A New Virtual Reconstruction of the Ndutu Cranium
Abstract
1. Introduction
2. Materials and Methods
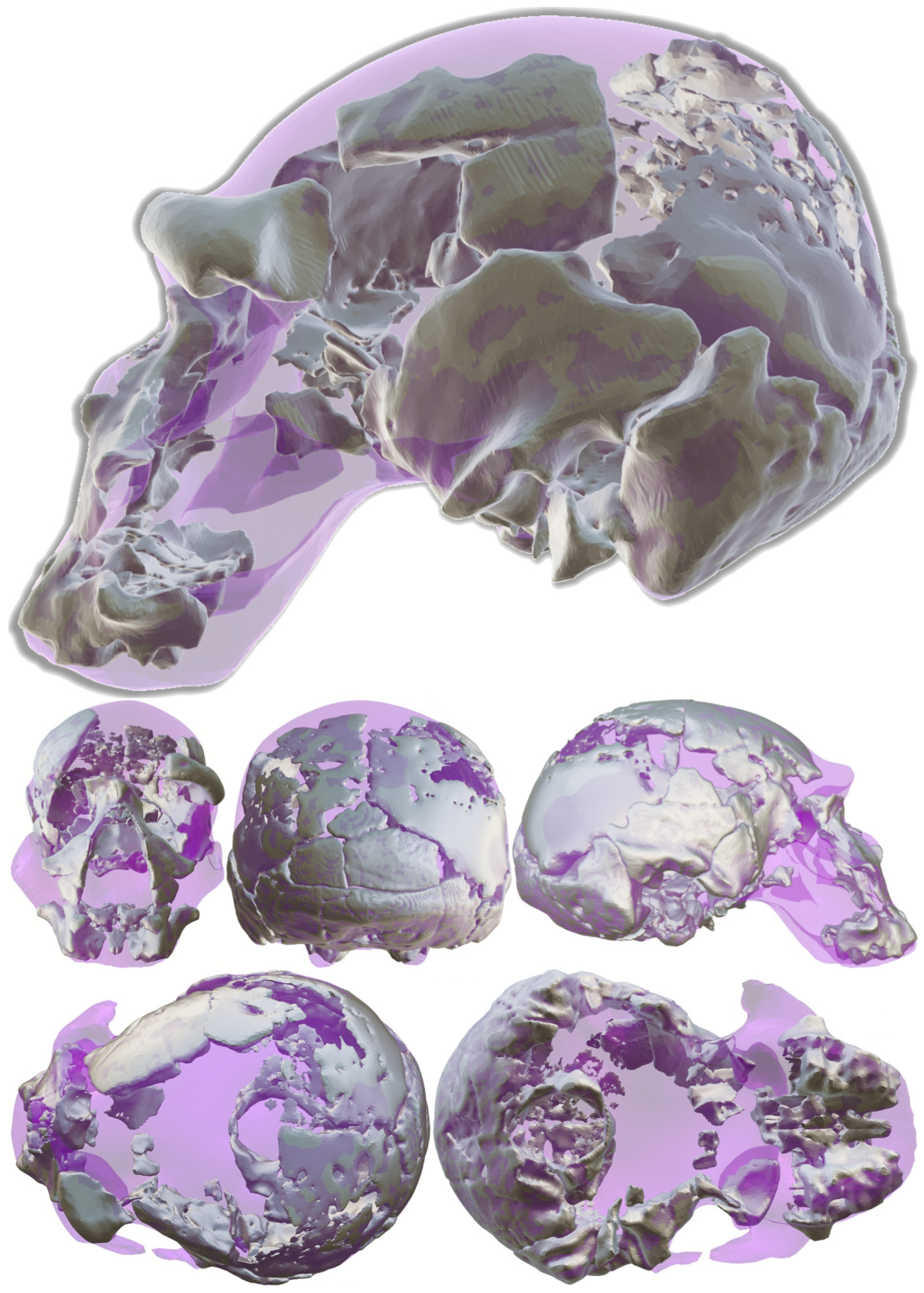
- Facial skeleton and dentitionIn its present condition, the Ndutu cranium’s face is represented by a disarticulated piece held in place with a generous amount of gypsum plaster. It gathers most of the maxillary bone, the antero-inferior portion of the zygomatic root, the greater part of the right lacrimal bone, and most of both nasals, as well as a small, attached fragment of the ethmoid representing the crista galli [3]. More accurately described as a near-continuous cluster of fragments, this piece boasts a flat midfacial region [13]. Here, the preserved nasal aperture opens tall and moderately wide, flanked by thin nasal margins. Above it, the inferior two thirds of the nasals sharply angle down and outward. To the sides, the orbital margins also slope infero-laterally quite markedly. On the better-preserved right side, this slope can be seen to yield above the infraorbital foramen [3]. Crisply defined lacrimal crests delimit tall and narrow lacrimal fossae [13].The inferior nasal margin is mostly missing, as so is the incisor portion. The left anterior part of the palate reveals a deep curve, to the side of which only a part of the left dental arcade from C1 TO M1 is present [3]. To Schwartz and Tattersall [13], the C1 root appears short, while the distance between the buccal and lingual roots of M1 seems long. Both premolars are double-rooted.
- Frontal boneThe frontal bone is represented by a detached left fragment of the supraorbital torus (most of which has been sculpted in plaster) and two frontal squama fragments that articulate with the parietals on either side of the cranial vault. Although the temporal crest is mostly visible on the left supraorbital torus fragment [3], its root can also be distinguished on the right frontal fragment, close to the orbit on its broken end. Overall, these lines are sharp and extremely low, allowing for a steep frontal rise that may be argued to be even more precipitous than estimated by Clarke [13].For its part, the anterior part of the left temporal line on the supraorbital torus fragment’s reverse is well-preserved from its departure close to the fronto-zygomatic suture to what is likely beyond the point of minimum frontal breadth. Furthermore, the left supraorbital torus fragment is shown in Clarke’s latest reconstruction to account for the central and lateral parts of the upper orbital rim and thus shows a piece of the lateral part of the orbital roof. Notably, it boasts a considerably large foramen near its right end.
