1. Introduction
The masterpiece this work deals with is the tomb of Lorenzo de’ Medici, Duke of Urbino, completed by Michelangelo around 1533. The tomb is located in the New Sacristy of Medici chapel in San Lorenzo in Florence (
Figure 1) and has been the subject of the final stage of the extensive restoration of the New Sacristy, which lasted a total of eight years (2013–2020). Systematically, the restoration interventions were supported by a constant dialogue between different disciplines, combining practice and sciences.
Memorial and documentary research remains the preparatory foundation for any restoration, especially when it affects works considered absolute masterpieces of humanity, such as this one. For this reason, a demanding documentary digging through historical photos, real documents of the life and uses of the place over the five hundred years of its existence, analysis of critics and latest historiography, was accompanied by in-depth scientific research, which is referred to in this article [
1,
2]. The guiding thread of the entire restoration was adhering to the key principles 2000). of compatibility and retractability, while addressing new technological challenges. The chemical–physical investigations carried out before the cleaning procedure allowed a correct diagnosis of the problems to be solved, addressing the methodological choices for the more appropriate approach to the architectural and sculptural elements of the New Sacristy.
An advanced scientific methodology was chosen for the tomb of Lorenzo, the final part of the restoration (2019–2020). The surface of the monument was altered and stained by consistent deposits of brown colour uneven in consistency and thickness. The front side was subjected to a drastic cleaning, while the lateral sides, the bottom and the back of sarcophagus were noticeably covered with highly aesthetic-altering deposits. Lorenzo’s tomb underwent a bio-cleaning procedure, a method of selective and gradual cleaning that uses more sustainable products in place of commonly used products that are often dangerous for operators, aggressive for works of art and no longer compatible with the environment. The cleaning process of a work of art is the first and most delicate phase of the restoration. Faced with the presence of resistant aged deposits, it is often necessary to use products that can cause damage to the artefact, if they are not selective. In challenging cases, there is also the difficulty of achieving good results with traditional methods. Bio-cleaning meets these difficulties and is proposed as a solution towards the ecological transition of restoration products. It concerns a process of removing coherent deposits of various origins (organic and inorganic)—even stratified—by using live bacteria cells or their products. The advantage of using live bacteria cells lies in the versatility of bacteria, which have the ability to produce a wide range of molecules and enzymes, including inducible enzymes, which are only produced by the cell under specific conditions or in the presence of certain substrates. Bacteria show enormous potential in inducing chemical transformations, both because they use the substrate directly as a carbon source and because they release active enzymes into the surrounding environment [
3]. Bio-cleaning combines the characteristics of efficacy, selectivity and gradualness, respect for the material, compatibility with the health of the operator and the environment, versatility. The procedure involves applying to the surfaces to be treated live bacterial cells immobilized within an inert support, creating a micro-pack that lasts “overnight”, but a few hours may also be sufficient. The application procedures can be diversified on a “tailor-made” basis. In the case of stratified deposits, packs can be applied in succession, using the competent strain for each layer [
4,
5]. In all of our case studies, the bacteria used are spontaneous environmental strains, wild types, isolated from harsh environments where, in order to survive, they have developed metabolic competencies of biotechnological interest. They are safe and not spore-forming to avoid leaving latent forms of life; furthermore, they must lack functions to interact with the original material of the opera. The introduction of these methods that use safe microorganisms and other natural substances is proving to be a feasible way to address problems in restoration phases: disinfection, cleaning, consolidation and protection [
6,
7,
8,
9,
10,
11].
A few general historical notes will be useful to better identify the object of intervention. The construction of a second sepulchral chapel for the “Magnifici” branch was carried out by Michelangelo starting in 1519 at the behest of Medici’s popes Leo X and Clement VII. The realization of the works has undergone alternating phases, conditioned by the historical and political events, up to the year 1534, when the ultimate departure of Michelangelo from Florence decreed the interruption of the works.
The sarcophagus houses the remains of Lorenzo, Duke of Urbino and his natural son, Duke Alessandro, buried in the tomb after a tragic death by killing in 1537. The sarcophagus is currently surmounted by two sculptures,
Crepuscolo and
Aurora (the Twilight and the Dawn, respectively) and was heavily stained by sewage and dirt of the human remains of Duke Alessandro, who was buried without being eviscerated. The problem of stains on Lorenzo-Alessandro’s sarcophagus became evident very early on, as reported by documents dating back to 1552 [
12,
13]. The very thick patina had never been removed in previous restorations to avoid damaging the original material by using aggressive products.
Today’s restoration set the goal of finding the right way to free the sarcophagus from these unsightly alterations, by approaching the problem with an advanced scientific methodology. Spectroscopic analysis (FTIR in ATR and in TR—total reflection—mode and XRF) and microscopic analyses on samples taken in correspondence with the brown patina revealed the chemical nature, thickness and extent of the alterations. As a result, analytical information guided the selection of potentially competent bacterial strains for a selective cleaning. Preliminary on-site tests on small test pieces made it possible to evaluate the efficacy of ten different strains and to choose the three best ones to proceed with the bio-cleaning of the sarcophagus. Diagnostic, bio-cleaning and the outcome of the restoration performed on the sarcophagus of Lorenzo Duke of Urbino will be discussed in this article.
2. Materials and Methods
2.1. Characterization of the Superficial Patina by Chemical Analyses
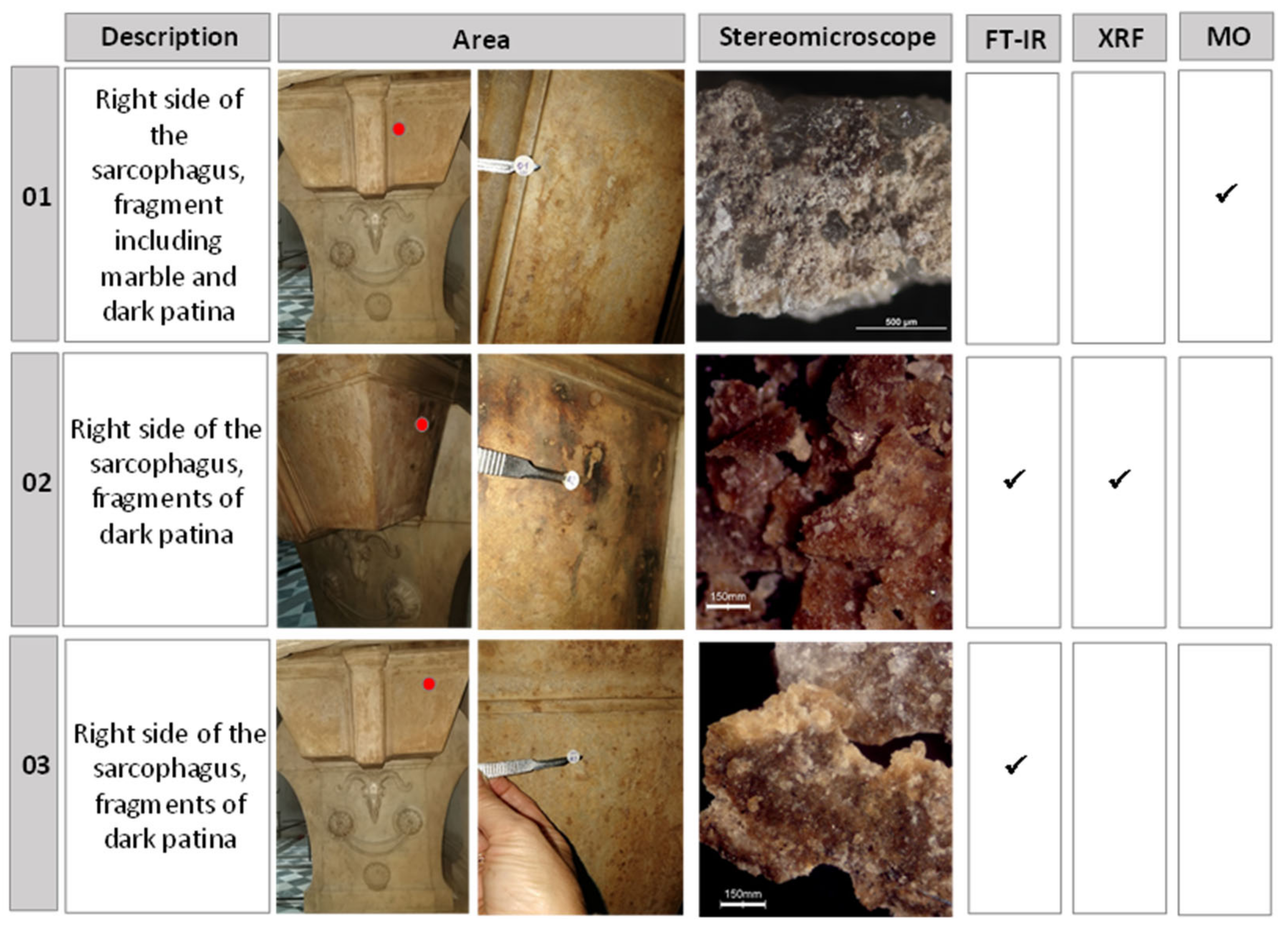
In order to guide cleaning operations and select competent bacterial strains, three micro-fragments of the brownish patina on the lower part of the sarcophagus were collected with a scalpel and analysed with different techniques (
Figure 2). A stereomicroscope Zeiss model Stemi 200 C equipped with an external halogen lamp was used to acquire fragments and powder images. Sample 01 was embedded in a bicomponent epoxyresin (Epofix, Struers DK) and polished with silica abrasive papers, to obtain a cross-section. The cross-section was observed in visible light with an optical microscope (MO) Nikon Eclipse E600 equipped with a halogen lamp to get information about the thickness of the brownish patina.
