Integrating Material Analysis, Radiocarbon Dating, and Technical Examination in the Dating and Provenance Study of a Copy of Raphael’s “The Great Holy Family of Francis I”
Abstract
1. Introduction
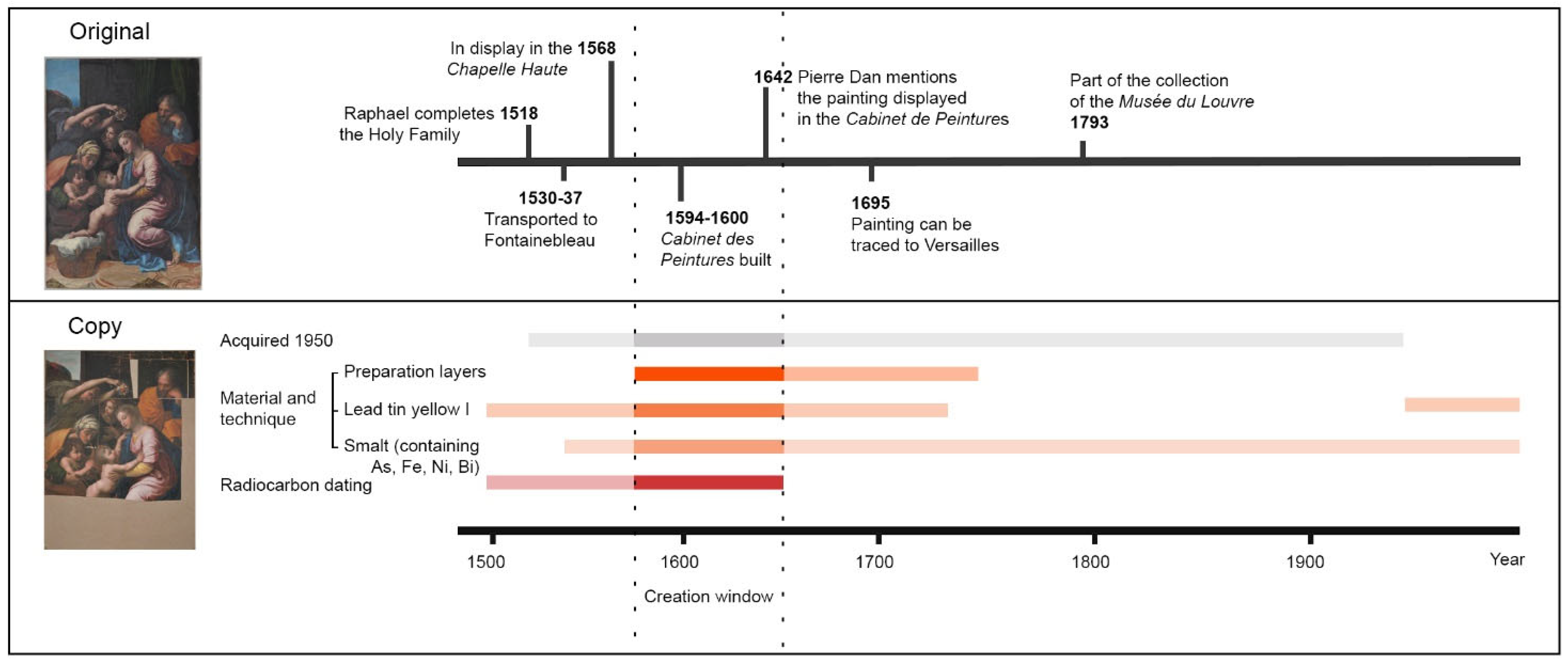
1.1. The Original Raphael “The Great Holy Family of Francis I”
“In the near foreground Mary, wearing a red dress and a blue mantle, sits on an undefined support on a marble ground. (…) she is slightly leaning forward and with both hands holds the naked Christ Child (…). On the left St. Elizabeth (…) clasps the young St. John who holds the reed cross between his arms and turns to the Child in adoration. Above this group an angel is leaning forward with outstretched arms to scatter flowers over the Virgin. (…) Behind Mary to the right stands Joseph, pensively resting his head on a praying desk draped with part of his mantle.”[1] (p. 170)