- Sphenoid boneThe preserved sphenoid encompasses the posterior portion of the right orbital surface of the right greater wing forming the roof of the right orbit, as well as a part of the right temporal surface, a smaller piece of the left temporal surface, and a small fragment of the lesser wing. On this area, several small ethmoid fragments were also noted by Clarke [3]. Conspicuously, most of the sphenoid body is missing and many of the inner fragments are disarticulated, which implies a degree of uncertainty in the width of the sphenoid.
- Parietal bonesIn their current state, the parietals are missing various sections of either their inner or outer tables. Indeed, they constitute the most deteriorated of the preserved parts of the Ndutu cranium, having sustained a great deal of erosion and both plastic and brittle deformation. This particularly affects the anterior part of the right parietal and the posterior end of the sagittal suture, as admitted by Clarke in 1990.Despite this, Clarke’s meticulous work allowed to recover what is known about this part of Ndutu’s braincase. According to his reconstruction, their parietals are noticeably thick, display a large diploic space, and appear quite bossed—a trait that has been largely exaggerated on the right side by taphonomic deformation. Still, as indicated by the left parietal contour, it can be agreed that the neurocranium would have been rather short, round, and wide [13].
- Temporal bonesBoth temporals are present, with some of their squamae and at least a hint of the root of both zygomatic processes being preserved. In addition to this, on the right, this is limited to the superior part of the acoustic meatus, the lateral segment of the glenoid fossa, the supramastoid crest, and most of the mastoid portion. Contrastingly, the better-preserved left temporal adds information on this anatomy, plus the presence of a remarkable tympanic plate and the styloid process.Ndutu’s temporal squamae project straight upward, supporting a wide neurocranium [4,14] and possibly arching quite high on the sides of the cranial vault. Their temporal anatomy also exhibits a striking articular eminence in front of a short and narrow glenoid fossa. Alongside this are short and stout mastoid processes and a modest occipito-mastoid crest.
- Occipital boneThe Ndutu occipital is nearly complete. It is most notably lacking the basilar process and the left occipital condyle, alongside a few squamous fragments. The foramen magnum is ovoid in shape and the condyles seem to have been forwardly positioned. As the squama extends posteriorly, it meets a rather faint occipital torus. Despite this, the occipito–nuchal angle is sharp (110° according to Clarke). On its end, the squama exhibits a moderately sized interparietal bone that, according to Tattersall [13], obscures the position of lambda. Projection of the lambdoid and sagittal sutures locate lambda close to the superior edge of this Wormian bone.
3. Results
3.1. Reassembly
3.2. Digital Alignment
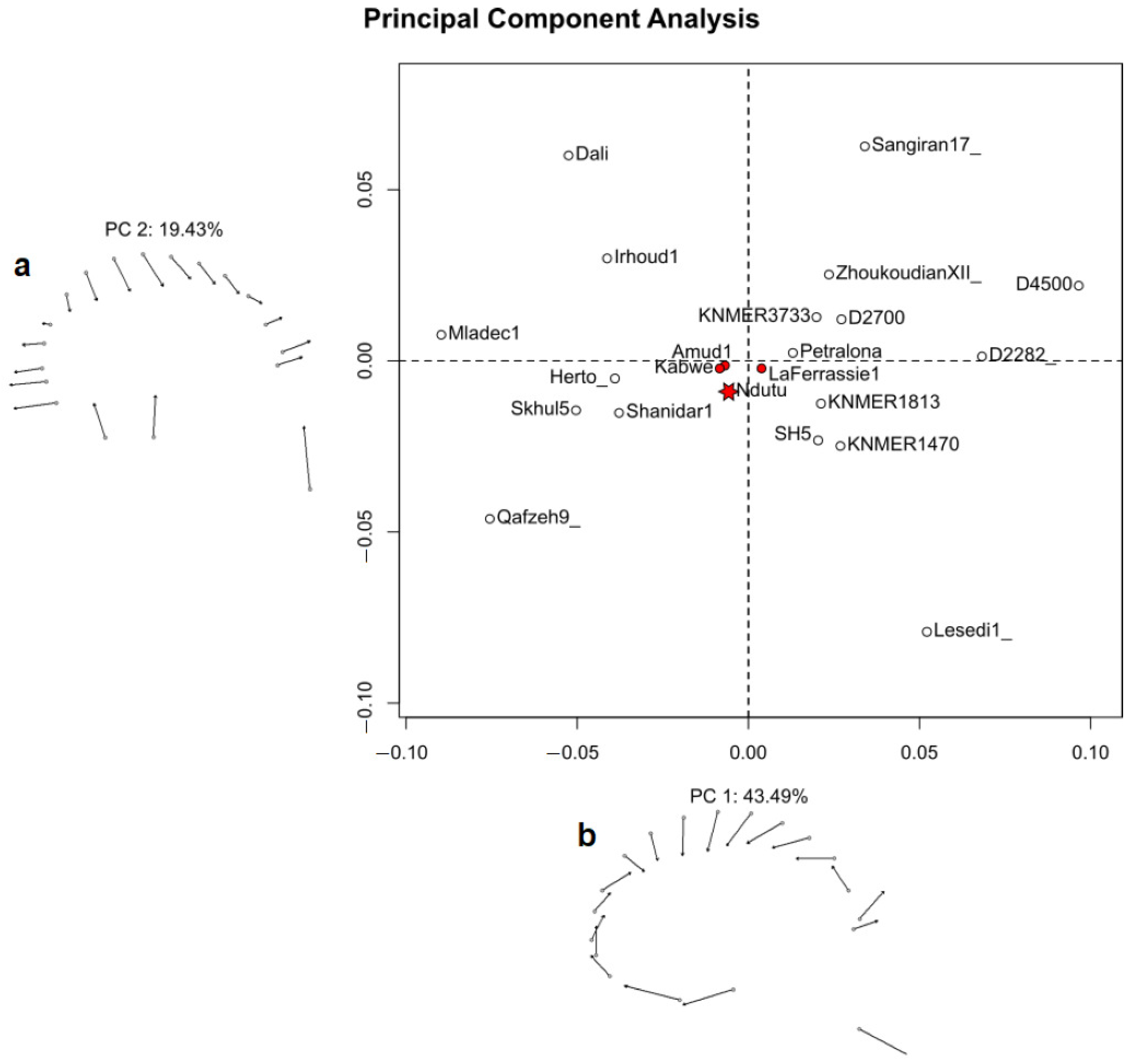
3.3. Geometric Morphometrics
4. Discussion
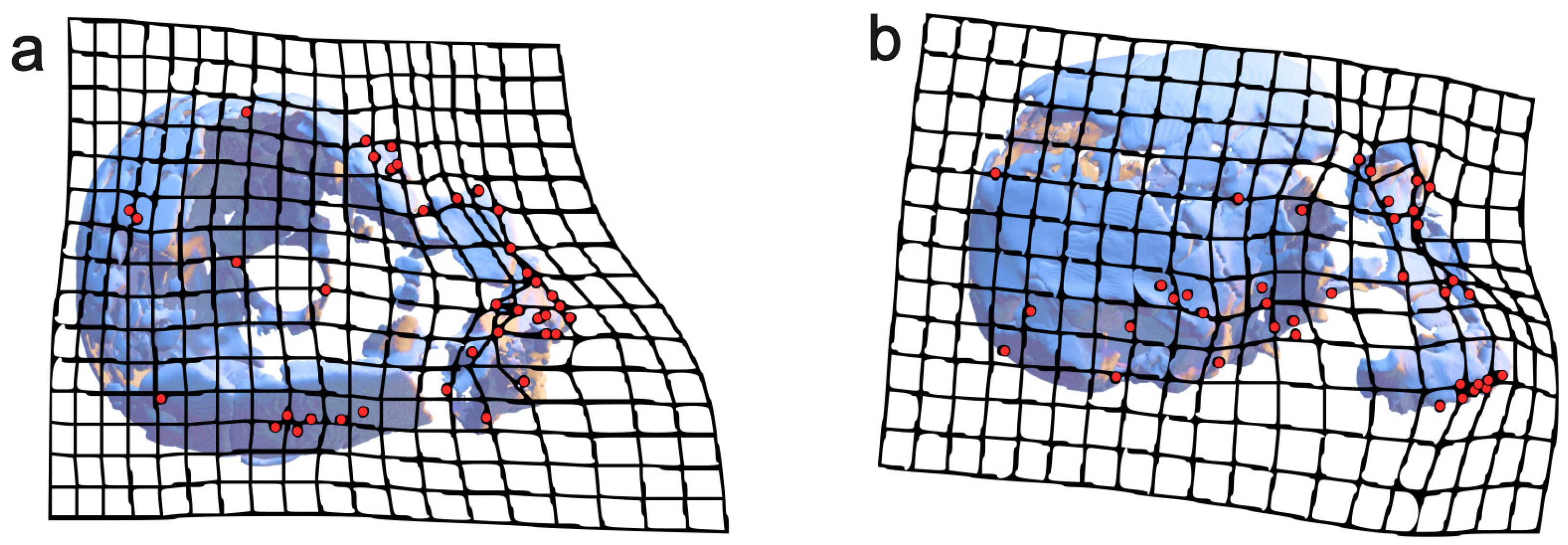
4.1. Digital Alignment
4.2. Shape Affinities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IPHES-CERCA | Institut Català de Paleoecologia Humana i Evolució Social |
| FOV | Field of view |
| H. | Homo |
| SH | Sima de los Huesos (site) |
| DTA | Digital Alignment Tool |
| ANOVA | Analysis of Variance |
| GPA | General Procrustes Analysis |
| PCA | Principal Component Analysis |
| TPS | Thin-plate spline |
References
- Mturi, A. New hominid from Lake Ndutu, Tanzania. Nature 1976, 262, 484–485. [Google Scholar] [CrossRef]
