Comparison of Bone Quality in Middle Ages and Late Modern Period Human Skeletons from Latvia
Abstract
1. Introduction
1.1. Bone Tissue Factors and Proteins
1.2. Bone Microstructure
1.3. Socioeconomic History of Riga in the 14–15th and 18–19th Centuries
2. Materials and Methods
2.1. Bone Samples
2.2. Micro-Computed Tomography
2.3. Immunohistochemistry
2.4. Statistics
3. Results
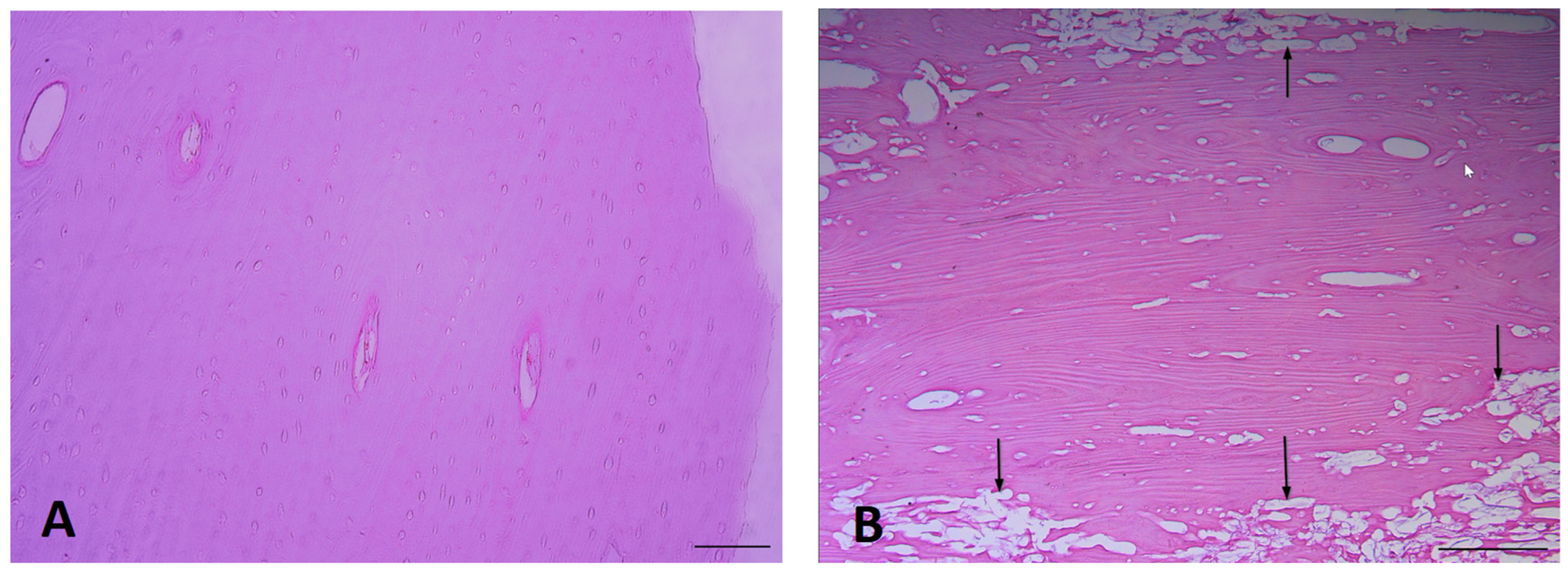
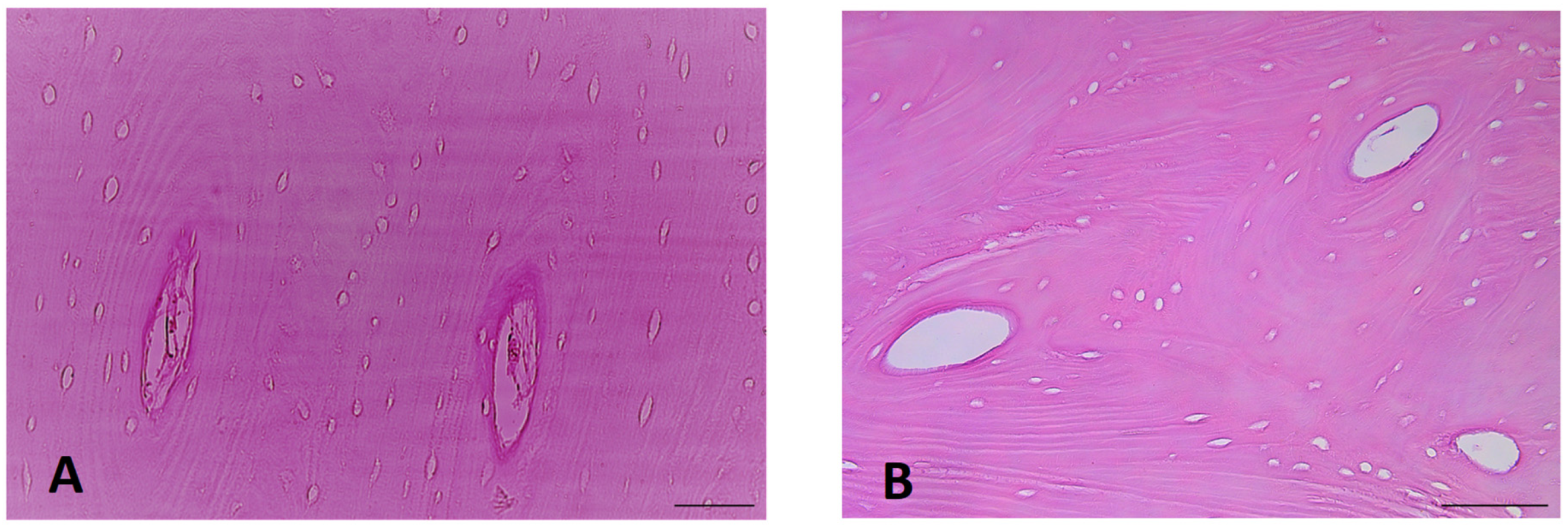
3.1. Exemplar Micrographs
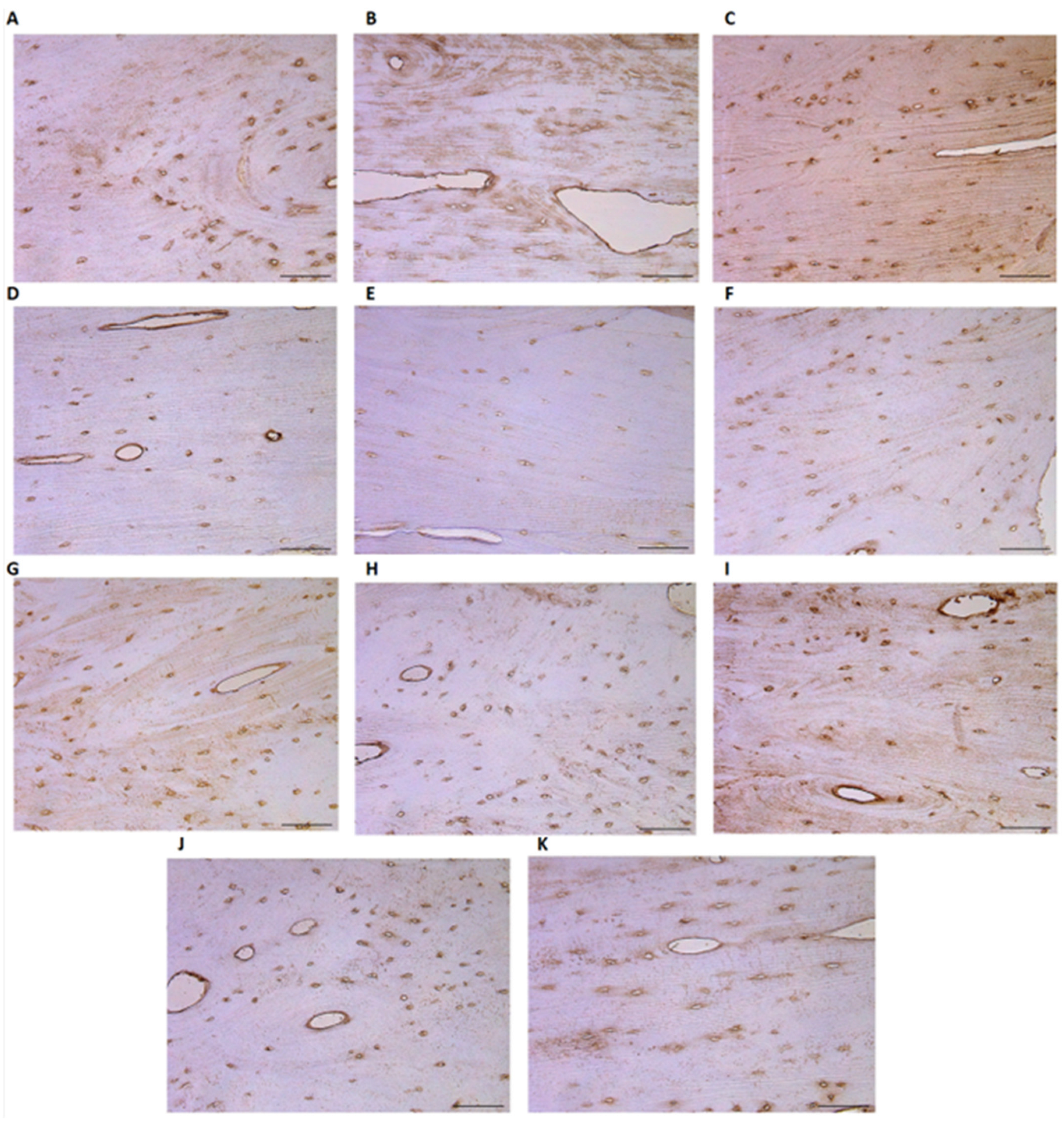
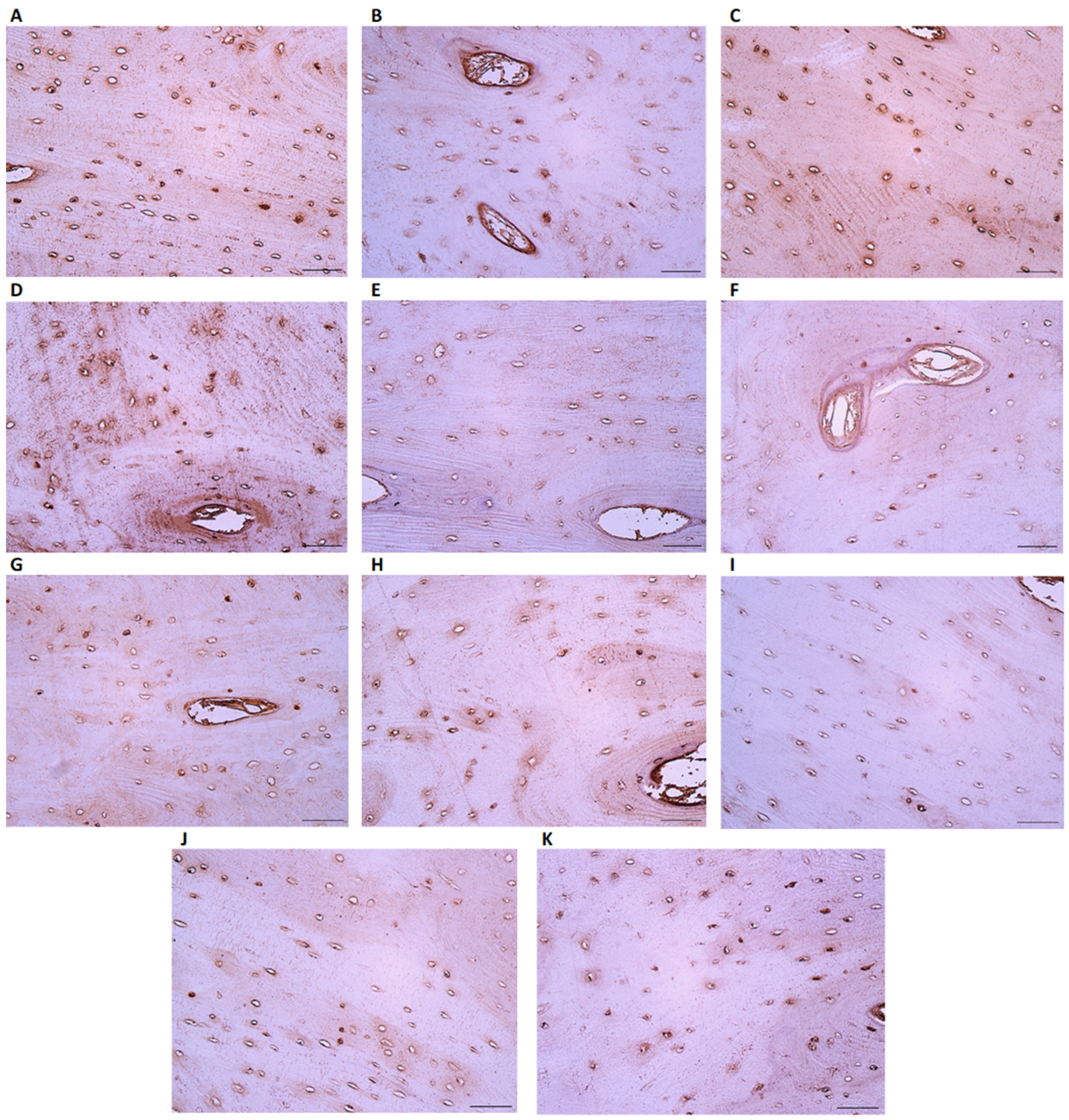
3.2. IHC-Positive Cell Presence in Bone Tissue
3.3. Micro-CT Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2001, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Senda, T.; Kubo, K.Y. The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Med. Mol. Morphol. 2015, 48, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.S.; Kayser, M.; Jones, C. The mineralized osteocyte: A living fossil. Am. J. Phys. Anthropol. 2008, 137, 449–456. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Tresguerres, F.G.F.; Torres, J.; López-Quiles, J.; Hernández, G.; Vega, J.A.; Tresguerres, I.F. The osteocyte: A multifunctional cell within the bone. Ann. Anat. 2020, 227, 151422. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral Biol. 2016, 18, 9–16. [Google Scholar]
- Heino, T.J.; Hentunen, T.A.; Väänänen, H.K. Osteocytes inhibit osteoclastic bone resorption through transforming growth factor-beta: Enhancement by estrogen. J. Cell. Biochem. 2002, 85, 185–197. [Google Scholar] [CrossRef]
- Bosetti, M.; Boccafoschi, F.; Leigheb, M.; Cannas, M.F. Effect of different growth factors on human osteoblasts activities: A possible application in bone regeneration for tissue engineering. Biomol. Eng. 2007, 24, 613–618. [Google Scholar] [CrossRef]
- Montero, A.; Okada, Y.; Tomita, M.; Ito, M.; Tsurukami, H.; Nakamura, T.; Doetschman, T.; Coffin, J.D.; Hurley, M.M. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J. Clin. Investig. 2000, 105, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Coffin, J.D.; Homer-Bouthiette, C.; Hurley, M.M. Fibroblast Growth Factor 2 and Its Receptors in Bone Biology and Disease. J. Endocr. Soc. 2008, 2, 657–671. [Google Scholar] [CrossRef]
- Paiva, K.B.S.; Granjeiro, J.M. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Prog. Mol. Biol. Transl. Sci. 2017, 148, 203–303. [Google Scholar]
