Authentication of a Bronze Bust of Napoleon I, Attributed to Renzo Colombo from 1885
Abstract
1. Introduction
2. Experimental Section
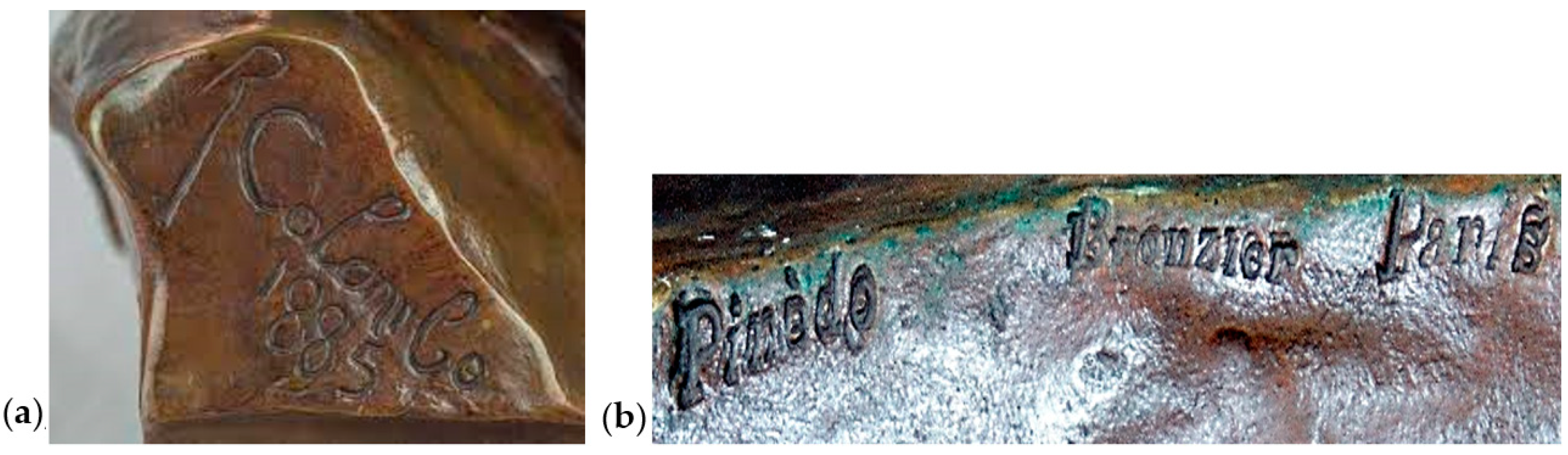
2.1. Presentation of the Bust
2.2. Conservation Status
2.3. Digital Imaging Methods and Techniques
2.4. Sampling and Processing of Material
2.5. Microscopic Methods and Techniques
3. Results and Discussions
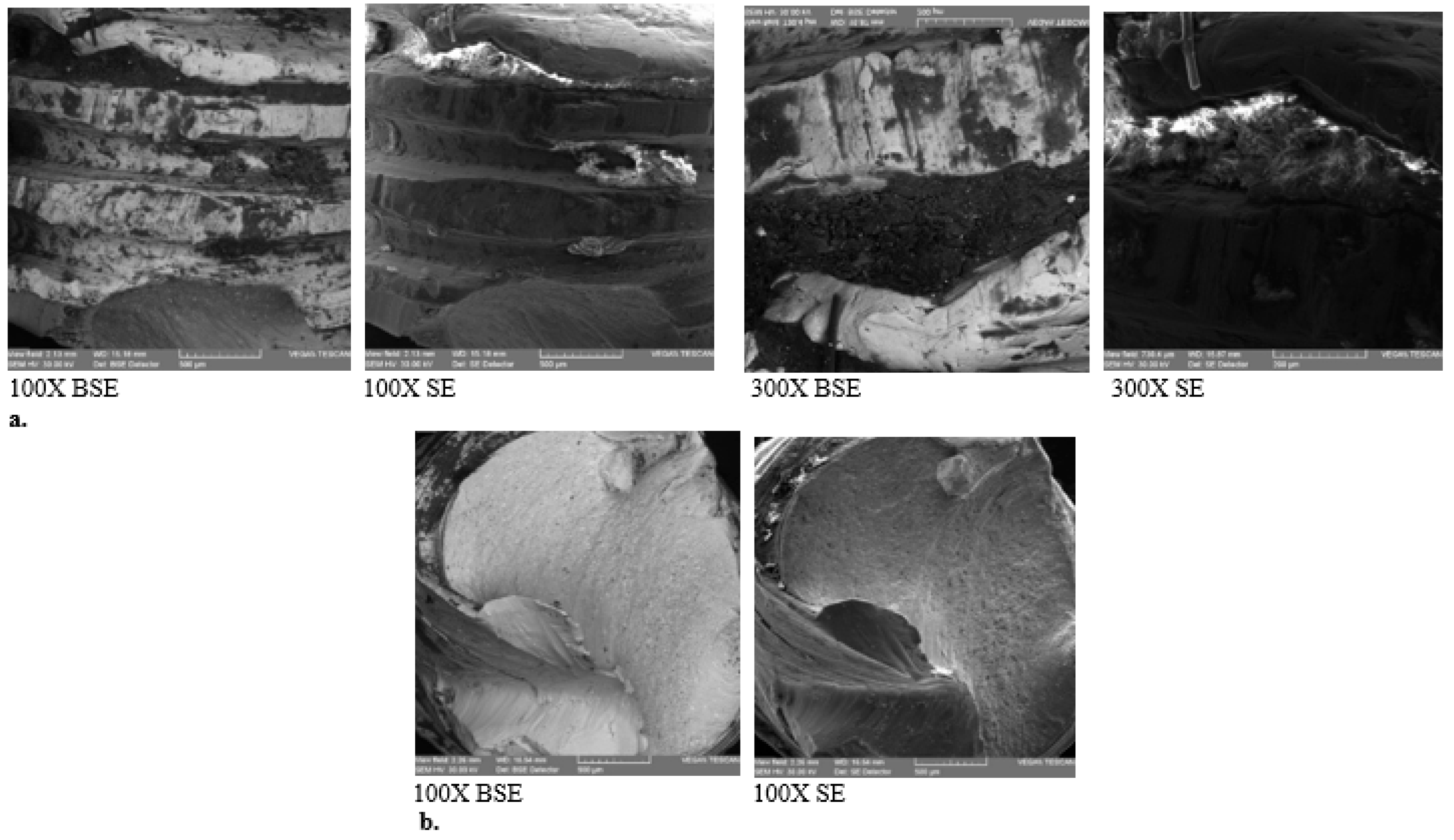
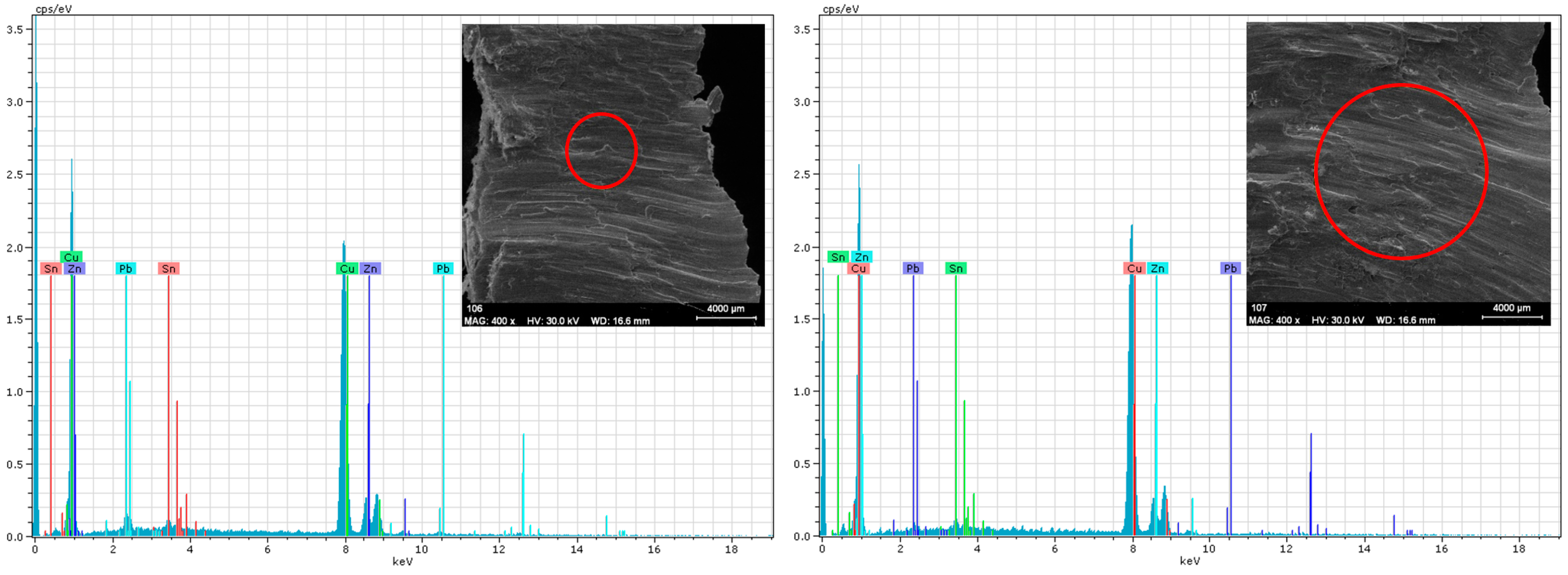
SEM-EDX Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sandu, I.; Cotiuga, V. Cercetarea Bunurilor de Patrimoniu Şi a Documentelor Falsificate; AIT Laboratory: Bucuresti, Romania, 2011. [Google Scholar]
- Sandu, I.; Vasilache, V. Conservarea Amprentelor Formă Şi a Urmelor Materiale; AIT Laboratory: Bucuresti, Romania, 2011. [Google Scholar]
- Matei, G. Investigarea Criminalistică a Infracţiunilor Privind Operele de Artă Şi Artefactele Arheologice; Universul Juridic: Bucuresti, Romania, 2019. [Google Scholar]
- Baker, P. Protecting Art, Protecting Artists and Protecting Consumers Conference; Autralian Institute of Criminology: Sydney, Australia, 1999. [Google Scholar]
- Arnau, F. Arta Falsificatorilor—Falsificatorii Artei (in Romanian); Meridiane: Bucuresti, Romania, 1970. [Google Scholar]
- Sandu, I.C.A.; Sandu, I.; Popoiu, P.; Van Saanen, A. Aspecte Metodologice Privind Conservarea Științifică a Patrimoniului Cutural; Corson: Iaşi, Romania, 2001. [Google Scholar]
- Sandu, I.; Sandu, I.C.A.; Van Saanen, A. Expertiza Științifică a Operelor de Artă; Universității Alexandru Ioan Cuza: Iaşi, Romania, 1998. [Google Scholar]
- Sandu, I. Degradarea Și Deteriorarea Bunurilor de Patrimoniu Cultural, Vol. I. Bunuri Din Material Anorganice; Universității Alexandru Ioan Cuza: Iaşi, Romania, 2008. [Google Scholar]
- Sandu, I.; Dima, A.; Sandu, I.G. Restaurarea Și Conservarea Artefactelor Metalice; Corson: Iaşi, Romania, 2002; p. 666. [Google Scholar]
- Sandu, I. New Materials and Advanced Procedures of Conservation Ancient Artifacts. Appl. Sci. 2023, 13, 8387. [Google Scholar] [CrossRef]
- Sandu, I. Modern Aspects Regarding the Conservation of Cultural Heritage Artifacts. Int. J. Conserv. Sci. 2022, 13, 1187–1208. [Google Scholar]
- Sandu, I.G.; Sandu, I.; Dima, A. Aspecte Moderne Privind Conservarea Patrimoniului Cultural, Vol. III. Autentificarea Și Restaurarea Artefactelor Din Materiale Anorganice; Performantica: Iaşi, Romania, 2006; p. 502. [Google Scholar]
- Dumitrescu, E. Artă Si Tehnologie: Sculptura în Bronz—Despre Turnarea Formelor Prin Metoda Cerii Pierdute; UNARTE: Bucuresti, Romania, 2010; pp. 93–98. [Google Scholar]
- Kjellberg, P. Bronzes of the Nineteenth Century: Dictionary of Sculptors, Early ed.; Schiffer Publishing Ltd.: Atglen, PA, USA, 1994; p. 684. ISBN 0887406297/9780887406294 (a se vedea pagina 220). [Google Scholar]
- Lami, S. Dictionnaire des Sculpteurs de L’École Français, Au Dix-Neuvième Siècle, Vol. I; Hachette Livre–BNF: Paris, France, 1970; p. 85. [Google Scholar]
- de Champeaux, A. Dictionnaire des Fondeurs, Ciseleurs, Modelleurs en Bronze, et Doreurs, Vol. I; Rouam, J., Ed.; Librairie de l’Art: Paris, France, 1886; p. 357. [Google Scholar]
- Stanley, C. The Technique of Early Greek Sculpture; Oxford at Clarendon Press: London, UK, 1933. [Google Scholar]
- Dame, C.C. Metal Casting of Sculpture; The Standard Arts Press: Butler, MD, USA, 1948. [Google Scholar]
- Edilberto, F. I Grandi Bronzi Antichi—Le Fonderie e le Tecniche Di Lavorazione Dall’eta Arcaica al Rinascimento; Nuova Imagine Editrice: Siena, Italy, 1999. [Google Scholar]
- Gallardo, J.M.; Cuevas, F.G.; Cintas, J.; Montes, J.M. La Fe Victoriosa Casting Features. Adv. Mater. Forum 2008, 587–588, 1014–1018. [Google Scholar] [CrossRef]
- Castelle, M.; Bormand, M.; Vandenberghe, Y.; Bourgarit, D. Two of a Kind: Shining New Light on Bronze Spiritelli Attributed to Donatello. Stud. Conserv. 2000, 65, 200–211. [Google Scholar] [CrossRef]
- Castelle, M.; Coquinot, Y.; Bourgarit, D. Casting cores of French bronze statues of the 16th and 17th centuries: Identification of regional practices and artistic choices. Microchem. J. 2016, 126, 121–131. [Google Scholar] [CrossRef]
