Abstract
Microbial contamination control in indoor environments, such as libraries and archives, represents a challenge. Essential oils (EOs), well-known for their antimicrobial properties, have been applied in pharmaceutical and food industry from many years. In the present study, Thymus vulgaris and Origanum vulgare EO antimicrobial efficacy on paper-born microorganisms, Staphylococcus epidermidis, Rhodotorula mucilaginosa and Alternaria alternata, was investigated to protect water-damaged paper documents and to control indoor air quality for operator’s health safety. T. vulgaris EO was the most effective: Minimum Inhibitory Concentration (MIC) values obtained for S. epidermidis and R. mucilaginosa, with a broth macro-dilution method, were 7.5 µg/mL and 5.63 µg/mL, respectively. T. vulgaris EO (0.75% v/v), nebulized immediately after the inoculation on agar plates or paper sheets, showed a high inhibition effect against the three biodeteriogenic microorganisms, also when lyophilized on paper sheets; in this last case, the EO has a higher efficacy when applied immediately after the freeze drying. Regarding the EO effect against A. alternata, the inhibition percentage of the mycelial growth, MGI, (81.4%), observed for nonsporulated mycelium, was higher than that for the sporulated one (51.4%). Finally, T. vulgaris EO (0.75% v/v) was effectively applied on a real contaminated book cover by means of EO impregnated contact sheets. Obtained results demonstrated that tested EOs were able to delay or completely inhibit paper-born microorganism growth for both flood-independent or -dependent contamination.
1. Introduction
The level of biological contamination of libraries and archives can be quite high and potentially dangerous not only for the stored materials that play the role of substrate for microorganism growth, but also for the occupants. In fact, the bioaerosol resulting from these contaminants can be inhaled in a passive way and/or breathed during the handling and the cleaning of dusty or mouldy books [1,2].
The microorganism number and type in libraries and archives depends on (i) the microclimatic conditions, i.e., the temperature and the air humidity, that may be affected by air recirculation and conditioning, (ii) the presence of macro- and micronutrients and (iii) the water activity (aw) of the different substrates. Besides, the composition of the bioaerosol is strictly related to the microorganisms that colonize the surfaces and the stored material [3].
The main paper biodeteriogenic microorganisms are xerophilic fungi able to tolerate dry environments, characterized by low values of water activity. In the first phase of colonization, bacteria are less relevant because they require high water activity values. Nevertheless, xerophilic fungi colonize the paper as pioneers, and in case the water activity value increases, the growth of other species, such as the cellulolytic fungi and bacteria, can also be sustained.
Both fungi and bacteria can persist on the library material for long periods, also for years, without producing biodeterioration phenomena. However, if the microclimatic conditions change, for example as a consequence of a flooding, favorable conditions are created for an increase in the microbial colonization and, consequently, for the decomposition of the organic matter [4,5]. In that case, the amounts and the type of the microorganisms or microbial metabolites can change: this can support the biodeterioration of the stored material, besides becoming a risk for the health of the library occupants [6].
Fungal species such as Aspergillus flavus, A. parasiticus, A. versicolor, Penicillium chrysogenum, P. expansum, and Stachybotrys chartarum are commonly isolated in archives and in libraries, especially those characterized by high humidity; the presence of these fungi is probably associated with the production of mycotoxins or other volatile organic microbial compounds [2]. Fungal species that need high values of water activity (aw) to survive are commonly found in those rooms, destined to the paper material storage: these fungi can produce intense smell (es. Trichoderma spp.), coloured mycelia (es. Chaetomium spp. and Epicoccum spp.), or toxic compounds (es. Stachybotrys spp.) [7].
The library and archive environments can become ideal also for cellulolytic fungi and other microbial species; the Ascomycetes (Chaetomium spp.), the Phycomycetes (Rhizopus and Mucor spp.), and the Deuteromycetes (Aspergillus, Penicillium, Trichoderma, Stachybotrys, Stemphylium, Alternaria, Mycothecium spp.) include fungal species which commonly biodegrade the cellulose in aerobic conditions. Among the bacteria, Streptomyces, Micromonospora, Bacillus, Cellulomonas, and Cytophaga spp. can be listed [8]. Two yeast genera (Candida spp. and Rhodotorula spp.) were isolated from photo on paper [9].
Montanari et al. (2012) report, as a typical example of library contamination, the one that can occur in a compact storage shelving system [5]. The presence of moulds and the signs of their growth can be evidenced on the binding of leather, parchment, or cotton fibre volumes. White and irregular fungal colonies are evident contamination signs on the exposed surfaces of the volumes located in the lower parts of the shelving blocks; commonly, the involved fungi belong to the genus Aspergillus spp.
The occurrence of accidental events caused by water (i.e., rainstorm, flooding or fire extinction systems) unavoidably causes the growth of biodeteriogenic microorganisms on paper documents: currently, the freezing represents one of the most efficacious treatments to control biodeterioration of the flooded material. In fact, the frozen paper material can be stored for a very long time without negative consequences. In these cases, freeze-drying represents an interesting possibility for recovering the documents, since the first phase of the process, i.e., the freezing of the material, has already been carried out. Freeze-drying is a well-known technology, which can give excellent results avoiding further damages to paper material because the operating temperatures are very low and there is no presence of liquid water (during the process) that can displace soluble components present in the material, such as dyes and glues [10,11]. In the scientific literature, results of freeze-drying treatment on the viability of paper-born microorganisms are not univocal and are sometimes contradictory. As an example, according to the work by Troiano et al. (2013) [12], lyophilization, in general, reduces significantly the microbial load present; on the contrary, Fissore et al. (2019) reported that growth of R. mucilaginosa was stimulated [13].