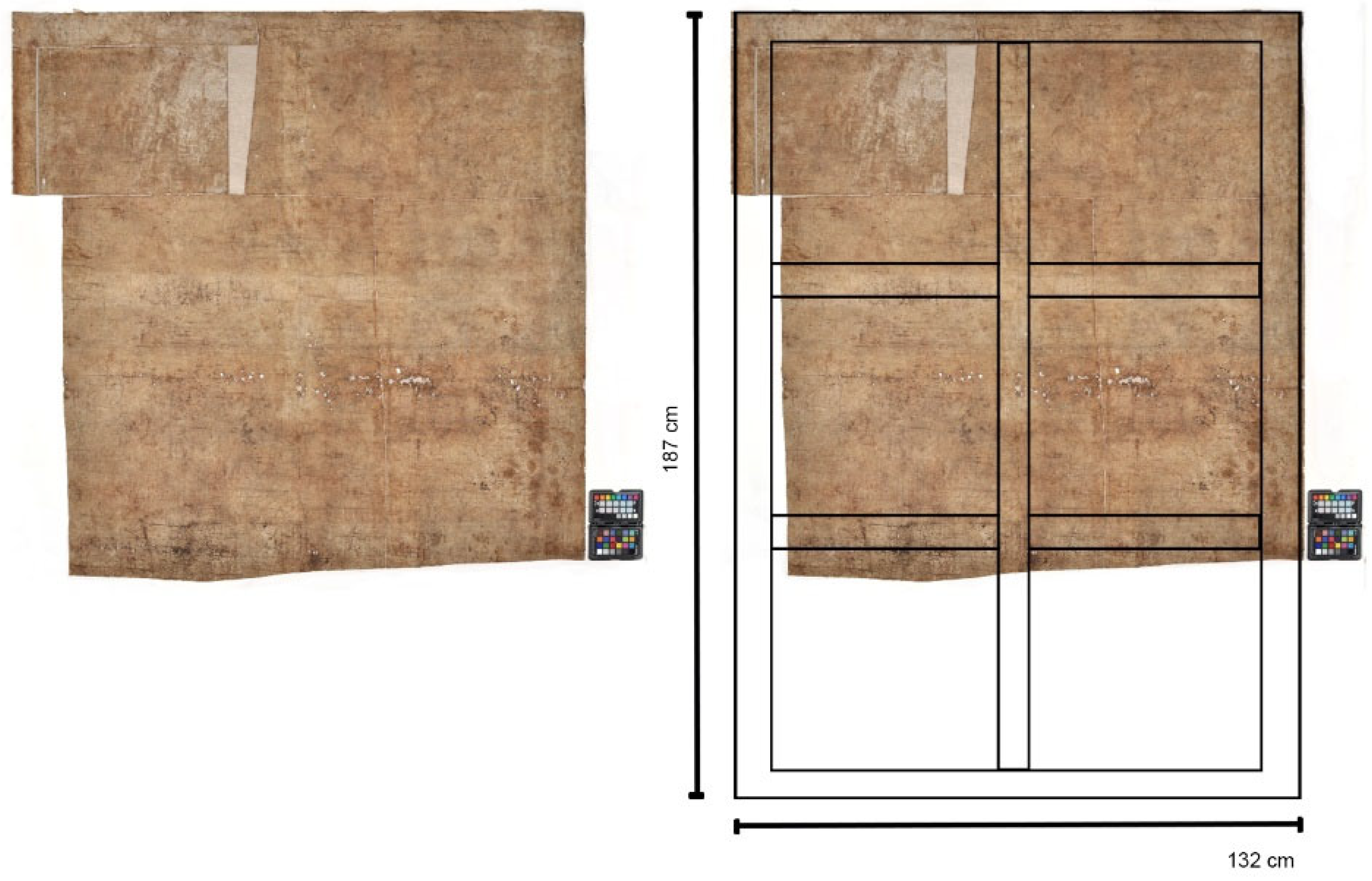
1.2. The Copy at CICS
1.3. Strategy for Provenance and Dating Studies
2. Materials and Methods
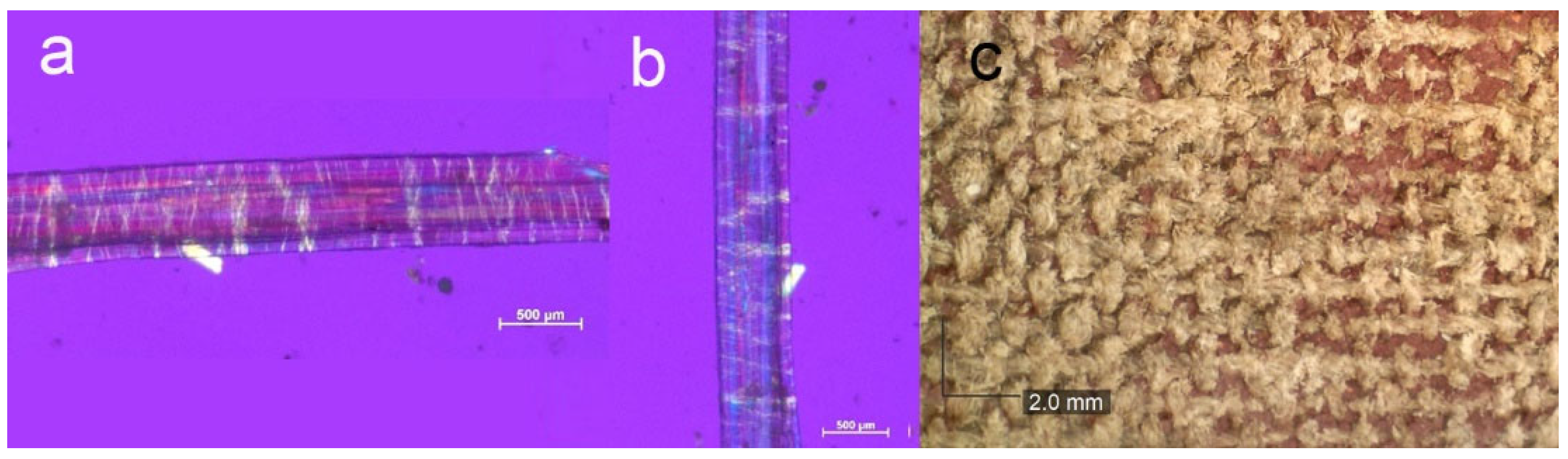
2.1. Canvas Fibre Identification
2.2. Stratigraphy Analysis in Cross Section
2.3. Raman Spectroscopy
2.4. SEM-EDX
2.5. Py-GC/MS
2.6. Radiocarbon Dating
2.7. Lead Isotope Analysis
3. Results and Discussion
3.1. Material Characterisation
3.1.1. Canvas Fibres and Sizing
3.1.2. Ground Layers
3.1.3. Paint Layers
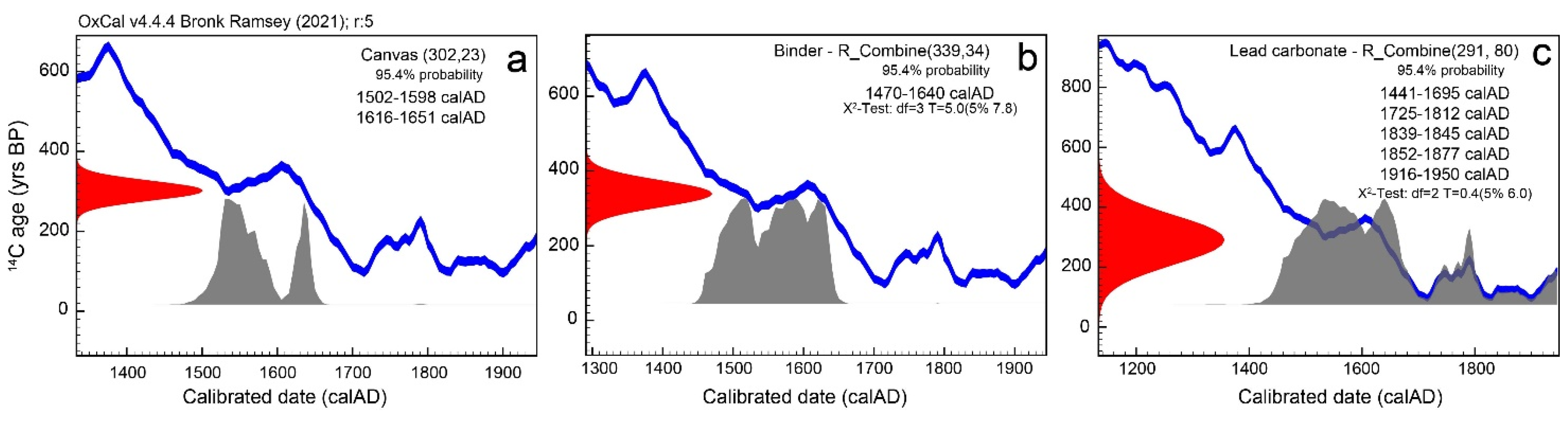
3.2. Radiocarbon Dating
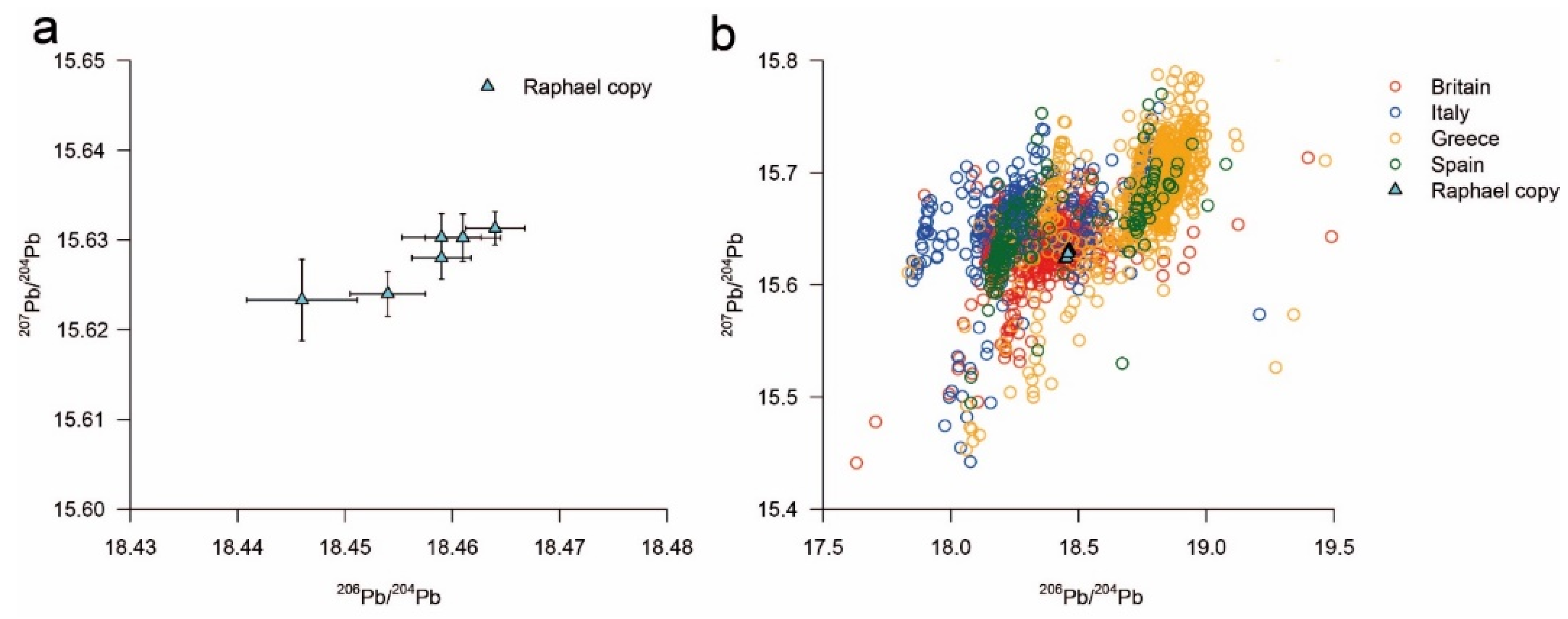
3.3. Lead Isotope Analysis
4. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer zur Capellen, J. Raphael. A Critical Catalogue of His Paintings. Volume II, The Roman Religious Paintings ca. 1508–1520; ARCOS: Landhut, Germany, 2005; pp. 170–177. [Google Scholar]
- Cox-Rearick, J. The Collection of Francis I. Royal Treasures; Fonds Mercator Paribas: Antwerp, Belgium, 1995. [Google Scholar]
- Fagnart, L. L’appartement des bains et le cabinet des peintures du château de Fontainebleau sous le règne d’Henri IV. In Henri IV. Art et Pouvoir; Nativel, C., Ed.; Presses universitaires François-Rabelais: Tours, France, 2016; pp. 53–65. [Google Scholar] [CrossRef]
- Dan, P. Le Trésor des Merveilles de la Maison Royale de Fontainebleau, Paris, Sébastien Cramoisy, 1642, Gallica Digital archive of the Bibliothèque Nationale de France. Available online: https://gallica.bnf.fr/ark:/12148/bpt6k96507143 (accessed on 27 August 2025).