- Agnoletti, S.; Brini, A.; Cagnini, A. Le Teste in Bronzo Della Cantoria Di Donatello: Aspetti Conoscitivi e Conservativi. OPD Restaur. 2013, 25, 201–212. [Google Scholar]
- Ammannatti, N.; Paita, A.; Toni, S. Analisi Morfologica e Composizionale di Frammenti Metallici Provenienti dal Monumento Equestre di Cosimo I; CR Europa Metalli Rapporto: Fornaci di Barga, Italy, 1993; No. 930507/0647. [Google Scholar]
- Bassett, J.; Scherf, G. Jean-Antoine Houdon: Sculptor and founder. In French Bronze Sculpture: Materials and Techniques 16th–18th Century; Archetype: Paris, France, 2014; pp. 107–124. [Google Scholar]
- Bourgarit, D.; Bewer, F.G.; Bresc-Bautier, G. Francesco Bordoni: Specificites techniques chez un fondeur sculpteur du XVIIeme siècle. In French Bronze Sculpture: Materials and Techniques 16th-18th Century; Archetype: Paris, France, 2014; pp. 1–17. [Google Scholar]
- Lombardi, G.; Vidale, M. From the shell to its content: The casting cores of the two bronze statues from Riace (Calabria, Italy). J. Archaeol. Sci. 1998, 25, 1055–1066. [Google Scholar] [CrossRef]
- Lanterna, G. Multidisciplinary scientific analysis on restoration of a Renaissance masterpiece: Verrocchio’s ‘‘L’Incredulita di san tommaso’‘, outdoor bronze group of Orsammichele Church in Florence. A case history. Termochemica Acta 1995, 269, 729–742. [Google Scholar] [CrossRef]
- Pouyet, E.; Ganio, M.; Motlani, A.; Saboo, A.; Casadio, F.; Walton, M. Casting Light on 20th-Century Parisian Artistic Bronze: Insights from Compositional Studies of Sculptures Using Hand-Held X-ray Fluorescence Spectroscopy. Heritage 2019, 2, 732–748. [Google Scholar] [CrossRef]
- Cartechini, L.; Rinaldi, R.; Kockelmann, W.; Bonamore, S.; Manconi, D.; Borgia, I.; Rocchi, P.; Brunetti, B.; Sgamellotti, A. Non-destructive characterization of compositional and textural properties of Etruscan bronzes: A multi-method approach. Appl. Phys. A-Mater. Sci. Process. 2006, 83, 631–636. [Google Scholar] [CrossRef]
- Ropret, P.; Kosec, T. Outdoor Bronze and Its Protection. Raman Spectrosc. Archaeol. Art Hist. 2019, 2, 196–212. [Google Scholar]
- Picciochi, R.; Ramos, A.C.; Mendonça, M.H.; Fonseca, I.T.E. Influence of the environment on the atmospheric corrosion of bronze. J. Appl. Electrochem. 2004, 34, 989–995. [Google Scholar] [CrossRef]
- Robbiola, L.; Blengino, J.M.; Fiaud, C. Morphology and mechanisms of formation of natural patinas on archaeological Cu-Sn alloys. Corros. Sci. 1998, 40, 2083–2111. [Google Scholar] [CrossRef]
- Robbiola, L.; Portier, R. A global approach to the authentication of ancient bronzes based on the characterization of the alloy–patina–environment system. J. Cult. Herit. 2006, 7, 1–12. [Google Scholar] [CrossRef]
- FitzGerald, K.P.; Nairn, J.; Skennerton, G.; Atrens, A. Atmospheric corrosion of cooper and the colour, structure and composition of natural patinas on copper. Corros. Sci. 2006, 48, 2480–2509. [Google Scholar] [CrossRef]
- de Oliveira, F.J.R.; Lago, D.C.B.; Senna, L.F.; de Miranda, L.R.M.; d’Elia, E. Study of patina formation on bronze specimens. Mater. Chem. Phys. 2009, 115, 761–770. [Google Scholar] [CrossRef]
- Chiavari, C.; Colledan, A.; Frignani, A.; Brunoro, G. Corrosion evaluation of traditional and new bronzes for artistic castings. Mater. Chem. Phys. 2006, 95, 252–259. [Google Scholar] [CrossRef]
- Ingo, G.M.; de Caro, T.; Riccucci, C.; Khosroff, S. Uncommon corrosion phenomena of archaeological bronze alloys. Appl. Phys. A Mater. Sci. Process. 2006, 83, 581–588. [Google Scholar] [CrossRef]
- Domenic-Carbo, A.; Domenic-Carbo, M.T.; Martinez-Lazaro, I. Electrochemical identification of corrosion products in archaeological artefacts. A case study. Microchim. Acta 2007, 162, 351–359. [Google Scholar] [CrossRef]
- Chelaru, J.D.; Soporan, V.F.; Nemeş, O. Time analysis of King Matthias the I Sculptural Group. Int. J. Conserv. Sci. 2010, 1, 69–74. [Google Scholar]
- Sabau, J.D.; Muresan, L.M.; Soporan, V.F.; Nemes, O.; Kolozsi, T. A Study on the Corrosion Resistance of Bronzes Covered with Artificial Patina. Int. J. Conserv. Sci. 2011, 2, 109–116. [Google Scholar]