Few scientific papers on the effect of freeze-drying on biodeteriogenic microorganisms were published, probably due to the fact that microorganisms respond to the treatment in a species-dependent manner depending on the growth phase as well [12]. In the work of Fissore et al. (2019) [13], the effect of freezing and freeze-drying on microorganism survival and growth was separately evaluated and the necessity to control the paper-born microorganisms, after the lyophilization, appeared mandatory. In this framework, the possibility to control the biodeteriogenic and toxigenic microflora growth by using essential oils appears particularly interesting [14].
Essential oils (EOs) are in fact well-known for their antimicrobial properties, and they were applied in several fields such as pharmaceutical and food industry. On the contrary, as reported by Díaz-Alonso et al. (2021) [15], very few papers on the application of EO in the field of cultural heritage were published in the last decade [16]. Therefore, the use of EOs as natural biocides in the control of biodeteriogenic microorganisms can still be considered a challenge. In the work of Díaz-Alonso et al. (2021) [15], the effectiveness of Melaleuca alternifolia (Tea tree) and Thymus vulgaris (Thyme) EOs in reducing air bacterial and fungi contamination in unventilated indoor spaces was evaluated. The vaporization of tea tree EO showed the best results allowing an air contamination reduction equal to 77.3% and 95.0% for fungi and bacteria, respectively. Palla et al. (2020), in order to contrast the biodeterioration induced by Aspergillus flavus or insect infestation (Anobium punctatum), exposed wooden artworks to the volatile components of Origanum vulgare or Thymus vulgaris EOs [17]. The authors concluded that these natural pesticides could be used as a valid alternative in the control of the biodeterioration processes avoiding any negative impact on the environment or operator health. The high antimicrobial activity of the wild thyme EO detected against B. subtilis, F. oxysporum and A. niger, microorganisms, which quite frequently infest archives, libraries and historical art craft objects, was also reported by Casiglia et al. (2019) [18].
The present study will thus be focused on the investigation of the effect of essential oils on contaminated paper considering two case studies, namely the contamination of paper that did not undergo any flooding and the contamination of freeze-dried paper, after flooding, as in this case, the response of the system may be different. The effect of freezing and drying on the survival of S. epidermidis, R. mucilaginosa and A. alternata on flooded paper has already been described in a previous work [13]. The process was not able to control the growth of S. epidermidis and R. mucilaginosa, while it inhibited A. alternata. Thus, in the present work, a preliminary screening with Thymus vulgaris and Origanum vulgare leaf oils was performed, by means of “broth dilution methods”, on S. epidermidis and R. mucilaginosa. The effect of Thymus vulgaris oil, vaporized immediately after the inoculum or at 24 h of incubation, was tested on the two unicellular microorganisms grown on agar media. Considering the positive effect of EO, the investigation was also extended to A. alternata, chosen as representative of the filamentous fungi that can be found in archives. Finally, the treatment was extended on a real contaminated book. After that, a system was set up in which flooding and “artificial” contamination, followed by freezing or lyophilization process, at different time of growth, were realized; contaminated paper sheets, removed from the paper blocks, after lyophilization or freeze-thawing, were treated with the EO immediately or after 24 h of incubation. Finally, the treatment with T. vulgaris oil was also applied on a real contaminated book.
2. Materials and Methods
The tested microorganisms (Figure 1) were the Gram-positive bacterium, S. epidermidis (LMG0474, BCCM, Belgium), the yeast R. mucilaginosa and the filamentous fungus A. alternata BNR, belonging to the private collection of the Biotechnological Laboratory of Politecnico di Torino. All the microorganisms have been described as paper biodeteriogens [8,9,19,20].
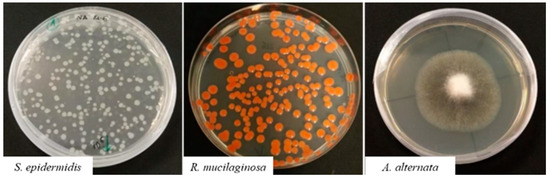
Figure 1.
Tested microorganisms.
The media utilized for the maintenance and cultivation of S. epidermidis (incubated at 37 ± 0.5 °C), R. mucilaginosa, and A. alternata (both incubated at 30 ± 0.5 °C) were as follows, respectively: the Nutrient agar Oxoid CM003 (NA: yeast extract 2 g/L, peptone 5 g/L, NaCl 5 g/L, Lab-Lemco powder 1 g/L, and agar 15 g/L), the Malt Extract agar (MEA: malt extract 20 g/L, peptone 2 g/L, glucose 20 g/L, and agar 20 g/L), and the Czapeck agar (CZ: NaNO3 3 g/L, K2HPO4 1 g/L, MgSO4 0.5 g/L, KCl 0.5 g/L, FeSO4 0.01 g/L, glucose 30 g/L, and agar 20 g/L). The same media, without the agar, were applied for liquid medium studies. Sterile PE Petri dishes, without venting, 90 mm Ø, were utilized for the all experiments with solid media.
2.1. EO Screening in Liquid Medium: Broth Dilution Method
Thymus vulgaris OE0970 and Origanum vulgare OE0375, both supplied by Witt Italia Spa, were the utilized essential oils (EOs); the EO suspensions were prepared, in sterile conditions, utilizing 1.5% v/v of polysorbate 20 (TEGO SML 20) as a diluent. A “96-well plate broth microdilution method” was applied to separately test the two EO antimicrobial susceptibility of S. epidermidis and R. mucilaginosa and to record the Minimum Inhibitory Concentration (MIC); with this purpose, the EUCAST protocols were followed with some modifications for bacteria and for fungi [21,22]. Two-fold serial dilutions of the EOs (0.75%, 0.375%, 0.1875%, 0.0938%, 0.0469%, 0.0234%, 0.0117%, and 0.0059%) were inoculated with standardized microbial inocula of each microorganism, prepared by means of the suspension of 3 colonies, grown for 24 h, in the appropriate medium as previously reported in Section 2, to obtain a final well concentration in the range 1 × 105 ÷ 2.5 × 105 cfu/mL. Each well was filled with a total volume of 300 µL; samples, biotic and abiotic controls, together with controls prepared to check the possible influence of TEGO 1.5% (v/v) were set-up as indicated in Table 1. Filled 96-well plates, sealed with Parafilm®® and enveloped with PE transparent films to prevent EO losses, were incubated in static conditions, at the appropriate temperature, for 24 h. At the end of the incubation, the MIC was read as the lowest EO concentration that completely inhibits microbial growth by the naked eye. Moreover, to verify uncertain results, due to the turbidity and opacity of the EO suspensions, the viable count on agar plate was also carried out [23]. A total of 10 µL were withdrawn from each sample well and spread over agar plates; after 24 h of incubation, the absence/or presence of microbial colonies was check to identify the MIC, as the lowest EO concentration that inhibit the microbial growth on agar medium.

