Non-Invasive Raman Analysis of 18th Century Chinese Export/Armorial Overglazed Porcelain: Identification of the Different Enameling Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods
2.2. Artefacts
| Artefact | Period | Description | Dimension (cm) | Figure | Procedure | References |
|---|---|---|---|---|---|---|
| Dish | 1710–1730 | Dutch decorated | D = 21 | Figure 2e | Non-invasive | |
| Dish | 1700–1720 | Imari style | D = 22 | Figure 2f | Sampling | |
| Ewer | 1720–1725 | Duvelaer family coat-of-arms | H ~25 | Figure 2a | Non-invasive | |
| Dish | circa 1730 | Philibert Orry coat-of-arms | D = 25 | Figure 2g | Non-invasive | [48,59] |
| Dish | 1730–1735 | La Bistrate-and-Poli family coat-of-arms | D = 22 | Figure 2d | Non-invasive | [60,63] |
| Dish | circa 1737 | Duc de Penthièvre coat-of-arms | D = 22.5 | Figure 2h | Non-invasive | [62] |
| Tureen | circa 1738 | Louis XV table set with the coat-of-arms of kings of France | Figure 2c | Non-invasive | [42,43,44,45,46,47,61] | |
| Coffee pot | 1730–1740 | Pronk’ design | H = 34 | Figure 1 and Figure 2b | Non-invasive | [58,65,66] |
| Plate (LA) | 1740–1750 | Mountainous river landscape decor (Famille rose) | D = 22 | Figure A1c and Figure 3c’ | Sampling | [26] |
| Plate (PEO) | circa 1760 | Citrus and peony pattern (Famille rose) | D = 23 | Figure A1 | Sampling | |
| Plate (Rose) | circa 1770 | Rose pattern (Famille rose) | D = 23 | Figure A1e | Sampling | |
| Cup saucer (BW f) | circa 1780 | Floral pattern (Blue-and-white) | D = 12 | Figure A1a | Sampling | [55] |
| Plate (BW ww) | circa 1780 | Weeping willow pattern (Blue-and-white) | D = 23 | Figure A1b | Sampling |
- A dish of rounded form made in China and later enameled in Holland assigned from the end of Kangxi or Yongzheng reign, with a Kakiemon-style design of two quails pecking at millet beneath flowering stems, a grasshopper perched above, the rim with four panels of quail within a cracked ice and prunus ground in a Famille verte palette (Figure 2e). This unusual but well-executed design once again demonstrates the confusion that Dutch decorators held over the source of oriental decoration, combining elements of Japanese and Chinese designs on one plate to produce an attractive, but distinctly European, effect;
- A Chinese ‘Imari’ plate, Kangxi period (1662–1722), ca. 1700/1720 (Figure 2f). Decorated in the Imari-style as original Japanese porcelain made at kilns at/near Arita, (Hizen province), with Buddhist wheel, scrolling lotus, chrysanthemum and peony sprays with their foliage;
- A rare Chinese ewer (Figure 2a), Kangxi period, probably ca. 1720/1725. Modeled after a European silver shape with an incurving bracket handle, spreading foot and molded human mask with a beard under the spout, enameled around the body camellia branch above molded elongated petals reserved on a seeded-green ground, the foot with similar decoration. The body is decorated with the coat-of-arms of the Duvelaer family. Members of this family lived in Holland, Austrian Low-Countries and France during the 18th century. The shape of a stick ewer was in vogue in France between 1700 and 1725. Afterwards, it was out of fashion (so it is almost impossible that it had been ordered after that date);
- A Chinese armorial dish for the French market (Figure 2g), Yongzheng period (1723-1735). With the coat-of-arms of Orry family, de gueule à un lion rampant et grimpant sur un rocher d’argent, in colors and gilt with a rocaille surrounded by a vividly enameled foliate border, the rim scalloped. Orry was named Contrôleur général des finances (Royal Finance Minister) in 1730 and combined this function with being the general director of the Bâtiments du roi (The royal buildings office) in 1736, after the death of the Duc d’Antin. Orry remained Contrôleur général until 1745, making him the longest continuously serving holder of the office in the 18th century.
- A Chinese export Famille rose and silvered armorial plate (Figure 2d) for the Belgian market (la Bistrate of Anvers) [60,63], Yongzheng period, i.e., before 1735. The center enameled and silvered with the arms of la Bistrate accollée with those of Proli (his wife’ Family) between greyhound supporters and below, a helmet, a coronet and a coat-of-arms, surrounded by a trellis-pattern in the well, the arms repeated individually around the everted rim between a du Paquier style design of linked feathers, strapwork, shells and hanging drapery. This service was made for Jean-Charles de la Bistrate (baptized in Anvers in 1715), seigneur de Loer and Neerwinde and chief almoner of Anvers who married Anne-Martine Proli (born in 1711), also of Anvers on October 7, 1736. Her father, Pierre Proli, was one of the seven directors of the Compagnie d’Ostende. Objects bearing the same coats-of-arms are also known [60,61,62,63]. La Bistrate’s wedding took place in October 1736. Stylistically, we would wish to assign it earlier, around 1730, but this is almost impossible since the marriage of the future spouse could not have been planned six years in advance. The order must have been made around 1734/1735, to allow for the time that the order reached China and then the service traveled from China to Europe;