- Casaletto, M.P.; Ingo, G.M.; Albini, M.; Lapenna, A.; Pierige, I.; Riccucci, C.; Faraldi, F. An integrated analytical characterization of corrosion products on ornamental objects from the necropolis of Colle Badetta-Tortoreto (Teramo, Italy). Appl. Phys. Amater. Sci. Process. 2010, 100, 801–808. [Google Scholar] [CrossRef]
- Mureşan, L.; Varvara, S.; Stupnišek-Lisac, E.; Otmačić, H.; Marušić, K.; Takenouti, H. Protection of bronze covered with patina by innoxiouus organic substances. Electrochim. Acta 2007, 52, 7770–7779. [Google Scholar] [CrossRef]
- Vlasa, A.; Varvara, S.; Mureşan, L. Electrochemical investigation of the influence of two thiadiazole derivatives on the patina of an artchaeological bronze artefact using a carbon paste electrode. Stud. Univ. Babeş-Bolyai 2007, 2, 63–71. [Google Scholar]
- Kipper, P. Patina for Silicon Bronze; Regal Printing: Hong Kong, 2003. [Google Scholar]
- Souissi, N.; Bousselmi, L.; Khosrof, S.; Triki, E. Electrochemical behavior of an archaeological bronze alloy in various aqueous media: New method for understanding artifacts preservation. Mater. Corrsion 2003, 54, 318–325. [Google Scholar] [CrossRef]
- Privitera, A.; Corbascio, A.; Calcani, G.; Della Ventura, G.; Ricci, M.A.; Sodo, A. Raman approach to the forensic study of bronze patinas J. Archaeol. Sci. Rep. 2021, 39, 103115. [Google Scholar] [CrossRef]
- Satovic, D.; Martinez, S.; Bobrowski, A. Electrochemical identification of corrosion products on historical and archaeological bronzes using the voltammetry of micro-particles attached to a carbon paste electrode. Talanta 2010, 81, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Kosec, T.; Curkovic, H.O.; Legat, A. Investigation of the corrosion protection of chemically and electrochemically formed patinas on recent bronze. Electrochim. Acta 2010, 56, 722–731. [Google Scholar] [CrossRef]
- Noli, F.; Misaelides, P.; Hatzidimitriou, A.; Pavlidou, E.; Kokkoris, M.M. Investigation of artificially produced and natural copper patina layers. J. Mater. Chem. 2003, 13, 114–120. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Ramírez-Barat, B.; Petiti, C.; Goidanich, S.; Doménech-Carbó, M.T.; Cano, E. Characterization of traditional artificial patinas on copper using the voltammetry of immobilized particles. J. Electroanal. Chem. 2020, 877, 114494. [Google Scholar] [CrossRef]
- Sandu, I.; Mircea, O.; Sandu, I.G.; Vasilache, V.; Sandu, A.V. Liesegang Effect Typology on Ancient Bronzes Discovered in Romania. Rev. Chim. 2014, 65, 311–319. [Google Scholar]
- Sandu, I.; Mircea, O.; Sandu, I.G.; Vasilache, V. The Liesegang Effect on Ancient Bronze Items Discovered in Romania. Int. J. Conserv. Sci. 2013, 4, 573–586. [Google Scholar]
- Sandu, I.G.; Mircea, O.; Vasilache, V.; Sandu, I. Influence of archaeological environment factors in alteration processes of copper alloy artifacts. Microsc. Res. Tech. 2012, 75, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Sandu, I.G.; Stoleriu; Sandu, I.; Brebu, M.; Sandu, A.V. Authentication of ancient bronze coins by the study of the archaeological patina. I. Composition and structure. Rev. Chim. 2005, 56, 981–994. [Google Scholar]
- Scott, D.A. Copper and Bronze in Art: Corrosion, Colorants and Conservation, 1st ed.; Paul Getty Conservation Institute: Los Angeles, CA, USA, 2002; pp. 139–141. [Google Scholar]
- Hughes, R.; Rowes, M. The Colouring, Bronzing and Patination of Metals, Crafts, 2nd ed.; Thames and Hudson: London, UK, 1991. [Google Scholar]
- Balta, I.Z.; Robbiola, L. Study of black patinas on copper and bronze obtained by using 19th century western traditional techniques of artificial patination. In Proceedings of the 8th International Conference on Non-destructive Investigations and Microanalysis for the Diagnostics and Conservation of the Cultural and Environmental Heritage, Lecce, Italy, 5–19 May 2005. [Google Scholar]
- Noli, F.; Misaelides, P.; Pavlidou, E.; Kokkoris, M. Investigation of copper patinas using ion beam analysis and scanning electron microscopy. J. Surf. Interface Anal. 2005, 37, 288–293. [Google Scholar] [CrossRef]