- Mottin, B.; Ravaud, E.; Bastian, G.; Eveno, M. Raphael and his Entourage in Rome: Laboratory Study of the Works in the Musée du Louvre. In Late Raphael. Exhibition Catalogue; Henry, T., Joannides, P., Eds.; Musée du Louvre: Paris, France; Museo Nacional del Prado: Madrid, Spain, 2012; pp. 351–364. [Google Scholar]
- Haugan, E.; Holst, B. Determining the fibrillar orientation of bast fibers with polarizes light microscopy: The modified Herzog test (red plate test) explained. J. Microsc. 2013, 252, 159–168. [Google Scholar] [CrossRef]
- Hajdas, I.; Cristi, C.; Bonani, G.; Maurer, M. Textiles and Radiocarbon Dating. Radiocarbon 2014, 56, 637–643. [Google Scholar] [CrossRef]
- Wacker, L.; Nemec, M.; Bourquin, J. A revolutionary graphitisation system: Fully automated, compact and simple. Nucl. Instrum. Methods B 2010, 268, 931–934. [Google Scholar] [CrossRef]
- Hendriks, L.; Kradolfer, S.; Lombardo, T.; Hubert, V.; Küffner, M.; Khandekar, N.; Hajdas, I.; Synal, H.-A.; Hattendorf, B.; Günther, D. Dual isotope system analysis of lead white in artworks. Analyst 2020, 145, 1310–1318. [Google Scholar] [CrossRef]
- Synal, H.A.; Stocker, M.; Suter, M. MICADAS: A new compact radiocarbon AMS system. Nucl. Instrum. Methods B 2007, 259, 7–13. [Google Scholar] [CrossRef]
- Wacker, L.; Fahrni, S.M.; Hajdas, I.; Molnar, M.; Synal, H.A.; Szidat, S.; Zhang, Y.L. A versatile gas interface for routine radiocarbon analysis with a gas ion source. Nucl. Instrum. Methods B 2013, 294, 315–319. [Google Scholar] [CrossRef]
- Wacker, L.; Christl, M.; Synal, H.A. Bats: A New Tool for AMS Data Reduction. Nucl. Instrum. Methods B 2010, 268, 976–979. [Google Scholar] [CrossRef]
- Ramsey, C.B. Radiocarbon Calibration and Analysis of Stratigraphy: The OxCal Program. Radiocarbon 1995, 37, 425–430. [Google Scholar] [CrossRef]
- Reimer, P.J.; Austin, W.E.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Ramsey, C.B.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 Cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Koller, M.; Kühn, H.; Roosen-Runge, H.; Straub, R.E. Das Staffeleibild der Neuzeit. In Reclams Handbuch der Künstlerischen Techniken 1, Farbmittel, Buchmalerei, Tafel- und Leinwandmalerei; Reclam, P., Jr., Ed.; GmbH Publishers: Stuttgart, Germany, 1984. [Google Scholar]
- Vanderbilt Carbonnel, K. A study of French painting canvas. J. Am. Inst. Conserv. 1980, 20, 3–20. [Google Scholar] [CrossRef]
- Higgit, C.; Spring, M.; Saunders, D. Pigment-medium Interactions in oil paint films containing red lead or lead-tin yellow. Natl. Gallery Tech. Bull. 2003, 24, 75–95. [Google Scholar]
- Boon, J.J.; van der Weerd, J.P.; Keune, K.; Noble, P.; Wadum, J. Mechanical and chemical changes in Old Master paintings: Dissolution, metal soap formation and remineralization processes in lead pigmented ground/intermediate paint layers of 17th century paintings. In Proceedings of the ICOM-CC 13th Triennial Conference Preprints, Rio de Janeiro, Brazil, 20–27 September 2002; James & James (Science Publishers) Ltd.: London, UK, 2002. [Google Scholar]
- Boon, J.J. Processes inside paintings that affect the picture: Chemical changes at, near and underneath the paint surface. In Reporting Highlights of the De Mayerne Programme. Research Programme on Molecular Studies in Conservation and Technical Studies in Art History MOLART Reports 13; Boon, J.J., Ferreira, E.S.B., Eds.; Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO): The Hague, The Netherlands, 2006. [Google Scholar]
- Gettens, R.J.; Fitzhugh, E.W.; Feller, R.L. Calcium Carbonate Whites. In Artists’ Pigments: A Handbook of Their History and Characteristics; Roy, A., Ed.; Archetype Publications Ltd.: London, UK, 1993; Volume 2, pp. 203–226. [Google Scholar]
