Open-Access Infrared Spectra Depository for the Damage Assessment of Parchment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parchment Samples
2.2. Instrumentation
3. Results and Discussion
3.1. FTIR of New Parchments
3.2. FTIR of Accelerated Aged Parchments
3.2.1. Visible Light Exposure
3.2.2. Humid Heat Exposure
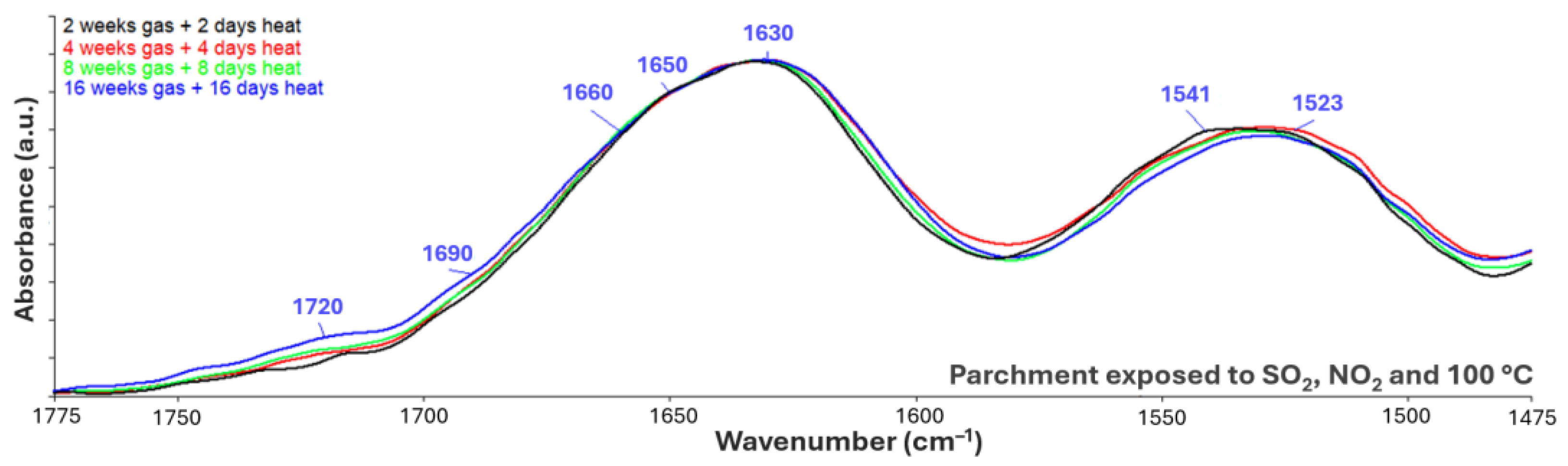
3.2.3. Exposure to Inorganic Gases, and Combinations of Light and Heat Ageing
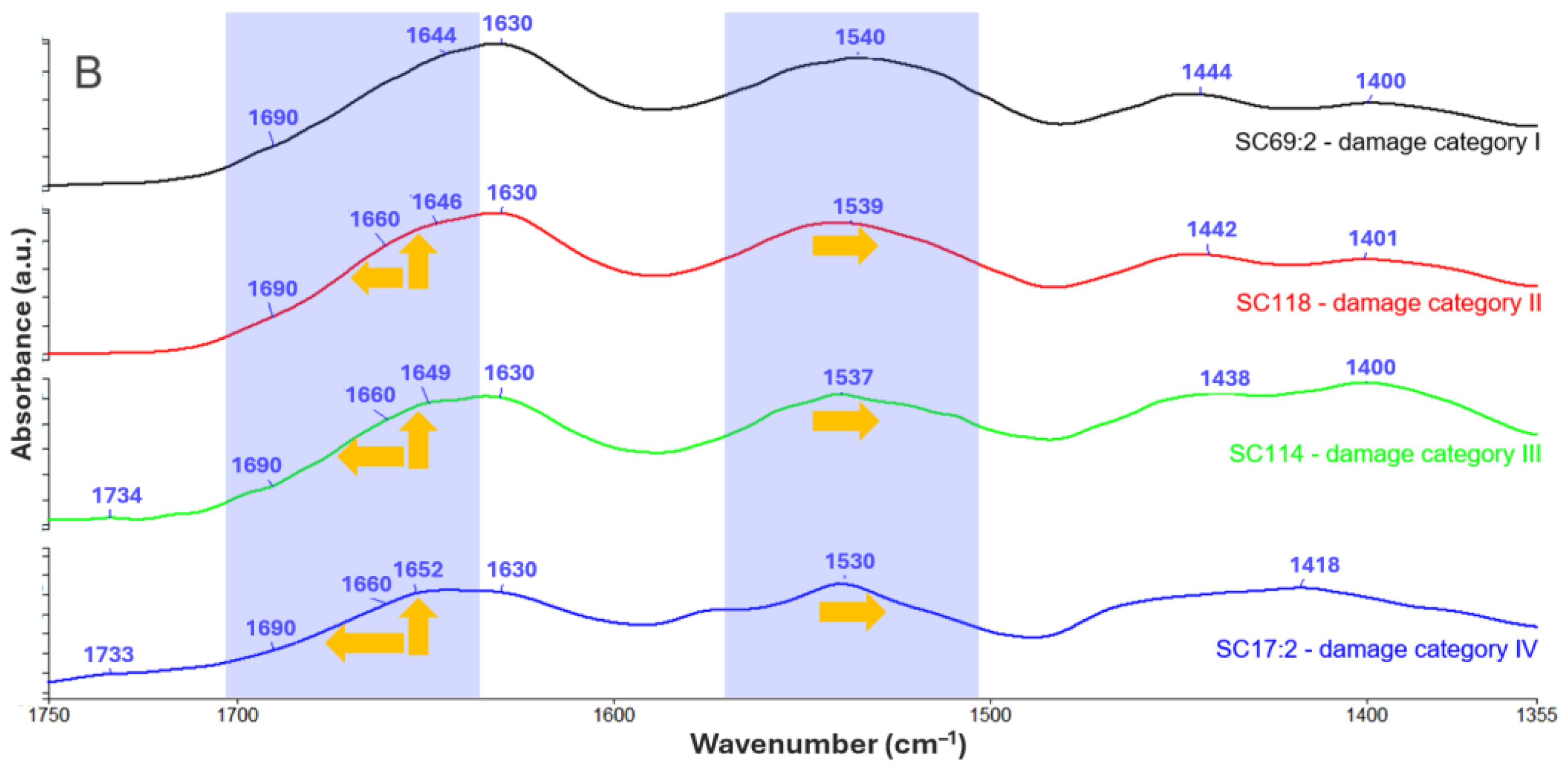
3.3. Historical Parchments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meir, B.I. Parchment. 1995. Available online: https://web.archive.org/web/20050422080330/http://faculty.biu.ac.il/~barilm/parchmen.html (accessed on 12 March 2025).