Fourier-transform infrared spectroscopy (FT-IR) analyses on samples 02–03 were performed in ATR (Attenuated Total Reflectance) mode to characterize organic and inorganic compounds. The instrument used was a portable Bruker Optics ALPHA FT-IR Spectrometer equipped with a SiC Globar source, a DTGS detector and a single reflection diamond ATR module. Each spectrum was the mean of 16 scans collected at 4 cm−1 resolution, in the spectral range of 4000–400 cm−1. The spectra were processed with OPUS 7.2 software and compared with the spectra of reference materials. The elemental composition of sample 02 was further investigated with p-XRF using a handheld Tracer III SD Bruker spectrometer, equipped with a rhodium anode and solid-state silicon detector energy dispersion system. XRF spectra were collected with the following set-up: 40 keV and 12 μA for 60 s. The measuring area was an elliptical spot of 4 mm × 7 mm. For data elaboration, ARTAX software was used after normalizing the spectra to the intensity of Rh Kα at 20.21 keV.
In addition, in order to investigate the distribution of compounds on the surface of the sarcophagus, non-invasive FT-IR analyses were performed with the same instrument, Bruker Optics ALPHA FT-IR Spectrometer, exploiting a reflection module and a video camera. All spectra were acquired, collecting 128 scans, with a resolution of 4 cm−1 in the 7500–375 cm−1 range and a measuring spot of 6 mm in diameter. The collected IR spectra were processed using OPUS 7.2 software. All the areas analysed with reflection FTIR were documented by a portable digital microscope, Dino-Lite mod. AM7013MZT (R4), equipped with 10–200× optical zoom, with white light LED as a light source. The images were acquired at 50× magnification.
2.2. Bacteria Selection, Growth and Screening Assays
The bacterial strains belong to the large laboratory microbial collection Microbial Resource Research Infrastructure Italian Node MIRRI-IT (
http://www.mirri-it.it/index.php/associated/enea/, accessed on 28 September 2022). The collection contains strains of environmental origin, isolated from polluted sites [
14,
15] industrial wastewaters and archaeological sites [
16]. All strains had been identified by rDNA 16S gene sequencing and the sequences are deposited in Genebank with a accession number. According to the results obtained by FTIR analysis, ten bacterial strains potentially suitable for interacting with the individual substances detected were identified within the laboratory collection.
The strains (stored at −80 °C) were revitalised and then cultured in TSB medium (Tryptic Soy Broth, Liofilchem, Teramo, Italy) at 28 °C in an orbitary shaker at 120 rpm for 48 h, then streaked on the assay media. The bacteria selected for the screening belong to different classes: Bacillales (Virgibacillus sp. NOT1), Gammaproteobacteria (Serratia ficaria SH7, Stenotrophomonas maltophilia TPID9, Pseudomonas stutzeri CONC11, Pseudomonas fluorescens LAM33, Acinetobacter calcoaceticus LAM21), Actinobacteria (Plantibacter sp. UI24, Cellulosimicrobium cellulans TBF11, Rhodococcus sp. ZCONT) and Sphingobacteria (Pedobacter sp. MCCZ). All the strains were non-hazardous and non-spore-forming. They are members of the biohazard level 1 group according to ATCC strains collection biosafety level (BSL), except for A. calcoaceticus LAM21, tested for lack of pathogenicity by antibiogram and currently under whole genome sequencing, pending to define if it is a new species.
The strains were then tested for desired metabolic functions (
Table 1). The production of extracellular proteases was assessed, as described in [
4], on TSA medium (Tryptic Soy Agar, Liofilchem, Teramo, Italy) supplemented with gelatine 1% (
w/
v). After bacterial streaking, the plates were incubated in a thermostat at 28 °C and the results were recorded at intervals of 24 h for 7 days. The capability to produce acidic metabolites (linked to the solubilisation of inorganic patinas, i.e., calcite and gypsum, in this case) was tested on Petri dishes containing TSA glazed on the surface with a sterile suspension of either CaCO
3 or CaSO
4·2H
2O at 1%
w/v in distilled water (pH 8.4). After sowing a spot, the plates were incubated at room temperature for up to 7 days and the clarification halo on gypsum or carbonate patinas was observed and recorded for up to 7 days. Pikovskaya’s medium (PVK) was used to select the strains capable of solubilising calcium phosphate. Bacterial strains streaked on the agar plates were incubated for up to 14 days; phosphate solubilisation was evaluated by halo formation around the bacterial colonies [
17]. Lipid hydrolysis due to esterase production was tested according to [
18] on a solid medium composed by: Peptone 10 g/L, NaCl 5 g/L, CaCl
2 2H
2O 0.1 g/L, agar 15 g/L, Tween80 10 mL/L. Esterase activity was revealed by a turbid zone around the colonies streaked on the plates. Lipases production was driven by a specific growth medium CYM, designed to optimize lipase production: Peptone 3 g/L, Yeast extract 1 g/L, NaCl 0.5 g/L, CaCl
2 2H
2O 1.36 g/L, Tween80 2%, olive oil 1%, pH 7 (modified from [
19]).