- Manega, P.C. Geochronology, Geochemistry and Isotopic Study of the Plio-Pleistocene Hominid Sites and the Ngorongoro Volcanic Highland in Northern Tanzania. Ph.D. Thesis, University of Colorado at Boulder, Boulder, CO, USA, 1993. [Google Scholar]
- Clarke, R.J. The Ndutu cranium and the origin of Homo sapiens. J. Hum. Evol. 1990, 19, 699–736. [Google Scholar] [CrossRef]
- Rightmire, G.P. The Lake Ndutu cranium and early Homo sapiens in Africa. Am. J. Phys. Anthropol. 1983, 61, 245–254. [Google Scholar] [CrossRef]
- Hublin, J.J. Northwestern African Middle Pleistocene hominids and their bearing on the emergence of Homo sapiens. In Human Roots, Africa and Asia in the Middle Pleistocene; Western Academic and Specialist Press: Barnsley, UK, 2002; pp. 99–121. [Google Scholar]
- Arsuaga, J.L.; Martınez, I.; Gracia, A.; Lorenzo, C. The Sima de los Huesos crania (Sierra de Atapuerca, Spain): A comparative study. J. Hum. Evol. 1997, 33, 219–281. [Google Scholar] [CrossRef]
- Stringer, C. The origin and evolution of Homo sapiens. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150237. [Google Scholar] [CrossRef] [PubMed]
- Grün, R.; Pike, A.; Mcdermott, F.; Eggins, S.; Mortimer, G.; Aubert, M.; Kinsley, L.; Joannes-Boyau, R.; Rumsey, M.; Denys, C.; et al. Dating the skull from Broken Hill, Zambia, and its position in human evolution. Nature 2020, 580, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Rightmire, G.P. Middle Pleistocene Homo Crania from Broken Hill and Petralona: Morphology, metric comparisons, and evolutionary relationships. In Human Paleontology and Prehistory; Springer: Berlin/Heidelberg, Germany, 2017; pp. 145–159. [Google Scholar]
- Roksandic, M.; Radović, P.; Wu, X.; Bae, C.J. Resolving the “muddle in the middle”: The case for Homo bodoensis sp. nov. Evol. Anthropol. Issues News Rev. 2022, 31, 20–29. [Google Scholar] [CrossRef]
- Lautenschlager, S. Reconstructing the past: Methods and techniques for the digital restoration of fossils. R. Soc. Open Sci. 2016, 3, 160342. [Google Scholar] [CrossRef]
- Eslami, D.; Di Angelo, L.; Di Stefano, P.; Pane, C. Review of computer-based methods for archaeological ceramic sherds reconstruction. Virtual Archaeol. Rev. 2020, 11, 34. [Google Scholar] [CrossRef]
- Schwartz, J.H.; Tattersall, I. The Human Fossil Record, Craniodental Morphology of Genus Homo (Africa and Asia); John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 2. [Google Scholar]
- Clarke, R.J. New cranium of Homo erectus from Lake Ndutu, Tanzania. Nature 1976, 262, 485–487. [Google Scholar] [CrossRef]
- Weber, G.W.; Seidler, H.; Magori, C.; Saanane, C.; Kamamba, D.; Thackeray, F.; Schrenk, F.; Recheis, W.; Nedden, D.Z.; Conroy, G.C. NDUTU, CD-ROM Including Data from CT-Scans; Department of Evolutionary Anthropology, University of Vienna: Vienna, Austria; Department of Antiquities, Dar-es-Salaam: Dar es Salaam, Tanzania, 2005. [Google Scholar]
- Materialise, N.V. Mimics Medical (Software); Materialise NV: Leuven, Belgium, 2017. [Google Scholar]
- Cignoni, P.; Callieri, M.; Corsini, M.; Dellepiane, M.; Ganovelli, F.; Ranzuglia, G. MeshLab: An Open-Source Mesh Processing Tool. In Proceedings of the Eurographics Italian Chapter Conference, Salerno, Italy, 2–4 July 2008; Scarano, V., Chiara, R.D., Erra, U., Eds.; The Eurographics Association: Vienna, Austria, 2008. [Google Scholar] [CrossRef]
- Agisoft LLC. AgiSoft PhotoScan Standard (Version 1.4.0) (Software); Agisoft LLC: St. Petersburg, Russia, 2017. [Google Scholar]
- Papaioannou, G.; Schreck, T.; Andreadis, A.; Mavridis, P.; Gregor, R.; Sipiran, I.; Vardis, K. From Reassembly to Object Completion. J. Comput. Cult. Herit. 2017, 10, 1–22. [Google Scholar] [CrossRef]