- Reed, R. The Making and Nature of Parchment; Elmete Press: Leeds, UK, 1975. [Google Scholar]
- Larsen, R.; Poulsen, D.V.; Juchauld, F.; Jerosch, H.; Odlyha, M.; de Groot, J.; Wang, Q.; Theodorakopoulos, C.; Wess, T.; Hiller, J.; et al. Damage assessment of parchment: Complexity and relations at different structural levels. 14th ICOM-CC Prepr. 2005, 1, 199–208. Available online: https://www.researchgate.net/profile/Elena-Badea/publication/230766446_Damage_assessment_of_parchment_complexity_and_relations_at_different_structural_levels/links/0deec5214c8a389d6a000000/Damage-assessment-of-parchment-complexity-and-relations-at-different-structural-levels.pdf (accessed on 12 March 2025).
- Odlyha, M.; Theodorakopoulos, C.; de Groot, J.; Bozec, L.; Horton, M. Thermoanalytical (macro to nano-scale) techniques and non-invasive spectroscopic analysis for damage assessment of parchment. In Improved Damage Assessment of Parchment (IDAP), Assessment, Data Collection and Sharing of Knowledge European Commission, Research Report no 18; Larsen, R., Ed.; Office for Official Publications of the European Communities: Luxembourg, 2007; pp. 73–85. Available online: https://www.researchgate.net/profile/Charis-Theodorakopoulos/publication/288527466_Thermoanalytical_macro_to_nano-scale_techniques_and_non-invasive_spectroscopic_analysis_for_damage_assessment_of_parchment/links/642307d9a1b72772e4319058/Thermoanalytical-macro-to-nano-scale-techniques-and-non-invasive-spectroscopic-analysis-for-damage-assessment-of-parchment.pdf (accessed on 12 March 2025).
- Thickett, D.; Emmerson, N.; Larsen, R.; Odlyha, M.; Watkinson, D. Analysing Objects to Tailor Environmental Preventive Conservation. Heritage 2022, 6, 212–235. [Google Scholar] [CrossRef]
- Kennedy, C.J.; Wess, T.J. The Structure of Collagen within Parchment—A review. Restaurator 2003, 24, 61–80. [Google Scholar] [CrossRef]
- Bechmann Hansen, D.; Nielsen, K.; Rasmussen, S.B. Detection of Radicals in Collagen and Parchment. In Microanalysis of Parchment; Archetype Publications Ltd.: London, UK, 2002; pp. 117–121. [Google Scholar]
- Larsen, R.; Poulsen, D.V.; Vest, M.; Jensen, A. Amino Acid Analysis of New and Historical Parchment. In Microanalysis of Parchment; Archetype Publications Ltd.: London, UK, 2002; pp. 93–98. Available online: https://adk.elsevierpure.com/da/publications/amino-acid-analysis-of-new-and-historical-parchments (accessed on 12 March 2025).
- Sakura, S.; Fujimoto, D.; Sakamoto, K.; Mizuno, A.; Motegi, K. Photolysis of Pyridinoline, a Cross-linking Amino Acid of Collagen, by ultraviolet light. Can. J. Biochem. 1982, 60, 525–529. [Google Scholar] [CrossRef]
- Hansen, E.F.; Lee, S.N.; Sobel, H. The Effects of Relative Humidity on Some Physical Properties of Modern Vellum. J. Am. Inst. Conserv. 1992, 31, 325–342. [Google Scholar] [CrossRef]
- Miles, C.A.; Burjanadze, T.V.; Bailey, J.A. The Kinetics of the Thermal Denaturation of Collagen. J. Mol. Biol. 1995, 245, 437–446. [Google Scholar] [CrossRef]
- Wright, N.T.; Humphrey, J.D. Denaturation of Collagen by Heating. Annu. Rev. Biomed. Eng. 2002, 4, 109–128. [Google Scholar] [CrossRef]
- Larsen, R. (Ed.) Introduction to damage and damage assessment of parchment. In Improved Damage Assessment of Parchment (IDAP) Assessment, Data Collection and Sharing of Knowledge; European Commission, Research Report no. 18; Office for Official Publications of the European Communities: Luxembourg, 2007; pp. 17–21. Available online: https://op.europa.eu/en/publication-detail/-/publication/0edae146-fe1f-4060-a0b1-b506b19de9c8 (accessed on 12 March 2025).