- Helwig, K. Iron Oxide Pigments. In Artists’ Pigments: A Handbook of Their History and Characteristics; Berrie, B.H., Ed.; Archetype Publications Ltd.: London, UK, 2007; Volume 4, pp. 39–109. [Google Scholar]
- Martens, W.; Frost, R.L.; Kloprogge, T.; Williams, P.A. Raman spectroscopic study of the basic copper sulphates—Implications for the copper corrosion and bronze disease. J. Raman Spect. 2003, 34, 145–151. [Google Scholar] [CrossRef]
- Gilbert, B.; Denoel, S.; Weber, G.; Allart, D. Analysis of green copper pigments in illuminated manuscripts by micro-Raman spectroscopy. Analyst 2003, 128, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Eastaugh, N.; Walsh, V.; Chaplin, T.; Siddal, R. Pigment Compendium; Elsevier Butterworth-Heinemann: Oxford, UK, 2008. [Google Scholar]
- Scott, D.A. Basic sulfates. In Copper and Bronze in Art; Scott, D.A., Ed.; Getty Publications: Hong Kong, China, 2002. [Google Scholar]
- Svarcova, S.; Hradil, D.; Hradilova, J.; Cermakova, Z. Pigments copper-based greens and blues. Archaeol. Anthropol. Sci. 2021, 13, 190. [Google Scholar] [CrossRef]
- Gettens, R.J.; Fitzburg, E.W. Malachite and Green Verditer. In Artists Pigments A Handbook of Their History and Characteristics; Roy, A., Ed.; Archetype Publications Ltd.: London, UK, 1993; Volume 2, pp. 183–201. [Google Scholar]
- Bersani, D.; Antonioli, G.; Lottici, P.; Casoli, A. Raman microspectrometric investigation of wall paintings in S.Giocanni Envagelista Abbey in Parma: A comparison between two artists of the 16th century. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Franquelo, M.L.; Perez-Rodriguez, J.L. A new approach to the determination of the synthetic or natural origin of red pigments through spectroscopic analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 166, 103–111. [Google Scholar] [CrossRef]
- Gettens, R.J.; Feller, R.L.; Chase, W.T. Vermilion and Cinnabar. In Artists Pigments A Handbook of Their History and Characteristics; Roy, A., Ed.; Archetype Publications Ltd.: London, UK, 1993; Volume 2, pp. 159–182. [Google Scholar]
- Sefcu, R.; Chlumska, S.; Hostasova, A. An investigation of the lead tin yellow type I and II and their use in Bohemian panel paintings from the Gothic period. Herit. Sci. 2015, 3, 16. [Google Scholar] [CrossRef]
- Kühn, H. 1993 Lead tin yellow. In Artists Pigments A Handbook of Their History and Characteristics; Roy, A., Ed.; Archetype Publications Ltd.: London, UK, 1993; Volume 2, pp. 83–112. [Google Scholar]
- Gettens, R.J.; Kühn, H.; Chase, W.T. Lead White. In Artists’ Pigments A Handbook of Their History and Characteristics; Roy, A., Ed.; Archetype Publications Ltd.: London, UK, 1993; Volume 2, pp. 67–82. [Google Scholar]
- Seldes, A.M. A note on the pigments and media in some Spanish Colonial paintings from Argentina. Stud. Conserv. 1994, 39, 272–276. [Google Scholar] [CrossRef]
- Gil, M.; Carvalho, M.L.; Longelin, S.; Ribeiro, I.; Valadas, S.; Mirão, J.; Candeias, A.E. Blue pigment colors from wall painting churches in danger (Portugal 15th to 18th century): Identification, diagnosis, and color evaluation. Appl. Spectrosc. 2011, 65, 782–789. [Google Scholar] [CrossRef]
- Berrie, B.H. Mining for Color: New Blues, Yellows, and Translucent Paint. Early Sci. Med. 2015, 20, 308–334. [Google Scholar] [CrossRef]
- Seccaroni, C.; Haldi, J.P. Impuresse ed elementi associate al cobalto. In Cobalto, Zaffera, Smalto dall Antichita al XVIII Secolo; Seccaroni, C., Haldi, J.P., Eds.; ENEA: Rome, Italy, 2016; pp. 65–102. [Google Scholar]
- Giannini, R.; Freestone, I.C.; Shortland, A.J. European Cobalt Sources Identified in the Production of Chinese Famille Rose Porcelain. J. Archaeol. Sci. 2017, 80, 27–36. [Google Scholar] [CrossRef]