- Palmas, G.; Pietroni, N.; Cignoni, P.; Scopigno, R. A computer-assisted constraint-based system for assembling fragmented objects. In Digital Heritage International Congress; The Eurographics Association: Vienna, Austria, 2013. [Google Scholar] [CrossRef]
- Profico, A.; Buzi, C.; Davis, C.; Melchionna, M.; Veneziano, A.; Raia, P.; Manzi, G. A New Tool for Digital Alignment in Virtual Anthropology. Anat. Rec. 2019, 302, 1104–1115. [Google Scholar] [CrossRef]
- Gower, J.C. Generalized procrustes analysis. Psychometrika 1975, 40, 33–51. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes Method for the Optimal Superimposition of Landmarks. Syst. Zool. 1990, 39, 40. [Google Scholar] [CrossRef]
- White, T.D.; Black, M.T.; Folkens, P.A. Human Osteology; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Stelzer, S.; Neubauer, S.; Hublin, J.J.; Spoor, F.; Gunz, P. Morphological trends in arcade shape and size in Middle Pleistocene Homo. Am. J. Phys. Anthropol. 2019, 168, 70–91. [Google Scholar] [CrossRef]
- McNulty, K.P. A geometric morphometric assessment of the hominoid supraorbital region: Affinities of the Eurasian Miocene hominoids Dryopithecus, Graecopithecus, and Sivapithecus. In Modern Morphometrics in Physical Anthropology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 349–373. [Google Scholar]
- Martin, R.; Saller, K. Lehrbuch der Anthropologie III; Gustav Fischer: Stuttgart, Germany, 1957. [Google Scholar]
- Smith, H.F.; Ritzman, T.; Otárola-Castillo, E.; Terhune, C.E. A 3-D geometric morphometric study of intraspecific variation in the ontogeny of the temporal bone in modern Homo sapiens. J. Hum. Evol. 2013, 65, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Contents; Cambridge University Press: Cambridge, UK, 1992; pp. v–xii. [Google Scholar]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Gunz, P.; Mitteroecker, P.; Bookstein, F.L. Semilandmarks in Three Dimensions. In Modern Morphometrics in Physical Anthropology; Slice, D.E., Ed.; Springer: Boston, MA, USA, 2005; pp. 73–98. [Google Scholar] [CrossRef]
- Gunz, P.; Mitteroecker, P. Semilandmarks: A method for quantifying curves and surfaces. Hystrix Ital. J. Mammal. 2013, 24, 103–109. [Google Scholar]
- Bardua, C.; Felice, R.N.; Watanabe, A.; Fabre, A.C.; Goswami, A. A Practical Guide to Sliding and Surface Semilandmarks in Morphometric Analyses. Integr. Org. Biol. 2019, 1, obz016. [Google Scholar] [CrossRef] [PubMed]
- Roth, V. On three-dimensional morphometrics, and on the identification of landmark points. In Contributions to Morphometrics; Museo Nacional de Ciencias Naturales: Madrid, Spain, 1993; Volume 41, p. 61. [Google Scholar]
- Zelditch, M.; Swiderski, D.; Sheets, H.D.; Fink, W. Geometric Morphometrics for Biologists: A Primer; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar] [CrossRef]
- Madrigal, L. Statistics for Anthropology; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Peterson, R.A. Finding Optimal Normalizing Transformations via bestNormalize. R J. 2021, 13, 310–329. [Google Scholar] [CrossRef]
- Adams, D.; Collyer, M.; Kaliontzopoulou, A.; Baken, E. Geomorph: Software for Geometric Morphometric Analyses. R Package Version 4.0. 2021. Available online: https://cran.r-project.org/package=geomrph (accessed on 27 December 2022).
- Community, B.O. Blender—A 3D Modelling and Rendering Package; Blender Foundation, Stichting Blender Foundation: Amsterdam, The Netherlands, 2018. [Google Scholar]
- 3D Slicer Image Computing Platform; BWH and 3D Slicer Contributors. 16 November 2022. Available online: https://www.slicer.org/ (accessed on 27 December 2022).
