 8 (SV) | 13.88 | Pentamethylmelamine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelova, L.V.; Shugar, A.; Maines, C.A.; Tanimoto, S.; Singer, M.; Dommermuth, J.; Duggan, H.; Duncan, T.T.; Finnefrock, A.C.; Mass, J.L. The Use and Deterioration of Intumescent Fire-Retardant Paint on Louise Nevelson’s Erol Beker Chapel of the Good Shepherd. Heritage 2025, 8, 128. https://doi.org/10.3390/heritage8040128
Angelova LV, Shugar A, Maines CA, Tanimoto S, Singer M, Dommermuth J, Duggan H, Duncan TT, Finnefrock AC, Mass JL. The Use and Deterioration of Intumescent Fire-Retardant Paint on Louise Nevelson’s Erol Beker Chapel of the Good Shepherd. Heritage. 2025; 8(4):128. https://doi.org/10.3390/heritage8040128
Chicago/Turabian StyleAngelova, Lora V., Aaron Shugar, Christopher A. Maines, Satoko Tanimoto, Martha Singer, Jean Dommermuth, Hannah Duggan, Teresa T. Duncan, Adam C. Finnefrock, and Jennifer L. Mass. 2025. "The Use and Deterioration of Intumescent Fire-Retardant Paint on Louise Nevelson’s Erol Beker Chapel of the Good Shepherd" Heritage 8, no. 4: 128. https://doi.org/10.3390/heritage8040128
APA StyleAngelova, L. V., Shugar, A., Maines, C. A., Tanimoto, S., Singer, M., Dommermuth, J., Duggan, H., Duncan, T. T., Finnefrock, A. C., & Mass, J. L. (2025). The Use and Deterioration of Intumescent Fire-Retardant Paint on Louise Nevelson’s Erol Beker Chapel of the Good Shepherd. Heritage, 8(4), 128. https://doi.org/10.3390/heritage8040128