- Kumar, G.; Roger, P.M. From Crosstalk between Immune and Bone Cells to Bone Erosion in Infection. Int. J. Mol. Sci. 2019, 20, 5154. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Schultz, T.H.; Schultz, M. Bone protects proteins over thousands of years: Extraction, analysis, and interpretation of extracellular matrix proteins in archeological skeletal remains. Am. J. Phys. Anthropol. 2004, 123, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Schultz, T.H.; Schultz, M. Well preserved non-collagenous extracellular matrix proteins in ancient human bone and teeth. Int. J. Osteoarch. 2007, 17, 91–99. [Google Scholar] [CrossRef]
- Schmidt-Schultz, T.H.; Schultz, M. Intact growth factors are conserved in the extracellular matrix of ancient human bone and teeth: A storehouse for the study of human evolution in health and disease. Biol. Chem. 2005, 386, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.I.; Craig, O.E.; Prigodich, R.V.; Nielsen-Marsh, C.M.; Jans, M.M.E.; Vermeer, C.; Collins, M.J. Diagenesis and survival of osteocalcin in archaeological bone. J. Arch. Sci. 2005, 32, 105–113. [Google Scholar] [CrossRef]
- Scott, A.B.; Alberto, J.; Taurozzi, A.J.; Hughes, N.; Pedersen, D.D.; Kontopoulos, I.; Mackie, M.; Collins, M.J. Comparing biological and pathological factors affecting osteocalcin concentrations in archaeological skeletal remains. J. Arch. Sci. 2020, 34, 102573. [Google Scholar] [CrossRef]
- Caruso, V.; Cummaudo, M.; Maderna, E.; Cappella, A.; Caudullo, G.; Scarpulla, V.; Cattaneo, C. A comparative analysis of microscopic alterations in modern and ancient undecalcified and decalcified dry bones. Am. J. Phys. Anthropol. 2018, 165, 363–369. [Google Scholar] [CrossRef]
- Miszkiewicz, J.J.; Mahoney, P. Ancient Human Bone Microstructure in Medieval England: Comparisons between Two Socio-Economic Groups. Anat. Rec. 2016, 299, 42–59. [Google Scholar] [CrossRef]
- Beresheim, A.C.; Pfeiffer, S.; Grynpas, M. Ontogenetic changes to bone microstructure in an archaeologically derived sample of human ribs. J. Anat. 2020, 236, 448–462. [Google Scholar] [CrossRef]
- Chappard, D.; Baslé, M.F.; Legrand, E.; Audran, M. Trabecular bone microarchitecture: A review. Morphologie 2008, 92, 162–170. [Google Scholar] [CrossRef]
- Beresheim, A.C.; Pfeiffer, S.K.; Grynpas, M.D.; Alblas, A. Sex-specific patterns in cortical and trabecular bone microstructure in the Kirsten Skeletal Collection, South Africa. Am. J. Hum. Biol. 2018, 30, e23108. [Google Scholar] [CrossRef] [PubMed]
- Kozma, C. Skeletal dysplasia in ancient Egypt. Am. J. Med. Genet. A 2008, 146, 3104–3112. [Google Scholar] [CrossRef] [PubMed]
- Kesterke, M.J.; Judd, M.A. A microscopic evaluation of Paget’s disease of bone from a Byzantine monastic crypt in Jordan. Int. J. Paleopathol. 2019, 24, 293–298. [Google Scholar] [CrossRef]