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Huttegger, S.M. The concept of morphospaces in evolutionary and developmental biology: Mathematics and metaphors. Biol. Theory 2009, 4, 54–67. [Google Scholar] [CrossRef]
- Suzuki, H.; Takai, F. The Amud Man and His Cave Site; Academic Press of Japan: Tokyo, Japan, 1970. [Google Scholar]
- Gunz, P.; Mitteroecker, P.; Neubauer, S.; Weber, G.W.; Bookstein, F.L. Principles for the virtual reconstruction of hominin crania. J. Hum. Evol. 2009, 57, 48–62. [Google Scholar] [CrossRef]
- Lieberman, D.E. 16. Epigenetic Integration, Complexity, and Evolvability of the Head: Rethinking the Functional Matrix Hypothesis. In Epigenetics; University of California Press: Berkeley, CA, USA, 2011; pp. 271–289. [Google Scholar]
- Müller, G.B. Evo–devo: Extending the evolutionary synthesis. Nat. Rev. Genet. 2007, 8, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Sardi, M.L.; Ventrice, F.; Ramírez Rozzi, F. Allometries Throughout the Late Prenatal and Early Postnatal Human Craniofacial Ontogeny. Anat. Rec. 2007, 290, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- González-José, R.; Escapa, I.; Neves, W.A.; Cúneo, R.; Pucciarelli, H.M. Cladistic analysis of continuous modularized traits provides phylogenetic signals in Homo evolution. Nature 2008, 453, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Cheverud, J.M. Phenotypic, genetic, and environmental morphological integration in the cranium. Evolution 1982, 36, 499–516. [Google Scholar] [CrossRef]
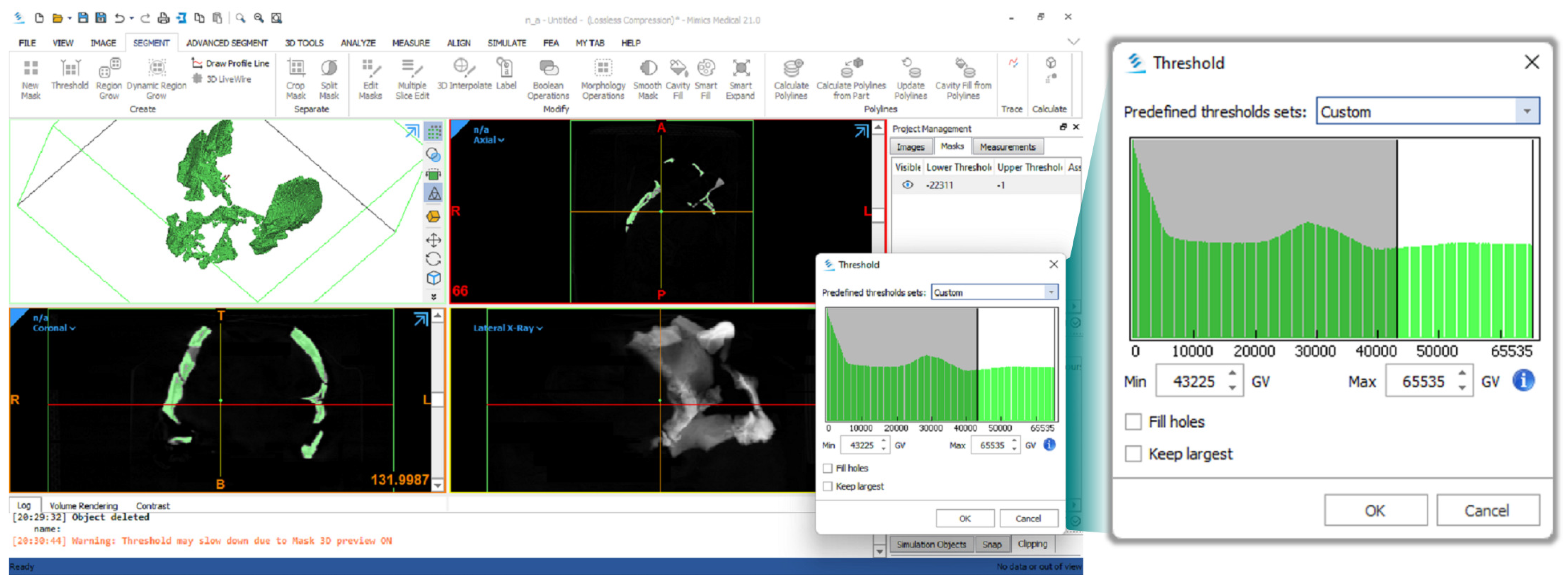
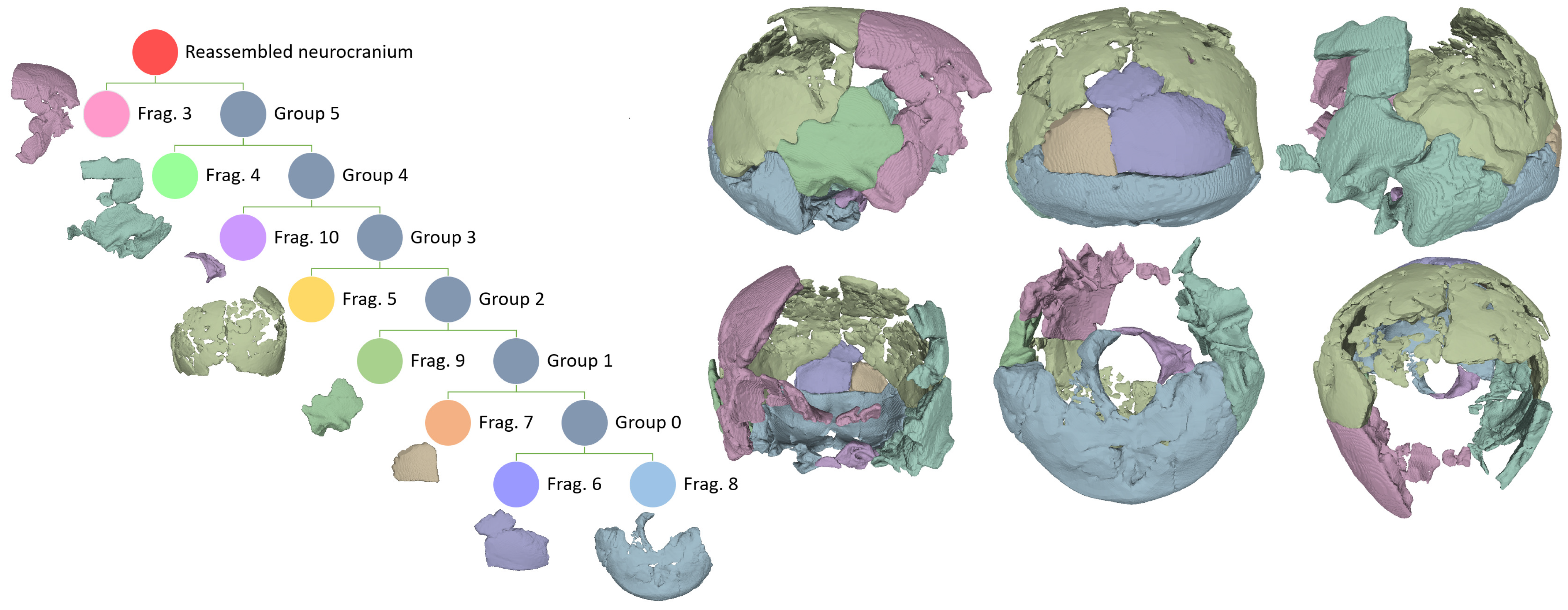


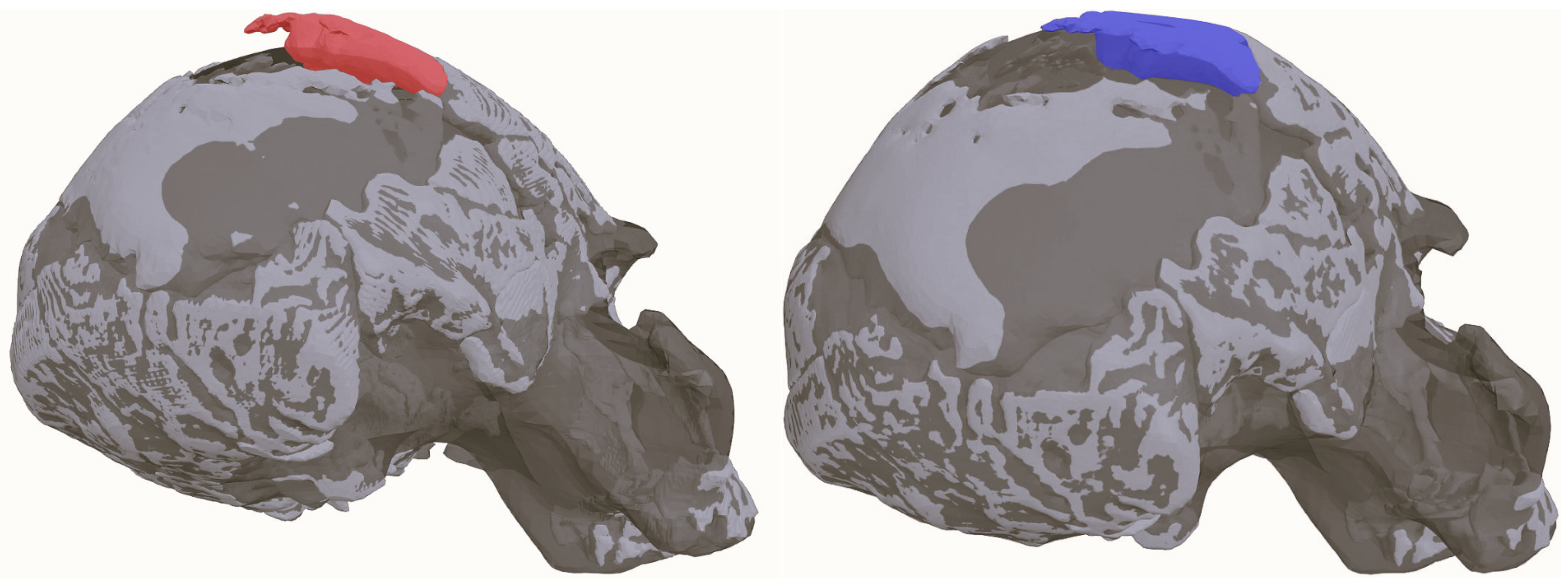


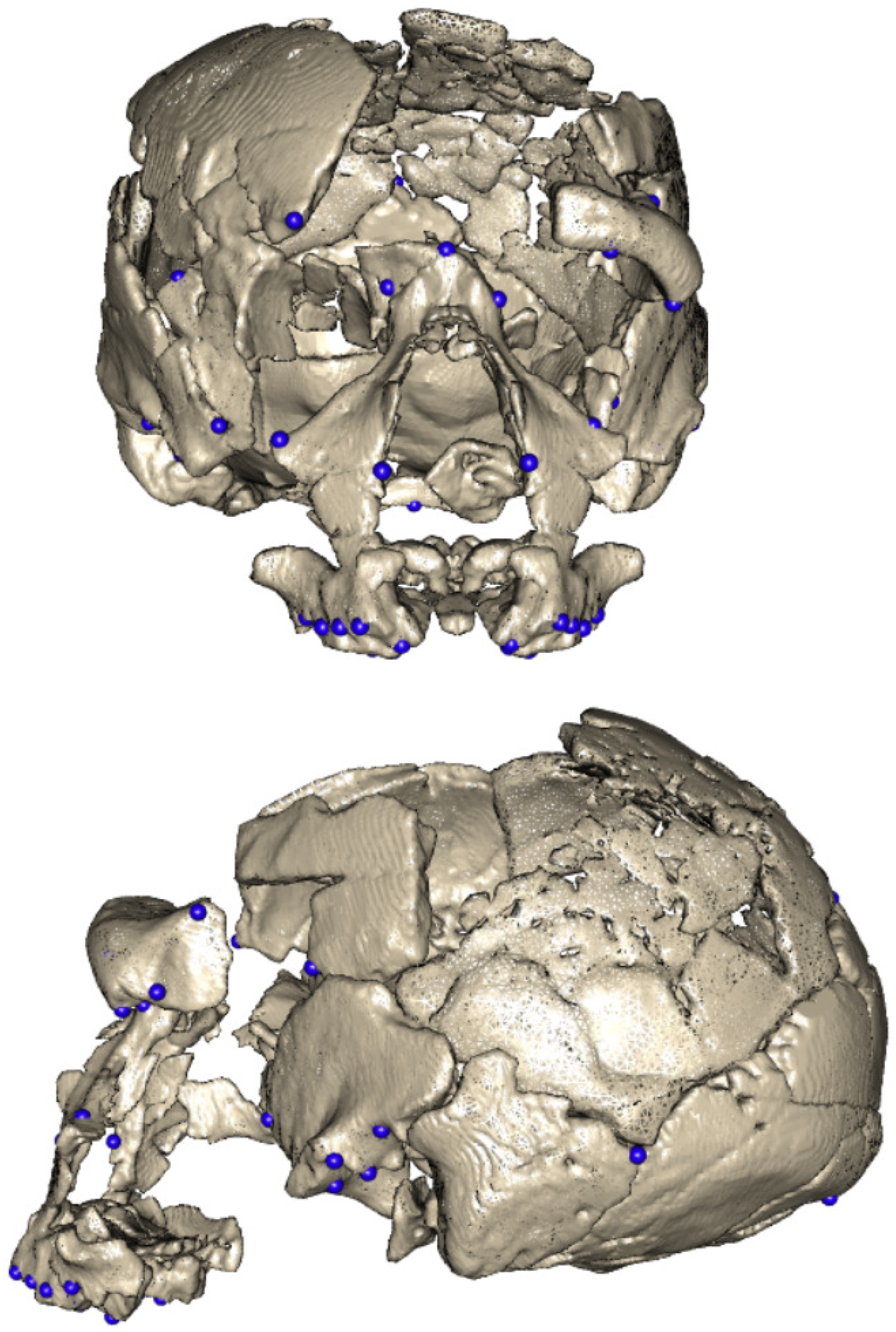

| Procedure | Software Package(s) |
|---|---|
| Segmentation of CT scan data | Materialise Mimics Medical 21.0.0.406 |
| Generation of disarticulated fragment models | MeshLab 2022.02 |
| Photogrammetry of fossil replicas for reference sample | Agisoft Photoscan Professional 1.40 build 5076 |
| Reassembly of articulated fragments of the neurocranium | Fragment Reassembler 1.0 |
| Mirroring of the facial skeleton | MeshLab 2022.02 |
| Landmark digitization | MeshLab 2022.02 |
| Surface semilandmark digitization | R (Morpho, Geomorph, and other packages), MeshLab 2022.02 |
| Digital alignment tool testing | R (Arothron and other packages) |
| Test results analysis | R (Rstatix, BestNormalize, and other packages) |
| Alignment of disarticulated fragments | R (Arothron package) |
| Completion of the Ndutu cranium via interpolation | R (Morpho, Geomorph, and other packages) |