- A Chinese armorial dish for the French market, Yongzheng period, ca. 1730 [62]. Decorated in the Famille verte palette, with the coat-of-arms of Penthièvre. The order depicted with the coat-of-arms is Knight of the Golden Fleece and the Holy Spirit as well as the Anchor of Admiral of the Fleet. The service was order for Louis-Jean-Marie de Bourbon, Duc de Penthièvre, de Chateuvillain, de Rambouillet, (1725–1793), his father, the Comte de Toulouse, son of King Louis XIV [62]. Stylistically, this service is early; the wing is in the style of the Famille verte, the decoration employing this red color is so particular that we date today around 1730, although the marine anchors give a broad dating of the order;
- A Royal armorial tureen for the French market, Qianlong period (1736–1795), ca. 1737–1740 (Figure 2c). Decorated with the royal coat-of-arms of France, the reserve and sides below the underglaze-blue shell-form handles with a Buddha’s hand citron or a gourd in a dish on a lotus blossom, and pads or branches, the rim with a floral-scroll border repeated around the chrysanthemum-decorated know three gilt fleurs-de-lus alternating with floral roundels. This service was delivered at Versailles, for the winter dining room of King Louis XV of France [42,43,44,45,46,61] in 1740 through the French East India Company for the Palace of Versailles;
- A rare Pronk’ design coffee pot and cover (Figure 1 and Figure 2b), Qianlong period, ca. 1740. Painted with a wide band of beetles, butterflies and moths above black-ground flowering vine, all between a mint-green scale pattern, the black-ground with gilt tassel or bell-flower motifs. Cornelis Pronk was a Dutch draughtsman [64,65,66], who was commissioned by the directors of the Dutch East India Company from circa 1734–1738 to produce designs to be transferred onto Chinese porcelain. Compared to the other known pieces [58], the porcelain handle has been replaced by a silver handle;
- Four plates, a cup and saucer were analyzed on their surfaces (non-invasively) and on sections prepared by diamond saw cutting: two blue-and-white porcelains, Qianlong period, circa 1780, one depicting a floral décor (Appendix A, Figure A1a), the second a weeping willow décor (Appendix A, Figure A1), and three Famille rose porcelains, depicting a ‘mountainous river landscape’ (Appendix A, Figure A1c,c’), circa 1740–1750, citrus and peony (Appendix A, Figure A1d), circa 1760, and a rose (Appendix A, Figure A1e), circa 1770, respectively.
3. Results
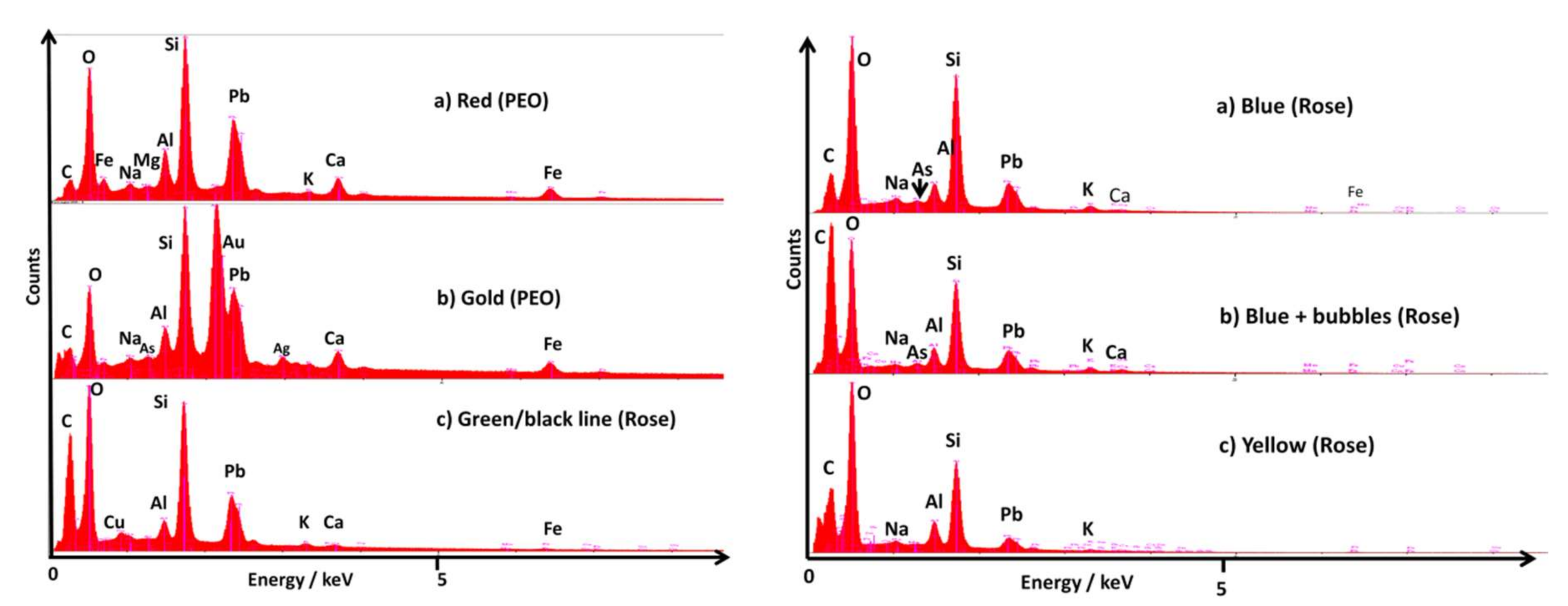
3.1. Compositions
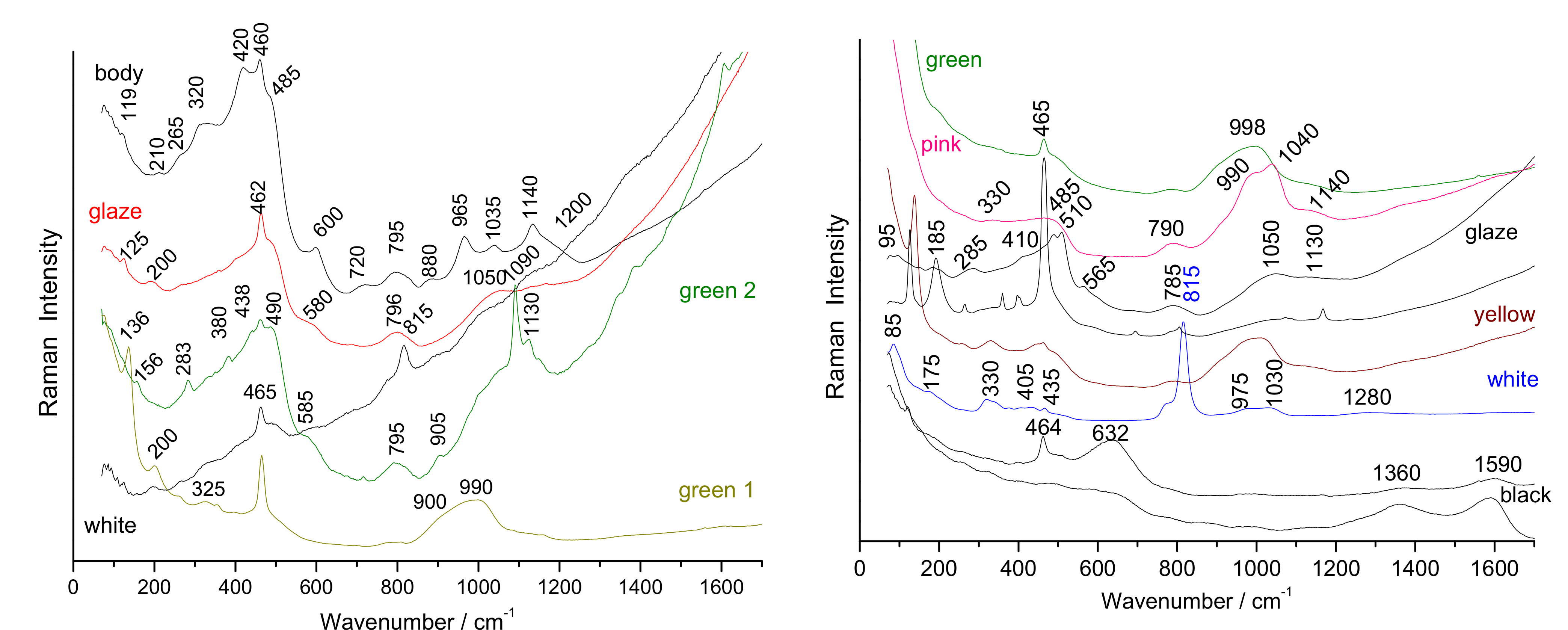
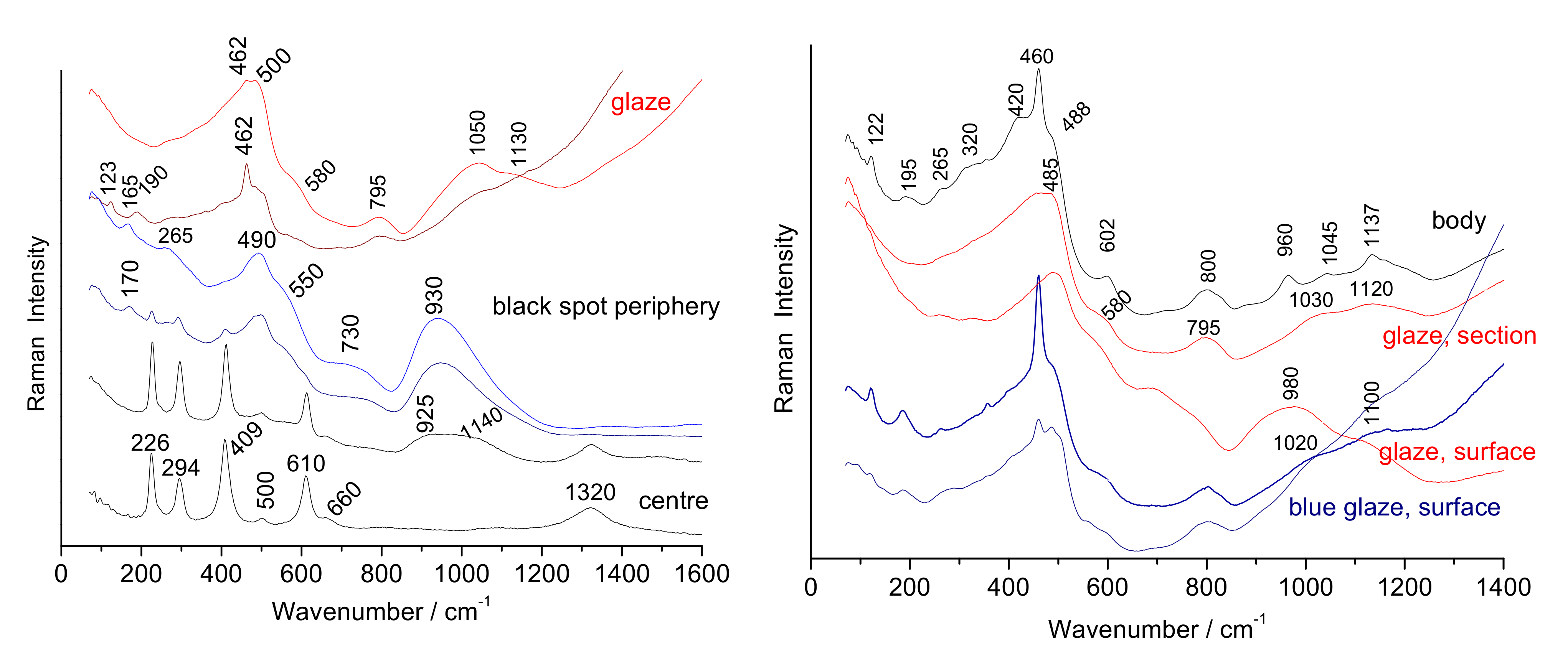
3.2. Characteristic Raman Signatures
3.2.1. Body
3.2.2. Glaze and Overglazes
3.3. Non-Invasive Analysis of Rare Objects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

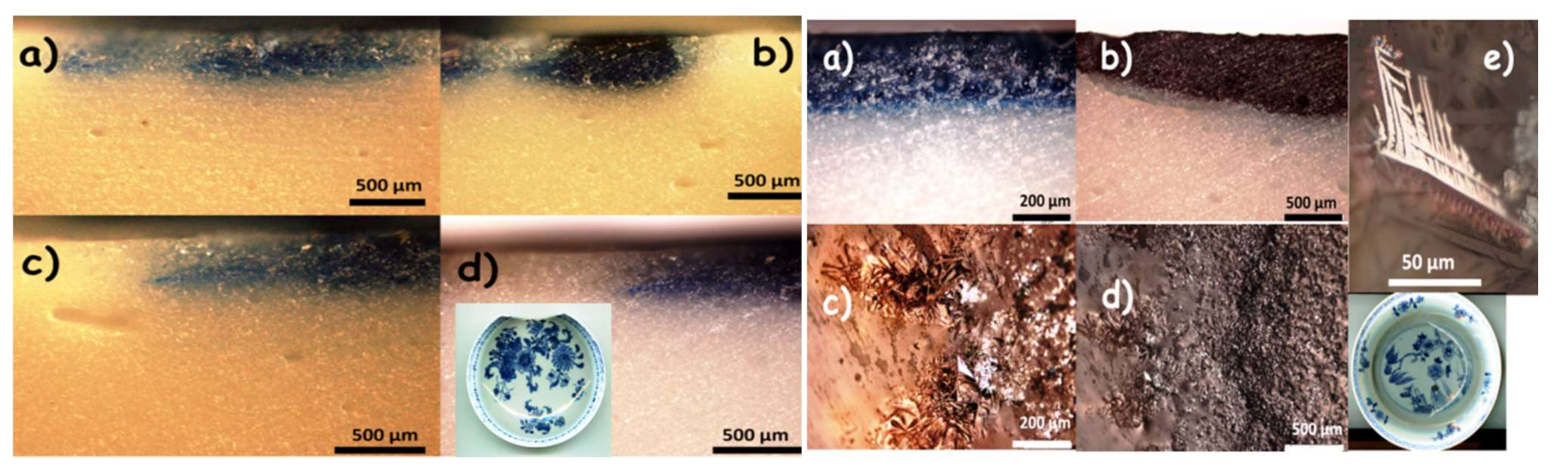
References
- d’Entrecolle, F.-X. Letters from Ching-Te-Chen in 1712 and 1722; New York State Institute for Glaze Research: Painted Post, NY, USA, 1983. [Google Scholar]
- Vogt, G. La porcelaine Chinoise. Bull. Soc. d’Encouragement pour l’Industrie Nationale 1900, 99, 530–612. [Google Scholar]
- Levy, E. Le Goût Chinois en Europe au XVIIIe Siècle: Catalogue du Musée des Arts Décoratifs, June–October 1910, Librairie Centrale des Beaux-Arts Paris 1910. Available online: https://gallica.bnf.fr/ark:/12148/bpt6k441547m/f1.texteImage (accessed on 28 November 2021).
- Lion-Goldschmidt, D. Les porcelaines chinoises du palais de Santos. Arts Asiatiques 1984, 39, 5–72. Available online: https://www.persee.fr/doc/arasi_0004-3958_1984_num_39_1_1616 (accessed on 28 November 2021). [CrossRef]
- Ayers, J.; Impey, O.; Mallet, J.V.G. Porcelain for Palaces: The Fashion for Japan in Europe 1650–1750; Oriental Ceramic Society: London, UK, 1990. [Google Scholar]
- Impey, O. Collecting Oriental Porcelain in Britain in the Seventeenth and Eighteenth Centuries. In The Burghley Porcelains, an Exhibition from the Burghley House Collection and Based on the 1688 Inventory and 1690 Devonshire Schedule; Japan Society: New York, NY, USA; Tokyo, Japan, 1990; pp. 36–43. [Google Scholar]
- Alayrac-Fielding, V. From the curious to the “artinatural”: The meaning of oriental porcelain in 17th and 18th-century English interiors. Miranda 2012, 7, 1–21. [Google Scholar] [CrossRef]
- Castelluccio, S. Le Gout pour les Porcelaines de Chine et du Japon à Paris aux XVIIe et XVIIIe Siècles; Monelle Hayot Editions: Saint-Rémy-en-l’Eau, France, 2013. [Google Scholar]
- van Campen, J.; Eliëns, T.M. Chinese and Japanese Porcelain for the Dutch Golden Age; Zwolle: Waanders Uitgevers, The Netherlands, 2014. [Google Scholar]
- Faÿ-Hallé, A.; Lahaussois, C. La Porcelaine Française au XVIIIe Siècle, Histoire, Motifs et Marques; Les Essentiels du Patrimoine-Massin: Paris, France, 2011. [Google Scholar]
- de Plinval de Guillebon, R. L’Eveil de la porcelaine à Paris. Rev. Soc. Amis Musée National de Céramique. 2005, pp. 55–68. Available online: http://www.amisdesevres.com/wp-content/uploads/2011/01/revue_19_55-68.pdf (accessed on 28 November 2021).