- Shaw, B.; Burrell, C.L.; Green, D.; Navarro-Martinez, A.; Scott, D.; Daroszewska, A.; van‘t Hof, R.; Smith, L.; Hargrave, F.; Mistry, S.; et al. Molecular insights into an ancient form of Paget’s disease of bone. Proc. Natl. Acad. Sci. USA 2019, 116, 10463–10472. [Google Scholar] [CrossRef] [PubMed]
- Bajon, K.; Smiszkiewicz-Skwarska, A.; Stolarczyk, H.; Zygmunt, A.; Rutkowski, M.; Sewerynek, E. Evaluation of bone mineral density on the basis of the results of studies of selected skeleton populations from the microregion of Brześć Kujawski. Endokrynol. Pol. 2006, 57, 494–500. [Google Scholar]
- Stride, P.J.; Patel, N.; Kingston, D. The history of osteoporosis: Why do Egyptian mummies have porotic bones? J. R. Coll. Phys. Edinb. 2013, 43, 254–261. [Google Scholar] [CrossRef]
- Chirchir, H.; Kivell, T.L.; Ruff, C.B.; Hublin, J.J.; Carlson, K.J.; Zipfel, B.; Richmond, B.G. Recent origin of low trabecular bone density in modern humans. Proc. Natl. Acad. Sci. USA 2015, 112, 366–371. [Google Scholar] [CrossRef]
- Chirchir, H.; Ruff, C.B.; Junno, J.A.; Potts, R. Low trabecular bone density in recent sedentary modern humans. Am. J. Phys. Anthropol. 2017, 162, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Gosman, J.H.; Ketcham, R.A. Patterns in ontogeny of human trabecular bone from SunWatch Village in the Prehistoric Ohio Valley: General features of microarchitectural change. Am. J. Phys. Anthropol. 2009, 138, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Pitfield, R.; Deter, C.; Mahoney, P. Bone histomorphometric measures of physical activity in children from medieval England. Am. J. Phys. Anthropol. 2019, 169, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Vinci, R.; Rebaudi, A.; Capparè, P.; Gherlone, E. Microcomputed and Histologic Evaluation of Calvarial Bone Grafts: A Pilot Study in Humans. Int. J. Periodontics Restor. Dent. 2011, 31, e29–e36. [Google Scholar]
- Zeids, T. Feodālā Rīga; Zinātne: Riga, Latvia, 1978; pp. 48–53. [Google Scholar]
- Celmiņš, A. Zemē Apslēptā Pilsēta: Izstade par 1991–1997. Gada Arheoloǧiskajiem Atrdumiem Rīgā = A City under the Ground: An Exhibition of Archaeological Finds from Riga, 1991–1997; Dizaina un drukas apgāds: Rīga, Latvia, 1998; ISBN 978-9984-9116-2-5. [Google Scholar]
- Šterns, I. Latvijas Vēsture 1290–1500; Daugava: Riga, Latvia, 1997; pp. 98–177. [Google Scholar]
- Krastiņš, J. Rīga 1860–1917; Zinātne: Riga, Latvia, 1978; pp. 22–45. [Google Scholar]
