1. Introduction
The overall protection of museum collections depends on a large group of collections care staff, exhibition designers, facilities personnel, preparators, custodial staff, and scientists to ensure that artworks are not exposed to unnecessary pollutants from display, storage, or shipping environments. The potential sources of pollution in museums are legion—outside air, museum furnishings, disinfectants and cleaning supplies, guests, food service, and the artworks themselves, to name but a few. One of the most important and pervasive sources of volatile organic compounds (VOCs) capable of damaging art objects are construction materials used in the creation of galleries, display cases, storage containers, and shipping crates. Emissions from materials used in tightly enclosed spaces are especially damaging since these containers are usually designed to have little air exchange and, therefore, higher concentrations of pollutants can be achieved over time if the source of pollution is internal. Micro-climate packaging of individual artworks is also becoming more prevalent with increasing risks due to pollution, climate disasters, terrorism, seasonal relative humidity and temperature fluctuations, and the more liberal use of gallery spaces for events involving food and drink.
As a result of these concerns and the need to protect art, many museums have policies in place that dictate how materials for use in proximity to artworks are selected and used. These protocols can vary widely in their complexity and sophistication. For smaller institutions, selection may be based on commercial labels, such as “archival” or “safe”, although it is widely recognized that these designations are not standardized, nor is the relevant testing of materials enforced. Choices may also be steered toward products that have performed well in the past, although the possibility of product reformulations is an ever-present concern. For institutions with conservation departments, active testing of products being considered for use is undertaken with minimal equipment or chemical facilities. These tests can involve accelerated aging experiments using artwork surrogates or microchemical spot tests specific for known pollutants with a high damage potential such as organic acids, sulfurous pollution, or reactive chlorides [
1,
2,
3]. Finally, a small number of museums have sophisticated science laboratories that allow for specific material characterization, as well as comprehensive analysis of VOCs, to inform construction material selection.
1.1. Recent Developments in Construction Material Assessments
Exhibition planners rely on many different approaches for the assessment of materials to be used in museum construction and exhibition spaces. The current museum “gold standard” is the accelerated metal corrosion test commonly referred to as the Oddy test [
3,
4,
5,
6]. Named after its creator, Andrew Oddy from the British Museum, the test exposes three high-purity metal coupons (copper, lead, and silver) to a material under study in a closed vessel at 60 °C and 100% relative humidity for 28 days. After the exposure period is completed, the coupons are examined visually for signs of corrosion and compared to a set of control coupons. Construction materials are then graded as acceptable for permanent use, adequate for temporary use, or unacceptable for use in proximity to artwork, based on the level of corrosion they induced in the metal coupons. The strength of the Oddy test lies in its breadth of sensitivity—any VOC capable of causing metal corrosion can be detected—and its visual indicator of negative impacts. Unfortunately, some important non-corrosive compounds, such as plasticizers and solvents, can go undetected. While this test is relatively easy to implement, the long incubation time rarely matches the timescale of exhibition decision-making, and the subjective grading of the corrosion level increases variability in the results [
6]. Although the test is often said to be inexpensive because it uses generally available laboratory glassware and reagents, what is often overlooked is the extensive and, therefore, costly, labor by an experienced staff member required to prepare a large number of tests and replicates. An additional minor concern is the increasing costs, both financial and environmental, of disposing of heavy metal waste from the discarded lead coupons, although larger museums may already have a hazardous waste program in place.
Other selective chemical tests for detecting museum pollutants that rely on a visual cue, such as a color change or gas evolution, have been described in detail elsewhere [
1,
4,
7,
8,
9,
10]. These tests typically expose a reagent solution or chemical test strip to a sample of the material in a closed and sometimes heated vessel. A distinct visual change signals the presence of a specific pollutant such as formaldehyde, sulfur, oxidants, or organic acid. Microchemical tests are often quick, generating a result immediately or within 1 h, provided the reagents are prepared and on hand when needed. As an example, the chromotropic acid test indicates the off-gassing of formaldehyde [
7]. A 2 g sample of material is enclosed in a jar with a small vessel containing the chromotropic acid reagent mixture and is heated for 30 min at 60 °C. If formaldehyde is emitted from the sample material, the container of the yellowish reagent solution turns a deep violet color, allowing for the identification of formaldehyde down to ppm concentrations. It is important to test at the same time both a known formaldehyde emitter (such as Delrin poly(oxymethylene) plastic) and a non-emitter (such as polyethylene plastic or an empty reactor) as positive and negative controls, respectively, to ensure the reagent is working correctly. While a number of pollutants can be identified by the microchemical approach, often multiple chemical tests are needed to assess a material thoroughly, and again some potentially dangerous VOCs could still be missed. Furthermore, the chemical reagents used can be hazardous or have a short shelf life, necessitating care and diligence in managing stock solutions.
Instrument-based methods of testing are becoming commonplace in larger museums equipped with modern scientific laboratories. Chromatographic techniques offer significant advantages over chemical testing in terms of speed and the comprehensive nature of the analysis. In addition, they are often regarded as a more objective approach to the assessment of material suitability for known pollutants and they do not rely on a visual interpretation of corrosion or subjective determination of color change reactions. Commercial protocols, such as BEMMA and ISO16000, make use of an adsorbent mixture to trap VOCs emitted from materials [
10]. Afterward, these compounds are thermally desorbed from the adsorbent media and analyzed by gas chromatography–mass spectrometry (GC-MS). Similarly, solid phase microextraction (SPME) employs a small adsorbent fiber housed in the barrel of a needle. The fiber is exposed in an environment selected for air quality monitoring and is then desorbed in the hot inlet of a GC-MS instrument [
11]. Trapped VOCs are separated, detected, and sometimes quantified by this approach.