- Cicileo, G.P.; Crespo, M.A.; Rosales, B.M. Comparative study of patinas formed on statuary alloys by means of electrochemical and surface analysis techniques. Corros. Sci. 2004, 46, 929–953. [Google Scholar] [CrossRef]
- Crippa, M.; Bongiorno, V.; Piccardo, P.; Carnasciali, M.M. A Characterisation Study on Modern Bronze Sculpture: The Artistic Patinas of Nado Canuti. Stud. Conserv. 2019, 64, 16–23. [Google Scholar] [CrossRef]
- Kwon, H. Corrosion Behaviors of Artificial Chloride Patina for Studying Bronze Sculpture Corrosion in Marine Environments. Coatings 2023, 13, 1630. [Google Scholar] [CrossRef]
- Gianni, L. Corrosion Behavior of Bronze Alloys Exposed to Urban and Marine Environment: An Innovative Approach to Corrosion Process Understanding and to Graphical Results Presentation. Ph.D. Thesis, University of Ghent, Ghent, Belgium, 2011; pp. 7–27. [Google Scholar]
- Sabbe, P.J.; Dowsett, M.G.; De Keersmaecker, M.; Hand, M.; Thompson, P.; Adriaens, A. Synthesis and surface characterization of a patterned cuprite sample: Preparatory step in the evaluation scheme of an X-ray-excited optical microscopy system. Appl. Surf. Sci. 2015, 332, 657–664. [Google Scholar] [CrossRef]
- Balta, I.Z.; Pederzoli, S.; Iacob, E.; Bersani, M. Dynamic secondary ion mass spectrometry and X-ray photoelectron spectroscopy on artistic bronze and copper artificial patinas. Appl. Surf. Sci. 2009, 255, 6378–6385. [Google Scholar] [CrossRef]
- Municchia, A.C.; Bellatreccia, F.; D’Ercoli, G.; Mastro, S.L.; Reho, I.; Ricci, M.A.; Sodo, A. Characterisation of artificial patinas on bronze sculptures of the Carlo Bilotti Museum (Rome). Appl. Phys. A 2016, 122, 1021. [Google Scholar] [CrossRef]
- Stranges, F.; La Russa, M.; Oliva, A.; Galli, G. Analysis of the Quintilii’s Villa Bronzes by Spectroscopy Techniques. J. Archaeol. 2014, 2014, 312981. [Google Scholar] [CrossRef]
- Wells, C. Scanning Electron Microscopy; McGraw-Hill: New York, NY, USA, 1974. [Google Scholar]
- Kuo, J. Electron Microscopy: Methods and Protocols, 2nd ed.; Humana Press: New Jersey, NJ, USA, 2007. [Google Scholar]
- Tanasa, P.O.; Sandu, I.; Vasilache, V.; Sandu, I.G.; Negru, I.C.; Sandu, A.V. Authentication of a Painting by Nicolae Grigorescu Using Modern Multi-Analytical Methods. Appl. Sci. 2020, 10, 3558. [Google Scholar] [CrossRef]
- Sandu, I.; Tanasa, O.; Negru, I.C.; Lupascu, M.-M.; Vasilache, V.; Chirila, M. Authentication of a Painting by Rene Magritte. Int. J. Conserv. Sci. 2022, 13, 1445–1462. [Google Scholar]
- Sandu, I.; Lupascu, E.; Sandu, I.C.A.; Ivashko, Y. Artefactometrical Assessment of Works of ArtbBy Summing the Impact Grids of Altmetric Quantification. Egypt. J. Archaeol. Restor. Stud. 2023, 13, 185–196. [Google Scholar] [CrossRef]







| Patina Hue | Composition of Solutions and Treatment Operations |
|---|---|
| Dark brown * | An aqueous mixture of potassium sulfide, barium sulfide, and liquid ammonia or 1 part antimony sulfide, 1 part sodium hydroxide (lye), and 32 parts water, brought to a temperature of 93 °C. applied to the hot piece or 1 part copper sulfate, 1 part potassium chlorate, and 16 parts water, all mixed at room temperature and applied to the cold piece. |
| Reddish brown * | Aqueous solution of yellow barium sulfide and water, heated to 65.5 °C or 5 parts copper sulfate, 2 parts acetic acid, 2 parts sodium hydroxide (lye), and 65 parts water, at 71.1 °C |
| Black * | Arsenic acid 2 parts, 4 parts muriatic acid, 1 part sulfuric acid and 64 parts water or 2 parts copper carbonate, 4 parts ammonium hydroxide, 1 part sodium carbonate, and 32 parts water; the solution is heated to 66–67 °C |
| Bluish green * | Mixture of sodium sulfate and iron nitrate dissolved in water (ratio 4:1:5) or 4 parts sodium sulfate, 1 part sodium sulfate, 1 part iron nitrate, and 64 parts water, the solution is heated to 80 °C and the piece is kept immersed until the desired color appears; or 1 part copper nitrate, 1 part ammonium chloride, 1 part calcium chloride, and 32 parts water, the mixture is used at room temperature; or 8 parts copper sulfate, 4 parts ammonium chloride, 4 parts sodium chloride, 1 part zinc chloride, 3 parts acetic acid, and 128 parts water at normal temperature (for a more rapid effect by gentle heating). |