- Edwards, H.G.M. Porcelain Analysis and Its Role in the Forensic Attribution of Ceramic Specimens; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Shih, C.F. Evidence of East-West exchange in the eighteenth century: The establishment of painted enamel art at the Qing Court in the reign of Emperor Kangxi. Natl. Palace Mus. Res. Q. 2007, 24, 45–94. [Google Scholar]
- Zhou, S.Z. Research on Painted Enamels Porcelain Ware from the Qing Court; Wenwu Chubanshe: Beijing, China, 2008. [Google Scholar]
- Curtis, E. Glass Exchange between Europe and China, 1550–1800; Ashgate: Farnham, UK, 2009. [Google Scholar]
- Lili, F. La Céramique Chinoise; China Intercontinental Press: Beijing, China, 2011. [Google Scholar]
- Shih, C.F. Radiant Luminance: The Painted Enamelware of the Qing Imperial Court; The National Palace Museum of Taipei: Taipei, Taiwan, 2012.
- Xu, X.D. Europe-China-Europe: The Transmission of the Craft of Painted Enamel in the Seventeenth and Eighteenth Centuries. In Goods from the East, 1600–1800 Trading Eurasia; Berg, M., Ed.; Palgrave Macmillan: London, UK, 2015; pp. 92–106. [Google Scholar]
- Zhang, F. The origin and development of traditional Chinese glazes and decorative ceramic colors. In Technology to Modern Science; Kingery, W.D., Ed.; Ceramics and Civilization Series; The American Ceramic Society: Colombus, OH, USA, 1985; Volume 1, pp. 163–180. [Google Scholar]
- Kingery, W.D.; Vandiver, P.B. The Eighteenth-Century Change in Technology and Style from the Famille-Verte Palette to the Famille-Rose Palette. In Technology and Style; Kingery, W.D., Ed.; Ceramics and Civilization Series; The American Ceramic Society: Colombus, OH, USA, 1986; Volume 2, pp. 363–381. [Google Scholar]
- Wood, N. Chinese Glazes: Their Origins, Chemistry and Recreation; A & C Black: London, UK, 1999. [Google Scholar]
- Kırmızı, B.; Colomban, P.H.; Quette, B. On-site analysis of Chinese Cloisonné enamels from fifteenth to nineteenth centuries. J. Raman Spectrosc. 2010, 41, 780–790. [Google Scholar]
- Van Pevenage, J.; Lauwers, D.; Herremans, D.; Verhaeven, E.; Vekemans, B.; De Clercq, W.; Vincze, L.; Moens, L.; Vandenabeele, P. A Combined Spectroscopic Study on Chinese Porcelain Containing Ruan-Cai Colours. Anal. Methods 2014, 6, 387–394. [Google Scholar] [CrossRef]
- Colomban, P.; Arberet, L.; Kırmızı, B. On-Site Raman Analysis of 17th and 18th Century Limoges Enamels: Implications on the European Cobalt Sources and the Technological Relationship between Limoges and Chinese Enamels. Ceram. Int. 2017, 43, 10158–10165. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Zhang, Y.; Zhao, B. Non-invasive Raman analyses of huafalang and related porcelain wares. Searching for evidence for innovative pigment technologies. Ceram. Int. 2017, 43, 12079–12088. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Ambrosi, F.; Ngo, A.-T.; Lu, T.-A.; Feng, X.-L.; Chen, S.; Choi, C.-L. Comparative analysis of wucai Chinese porcelains using mobile and fixed Raman microspectrometers. Ceram. Int. 2017, 43, 14244–14256. [Google Scholar] [CrossRef] [Green Version]
- Giannini, R.; Freestone, I.; Shortland, A. European cobalt sources identified in the production of Chinese Famille rose porcelain. J. Archaeol. Sci. 2017, 80, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Lu, T.-A.; Milande, V. Non-invasive on-site Raman study of blue-decorated early soft-paste porcelain: The use of arsenic-rich (European) cobalt ores—Comparison with huafalang Chinese porcelains. Ceram. Int. 2018, 44, 9018–9026. [Google Scholar] [CrossRef]
- Montanari, R.; Alberghina, M.F.; Casanova Municchia, A.; Massa, E.; Pelagotti, A.; Pelosi, C.; Schiavone, S.; Sodo, A. A polychrome Mukozuke (1624–1644) porcelain offers a new hypothesis on the introduction of European enameling technology in Japan. J. Cult. Herit. 2018, 32, 232–237. [Google Scholar] [CrossRef]
- Montanari, R.; Murakami, N.; Alberghina, M.F.; Pelosi, C.; Schiavone, S. The Origin of overglaze-blue enameling in Japan: New discoveries and a reassessment. J. Cult. Herit. 2019, 37, 94–102. [Google Scholar] [CrossRef]
- Montanari, R.; Murakami, N.; Colomban, P.; Alberghina, M.F.; Pelosi, C.; Schiavone, S. European Ceramic technology in the Far East: Enamels and pigments in Japanese art from the 16th to the 20th century and their reverse influence on China. Herit. Sci. 2020, 8, 48. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B.; Zhao, B.; Clais, J.-B.; Yang, Y.; Droguet, V. Non-invasive on-site Raman study of pigments and glassy matrix of the 17th–18th century painted enamelled Chinese metal wares: Comparison with French enamelling technology. Coatings 2020, 10, 471. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B. Non-invasive on-site Raman study of polychrome and white enamelled glass artefacts in imitation of porcelain assigned to Bernard Perrot and his followers. J. Raman Spectrosc. 2020, 51, 133–146. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B.; Gougeon, C.; Gironda, M.; Cardinal, C. Pigments and glassy matrix of the 17th–18th century enamelled French watches: A non-invasive on-site Raman and pXRF study. J. Cult. Herit. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Colomban, P.; Gironda, M.; Vangu, D.; Kırmızı, B.; Zhao, B.; Cochet, V. The technology transfer from Europe to China in the 17th–18th centuries: Non-invasive on-site XRF and Raman analyses of Chinese Qing Dynasty enameled masterpieces made using European ingredients/recipes. Materials 2021, 14, 7434. [Google Scholar] [CrossRef]
- Howard, D. Chinese Armorial Porcelain; Faber & Faber: London, UK, 1952; Volume 1. [Google Scholar]
- Jourdain, M.; Soame, J. Chinese Export Art in the 18th Century; Spring Books: Feltham, UK, 1967. [Google Scholar]
- Le Corbeiller, C. China trade armorial porcelain in America. Antiques 1977, 112, 1124–1129. [Google Scholar]
- Godden, G.A. Oriental Export Market Porcelain and Its Influence on European Wares; Grenada: London, UK; New York, NY, USA, 1979. [Google Scholar]
- Le Corbeiller, C.; Frelinghuysen, A.C. Chinese Export Porcelain. Metrop. Mus. Art Bull. 2003, 60, 1–60. [Google Scholar] [CrossRef]
- Howard, D.S. Chinese Armorial Porcelain; Heirlomm & Howard: London, UK, 2003; Volume 2, p. 155. [Google Scholar]
- Lebel, A. Armoiries Françaises et Suisses sur la Porcelaine de Chine au XVIIIe Siècle; Antoine Lebel: Bruxelles, Belgium, 2009. [Google Scholar]
- Castellucio, S. Le Roi et la Compagnie Française des Indes Orientales, in La Chine à Versailles, Art et Diplomatie au XVIIIe Siècle; De Rocherunne, M.L., Ed.; Somogy Editions d’Arts: Paris, France, 2014; pp. 94–119. [Google Scholar]
- Un Cabinet Dédié aux Porcelaines. Available online: https://www.chateauversailles.fr/actualites/vie-domaine/cabinet-porcelaines#les-collections-presentees (accessed on 12 November 2021).
- Sothebys. Available online: https://www.sothebys.com/en/auctions/ecatalogue/2008/the-collection-of-khalil-rizk-n08411/lot.4.html?locale=en (accessed on 12 November 2021).
- Christies. A Chinese Export French Royal Ecuelle and Cover. Available online: https://www.christies.com/lot/lot-5648195 (accessed on 12 November 2021).
- Christies. A Set of Three Graduated French Royal Armorial Ecuelles and Covers. Available online: https://www.christies.com/lot/lot-a-set-of-three-graduated-french-royal-6187630/?from=salesummary&intObjectID=6187630&lid=1 (accessed on 12 November 2021).
- Christies. A Set of French Market Armorial Dishes. Available online: https://www.christies.com/en/lot/lot-6187628 (accessed on 12 November 2021).
- Gotheborg Armorial Porcelain. Available online: https://gotheborg.com/glossary/armorial.shtml (accessed on 28 November 2021).
- Colomban, P.; Treppoz, F. Identification and differentiation of ancient and modern European porcelains by Raman macro- and micro-spectroscopy. J. Raman Spectrosc. 2001, 32, 93–102. [Google Scholar] [CrossRef]
- Colomban, P.; Sagon, G.; Faurel, X. Differentiation of antique ceramics from the Raman spectra of their coloured glazes and paintings. J. Raman Spectrosc. 2001, 32, 351–360. [Google Scholar] [CrossRef]
- Colomban, P.; Robert, I.; Roche, C.; Sagon, G.; Milande, V. Identification des porcelaines “tendres” du 18ème siècle par spectroscopie Raman: Saint-Cloud, Chantilly, Mennecy et Vincennes/Sèvres. Rev. d’Archéomètrie 2004, 28, 153–167. Available online: https://www.persee.fr/doc/arsci_0399-1237_2004_num_28_1_1070 (accessed on 20 December 2021).
- Colomban, P.; Milande, V.; Le Bihan, L. On-site Raman analysis of Iznik pottery glazes and pigments. J. Raman Spectrosc. 2004, 35, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Milande, V. On-site Raman analysis of the earliest known Meissen porcelain and stoneware. J. Raman Spectrosc. 2006, 37, 606–613. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Ngo, A.-T.; Edwards, H.G.M.; Prinsloo, L.C.; Esterhuizen, L.V. Raman identification of the different glazing technologies of Blue-and-White Ming porcelains. Ceram. Int. 2021, 48, 1673–1681. [Google Scholar] [CrossRef]
- Colomban, P.; Edwards, H.G.M.; Fountain, C. Raman spectroscopic and SEM/EDXS analyses of high translucent Nantgarw porcelain. J. Eur. Ceram. Soc. 2020, 40, 4664–4675. [Google Scholar] [CrossRef]
- Neri, E.; Morvan, C.; Colomban, P.; Guerra, M.F. Late Roman and Byzantine mosaic opaque ‘glass-ceramics’ tessarae (5th–9th century). Ceram. Int. 2016, 42, 18859–18869. [Google Scholar] [CrossRef] [Green Version]
- Christies. A Very Rare ‘Pronk’ Coffee-Pot and Cover. Available online: https://www.christies.com/en/lot/lot-5171857 (accessed on 12 November 2021).