- Habbal, O. The Science of Anatomy: A historical timeline. Sultan Qaboos Univ. Med. J. 2017, 17, e18–e22. [Google Scholar] [CrossRef]
- Walker, E.C.; McGregor, N.E.; Chan, A.S.M.; Sims, N.A. Measuring Bone Volume at Multiple Densities by Micro-computed Tomography. Bio Protoc. 2021, 11, e3873. [Google Scholar] [CrossRef]
- Idleburg, C.; Lorenz, M.R.; DeLassus, E.N.; Scheller, E.L.; Veis, D.J. Immunostaining of Skeletal Tissues. Methods Mol. Biol. 2021, 2221, 261–273. [Google Scholar]
- Meyerholz, D.K.; Beck, A.P. Principles and approaches for reproducible scoring of tissue stains in research. Lab. Investig. 2018, 98, 844–855. [Google Scholar] [CrossRef]
- Pilmane, M.; Sidhoma, E.; Akota, I.; Kazoka, D. Characterization of Cytokines and Proliferation Marker Ki67 in Cleft Affected Lip Tissue. Medicina 2019, 55, 518. [Google Scholar] [CrossRef] [PubMed]
- Augat, P.; Schorlemmer, S. The Role of Cortical Bone and Its Microstructure in Bone Strength. Age Ageing 2006, 35, ii27–ii31. [Google Scholar] [CrossRef] [PubMed]
- Ducher, G.; Reduce, S.; Courteix, D.; Benhamou, C.L. Cortical and trabecular bone at the forearm show different adaptation patterns in response to tennis playing. J. Clin. Densitom. 2004, 7, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Ohlsson, C.; Sundh, D.; Mellström, D.; Lorentzon, M. Association of physical activity with trabecular microstructure and cortical bone at distal tibia and radius in young adult men. J. Clin. Endocrinol. Metab. 2010, 95, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Sundh, D.; Mellström, D.; Lorentzon, M. Current Physical Activity Is Independently Associated with Cortical Bone Size and Bone Strength in Elderly Swedish Women. J. Bone Miner. Res. 2017, 32, 473–485. [Google Scholar] [CrossRef]
- Vilayphiou, N.; Boutroy, S.; Sornay-Rendu, E.; Van Rietbergen, B.; Chapurlat, R. Age-related changes in bone strength from HR-pQCT derived microarchitectural parameters with an emphasis on the role of cortical porosity. Bone 2016, 83, 233–240. [Google Scholar] [CrossRef]
- Chen, H.; Kubo, K.Y. Bone three-dimensional microstructural features of the common osteoporotic fracture sites. World J. Orthop. 2014, 5, 486–495. [Google Scholar] [CrossRef]
- Samakkarnthai, P.; Sfeir, J.G.; Atkinson, E.J.; Achenbach, S.J.; Wennberg, P.W.; Dyck, P.J.; Tweed, A.J.; Volkman, T.L.; Amin, S.; Farr, J.N.; et al. Determinants of Bone Material Strength and Cortical Porosity in Patients with Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2020, 105, e3718-29. [Google Scholar] [CrossRef]