| Ancient green * | Hot aqueous solution of copper sulfate, ammonium chloride (12:1) or 14 parts ammonium chloride, 3 parts iron chloride, 8 parts sodium chloride, 8 parts green pigment, 4 parts potassium bitartrate, and 128 parts water; the solution should be heated to 93 °C and applied to the heated piece (the higher the concentration, the more intense the color) |
| Raw greens * | Aqueous mixture of sodium chloride, liquid ammonia, ammonium chloride, and vinegar. |
| Yellow greens * | Mixture of ammonium chloride, copper acetate, and water. |
| Blue * | 4 parts sodium sulfate, 3 parts lead acetate and 64 parts water, all heated to 82 °C, or use a mixture of 1 part ammonium chloride and 1 part copper nitrate dissolved in 32 parts water |
| Copper | Zinc | Tin | Color |
|---|---|---|---|
| 84.42 | 11.28 | 4.30 | Reddish yellow |
| 84.00 | 11.00 | 5.00 | Reddish orange |
| 83.05 | 13.03 | 3.92 | Reddish orange |
| 83.00 | 12.00 | 5.00 | Reddish orange |
| 81.05 | 15.32 | 3.63 | Reddish orange |
| 81.00 | 15.00 | 4.00 | Yellow orange |
| 78.09 | 18.47 | 3.44 | Yellow orange |
| 73.58 | 23.27 | 3.15 | Yellow orange |
| 73.00 | 23.00 | 4.00 | Pale orange |
| 70.36 | 26.88 | 2.76 | Pale yellow |
| 70.00 | 27.00 | 3.00 | Pale yellow |
| 65.95 | 31.56 | 2.49 | Pale yellow |
| Type of Base Alloy | Elemental Concentration (%) | |||
|---|---|---|---|---|
| Cu | Sn | Pb | Zn | |
| Egyptian bronze | 85.85 | 15.15 | - | - |
| Greek statuary bronze | 88.50–89.50 | 6.00–9.20 | 3.50 | - |
| Ancient Japanese and Chinese bronzes | 80.00 | 4.00 | 10.00 | 4.00 |
| Roman bronzes | 78.05–89.00 | 2.05–6.00 | 5.00 | - |
| Ancient monetary bronzes | 74.09 | 25.91 | - | - |
| Italian Renaissance bronzes | 75.00 | 25.00 | - | - |
| European Renaissance bronzes | 86.00 | 12.00 | 2.00 | - |
| French bronzes—17th century | 90.00–91.60 | 2.00–1.70 | 1.00–1.37 | 7.00–5.33 |
| French bronzes for monuments | 87.80 | 5.10 | 0.58 | 6.52 |
| Modern-age bronzes | 86.60 | 6.60 | 3.30 | 3.30 |
| Nr. Crt. | Auction House | Date/Time of Sale | Lot | Value | Statue Size |
|---|---|---|---|---|---|
| 1. | Christie’s— similar patterns, light brown patina | 31 May 2000/8369 | 171 | 9.400 USD | 21” H |
| 27 November 2002/1099 | 9519 | 4700 GBP | 21 5/8” H | ||
| 10–11 July 2002/1099 | 1099 | 4780 USD | 21 1/2” H | ||
| 19–20 October 2011/2473 | 12 | 5.250 USD | 21 5/8” H (Bronze Garanti au Titre cast variant). | ||
| 1 April 2003 | 766 | 2.868 USD | 31.8 cm, 12½” H | ||
| 20 October 2011 | 1–23 | 5.530 USD | 55 cm, 21 5/8” H | ||
| 2 December 2004 | 2162 | 1.016 USD | 19.5 cm., 7¾” H | ||
| 22 May 1996 | 12 | 5.500 USD | 55.88 cm | ||
| 2. | Sotheby’s, London—similar designs, yellow brown patina | 22 May 1996 | 88 | 4.800 USD | 55.88 cm |
| 10 May 2005/L03503 | 298 | 11,400 GBP | 22” H (Pinédo variant) | ||
| 28 November 2006/N08232 | 52 | 5100 USD | 23” H (Bronze Garanti au Titre cast variant) | ||
| 29 November 2006/N08233 | 53 | 4800 USD | 22” H (Pinédo variant) | ||
| 20 April 2007/N08305 | 271 | 5400 USD | 71 cm, 28” H | ||
| 22 April 2010/N08627 | 82 | 9375 USD | 55.9 cm, 22” H | ||
| 2 June 2010/ | 271 | 6875 GBP | 58.5 cm, 23” H | ||
| 1 April 2010/N08623 | 283 | 7500 USD | 22” H | ||
| 3. | Cowan’s, Cincinnati—dark brown patina | 11 July 2014 | 733 | - | 52.07 cm |
| 11 October 2014 | 893 | - | 54.61 cm | ||
| 4. | Heritage Auctions, Dallas—dark brown patina | 12 September 2015 | 347 | - | 55.88 cm |
| 5. | Heritage Auctions, Dallas; Historia Auktionshaus, Berlin—light brown patina | 25 February 2015 | 3803 | - | 32 cm |
| 6. | Weschler’s, Washington—golden brown patina | 5 December 2015 | 163 | - | 55.88 cm |
| 7. | Freeman’s, Philadelphia—gray–brown patina | 20 May 2014 | 347 | - | 55.88 × 38.1 × 27.94 cm |
| 26 October 2011 | 399 | - | 31.6 cm | ||
| 8. | Erich Pillon Encheres, Versailles—grayish brown patina | 16 March 2014 | 13 | - | 32 cm |