- Galerie Nicolas Fournery. Paire de Petits Plats Aux Armes de Philibert Orry, Contrôleur Général des Finances, en Porcelaine de Chine D’époque Yongzheng. Available online: https://www.galerienicolasfournery.fr/collection/paire-de-petits-plats-aux-armes-de-philibert-orry-de-vignory-controleur-general-des-finances-en-porcelaine-de-chine-depoque-yongzheng/ (accessed on 12 November 2021).
- lot-5033522. Available online: https://www.christies.com/en/lot/lot-5033522 (accessed on 12 November 2021).
- Musee Lorient. Available online: https://musee.lorient.bzh/collections/une-semaineune-oeuvre/bourdaloue/ (accessed on 12 November 2021).
- Noblesse & Royautés. Vente d’un Plat de Service du Duc de Penthièvre. Available online: http://www.noblesseetroyautes.com/vente-dun-plat-de-service-du-duc-de-penthievre/ (accessed on 12 November 2021).
- Galerie Nicolas Fournery. Assiette à Décor Armorié pour le Marché Belge (Bistrate d’Anvers) en Porcelaine de Chine D’époque Yongzheng. Available online: https://www.galerienicolasfournery.fr/collection/coupe-a-decor-armorie-pour-le-marche-hollandais-bistrate-danvers-en-porcelaine-de-chine-depoque-yongzheng/ (accessed on 12 November 2021).
- Wikipedia. Cornelis Pronk. Available online: https://fr.wikipedia.org/wiki/Cornelis_Pronk (accessed on 12 November 2021).
- Jörg, C.J.A. Pronk Porcelain: Porcelain after Designs by Cornelis Pronk; Groninger Museum: La Haye, The Netherlands, 1980. [Google Scholar]
- Jörg, C.J.A. Chinese Export Porcelain. Chine de Commande from the Royal Museum of Art and History in Brussels; Urban Council: Hong Kong, China, 1989. [Google Scholar]
- Lunsingh Scheurleer, D.F. Chinese Export Porcelain-Chine de Commande; Pitman Publishing Corporation: New York, NY, USA, 1974. [Google Scholar]
- Howard, D.; Ayers, J. China for The West Chinese Porcelain and Other Decorative Arts for Export Illustrated from the Mottahedeh Collection; Sotheby Parke Benet: London, UK, 1978. [Google Scholar]
- Kroes, J. Chinese Armorial Porcelain for the Dutch Market; Waanders BV: Uitgeverij, The Netherlands, 2007. [Google Scholar]
- Krahl, R.; Harrison-Hall, J. Chinese Ceramics—Highlights of the Sir Percival David Collection; British Museum Press: London, UK, 2009. [Google Scholar]
- Colomban, P.; Calligaro, T.; Vibert-Guigue, C.; Nguyen, Q.L.; Edwards, H.G.M. Dorures des céramiques et tesselles anciennes: Technologies et accrochage. ArchéoSciences 2005, 29, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Maggetti, M.; d’Albis, A. Non-invasive Raman identification of crystalline and glassy phases in a 1781 Sèvres Royal Factory soft paste porcelain plate. J. Eur. Ceram. Soc. 2018, 38, 5228–5233. [Google Scholar] [CrossRef]
- Michel, D.; Colomban, P.; Abolhassani, S.; Voyron, F.; Kahn-Harari, A. Germanium mullite: Structure and vibrational spectra of gels, glasses and ceramics. J. Eur. Ceram. Soc. 1996, 16, 161–168. [Google Scholar] [CrossRef]
- Colomban, P.; Paulsen, O. Non-destructive Raman Determination of the Structure and Composition of Glazes by Raman Spectroscopy. J. Am. Ceram. Soc. 2005, 88, 390–395. [Google Scholar] [CrossRef]
- Colomban, P. Polymerization degree and Raman identification of ancient glasses used for jewellery, ceramic enamels and mosaics. J. Non-Crystall. Solids 2003, 323, 180–187. [Google Scholar] [CrossRef]
- Colomban, P.; Tournié, A.; Bellot-Gurlet, L. Raman identification of glassy silicates used in ceramic, glass and jewellery: A tentative differentiation guide. J. Raman Spectrosc. 2006, 37, 841–852. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P. Non-destructive Raman analysis of ancient glasses and glazes. In Modern Methods for Analysing Archaeological and Historical Glass, 1st ed.; Janssens, K., Ed.; John Wiley & Sons: London, UK, 2012; pp. 275–300. [Google Scholar]
