Abstract
Recently, a notable change has occurred in how street art murals are perceived by art history and the general public, with a growing recognition of their social and cultural significance and a new focus on preserving the most representative modern urban murals for future generations. An interesting case study is constituted by the “UBUNTU” mural (Ivan Pontevia and Daniele Castagnetti, Reggio Emilia, 2018), whose appearance has radically changed in a few years. Indeed, the intense and direct exposure to sunlight as well as the environmental and polluting agents have induced the bleaching and fading of the original highly fluorescent hues. To investigate the degradation processes that are occurring, five micro-samples were collected from different fluorescent-coloured areas and analysed by a combined approach based on µ-Raman and Surface Enhanced Raman Spectroscopy (SERS), High Performance Liquid Chromatography coupled with Diode Array Detector and mass spectrometry (HPLC-DAD and HPLC-MS), and Pyrolysis Gas Chromatography coupled with Mass Spectrometry (Py-GC-MS). The analytical protocol applied allowed us to disclose the painting materials used by the artist and fully characterise the ageing phenomena occurring in the mural that are possibly responsible for its colour ephemerality.
1. Introduction
The recognition of street art as an integral part of the cultural heritage has emerged only in recent years. The fleeting character, free access, and exposure to the environment and anthropic action make public paintings vulnerable to overpainting and degradation [1]. Therefore, in the last decade, several studies have reported the characterization of spray-paint formulations [2,3,4,5] to disclose the chemical composition of street-art paint materials, to better understand the mutual interaction of the materials and to evaluate the role played by UV light and environmental parameters on their ageing processes [6,7,8,9,10]. In order to slow down or prevent the extensive degradation of murals, the efficiency of several kinds of protective agents was tested [4,11,12]. Nevertheless, studies focused on the analysis of street art murals using in situ or micro-invasive spectroscopic techniques [13,14,15,16,17] as well as pyrolysis, chromatographic, and mass spectrometric methods [15,18] are still relatively limited.
Considering the challenges in defining conservation strategies in this new research field, an integrated protocol for the diagnostics of modern paint materials of street artworks and for a long-term sustainable conservation is highly needed. The optimisation and integration of analytical methods is one of the main goals of the PRIN2020 project “SUPERSTAR—Sustainable Preservation Strategies for Street Art” (2022–2025), an Italian network project [19].
“UBUNTU” is a mural painting (32 m × 2 m, [20]) created by Ivan Pontevia and Daniele Castagnetti in collaboration with the Municipality of Reggio Emilia and Officina Educativa on the external wall of Dalla Chiesa middle school (Reggio Emilia, Italy) in July 2018. “UBUNTU” is included among the selected artworks for the Erasmus+ project “CAPUS—Conservation of Art in Public Spaces” [20], aiming at investigating the history of the artworks, characterizing the paint materials and degradation processes, and selecting the methods and materials for conservation [21]. Already a few years after the creation of the mural, severe fading and colour alteration phenomena are observed (Figure 1), mainly in the fluorescent sprays, radically changing and compromising the appearance of the mural. A previous study was conducted within the CAPUS project, aimed at characterizing the composition of paint mock-ups prepared with unaged, naturally aged and artificially aged commercial spray series (MNT94 and Montana Cans) to mimic the degradation processing occurring on the artworks, evaluating the effect of different environmental conditions [4]. The materials were specifically selected amongst those possibly used by Ivan Pontevia, Daniele Castagnetti and other contemporary street artists who were the authors of other murals in Reggio Emilia. Conversely, the present work is focused on actual painting samples taken from the areas of the flag of the “UBUNTU” mural, which appeared as the most faded. Previous studies of the archive documents (e.g., invoices and pictures of the realisation phases) pointed specifically at the use of fluorescent spray paints from MNT94 (Montana Colors, Barcelona, Spain) [4].
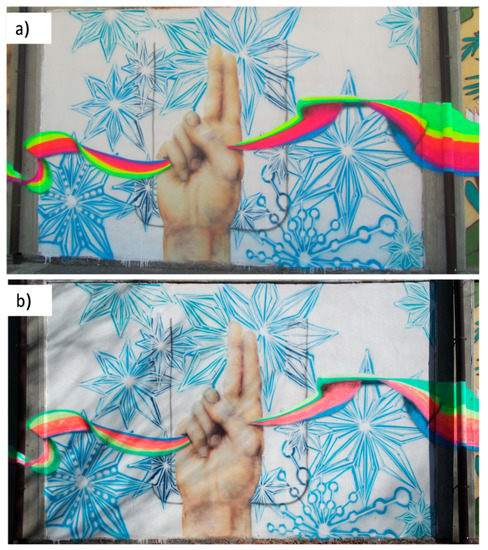
Figure 1.
Ubuntu (a) after its production in 2018, and (b) after one year of exposure to the environment in 2019. Adapted from [4].