Table 1.
Composition and amounts, in µL, of the 96-well plates. “2X Medium” is the double concentrated ME broth; “Inoculum” refers to each single microorganism, S. epidermidis and R. mucilaginosa, 1 × 106 CFU/mL concentrated; “EO” refers to each single suspension of Thymus vulgaris and Origanum vulgaris, concentrated from 0.75% to 0.0059% (v/v), as indicated in the text.
The EO concentrations of 0.750%, 0.563%, and 0.375% (v/v) were tested with a “broth macrodilution method” in a dynamic system—in 10 mL sterile glass tubes, maintained in continuous agitation, on a rotator loopster, at 20 rpm, for 24 h. Each tube was filled with a total volume of 5.1 mL; sample, abiotic and biotic control samples were prepared maintaining the same proportions as indicated in Table 1. At the end of the incubation, a withdrawal of 10 µL from each tube was spread over agar plates and incubated for 24 h. After that, the absence/or presence of microbial colonies was checked to identify the MIC.
2.2. EO Application on Solid Media and on Inoculated Paper Sheets
Considering the higher antimicrobial activity of T. vulgaris (0.75% v/v), it was used for the following tests on microorganisms inoculated on agar plates or paper sheets. The suspension of 0.75% Thymus vulgaris EO, diluted with 1.5% TEGO SML20, was used to directly treat S. epidermidis and R. mucilaginosa, inoculated to obtain separated colonies, and A. alternata, nonsporulating mycelium, grown on solid media (for the description of the inocula refer to Section 2.1). The EO treatment was carried out immediately after the inoculum or after 24 h of incubation; untreated samples were used as controls. The EO suspension was vaporized as described in the Supplementary Material Section (Figure S1). The same EO treatment was also carried out on inoculated paper sheets after lyophilization or freeze thawing; the two paper sheets, contaminated either with S. epidermidis, R. mucilaginosa, A. alternata, or with the mixed inoculum, were removed from the paper block (see Section 2.4) and deposited on the appropriate agar medium. Moreover, in this case, the EO treatment was carried out immediately after the deposition in the Petri dish or after 24 h incubation, while untreated samples were used as controls. In order to prevent EO losses, all the Petri dishes were sealed with Parafilm®® and enveloped with PE transparent films; after that, they all were incubated at the selected temperature and microbial growth was monitored for different incubation times depending on the microorganism.
Regarding the unicellular microorganisms, the possible increase in colony number was detected by the naked eye. In the specific case of S. epidermidis, which is characterized by clear whitish colonies, not easily detectable, the replica plating of the paper sheet, inoculated and treated with the EO, was carried out after 72 h of incubation; after 1 h, the paper was removed and the agar plates were incubated. By this way, the bacterial colony detection resulted more accurately. The microbial growth was visually monitored every 24 h and for 5 days from the deposition of the contaminated paper sheets on the surface of the agar. Moreover, for A. alternata colonies, at the same time, two perpendicular diameters of the colony were measured until the stop of the mycelium growth, or when the colony reached the edge of the Petri dishes. The mean diameter values were then used to calculate the inhibition percentage of mycelial growth (MGI%) as reported in Equation (1):
where dc is the colony diameter in the control sheet and dt is the colony diameter in the treated sheet.
MGI (%) = [(dc − dt)/dc] × 100
2.3. EO Application on a Real Contaminated Book-Cover
The 0.75% (v/v) Thymus vulgaris EO suspension was also utilized to treat the contaminated cover of a real book. Before the EO treatment, the vitality of the microbial contaminants was checked by means of the replica plating of the colonies present on the cover. A sterile wooden stamp covered with sterile filter paper was applied, for 1 h, to the cover and then transferred to an MEA plate, hereafter incubated for 72 h at 30 °C. After that, pure colonies were isolated and treated with the EO treatment in three different ways: (a) the EO was directly nebulised on a contaminated area, delimited by means of a plastic mask (6 cm × 3 cm) (b) a paper sheet, impregnated with 0.75% EO, and was deposited on the contaminated cover for 2 h or for (c) 24 h. The treated book was placed in a box, completely sealed with PE transparent film, and incubated at 30 °C for 24 h; then, the EO treatments were repeated and the book incubated again for 72 h. The EO influence on the contaminant microorganisms was checked by the naked eye.
2.4. Simulation of Flooded Contaminated Books
In order to simulate real flooded contaminated books, the procedure described by Fissore et al. (2019) [13] was applied, with some modifications. Microbial inocula were carried out on single sterile paper squares (60 mm × 60 mm of modern blank paper double A, 80 g cm−2) deposited on the suitable agar medium. S. epidermidis and R. mucilaginosa were inoculated by means of replica plating of separated colonies, 1 ± 0.5 mm Ø, evenly grown on agar plates (to obtain separated colonies, 100 µL of a 1 × 105 microbial suspension was spread on solid medium and incubated for 24 h); paper sheets were then incubated for 48 h. A. alternata was inoculated depositing, in the middle of the sheet, a mycelial plug, 5 mm Ø, withdrawn from a seven-day-old colony; the incubation was prolonged for 48 or 120 h to obtain, respectively, sporulating and nonsporulating mycelium. Moreover, mixed inocula of the three microorganisms (ratio 1:1:1) were also prepared depositing, in the middle of the paper sheet, a suspension, 30 µL, of hyphal fragments and cells, having an optical density at 650 nm (OD650) of 0.8 ÷ 1. The mixed paper inoculum, deposited on MEA plates, where all the tested microorganisms showed an optimal growth, was incubated at 30 °C for 120 h.