| Retrodeformation of the anterior fragment of the right parietal | R (Morpho, Geomorph, and other packages) |
| Comparison with previous reconstruction | R (Morpho, Geomorph, and other packages) |
| Digitization of curve semilandmarks | 3DSlicer 5.2.1 (SlicerMorph) |
| Shape analysis | R (Morpho, Geomorph, and other packages) |
| Fragment No. | CT Scan No. | Identification | Articulates with No. |
|---|---|---|---|
| 1 | 1 | Left circum-nasal area, medial wall of the right orbit, and anterior portion of the left palate, with a disarticulated fragment of the right upper lateral border of the nasal aperture. | Disarticulated |
| 2 | 1 | Left lateral supraorbital. | Disarticulated |
| 3 | 1 | Right lateral frontal squama and fused lower anterior right parietal, right greater wing of the sphenoid, lower anterior temporal, and a disarticulated fragment of the left lesser wing of the sphenoid. | 5, 9 |
| 4 | 1 | Left lateral frontal squama fragments of the upper posterior and (disarticulated) lower posterior lateral surface of the left greater wing of the sphenoid; squamous and mastoid portions of the left temporal. | 5, 8 |
| 5 | 2 | Left and right parietals (excluding the anterior third of the left one and the antero-medial portion of the right one), including several disarticulated inner and outer tabula fragments. | 3, 4, 8, 9 |
| 6 | 3 | Distal medial occipital squama including interparietal bone. | 5, 7, 8 |
| 7 | 4 | Single fragment of the left part of the distal occipital squama. | 5, 6, 8 |
| 8 | 5 | Restored occipital bone including the nuchal plane, the base of the occipital planum, and the posterior and right lateral borders of the foramen magnum, fused to the mastoid portion of the right temporal. | 4, 5, 6, 7, 9, 10 |
| 9 | 6 | Right temporal squama | 3, 5, 8 |
| 10 | 7 | Anterior and left lateral borders of the foramen magnum. | 8 |
| 11 | 8 | Unmatched parietal fragment cluster. | Disarticulated |
| Specimen Name | Digital Capture | CT Scan Resolution | Vertices | Faces | Specimen Type | Source |
|---|---|---|---|---|---|---|
| Amud 1 | Photogrammetry | 2,230,885 | 4,461,770 | Replica | Paleoanthropology Laboratory, IPHES-CERCA (own data) | |
| D2282 | Photogrammetry | 1,498,772 | 2,996,828 | Replica | Paleoanthropology Laboratory, IPHES-CERCA (own data) | |
| D2700 | Photogrammetry | 1,285,589 | 2,570,089 | Replica | Paleoanthropology Laboratory, IPHES-CERCA (own data) | |
| D4500 | Photogrammetry | 1,109,845 | 2,219,686 | Replica | Paleoanthropology Laboratory, IPHES-CERCA (own data) | |