2.3. Preparation of Bacterial Micro-Pack and Preliminary Test on Site
The bacterial suspensions were obtained by inoculating 0.50 mL of overnight culture into 100 mL of liquid medium. Except for the strain
S. ficaria SH7, all of the strains were cultured in TSB medium up to the stationary phase, when growth ceases but cells remain metabolically active. The cultures were then centrifuged at 3000 rpm for 15 min, and the cell pellet was washed and suspended in sodium pyrophosphate 0.05% (
w/
v). Cell suspensions were added with Vanzan
®NF (Vanderbilt Minerals LLC, Norwolk, CT, USA) or Laponite
®RD, to immobilise the cells and create a micro-pack, easy to apply and remove without leaving residues on the marble surface [
4]. Vanzan is a high molecular weight exocellular polysaccharide derived from the bacterium
Xanthomonas campestris, widely used as a rheology control agent for aqueous systems. It increases viscosity, helps to stabilise emulsions and prevents the settling of solids in a wide variety of applications. Laponite is a colloidal clay consisting of a mixture of silicates of sodium, magnesium and lithium. Both products had already been tested as immobilization agents in previous bio-cleaning applications [
4,
20]. These supportants showed high compatibility with the viability of bacterial cells, both for pH suitability and water retention, without, however, being a useful carbon source for growth. They are inert supports, unable to interfere with the selective action of bacteria towards the target substrates or compete as a carbon source. The Laponite micro-packs were prepared by gently mixing 9% of powder with the bacterial suspensions until a firm gel was obtained. The Vanzan micro-packs were prepared by vigorously mixing 6% of the powder with the bacterial suspensions. The jellified bacterial suspensions were kept at 4 °C for 4–12 h to achieve the optimal degree of swelling and to make bacteria hungry by exhausting the residual internal growth reserves. Hungry bacteria express appropriate cellular responses by attacking effectively the compounds to be removed [
21]. The strain
S. ficaria SH7 requires different preparation. It is grown in CYM up to the stationary phase and it is added with Vanzan without separating the cells by centrifugation, in order to keep the concentration and the activity of lipase enzymes produced during the growth. The micro-pack is usually applied with a spatula over a sheet of Japanese paper (to be easily removed without leaving any residues) and then covered with plastic film to protect from evaporation; the contact time was overnight—18 to 24 h—and the packs were repeated as needed. In contrast, depending on the specific condition, the micro-pack can be applied in direct contact with the surface. The choice for application procedure was assessed on a case-by-case basis. Direct contact was chosen for larger and dirtier areas of the sarcophagus.
The bio-cleaning was carried out in two stages, approximately one year apart, due to the COVID-19 closures of the Museum.
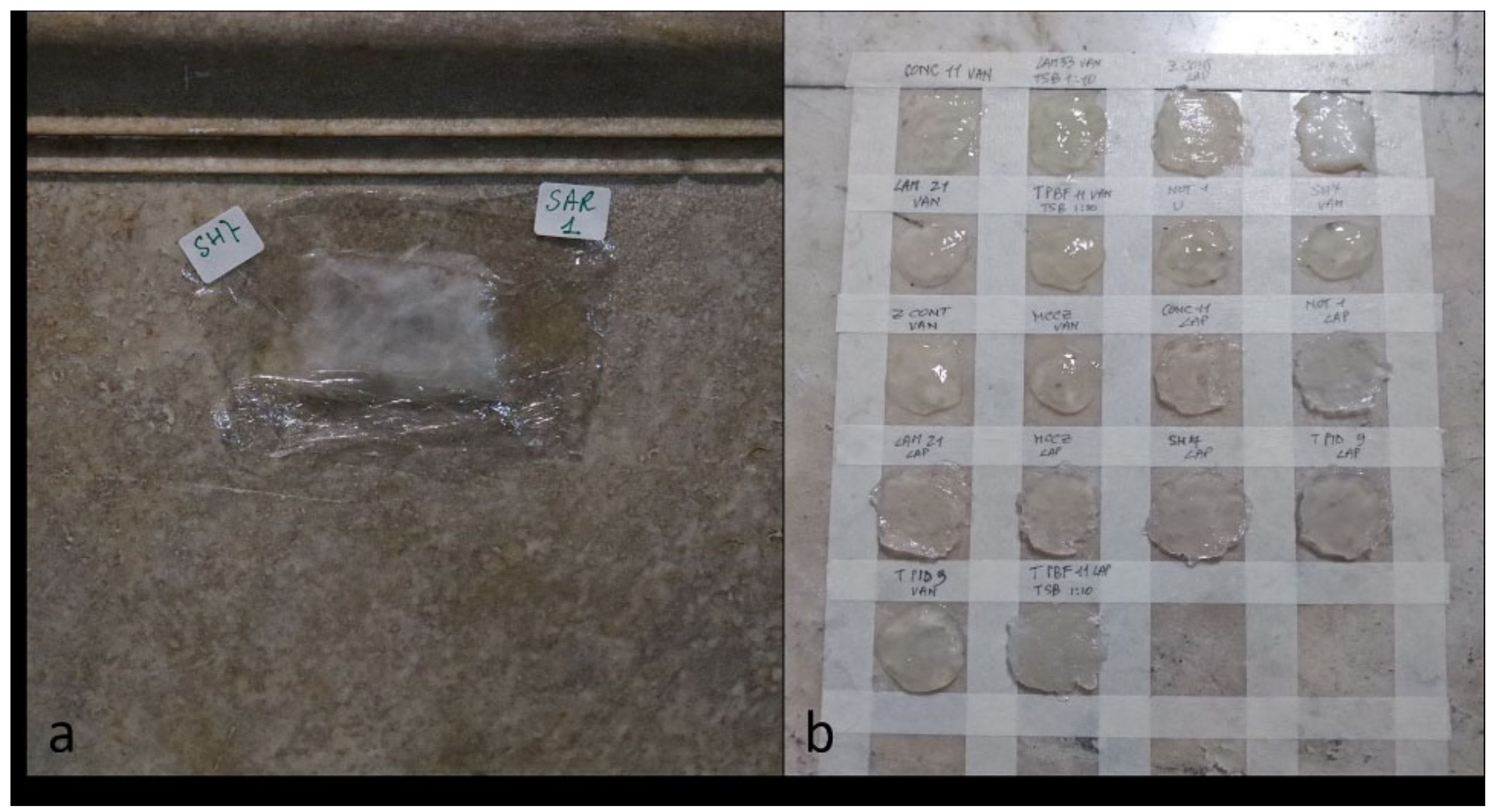
In November 2019, preliminary tests were set up, both on small portions of the sarcophagus (
Figure 3a) and on the back of the altar, altered by a large yellow-brownish stain due to old treatments. All strains were applied on a palette to obtain a close comparative result (
Figure 3b). The aim was to find the optimal condition, combining good cleaning and respect for the natural patina of the material. Each bacterial strain was then tested both in Vanzan and in Laponite. As a reference test, some areas were treated with packs of Vanzan or Laponite bacteria-free, in order to verify any action due to the supportants (negative controls).
2.4. Bio-Cleaning Intervention on the Sarcophagus of Lorenzo Duke of Urbino
Following the preliminary test the bio-cleaning intervention was implemented on the sarcophagus in mid-October 2020 by using the three selected strains. According to the methods described in 2.2 and 2.3, large amounts (up to 10 L) of each selected bacterial strain were cultured in the lab, and then suspended in Vanzan or Laponite. The fresh gel was transported from the lab to Florence in a container at 4 °C. The micro-packs, each containing a single strain, were applied separately, based on the map of substances analyses detected, especially insisting on the most affected areas, namely the lateral sides and the bottom side. Each strain was applied for the selective cleaning of the relevant substance (
Table 1). The procedure involved some simple steps: (1) spreading a veil of Japanese paper, moistened with a soft brush on the target surface; (2) applying the bacterial strain immobilized in the gel with a plastic or wooden spatula; (3) covering the gel with a plastic film; (4) leaving in contact for the defined time, inspecting from time to time to check the progress of the process; (5) removing the micro-pack; (6) finishing the cleaning with a soft swab or sponge moistened with tap water, to remove any residues. The operations in situ do not require special precautions, there is no need to operate in sterile conditions. Step 1 was skipped for most parts of the sarcophagus. In this case, the finishing step was carried out by using a flexible spatula and the final rinse required attention to remove any gel residue. Each micro-pack (
Figure 4 and
Figure 5), was kept in contact with the surface for about 18 h (overnight), and the application was repeated twice, using a fresh micro-pack each time. In the different areas, the bio-cleaning was carried out up to the result as the Museum team considered it fitting.