Small blocks (60 mm × 60 mm, 10 ± 1 mm high) of modern paper were soaked in distilled water and then drained. After that, for each microorganism or for the mixed inoculum, two inoculated paper squares were respectively positioned on the surface (in the case of A. alternata inoculated sheets, the mycelial plug was removed before composing the paper block) and in the middle of a soaked paper block. Set-up blocks were opportunely packed to be frozen or lyophilized as reported by Fissore et al. (2019) [13]. Freeze drying was carried out in a lab-scale REVO freeze dryer (Millrock, Kingston, NY, USA) at 0 °C and 200 bar for 72 h, thus ensuring the total removal of frozen water. Freezing was carried out in a domestic freezer, whose temperature was set at (about) −20 °C. Although freezing could be carried out in the same equipment where the ice sublimation will take place (as it is generally done for pharmaceutical applications), in this part of the study, we aimed at mimicking the situation in which books are frozen immediately after flooding, and then drying is carried out. Additional tests were carried out considering the treatment of the paper material after a freeze-thawing test; this aimed at mimicking the situation where the soaked paper is frozen, to stop microbial deterioration, and then thawed to go on with traditional (hot) air drying. Moreover, in this case, freezing was carried out at −20 °C in a domestic apparatus.
3. Results
3.1. Antimicrobial Activity of EOs
Initially, the inhibition effect of T. vulgaris and O. vulgare EOs was evaluated on S. epidermidis and R. mucilaginosa. To this end, a microbroth dilution method in 96 well plates, in static condition, and a broth macrodilution method in test tubes, in dynamic conditions, were used. In the tests, carried out in static conditions, the concentration of both EOs was in the range 0.047–0.750% (v/v). The results are reported in Table S1 and shown in Figure 2.
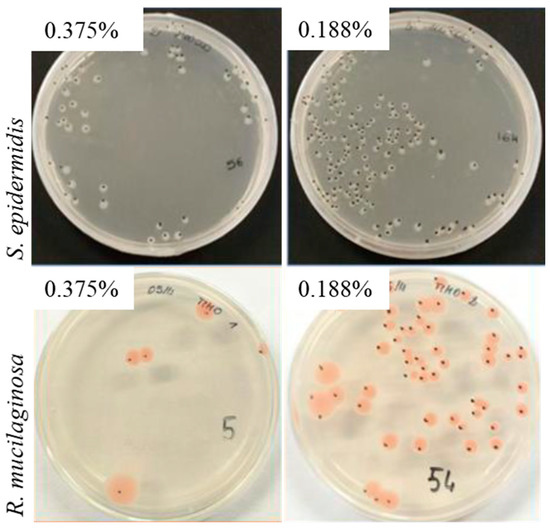
Figure 2.
CFU count plates of S. epidermidis and R. mucilaginosa both treated with 0.375% and 0.188% (v/v) Thymus vulgaris EO.
As an example, the S. epidermidis CFU number at 0.188% (8 × 105) was more than two orders of magnitude higher than that obtained at 0.75% (7 × 103); the corresponding CFU values for R. mucilaginosa were 6 × 104 and 2 × 103. Two different EOs concentration were evaluated in broth macrodilution tests—the highest already tested in microdilution (0.750%) and a lower one (0.563%). The MIC value of Thymus vulgaris EO obtained for S. epidermidis was 7.5 µg/mL; on the contrary, a lower MIC value (5.63 µg/mL) was obtained for R. mucilaginosa. Different from the results obtained in 96 multiwell plates, in broth microdilution tests, an MIC value of 7.5 µg/mL was obtained for R. mucilaginosa in the presence of O. vulgare EO; for S. epidermidis, a MIC value was not found.
3.2. Antimicrobial Activity of Thymus vulgaris EO on Agar Plates
All of the biodeteriogenic microorganisms were tested on agar media. S. epidermidis, R. mucilaginosa and A. alternata were cultured on NA, MEA and CZ, respectively. The same media were used when the microorganisms were inoculated on paper sheets. In all the cases, Thymus vulgaris EO (0.75%) was nebulised (10 sprays at a distance of 12 cm) immediately after inoculation t(0) and at 24 h of growth t(24). The effect of EO treatment was evaluated at 48 h of growth for bacterium, at 72 h for the yeast, and at 168 h for A. alternata. As it is possible to observe in Figure 3, the effect of EO on growth was particularly evident for the filamentous fungus, both treated at t(0) and t(24) (H and I), while for the bacterium, differences were not so evident (B and C). Finally, in the case of R. mucilaginosa, after 72 h of growth, colonies treated at t(24) were larger than those treated at t(0) with the same EO amount (F and E); it is possible to observe that, in both cases, colonies are not red-colored because carotenoids were not produced.
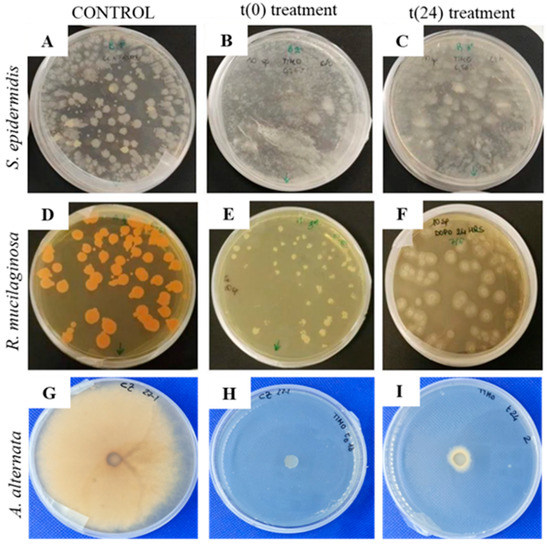
Figure 3.