- de Groot, J. Damage Assessment of Collagen in Historical Parchment with Microscopy Techniques. Ph.D. Thesis, Birkbeck College, University of London, London, UK, 2007. [Google Scholar]
- Garp, T.; Nielsen, K.; Boghosian, S. Study of the chemical breakdown of collagen and parchment by Raman spectroscopy. In Microanalysis of Parchment; Larsen, R., Ed.; Archetype Publications Ltd.: London, UK, 2002; pp. 109–116. [Google Scholar]
- Odlyha, M.; Theodorakopoulos, C.; de Groot, J.; Bozec, L.; Horton, M. Fourier Transform Infra-Red Spectroscopy (ATR/FTIR) and Scanning Probe Microscopy of parchment. ePS 2009, 6, 138–144. Available online: https://www.researchgate.net/profile/Laurent-Bozec/publication/49584102_Fourier_transform_infra-red_spectroscopy_ATRFTIR_and_scanning_probe_microscopy_of_parchment/links/00b7d52d685c56f8eb000000/Fourier-transform-infra-red-spectroscopy-ATR-FTIR-and-scanning-probe-microscopy-of-parchment.pdf (accessed on 12 March 2025).
- Boyatzis, S.C.; Velivasaki, G.; Malea, E. A study of the deterioration of aged parchment marked with laboratory iron gall inks using FTIR-ATR spectroscopy and micro hot table. Herit. Sci. 2016, 4, 13. [Google Scholar] [CrossRef]
- Malea, E.; Boyatzis, S.C.; Karlis, D.; Palles, D.; Boghosian, S.; Zervos, S. The Complementary Use of Raman, ATR-FTIR Spectroscopy, and Chemometrics for Investigating the Deterioration of Artificially Aged Parchment. J. Raman Spectrosc. 2024, 55, 1266–1280. [Google Scholar] [CrossRef]
- Malissa, A.; Cappa, F.; Schreiner, M.; Marchetti-Deschmann, M. Spectral Features Differentiate Aging- Induced Changes in Parchment—A Combined Approach of UV/VIS, μ-ATR/FTIR and μ-Raman Spectroscopy With Multivariate Data Analysis. Molecules 2023, 28, 4584. [Google Scholar] [CrossRef]
- Cappa, F.; Paganoni, I.; Carsote, C.; Schreiner, M.; Badea, E. Studies on the effect of dry-heat ageing on parchment deterioration by vibrational spectroscopy and micro hot table method. Polym. Degrad. Stab. 2020, 182, 109375. [Google Scholar] [CrossRef]
- Cappa, F.; Paganoni, I.; Carsote, C.; Badea, E.; Schreiner, M. Studies on the effects of mixed light-thermal ageing on parchment by vibrational spectroscopy and micro hot table method. Herit. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Infrared Spectra Database—Depository of FTIR Spectra of IDAP Parchments. 2023. Available online: https://figshare.northumbria.ac.uk/collections/Infrared_spectra_database_-_Depository_of_FTIR_spectra_of_IDAP_parchments/6742791 (accessed on 12 March 2025).
- New Parchments FTIR: Measured Prior to Deterioration. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/New_Parchments_FTIR_measured_prior_to_deterioration/23695608/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: Light Aged 170 klux ×4–32 Hours. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_Light_Aged_170_klux_x_4-32_hours/23696043/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: 40%RH—80 °C ×2–16 Days and Visible Light 170 klux ×4–32 Hours. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_40_RH_-_80_C_x2-16_days_and_Visible_Light_170_klux_x_4-32_hours/23696277/1 (accessed on 12 March 2025).
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Accelerated Aged Parchment FTIR: 80%RH and 60 °C ×1–32 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_80_RH_and_60_C_x1-32_days/23704272/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: 60%RH and 80 °C ×1–32 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_60_RH_and_80_C_x1-32_days/23704401/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: 80%RH and 80 °C ×1–32 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_80_RH_and_80_C_x1-32_days/23704410/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: 60%RH and 60 °C ×1–32 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_60_RH_and_60_C_x1-32_days/23704194/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: 60%RH and 40 °C ×1–16 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_60_RH_and_40_C_x1-16_days/23696535/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: 80%RH and 40 °C ×1–32 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_80_RH_and_40_C_x1-32_days/23703882/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: 40%RH and 80 °C ×1–32 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_40_RH_and_80_C_x1-32_days/23704341/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: 40%RH and 80 °C ×2–16 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_40_RH_and_80_C_x2-16_days/23695842/1 (accessed on 12 March 2025).