The five samples were analysed by non-invasive and micro-invasive techniques (µ-Raman, SERS, HPLC-DAD, HPLC-MS and Py-GC-MS) to determine the composition and degradation of the paint materials used and to investigate, at a molecular level, the causes of the fast bleaching of this street art mural. The possible presence of rutile, which can exert a photocatalytic action towards photo-oxidation [22], as hypothesized for “Oriental Carpet” by H101 [13], was also assessed.
2. Materials and Methods
2.1. Sampling
Five samples were collected from each of the five hues of the fluorescent flag depicted in Figure 1: blue (S1B), green (S2G), pink (S3P), yellow (S4Y) and orange (S5O). Samples were collected in 2021 and have been analysed by µ-Raman, SERS, HPLC-DAD, HPLC-MS and Py-GC-MS.
2.2. µ-Raman Spectroscopy and SERS Analysis
For both µ-Raman and SERS analyses, an InVia Raman microscope with CCD (Renishaw; England) with grating 1800 and 1200 groove/mm was used. The 532 nm line of a NgYAG laser (50 mW maximum output) was used for µ-Raman, while the 785 nm diode laser (1 mW maximum output) was used for SERS. For µ-Raman, a resolution of 5 cm−1 (1800 rulings/mm grating), an integration time of 10 s, and 10 scans were used, while for SERS, the resolution was 5 cm−1 (1200 rulings/mm grating), the integration time 10 s, and 1 scan was used.
Prior to SERS analyses, the silver colloid was prepared following the Lee and Meisel procedure, then concentrated by centrifugation for 5′ at 10,000× g, after which the supernatant was removed. A few mg of sample were dissolved in 50 μL acetone. The measurements were performed while focusing through a drop constituted of 5 μL of silver colloid, 1 μL of the dye solution in acetone, and then 1 μL of 0.5 M potassium nitrate deposited on a slide.
2.3. Liquid Chromatography with Spectrophotometric (HPLC-DAD) and Mass Spectrometric Detection (HPLC-MS) Analyses
For HPLC-DAD analyses, a PU-2089 pump coupled to a spectrophotometric diode array detector MD-2010 (Jasco international Co.; Tokyo, Japan) was used. The spectral acquisition range was 200–650 nm, and the resolution was 4 nm. For the HPLC-MS analyses, an ESI-Q-ToF (Agilent Technologies, Lexington, MA, USA) was used. The ESI conditions were: drying gas N2, purity > 98%, temperature 350 °C, flow 10 L/min; sheath gas N2, temperature 375 °C, flow 11 L/min; capillary voltage 4.5 kV; and nebulizer gas pressure 35 psi. The MS parameters were: fragmentor voltage 175 V, nozzle voltage 1000 V, skimmer voltage 65 V and octapole RF voltages 750 V. The high-resolution mass spectrometric (MS) and tandem mass spectrometric (MS/MS) acquisition ranges were set from 100 to 1000 m/z in negative and positive mode, with an acquisition rate of 1.04 spectra/s. For the MS/MS experiments, 30 V was applied in the collision cell to obtain CID fragmentation (collision gas N2, purity 99.999%). The FWHM (full width half maximum) of the quadrupole mass bandpass during MS/MS precursor isolation was set at 4 m/z. The Agilent tuning mix HP0321 was used to calibrate the mass axis daily. The separation and eluents for the HPLC–DAD and HPLC-MS systems were water and acetonitrile (ACN), both HPLC grade (Sigma Aldrich, St Louis, MO, USA), while for HPLC-ESI-Q-ToF analyses, they were water and acetonitrile, both LC–MS grade (Sigma-Aldrich, St Louis, MO, USA). All eluents were added with 0.1% v/v formic acid (FA; 98% purity, J.T. Baker, Phillipsburg, NJ, USA). The chromatographic separation was carried out using an analytical reversed-phase column Poroshell 120 EC-C18 (3.0 × 75 mm, particle size 2.7 μm) with a Zorbax pre-column (4.6 × 12.5 mm, particle size 5 μm), both Agilent Technologies, and the selected flow rate was 0.4 mL/min. The elution programme was as follows: 15% B (0.1% FA in ACN) for 2.6 min, then to 50% B in 13.0 min, to 70% B in 5.2 min, to 100% B in 0.5 min and then held for 6.7 min. Re-equilibration took 11 min and column temperature was set at 30 °C.
Prior to the analyses, 0.3 mg of sample was dissolved in 300 μL of DMSO, heated in an ultrasonic bath for 10′ at 60 °C, filtered with PTFE syringe filters, and 10 μL and 5 μL were injected in HPLC-DAD and HPLC-MS, respectively.