S. epidermis, R. mucilaginosa and A. alternata grown on agar media: t(0) (labels B,E,H) and t(24) (labels C,F,I) treatments with Thymus vulgaris EO (0.75% v/v) compared to the untreated controls (24, 72 and 168 h for the bacterium, the yeast and the filamentous fungus, respectively) (labels A,D,G).
3.3. EO Application on a Contaminated Book
The Thymus vulgaris oil treatment was also applied on a real contaminated book. Firstly, the microorganisms, isolated from the book surface, by means of the replica plating technique (Figure 4) were treated with the T. vulgaris (0.75%) on agar media. Then, once the EO efficacy was verified, it was directly tested on the contaminated book cover by means of two different application methods: spraying and contact sheets (see Material and Methods section). In Figure 5, the images before and after treatment, at 72 h of incubation, are shown and compared. The direct contact between EO impregnated sheets (Figure 5, labels 5 and 6) and the contaminated surface allowed a total growth inhibition, regardless of the contact time.
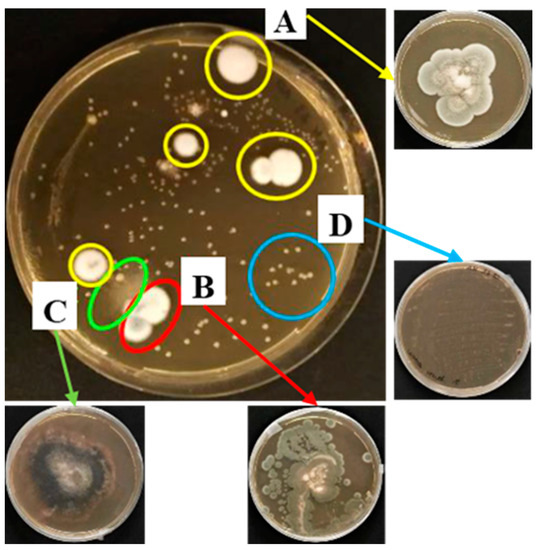
Figure 4.
Microorganism growth on agar medium after 72 h of incubation: the corresponding isolates (labels A–D) are indicated by the coloured arrows.
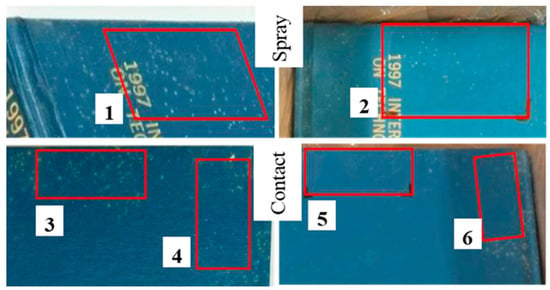
Figure 5.
Contaminated book-cover treated with Thymus vulgaris EO (0.75% v/v). Pre-treatment with EO (labels 1, 3, and 4), post-treatment with EO (labels 2, 5, and 6), 2-h contact time (labels 3 and 5), 24-h contact time (labels 4 and 6).
3.4. Antimicrobial Activity of EO on Freeze-Dried Paper Sheets
At the end of the lyophilization process, inoculated sheets were extracted from the paper block, deposited on agar plates and then treated, at t(0) and t(24), with Thymus vulgaris oil (0.75%) in the same conditions previously described (see Section 3.2). After five days of incubation, at the selected temperature for each microorganism, the microbial growth was evaluated.
R. mucilaginosa on paper sheets, incubated on MEA at 37 °C, is showed in Figure 6. The yeast growth was not influenced by the lyophilization process alone (Figure 6A), while the EO treatment (Figure 6B,C) inhibited the microbial growth and the synthesis of carotenoids.
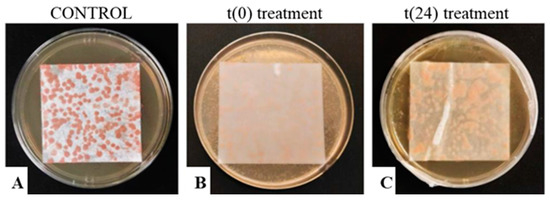
Figure 6.
R. mucilaginosa, grown on paper, treated with Thymus vulgaris EO (0.75% v/v) at t(0) (label B) and t(24) (label C) after the lyophilization process. The untreated control is also shown (label A).
Sheets contaminated with S. epidermidis, incubated on NA plates at 37 °C, are shown in Figure 7. Because the color of the S. epidermidis colonies was very similar to that of the paper, in order to observe the growth on the sheets, it was necessary to apply the replica-plating technique at 72 h. In addition, as already reported for R. mucilaginosa, the growth of S. epidermidis was not influenced by the lyophilization process; in this case, the effect of the EO treatment was lower than that observed for the yeast.
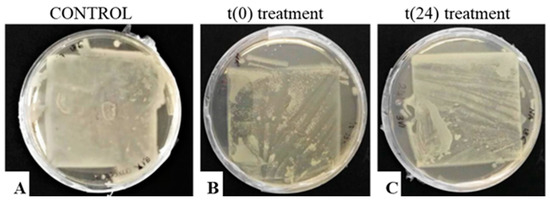
Figure 7.
“Replica-plating” of S. epidermidis colonies grown on lyophilized paper sheets: treatment with Thymus vulgaris oil (0.75% v/v) at t(0) (label B) and t(24) (label C). The untreated control is also shown (label A).
The treatment method, optimized on unicellular microorganisms, was also applied to A. alternata. After freeze drying, the contaminated sheets were deposited on CZ agar plates, treated with Thymus vulgaris oil (0.75% v/v) and incubated for two weeks at 30 °C. The mycelium, with or without sporulation, treated immediately after the drying, showed a total inhibition of growth during the evaluation period (Figure 8). As an example, in Figure 9, images related to A. alternata nonsporulated and sporulated mycelium was reported. EO treatment, carried out on both types of mycelia, after 24 h of incubation, inhibited the fungal growth for 168 h. After 192 h of incubation, the growth starts again with a rate always lower than that of the control.