- Gratuze, B. Provenance Analysis of Glass Artefacts. In Modern Methods for Analysing Archaeological and Historical Glass; Janssens, K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume I, pp. 311–343. [Google Scholar]
- Gratuze, B.; Soulier, I.; Barrandon, J.N.; Foy, D. The origin of cobalt blue pigments in French glass from the thirteenth to the eighteenth centuries. In Trade and Discovery: The Scientific Study of Artefacts from Post-Medieval Europe and Beyond; British Museum: London, UK, 1995; Volume 109, pp. 123–132. [Google Scholar]
- Mühlenthaler, B.; Theissen, J. Smalt. In Artists Pigments A Handbook of Their History and Characteristics; Roy, A., Ed.; Archetype Publications Ltd.: London, UK, 1993; Volume 2, pp. 113–130. [Google Scholar]
- Stege, H. Out of the Blue? Considerations on the early use of smalt as blue pigment in Europe easel painting. ZKK 2004, 18, 121–142. [Google Scholar]
- Robinet, L.; Spring, M.; Pages-Camagna, S. Vibrational Spectroscopy correlated with elemental analysis for the investigation of smalt pigments and its alteration in paintings. Anal. Methods 2013, 5, 4628. [Google Scholar] [CrossRef]
- Berrie, B.H. Prussian Blue. In Artists Pigments. A Handbook of Their History and Characteristics; Fitzhugh, E.W., Ed.; Archetype Publications Ltd.: London, UK, 1997; Volume 3, pp. 191–218. [Google Scholar]
- Hendriks, L.; Hajdas, I.; Ferreira, E.S.B.; Scherrer, N.C.; Zumbühl, S.; Smith, G.D.; Welte, C.; Wacker, L.; Synal, H.; Günther, D. Uncovering modern paint forgeries by radiocarbon dating. Proc. Natl. Acad. Sci. USA 2019, 116, 13210–13214. [Google Scholar] [CrossRef]
- Hendriks, L.; Hajdas, I.; Ferreira, E.S.B.; Scherrer, N.C.; Zumbühl, S.; Küffner, M.; Wacker, L.; Synal, H.-A.; Günther, D. Combined 14C Analysis of Canvas and Organic Binder for Dating a Painting. Radiocarbon 2018, 60, 207–218. [Google Scholar] [CrossRef]
- Messager, C.; Beck, L.; de Viguerie, L.; Jaber, M. Thermal Analysis of Carbonate Pigments and Linseed Oil to Optimize CO2 Extraction for Radiocarbon Dating of Lead White Paintings. Microchem. J. 2020, 154, 104637. [Google Scholar] [CrossRef]
- D’imporzano, P.; Batur, K.; Keune, K.; Koornneef, J.M.; Hermens, E.; Noble, P.; van Zuilen, K.; Davies, G.R. Lead isotope heterogeneity in lead white: From lead white raw pigment to canvas. Microchem. J. 2021, 163, 105897. [Google Scholar] [CrossRef]
- Keisch, B.; Callahan, R.C. Lead isotope ratios in artists’ lead white: A progress report. Archaeometry 1976, 18, 181–193. [Google Scholar] [CrossRef]
- van Loon, A.; Vandivere, A.; Delaney, J.K.; Dooley, K.A.; De Meyer, S.; Vanmeert, F.; Gonzalez, V.; Janssens, K.; Leonhardt, E.; Haswell, R.; et al. Beauty is skin deep: The skin tones of Vermeer’s Girl with a Pearl Earring. Herit. Sci. 2019, 7, 1–20. [Google Scholar] [CrossRef]
- Fortunato, G.; Ritter, A.; Fabian, D. Old Masters’ lead white pigments: Investigations of paintings from the 16th to the 17th century using high precision lead isotope abundance ratio. Analyst 2005, 130, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Fabian, D.; Fortunato, G. Tracing white: A study of lead white pigments found in seventeenth-century paintings using high precision lead isotope abundance ratios. In Trade in Artists’ Materials. Markets and Commerce in Europe to 1700; Kirby, J., Nash, S., Cannon, J., Eds.; Archetype: London, UK, 2010; pp. 426–443. [Google Scholar]
- Stevenson, R.K.; Moffatt, E.A.; Corbeil, M.C.; Poirier, A. Pb and Sr Isotopes and the Provenance of the Painting Materials of Cornelius Krieghoff in 19th-Century Canada. Archaeometry 2016, 58, 673–687. [Google Scholar] [CrossRef]