| Dali | Laser scan | 367,362 | 732,191 | Replica | Muséum National d’Histoire Naturelle | |
| BOU-VP-16/1 (Herto) | Photogrammetry | 2,178,417 | 4,356,086 | Replica | Paleoanthropology Laboratory, IPHES-CERCA (own data) | |
| Irhoud 1 | Photogrammetry | 1,215,368 | 2,430,726 | Replica | Paleoanthropology Laboratory, IPHES-CERCA (own data) | |
| Kabwe | CT scan | x = 0.108868 mm, y = 0.108868 mm, z = 0.108868 mm | 1,073,374 | 2,052,377 | Replica | Morphosource.org (Duke University) |
| KNM ER 1470 | Photogrammetry | 49,989 | 100,006 | Replica | AfricanFossils.org (Turkana Basin Institute) | |
| KNM ER 1813 | Laser scan | 49,966 | 99,928 | Replica | AfricanFossils.org (Turkana Basin Institute) | |
| KNM ER 3733 | Structured light scan | 49,984 | 99,972 | Replica | AfricanFossils.org (Turkana Basin Institute) | |
| La Chapelle-aux-Saints | CT scan | x = 0.123 mm, y = 0.123 mm, z = 0.123 mm | 719,164 | 1,430,560 | Original Fossil | Muséum National d’Histoire Naturelle |
| La Ferrasie 1 | Laser scan | 648,417 | 839,550 | Replica | Muséum National d’Histoire Naturelle | |
| Lesedi 1 | Laser scan | 1,944,690 | 3,889,412 | Original Fossil | Morphosource.org (University of the Witswatersrand) | |
| Sima de los Huesos 5 | Photogrammetry | 1,418,721 | 2,837,354 | Replica | Paleoanthropology Laboratory, IPHES-CERCA (own data) | |
| Mladeč 1 | CT scan | x = 0.4668 mm, y = 0.4668 mm, z = 0.75 mm | 234,610 | 468,560 | Original Fossil | Natural History Museum Vienna |
| Petralona 1 | Photogrammetry | 1,821,546 | 3,640,857 | Replica | Paleoanthropology Laboratory, IPHES-CERCA (own data) | |
| Qafzeh 9 | Photogrammetry | 1,648,506 | 3,296,880 | Replica | Paleoanthropology Laboratory, IPHES-CERCA (own data) | |
| Sangiran 17 | CT scan | x = 0.107417 mm, y = 0.107417 mm, z = 0.107417 mm | 1,498,652 | 2,953,295 | Replica | Morphosource.org (Duke University) |
| Shanidar 1 | Photogrammetry | 1,741,950 | 3,488,595 | Replica | Paleoanthropology Laboratory, IPHES-CERCA (own data) | |
| Skhul 5 | CT scan | x = 0.488281 mm, y = 0.488281 mm, z = 0.5 mm | 642,964 | 1,279,624 | Original Fossil | Peabody Museum (Harvard University) |
| Zhoukoudian XII | Photogrammetry | 184,371 | 386,742 | Replica | Paleoanthropology Laboratory, IPHES-CERCA (own data) |
| Effect | DFn | DFd | F Statistic | p Value | Ges |
|---|---|---|---|---|---|
| semilandmarks | 1 | 80 | 5.945 | 0.017 1 | 0.069 |
| symmetry | 1 | 80 | 0.824 | 0.367 | 0.010 |
| semilandmarks:symmetry | 1 | 80 | 0.906 | 0.344 | 0.011 |
| Effect | DFn | DFd | F Statistic | p Value | Ges |