2.4. Pyrolysis-Gas Chromatography-Mass Spectrometry Analyses
For analytical pyrolysis, a Multi-Shot Pyrolyzer EGA/Py-3030D (Frontier Laboratories; Fukushima, Japan) coupled to a 8890 gas chromatograph, combined with a 5977B mass selective
single quadrupole mass spectrometer detector (Agilent Technologies, Palo Alto, CA, USA), was used. The separation took place on a HP-5MS fused silica capillary column (stationary phase
5% diphenyl-95% dimethyl-polysiloxane, 30 m × 0.25 mm i.d., 0.25 μm, J&W Scientific, Agilent Technologies) preceded by 2 m of deactivated fused silica pre-column with an
internal diameter of 0.32 mm. The Py furnace temperature was set at 600 °C, Py-GC interface temperature at 280 °C, GC injector temperature at 280 °C, and split ratio at
1:10. The chromatographic conditions selected were: 40 °C for 5 min, 10 °C/min to 310 °C for 20 min. Helium (purity 99.9995%) gas flow was set in constant flow mode at
1.2 mL/min. The MS parameters were: electron impact ionization (EI, 70 eV) in positive mode, ion source temperature 230 °C, and scan range 50–700 m/z.
3. Results and Discussion
The results obtained by the different techniques are presented in the following paragraphs and summarised in Table 1.

Table 1.
Summary of the materials detected in the samples. Materials found in traces are reported as (tr). PB15 = Pigment Blue 15; PG 7 = Pigment Green 7; BR1 = Basic Red 1; BV10 = Basic Violet 10; PY74 = Pigment Yellow 74; MMA-nBA = methyl methacrylate/n-butyl acrylate; nBMA = n-butyl methacrylate; PVAc = polyvinyl acetate.
3.1. Raman Spectroscopy and SERS Analysis
µ-Raman analyses performed on blue, green and yellow spray paints revealed the presence of phthalocyanines, synthetic organic pigments featuring brilliant hues and exhibiting fluorescence, such as Pigment Blue 15 (PB15; C.I. 74160; main peaks at 678, 745, 1339, 1446 and 1527 cm−1 [23]) in samples S1B and S2G (Figure 2a), and Pigment Green 7 (PG7; C.I. 74160; main peaks at 682, 734, 772, 814, 1210, 1279, 1336 and 1535 cm−1 [23]) in samples S2G and S4Y (Figure 2b). The spectra of samples S3P, S4Y and S5O show peaks due to titanium dioxide (TiO2) in the rutile form (main peaks at 445 and 607 cm−1), usually used as thickener or filler in commercial spray paints [4].
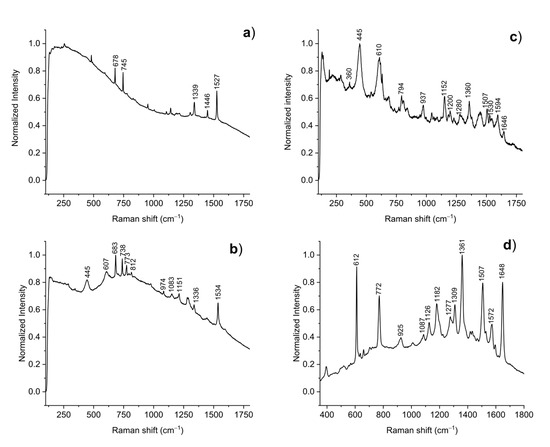
Figure 2.
Micro-Raman performed on samples (a) S1B, (b) S4Y, and (c) S5O collected from Ubuntu. (d) SERS spectrum collected for sample S5O.
The application of SERS provided complementary information to that obtained from µ-Raman. Spray formulations of samples S3P, S4Y and S5O all contained xanthenes, a class of highly fluorescent synthetic organic pigments. Raman spectra (see Figure 2c) pointed at the use of Rhodamine B (BV10; C.I. 45170; main peaks at 622, 1200, 1360, 1507, 1530 and 1646 cm−1 [24]); conversely, SERS (Figure 2d) suggested the use of Rhodamine 6G (BR1; C.I. 45160; main peaks at 612, 772, 1182, 1361, 1504 and 1648 cm-1). While the presence of red xanthenes is expected in pink and orange samples, their occurrence in yellow sample S4Y can be explained by a contamination due to the adjacent orange area of the mural.
In samples S2G and S4Y, the triarylmethane dye Crystal Violet (PV39; C.I. 42555; main peaks at 423, 806, 1178, 1371, 1540, 1590 and 1622 cm−1 [25]) was found (Supplementary Materials, Figure S1). Its presence can be due to the intentional addition of minor amounts of such a colorant to the spray formulations, but an environmental contamination cannot be ruled out, since triarylcarbonium dyes display an intense response in Raman SERS and can thus be detected even at extremely low concentrations.