- Duval, A. Les préparations colorées des tableaux de l’Ecole Française des dixseptième et dixhuitième siècles. Stud. Conserv. 1992, 37, 239–258. [Google Scholar]
- Stols-Witlox, M. Grounds 1400–1900. In Conservation of Easel Paintings, 2nd ed.; Stoner, J.H., Rushfiled, R., Eds.; Routledge: London, UK, 2012; Chapter 7; pp. 161–188. [Google Scholar]
- Stols-Witlox, M. “By no means a trivial matter”. The influence of the colour of ground layers on artists’ working methods and on the appearance of oil paintings, according to historical recipes from North West Europe, c. 1550–1900. Oud Holl.-J. Art Low Ctries. 2015, 128, 171–186. [Google Scholar] [CrossRef]
- Martin, E. Grounds on canvas 1600-1640 in various European artistic centres. In Preparation for Painting: The Artist’s Choice and Its Consequences g, Proceedings of the 2007 ICOM-CC Interim Meeting, London, UK, 31 May –1 June 2007; Townsend, J.H., Doherty, T., Heydenreich, G., Eds.; Archetype Publications Ltd.: London, UK, 2008. [Google Scholar]
- Salvant, J.; Eveno, M.; Betelu, C.; Negrello, C.; Bastian, G.; Faroult, G.; Ravaud, E. Investigating the transition period from colored to white preparatory layers in 18th-century French canvas paintings: A retrospective study. In Transcending Boundaries: Integrated Approaches to Conservation. Proceedings of the ICOM-CC 19th Triennial Conference Preprints, Beijing, China, 17–21 May 2021; Bridgland, J., Ed.; International Council of Museums: Paris, France, 2021. [Google Scholar]
- Heydenreich, G. Lucas Cranach the Elder. Painting Materials, Techniques and Workshop Practice; Amsterdam University Press: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Voermann, I. Die Kopie als Element Fürstlicher Gemäldesammlungen des 19 Jahrhundert; Lukas Publishers: Berlin, Germany, 2012. [Google Scholar]
- Putzger, A. Kult und Kunst-Kopie und Original. Altarbilder von Rogier van der Weyden, Jan van Eyck und Albrecht Dürer in Ihrer Frühneuzeitlichen Rezeption; Bild+Bild; Reimer: Berlin, Germany, 2021; Volume 5. [Google Scholar]
- Gaggetta, C. The faithful copy as a medium of appropriation and propaganda. French commissions after Leonardo’s “Last Supper”. In Nichts Neues Schaffen. Perspektiven auf die Treue Kopie 1300–1900; Putzger, A., Heisterberg, M., Müller-Bechtel, S., Eds.; De Gruyter Brill: Berlin, Germany, 2018; pp. 133–152. [Google Scholar] [CrossRef]


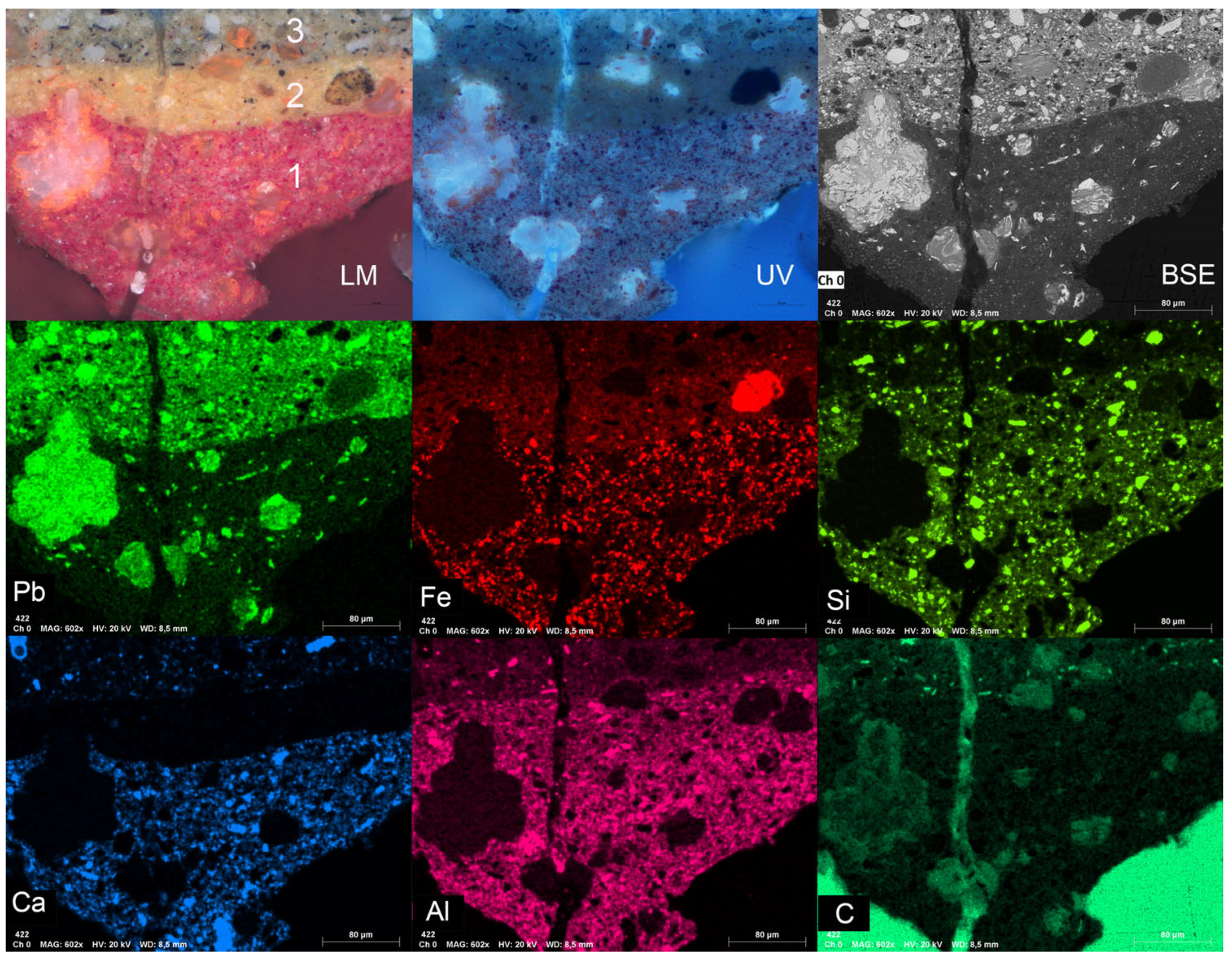
| Layer | Light Microscopy | SEM-EDX | Raman Peaks (cm−1) | Material Identification |
|---|---|---|---|---|
| 3. Grey/beige ground | white particles | Pb, C, O | 1050 | Lead carbonate |
| black particles | C | 1599, 1302 | Carbon black | |
| orange particles | Pb, O | 549, 391, 314, 223, 152, 122 | Minium (Pb3O4) | |
| white particles (foraminifera) | Ca, C, O | -- | Calcium carbonate | |
| white translucent particles | Si, O | -- | Quartz | |
| 2. Yellow/orange ground | white particles | Si, Al, O | -- | Aluminium silicates |
| white translucent particles | Si, O | -- | Quartz | |
| yellow particles | Fe, O | -- | Yellow Ochre | |
| orange particles | Pb, O | 549, 391, 310, 225, 151, 122 | Minium (Pb3O4) | |
| 1. Red ground | red particles (1–3 µm), non-fluorescent | Fe, O | 610, 501, 410, 293, 225 | Iron oxide (Haematite) Fe2O3 |
| orange particles in the matrix and associated with the metal soap aggregates | Pb, O | 550, 391, 310, 225, 151, 122 | Minium (Pb3O4) | |
| white particles | Ca, C, O | -- | Calcium carbonate | |
| white particles | Al, Si, O | -- | Aluminium silicates | |
| white particles | Si, O | -- | Quartz | |
| translucent fluorescent phase | Pb, C, O | -- | Lead carboxylates |