3. Results
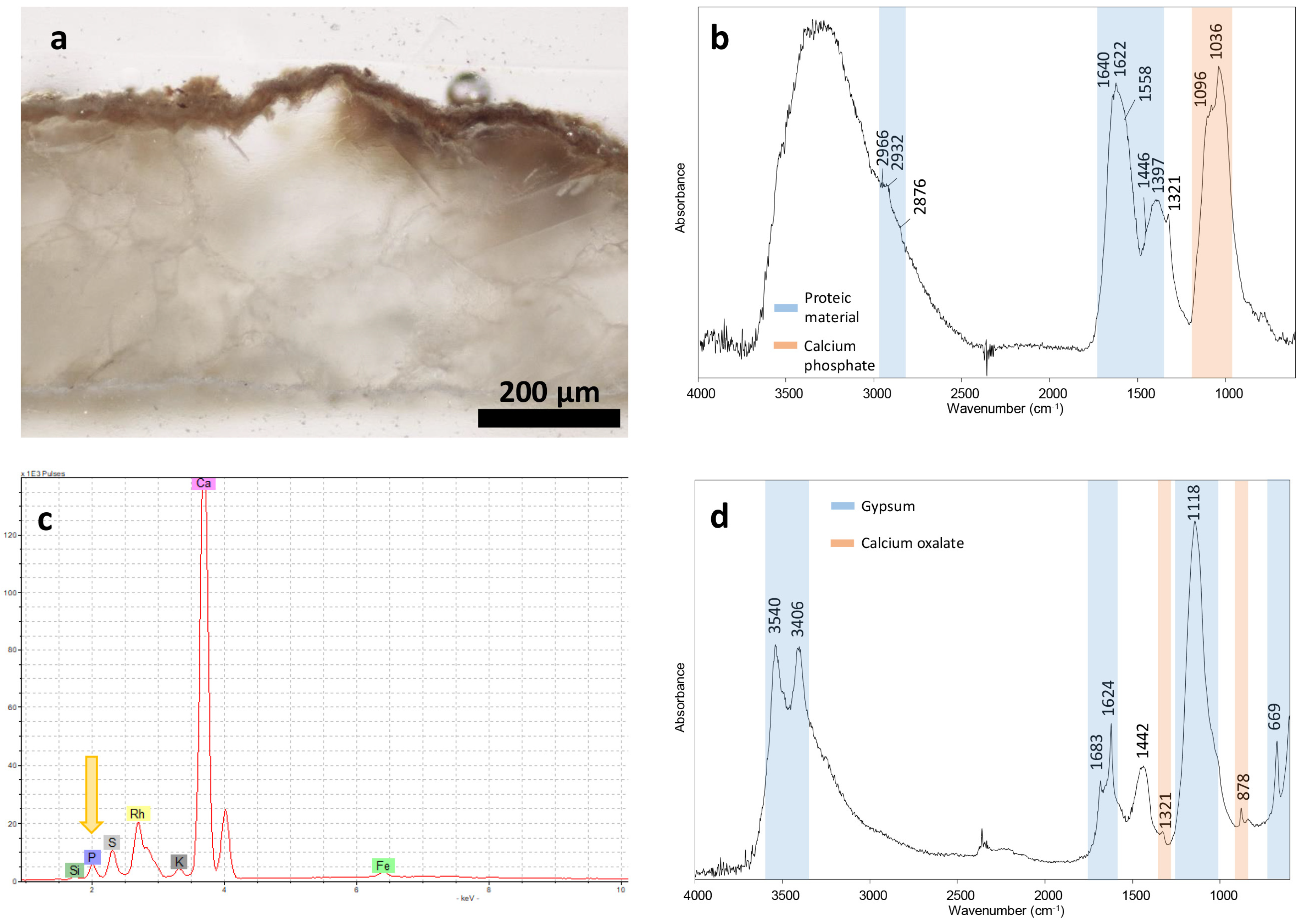
An optical microscope observation of the sample’s cross-section revealed a dark patina with a thickness of 35–40 μm, well adherent to the marble (
Figure 6a). In Sample 02, the FT-IR analysis (
Figure 6b) showed the presence of calcium phosphate by the strong band at 1036 cm
−1 with a shoulder at 1096 cm
−1 and of proteic material, as suggested by the typical bands of amide I and amide II at 1640 and 1558 cm
−1, respectively. In this spectrum, the latter overlap with the intense vibrational band of calcium oxalate at 1622 cm
−1. The presence of phosphorus-based compounds was confirmed by XRF as well, registering P counts in the spectrum (
Figure 6c). Instead, calcium sulphate dihydrate (gypsum) and calcium oxalate were found in Sample 03 (
Figure 6d).
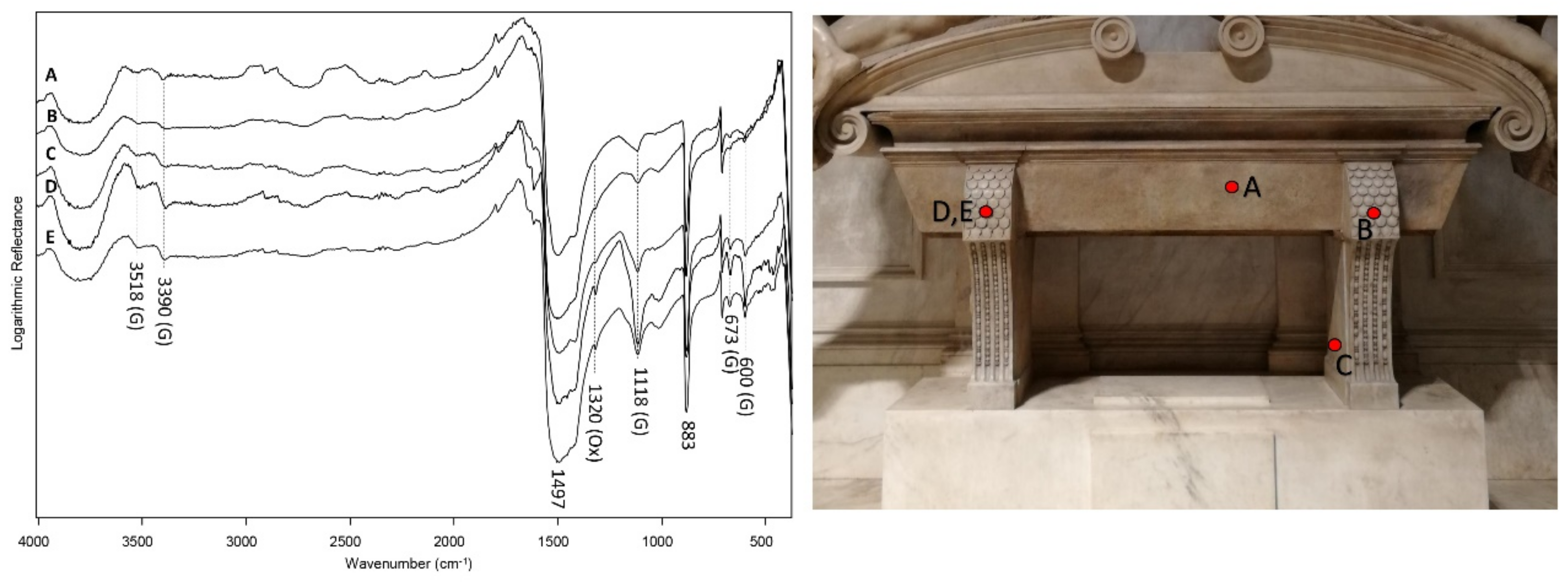
The latter compounds were also detected by the analyses in reflection mode on the surface of the sarcophagus (
Figure 7 and
Figure 8), as shown by the vibration bands at 3518, 3390, 1118, 673 and 600 cm
−1 (gypsum) and 1320 cm
−1 (calcium oxalate).
The results of the chemical investigations have guided the choice of bacterial strains. The appropriate strains were chosen among the laboratory collection, on the basis of a previous metabolic characterization and have been re-tested for their effectiveness against the target substances, namely: protein material, calcium phosphates, gypsum and calcium oxalate. In
Table 1 are the reported results.