- Labet, V.; Colomban, P. Vibrational properties of silicates: A cluster model able to reproduce the effect of “SiO4” polymerization on Raman intensities. J. Non-Crystall. Solids 2013, 370, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P. Glaze and enamels. In Encyclopedia of Glass Science, Technology, History and Culture, 1st ed.; Richet, P., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1309–1326. [Google Scholar]
- Maggetti, M.; d’Albis, A. Phase and compositional analysis of a Sèvres soft paste porcelain platefrom 1781, with a review of early porcelain techniques. Eur. J. Min. 2017, 29, 347–367. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.K.; Torell, L.M.; Börjesson, L.; Doweidar, H. Structural changes of Be2O3 through the liquid-glass transition range: A Raman-scattering study. Phys. Rev. B 1992, 45, 12797. [Google Scholar] [CrossRef]
- Ciceo-Lucatel, R.; Ardelean, R. FT-IR and Raman study of silver lead borate-based glasses. J. Non-Crystall. Solids 2007, 353, 2020–2024. [Google Scholar] [CrossRef]
- Satyanarayana, T.; Kityk, I.V.; Piasecki, M.; Bragiel, P.; Brik, M.G.; Gandhi, Y.; Veeraiah, N. Structural investigations on PbO–Sb2O3–B2O3:CoO glass ceramics by means of spectroscopic and dielectric studies. J. Phys. Condens. Matter 2009, 21, 245104. [Google Scholar] [CrossRef]
- Moshkina, E.; Gudim, I.; Temerov, V.; Krylov. Temperature-dependent absorption lines observation in Raman spectra of SmFe3(BO3)4 ferroborate. J. Raman Spectrosc. 2018, 49, 1732–1735. [Google Scholar] [CrossRef]
- Préaud, T.; d’Albis, J. La Porcelaine de Vincennes; AdamBiro: Paris, France, 1991. [Google Scholar]
- Bourgeois, E. Les Archives de la Manufacture de Sèvres 1741–1905. Unpuplished Report; Manufacture Nationale: Sèvres, France, 1905; 37p. [Google Scholar]
- Doru, T. Chimie Appliquée et Technologie Chimique en France au Milieu du XVIIIe Siècle. Oeuvre et Vie de Jean Hellot. Ph.D. Thesis, Ecole Pratique des Hautes Etudes, Université de Paris-Sorbonne, Paris, France, 1977. [Google Scholar]
- Wisniak, J. Jean Hellot. A pioneer of chemical technology. Revista CENIC Ciencias Quimicas 2009, 40, 111–121. [Google Scholar]
- Brongniart, A. Sur les couleurs obtenues des oxides métalliques, et fixées par la fusion sur les différents corpsvitreux. J. Mines 1802, 12, 58–79. [Google Scholar]
- Brongniart, A. Traité des Arts Céramiques ou des Poteries Considéréesdans leur Histoire, leur Pratique et Leur Théorie, 2nd ed.; Béchet Jeune: Paris, France, 1854. [Google Scholar]
- D’Albis, A. Steps in the manufacture of the soft-paste porcelain of Vincennes, according to the books of Hellot. In Ancient Technology to Modern Science; Kingery, W.D., Ed.; Ceramics and Civilization I; American Ceramic Society: Columbus, OH, USA, 1985; pp. 257–271. [Google Scholar]
- D’Albis, A. Methods of manufacturing porcelain in Francein the later seventeenth and early eighteenth centuries. In Discovering the Secrets of Soft-Paste Porcelain at the Saint-Cloud Manufactory ca. 1690–1766; Rondot, B., Ed.; Yale University Press: New Haven, CT, USA; London, UK,, 1999; pp. 35–42. [Google Scholar]
- D’Albis, A. Traité de la Porcelaine de Sèvres; Faton: Dijon, France, 2003. [Google Scholar]
- Pinto, A.; Sciau, P.; Zhu, T.Q.; Zhao, B.; Groenen, E.S. Raman study of Ming porcelain dark spots: Probing Mn-rich spinels. J. Raman Spectrosc. 2019, 50, 711–719. [Google Scholar] [CrossRef]
- Colomban, P.; Simsek Franci, G.; Kirmizi, B. Cobalt and Associated Impurities in Blue (and Green) Glass, Glaze and Enamel: Relationships between Raw Materials, Processing, Composition, Phases and International Trade. Minerals 2021, 11, 633. [Google Scholar] [CrossRef]
- Sakellariou, K.; Miliani, C.; Morresi, A.; Ombelli, M. Spectroscopic investigation of yellow majolica glazes. J. Raman Spectrosc. 2004, 35, 61–67. [Google Scholar] [CrossRef]
- Sandalinas, C.; Ruiz-Moreno, S. Lead-tin-antimony yellow-Historical manufacture, molecular characterization and identification in seventeenth-century Italian paintings. Stud. Conserv. 2004, 49, 41–52. [Google Scholar] [CrossRef]
- Sandalinas, C.; Ruiz-Moreno, S.; Lopez-Gil, A.; Miralles, J. Experimental confirmation by Raman spectroscopy of a Pb-Sn-Sb triple oxide yellow pigment in sixteenth-century Italian pottery. J. Raman Spectrosc. 2006, 37, 1146–1153. [Google Scholar] [CrossRef]