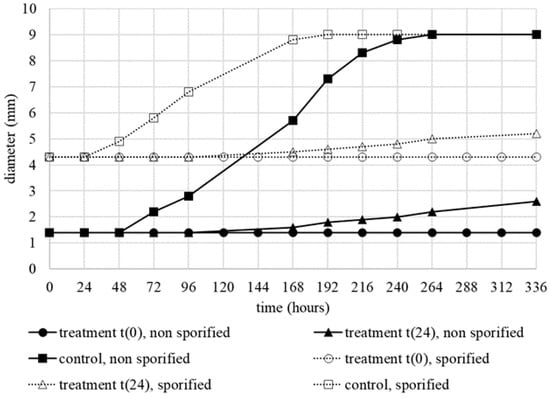
Figure 8.
Diameter of nonsporulated (continuous lines) and sporulated (dotted lines) of A. alternata mycelium after different EO treatments (t(0) and t(24)).
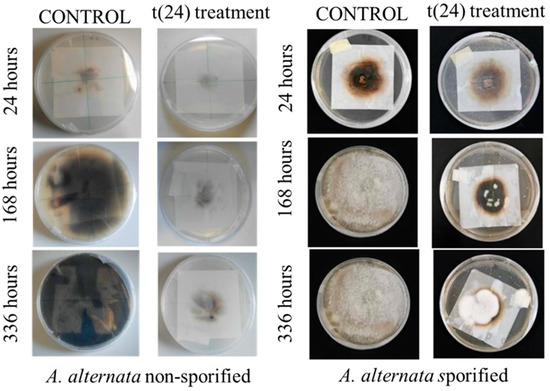
Figure 9.
Growth of A. alternata nonsporulated (left) and sporulated (right) mycelium: comparison between the control and the EO treated samples t(24).
In Table 2, main growth parameters of A. alternata, with mycelium sporified or not, have been reported and compared. Regarding the lyophilization effect, a double inhibition time of 48 h was observed for nonsporified mycelium with respect to that observed for the sporified one. The MGI maximum value was obtained when the essential oil was sprayed immediately after drying: 51.4% and 81.4% for A. alternata with mycelium sporulated or nonsporulated, respectively.

Table 2.
Comparison of the main growth parameters for nonsporified and sporified A. alternata after the lyophilization and the EO treatments.
3.5. Antimicrobial Activity of EO on Paper Sheets after Freezing and after Lyophilization
Paper sheets with mixed contamination (i.e., R. mucilaginosa, S. epidermidis and A. alternate inoculated together) were treated with T. vulgaris (0.75%). The frozen and thawed paper sheets and the lyophilized ones were deposited on MEA plates and incubated at 30 °C. After that, the microbial growth was evaluated for two weeks, as previously described. At the end of the incubation time, the samples frozen and thawed, immediately treated with EO, showed a total growth inhibition of all the incubated microorganisms (Figure 10C) with respect to the control (Figure 10A). Concerning the samples treated at 24 h, the inhibition was observed until 216 h after the treatment, when the mycelium growth started again at a low rate (Figure 10B).
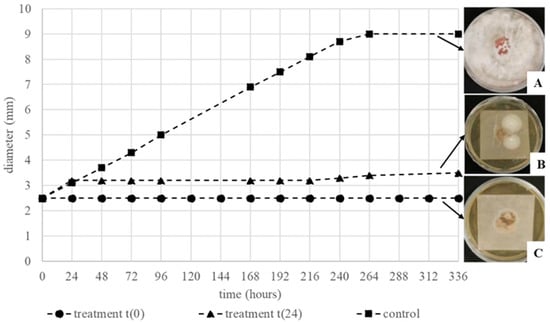
Figure 10.
Diameter of A. alternata mycelium (cm), in the mixed inoculum, after the freezing and the t(0) and t(24) treatments. The appearance of the different mixed inocula after 14 days of incubation are shown on the right of the graphic (A–C).
As far as the effect of Thymus vulgaris oil on mixed contaminated paper sheets, treated after the lyophilization process, it was possible to observe an inhibition effect in all the samples, independently from the time of the treatment (Figure 11B,C). Nevertheless, as previously reported for samples frozen and thawed, in this case, the effect was also higher when EO treatment was applied earlier. In fact, in the sheets treated at 24 h, after 264 h, a growing fungal colony was observed (Figure 11B). The behavior of A. alternata growth reported in Figure 11 shows that in this case it was also observed that the duration of the inhibition was lower as in frozen and thawed samples.
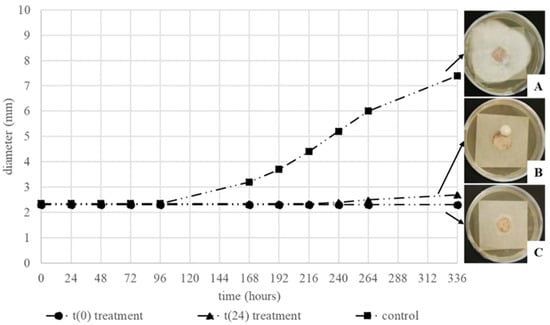
Figure 11.
Diameter of A. alternata mycelium (cm), in the mixed inoculum, after the lyophilization and the t(0) and t(24) treatments. The appearance of the different mixed inocula after 14 days of incubation are shown on the right of the graphic (A–C).
4. Discussion
In the preliminary test (microbroth test in static conditions), both O. vulgare and T. vulgaris EOs exhibited an inhibition effect on the unicellular microorganisms; a higher activity for T. vulgaris EO was observed. A MIC value was not obtained for O. vulgare, notwithstanding an observed lower growth rate. The MIC for T. vulgaris EO against S. epidermidis was 7.5 µg/mL, in accordance with values reported in the literature (see Table 3).