More recently, the present authors pioneered the direct thermal desorption (DTD) analysis of museum construction materials using a commonly available commercial microfurnace pyrolyzer (PY) available in many museum laboratories coupled with GC-MS [
12,
13,
14]. In this test, several milligrams of material are introduced to the pyrolysis oven operated at a lower temperature (115–180 °C) for 30 s to 1 min. All desorbed VOCs are immediately carried to the GC inlet and are trapped on the GC column using a cryogenic focusing apparatus; a significant advantage is that no selective intermediate adsorbent phase is used, and all emitted gases are collected on the column for analysis. To concentrate the analytes and increase sensitivity, a longer desorption time or higher temperature can be utilized. After the desorption time has elapsed, the cryotrap is shut off, and the trapped VOCs are passed into the GC-MS for separation and identification. Relative quantitation is possible with DTD-GC-MS in order to compare the amount of off-gassing from samples when multiple versions of materials are being tested [
12]. When using a “chemical intuition” approach to evaluating the detected VOCs’ ability to harm artwork materials, the results from DTD-GC-MS were shown to be consistent with results obtained from Oddy testing for common museum materials [
13]. DTD-GC-MS was recently used to assess a range of rigid poly(vinyl chloride) (PVC) materials for their use in museum display cases showing differences between premium and economy versions; surprisingly, the economy samples performed better [
14].
1.2. A New Materials Testing Methodology
Previously at the Indianapolis Museum of Art (IMA) at Newfields, material vetting for gallery and display case construction relied on a modified version of the Oddy test that did not conform to the procedure as described by the British Museum [
6]. Reused thick metal alloy coupons were employed to save costs, test materials were tied directly to the coupons, and a leaky foil-covered one-liter beaker served as the reaction vessel. Over the past ten years—motivated in part by the creation of a separate conservation science laboratory within the collection care division—a significant effort has gone into developing a more rigorous testing methodology that achieves an efficient and thorough assessment of museum construction materials. This process relies on rapid instrumental measurements of desorbed volatiles, supplemented when necessary by microchemical techniques, and as a last resort traditional accelerated corrosion testing, i.e., Oddy testing. While each of these assessments has a history of application in construction materials assessments in museums, it is their combination in the sequential methodology presented here that is novel. This report describes this emerging methodology at the museum for material suitability testing, highlighting the premier role that DTD-GC-MS plays in the overall process of ensuring the safety of the museum’s collections. The use of this procedure in the preparations for a new, nontraditional, and multisensory museum exhibition, The LUME Indianapolis, demonstrates the successes and struggles of the new methodology.
3. Results
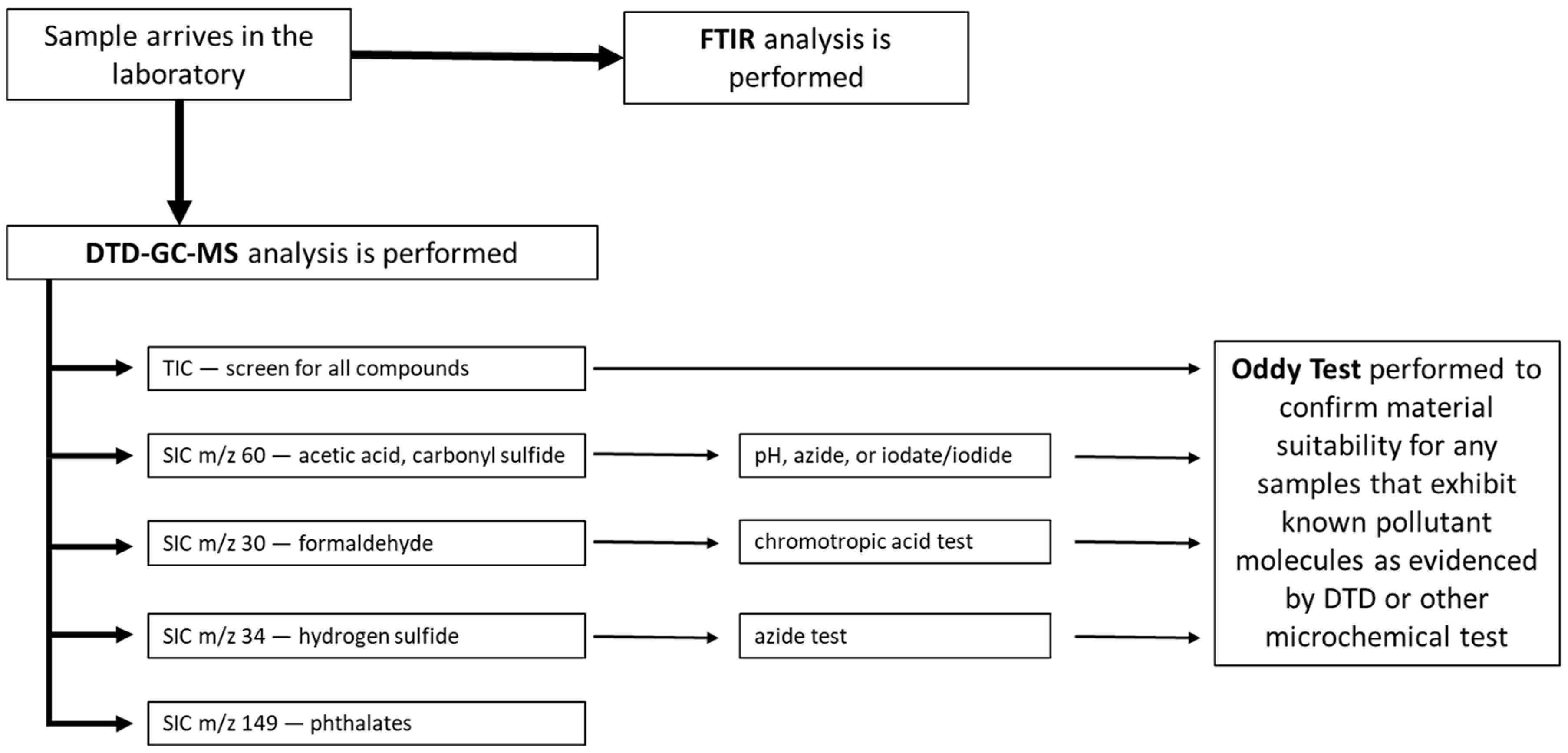
Figure 1 shows schematically the current approach to material suitability testing at the IMA. Testing is conducted within the scientific research department by cultural heritage chemists and scientific research fellows. It is important to note that this is not always the case; construction material testing at museums can be the responsibility of conservators, collection managers, and even interns or volunteers. This can be true even when an in-house scientific laboratory is available, depending on the priorities and mandates of the various personnel. The DTD-GC-MS approach, however, relies on a broad understanding of chemical reactivity, referred to as “chemical intuition” [
13] as described below, and so personnel with advanced chemistry training are currently best suited to undertake the assessments.