| 9. | Schuler Auktionen, Zurich—golden brown patina | 10 December 2012 | 3079 | - | 45 cm |
| 18 May 2013 | 3059 | - | 45 cm | ||
| 10. | Jackson’s International Auctioneers & Appraisers, Cedar Falls—chocolate brown patina | 15 Nov 2011 | 322 | - | 55.5 cm |
| 17 July 2007 | 952 | - | 20.32 cm | ||
| 11. | Uppsala Aucktionskammare, Uppsala—grayish brown patina | 09 December 2001 | 258 | - | 60.96 cm |
| 12. | Pook & Pook, Inc., Downingtown—chocolate brown patina | 23 March 2007 | 58 | - | 55.88 cm |
| 21 November 2008 | 108 | - | 54.61 cm | ||
| 26 October 2007 | 165 | - | 31.12 cm |
| Detail No. | Replica Presented by Christie’s Auction House | Studied Replica | Detail No. | Replica Presented by Christie’s Auction House | Studied Replica |
|---|---|---|---|---|---|
| 1. |  |  | 2. |  |  |
| 3. |  |  | 4. |  |  |
| 5. |  |  | 6. |  |  |
| 7. |  |  | 8. |  |  |
| 9. |  |  | 10. |  |  |
| 11. |  |  | 12. |  |  |
| 13. |  |  | 14. |  |  |
| 15. |  |  | 16. |  |  |
| 17. |  |  | 18. |  |  |
| 19. |  |  | 20. |  |  |
| 21. |  |  | 22. |  |  |
| 23. |  |  |
| Sample | Chemical Element | X-ray Spectra | Normal Wt (%) | Normal At (%) | Error (%) |
|---|---|---|---|---|---|
| S1 (zone 123, 400× SE, central base alloy, area in Figure 6, B) | Copper | Series-K | 75.695 | 82.484 | 2.444 |
| Zinc | Series-K | 11.814 | 12.511 | 0.488 | |
| Tin | Series-K | 3.333 | 1.944 | 0.383 | |
| Lead | Series-K | 9.156 | 3.060 | 0.521 | |
| Total | 100 | 100 | |||
| S2 (zone 124 400× SE, bottom base alloy, area in Figure 6, D) | Copper | Series-K | 77.196 | 83.348 | 2.445 |
| Zinc | Series-K | 11.521 | 12.089 | 0.484 | |
| Tin | Series-K | 3.347 | 1.934 | 0.368 | |
| Lead | Series-K | 7.933 | 2.627 | 0.503 | |
| Total | 100 | 100 | |||
| S3 (zone 125, 200× central base alloy, area in Figure 6, B) | Copper | Series-K | 71.382 | 80.503 | 2.238 |
| Zinc | Series-K | 11.235 | 12.314 | 0.501 | |
| Tin | Series-K | 4.539 | 2.740 | 0.708 | |
| Lead | Series-K | 12.841 | 4.441 | 0.776 | |
| Total | 100 | 100 | |||
| S4 (zone 118, center patina up, area in Figure 6, C) | Copper | Series-K | 42.000 | 19.406 | 1.196 |
| Zinc | Series-K | 6.674 | 2.997 | 0.240 | |
| Lead | Series-K | 4.092 | 0.579 | 0.212 | |
| Tin | Series-K | 3.562 | 0.881 | 0.239 | |
| Iron | K-series | 0.488 | 0.256 | 0.052 | |
| Calcium | K-series | 0.095 | 0.069 | 0.034 | |
| Sodium | K-series | 7.049 | 9.003 | 4.648 | |
| Aluminum | K-series | 0.334 | 0.363 | 0.062 | |
| Silicon | K-series | 0.302 | 0.316 | 0.054 | |
| Carbon | K-series | 4.487 | 10.970 | 2.337 | |
| Sulfur | K-series | 0.851 | 0.779 | 0.093 | |
| Chlorine | K-series | 0.725 | 0.600 | 0.070 | |
| Phosphorus | K-series | 0.064 | 0.060 | 0.036 | |
| Oxygen | K-series | 29.269 | 53.713 | 5.054 | |
| Total | 100 | 100 | |||
| S5 (zone 119, center patina top right, area in Figure 6, A) | Copper | Series-K | 52.859 | 27.661 | 1.658 |
| Zinc | Series-K | 8.381 | 4.261 | 0.333 | |
| Lead | Series-K | 4.809 | 0.771 | 0.291 | |
| Tin | Series-K | 2.789 | 0.781 | 0.220 | |
| Iron | K-series | 0.449 | 0.267 | 0.058 | |
| Calcium | K-series | 0.049 | 0.041 | 0.033 | |
| Aluminum | K-series | 0.169 | 0.208 | 0.056 | |
| Silicon | K-series | 0.213 | 0.252 | 0.055 | |
| Carbon | K-series | 5.894 | 16.320 | 3.128 | |
| Sulfur | K-series | 0.463 | 0.480 | 0.078 | |
| Chlorine | K-series | 0.597 | 0.560 | 0.075 | |
| Phosphorus | K-series | 0.082 | 0.088 | 0.040 | |
| Oxygen | K-series | 23.239 | 48.302 | 5.185 | |
| Total | 100 | 100 | |||
| S6 (zone 120, central crust patina, mobile, area in Figure 6, A) | Copper | Series-K | 33.718 | 13.682 | 1.042 |
| Zinc | Series-K | 5.678 | 2.239 | 0.220 | |
| Lead | Series-K | 3.951 | 0.491 | 0.208 | |
| Tin | Series-K | 3.119 | 0.677 | 0.191 | |
| Sodium | K-series | 8.315 | 9.326 | 5.303 | |
| Aluminum | Series-K | 0.653 | 0.624 | 0.084 | |
| Carbon | K-series | 5.732 | 12.307 | 1.588 | |
| Sulfur | K-series | 1.287 | 1.035 | 0.108 | |
| Chlorine | Series-K | 0.762 | 0.554 | 0.071 | |
| Phosphorus | Series-K | 0.276 | 0.230 | 0.058 | |
| Oxygen | Series-K | 36.504 | 58.831 | 6.049 | |
| Total | 100 | 100 | |||