All strains are actually able to degrade at least one of the components of the deposit and overall, all the functions required for the bio-cleaning of target substances are expressed. Several strains are multifunctional. The negative controls showed no cleaning effect. All the strains were, therefore, used for the exploratory tests performed on the palette in the back of the altar. The responses rated as the more successful by the scientific committee of the Museum were the followings:
S. ficaria SH7 with CYM in Vanzan,
P. stutzeri CONC11 in Laponite and
Rhodococcus sp. ZCONT in Vanzan (
Figure 9). These bacterial strains under the conditions selected were used to implement the bio-cleaning intervention on the sarcophagus, which took place about a year later.
Based on the chemical analysis map (
Figure 7), and in accordance with the methods described in
Section 2.4, the three strains have been applied individually on different portions of the sarcophagus.
S. ficaria SH7 in Vanzan was applied on the largest and most compromised parts, with a prominent role in removing phosphate, protein and fat compounds;
Rhodococcus sp. ZCONT in Vanzan was applied to smaller portions with lipid residues and
P. stutzeri CONC11 in Laponite was applied for the degradation of protein material and in the lateral areas as a second application after SH7, to improve the homogeneity of the cleaning. After the removal of micro-packs, the results were evident from the first application and already appreciable with the naked eye. The second micro-pack application brought the cleaning to a level deemed satisfactory by the Museum’s experts (
Figure 10 and
Figure 11).
4. Discussion
The challenge of this restoration was to take care of Michelangelo’s masterpiece, conveying the artistic message of the “passing of time” carved in marble. To this aim, the methodological approach was a selective and gradual cleaning, which would return to a harmonious reading of Michelangelo’s masterpieces and technique. Sculptures and the marble coverings of the architectural elements were intact but had undergone, over the centuries, changes in their chromatic perception. The initial visual approach was supported by photographic documentation in visible light and photographic investigations by ultraviolet and infrared-induced luminescence (data not shown). During the first cleaning phase (from 2013 to 2019), the removal of incoherent deposits was done using a low-power vacuum cleaner and soft brushes with mild solvents. Cleaning was constantly monitored by colorimetric and reflectance measurements on the marble confirming the correctness of the methodological choice.
The cleaning of the sarcophagus of Lorenzo Duke of Urbino was faced in the last phase (2019–2020). The unique history and the mediocre state of the conservation of the marble required the contribution of chemical-physical analyses and a selective and gradual cleaning intervention that did not interfere with the material consistency of the marble, opting for bio-cleaning with living bacteria.
The reason for using living cells and not purified molecules, such as enzymes, lies in cellular homeostasis. Bacteria self-regulate, adjusting to the ever-changing environmental conditions that surround them [
22]. The main homeostatic processes that guarantee the survival of bacteria, even in suboptimal or adverse conditions, include pH homeostasis [
23] iron and metal homeostasis [
24,
25] membrane lipid homeostasis [
26], cell size [
27] timescales in bacterial growth [
28] and protein content [
29]. The homeostatic capacity of the cells offers an important advantage for in situ applications, both in the open air and indoors, allowing it to operate freely in very different conditions, without having to comply with the stringent operational measures of temperature and pH, as the use of purified enzymes requires. Active molecules (enzymes, organic acids, etc.) are harboured and continue to be produced by the cells. That is, we work directly with the factories of the target molecules, which are necessary for selective bio-cleaning. The bacteria—prepared as described—maintain their vitality and effectiveness for a few days, allowing for the repetition of the applications in succession, if necessary, until the desired result is achieved.
With regard to the substances detected, while the presence of gypsum resulting from FT-IR analyses can be referred to as atmospheric deposition, the proteins and calcium phosphate may be related to body decomposition processes, due to the poor preservation of the uneviscerated body of Duke Alessandro inside the sarcophagus. Degradation of surface organic materials (such as old natural protective coatings) may be responsible for the small amounts of calcium oxalate detected [
30,
31].
Following the screening on the test panel, the three bacterial strains used for the bio-cleaning of the sarcophagus had been chosen considering the quality of the cleaning in accordance with the conservator’s criteria. The metabolic features of the strains first determined the success of the cleaning, however other factors contributed positively. As described, the micro-packs are normally applied to the surface by interposing a Japanese paper, to make both application and removal easier and to allow for inspection during the contact time, when monitoring is necessary. In this case, it was chosen to apply the gel with bacteria in direct contact with the surface, due to the consistent thickness of the patina, especially on the sarcophagus base. This mode also makes it possible to modulate the application, according to the different distribution and consistency of the dirt layers. We observed that the direct contact actually helped bacteria to better penetrate the coherent deposit layers, favouring the in-depth action. After the removal of the pack, the gel appeared dark, having partly incorporated the dark deposits. Two consecutive fresh overnight applications were sufficient to obtain a quality of cleaning deemed suitable by the conservators: selective, gradual and respectful of the original material. Selective because each strain was able to transform target compounds; gradual as the microbial metabolism follows metabolic rhythms; respectful of the material as the selected strains lacked the functions of solubilizing or precipitating calcium carbonates, the main components of the marble. As shown in the representative pictures in
Figure 12, the observations of the marble performed with a digital microscope before, during and after bio-cleaning confirmed the effective removal of the brownish patina in full respect of the surface. No microbiological monitoring was performed after the bio-cleaning. Previous studies have indeed proved that monitoring at intervals of up to 4 months after treatment detected only transient environmental microorganisms, without any correlation with those used in bio-cleaning [
4]. Indeed, the bacteria remain effectively incorporated in the gels which allow an excellent removal of the bacteria.
The unique history of the monument and the peculiarity of the cleaning problem faced make the comparison with other works difficult. Most of the bio-cleaning on marble involved the removal of sulphate and nitrate crusts [
32] a critical comparison of delivery systems [
33] the removal of waxes and fats [
34]. In Michelangelo’s case, the deposits were mainly made up of calcium phosphates and proteins. Selecting the appropriate bacteria was the hardest task.
S.ficaria SH7 has been the main protagonist with the most problematic areas encountered on the sarcophagus. It is a strain isolated from the mining waste in an abandoned mine of blende and galena in Sardinia, thus influenced by the habitat rich in heavy metals and poor in nutrients. It is tolerant to heavy metals and promoter of plant growth. With regard to the functions of specific interest in bio-cleaning, it is a multifunctional strain but lacks the functions to damage the marble matrix. It combines the ability to metabolise phosphates, proteins and fats. We can metaphorically say that the micro-pack with
S.ficaria SH7 turned out to be a “metabolic bomb”, which managed to remove dirt that had persisted over 500 years, whose cleaning would have required aggressive and partly toxic products. After all, we can say that being able to eliminate that dark patina was a successful bet [
35].
A summary of the restoration design, scientific investigations and bio-cleaning is, respectively, shown in three short videos (Toccare Michelangelo 1, 2, 3,
Supplementary Materials). A graphic scheme of the biocleaning approach is also available in
Supplementary Materials.
5. Conclusions
The restoration of Michelangelo’s New Sacristy lasting eight years (2013–2020) has been a multidisciplinary experience, open to professionals with different backgrounds from Sciences to Humanities, leading to a better understanding of Michelangelo’s executive technique.
The documentary archival research that accompanied the restoration has shed new light on the life and uses of the place a symbol of art Renaissance, over the five hundred years of its existence.
An analytical campaign addressed the cleaning methodological choices that made this place legible and enjoyable, while maintaining a harmonious level of cleanliness without pushing to the extreme.
The restoration path was based on the principles of respect for the material and turned to the most advanced principles of sustainability and the most innovative applications in restoration.
Bio-cleaning with living bacteria was the methodological choice for the sarcophagus of Lorenzo Duke of Urbino’s restoration (2019–2020). Coherent deposits for centuries had altered portions of the masterpiece, in the absence of a real solution capable of respecting the precious material.
Chemical–physical analyses allowed for a scientific diagnosis, detecting the presence of mainly proteins and calcium phosphate in the deposits, probably related to body the decomposition processes of Duke Alessandro, Lorenzo’s son, quickly buried in the sarcophagus without evisceration.