- Rosi, F.; Manuali, V.; Miliani, C.; Brunetti, B.G.; Sgamellotti, A.; Grygar, T.; Hradil, D. Raman scattering features of lead pyroantimonate compounds. Part I: XRD and Raman characterization of Pb2Sb2O7 doped with tin and zinc. J. Raman Spectrosc. 2009, 40, 107–111. [Google Scholar] [CrossRef]
- Ricciardi, P.; Colomban, P.H.; Tournié, A.; Milande, V. Non-destructive on-site identification of ancient glasses: Genuine artefacts, embellished pieces or forgeries? J. Raman Spectrosc. 2009, 40, 604–617. [Google Scholar] [CrossRef]
- Pereira, M.; de Lacerda-Aroso, T.; Gomes, M.J.M.; Mata, A.; Alves, L.C.; Colomban, P.H. Ancient Portuguese ceramic wall tiles (“Azulejos”): Characterization of the glaze and ceramic pigments. J. Nano Res. 2009, 8, 79–88. [Google Scholar] [CrossRef]
- Pelosi, C.; Agresti, G.; Santamaria, U.; Mattei, E. Artificial yellow pigments: Production and characterization through spectroscopic methods of analysis. e-Preserv. Sci. 2010, 7, 108–115. [Google Scholar]
- Rosi, F.; Manuali, V.; Grygar, T.; Bezdicka, P.; Brunetti, B.G.; Sgamellotti, A.; Burgio, L.; Seccaroni, C.; Miliani, C. Raman scattering features of lead pyroantimonate compounds: Implication for the non-invasive identification of yellow pigments on ancient ceramics. Part II. In situcharacterisation of Renaissance plates by portable micro-Raman and XRF studies. J. Raman Spectrosc. 2011, 42, 407–414. [Google Scholar] [CrossRef]
- Cartechini, L.; Rosi, F.; Miliani, C.; D’Acapito, F.; Brunetti, B.G.; Sgamellotti, A. Modified Naples yellow in Renaissance majolica: Study of Pb-Sb-Zn and Pb-Sb-Fe ternary pyroantimonates by X-ray absorption spectroscopy. J. Anal. At. Spectrom. 2011, 26, 2500–2507. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B.; Zhao, B.; Clais, J.-B.; Yang, Y.; Droguet, V. Investigation of the Pigments and Glassy Matrix of Painted Enamelled Qing Dynasty Chinese Porcelains by Noninvasive On-Site Raman Microspectrometry. Heritage 2020, 3, 50. [Google Scholar] [CrossRef]
- Manoun, B.; Azdouz, M.; Azrour, M.; Essehli, R.; Benmokhtar, S.; El Ammari, L.; Ezzahi, A.; Ider, A.; Lazor, P. Synthesis, Rietveld refinements and Raman spectroscopic studies of tricationic lacunar apatites Na1-xKxPb4(AsO4)3 (0 < x <1). J. Mol. Struct. 2011, 986, 1–9. [Google Scholar]
- Froment, F.; Tournié, A.; Colomban, P. Raman identification of natural red to yellow pigments: Ochre and iron-containing ores. J. Raman Spectrosc. 2008, 39, 560–568. [Google Scholar] [CrossRef]
- Zeynal, O.A.; Jahangirli, Z. First-principles lattice dynamics and Raman scattering in ionic conductor β-Ag2S: Lattice dynamics and Raman scattering in ionic conductor β-Ag2S. Phys. Stat. Sol. B 2016, 253, 2049–2055. [Google Scholar] [CrossRef]
- Sadovnikov, E.; Vovkotrub, A.; Rempel, S. Micro-Raman Spectroscopy of Nanostructured Silver Sulfide. Doklady Phys. Chem. 2018, 480, 81–84. [Google Scholar] [CrossRef]
- Colomban, N.Q.; Liem, G.; Sagon, H.X.; Tinh, T.B.; Hoành, P. Microstructure, composition and processing of 15th century Vietnamese porcelains and celadons. J. Cult. Herit. 2003, 4, 187–197. [Google Scholar] [CrossRef]




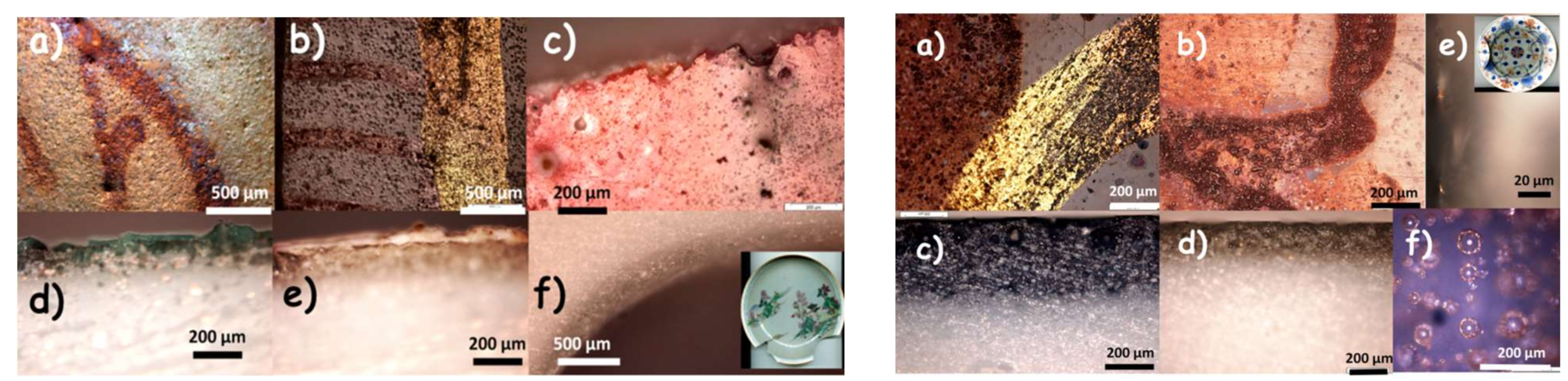
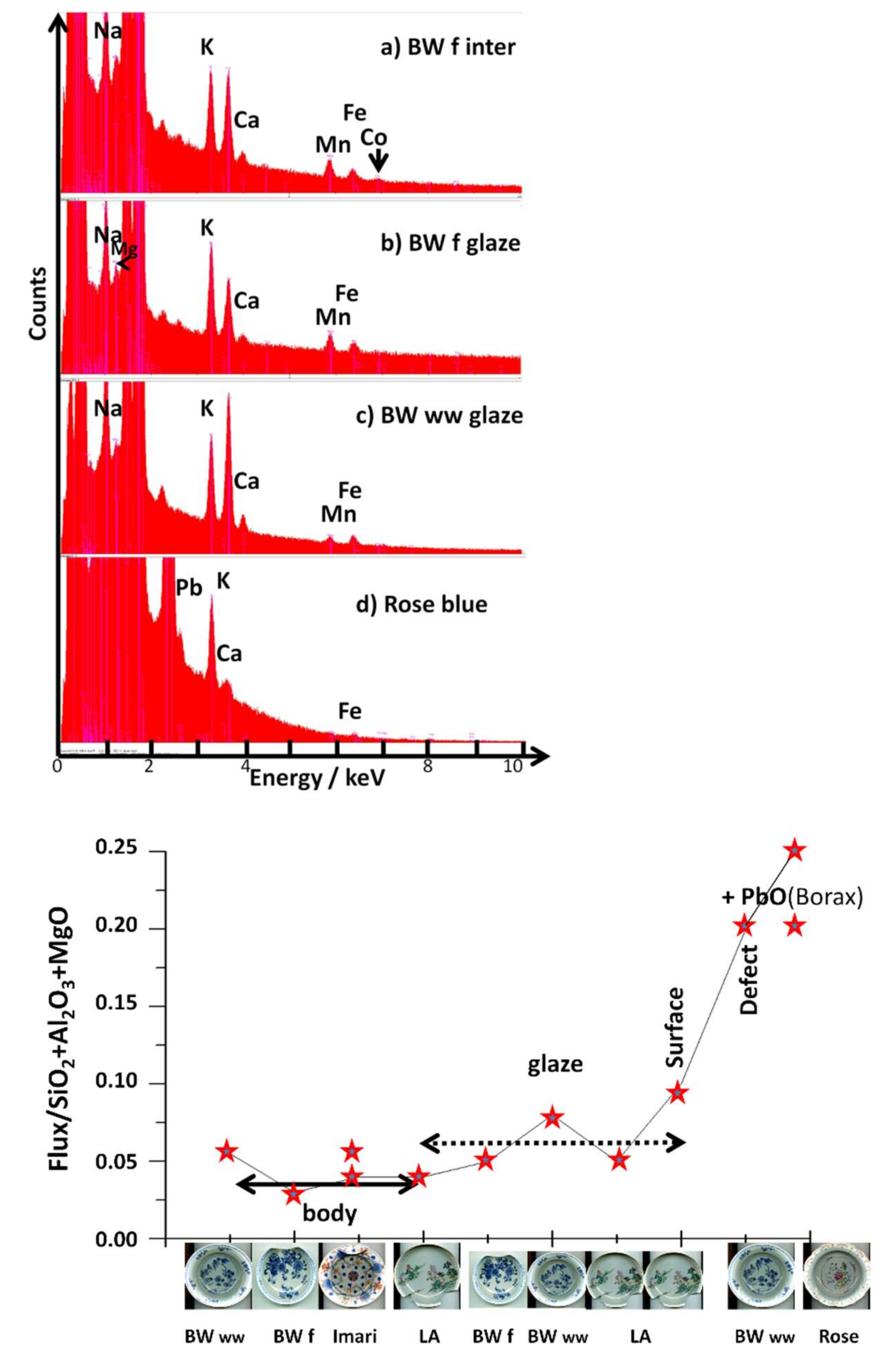
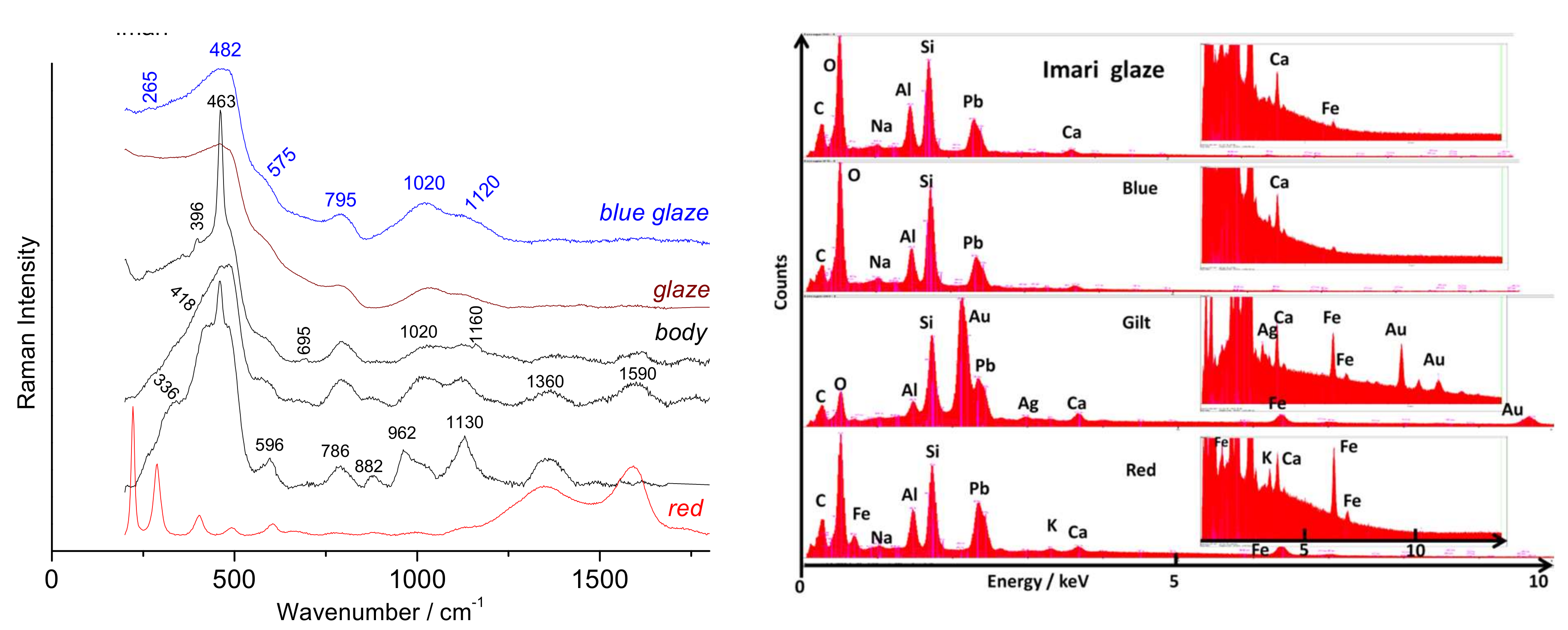
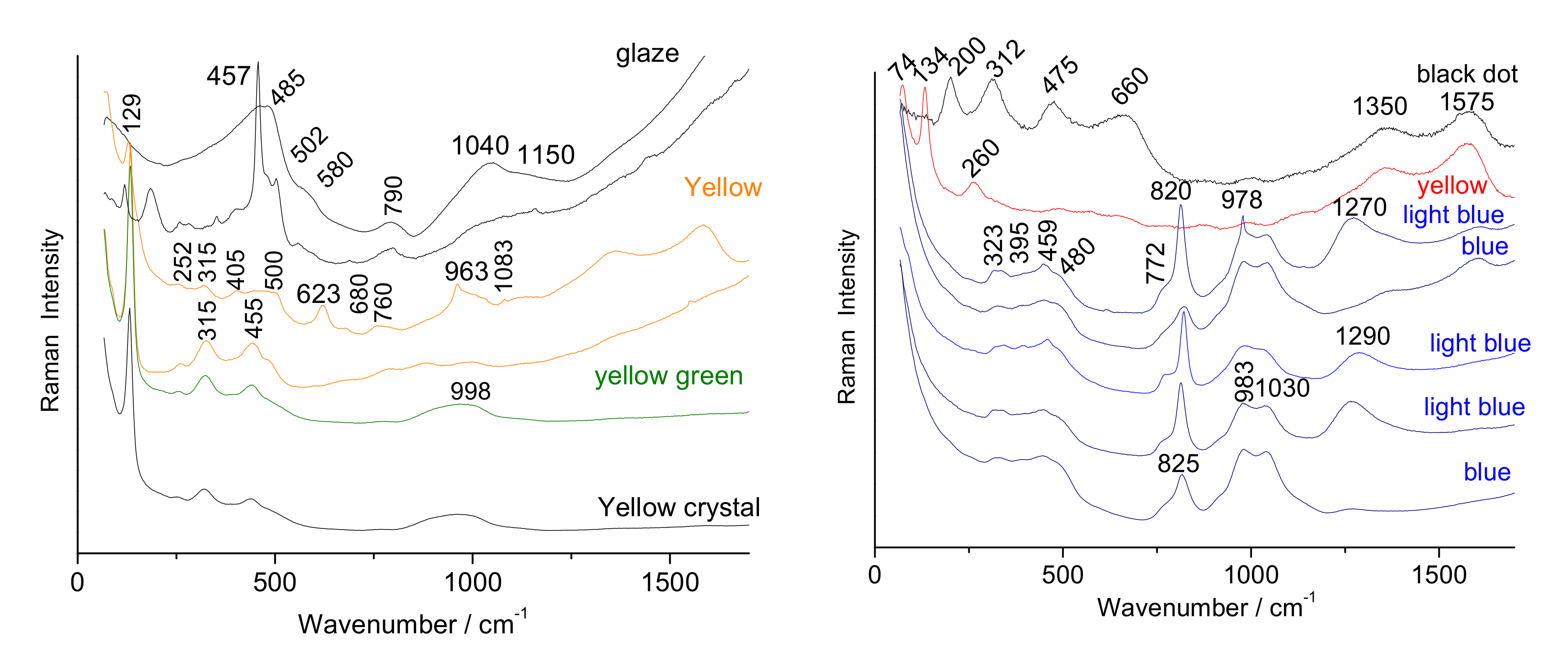
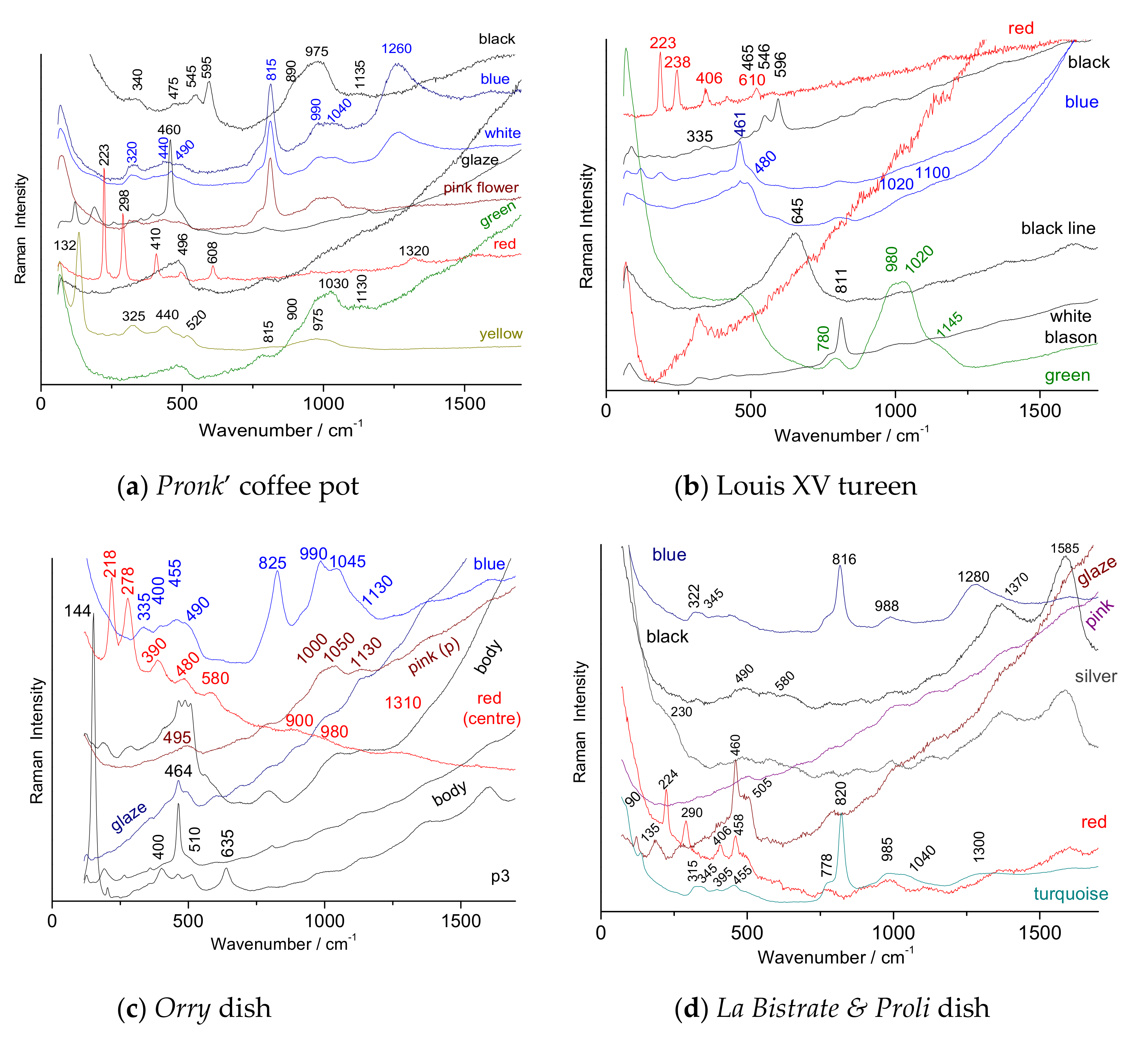
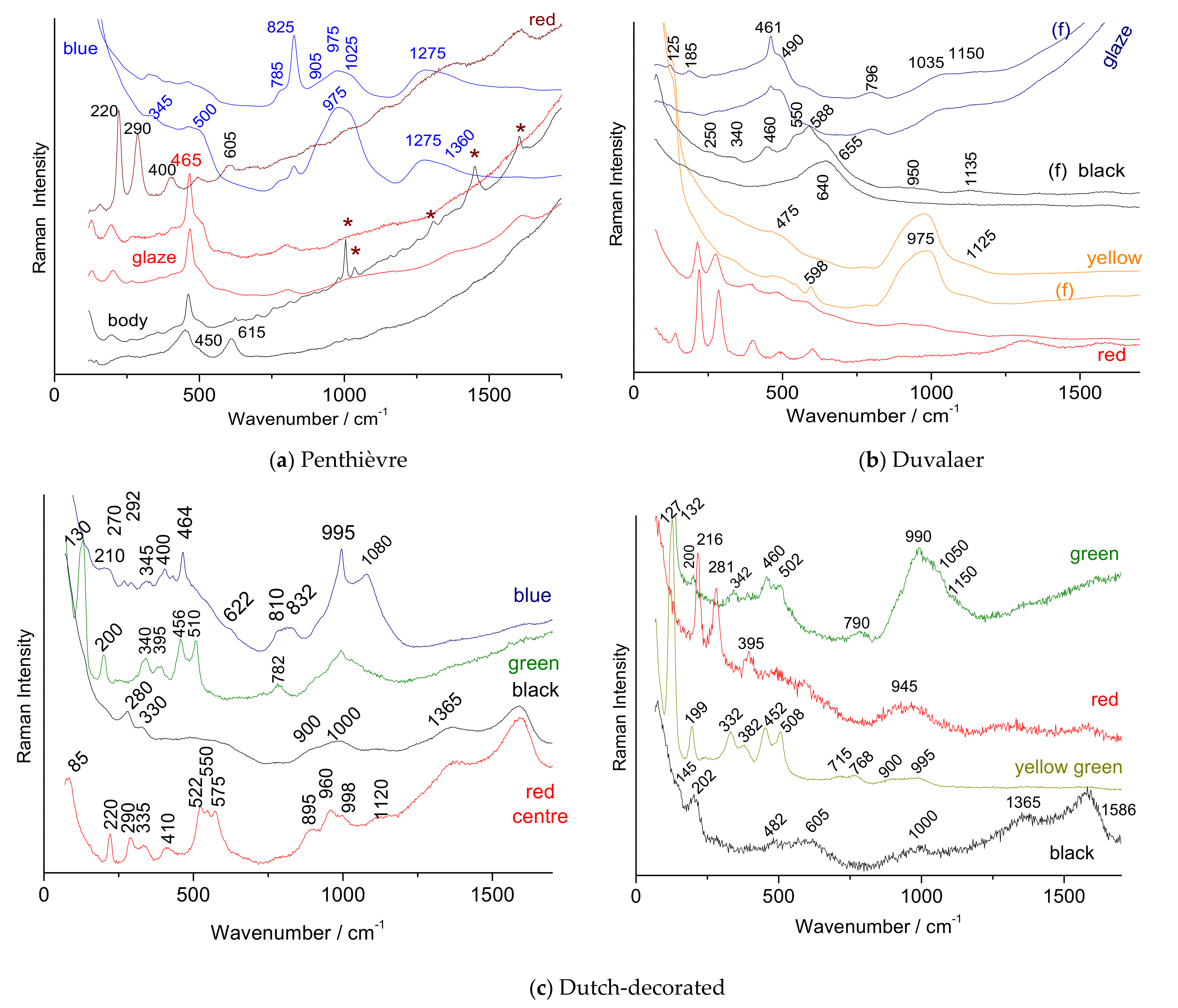
| Artefact (Magnification) | Spot | SiO2 | Al2O3 | MgO | TiO2 | Na2O | K2O | CaO | MnO2 | Fe2O3 | CoO | CuO | ZnO | PbO | Au |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW ww (200×) | body | 65.6 | 28.2 | 0.6 | 0.02 | 2.9 | 1.6 | 0.7 | 0.8 | 0.3 | 0.02 | nd | nd | nd | nd |
| BW f (200×) | body | 66.2 | 29.9 | 0.5 | 0.03 | 1.35 | 1.1 | 0.5 | 0.9 | 0.2 | 0.02 | 0.06 | 0.05 | nd | nd |
| Imari (150×) | body | 66.8 | 27.8 | 0.8 | 0.05 | 1.9 | 1.7 | 0.4 | 0.05 | 0.3 | 0.03 | 0.05 | 0.05 | nd | nd |