| S7 (zone 121, mobile crystallites, agglomerates, area in Figure 6, A) | Copper | Series-K | 33.683 | 14.116 | 1.110 |
| Zinc | Series-K | 5.490 | 2.236 | 0.242 | |
| Tin | Series-K | 4.672 | 1.048 | 0.355 | |
| Lead | Series-K | 5.508 | 0.707 | 0.311 | |
| Iron | Series-K | 0.674 | 0.321 | 0.065 | |
| Chrome | Series-K | 0.332 | 0.170 | 0.050 | |
| Calcium | Series-K | 0.132 | 0.087 | 0.039 | |
| Magnesium | Series-K | 0.672 | 0.736 | 0.114 | |
| Aluminum | Series-K | 1.259 | 1.243 | 0.139 | |
| Silicon | Series-K | 1.124 | 1.066 | 0.114 | |
| Carbon | K-series | 6.494 | 14.399 | 2.342 | |
| Sulfur | K-series | 1.376 | 1.142 | 0.130 | |
| Chlorine | Series-K | 1.226 | 0.921 | 0.105 | |
| Phosphorus | Series-K | 0.464 | 0.399 | 0.069 | |
| Oxygen | Series-K | 36.888 | 61.402 | 8.730 | |
| Total | 100 | 100 | |||
| S8 (zone 122, 400× BSE, central patina crystallite, area in Figure 6, A) | Copper | Series-K | 38.915 | 17.185 | 1.239 |
| Zinc | Series-K | 5.583 | 2.396 | 0.241 | |
| Tin | Series-K | 2.952 | 0.697 | 0.274 | |
| Lead | Series-K | 4.132 | 0.559 | 0.254 | |
| Iron | Series-K | 0.305 | 0.153 | 0.050 | |
| Sodium | Series-K | 9.007 | 10.995 | 6.835 | |
| Magnesium | Series-K | 0.211 | 0.108 | 0.045 | |
| Calcium | Series-K | 0.109 | 0.076 | 0.038 | |
| Aluminum | Series-K | 0.791 | 0.823 | 0.108 | |
| Silicon | Series-K | 0.424 | 0.424 | 0.070 | |
| Carbon | K-series | 4.402 | 10.285 | 1.866 | |
| Sulfur | K-series | 0.743 | 0.650 | 0.095 | |
| Phosphorus | Series-K | 0.178 | 0.162 | 0.051 | |
| Chlorine | Series-K | 1.108 | 0.877 | 0.099 | |
| Oxygen | Series-K | 31.132 | 54.604 | 5.881 | |
| Total | 100 | 100 | |||
| S9 (zone 127, screw, external thread 100× coated, area in Figure 6, D) | Copper | Series-K | 49.719 | 24.426 | 1.549 |
| Zinc | Series-K | 7.597 | 3.627 | 0.308 | |
| Tin | Series-K | 2.524 | 0.664 | 0.179 | |
| Lead | Series-K | 4.120 | 0.620 | 0.267 | |
| Iron | Series-K | 0.480 | 0.268 | 0.059 | |
| Aluminum | Series-K | 0.961 | 1.112 | 0.128 | |
| Silicon | Series-K | 2.920 | 3.246 | 0.228 | |
| Carbon | Series-K | 8.308 | 21.595 | 2.864 | |
| Phosphorus | Series-K | 0.142 | 0.143 | 0.049 | |
| Sulfur | Series-K | 0.485 | 0.472 | 0.086 | |
| Chlorine | Series-K | 0.508 | 0.447 | 0.077 | |
| Oxygen | Series-K | 22.228 | 43.373 | 5.230 | |
| Total | 100 | 100 | |||
| S10 (zone 128, screw with thread recess deposition, 100×, area in Figure 6, D) | Copper | Series-K | 2.362 | 0.893 | 0.188 |
| Zinc | Series-K | 4.012 | 1.517 | 0.315 | |
| Tin | Series-K | 5.146 | 1.072 | 0.904 | |
| Lead | Series-K | 6.626 | 0.791 | 0.591 | |
| Iron | Series-K | 10.355 | 4.587 | 0.514 | |
| Calcium | Series-K | 0.840 | 0.518 | 0.638 | |
| Magnesium | Series-K | 0.898 | 0.914 | 0.190 | |
| Bariu | Series-K | 9.445 | 1.701 | 0.528 | |
| Aluminum | Series-K | 3.565 | 3.269 | 0.378 | |
| Silicon | Series-K | 7.873 | 6.934 | 0.599 | |
| Carbon | Series-K | 7.696 | 15.851 | 3.899 | |
| Sulfur | Series-K | 3.072 | 2.370 | 0.381 | |
| Phosphorus | Series-K | 0.351 | 0.281 | 0.087 | |
| Oxygen | Series-K | 38.529 | 59.572 | 10.680 | |
| Total | 100 | 100 | |||
| S11 (zone 129, thread recess deposition, 300×, area in Figure 6, D) | Copper | Series-K | 5.850 | 2.434 | 0.346 |
| Zinc | Series-K | 2.306 | 0.932 | 0.221 | |
| Tin | Series-K | 8.595 | 1.914 | 1.024 | |
| Lead | Series-K | 13.646 | 1.741 | 0.895 | |
| Iron | Series-K | 3.577 | 1.693 | 0.235 | |
| Calcium | Series-K | 3.774 | 2.490 | 1.202 | |
| Potassium | Series-K | 1.557 | 1.053 | 0.166 | |
| Magnesium | Series-K | 1.445 | 1.572 | 0.203 | |
| Aluminum | Series-K | 4.000 | 3.920 | 0.327 | |
| Silicon | Series-K | 11.275 | 10.615 | 0.632 | |
| Carbon | Series-K | 5.812 | 12.793 | 9.390 | |
| Sulfur | Series-K | 2.285 | 1.884 | 0.224 | |
| Chlorine | Series-K | 1.408 | 1.050 | 0.170 | |
| Phosphorus | Series-K | 1.312 | 1.120 | 0.145 | |
| Oxygen | Series-K | 33.151 | 54.783 | 17.017 | |
| Total | 100 | 100 |
| Sample | Main Alloy Components | |||
|---|---|---|---|---|
| Cu (%) | Zn (%) | Sn (%) | Pb (%) | |
| S1 (freshly cut area with homogeneous surface) | 49.44 */25.00 ** | 9.38 */4.60 ** | 2.45 */0.66 ** | 5.13 */0.79 ** |