Materials being considered for use in the museum are normally submitted to the lab staff by conservators, designers, fabricators, or project planners. Ideally, all materials used in proximity to artwork should be properly vetted before use. In practice, however, materials used successfully in the past are often not resubmitted when new batches are ordered, and materials are frequently used without testing when deadlines are tight or if the scale of a project outpaces the sample throughput of the laboratory. In the past, the long incubation period for the Oddy test limited its application, but newer accelerated testing protocols, often only 30 min in duration, should reduce these instances of foregoing testing. Far more difficult to correct are those instances when the testing policy is circumvented, e.g., when outside contractors or fabricators fail to submit specimens of all the materials to be used or when new or temporary staff are unaware of the material testing requirements.
Ideally, multiple examples of a material or component (paint, adhesive, gasket, etc.) from different vendors should be submitted for consideration so that a comparison can be made in a single bout of testing, allowing the best-performing sample to be selected for the project. This situation, however, rarely occurs, and usually, a single example arrives for testing. Sourcing multiple examples of a component requires extensive research of alternative manufacturers and significant effort from design and fabrication staff who often already have a clear design aesthetic and plan in mind. However, when only one example is tested, it can fail, restarting the search for a substitute product, and this repetitive process wastes critical time for fast-moving projects. Another significant issue is that the sample sent for testing from a manufacturer is sometimes not exactly the product delivered ultimately for use. This can arise due to changes in availability or colors, the use of brand-specific names as a generic term for a type of material, e.g., “Sintra” for all rigid PVC foamboard [
14], or last-minute changes to the design aesthetic. In these instances, the results of the testing could be irrelevant if the component actually used in the construction project has a different formulation.
Once samples arrive in the laboratory, two instrumental analyses are conducted simultaneously. ATR-FTIR spectroscopy is performed on single-component products or on laminate products that can be easily disassembled in order to identify the core materials. For materials with layers that are difficult to delaminate cleanly, an FTIR microscope allows small homogeneous fragments removed with a scalpel to be analyzed separately. This step, while not mandatory for suitability testing, helps to inform later analyses based on known or likely formulation components for the particular material identified. For instance, a soft PVC layer will likely emit plasticizers and possibly some HCl in the volatiles analysis, and so any PVC product identified by FTIR will be scrutinized for these emissions.
Concurrent with FTIR analysis, the material is subjected to DTD-GC-MS to assess the VOCs emitted at 180 °C. With the appropriate chromatographic conditions and mass ranges (29 to 120 m/z for very volatile compounds and 45 to 300 for more traditional VOC analysis), a broad spectrum of gases of concern to conservators can be detected using this DTD-GC-MS method.
Once the DTD total ion chromatogram (TIC) has been generated, each major peak is identified based on its mass spectrum, generating a table of VOC emissions. Not all volatiles will be of concern to the preservation of museum artworks and artifacts, but certain compounds are known to have chemical reactivity with important artists’ materials. For instance, hydrogen sulfide is known to tarnish metals and to blacken lead and copper-based pigments [
1,
8]. Acetic acid can catalyze depolymerization reactions, react with colorants, and corrode metals [
1,
7,
8]. Formaldehyde crosslinks proteins and has been shown to react with some synthetic dyes [
17]. To ensure these known pollutants are identified when present in even trace quantities, a selected ion chromatogram (SIC) is generated for the principal mass fragment for a short list of the most egregious museum pollutants. The SIC effectively isolates the molecules of interest and removes much of the background to allow for better detection. These specifically selected ions are listed in the flowchart in
Figure 1.
Many of the compounds identified in the TIC chromatogram, however, will not be known to the analyst, and no information will exist on their interaction with artists’ materials. In these instances, the scientist must use their “chemical intuition” to flag VOCs that have chemical functional groups that are likely to be reactive to broad classes of artists’ materials. Chemical intuition applied to DTD-GC-MS has been previously described [
13], and it relies on a strong familiarity with chemical structure and reactivity. As an example, a thiol compound of modest molecular weight could be anticipated to volatilize and react similarly to the known pollutants H
2S, COS, and CS
2. Such a compound would potentially endanger metals or metallic pigments present in an enclosure with the thiol-bearing material. The identification of such a compound would lead to a search for an alternative construction material that does not emit the same VOC; this was shown recently for rigid PVC plastics, where some versions of the sheet plastic released 2-ethylhexyl thioglycolate while other economy versions did not [
14]. Similarly, an organic acid with a reasonable vapor pressure (e.g., butanoic acid) or an easily oxidized aldehyde of similarly high volatility (butanal) might be avoided if alternative materials without these emissions are available since volatile organic acids are known to react with many artists’ materials. Furthermore, lower volatility materials, such as phthalate plasticizers, which are known to migrate through contact from industrial plastics into other materials causing softening or dirt accumulation [
18,
19], could be specified for only temporary use in situations where non-contact with artworks can be assured. Chemical intuition is dependent on the chemistry knowledge of the analyst and therefore not entirely objective; however, the entire list of detected VOCs could be further researched by the novice to determine the risk potential each compound might pose to cultural heritage objects.
When a known pollutant can only be identified using selected ion monitoring, the question arises whether the low concentration of the pollutant that was detected is sufficient to cause damage. An initial step is to confirm the presence of the specific pollutant using a microchemical test. These confirmatory tests can be completed easily and quickly using stock reagents prepared periodically and stored in a refrigerator. The tests utilized at the IMA include the iodide–iodate test for organic acids [
1,
2,
7], the sodium azide test for reducible sulfides [
1,
2], and the chromotropic acid test for formaldehyde [
1,
2,
7]. As performed in the lab with only visual indicators, these tests are only semi-quantitative in the sense that reactions can be judged as weakly or strongly positive and mainly serve a confirmatory purpose.
If confirmed through microchemical testing, the presence of any of these classes of known pollutants does not necessarily exclude a construction material from consideration for use in the museum, especially when substitute products or alternative approaches are deemed nonexistent, less satisfactory, or prohibitively expensive. The same is true for materials that yield suspect VOCs based on chemical intuition, but for which there are no detailed studies proving their deleterious effect on artists’ materials. In these instances, and as a last resort, an Oddy test may be warranted to provide visual evidence of the impact of the material’s emissions on metal coupons under aggressive environmental conditions.