3.2. HPLC-DAD-MS
All the samples were analysed by HPLC-DAD and HPLC-MS, but only the pigments in samples S1B (blue), S3P (pink), S4Y (yellow), and S5O (orange) were soluble in the tested organic solvents, being phthalocyanines that are extremely difficult to extract. The HPLC-DAD chromatograms of sample S1B only featured one peak, ascribed to Pigment Yellow 74 (PY74; C.I. 11741; [M−] = 385.114), an azo pigment widely used in spray cans [4,13], possibly present as a contaminant in the blue layer. Conversely, samples S3P (pink) and S5O (orange) display several peaks, whose maximum of absorbance (500–550 nm) and shape of UV-Vis spectra suggest the use of xanthenes [26]. The application of HPLC-MS acquired in positive ionization mode (Figure 3) allowed us to unequivocally ascribe to each peak the corresponding xanthene compound, while the acquisition in negative mode revealed the presence of traces of Pigment Yellow 74, in the extracts of both samples. The molecular profile of samples S3P (pink) and S5O (orange) featured the peaks of Rhodamine B (Rh B [M+] = 443.241) and Rhodamine 6G (Rh 6G; [M+] = 443.241) and their relative de-ethylated forms (DeRh B and DeRh 6G [M+] = 415.205, BisDeRh B and BisDeRh 6G [M+] = 387.172, TrisDeRh B [M+] = 359.141). The two rhodamines and their de-ethylated products are isomeric and elute close in time; thus, their unequivocal identification can only be performed on the basis of the interpretation of the tandem mass spectra and in comparison with analytical standards [27,28]. De-ethylated forms are due to the N-dealkylation process occurring in some xanthene and triarylmethane dyes with photo-ageing [28,29,30,31]. Some degraded forms of RhB have also been detected by Laser Desorption Mass Spectrometry (LDI-MS) in red pen inks [32], LDI-MS and Matrix-Assisted LDI (MALDI-MS) being versatile, rapid and sensitive methods used for the analysis of synthetic organic pigments and degradation processes in reference materials [33,34] and contemporary paintings [35,36]. Considering the distribution of the de-ethylated forms of Rh B and Rh 6G, an equivalent lightfastness for the two pigments in this matrix can be suggested. The observed fading phenomenon might be the result of the partial decrease of the relative amounts of Rh B and Rh 6G in favour of lesser amount of de-ethylated products, whose maxima of absorbance in the corresponding UV-Vis spectra are blue-shifted with respect to the original molecules, thus influencing the final hue [28,37]. Although the two samples have the same qualitative composition and the two colourants have been reported to be occasionally used together [29], the differences in the relative intensity of the xanthenes may explain the slightly different hues of the two sprays. Indeed, sample S3P (pink) was taken from a pink area and is richer in Rh B (λabs max = 556 nm), while sample S5O (orange) is more orangish and, consistently, richer in Rh 6G (λabs max = 527 nm). The composition in terms of synthetic organic pigments differs with respect to that of reference spray materials analysed in [4], and thus a direct comparison and correlation between the artificial ageing experiments performed on mock-ups and the case study cannot be drawn at this stage. The degree of observed degradation might also have been promoted by the presence of rutile, detected in the pigments by Raman spectroscopy, acting as a photo-catalyst [22].
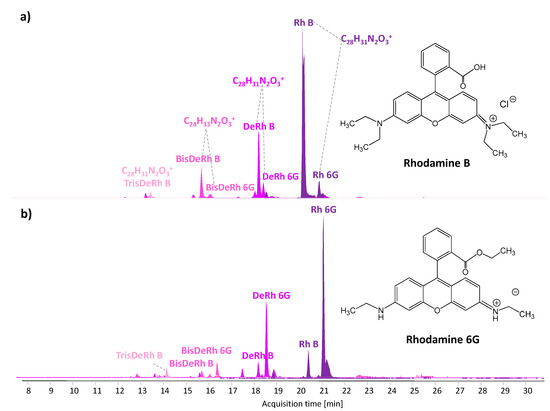
Figure 3.
HPLC-MS-extracted ion chromatograms (positive ionization mode) highlighting the presence of Rhodamine B and Rhodamine 6G along with their photo-ageing products in the extracts of (a) sample S3P and (b) sample S5O.
With regard to the yellow sample S4Y, the HPLC-DAD chromatogram displays a small peak at 19.2 min with a maximum of absorbance at 450 nm, typical of an orange-yellow compound (Supplementary Materials, Figure S2). Unfortunately, such a peak did not correspond to any of our standards or reference materials, nor with literature data [38]. Unexpectedly, a matching peak was not found in HPLC-MS chromatograms, and thus the identification of the yellow fluorescent component of sample S4Y remains unknown.