- Porrelli, D.; Abrami, M.; Pelizzo, P.; Formentin, C.; Ratti, C.; Turco, G.; Grassi, M.; Canton, G.; Grassi, G.; Murena, L. Trabecular bone porosity and pore size distribution in osteoporotic patients—A low field nuclear magnetic resonance and microcomputed tomography investigation. J. Mech. Behav. Biomed. Mater. 2022, 125, 104933. [Google Scholar] [CrossRef]
- Cowell, S.; Knauper, V.; Stewart, M.L.; D’Ortho, M.P.; Stanton, H.; Hembry, R.M.; Lopez-Otin, C.; Reynolds, J.J.; Murphy, G. Induction of matrix metalloproteinase activation cascades based on membrane-type 1 matrix metalloproteinase: Associated activation of gelatinase A, gelatinase B and collagenase 3. Biochem J. 1998, 331, 453–458. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Hammani, K.; Blakis, A.; Morsette, D.; Bowcock, A.M.; Schmutte, C.; Henriet, P.; DeClerck, Y.A. Structure and characterization of the human tissue inhibitor of metalloproteinases-2 gene. J. Biol. Chem. 1996, 271, 25498–25505. [Google Scholar] [CrossRef]
- Ryan, M.E.; Ramamurthy, S.; Golub, L.M. Matrix metalloproteinases and their inhibition in periodontal treatment. Curr. Opin. Periodontol. 1996, 3, 85–96. [Google Scholar] [PubMed]
- Paiva, K.B.S.; Granjeiro, J.M. Bone tissue remodeling and development: Focus on matrix metalloproteinase functions. Arch. Biochem. Biophys. 2014, 561, 74–87. [Google Scholar] [CrossRef]
- Miller, B.; Spevak, L.; Lukashova, L.; Javaheri, B.; Pitsillides, A.A.; Boskey, A.; Bou-Gharios, G.; Carriero, A. Altered Bone Mechanics, Architecture and Composition in the Skeleton of TIMP-3-Deficient Mice. Calcif. Tissue Int. 2017, 100, 631–640. [Google Scholar] [CrossRef]
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the bone and immune system. Endocr. Rev. 2008, 29, 403–440. [Google Scholar] [CrossRef]
- Liao, R.; Feng, Z.; Li, W.; Liu, R.; Xu, X.; Yao, S.; Tian, J. Interleukin-1 induces receptor activator of nuclear factor-κB ligand-independent osteoclast differentiation in RAW264.7 cells. Exp. Ther. Med. 2021, 21, 640. [Google Scholar] [CrossRef]
- Lorenzo, J.A.; Sousa, S.L.; Van Den Brink-Webb, S.E.; Korn, J.H. Production of both interleukin-1 α and β by newborn mouse calvaria cultures. J. Bone Miner. Res. 1990, 5, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Pilbeam, C.C.; Vargas, S.J.; Morse, E.E.; Lorenzo, J.A.; Raisz, L.G. Ovariectomy enhances and estrogen replacement inhibits the activity of bone marrow factors that stimulate prostaglandin production in cultured mouse calvaria. J. Clin. Investig. 1995, 96, 539–548. [Google Scholar] [CrossRef]