3.1. THE LUME Indianapolis
This testing methodology is demonstrated here through a case study of material selection for a unique exhibition at the IMA. THE LUME Indianapolis, which involved converting the entire top floor of the museum into a largescale, immersive, multisensory digital projection experience, was the largest show in the museum’s history and required extensive renovation of the gallery spaces. In its inaugural experience, the exhibition surrounded guests with animated digital projections of van Gogh’s paintings and drawings set to music and augmented with a faintly dispersed scent to celebrate the artist, as seen in
Figure 2. Textures were also reproduced in a life-size 3D replica of van Gogh’s 1888 painting from Arles,
The Bedroom. The sense of taste is engaged through a café located halfway through the exhibition where guests can purchase food and drink to carry with them through portions of the show. The final gallery of the exhibition space treats visitors to three actual masterworks by van Gogh and his contemporaries.
Significant alterations to existing gallery spaces were required to accommodate the immersive environment including projection galleries, an interactive play space, an impressionist style café, a life-size 3D replica of a van Gogh painting, and a gift shop. Although far too many components were included in the exhibition to fully test every material used in the spaces prior to the construction of the galleries, certain materials that would be used extensively were assessed for their potential to emit VOCs. Flooring, paints, adhesive films, and even an aerosol scent were tested for potential application in over 25,000 sq. ft. of gallery space, requiring careful vetting to ensure that harmful pollutants were avoided as much as possible to protect artworks in adjacent galleries and the three post-impressionist paintings exhibited within THE LUME Indianapolis experience itself. Moreover, the transformed space is anticipated to remain largely intact for at least 5 years, as new projection shows of digital art are commissioned annually.
3.2. Flooring Materials
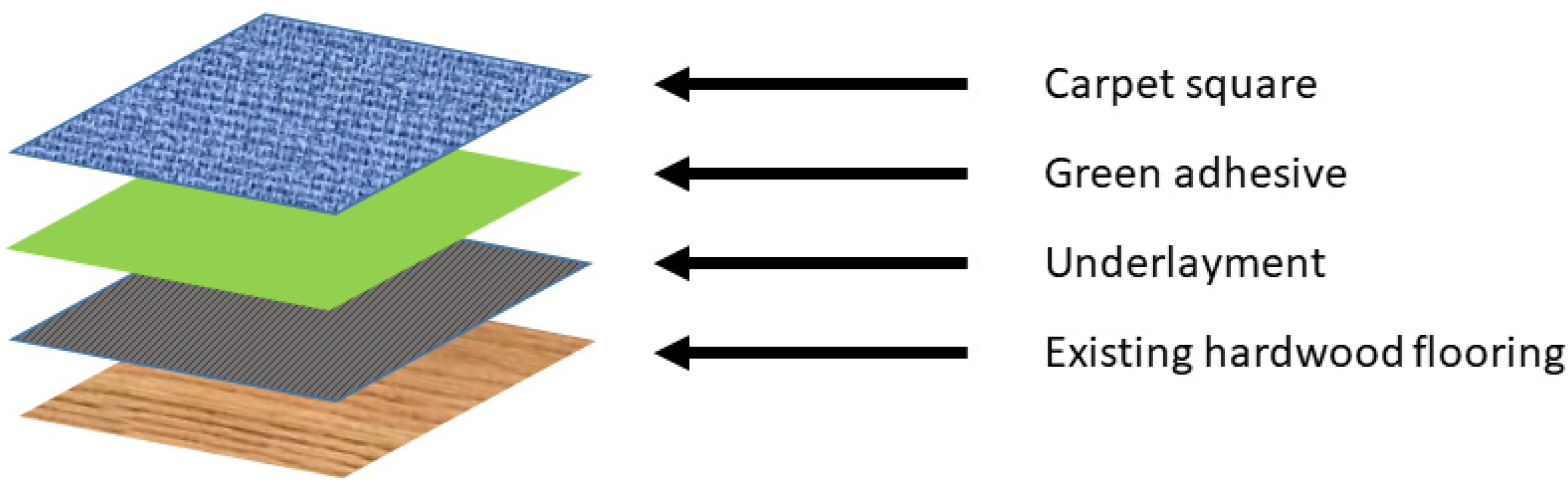
To demonstrate the utility of the DTD-based methodology, suitability testing was conducted on various flooring options considered during the design phase of the exhibition. A durable, reflective floor was required to provide a projection surface for the immersive experience that would extend onto floors and walkways. However, the galleries being renovated contained expensive natural-colored oak hardwood floors that needed to be preserved for potential future use if the galleries reverted later. The original plan was to use a commercial flooring system, shown schematically in
Figure 3, that involved taping a thin polymer sheet underlayment onto the existing hardwood floors of the gallery as a protective layer. Then, a tacky green colored pressure-sensitive adhesive would be applied to the underlayment to secure carpet squares or other flooring tiles onto the floor. In this way, damage to the hardwood floors would be minimized, and the flooring could be more easily replaced or removed as needed during the exhibition. Once the show ended, the hardwood flooring could be re-exposed, perhaps without the need for extensive refinishing and revarnishing of the surfaces.