Following the analytical results, competent bacterial strains were selected among the microbial laboratory collection ENEA-MIRRI. As a result of exploratory tests, three bacterial strains were used for bio-cleaning: S. ficaria SH7, P. stutzeri CONC11 and Rhodococcus sp. ZCONT. The strains had good metabolic skills for the removal of target substances, but lacked the functions to interact with the marble matrix (precipitation or solubilization of calcium carbonates). Micro-packs of bacteria immobilised in a supportant gel have been separately applied to the sarcophagus surfaces. Two successive overnight applications satisfactorily removed the aged deposits within 48 h. The multifunctional strain S. ficaria SH7 immobilised in Vanzan was the main protagonist for the most problematic areas to be treated.
In accordance with the conservatory’s criteria, a good cleaning must respond to the qualities of selectivity, gradualness and respect for the material. The bio-cleaning discussed in this work responded to these requirements and, once again, demonstrates that the biological route represents a valid alternative to the use of products that are often toxic for restorers and aggressive towards works of art. Bio-cleaning with living bacteria is a technology that can allow the transition to a more sustainable restoration, using both a knowledge-based approach and renewable products. The bio-restoration approach is in full agreement with the mandatory health, energy and environmental requirements, which also inspire the need for an ecological transition.
Last but not least, an important factor that made the success of this work possible should be emphasized: an integrated dialogue and synthesis between the disciplines of art, history and science, that can represent a reference model for an innovative and sustainable restoration approach.
Author Contributions
Conceptualization: M.B. and A.R.S.; methodology: D.M. (Donata Magrini), D.M. (Daniela Manna), M.V., C.A., B.S. and S.V.; formal analysis: B.S.; investigation: B.S., D.M. (Donata Magrini) and S.V.; data curation: B.S., D.M. (Donata Magrini), S.V., M.V., D.M. (Daniela Manna), M.B., A.R.S. and C.A.; writing—original draft preparation: A.R.S., C.A., B.S., D.M. (Donata Magrini) and S.V.; writing—review and editing, C.A., A.R.S., B.S., D.M. (Donata Magrini) and S.V. All authors have read and agreed to the published version of the manuscript.
Funding
The restoration was funded by the Italian Ministry of Culture. The diagnostic analysis was funded by Bargello Museum. The bio-cleaning and the entire related process was carried out with ENEA ordinary funds.
Acknowledgments
Thanks to Flavia Tasso, Giada Migliore and Patrizia Paganin of the OEM laboratory of ENEA-Casaccia, for taking care of the bacterial strains in the ENEA-MIRRI collection and for making them ready for this job.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frey, C. Die Literarische Nachlass Giorgio Vasaris; George Muller: Munich, Germany, 1923; Volume 3, pp. 695–696. [Google Scholar]
- Rosenberg, R. Beschreibungen und Nachzeichnungen der Skulpturen Michelangelos: Eine Geschichte der Kunstbetrachtung; Dt. Kunstverl: Munich, Germany, 2000; p. 32. [Google Scholar]
- Palla, F.; Tartamella, E. Chromatic alteration on marble surfaces analysed by molecular biology tools. Conserv. Sci. Cult. Herit. 2007, 7, 111–127. [Google Scholar]
- Mazzoni, M.; Alisi, C.; Tasso, F.; Cecchini, A.; Marconi, P.; Sprocati, A.R. Laponite micro-packs for the selective cleaning of multiple coherent deposits on wall paintings: The case study of Casina Farnese on the Palatine Hill (Rome, Italy). Int. Biodeterior Biodegrad. 2014, 94, 1–11. [Google Scholar] [CrossRef]
- Sprocati, A.R.; Alisi, C.; Tasso, F. Biotechnology Process for the Removal of Cohesive Deposits of Organic and Inorganic Origin from Materials and Works of Historical—Artistic Interest. European Patent EP 3046779 B1, 17 September 2014. [Google Scholar]
- Ortega-Villamagua, E.; Gudiño-Gomezjurado, M.; Palma-Cando, A. Microbiologically Induced Carbonate Precipitation in the Restoration and Conservation of Cultural Heritage Materials. Molecules 2020, 25, 5499. [Google Scholar] [CrossRef]
- Rugnini, L.; Migliore, G.; Tasso, F.; Ellwood, N.W.T.; Sprocati, A.R.; Bruno, L. Biocidal activity of phyto-derivative products used on phototrophic biofilms growing on stone surfaces of the Domus Aurea in Rome (Italy). Appl. Sci. 2020, 10, 6584. [Google Scholar] [CrossRef]
- Ortega-Morales, B.O.; Gaylarde, C.C. Bioconservation of Historic Stone Buildings—An Updated Review. Appl. Sci. 2021, 11, 5695. [Google Scholar] [CrossRef]
- Sprocati, A.R.; Alisi, C.; Migliore, G.; Marconi, P.; Tasso, F. Sustainable Restoration through Biotechnological Processes: A Proof of Concept. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Ranalli, G.; Bosch-Roig, P.; Crudele, S.; Rampazzi, L.; Corti, C.; Zanardini, E. Dry biocleaning of artwork: An innovative methodol-ogy for Cultural Heritage recovery? Microb. Cell 2021, 8, 91–105. [Google Scholar] [CrossRef]
- Bietti, M.; Manna, D.; Vincenti, M.; Sprocati, A.R.; Alisi, C. Il restauro della Sagrestia Nuova di Michelangelo. Un modello di integrazione tra arte, metodo e scienza. Kermes 2022, 122, 57–66. [Google Scholar]
- Doni, A.F.; Leo, S. I Marmi, 1552; Leo S. Olschki Editore: Florence, Italy, 2017; p. 24. [Google Scholar]
- Lapini, A.; Corazzini, G.O. Diario Fiorentino di Agostino Lapini dal 252 al 1596; G.C. Sansoni: Florence, Italy, 1900; p. 101. [Google Scholar]
- Sprocati, A.R.; Alisi, C.; Tasso, F.; Segre, L.; Cremisini, C. Investigating heavy metal resistance, bioaccumulation and metabolic profile of a metallophile microbial consortium native to an abandoned mine. Sci. Total Environ. 2006, 366, 649–658. [Google Scholar] [CrossRef]
- Sprocati, A.R.; Alisi, C.; Tasso, F.; Fiore, A.; Marconi, P.; Langella, F.; Haferburg, G.; Nicoara, A.; Neagoe, A.; Kothe, E. Bioprospecting at former mining sites across Europe: Microbial and functional diversity in soils. Environ. Sci. Pollut. Res. 2014, 21, 6824–6835. [Google Scholar] [CrossRef]