| S2 (freshly cut area with non-homogeneous surface) | 54.28 */32.44 ** | 4.43 */2.57 ** | 3.28 */1.05 ** | 10.54 */1.93 ** |
| S3 (freshly cut area) | 74.15 */76.87 ** | 10.79 */10.87 ** | 3.55 */2.00 ** | 7.62 */2.42 ** |
| S4 (old patina area) | 33.67 */12.92 ** | 5.74 */2.14 ** | 3.32 */0.68 ** | 2.88 */0.34 ** |
| Sample | Chemical Element | X-ray Spectra | Normal Wt (%) | Normal At (%) | Error (%) |
|---|---|---|---|---|---|
| S1 (central zone, cross-section, area in Figure 6, D) | Copper | Series-K | 80.532 | 85.093 | 2.614 |
| Zinc | Series-K | 11.900 | 12.220 | 0.471 | |
| Tin | Series-K | 0.970 | 0.549 | 0.142 | |
| Lead | Series-K | 6.597 | 2.138 | 0.404 | |
| Total | 100 | 100 | |||
| S2 (marginal zone, cross-section, area in Figure 6, D) | Copper | Series-K | 77.589 | 83.710 | 2.746 |
| Zinc | Series-K | 11.762 | 12.333 | 0.617 | |
| Tin | Series-K | 1.753 | 1.013 | 0.454 | |
| Lead | Series-K | 8.896 | 2.944 | 0.722 | |
| Total | 100 | 100 |
| Sample | Chemical Element | X-ray Spectra | Normal Wt (%) | Normal At (%) | Error (%) |
|---|---|---|---|---|---|
| S1 (cleaned area, Figure 6, C) | Copper | Series-K | 49.436 | 24.975 | 2.049 |
| Zinc | Series-K | 9.376 | 4.603 | 0.512 | |
| Tin | Series-K | 2.451 | 0.663 | 0.350 | |
| Lead | Series-K | 5.125 | 0.794 | 0.474 | |
| Iron | Series-K | 2.227 | 1.280 | 0.170 | |
| Aluminum | Series-K | 2.260 | 2.688 | 0.332 | |
| Silicon | Series-K | 1.326 | 1.516 | 0.203 | |
| Carbon | Series-K | 11.565 | 30.910 | 4.129 | |
| Oxygen | Series-K | 16.234 | 32.571 | 6.200 | |
| Total | 100 | 100 | |||
| S2 (unfinished area, Figure 6, C) | Copper | Series-K | 54.282 | 32.441 | 2.054 |
| Zinc | Series-K | 4.420 | 2.567 | 0.339 | |
| Tin | Series-K | 3.277 | 1.048 | 0.449 | |
| Lead | Series-K | 10.544 | 1.933 | 0.799 | |
| Iron | Series-K | 0.963 | 0.655 | 0.131 | |
| Silicon | Series-K | 2.549 | 3.447 | 0.311 | |
| Aluminum | Series-K | 2.150 | 3.026 | 0.328 | |
| Carbon | Series-K | 3.933 | 12.435 | 2.434 | |
| Oxygen | Series-K | 17.882 | 42.448 | 6.716 | |
| Total | 100 | 100 | |||
| S3 (rough area, Figure 6, C) | Copper | Series-K | 74.146 | 76.869 | 2.169 |
| Zinc | Series-K | 10.788 | 10.868 | 0.388 | |
| Tin | Series-K | 3.545 | 1.968 | 0.315 | |
| Lead | Series-K | 7.606 | 2.418 | 0.378 | |
| Iron | Series-K | 1.119 | 1.320 | 0.082 | |
| Silicon | Series-K | 2.796 | 6.557 | 0.215 | |
| Oxygen | |||||
| Total | 100 | 100 | |||
| S4 (patina, Figure 6, C) | Copper | Series-K | 33.670 | 12.921 | 1.133 |
| Zinc | Series-K | 5.741 | 2.141 | 0.230 | |
| Tin | Series-K | 3.316 | 0.681 | 0.194 | |
| Lead | Series-K | 2.880 | 0.339 | 0.163 | |
| Iron | Series-K | 0.552 | 0.241 | 0.052 | |
| Carbon | Series-K | 10.750 | 21.825 | 2.503 | |
| Silicon | Series-K | 2.268 | 1.969 | 0.171 | |
| Aluminum | Series-K | 2.178 | 1.969 | 0.187 | |
| Phosphorus | Series-K | 0.559 | 0.440 | 0.066 | |
| Chlorine | Series-K | 0.687 | 0.472 | 0.067 | |
| Oxygen | Series-K | 37.399 | 57.002 | 6.654 | |
| Total | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandu, I.; Drobota, V.; Drob, A.; Sandu, A.V.; Vasilache, V.; Iurcovschi, C.T.; Sandu, I.G. Authentication of a Bronze Bust of Napoleon I, Attributed to Renzo Colombo from 1885. Heritage 2024, 7, 5748-5773. https://doi.org/10.3390/heritage7100270
Sandu I, Drobota V, Drob A, Sandu AV, Vasilache V, Iurcovschi CT, Sandu IG. Authentication of a Bronze Bust of Napoleon I, Attributed to Renzo Colombo from 1885. Heritage. 2024; 7(10):5748-5773. https://doi.org/10.3390/heritage7100270
Chicago/Turabian StyleSandu, Ion, Vasile Drobota, Ana Drob, Andrei Victor Sandu, Viorica Vasilache, Cosmin Tudor Iurcovschi, and Ioan Gabriel Sandu. 2024. "Authentication of a Bronze Bust of Napoleon I, Attributed to Renzo Colombo from 1885" Heritage 7, no. 10: 5748-5773. https://doi.org/10.3390/heritage7100270
APA StyleSandu, I., Drobota, V., Drob, A., Sandu, A. V., Vasilache, V., Iurcovschi, C. T., & Sandu, I. G. (2024). Authentication of a Bronze Bust of Napoleon I, Attributed to Renzo Colombo from 1885. Heritage, 7(10), 5748-5773. https://doi.org/10.3390/heritage7100270