- Paschalis, E.P.; Verdelis, K.; Doty, S.B.; Boskey, A.L.; Mendelsohn, R.; Yamauchi, M. Spectroscopic characterization of collagen crosslinks in bone. J. Bone Miner. Res. 2001, 16, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Badea, E.; Della Gatta, G.; Usacheva, T. Effects of temperature and relative humidity on fibrillar collagen in parchment: A micro differential scanning calorimetry (micro DSC) study. Polym. Degrad. Stab. 2012, 97, 346–353. [Google Scholar] [CrossRef]
- Accelerated Aged Parchment FTIR: NO2 Gas 50 ppm ×2–16 Weeks. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_NO2_sub_sub_gas_50ppm_x2-16_weeks/23685183/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: NO2 Gas 50 ppm ×2–16 Weeks & Light: 170 klux ×4–32 Hours. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_NO2_gas_50ppm_x2-16_weeks_light_170klux_x4-32_hours/23685522/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: NO2 Gas: 50 ppm ×2–16 Weeks & Heat: 100 °C ×2–16 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_NO2_gas_50ppm_x2-16_weeks_heat_100_C_x2-16_days/23686110/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: NO2 Gas ×2–16 Weeks, Light:170 klux ×4–32 Hours, Heat: 100 °C ×2–16 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_NO2_gas_x2-16_weeks_light_170klux_x4-32_hours_heat_100_strong_strong_C_x2-16_days/23686680/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: SO2 Gas 50 ppm ×2–16 Weeks. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_SO2_gas_50ppm_x2-16_weeks/23688753/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: SO2 Gas 50 ppm ×2–16 Weeks & Light: 170 klux ×4–32 Hours. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_SO2_gas_50ppm_x2-16_weeks_light_170klux_x4-32_hours/23691924/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: SO2 Gas: 50 ppm ×2–16 Weeks & Heat: 100 °C ×2–16 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_SO2_gas_50ppm_x2-16_weeks_heat_100_C_x2-16_days/23691945/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: SO2 Gas ×2–16 Weeks, Light:170 klux ×4–32 Hours, Heat: 100 °C ×2–16 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_SO2_gas_x2-16_weeks_light_170klux_x4-32_hours_heat_100_strong_strong_C_x2-16_days/23691951/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: SO2 and NO2 Gas 50 ppm ×2–16 Weeks. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_SO2_NO2_gas_50ppm_x2-16_weeks/23691984/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: SO2 and NO2 Gas 50 ppm ×2–16 Weeks & Light: 170 klux ×2–16 Hours. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_SO2_and_NO2_gas_50ppm_x2-16_weeks_light_170klux_x2-16_hours/23691996/1 (accessed on 12 March 2025).
- Accelerated Aged Parchment FTIR: SO2 and NO2 Gas: 50 ppm ×2–16 Weeks & Heat: 100 °C ×2–16 Days. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/Accelerated_Aged_Parchment_FTIR_SO2_NO2_gas_50ppm_x2-16_weeks_heat_100_C_x2-16_days/23691999/1 (accessed on 12 March 2025).
- ATR/FTIR Spectra of Historical Parchments—Damage Category I. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/ATR_FTIR_spectra_of_historical_parchments_-_damage_category_I/23904270/1 (accessed on 12 March 2025).
- ATR/FTIR Spectra of Historical Parchments—Damage Category II. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/ATR_FTIR_spectra_of_historical_parchments_-_damage_category_II/23904528/1 (accessed on 12 March 2025).
- ATR/FTIR Spectra of Historical Parchments—Damage Category III. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/ATR_FTIR_spectra_of_historical_parchments_-_damage_category_III/23904534/1 (accessed on 12 March 2025).
- ATR/FTIR Spectra of Historical Parchments—Damage Category IV. 2023. Available online: https://figshare.northumbria.ac.uk/articles/figure/ATR_FTIR_spectra_of_historical_parchments_-_damage_category_IV/23904567/1 (accessed on 12 March 2025).
- Galatis, P.; Boyatzis, S.; Theodorakopoulos, C. Removal of a synthetic soiling mixture on mastic, shellac & Laropal® K80 coatings using two hydrogels. ePS 2012, 9, 72–83. Available online: https://d1wqtxts1xzle7.cloudfront.net/31876413/Galatis-22-07-2012-libre.pdf?1391584876=&response-content-disposition=inline%3B+filename%3DAn_Investigation_of_the_Selective_Remova.pdf&Expires=1737632375&Signature=V5I4ox0iKkbrzISxH-8aIt2S5Wip1Ku9C5Y7k6-2gf6LUz7xHcIZ3D0EJtoEPWdSBjN9pJA4Nup2H1UoD7~rsVGDS0672Vuoie4EEXq~D1ttYskmGQudIG12GusTXXTTNrMljWuw6IEKe~5WEijbaMSwMgAcf-pMUhSfnlWK0T7O1TZiHK6NE8enpY~~E7TLsUzk-vQMKcDPH0kKv~PBHA22fx-cW5W1i4k-vCbln3pFe0eRt~nCYQip6zFuhv0sDJC2MjQTS4uNfSnWy-JUBkt1MjIZOpMbLMUCIQyFt0v0B-HoiRHAkVDX-LN4at9ANALEoWYEtqMp5a1mLSnS2g__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 12 March 2025).