| Layer | Light Microscopy | SEM-EDX | Raman Peaks cm−1 | Material Identification |
|---|---|---|---|---|
| Yellow paint layer (sample 4) | yellow particle | Pb, Sn, O | 196, 129 | Lead tin yellow type I (Pb2SnO4) |
| red particle | Hg, S | 344, 290, 254 | Cinnabar | |
| Blue paint layer 4 (sample 2) | blue particle | O, Si, K, Co, Fe, As, Bi, Ni (Al, Cl) | -- | Smalt |
| Blue paint layer 5 (sample 3) | blue particle | Cu, C, O | -- | Azurite (Cu3(CO3)2(OH)2) |
| blue particle | O, Si, C, Co, Fe, As, K, Bi, Ni (Al) | -- | Smalt | |
| Blue paint layer 6 (sample 3) | blue particle | O, C, Al, Si, Na, S, Ca, K | -- | Ultramarine |
| Green paint (sample 1) | green particle | Cu, C, O | 1085 | Copper carbonate Malachite (Cu2CO3(OH)2) |
| green bluish particle | Cu, C, O, S | 445, 972 | Hydrated copper sulphate Posnjakite Cu4(SO4)(OH)6 H2O | |
| Green paint layer 4 (sample 7) | green particle | Cu, O, C, S | -- | Copper carbonate copper sulphate, |
| green particle | Cu, O, C | -- | Copper carbonate Malachite (Cu2CO3(OH)2) | |
| White layer (sample 5) | white particle | Pb, C, O | -- | Lead carbonate |
| white translucent particle | Si, O, (Ca, Al) | -- | Quartz | |
| Light red/pink layer (sample 5) | white particle | Pb, C, O | -- | Lead carbonate |
| red particle | Hg, S | -- | Cinnabar | |
| Overpaint (sample 2) | blue particle | Fe, K, O, (Al, Ca) | 2154, 2092, 950, 592, 537 | Prussian blue |
| Sample Code | MC-ICPMS label | Mean 208Pb/204Pb | RSD% | Mean 206Pb/204Pb | RSD% | Mean 207Pb/204Pb | RSD% | Hg ∆ |
|---|---|---|---|---|---|---|---|---|
| 92174.2.1 | SK158 | 38.392 | 0.028 | 18.446 | 0.029 | 15.62331 | 0.023 | 0.05% |
| 92174.2.2 | SK159 | 38.422 | 0.015 | 18.464 | 0.012 | 15.63130 | 0.013 | 0.5% |
| 92174.3.1 | SK160 | 38.414 | 0.020 | 18.459 | 0.017 | 15.63029 | 0.017 | 1.2% |
| 92174.1.1 | SK188 | 38.401 | 0.019 | 18.454 | 0.016 | 15.624 | 0.023 | 0.5% |
| 92174.4.1 | SK206 | 38.416 | 0.019 | 18.461 | 0.017 | 15.63025 | 0.016 | 0.6% |
| 92174.1.1 * | SK226 | 38.412 | 0.015 | 18.459 | 0.015 | 15.628 | 0.016 | 0.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, E.S.B.; Hoffmann, C.; Hendriks, L.; Hajdas, I.; Kradolfer, S.; Günther, D.; Hünerfauth, K.; Reinhardt, J.; Portsteffen, H.; Müller-Bechtel, S. Integrating Material Analysis, Radiocarbon Dating, and Technical Examination in the Dating and Provenance Study of a Copy of Raphael’s “The Great Holy Family of Francis I”. Heritage 2025, 8, 424. https://doi.org/10.3390/heritage8100424
Ferreira ESB, Hoffmann C, Hendriks L, Hajdas I, Kradolfer S, Günther D, Hünerfauth K, Reinhardt J, Portsteffen H, Müller-Bechtel S. Integrating Material Analysis, Radiocarbon Dating, and Technical Examination in the Dating and Provenance Study of a Copy of Raphael’s “The Great Holy Family of Francis I”. Heritage. 2025; 8(10):424. https://doi.org/10.3390/heritage8100424
Chicago/Turabian StyleFerreira, Ester S. B., Charlotte Hoffmann, Laura Hendriks, Irka Hajdas, Stefan Kradolfer, Detlef Günther, Katharina Hünerfauth, Juliane Reinhardt, Hans Portsteffen, and Susanne Müller-Bechtel. 2025. "Integrating Material Analysis, Radiocarbon Dating, and Technical Examination in the Dating and Provenance Study of a Copy of Raphael’s “The Great Holy Family of Francis I”" Heritage 8, no. 10: 424. https://doi.org/10.3390/heritage8100424
APA StyleFerreira, E. S. B., Hoffmann, C., Hendriks, L., Hajdas, I., Kradolfer, S., Günther, D., Hünerfauth, K., Reinhardt, J., Portsteffen, H., & Müller-Bechtel, S. (2025). Integrating Material Analysis, Radiocarbon Dating, and Technical Examination in the Dating and Provenance Study of a Copy of Raphael’s “The Great Holy Family of Francis I”. Heritage, 8(10), 424. https://doi.org/10.3390/heritage8100424