Table 3.
MIC values of different Thymus EOs against S. epidermidis and R. mucilaginosa, reported in the literature, compared to those found in the present work.
Results obtained in broth dilution method have been confirmed with tests carried out on agar plates, for which A. alternata was considered together with unicellular microorganisms, using 0.75% T. vulgaris. The inhibition on the growth of all the microorganisms was higher when the samples were treated at t(0) even if some inhibition effect were observed in the case of delayed treatments. As an example, in R. mucilaginosa, a depigmentation was observed (Figure 3), probably related to an influence of EO on the carotenoid synthesis pathway, as reported in a previous work for the fungicide naftifine [26]. The same behavior was observed for all the microorganisms cultured on paper sheets (data not shown); in this case, the antimicrobial activity was higher, probably in relation to the absorption of the EO on paper which guarantees a longer contact time with the microorganism.
Finally, when the selected EO was applied on a real contaminated book cover, the inhibition was lower in the area where the EO was sprayed with respect to that obtained by means of contact sheets. In the last case, the presence of the paper probably reduces the evaporation rate of the volatile components of the oil (see Section 3.3 and Figure 5). Therefore, when moving to a real case, a suitable dispersion system has to be designed to cope with this issue.
Concerning the EO treatment after flooding and lyophilization, it has to be pointed out that the lyophilization process did not control the growth of R. mucilaginosa, as previously reported by Fissore et al. (2019) [13]. Considering the effect of T. vulgaris EO (0.75%), when the contaminated sheets were treated immediately after lyophilization t(0), the microbial growth was lower than that observed for the sheets treated later t(24). Additionally, for S. epidermidis, the major inhibition effect also was observed when the contaminated sheets were treated immediately after the freeze-drying process (see Figure 7).
The not sporulated mycelium of A. alternata was inhibited by the lyophilization for a double time with respect to the sporulated one due to a higher spore resistance to thermal shock and to low aw values, as reported by Troiano et al. (2013) and Lucchese (2019) [12,27]. In the present work, the results reported by Salehi et al. (2018), Tullio et al. (2007), and Soylu et al. (2015) [24,28,29] on the efficacy of T. vulgaris oil on A. alternata, were confirmed.
Thymus vulgaris EO (0.75%) was also demonstrated to be effective on paper sheets with mixed contamination, after freezing and after freeze-drying. The EO was able to inhibit the microbial growth, independently from the treatment process, i.e., after freeze and thaw or after lyophilization. Comparing the results obtained for A. alternata growth, it was confirmed that lyophilization was more effective than the sole freezing in the control of microbial growth (see Figure 10 and Figure 11), as previously reported [13].
In conclusion, the treatment with T. vulgaris EO represents a real opportunity to control the growth of microorganisms involved in paper biodeterioration. With this in mind, the obtained results were exploited in the risk-based decision-making process for the safety of librarian heritage, adopting EOs as prevention measures but also for protection after accidental events, as discussed in [30].
5. Conclusions
The antimicrobial efficacy of Thymus vulgaris and Origanum vulgare oils on the paper-born microorganisms S. epidermidis, R. mucilaginosa and A. alternata was demonstrated in both liquid and solid cultures. T. vulgaris essential oil, at a concentration of 0.75%, was also able to inhibit the growth of the tested biodeteriogenic microorganisms, inoculated on flooded paper sheets, after a lyophilization treatment. In all the cases, independently from the contamination protocol (single or mixed inoculum), the antimicrobial effect was higher when the EO was applied earlier. Finally, the treatment with T. vulgaris oil was also effective on a real contaminated book. The new data collected will allow us to improve risk reduction assessment for librarian heritage. As a matter of fact, treatments with the EOs, coupled or not with a lyophilization process, will respectively allow us to preserve damaged books or to prevent/reduce the microbial contamination. Moreover, these treatments will allow us to simultaneously handle a large number of books or documents provided that a suitable concentration of EO is obtained in the environment where books and documents are stored. The results presented in this paper have to be intended as a proof of concept, and their scale-up to a “real” archive has yet to be addressed, and it is the topic of ongoing studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/heritage5030114/s1, Set-up of EO nebulization process; Figure S1: Diameter of the wet area as a function of the spraying distance (A); amount of nebulized EO as a function of the number of sprays (B). Table S1: CFU/mL values for S. epidermidis and R. mucilaginosa in the presence of Thymus vulgaris and Oregano vulgaris EOs.