- Chillè, C.; Papadakis, V.M.; Theodorakopoulos, C. An analytical evaluation of Er:YAG laser cleaning tests on a nineteenth century varnished painting. Microchem. J. 2020, 158, 105086. [Google Scholar] [CrossRef]
- Chillè, C.; Sala, F.; Wu, Q.; Theodorakopoulos, C. A study on the heat distribution and oxidative modification of aged dammar films upon Er:YAG laser irradiation. J. Inst. Conserv. 2020, 43, 59–78. [Google Scholar] [CrossRef]
- Odlyha, M.; Theodorakopoulos, C.; Campana, R. Studies on Woolen Threads from Historical Tapestries. AUTEX Res. J. 2007, 7, 9–18. [Google Scholar] [CrossRef]
- Theodorakopoulos, C.; Zafiropulos, V.; Boon, J.J.; Boyatzis, S.C. Spectroscopic Investigations on the Depth-Dependent Degradation Gradients of Aged Triterpenoid Varnishes. Appl. Spectrosc. 2007, 61, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Theodorakopoulos, C.; Risdonne, V.; Freese, S.; Diraoui, S.; Jonas, U. Cleaning testing of nineteenth-century plaster surface models with thin polyacrylamide-based gel layers attached to flexible polyethylene films. Herit. Sci. 2023, 11, 95. [Google Scholar] [CrossRef]
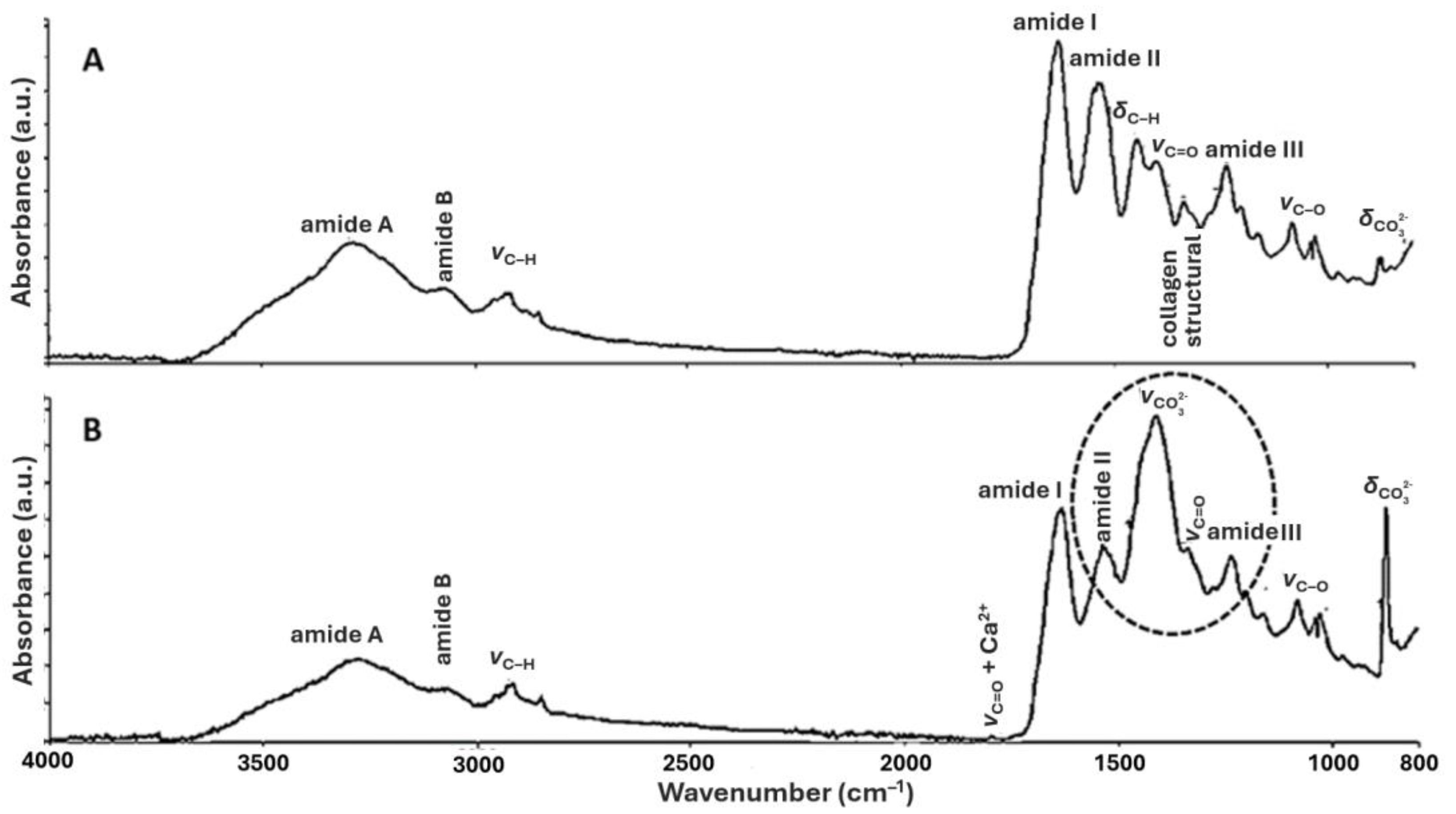
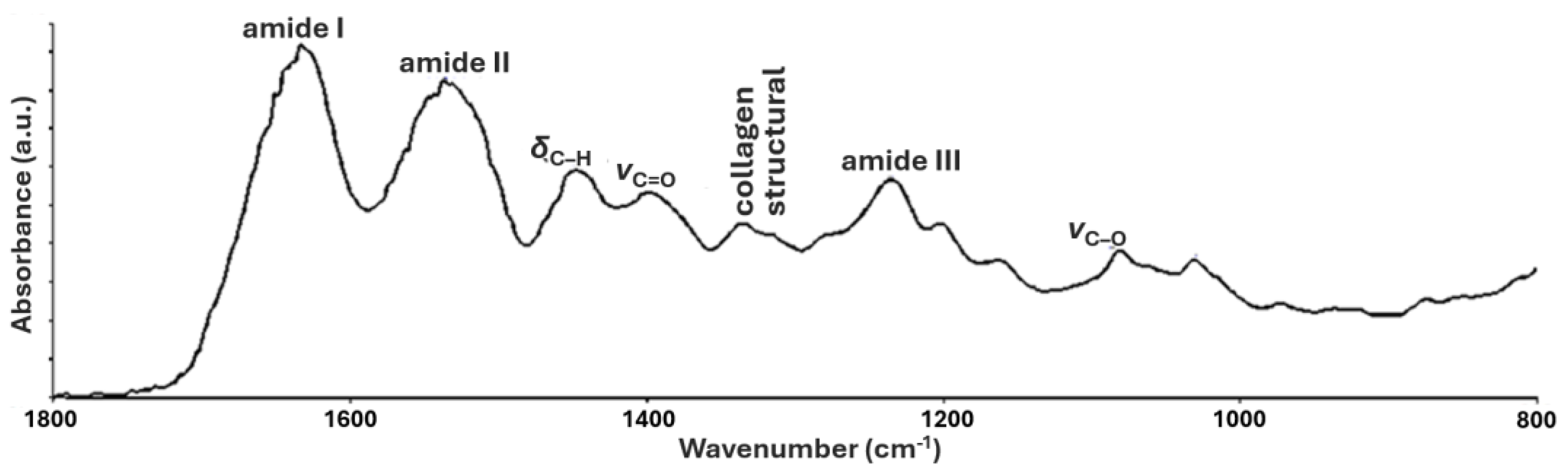
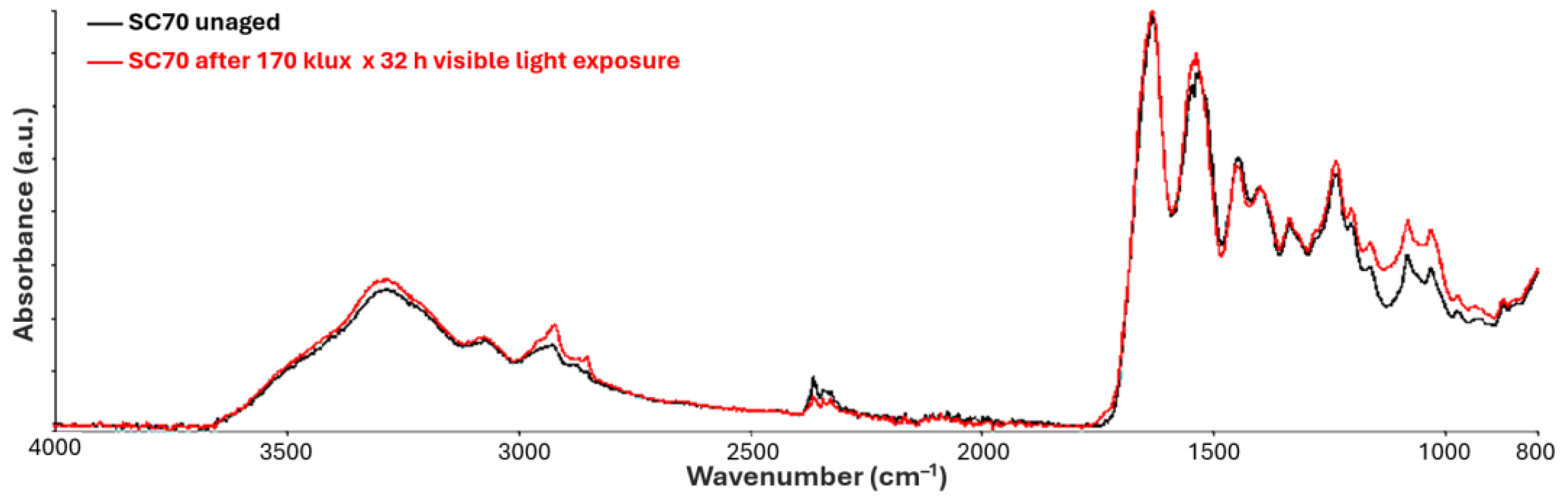

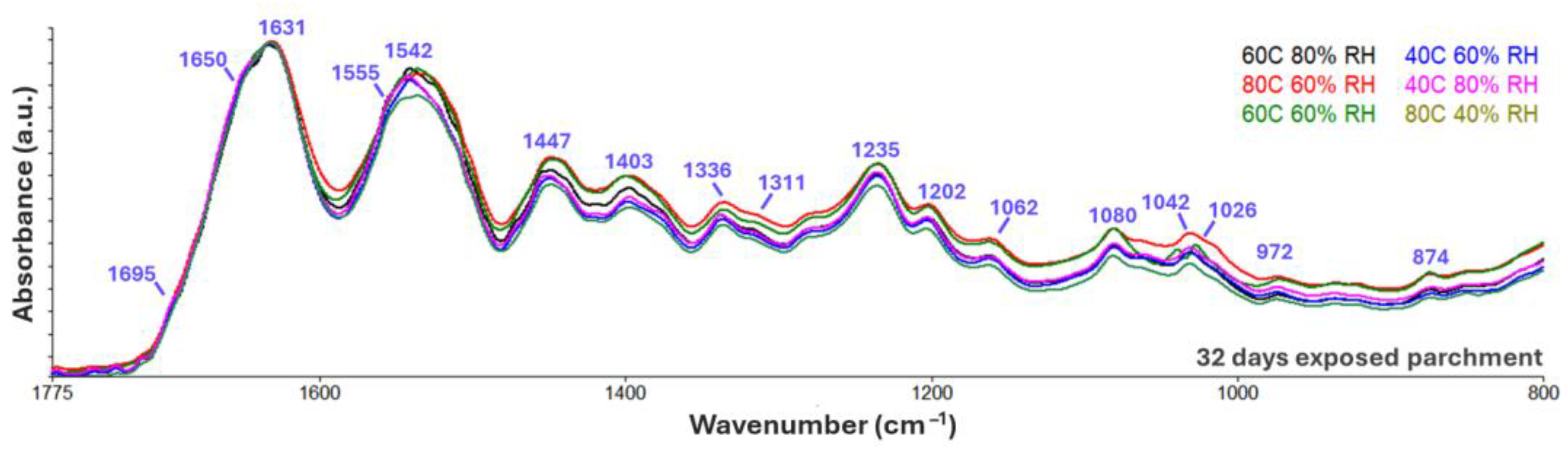

| Frequencies (cm−1) | Assignments |
|---|---|
| 3283 | Amide A: >95% ν 1(NH) in Fermi resonance with first overtone of amide II/overlaps with ν(OH) and νas(OH) of the water matrix in collagen |
| 3073 | Amide B: ν(NH) in Fermi resonance with amide II overtone |
| 2948, 2878 | νas(CH3) and ν(CH3), respectively |
| 2928, 2865 | νas(CH2) and ν(CH2), respectively |
| 1795 | overtone of ν(CO32−) and Ca2+ of CaCO3 |
| 1720 | ν(C=O) in –COOH |
| 1630 | Amide I: 80% ν(C=O) and 20% [ν(CN), δ(NH)] |
| 1580 | νas(C=O) in –COO− in aspartic and glutamic acids |
| 1536 | Amide II: 40–60% δ(NH), and ν(CN) |
| 1451 | δ(CH2) and δ(CH3)—overlaps with the νas(CO32−) band |
| 1447 | νas(CO32−) in CaCO3 |
| 1401 | ν(C=O) in –COO—overlaps with the νas(CO32−) band |
| 1338 | Sidechain vibration of collagen molecules |