| LA (150×) | body | 67.1 | 27.6 | 0.8 | 0.02 | 2.1 | 1.5 | 0.4 | 0.05 | 0.4 | 0.02 | 0.05 | 0.05 | nd | nd |
| BW f (200×) | Glaze(blue) | 71.4 | 21.6 | 0.5 | 0.1 | 1.3 | 1.9 | 1.4 | 0.9 | 0.5 | 0.1 | 0.15 | 0.15 | nd | nd |
| BW ww (200×) | Glaze(blue) | 69.1 | 21.8 | 0.5 | 0.02 | 3.1 | 1.4 | 3.1 | 0.5 | 0.3 | 0.1 | nd | nd | nd | nd |
| LA (150×) | Glaze (blue) | 70.7 | 22.2 | 0.5 | 0.05 | 2.1 | 1.4 | 1.4 | 0.9 | 0.5 | 0.1 | 0.1 | 0.07 | nd | nd |
| LA (400×) | Glaze (blue surf) | 73.2 | 14.9 | 0.7 | 0.05 | 1.1 | 4.5 | 4.5 | 0.05 | 0.7 | 0.08 | 0.1 | 0.09 | nd | nd |
| BW ww (1000×) | Defect (white) | 59.2 | 17.8 | 1.7 | 0.03 | 1. | 0.5 | 15.3 | 0.38 | 3.5 | 0.1 | 0.1 | 0.2 | nd | nd |
| BW ww (1000×) | Defect (black) | 30.3 | 11.4 | 1.2 | 0.04 | 0.5 | 0.2 | 10.7 | 0.7 | 43.2 | 1.2 | 0.1 | 0.2 | nd | nd |
| Imari (50×) | Glaze (close to gilding) | 66.6 | 24.5 | 0.2 | 0.15 | 1.5 | 0.4 | 2.2 | 0.13 | 0.8 | 0.07 | 0.26 | 0.13 | 1.5 | 1.5 |
| Rose (50×) | Glaze1B? | 62.7 | 22.1 | 0.5 | nd | 4.3 | 1.2 | 0.9 | 0.03 | 0.5 | 0.02 | nd | nd | 8 | nd |
| Rose (50×) | Glaze 2 | 64.6 | 15.4 | 0.5 | nd | 3.1 | 1 | 2.8 | nd | 0.3 | nd | 0.1 | nd | 12.2 | nd |
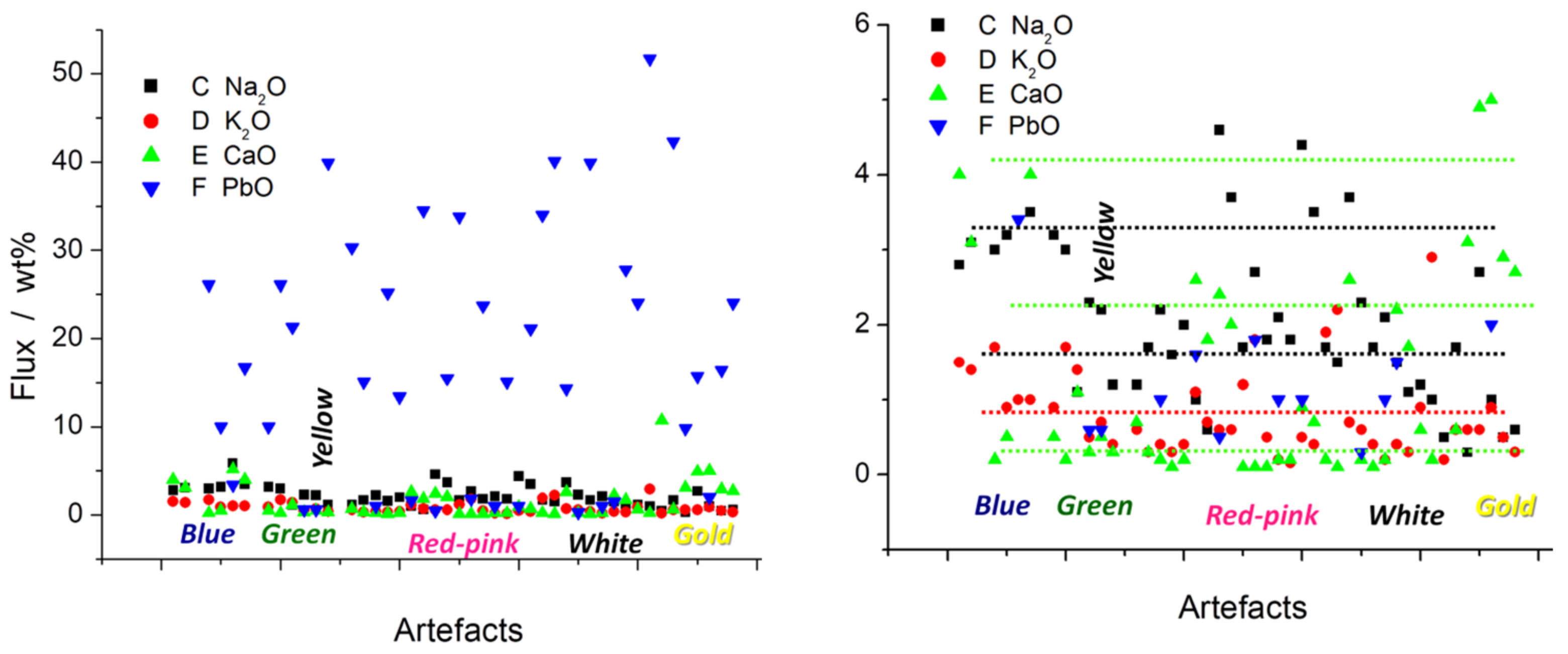
| Artefact (Magnification) | Spot | SiO2 | Al2O3 | MgO | TiO2 | Na2O | K2O | CaO | MnO2 | Fe2O3 | CoO | CuO | ZnO | As2O5 | PbO | Au[Ag] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW ww (200×) | Blue | 71.7 | 18.1 | 0.5 | 0.1 | 2.8 | 1.5 | 4.0 | 0.7 | 0.5 | 0.1 | nd | nd | nd | nd | nd |
| Blue 3 | 69.1 | 21.8 | 0.55 | 0.02 | 3.1 | 1.4 | 3.1 | 0.5 | 0.3 | 0.1 | nd | nd | nd | nd | nd | |
| Rose (50×) | Blue | 62.3 | 4.9 | nd | nd | 3 | 1.7 | 0.2 | 0.1 | 0.3 | 0.03 | nd | nd | 1.2 | 26.1 | nd |
| Blue inter | 60.2 | 22 | nd | nd | 3.2 | 0.9 | 0.5 | 0.05 | 0.5 | 0.1 | 1.6 | nd | 1.1 | 10 | nd | |
| Imari [Pb L] (50×) [Pb M] | Blue “ | 57.9 45.1 | 20.6 16.5 | 0.3 nd | 0.25 nd | 5.9 3.5 | 1 1 | 5.2 4 | 0.35 nd | 1.3 nd | 0.1 nd | 0.35 nd | 0.25 nd | 0 0.8 | 3.4 16.7 | 1. nd |
| Rose (50×) [Pb L] [Pb M] [Pb M] | Blue “ defect | 60.2 62.3 61.2 | 22 4.9 11 | nd nd nd | nd nd nd | 3.2 3 1.1 | 0.9 1.7 1.4 | 0.5 0.2 1.1 | 0.05 0.07 0.1 | 0.5 0.3 0.4 | 0.1 0.03 0.06 | 1.6 nd nd | nd nd nd | 1.1 1.2 2.3 | 10 26.1 21.3 | nd nd nd |
| LA (35×) | Green | 86.6 | 8.4 | nd | 0.06 | 2.3 | 0.5 | 0.3 | 0.06 | 0.3 | 0.02 | 1.2 | nd | 0.7 | 0.6 | nd |
| PEO (50×) | Green “ | 88.2 49.4 | 6.1 3.5 | nd nd | 0.04 0.02 | 2.2 1.2 | 0.7 0.4 | 0.5 0.3 | nd nd | 0.5 0.3 | nd nd | 0.2 4.4 | nd nd | 0.9 0.5 | 0.6 39.9 | nd nd |
| Rose (50×) | Green | 55.3 | 6.4 | nd | nd | 1.2 | 0.6 | 0.7 | 0.1 | 0.5 | 0.08 | 4.6 | nd | 0.3 | 30.3 | nd |
| Yellow- green | 71.6 | 10.3 | nd | nd | 1.7 | 0.3 | 0.3 | nd | 0.2 | nd | 0.1 | nd | nd | 15.1 | nd | |
| LA (35×) | Yellow “ | 85.3 64.2 | 10.2 7.7 | nd nd | 0.02 0.01 | 2.2 1.6 | 0.4 0.3 | 0.2 0.1 | 0.07 0.05 | 0.3 0.2 | 0.04 0.03 | 0.15 0.09 | nd nd | 0.6 0.4 | 1 25.2 | nd nd |
| Rose (50×) | Yellow (a) | 67.2 | 15 | 0.3 | nd | 2 | 0.4 | 0.2 | nd | 0.7 | nd | 0.2 | nd | nd | 13.4 | nd |
| Imari (50×) | Red “ | 59.8 38.8 | 22.1 15.2 | 0.02 0.01 | 0.2 0.15 | 1 0.6 | 1.1 0.7 | 2.6 1.8 | 0.1 0.08 | 9.9 6.3 | 0.3 0.2 | 0.2 0.15 | 0.18 nd | 0 0.7 | 1.6 34.5 | 1 0.6 |
| LA (35×) | Red/gold “ | 64.6 54 | 22.6 21.2 | 0.5 nd | nd 0.04 | 4.6 3.7 | 0.6 0.6 | 2.4 2 | 0.1 0.1 | 0.6 0.5 | 0.1 0.1 | nd nd | nd nd | 2 2.2 | 0.5 15.5 | 2 0.5 |
| Red/ | 58.8 | 2.3 | nd | 0.05 | 1.7 | 1.2 | 0.1 | nd | 0.3 | nd | nd | nd | 0.8 | 33.8 | 1 | |
| Red/pink | 87.6 66.5 | 3.5 4.3 | nd nd | nd 0.1 | 2.7 1.8 | 1.8 0.5 | 0.1 0.1 | 0.1 0.05 | 0.5 0.4 | nd 0.03 | nd nd | nd nd | 1.1 2.5 | 1.8 23.7 | 2 2 | |
| Pink-white | 80.4 68.8 | 7.5 6.4 | nd nd | 0.03 0.03 | 2.1 1.8 | 0.2 0.16 | 0.2 0.2 | nd nd | 0.6 0.5 | nd nd | nd nd | nd nd | 10.0 7 | 1 15.1 | nd nd | |
| Dark pink | 73.2 57.7 | 15.1 12.1 | nd nd | 0.1 0.1 | 4.4 3.5 | 0.5 0.4 | 0.9 0.7 | 0.13 0.1 | 0.3 0.2 | nd nd | nd nd | nd nd | 4.7 3.9 | 1 21.1 | 1 1 | |
| LA (50×) | Pink | 56.2 | 2.7 | nd | nd | 1.7 | 1.9 | 0.2 | nd | 0.3 | nd | nd | nd | 1.4 | 34 | 1 |