- Sprocati, A.R.; Alisi, C.; Tasso, F.; Vedovato, E.; Barbabietola, N.; Cremisini, C. A microbiological survey of the Etruscan Mercareccia Tomb (Italy): Contribution of microorganisms to deterioration and restoration. In Proceedings of the 9th International Conference on NDT of Art, Jerusalem, Israel, 26–29 May 2008. [Google Scholar]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Lelliott, R.A.; Stead, D.E. Methods for the Diagnosis of Bacterial Diseases of Plants; Blackwell Science: Oxfod, UK, 1987; 216p. [Google Scholar]
- Ebrahimpour, A.; Rahman, R.; Kamarudin, N.; Basri, M.; Salleh, A. Lipase production and growth modelling of a novel thermophilic bacterium: Aneurinibacillus thermoaerophilus strain AFNA. Electron. J. Biotechnol. N. Am. 2011, 14, 2206. [Google Scholar] [CrossRef]
- Crisci, L.; Alisi, C.; Pratelli, M.; Santamaria, U.; Sprocati, A.R. Il Biorestauro: Messa a punto di una procedura su misura per l’opera. In Tra Cielo e Terra. La Madonna della Cintola di Vincenzo Pagani; Edizioni Musei Vaticani: Vatican City, Vatican, 2022; pp. 189–207. [Google Scholar]
- Ferenci, T. Hungry bacteria—Definition and properties of a nutritional state. Environ. Microbiol. 2001, 3, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Meunier, A.; Cornet, F.; Campos, M. Bacterial cell proliferation: From molecules to cells. FEMS Microbiol. Rev. 2021, 45, fuaa046. [Google Scholar] [CrossRef] [PubMed]
- Slonczewski, J.L.; Fujisawa, M.; Dopson, M.; Krulwich, T.A. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv. Microb. Physiol. 2009, 55, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Chandrangsu, P.; Rensing, C.; Helmann, J. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 2017, 15, 338–350. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Rock, C. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- Si, F.; Le Treut, G.; Sauls, J.T.; Vadia, S.; Levin, P.A.; Jun, S. Mechanistic origin of cell-size control and homeostasis in bacteria. Curr. Biol. 2019, 29, 1760–1770. [Google Scholar] [CrossRef]
- Stawsky, A.; Vashistha, H.; Salman, H.; Brenner, N. Multiple timescales in bacterial growth homeostasis. iScience 2022, 25, 103678. [Google Scholar] [CrossRef]
- Wong, P.; Houry, W.A. Chaperone networks in bacteria: Analysis of protein homeostasis in minimal cells. J. Struct. Biol. 2004, 146, 79–89. [Google Scholar] [CrossRef]
- Otero, V.; Vilarigues, M.; Carlyle, L.; Cotte, M.; De Nolf, W.; Melo, J. A little key to oxalate formation in oil paints: Protective patina or chemical reactorPhotochem. Photobiol. Sci. 2018, 17, 266. [Google Scholar] [CrossRef] [PubMed]
- Pinna, D.; Bracci, S.; Magrini, D.; Salvadori, B.; Andreotti, A.; Colombini, M.P. Deterioration and discoloration of historical protective treatments on marble. Environ. Sci. Pollut. Res. 2022, 29, 20694–20710. [Google Scholar] [CrossRef] [PubMed]
- Troiano, F.; Gulotta, D.; Balloi, A.; Polo, A.; Toniolo, L.; Lombardi, E.; Daffonchio, D.; Sorlini, C.; Cappitelli, F. Successful combination of chemical and biological treatments for the cleaning of stone artworks. Int. Biodeterior. Biodegrad. 2013, 85, 294–304. [Google Scholar] [CrossRef]
- Bosch-Roig, P.; Lustrato, G.; Zanardini, E.; Ranalli, G. Biocleaning of Cultural Heritage stone surfaces and frescoes: Which delivery system can be the most appropriate? Ann. Microbiol. 2015, 65, 1227–1241. [Google Scholar] [CrossRef]
- Sprocati, A.R.; Alisi, C.; Tasso, F.; Marconi, P.; Migliore, G. Formule microbiche per l’arte. Kermes 2017, 100, 23–26. [Google Scholar]
- Horowitz, J. Send in the Bugs. The Michelangelo’s Need Cleaning. The New York Times, 30 May 2021. [Google Scholar]
Figure 1.
The New Sacristy, overall view with the Tomb of Lorenzo Duca d’Urbino before the restoration (photo Andrea Jemolo).
Figure 1.
The New Sacristy, overall view with the Tomb of Lorenzo Duca d’Urbino before the restoration (photo Andrea Jemolo).
Figure 2.
Description and stereomicroscope images of fragments and powders taken from the sarcophagus, with pictures showing the areas of sampling and indication of the analytical techniques applied for the investigation.
Figure 2.
Description and stereomicroscope images of fragments and powders taken from the sarcophagus, with pictures showing the areas of sampling and indication of the analytical techniques applied for the investigation.
Figure 3.
Micro-pack application on the side of the sarcophagus (a); on the back of the altar (b).
Figure 3.
Micro-pack application on the side of the sarcophagus (a); on the back of the altar (b).
Figure 4.
Front side of the sarcophagus covered with a micro-pack of S. ficaria SH7 in vanzan.
Figure 4.
Front side of the sarcophagus covered with a micro-pack of S. ficaria SH7 in vanzan.
Figure 5.
Lateral left side of the sarcophagus covered with micro-pack with different bacteria.
Figure 5.
Lateral left side of the sarcophagus covered with micro-pack with different bacteria.
Figure 6.
Results of the characterization of samples 01–03 taken from the sarcophagus: (a) cross-section of Sample 01 observed under optical microscope in reflected light; (b) FT-IR spectrum of Sample 02; (c) XRF; and (d) FT-IR spectrum of Sample 03 (the arrow points at the Kα line of phosphorus).
Figure 6.
Results of the characterization of samples 01–03 taken from the sarcophagus: (a) cross-section of Sample 01 observed under optical microscope in reflected light; (b) FT-IR spectrum of Sample 02; (c) XRF; and (d) FT-IR spectrum of Sample 03 (the arrow points at the Kα line of phosphorus).
Figure 7.
Reflection FT-IR spectra acquired on the surface of the sarcophagus, in correspondence with the points shown in the picture. The attribution of vibration bands to the compounds is indicated by the letters in parentheses: gypsum (G) and oxalate (Ox).
Figure 7.
Reflection FT-IR spectra acquired on the surface of the sarcophagus, in correspondence with the points shown in the picture. The attribution of vibration bands to the compounds is indicated by the letters in parentheses: gypsum (G) and oxalate (Ox).
Figure 8.
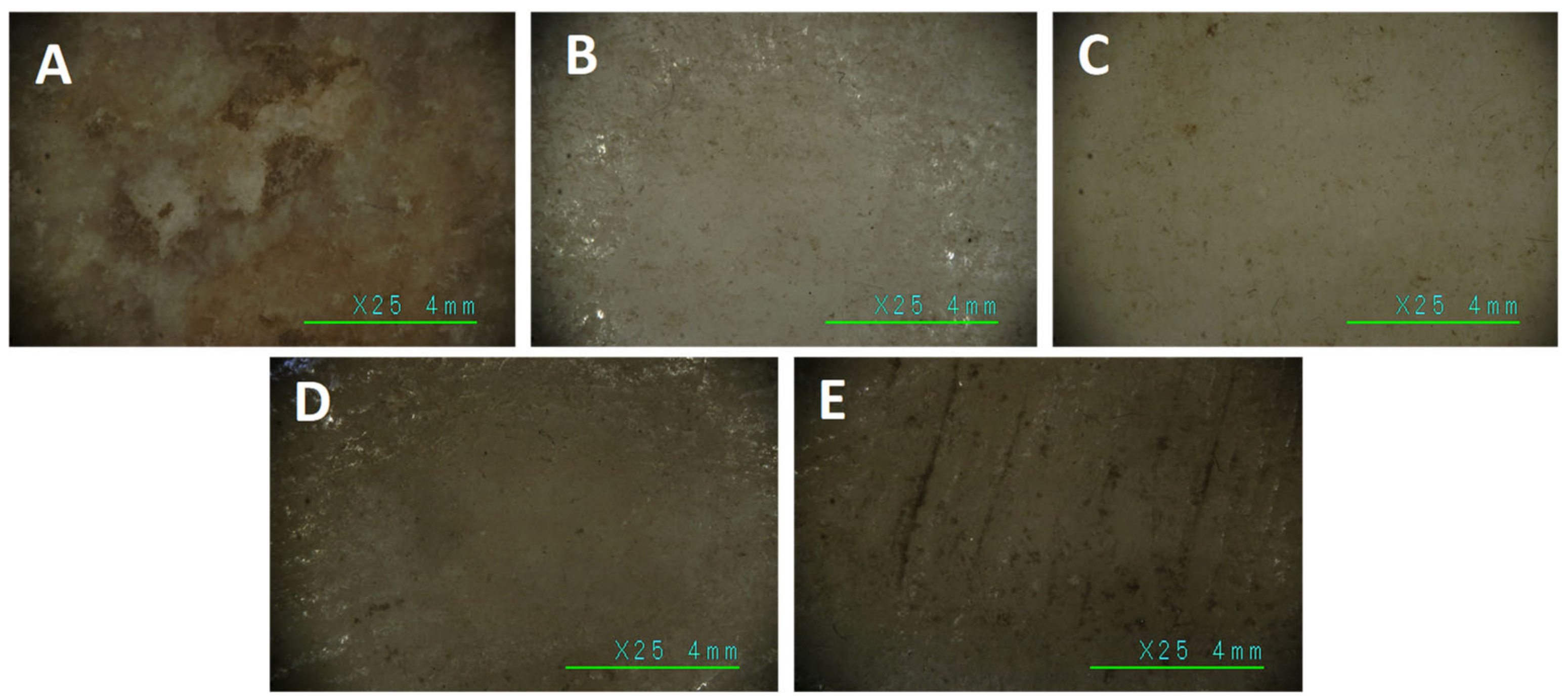
Images at high magnification (25×) of the areas (A–E) investigated with reflection FT-IR before treatments.