|---|---|---|---|---|---|
| fragment choice | 1 | 80 | 25.342 | a | 0.241000 |
| semilandmarks | 1 | 80 | 0.410 | 0.005000 | |
| fragment choice:semilandmarks | 1 | 80 | 0.067 | 0.000838 |
| Fragment of Choice | Variable | y | Level 1 | Level 2 | df | Statistic | p Value 1 |
|---|---|---|---|---|---|---|---|
| Facial skeleton | semilandmarks | mean Euclidean distance | no | yes | 80 | −0.636 | 0.527 2 |
| Left supraorbital fragment | semilandmarks | mean Euclidean distance | no | yes | 80 | −0.270 | 0.788 2 |
| Landmarks |
|---|
| Left buccal M2 |
| Left buccal M1 |
| Left buccal P4 |
| Left buccal P3 |
| Left buccal C |
| Left lingual M1 |
| Left lingual P4 |
| Left lingual P3 |
| Left lingual C |
| Left/right alare |
| Left/right zygoorbitale |
| Mid-torus inferior |
| Left/right maxillofrontale |
| Nasion |
| Left frontomalare orbitale |
| Left frontomalare temporale |
| Left/right frontotemporale |
| Left/right sphenion |
| Left/right superior infratemporal fossa |
| Left/right porion |
| Left/right asterion |
| Lambda |
| Inion |
| Left/right posterior glenoid point |
| Left/right lateral glenoid point |
| Left/right anterior glenoid point |
| Opisthion |
| Basion |
| Landmarks |
|---|
| Prosthion |
| Nasion |
| Glabella |
| 8 midline curve semilandmarks on the cranial vault |
| Lambda |
| 4 midline curve semilandmarks on occipital squama |
| Inion |
| Opisthion |
| Basion |
| Landmarks |
|---|
| Left midtorus inferior |
| Left frontomalare orbitale |
| Left frontomalare temporale |
| Left/right frontotemporale |
| Left/right sphenion |
| Left/right superior infratemporal fossa |
| Left/right porion |
| Left/right asterion |
| Lambda |
| Inion |
| Left/right posterior glenoid point |
| Left/right lateral glenoid point |
| Left/right anterior glenoid point |
| Opisthion |
| Glabella |
| 20 surface semilandmarks on the cranial vault |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montiel, G.; Lorenzo, C. A New Virtual Reconstruction of the Ndutu Cranium. Heritage 2023, 6, 2822-2850. https://doi.org/10.3390/heritage6030151
Montiel G, Lorenzo C. A New Virtual Reconstruction of the Ndutu Cranium. Heritage. 2023; 6(3):2822-2850. https://doi.org/10.3390/heritage6030151
Chicago/Turabian StyleMontiel, Gustavo, and Carlos Lorenzo. 2023. "A New Virtual Reconstruction of the Ndutu Cranium" Heritage 6, no. 3: 2822-2850. https://doi.org/10.3390/heritage6030151
APA StyleMontiel, G., & Lorenzo, C. (2023). A New Virtual Reconstruction of the Ndutu Cranium. Heritage, 6(3), 2822-2850. https://doi.org/10.3390/heritage6030151