3.3. Py-GC-MS
The chemical composition of the binders of all the spray paints was characterised using analytical pyrolysis coupled with GC/MS. The Py-GC-MS chromatograms obtained for sample S1B (a) and sample S5O (b) are shown in Figure 4. Specifically, the identification of pyrolysis markers allows us to determine not only the type of binders but also their molecular composition. Samples S2G, S3P and S5O contained the same acrylic resin made of a copolymer based on methyl methacrylate/n-butyl acrylate (MMA-nBA) and n-butyl methacrylate (nBMA). These results were in agreement with the composition of the binders detected in the reference materials previously analysed and reported in [4]. The binder detected for sample S4Y was characterised by the presence of the copolymer MMA-nBA only. Finally, sample S1B featured a more complex formulation: a mixture of acrylic resin (MMA-nBA), a styrene-modified alkyd resin, and polyvinyl acetate (PVAc).
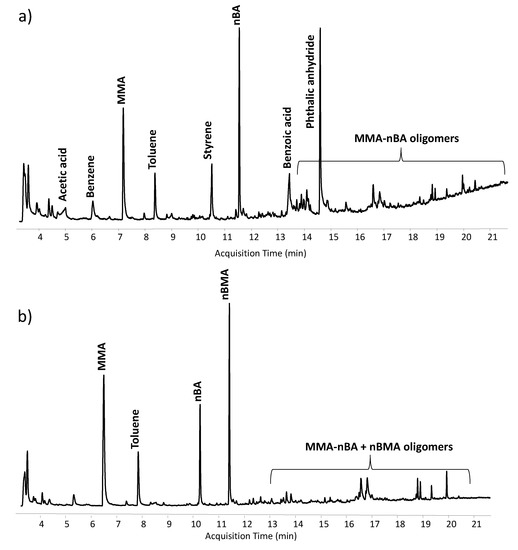
Figure 4.
Py-GC-MS chromatograms obtained for (a) sample S1B and (b) sample S5O.
Acrylic resins have emerged as a cornerstone of modern paint formulations, revolutionising the industry with their exceptional versatility and performances. These resins, derived from acrylic monomers, offer a wide range of advantages when used as binders in paints. Acrylic-based paints exhibit a remarkable adhesion to various surfaces, including metal, wood, and plastics, ensuring long-lasting and durable coatings. Acrylic resins represent one of the primary groups of paint binders used in street art. In particular, the copolymers detected in these paint samples are compatible with the formulation of water-based acrylic resins that have gradually replaced the traditional solvent-based resins since the 1970s [39,40,41]. Acrylic resins are characterised by a high stability towards photo-ageing, and no specific degradation product has been detected in the analysed samples.
Concerning alkyd resins, these polymers are derived from the combination of polyols and polyacids, resulting in a versatile binder that exhibits exceptional adhesion, gloss, and resistance properties. Alkyd resins are known for their ability to create a protective film on various surfaces, such as wood, metal, and concrete, making them suitable for a wide range of applications. The alkyd resins detected in the samples from “UBUNTU” belong to the modern class of modified-alkyd resins. When compared to the first alkyd resins introduced in the 1940s, closely related to siccative oil in their structure, the modern ones are much more complex materials, including extensive modifications in the polymer structure, such as the addition of nitrocellulose and styrene, resulting in improved chemical and mechanical properties of the paint layer [3,42].
Finally, PVAc-based binders are generally used in emulsions and are characterised by a good compatibility with several pigments and substrates, making them suitable for a wide range of applications [40].
4. Conclusions
The multi-analytical approach adopted allowed us to successfully characterise the binders, organic pigments, and inorganic pigments in the spray paints used in the flag of the “UBUNTU” mural. The results are summarised in Table 1.
The positive identification of the organic and inorganic pigments along with degradation products was only possible thanks to the combination of spectroscopic (µ-Raman and SERS) and chromatographic (HPLC-DAD-MS) techniques. Raman was crucial for the identification of inorganic pigments and organic pigments that were insoluble in the adopted extraction conditions (i.e., phthalocyanines), while SERS and HPLC-DAD-MS were suitable for detecting fluorescent xanthene organic pigments, such as Rhodamines. In particular, the selectivity and sensitivity of HPLC-DAD-MS allowed us to detect photo-oxidation products of the original dyes, highlighting that an early degradation is already occurring, consistent with the observed fading.
Conversely, no degradation was observed for the binding media, consistently with their composition based on acrylic or modern, modified alkyd resins, suitable for outdoor applications. More specifically, the binders were effectively detected and characterised by Py-GC-MS. The green, pink, and orange paints share the same composition in terms of medium (acrylic resin based on MMA-nBA and nBMA) and were possibly purchased from the same producer, belonging to the same series, possibly Montana MTN 94 Fluorescent sprays, while the blue contains a mixture of materials, such as an acrylic resin (MMA-nBA), a styrene-modified alkyd with PVAc. Even if the superimposition of different layers cannot be ruled out in this case, the spray paint used does not belong to the same set as those discussed above. Finally, the yellow sample S5Y features another different composition in terms of binding medium, based on an acrylic resin (MMA-nBA).