- Ross, F.P.; Chappel, J.; Alvarez, J.I.; Sander, D.; Butler, W.T.; Farach-Carson, M.C.; Mintz, K.A.; Robey, P.G.; Teitelbaum, S.L.; Cheresh, D.A. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J. Biol. Chem. 1993, 268, 9901–9907. [Google Scholar] [CrossRef] [PubMed]
- Ek-Rylander, B.; Andersson, G. Osteoclast migration on phosphorylated osteopontin is regulated by endogenous tartrate-resistant acid phosphatase. Exp. Cell Res. 2010, 316, 443–451. [Google Scholar] [CrossRef]
- Dong, M.; Yu, X.; Chen, W.; Guo, Z.; Sui, L.; Xu, Y.; Shang, Y.; Niu, W.; Kong, Y. Osteopontin Promotes Bone Destruction in Periapical Periodontitis by Activating the NF-κB Pathway. Cell. Physiol. Biochem. 2018, 49, 884–889. [Google Scholar] [CrossRef]
- Luukkonen, J.; Hilli, M.; Nakamura, M.; Ritamo, I.; Valmu, L.; Kauppinen, K.; Tuukkanen, J.; Lehenkari, P. Osteoclasts secrete osteopontin into resorption lacunae during bone resorption. Histochem. Cell Biol. 2019, 151, 475–487. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Guo, S.; Zhang, W.; Wang, J.; Lin, Y. Dynamic expression of matrix metalloproteinases 2, 9 and 13 in ovariectomy-induced osteoporosis rats. Exp. Ther. Med. 2018, 16, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.P.H.; Xu, J.; Xue, M.; Jackson, C.J. Matrix metalloproteinases in bone development and pathology: Current knowledge and potential clinical utility. Met. Med. 2016, 3, 93–102. [Google Scholar] [CrossRef]
- Samanna, V.; Ma, T.; Mak, T.W.; Rogers, M.; Chellaiah, M.A. Actin polymerization modulates CD44 surface expression, MMP-9 activation, and osteoclast function. J. Cell. Physiol. 2007, 213, 710–720. [Google Scholar] [CrossRef]
- Zhang, P.; Zhong, M. Effects of 17beta-estradiol and progesterone on the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in rat osteoblasts. Acad. J. First Med. Coll. PLA 2001, 21, 929–931. [Google Scholar]
- Bachmeier, B.E.; Iancu, C.M.; Jochum, M.; Nerlich, A.G. Matrix metalloproteinases in cancer: Comparison of known and novel aspects of their inhibition as a therapeutic approach. Exp. Rev. Anticancer Ther. 2005, 5, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Akhurst, R.J. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat. Cell Biol. 2007, 9, 1000–1004. [Google Scholar] [CrossRef]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β family: Context-dependent roles in cell and tissue physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef]

| TGFB | OPN | MMP2 | TIMP2 | OPG | OCN | bFGF | Runx2 | IL-1a | IL-10 | BMP2/4 | βDef.2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control group | + | + | + | +/++ | + | + | + | 0 | 0/+ | + | + | 0/+ | |