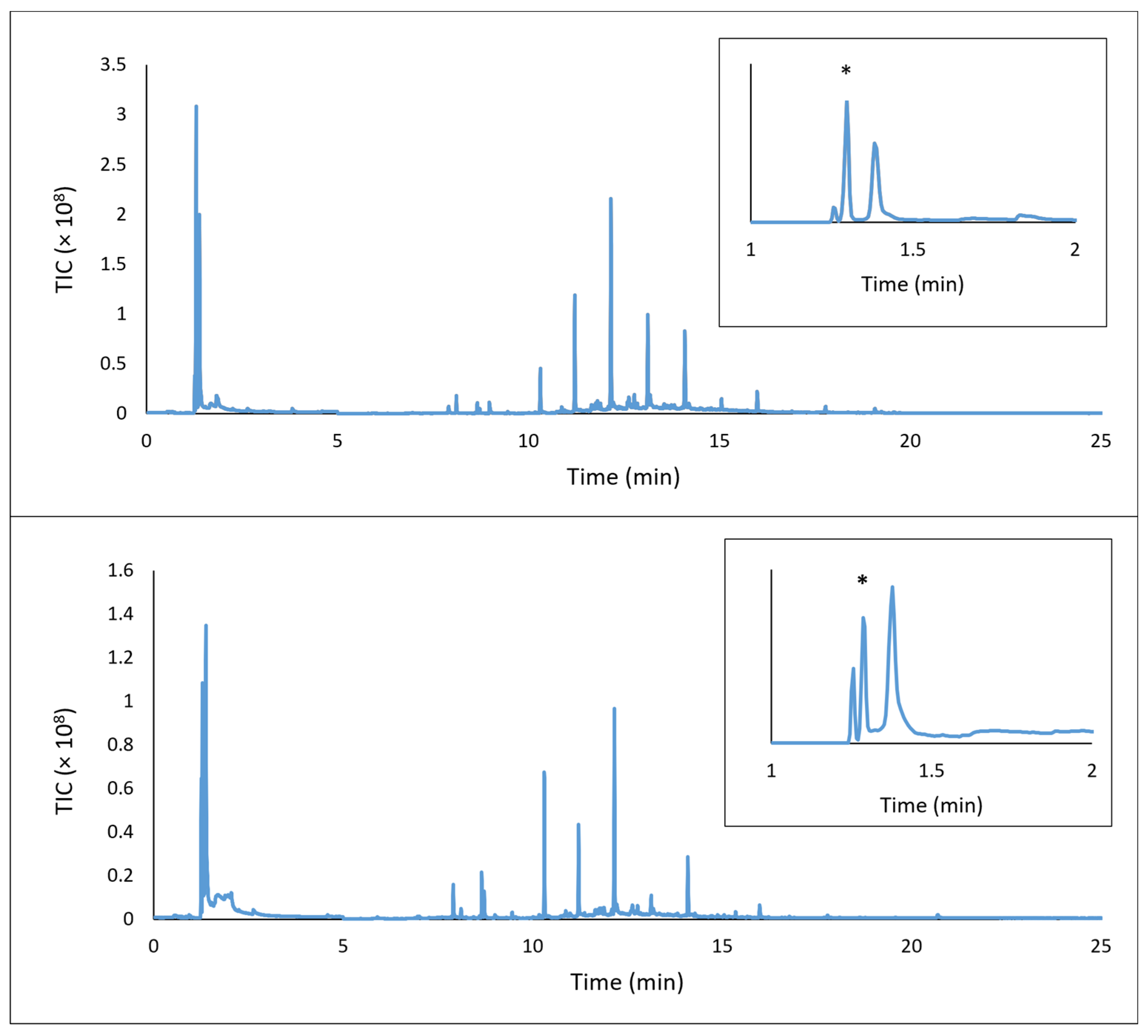
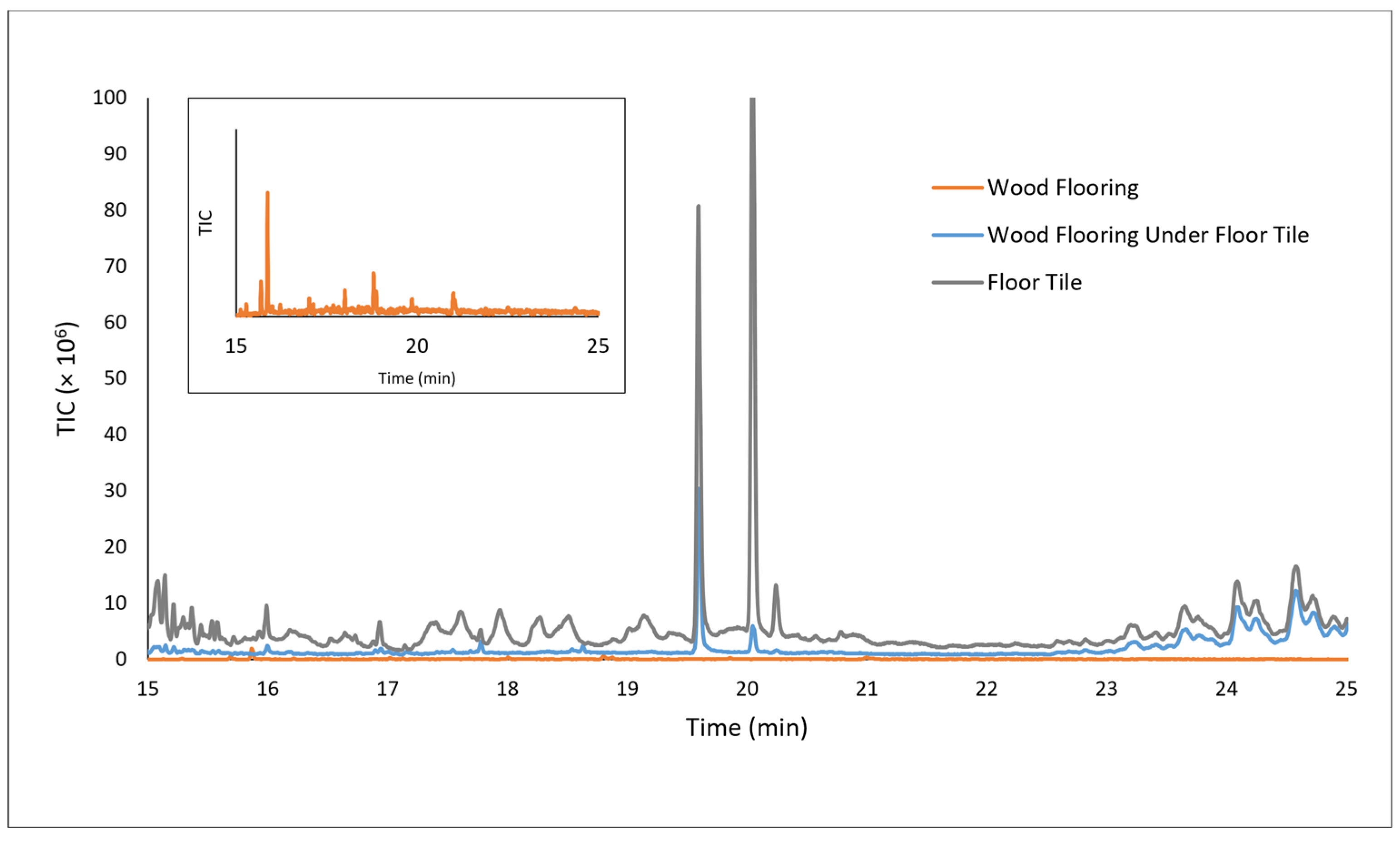
A chromatogram for the DTD analysis of the flooring underlayment onto which floor tiles would be glued is shown in
Figure 4. The original sample provided by the vendor (top) was shown to emit a series of hydrocarbons of increasing chain length between 10 and 20 min retention time in the chromatogram, consistent with the FTIR analysis that showed that one side of the underlayment contained a fibrous mat of polyethylene and polypropylene,
Supplementary Materials, Figure S1. These hydrocarbon emissions would not be a major concern for most artworks. Formaldehyde, which elutes from the GC column at 1.30 min (inset), was detected by the DTD methodology in the TIC chromatogram due to the immediate transfer and trapping of this very volatile emission on the GC column. Selected ion monitoring was not necessary in this case to observe the signal, as it was significant.