| 1286, 1202 | Amide III with δ(CH2) from glycine backbone and proline sidechain |
| 1236 | Amide III: 30% ν(CN), 30% δ(NH), ~20% ν(C–C), ~10% δ(CH) |
| 1163 | ν(C–O) in carbohydrates |
| 1084, 1031 | ν(C–O) in carbohydrates and proteoglycans |
| 1062 | ν(C–O) in carbohydrates and ν(SO3−) of sulphated glycosaminoglycans |
| 874 | δ(C–O) in calcite |
| Treatment | Duration (Days) | Sample Code | Database Reference | |
|---|---|---|---|---|
| Temperature | RH (%) | |||
| 60 | 80 | 1 | SC90 | [27] |
| 2 | SC91 | |||
| 4 | SC92 | |||
| 8 | SC93 | |||
| 16 | SC94 | |||
| 32 | SC95 | |||
| 80 | 60 | 1 | SC96 | [28] |
| 2 | CS97 | |||
| 4 | SC98 | |||
| 8 | SC99 | |||
| 16 | SC100 | |||
| 32 | SC101 | |||
| 80 | 80 | 1 | SC102 | [29] |
| 2 | SC103 | |||
| 4 | SC104 | |||
| 8 | SC105 | |||
| 16 | SC106 | |||
| 32 | SC107 | |||
| 60 | 60 | 1 | SC108 | [30] |
| 2 | SC109 | |||
| 4 | SC110 | |||
| 8 | SC111 | |||
| 16 | SC112 | |||
| 32 | SC113 | |||
| 40 | 60 | 1 | SC132 | [31] |
| 2 | SC133 | |||
| 4 | SC134 | |||
| 8 | SC135 | |||
| 16 | SC136 | |||
| 32 | SC137 | |||
| 40 | 80 | 1 | SC138 | [32] |
| 2 | SC139 | |||
| 4 | SC140 | |||
| 8 | SC141 | |||
| 16 | SC142 | |||
| 32 | SC143 | |||
| 80 | 40 | 1 | SC150 | [33] |
| 2 | SC151 | |||
| 4 | SC152 | |||
| 8 | SC153 | |||
| 16 | SC154 | |||
| 32 | SC155 | |||
| Treatment | Sample Code | Database Reference | |||||
|---|---|---|---|---|---|---|---|
| Gas (50 ppm) | Exposure to Gas (Weeks) | Heat (100 °C) | Exposure to Heat (Days) | Visible Light (170 klux) | Exposure to Light (Hours) | ||
| NO2 | 2 | n/a | – | n/a | – | CR14 | [37] |
| 4 | – | – | CR15 | ||||
| 8 | – | – | CR16 | ||||
| 16 | – | – | CR17 | ||||
| 2 | n/a | – | yes | 4 | CR18 | [38] | |
| 4 | – | 8 | CR19 | ||||
| 8 | – | 16 | CR20 | ||||
| 16 | – | 32 | CR21 | ||||
| 2 | yes | 2 | n/a | – | CR22 | [39] | |
| 4 | 4 | – | CR23 | ||||
| 8 | 8 | – | CR24 | ||||
| 16 | 16 | – | CR25 | ||||
| 2 | yes | 2 | yes | 4 | CR26 | [40] | |
| 4 | 4 | 8 | CR27 | ||||
| 8 | 8 | 16 | CR28 | ||||
| 16 | 16 | 32 | CR29 | ||||