| Mean | 1.25 | 1 | 1 | 1.5 | 1 | 1 | 1 | 0 | 0.25 | 1.25 | 1.25 | 0.5 | |
| SD | 0.35 | 0.71 | 0.71 | 0 | 0.71 | 0.71 | 0 | 0 | 0.35 | 0.35 | 0.35 | 0.71 | |
| Patient group | 0/+ | + | + | + | + | 0/+ | + | 0 | + | + | + | + | |
| Mean | 0.56 | 0.94 | 1.06 | 0.94 | 1 | 0.69 | 0.81 | 0.06 | 0.88 | 0.75 | 0.75 | 0.75 | |
| SD | 0.18 | 0.32 | 0.32 | 0.32 | 0.38 | 0.26 | 0.26 | 0.18 | 0.23 | 0.27 | 0.27 | 0.38 |
| Factor | Mann–Whitney U | Z-Score | p-Value |
|---|---|---|---|
| TIMP2 | 1 | −1984 | 0.047 |
| IL-1a | 1 | −2092 | 0.036 |
| Bone Name/Group | Sex | Age | Time Period | Mean Bone Volume to Trabecular Volume Ratio (%/0.5 cm3) | Mean Thickness of Trabecular Bone (µm) | Mean Diameter of Bone Pores (µm) | Mean Thickness of Cortical Bone (µm) |
|---|---|---|---|---|---|---|---|
| Ulna | F | 15–18 | Late Modern Period | 22.02 | 144.22 | 471.10 | 4690.98 |
| Radius | M | 20–25 | Late Modern Period | 20.35 | 150.63 | 614.98 | 3590.32 |
| Radius | F | 20–25 | Late Modern Period | 22.58 | 169.78 | 537.43 | 3585.83 |
| Radius | M | 20–25 | Late Modern Period | 23.76 | 161.50 | 558.43 | 3233.28 |
| Radius | F | 20–25 | Late Modern Period | 17.39 | 120.06 | 462.49 | 2903.22 |
| Ulna | M | 40–55 | Late Modern Period | 31.47 | 187.36 | 411.45 | 3518.14 |
| Ulna | M | 40–55 | Late Modern Period | 30.02 | 210.51 | 383.73 | 3555.34 |
| Ulna | F | 55+ | Late Modern Period | 30.39 | 164.81 | 445.10 | 3362.52 |
| Ulna | M | 55+ | Late Modern Period | 31.68 | 203.51 | 402.85 | 3564.94 |
| Ulna | F | 15–18 | Middle Ages | 23.46 | 175.46 | 375.77 | 3188.85 |
| Humerus | F | 15–18 | Middle Ages | 25.16 | 191.00 | 478.27 | 4575.80 |
| Radius | F | 15–18 | Middle Ages | 21.86 | 159.90 | 435.74 | 2930.25 |
| Humerus | F | 15–18 | Middle Ages | 22.01 | 144.00 | 471.00 | 4691.20 |
| Humerus | M | 18–20 | Middle Ages | 29.18 | 214.60 | 542.20 | 5044.80 |
| Ulna | M | 20–25 | Middle Ages | 27.39 | 154.25 | 421.55 | 4195.58 |
| Humerus | M | 20–25 | Middle Ages | 26.08 | 160.47 | 467.47 | 5523.00 |
| Radius | M | 20–25 | Middle Ages | 22.18 | 138.66 | 543.64 | 2956.02 |
| Ulna | M | 20–25 | Middle Ages | 19.66 | 143.01 | 545.34 | 2321.58 |
| Radius | M | 20–25 | Middle Ages | 19.07 | 137.99 | 546.79 | 2301.98 |
| Radius | F | 20–25 | Middle Ages | 16.78 | 151.90 | 518.59 | 2493.96 |
| Humerus | F | 30–35 | Middle Ages | 26.93 | 185.27 | 491.93 | 3454.60 |
| Ulna | F | 30–35 | Middle Ages | 26.91 | 176.19 | 447.43 | 3311.75 |
| Radius | M | 40–45 | Middle Ages | 24.09 | 160.64 | 492.41 | 3982.52 |
| Humerus | M | 40–45 | Middle Ages | 28.50 | 177.33 | 493.20 | 6267.80 |