With regard to the yellow fluorescent sample, its main coloured component remains unknown. Chemical data on fluorescent pigments are indeed very scant, manufacturers usually do not declare the composition of their formulations, and only a few studies are present in the literature [6,24,43], often reporting only the commercial, non-informative names of the ingredients. Therefore, further investigations are needed to widen the database of fluorescent pigments, which are highly fugitive and prone to changing their appearance [43], and of possible optical brighteners used to enhance the fluorescent effect of the formulations.
In perspective, the study of cross-sections of the samples might have highlighted the distribution of the materials in the layers, allowing us to better observe the discolouration of the paint. More specifically, the study of a cross-section sample may have helped us verify whether the bleaching effect is also due to a higher concentration of rutile in the upper layer occurred by micro-cracks in the paint layers or through a diffusive phenomenon from inner layers, as hypothesized for “Oriental Carpet” by H101 [13].
Notwithstanding some limitations, the overall results of the reported analytical investigation provided new and interesting information that will support curators in monitoring the state of conservation of the mural, promoting a sustainable preservation of this peculiar work of art. Finally, the new questions raised during this small-scale diagnostic campaign will also guide the future application of multi-analytical protocols to mural paintings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/heritage6080299/s1, Figure S1: Normalized SERS spectra of sample S4Y (blue line) and S2G (orange line); Figure S2. HPLC-DAD chromatogram at 450 nm of the extract of sample S4Y, along with the UV-Vis spectrum acquired for the peak at 19.2 min, smoothed with Means-Movement (5 points).
Author Contributions
Conceptualization, F.S., I.D., F.M., I.S., S.L. and J.L.N.; methodology, F.S., B.C. and J.L.N.; formal analysis, F.S., B.C. and J.L.N.; investigation, I.S.; writing—original draft preparation, I.D. and F.S.; writing—review and editing, S.L. and F.M.; visualization, F.S. and B.C.; supervision, F.M.; project administration, F.M.; funding acquisition, F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the PRIN2020 project “SUPERSTAR -Sustainable Preservation Strategies for Street Art” (2022–2025), an Italian network project, https://prin2020superstar.dcci.unipi.it/ (accessed on 02 June 2023) funded by the Italian Ministry of University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, I.D., upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Learner, T.; Sanchez-Pons, M.; Shank, W. (Eds.) Conservation Issues in Modern and Contemporary Murals; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2015. [Google Scholar]
- Cortea, I.M.; Ratoiu, L.; Rădvan, R. Characterization of spray paints used in street art graffiti by a non-destructive multi-analytical approach. Color Res. Appl. 2021, 46, 183–194. [Google Scholar] [CrossRef]
- Germinario, G.; van der Werf, I.D.; Sabbatini, L. Chemical characterisation of spray paints by a multi-analytical (Py/GC–MS, FTIR, μ-Raman) approach. Microchem. J. 2016, 124, 929–939. [Google Scholar] [CrossRef]
- Cimino, D.; Lamuraglia, R.; Saccani, I.; Berzioli, M.; Izzo, F.C. Assessing the (In)Stability of Urban Art Paints: From Real Case Studies to Laboratory Investigations of Degradation Processes and Preservation Possibilities. Heritage 2022, 5, 581–609. [Google Scholar] [CrossRef]
- Pellis, G.; Bertasa, M.; Ricci, C.; Scarcella, A.; Croveri, P.; Poli, T.; Scalarone, D. A multi-analytical approach for precise identification of alkyd spray paints and for a better understanding of their ageing behaviour in graffiti and urban artworks. J. Anal. Appl. Pyrolysis 2022, 165, 105576. [Google Scholar] [CrossRef]