Supplementary Materials, Figure S2 shows the full TIC chromatogram of the initial underlayment sample with major peaks identified. As previously mentioned, formaldehyde is a major concern for both human health and the safety of artworks. The presence of formaldehyde was confirmed using the chromotropic acid test, shown in
Figure 5, which yielded a deep violet color after the prescribed 30 min of heating. Moreover, a replicate underlayment test jar that was left for half an hour on the lab bench without being heated also turned deep purple while the accompanying control test remained unchanged. Although not quantitative, this result suggested the underlayment was a copious source of formaldehyde pollution.
Due to the presence of formaldehyde in the original underlayment, the exhibition designers requested from the vendor a new sample of underlayment that was purportedly prepared with a “formaldehyde-free” formulation (
Figure 4, bottom plot). However, this material also contained formaldehyde according to DTD-GC-MS analysis, as shown in
Supplementary Materials, Figure S3, along with the same longer chain aldehydes and hydrocarbons. It is unknown whether confusion at the vendor led to the same material being resent or if issues further into the supply chain and manufacturing led to an incorrect designation being applied to the product. Regardless, the ability to quickly retest the material without relying on vendor assurances or month-long Oddy testing was key in identifying the error.
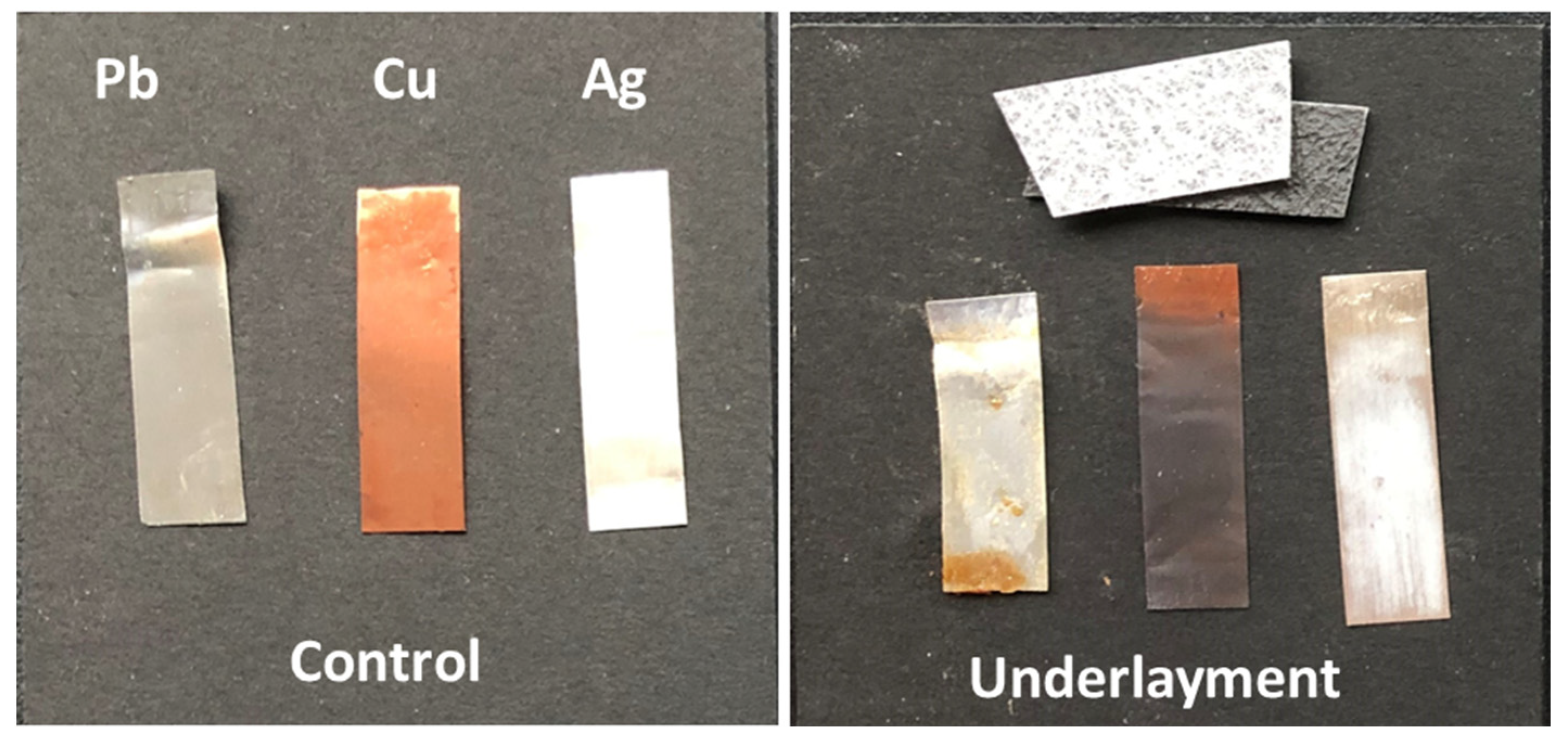
Since the DTD-GC-MS and microchemical tests did not determine the concentration of formaldehyde emitted and a second option for the underlayment was not submitted by the designers, an Oddy test was performed on the material to assess its potential deleterious effect. The resulting metal coupons are shown in
Figure 6. The coupon grading led to an unmitigated “unsuitable” rating with the darkening of the copper coupon and, curiously, the lead coupon dripping with a thick brown viscous liquid, smelling faintly of caramel. This same type of catastrophic material failure has been seen when testing Delrin polymer [
20], a polyoxymethylene homopolymer that is known to depolymerize into formaldehyde [
13]. The Oddy test confirmed that the formaldehyde levels in the underlayment are of significant concern when used over such a large swath of the gallery floor. In addition, the green adhesive for the carpet tiles, identified by FTIR as a pressure-sensitive acrylic emulsion, as shown in
Supplementary Materials, Figure S4, was also examined by DTD and found concerning due to the emission of acetic acid, as shown in
Supplementary Materials, Figure S5. The presence of formaldehyde in the underlayment, along with its dramatic failure in the Oddy test, prompted gallery designers to pursue an entirely different path for flooring, focusing instead on interlocking rubbery floor tiles that would not require an underlayment or adhesive to protect the existing hardwood floors.