| Rose (50×) | Pink | 52.4 | 2.1 | nd | nd | 1.5 | 2.2 | 0.1 | nd | 0.2 | nd | nd | nd | 0.4 | 40.1 | 1 |
| Pink-red | 61.6 | 14.7 | 0.5 | nd | 3.7 | 0.7 | 2.6 | nd | 0.2 | nd | 1.5 | nd | nd | 14.3 | 0.5 | |
| PEO (100×) | White | 72.8 52.4 | 11.8 8.7 | nd nd | 0.02 0.02 | 2.3 1.7 | 0.6 0.4 | 0.2 0.1 | nd nd | nd 0.1 | nd | nd | nd | 11.9 9.3 | 0.3 39.9 | nd nd |
| LA (35×) | White | 80.4 | 7.5 | nd | 0.02 | 2.1 | 0.2 | 0.2 | nd | 0.6 | nd | nd | nd | 10.0 | 1 | nd |
| Imari (50×) | White “ | 66.4 47.9 | 24.4 18.7 | 0.2 0.1 | 0.15 0.1 | 1.5 1.1 | 0.4 0.3 | 2.2 1.7 | 0.13 0.1 | 0.8 0.6 | 0.07 0.04 | 0.26 0.2 | 0.13 0.1 | 0 0.8 | 1.5 27.8 | 1.6 nd |
| LA (50×) | White 1 | 60 | 5.9 | nd | nd | 1.2 | 0.9 | 0.6 | nd | 0.7 | nd | nd | nd | 6 | 24 | nd |
| White 2 | 38 | 1.7 | nd | nd | 1 | 2.9 | 0.2 | nd | 0.4 | nd | nd | nd | 4.1 | 51.7 | nd | |
| BW ww (1000×) | Black defect | 30.3 | 11.4 | 1.2 | 0.04 | 0.5 | 0.2 | 10.7 | 0.7 | 43.2 | 1.2 | 0.1 | 0.2 | nd | nd | nd |
| Rose (50×) | Black (b) | 47 | 2.5 | nd | nd | 1.7 | 0.6 | 0.6 | nd | 0.6 | nd | 4.7 | nd | nd | 42.3 | 2 |
| Imari (50×) | Gilding | 25.3 | 4.4 | 0.02 | 0.1 | 0.3 | 0.6 | 3.1 | 0.2 | 5.6 | 0.3 | 0.45 | 0.4 | 3 | 9.8 | 46[3] |
| LA (35×) | Gilding | 60.1 | 13.7 | 0.05 | 2.7 | 0.6 | 4.9 | 1 | nd | nd | nd | 1 | 15.7 | |||
| LA (35×) | Gilding S | 24.1 11.1 | 4.5 2.3 | nd nd | 0.3 0.1 | 1 0.5 | 0.9 0.5 | 5.0 2.9 | 0.1 0.06 | 2.6 1.4 | nd 0.1 | nd nd | nd nd | 1.5 0.1 | 2 16.4 | 53[6] 60[3] |
| PEO (50×) | Gilding | 23.2 | 4.1 | nd | nd | 0.6 | 0.3 | 2.7 | 0.2 | 2.7 | nd | nd | nd | 0.5 | 24 | 38.9[2.9] |
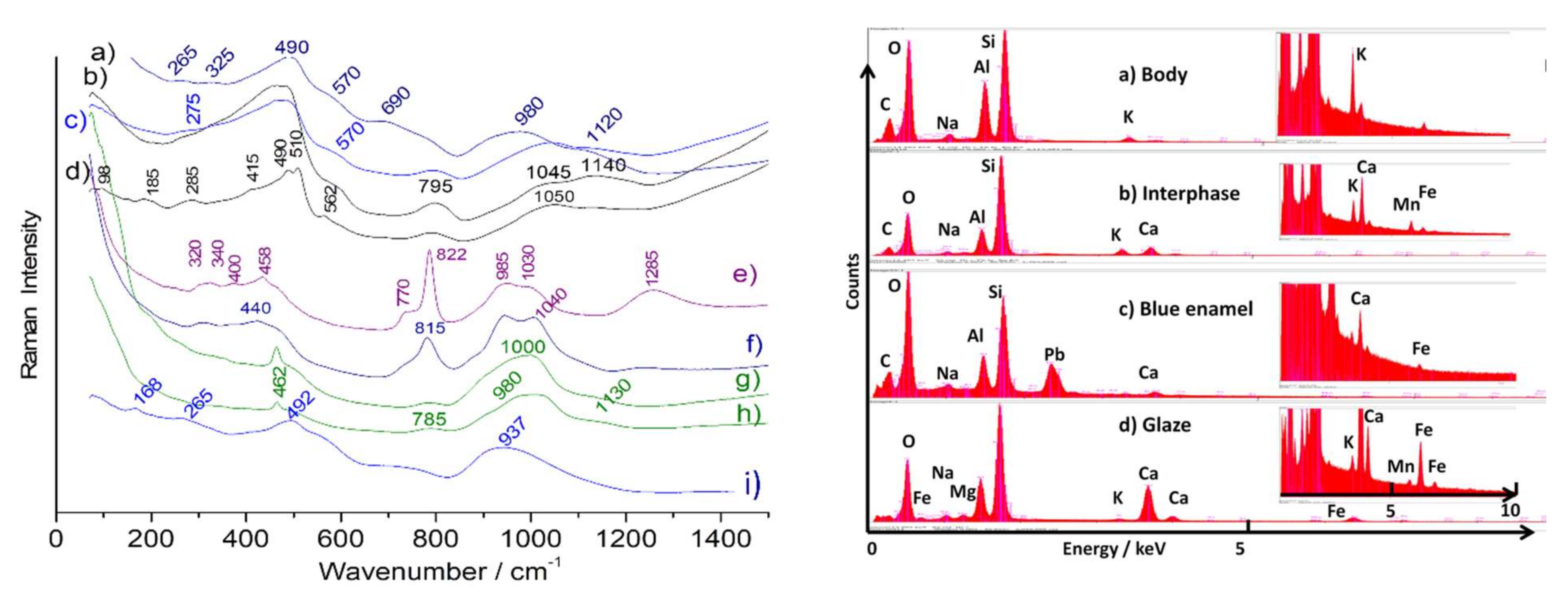
| Artefact | Period | Body | Glaze (cm−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Colourless | Blue | White | Green | Red | Pink | Black | Yellow | Siver | |||
| Dutch decorated dish | 1710–1730 | 975 (Ca-based) Wollastonite (995) | No As Wollastonite (995) | Complex NY (130,510) | Hematite + other phase (522–575) | Complex NY (130, 510) | |||||
| Duvelaer ewer | 1720–1725 | K-Na (1035–1150) | 2 types of hematite (road & narrow) + NY | 2 spinel phases (588 & 640) | (975) NY (125) (598 spinel?) | ||||||
| Philibert Orry’ dish | circa 1730 | Feldspar Quartz anatase | (1050) K-Na | As (820, broad) | Hematite (broad) | (1000–1050) | (975) Spinel (595) | ||||
| La Bistrate and Poli dish | 1730–1735 | K-Na (1035–1150) | As-apatite (816) Borax | Hematite (narrow) | (230) Ag2S | ||||||
| Duc de Penthièvre dish | circa 1737 | K-Na | As-apatite (825) Borax (1275) | Hematite (medium) | carbon | ||||||
| Louis XV tureen | circa 1738 | (1020–1100) underglaze | As-apatite (811) | (980–1020) | Hematite (narrow) | Spinel (595) Spinel (645,) | |||||
| Pronk coffee pot | 1730–1740 | quartz | As-apatite (815) Borax (1260) | As-apatite (815) | (975–1030) As (815) | Hematite (narrow) | As-apatite (815) | Spinel (595 (975)) | Pb2Sn2O6 (132) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomban, P.; Ngo, A.-T.; Fournery, N. Non-Invasive Raman Analysis of 18th Century Chinese Export/Armorial Overglazed Porcelain: Identification of the Different Enameling Techniques. Heritage 2022, 5, 233-259. https://doi.org/10.3390/heritage5010013
Colomban P, Ngo A-T, Fournery N. Non-Invasive Raman Analysis of 18th Century Chinese Export/Armorial Overglazed Porcelain: Identification of the Different Enameling Techniques. Heritage. 2022; 5(1):233-259. https://doi.org/10.3390/heritage5010013
Chicago/Turabian StyleColomban, Philippe, Anh-Tu Ngo, and Nicolas Fournery. 2022. "Non-Invasive Raman Analysis of 18th Century Chinese Export/Armorial Overglazed Porcelain: Identification of the Different Enameling Techniques" Heritage 5, no. 1: 233-259. https://doi.org/10.3390/heritage5010013
APA StyleColomban, P., Ngo, A.-T., & Fournery, N. (2022). Non-Invasive Raman Analysis of 18th Century Chinese Export/Armorial Overglazed Porcelain: Identification of the Different Enameling Techniques. Heritage, 5(1), 233-259. https://doi.org/10.3390/heritage5010013