| Radius | M | 40–45 | Middle Ages | 21.61 | 147.39 | 473.33 | 4299.95 |
| Humerus | M | 40–50 | Middle Ages | 25.04 | 170.33 | 558.67 | 4073.40 |
| Ulna | M | 40–55 | Middle Ages | 28.82 | 180.85 | 447.79 | 3320.23 |
| Ulna | M | 45–45 | Middle Ages | 26.04 | 199.49 | 409.80 | 4195.33 |
| Humerus | M | 45–50 | Middle Ages | 25.25 | 121.20 | 440.47 | 3718.20 |
| Ulna | M | 45–50 | Middle Ages | 27.62 | 171.27 | 391.52 | 4352.84 |
| Ulna | M | 55+ | Middle Ages | 31.07 | 157.65 | 524.62 | 4026.97 |
| Radius | F | 55+ | Middle Ages | 22.64 | 178.68 | 518.57 | 2018.25 |
| Ulna | F | 55+ | Middle Ages | 29.09 | 155.45 | 443.43 | 3680.35 |
| Radius | F | 55+ | Middle Ages | 19.12 | 131.28 | 536.06 | 2473.80 |
| Humerus | M | 55+ | Middle Ages | 26.81 | 166.33 | 426.33 | 5589.60 |
| Humerus | F | 55+ | Middle Ages | 28.09 | 160.00 | 516.07 | 6117.20 |
| Ulna | F | 55+ | Middle Ages | 30.73 | 169.69 | 561.16 | 4179.27 |
| Ulna | F | 55+ | Middle Ages | 24.23 | 176.83 | 480.08 | 3689.85 |
| Radius | F | 55+ | Middle Ages | 18.54 | 127.77 | 467.11 | 4073.07 |
| Ulna | M | 55+ | Middle Ages | 28.47 | 178.95 | 433.84 | 3628.81 |
| Ulna | M | 55+ | Middle Ages | 31.60 | 149.97 | 389.56 | 4681.52 |
| Ulna | M | 55+ | Middle Ages | 33.85 | 222.26 | 347.59 | 3537.82 |
| Correlation Type | Variable 1 | Variable 2 | r | p-Value |
|---|---|---|---|---|
| Positive | Strong correlation | OPN | BV/TV | 0.772 |
| OPN | MMP2 | 0.730 | ||
| OPN | βFGF | 0.716 | ||
| TGFβ | MMP2 | 0.716 | ||
| TIMP2 | Th. of CB | 0.687 | ||
| Negative | Strong correlation | TGFβ | Diameter of pores | −0.845 |
| MMP2 | Diameter of pores | −0.678 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šerstņova, K.; Edelmers, E.; Zolovs, M.; Pilmane, M. Comparison of Bone Quality in Middle Ages and Late Modern Period Human Skeletons from Latvia. Heritage 2023, 6, 5329-5346. https://doi.org/10.3390/heritage6070281
Šerstņova K, Edelmers E, Zolovs M, Pilmane M. Comparison of Bone Quality in Middle Ages and Late Modern Period Human Skeletons from Latvia. Heritage. 2023; 6(7):5329-5346. https://doi.org/10.3390/heritage6070281
Chicago/Turabian StyleŠerstņova, Ksenija, Edgars Edelmers, Maksims Zolovs, and Māra Pilmane. 2023. "Comparison of Bone Quality in Middle Ages and Late Modern Period Human Skeletons from Latvia" Heritage 6, no. 7: 5329-5346. https://doi.org/10.3390/heritage6070281
APA StyleŠerstņova, K., Edelmers, E., Zolovs, M., & Pilmane, M. (2023). Comparison of Bone Quality in Middle Ages and Late Modern Period Human Skeletons from Latvia. Heritage, 6(7), 5329-5346. https://doi.org/10.3390/heritage6070281