Figure 8.
Images at high magnification (25×) of the areas (A–E) investigated with reflection FT-IR before treatments.
Figure 9.
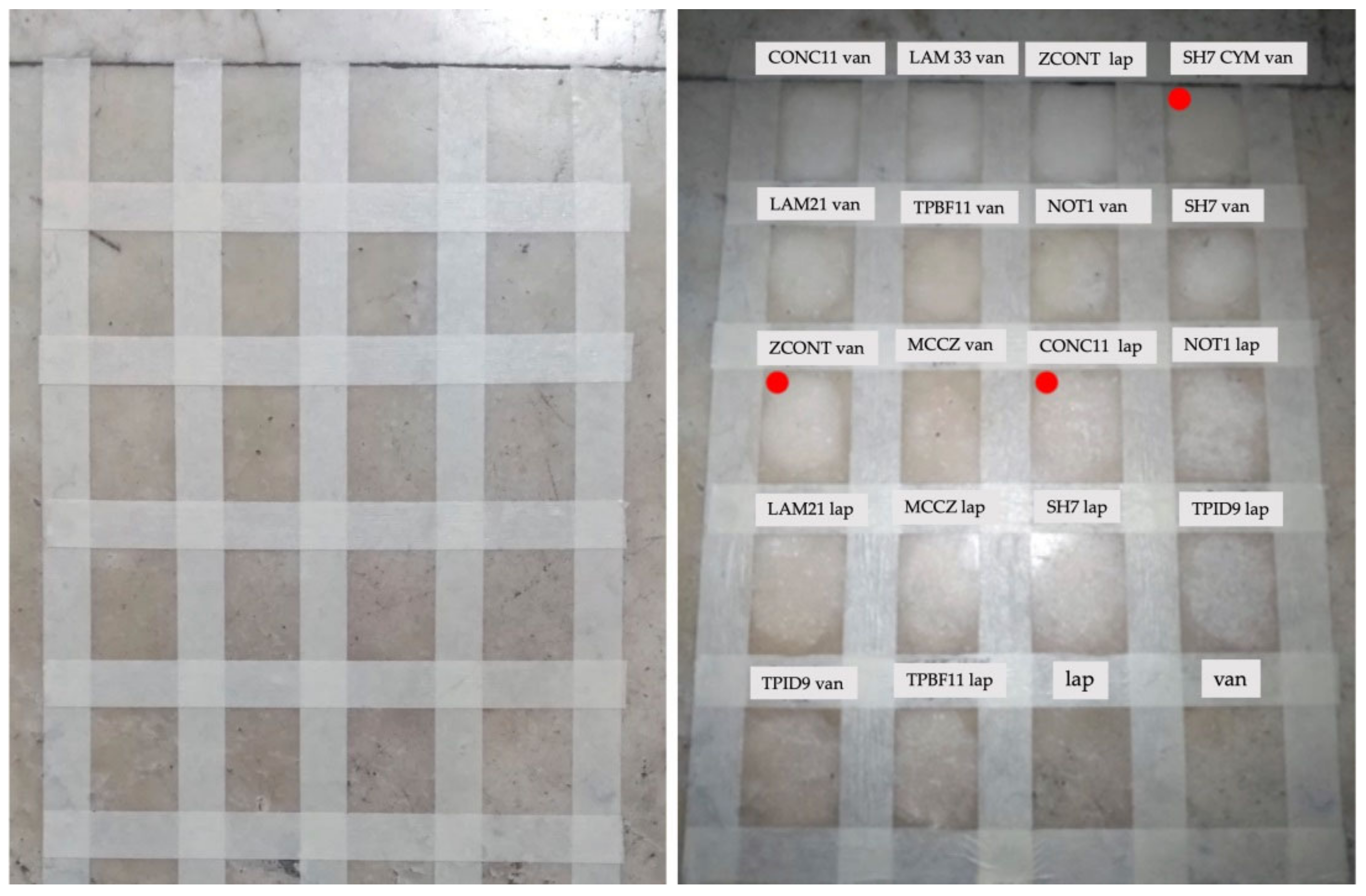
Result of preliminary tests on site before and after the removal of micro-packs containing different bacteria. The red dot indicates the bacterial strains chosen by the quality of the cleaning.
Figure 9.
Result of preliminary tests on site before and after the removal of micro-packs containing different bacteria. The red dot indicates the bacterial strains chosen by the quality of the cleaning.
Figure 10.
Right side of the sarcophagus, before (a) and after (b) the bio-cleaning treatment.
Figure 10.
Right side of the sarcophagus, before (a) and after (b) the bio-cleaning treatment.
Figure 11.
Sarcophagus base, before (a) and after (b) the bio-cleaning treatment.
Figure 11.
Sarcophagus base, before (a) and after (b) the bio-cleaning treatment.
Figure 12.
Images at high magnification of an area of the sarcophagus before (a), after partial (b) and complete (c) treatment with Rhodococcus sp ZCONT.
Figure 12.
Images at high magnification of an area of the sarcophagus before (a), after partial (b) and complete (c) treatment with Rhodococcus sp ZCONT.
Table 1.
Characteristics of bacterial strains undergone metabolic test in vitro for solubilisation (calcium carbonates/sulphates, phosphates) and degradation (protein and lipids): − (negative) + (positive: arbitrary scale of values describing the biochemical response).
Table 1.
Characteristics of bacterial strains undergone metabolic test in vitro for solubilisation (calcium carbonates/sulphates, phosphates) and degradation (protein and lipids): − (negative) + (positive: arbitrary scale of values describing the biochemical response).
| STRAIN CODE | GenBank Accession Number | Origin | CaCO3 sol | CaSO4 sol | PO4 sol | Protein Degrad. | Lipid Hydrol. |
|---|
| Virgibacillus sp. NOT1 | JX417495 | Parchment XVII century | − | − | − | +++ | − |
| Serratia ficaria SH7 | KP780180 | Mining soil—Ingurtosu, Italy | − | − | ++ | +++ | +++ |
| Plantibacter sp. UI24 | JX133202 | − | ++ | − | − | − |
| Cellulosimicrobium cellulans TBF11 | EU249577 | Etruscan tomb—Tarquinia, Italy | + | +++ | − | − | − |
| Stenotrophomonas maltophilia TPID9 | EU263112 | − | − | ++ | + | − |
| Rhodococcus sp. ZCONT | KY697119 | Laboratory contaminant, Italy | − | − | − | − | ++ |
| Pseudomonas stutzeri CONC11 | EU275358 | Tannery muds— Naples, Italy | − | − | − | +++ | − |
| Pedobacter sp. MCCZ | JF279930 | Industrial site—Bagnoli, Italy | − | − | ++ | ++ | ++ |
| Acinetobacter calcoaceticus LAM21 | KJ534248 | − | − | ++ | − | ++ |
| Pseudomonas fluorescens LAM33 | EU019991 | + | − | +++ | − | − |
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).