Initially, six different commercial floor tiles were assessed. Based on FTIR analysis, an example of which is shown in
Supplementary Materials, Figure S6, these tiles consisted primarily of plasticized PVC with a calcite filler. Analysis by DTD-GC-MS, shown in
Supplementary Materials, Figure S7, revealed that none of the samples were found to emit formaldehyde, acetic acid, or the anticipated hydrochloric acid during thermal extraction, though some did emit longer chain and lower volatility organic acids, aldehydes, and phosphate fire retardants (such as isodecyl diphenyl phosphate). The presence of calcite, as demonstrated by FTIR, may have reduced the generation of HCl in the polymer. The DTD analysis showed that various phthalates (SIC = 149
m/
z) and long-chain esters (identified in the TIC) are present to keep the flooring pliable, and these components caused concern that they might migrate into the floor varnish or hardware flooring and cause softening or staining over time.
An impromptu test was devised to study whether plasticizer migration would be problematic since any softening of the varnish on the hardwood floors, or discoloration of the hardwoods themselves, would be an issue if the interlocking flooring was removed in the future. A sample of the flexible floor tile was pressed onto the surface of a plank of the varnished hardwood flooring that had been previously removed in a renovation. The two were held in close contact under pressure using a twisted wire. Shown in
Figure 7 are the chromatograms obtained for the DTD-GC-MS analysis of a flooring sample (gray plot), a surface scraping of hardwood flooring after being aged in close contact to the flooring sample in a 60 °C oven for 2 weeks (blue plot), and a surface scraping of hardwood flooring after being similarly aged with no floor tile contact (orange plot). The peaks at 19.60 min and 20.05 min represent bis(2-ethylhexyl) adipate and isodecyl diphenyl phosphate, a plasticizer and plasticizing fire retardant, respectively, that are commonly used in PVC products. These compounds were not present in the wood sample prior to being exposed to the floor tile and only appear after close contact with the floor tile under the accelerated aging conditions. This demonstrated that migration of the plasticizers from the floor sample into the hardwood floors is a concern, although discoloration of the flooring was not observed, and any softening of the hardwood’s varnished surface was not noticeable under these conditions. Considering the absence of known pollutants and the relatively low volatility of the VOCs identified in the analysis of the floor tiles, this approach appeared to satisfy the design needs for a modular, reflective temporary flooring material.
Following those tests, a thicker, heavier, and more rubbery white interlocking floor mat was sourced by the gallery designers, and a sample tile was sent to the lab for assessment. The infrared spectrum of this material was dominated by an inorganic chalk filler, although weak peaks indicating the presence of polyethylene were also visible, as shown in
Supplementary Materials, Figure S8. Considering the filler’s interferences observed in the FTIR analysis, a small sample of the white mat was pyrolyzed at 600 °C using the same system utilized for the DTD-GC-MS experiment. The pyrogram of the flooring material showed that this mat was not PVC like the previous examples, but instead matched that of polyethylene, as shown by Tsuge et al. [
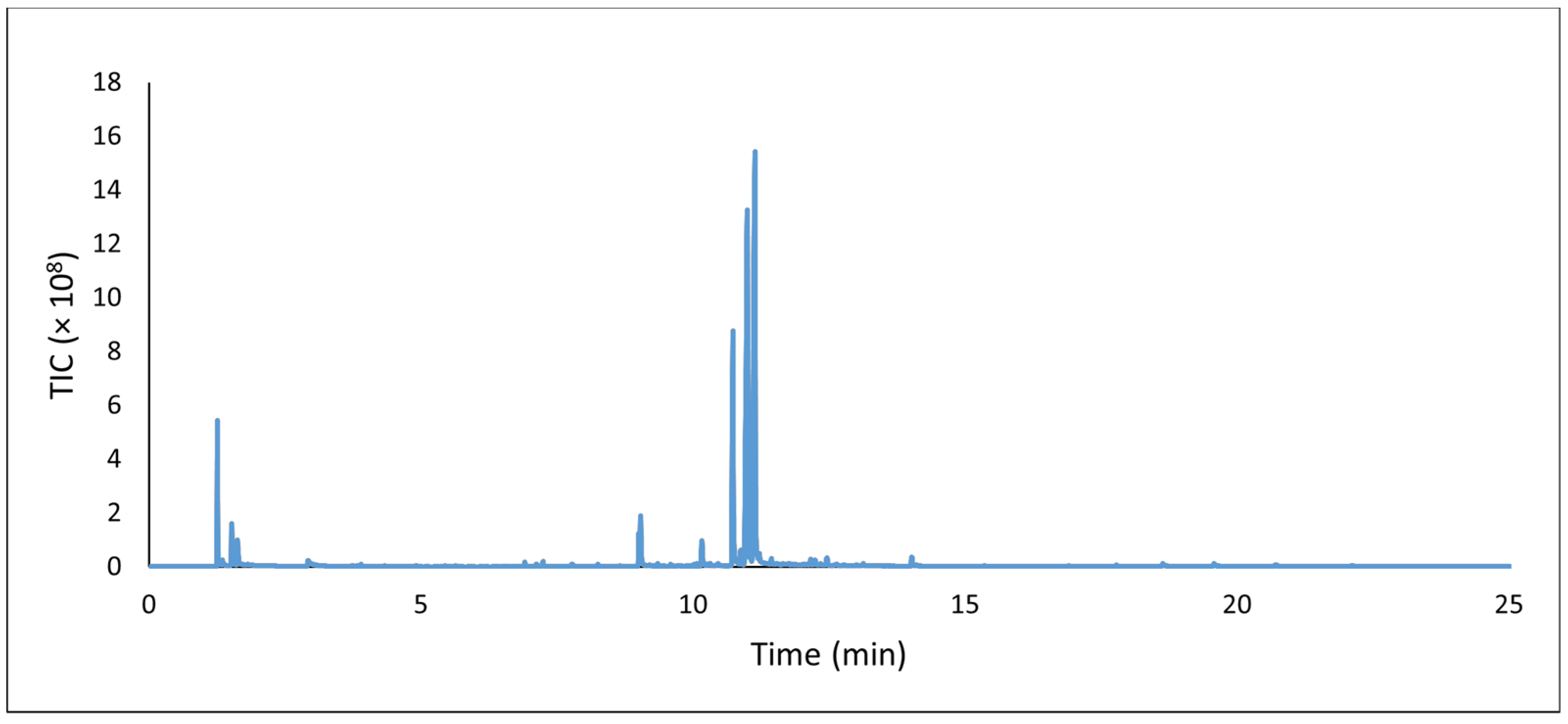
15], pg. 12. Off-gassing analysis of the white polyethylene mat by DTD-GC-MS,
Figure 8, indicated no formaldehyde, acetic acid, phthalate plasticizers, or other compounds of concern. With such a seemingly benign composition, this flooring material was ultimately chosen for installation in the galleries based on its functional properties, appearance, and low conservation concern. Unfortunately, delays in the production and supply chain delivery of the selected floor tiles during the 2021 COVID-19 pandemic risked the completion of the gallery remodeling in time to meet the publicized opening of the new exhibition; thus, an interim strategy was necessary.