- Connors-Rowe, S.A.; Morris, H.R.; Whitmore, P.M. Evaluation of Appearance and Fading of Daylight Fluorescent Water Colors. J. Am. Inst. Conserv. 2005, 44, 75–94. [Google Scholar] [CrossRef]
- Pintus, V.; Wei, S.; Schreiner, M. UV ageing studies: Evaluation of lightfastness declarations of commercial acrylic paints. Anal. Bioanal. Chem. 2012, 402, 1567–1584. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Silva, M.F.; Aura-Castro, E.; Fuster-López, L.; Kröner, S.; Martínez-Bazán, M.L.; Más-Barberá, X.; Mecklenburg, M.F.; Osete-Cortina, L.; Doménech, A.; et al. Study of behaviour on simulated daylight ageing of artists’ acrylic and poly(vinyl acetate) paint films. Anal. Bioanal. Chem. 2011, 399, 2921–2937. [Google Scholar] [CrossRef]
- Ciccola, A.; Serafini, I.; Guiso, M.; Ripanti, F.; Domenici, F.; Sciubba, F.; Postorino, P.; Bianco, A. Spectroscopy for contemporary art: Discovering the effect of synthetic organic pigments on UVB degradation of acrylic binder. Polym. Degrad. Stab. 2019, 159, 224–228. [Google Scholar] [CrossRef]
- Rivas, T.; Alonso-Villar, E.M.; Pozo-Antonio, J.S. Forms and factors of deterioration of urban art murals under humid temperate climate; influence of environment and material properties. Eur. Phys. J. Plus 2022, 137, 1257. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Alonso-Villar, E.M.; Rivas, T.; Márquez, I. Evaluation of a protective acrylic finish applied to surfaces painted with acrylic paints for outdoor or indoor uses. Dye. Pigment. 2023, 212, 111141. [Google Scholar] [CrossRef]
- Alonso-Villar, E.M.; Rivas, T.; Pozo-Antonio, J.S.; Pellis, G.; Scalarone, D. Efficacy of Colour Protectors in Urban Art Paintings under Different Conditions: From a Real Mural to the Laboratory. Heritage 2023, 6, 3475–3498. [Google Scholar] [CrossRef]
- Rousaki, A.; Vandenabeele, P.; Berzioli, M.; Saccani, I.; Fornasini, L.; Bersani, D. An in-and-out-the-lab Raman spectroscopy study on street art murals from Reggio Emilia in Italy. Eur. Phys. J. Plus 2022, 137, 252. [Google Scholar] [CrossRef]
- Bosi, A.; Ciccola, A.; Serafini, I.; Guiso, M.; Ripanti, F.; Postorino, P.; Curini, R.; Bianco, A. Street art graffiti: Discovering their composition and alteration by FTIR and micro-Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117474. [Google Scholar] [CrossRef] [PubMed]
- La Nasa, J.; Campanella, B.; Sabatini, F.; Rava, A.; Shank, W.; Lucero-Gomez, P.; De Luca, D.; Legnaioli, S.; Palleschi, V.; Colombini, M.P.; et al. 60 years of street art: A comparative study of the artists’ materials through spectroscopic and mass spectrometric approaches. J. Cult. Herit. 2021, 48, 129–140. [Google Scholar] [CrossRef]
- Cucci, C.; Bartolozzi, G.; Vita, M.D.; Marchiafava, V.; Picollo, M.; Casadio, F. The Colors of Keith Haring: A Spectroscopic Study on the Materials of the Mural Painting Tuttomondo and on Reference Contemporary Outdoor Paints. Appl. Spectrosc. 2016, 70, 186–196. [Google Scholar] [CrossRef]
- Magrini, D.; Bracci, S.; Cantisani, E.; Conti, C.; Rava, A.; Sansonetti, A.; Shank, W.; Colombini, M. A multi-analytical approach for the characterization of wall painting materials on contemporary buildings. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 39–45. [Google Scholar] [CrossRef]
- Nasa, J.L.; Orsini, S.; Degano, I.; Rava, A.; Modugno, F.; Colombini, M.P. A chemical study of organic materials in three murals by Keith Haring: A comparison of painting techniques. Microchem. J. 2016, 124, 940–948. [Google Scholar] [CrossRef]
- PRIN2020 SUPERSTAR, (n.d.). Available online: https://prin2020superstar.dcci.unipi.it/ (accessed on 19 May 2023).
- CAPuS Project, (n.d.). Available online: http://www.capusproject.eu/ (accessed on 19 May 2023).