Author Contributions
Conceptualization, D.F. and F.B.; methodology, D.F. and F.B.; formal analysis, C.M. and M.D.; investigation, C.M., F.B. and D.F.; data curation, M.D., F.B. and D.F.; writing—original draft preparation, F.B., D.F. and C.M.; writing—review and editing, F.B. and D.F.; supervision, M.D., F.B. and D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pasquarella, C.; Saccani, E.; Sansebastiano, G.E.; Ugolotti, M.; Pasquariello, G.; Albertini, R. Proposal for a biological environmental monitoring approach to be used in libraries and archives. Ann. Agric. Environ. Med. 2012, 19, 209–212. [Google Scholar] [PubMed]
- Skóra, J.; Gutarowska, B.; Pielech-Przybylska, K.; Stępień, L.; Pietrzak, K.; Piotrowska, M.; Pietrowski, P. Assessment of microbiological contamination in the work environments of museums, archives and libraries. Aerobiologia 2015, 31, 389–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadaifciler, D.G. Bioaerosol assessment in the library of Istanbul University and fungal flora associated with paper deterioration. Aerobiologia 2016, 33, 151–166. [Google Scholar] [CrossRef]
- Kalwasińska, A.; Burkowska, A.; Wilk, I. Microbial air contamination in indoor environment of a university library. Ann. Agric. Environ. Med. 2012, 19, 25–29. [Google Scholar] [PubMed]
- Montanari, M.; Melloni, V.; Pinzari, F.; Innocenti, G. Fungal biodeterioration of historical library materials stored in compactus movable shelves. Int. Biodet. Biodeg. 2012, 75, 83–88. [Google Scholar] [CrossRef]
- Michaelsen, A.; Pinar, G.; Pinzari, F. Molecular and microscopical investigation of the microflora inhabiting a deteriorated Italian manuscript dated from the thirteenth century. Microb. Ecol. 2010, 60, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Pinzari, F. Microbial ecology of indoor environments: The ecological and applied aspects of microbial contamination in archives, libraries and conservation environments. In Sick Building Syndrome, 1st ed.; Abdul-Wahab, S.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 1, pp. 153–178. [Google Scholar] [CrossRef]
- Gallo, F. Aerobiological research and problems in libraries. Aerobiologia 1993, 9, 117–130. [Google Scholar] [CrossRef]
- Borrego, S.; Guiamet, P.; Vivara, I.; Battistoni, P. Fungi involved in biodeterioration of documents in paper and effect on substrate. Acta Microsc. 2018, 27, 37–44. [Google Scholar]
- McCleary, J.P. Vacuum Freeze-Drying, a Method Used to Salvage Water-Damaged Archival and Library Materials: A RAMP Study with Guidelines; General Information Programme and UNISIST: Paris, France, 1987. [Google Scholar]
- Fissore, D.; Mussini, P.; Sassi, L.; Barresi, A. La liofilizzazione: Una tecnica efficace per il recupero di materiale archivistico a seguito di allagamento. Archivi 2017, 12, 28–46. [Google Scholar]
- Troiano, F.; Barbabietola, N.; Colaizzi, P.; Montanari, M.; Pinzari, F. La liofilizzazione quale intervento di recupero di volumi alluvionati ed attaccati da microfunghi. In Colore e Conservazione: Materiali e Metodi nel Restauro delle Opere Policrome Mobili; Lodi, C., Sburlino, C., Eds.; Il Prato: Parma, Italy, 2013. [Google Scholar]
- Fissore, D.; Lucchese, M.; Mollea, C.; Barresi, A.; Bosco, F. On the effect of freeze-drying on paper-borne microorganisms. In Proceedings of the 7th European Drying Conference, Turin, Italy, 10–12 July 2019; AXEA: Turin, Italy, 2019; pp. 303–309. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; d’Acierno, A.; Coppola, R.; Ayala-Zavala, F.J.; da Cruz, A.G.; De Feo, V. Essential Oils and Microbial Communication. In Essential Oils, 1st ed.; El-Shemy, H.A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Alonso, J.; Bernardos, A.; Regidor-Ros, J.L.; Martínez-Manez, R.; Bosch-Roig, P. Innovative use of essential oil cold diffusion system for improving air quality on indoor cultural heritage spaces. Int. Biodet. Biodeg. 2021, 162, 105–125. [Google Scholar] [CrossRef]
- Borrego, S.; Lavin, P.; Perdomo, I.; Gómez de Saravia, S.; Guiamet, P. Determination of Indoor Air Quality in Archives and Biodeterioration of the Documentary Heritage. Int. Schol. Res. Not. Vol. 2012, 2012, 680598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential Oils as Natural Biocides in Conservation of Cultural Heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef] [Green Version]
- Casiglia, S.; Bruno, M.; Scandolera, E.; Senatore, F.; Senatore, F. Influence of harvesting time on composition of the essential oil of Thymus capitatus (L.) Hoffmanns. & Link. growing wild in northern Sicily and its activity on microorganisms affecting historical art crafts. Arab. J. Chem. 2019, 12, 2704–2712. [Google Scholar] [CrossRef] [Green Version]
- Zyska, B. Fungi isolated from library materials: A review of the literature. Int. Biodet. Biodeg. 1997, 40, 43–51. [Google Scholar] [CrossRef]
- Karbowska-Berent, J.; Gorny, R.L.; Strzelczyk, A.B.; Wlazlo, A. Airborne and dust borne microorganisms in selected Polish libraries and archives. Build. Environ. 2011, 46, 1872–1879. [Google Scholar] [CrossRef]
- Eucast(A) Reading Guide for Broth Microdilution. 2022. Version 4.0. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2022_manuals/Reading_guide_BMD_v_4.0_2022.pdf (accessed on 10 January 2022).
- Eucast(B) Eucast Definitive Document E.DEF 7.3.2 Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts. 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf (accessed on 10 May 2022).
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.; Shukla, I.; Sharifi, M.; Contreras, M.M.; Carretero, A.S.; Fathi, H.; Nasrabadi, N.; Kobarfard, F.; Sharifi, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Ahmed, M.; Aissat, S.; Meslem, A.; Djebli, N. Preliminary Investigation on Antimycotic Synergism of Raw Honey and Essential Oil of Thyme (Thymus vulgaris L.). Med. Aromat. Plants 2014, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Moț, A.C.; Pârvu, M.; Pârvu, A.E.; Roşca-Casian, O.; Dina, N.E. Reversible naftifine-induced carotenoid depigmentation in Rhodotorula mucilaginosa (A. Jörg.) F.C. Harrison causing onychomycosis. Sci. Rep. 2017, 7, 11125. [Google Scholar] [CrossRef] [Green Version]
- Lucchese, M. Effetto Della Liofilizzazione Sul Biodeterioramento Della Carta Alluvionata. Master’s Thesis, Politecnico di Torino, Turino, Italy, 2019. [Google Scholar]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef]
- Soylu, E.M.; Kose, F. Antifungal activities of essential oils against citrus black rot disease agent Alternaria alternata. J. Essential Oil Bearing Plants 2015, 18, 894–903. [Google Scholar] [CrossRef]
- Bosco, F.; Demichela, M.; Barresi, A.A.; Fissore, D. Microbial contamination of libraries and archives: Risk assessment and contamination control. IOP Conf. Ser. Mater. Sci. Eng. 2020, 949, 012030. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).