To provide a seamless, reflective, and durable floor surface, a white epoxy-modified high-traffic acrylic floor paint from Benjamin Moore was used to prepare the floor areas in time for the exhibition opening. The painted floor could then be overlaid with the white interlocking floor tiles when they ultimately became available. If the floor reverted to its original state in the future, the floor tiles could then be deinstalled, and the hardwoods lightly sanded to remove the acrylic paint prior to revarnishing. Although far from ideal, the rapidly approaching gallery opening did not allow for thorough testing of the paint to occur before the floors were coated; the lab received a sample of the floor paint only after its application. However, the gallery spaces being painted have a dedicated air handler, so any VOCs from the drying paint would not be introduced into the other areas of the museum. To reduce the exposure of the three exhibition artworks to potential off-gassing from the paint, the floors in the final gallery space that would contain the actual post-impressionist paintings were left uncoated since projection was not used in this space. Furthermore, these paintings were housed in microclimate cases and were only installed after 2 weeks of off-gassing from the painted floors in the adjacent projection gallery.
DTD-GC-MS analysis of the floor paint was performed afterward on a sample that had cured for more than 2 weeks at room temperature and humidity. Solvents and additives, such as fungicides, were detected in the TIC, but none of these compounds were known or expected to have a deleterious effect on artwork. However, small amounts of low molecular weight aldehydes such as acetaldehyde, propanal, and butanal were also detected in the TIC, and the SIC
m/
z 60 showed the presence of acetic acid, as shown in
Supplementary Materials, Figure S9. While not an ideal solution, this interim approach of painting the floors satisfied the immediate needs of the exhibition in a cost-effective and timely manner, and the potential impact of the acids and aldehydes was reduced by the dedicated HVAC air handler and microclimate cases.
The newly manufactured flooring material arrived several months after the opening of the exhibition. Since significant time had elapsed between the analysis of the manufacturer’s sample and the arrival of the ordered tiles, the new tiles were re-tested using the rapid DTD protocol. Initially, formaldehyde was suspected in the new batch based on a weak signal in the SIC m/z 30, which had not been detected in the initial sample. Fortunately, microchemical testing with chromotropic acid returned a negative result, clearing the new tiles for use in the gallery. This re-testing procedure took a total of 60 min (30 min for the DTD-GC-MS and then 30 min for the microchemical test) and allowed approval of the flooring system without the 28-day Oddy test.
3.3. Aerosol Scent
One of the key design aspects of the multisensory exhibition was to use “scent marketing” approaches to evoke a sense of the French countryside in which van Gogh, the featured artist, was painting in the late 1800s. This idea was also one of the most controversial aspects of the gallery design from a conservation perspective since it would involve the intentional introduction of bespoke scents to accompany different galleries. In general, collections care staff seek to eliminate VOCs from exhibition spaces to protect the artwork, but in this case, appealing to all senses—sight, sound, taste, touch, and scent—was deemed essential to the experience.
DTD-GC-MS was used to assess the compounds formulated into a designer scent for the inaugural show of THE LUME Indianapolis. A sample of the product was delivered to the lab on paper scent sticks, and a small section was removed and thermally desorbed into the GC-MS, as shown in
Supplementary Materials, Figure S10. The proprietary formulation that was geared to evoke the smell of the French countryside yielded primarily terpenes and esters consistent with those reported on the SDS provided by the company. Although none of the compounds identified had documented negative impacts on artwork materials, one concern raised was the ability of the formulation to act as a plasticizer; the polyethylene bag that the samples were delivered in had curled and warped in the areas where the scent sticks had been in contact, as shown in
Supplementary Materials, Figure S11. Although this raised concerns regarding the intended deployment of the scent delivery units inside insulated air ducts, the presence of three post-impressionist artworks, including a van Gogh painting, in the final gallery space of THE LUME Indianapolis floorplan raised a particular alarm with conservation staff.
To accommodate the multisensory experience, concessions were made to both the original exhibition design concept and the conservation policy against intentionally introducing VOCs into gallery spaces. Fortunately, the galleries dedicated to the exhibition have their own air handling system that does not combine with HVAC units serving other parts of the museum building. By placing aerosol units high on structural support columns outside of ductwork and setting the discharge rate to its lowest setting, a faint scent could be delivered during the introductory gallery of the exhibition experience without jeopardizing the museum’s collections to VOCs for which the impacts at relevant concentrations were unknown. Although they were located in a distanced gallery with a different air handler, all three of the post-impressionist paintings were sealed in their own microclimate cases to protect them against any migrating scent, as well as food or drink from the exhibition’s café that might make its way outside of the allowable areas. Furthermore, in the event that the scent could be detected outside of the 4th-floor galleries housing THE LUME Indianapolis, the use of the scent would be reassessed and possibly removed. The inaugural exhibition was successfully completed with no ill effects noted, and the faint cypress smell was not detected outside of its intended gallery space.