| SO2 | 2 | n/a | – | n/a | – | CR30 | [41] |
| 4 | – | – | CR31 | ||||
| 8 | – | – | CR32 | ||||
| 16 | – | – | CR33 | ||||
| 2 | n/a | – | yes | 4 | CR34 | [42] | |
| 4 | – | 8 | CR35 | ||||
| 8 | – | 16 | CR36 | ||||
| 16 | – | 32 | CR37 | ||||
| 2 | yes | 2 | n/a | – | CR38 | [43] | |
| 4 | 4 | – | CR39 | ||||
| 8 | 8 | – | CR40 | ||||
| 16 | 16 | – | CR41 | ||||
| 2 | yes | 2 | yes | 4 | CR42 | [44] | |
| 4 | 4 | 8 | CR43 | ||||
| 8 | 8 | 16 | CR44 | ||||
| 16 | 16 | 32 | CR45 | ||||
| NO2 + SO2 | 2 | n/a | – | n/a | – | CR46 | [45] |
| 4 | – | – | CR47 | ||||
| 8 | – | – | CR48 | ||||
| 16 | – | – | CR49 | ||||
| 2 | n/a | – | yes | 4 | CR50 | [46] | |
| 4 | – | 8 | CR51 | ||||
| 8 | – | 16 | CR52 | ||||
| 16 | – | 32 | CR53 | ||||
| 2 | yes | 2 | n/a | – | CR54 | [47] | |
| 4 | 4 | – | CR55 | ||||
| 8 | 8 | – | CR56 | ||||
| 16 | 16 | – | CR57 | ||||
| FTIR Damage Category | Sample Code | Type | Date | Animal | Origin | Database Reference |
|---|---|---|---|---|---|---|
| Damage Category I | SC59:2 | singe sheet | 1985 | unknown | RLC 1 | [48] |
| SC69:2 | single sheet | 2002 | calf | SCC | ||
| SC70:2 | single sheet | 2002 | calf | |||
| SC76:1 | single sheet | unknown | unknown | |||
| SC115 | single sheet | 1769 | unknown | NAS | ||
| SC117 | single sheet | 1827 | unknown | |||
| SC123 | single sheet | 1765 | unknown | |||
| SC163 | unknown | Unknown | unknown | ASF | ||
| SC165 | unknown | Unknown | unknown | |||
| Damage Category II | SC16 | bookbinding | unknown | unknown | SCC | [49] |
| SC17:1 | unknown | unknown | unknown | |||
| SC31:1 | unknown | unknown | unknown | |||
| SC32 | unknown | unknown | unknown | |||
| SC35 | bookbinding | unknown | calf | |||
| SC38:2 | unknown | unknown | Unknown | |||
| SC58:1 | unknown | unknown | Unknown | |||
| SC58:2 | unknown | unknown | unknown | |||
| SC59:1 | singe sheet | 1985 | unknown | |||
| SC70:1 | single sheet | 2002 | calf | |||
| SC76:1 | unknown | unknown | unknown | |||
| SC77:1 | unknown | unknown | unknown | |||
| SC116 | unknown | unknown | unknown | NAS | ||
| SC118 | single sheet | 1824 | unknown | |||