- Ricci, C.; Croveri, P.; Scarcella, A.; Sunara, S.M.; Tabak, T.; Bertasa, M.; Scalarone, D. Tools to Document and Disseminate the Conservation of Urban Art. In Document|Archive|Disseminate Graffiti-Scapes, Proceedings of the goINDIGO 2022 International Graffiti Symposium, Vienna, Austria, 11–13 May 2022; Project INDIGO: Vienna, Austria, 2022; pp. 188–202. [Google Scholar]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Fremout, W.; Saverwyns, S. Identification of synthetic organic pigments: The role of a comprehensive digital Raman spectral library. J. Raman Spectrosc. 2012, 43, 1536–1544. [Google Scholar] [CrossRef]
- Campanella, B.; Botti, J.; Cavaleri, T.; Cicogna, F.; Legnaioli, S.; Pagnotta, S.; Poggialini, F.; Poli, T.; Scalarone, D.; Palleschi, V. The shining brightness of daylight fluorescent pigments: Raman and SERS study of a modern class of painting materials. Microchem. J. 2020, 152, 104292. [Google Scholar] [CrossRef]
- Cañamares, M.V.; Chenal, C.; Birke, R.L.; Lombardi, J.R. DFT, SERS, and Single-Molecule SERS of Crystal Violet. J. Phys. Chem. C 2008, 112, 20295–20300. [Google Scholar] [CrossRef]
- Petroviciu, I.; Teodorescu, I.C.; Vasilca, S.; Albu, F. Transition from Natural to Early Synthetic Dyes in the Romanian Traditional Shirts Decoration. Heritage 2023, 6, 505–523. [Google Scholar] [CrossRef]
- Ferreira, B.R.V.; Correa, D.N.; Eberlin, M.N.; Vendramini, P.H. Fragmentation Reactions of Rhodamine B and 6G as Revealed by High Accuracy Orbitrap Tandem Mass Spectrometry. J. Braz. Chem. Soc. 2017, 28, 136–142. [Google Scholar] [CrossRef]
- Sabatini, F.; Giugliano, R.; Degano, I. Photo-oxidation processes of Rhodamine B: A chromatographic and mass spectrometric approach. Microchem. J. 2018, 140, 114–122. [Google Scholar] [CrossRef]
- Sabatini, F.; Degano, I.; van Bommel, M. Investigating the in-solution photodegradation pathway of Diamond Green G by chromatography and mass spectrometry. Color. Technol. 2021, 137, 456–467. [Google Scholar] [CrossRef]
- Confortin, D.; Neevel, H.; Brustolon, M.; Franco, L.; Kettelarij, A.J.A.J.; Williams, R.M.R.M.; van Bommel, M.R. Crystal violet: Study of the photo-fading of an early synthetic dye in aqueous solution and on paper with HPLC-PDA, LC-MS and FORS. J. Phys. Conf. Ser. 2010, 231, 012011. [Google Scholar] [CrossRef]
- Groeneveld, I.; Bagdonaite, I.; Beekwilder, E.; Ariese, F.; Somsen, G.W.; Van Bommel, M.R. Liquid core waveguide cell with in situ absorbance spectroscopy and coupled to liquid chromatography for studying light-induced degradation. Anal. Chem. 2022, 94, 7647–7654. [Google Scholar] [CrossRef]
- Dunn, J.; Siegel, J.; Allison, J. Photodegradation and Laser Desorption Mass Spectrometry for the Characterization of Dyes Used in Red Pen Inks. Sci. J. Forensic 2003, 48, JFS2002359. [Google Scholar] [CrossRef]
- Lomax, S.Q.; Lomax, J.F.; Graham, T.K.; Moore, T.J.T.; Knapp, C.G. Historical azo pigments: Synthesis and characterization. J. Cult. Herit. 2018, 35, 218–224. [Google Scholar] [CrossRef]
- Stenger, J.; Kwan, E.E.; Eremin, K.; Speakman, S.; Kirby, D.; Stewart, H.; Huang, S.G.; Kennedy, A.R.; Newman, R.; Khandekar, N. Lithol red salts: Characterization and deterioration. e-Preserv. Sci. 2010, 7, 147–157. [Google Scholar]
- Kirby, D.P.; Khandekar, N.; Sutherland, K.; Price, B.A. Applications of laser desorption mass spectrometry for the study of synthetic organic pigments in works of art. Int. J. Mass Spectrom. 2009, 284, 115–122. [Google Scholar] [CrossRef]
- Lomax, S.Q.; Lomax, J.F.; Luca-Westrate, A.D. The use of Raman microscopy and laser desorption ionization mass spectrometry in the examination of synthetic organic pigments in modern works of art. J. Raman Spectrosc. 2014, 45, 448–455. [Google Scholar] [CrossRef]
- Watanabe, T.; Takizawa, T.; Honda, K. Photocatalysis through excitation of adsorbates. 1. Highly efficient N-deethylation of rhodamine B adsorbed to cadmium sulfide. J. Phys. Chem. 1977, 81, 1845–1851. [Google Scholar] [CrossRef]
- Souto, C.S.C.N. Analysis of Early Synthetic Dyes with HPLC-DAD-MS; Universidade de Lisboa: Lisbon, Portugal, 2010. [Google Scholar]
- Weiss, K.D. Paint and coatings: A mature industry in transition. Prog. Polym. Sci. 1997, 22, 203–245. [Google Scholar] [CrossRef]
- Borgioli, L. I Leganti Nell’arte Contemporanea; Nardini Editore: Florence, Italy, 2020. [Google Scholar]
- Jablonski, E.; Learner, T.; Hayes, J.; Golden, M. Conservation concerns for acrylic emulsion paints. Stud. Conserv. 2003, 48, 3–12. [Google Scholar] [CrossRef]
- Ploeger, R.; Scalarone, D.; Chiantore, O. The characterization of commercial artists’ alkyd paints. J. Cult. Herit. 2008, 9, 412–419. [Google Scholar] [CrossRef]
- Colombini, A.; Kaifas, D. Characterization of some orange and yellow organic and fluorescent pigments by Raman spectroscopy. e-Preserv. Sci. 2010, 7, 14–21. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).