| SC119 | single sheet | 1832 | unknown | |||
| SC164 | unknown | unknown | unknown | ASF | ||
| SC165:1 | unknown | unknown | unknown | |||
| SC166 | unknown | unknown | unknown | |||
| SC168 | unknown | unknown | unknown | |||
| SC173:2 | unknown | unknown | unknown | |||
| SC175:1 | unknown | unknown | unknown | |||
| Damage Category III | SC18 | bookbinding | unknown | sheep | SCC | [50] |
| SC24 | unknown | unknown | unknown | |||
| SC38:1 | unknown | unknown | unknown | |||
| SC56:1 | unknown | unknown | unknown | |||
| SC72:1 | unknown | unknown | unknown | |||
| SC72:2 | unknown | unknown | unknown | |||
| SC73:2 | bookbinding | unknown | unknown | |||
| SC75:1 | unknown | unknown | unknown | |||
| SC114 | single sheet | 1765 | unknown | NAS | ||
| SC124 | unknown | unknown | unknown | |||
| SC125 | unknown | unknown | unknown | |||
| Damage Category IV | SC17:2 | unknown | unknown | unknown | SCC | [51] |
| SC75:2 | unknown | unknown | unknown | |||
| SC120 | unknown | unknown | unknown | NAS | ||
| SC122 | bookbinding | unknown | unknown | |||
| SC169 | unknown | unknown | unknown | ASF | ||
| SC172 | unknown | unknown | unknown | |||
| SC173:1 | unknown | unknown | unknown | |||
| SC175:2 | unknown | unknown | unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodorakopoulos, C.; Odlyha, M. Open-Access Infrared Spectra Depository for the Damage Assessment of Parchment. Heritage 2025, 8, 110. https://doi.org/10.3390/heritage8030110
Theodorakopoulos C, Odlyha M. Open-Access Infrared Spectra Depository for the Damage Assessment of Parchment. Heritage. 2025; 8(3):110. https://doi.org/10.3390/heritage8030110
Chicago/Turabian StyleTheodorakopoulos, Charis, and Marianne Odlyha. 2025. "Open-Access Infrared Spectra Depository for the Damage Assessment of Parchment" Heritage 8, no. 3: 110. https://doi.org/10.3390/heritage8030110
APA StyleTheodorakopoulos, C., & Odlyha, M. (2025). Open-Access Infrared Spectra Depository for the Damage Assessment of Parchment. Heritage, 8(3), 110. https://doi.org/10.3390/heritage8030110





