Preliminary Thermal Investigations of Calcium Antimonate Opacified White Glass Tesserae
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Identification of Thermal Events
3.2. Linear Regression Model
3.3. Identification of Crystalline Phases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shortland, A.J. The use and origin of antimonate colorants in early Egyptian glass. Archaeometry 2002, 44, 517–530. [Google Scholar] [CrossRef]
- Tite, M.; Shortland, A.; Paynter, S. The beginnings of vitreous materials in the Near East and Egypt. Acc. Chem. Res. 2002, 35, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Turner, W.E.S.; Rooksby, H.P. A study of the opalising agents in ancient opal glasses throughout three thousand four hundred years. Glastech. Ber. 1959, 8, 17–28. [Google Scholar]
- Rutten, F.J.M.; Briggs, D.; Henderson, J.; Roe, M.J. The application of time-of-flight secondary ion mass spectrometry (Tof-SIMS) to the characterization of opaque ancient glasses. Archaeometry 2009, 51, 966–986. [Google Scholar] [CrossRef]
- Duckworth, C.N.; Henderson, J.; Rutten, F.J.M.; Nikita, K. Opacifiers in Late Bronze Age glasses: The use of ToF-SIMS to identify raw ingredients and production techniques. J. Archaeol. Sci. 2012, 39, 2143–2152. [Google Scholar] [CrossRef][Green Version]
- Licenziati, F.; Calligaro, T. Study of mosaic glass tesserae from Delos, Greece using a combination of portable μ-Raman and X-ray fluorescence spectrometry. J. Archaeol. Sci. Rep. 2016, 7, 640–649. [Google Scholar] [CrossRef]
- Venclová, N.; Křížová, Š.; Dillingerová, V.; Vaculovič, T. Hellenistic cast monochrome glass vessels from Staré Hradisko, 2nd–1st cent. BCE. J. Archaeol. Sci. Rep. 2018, 22, 540–549. [Google Scholar] [CrossRef]
- Boschetti, C.; Henderson, J.; Evans, J.; Leonelli, C. Mosaic tesserae from Italy and the production of Mediterranean coloured glass (4rd century BCE–4th century CE). Part I: Chemical composition and technology. J. Archaeol. Sci. Rep. 2016, 7, 303–311. [Google Scholar] [CrossRef]
- Boschetti, C.; Mantovani, V.; Leonelli, C. Glass coloring and recycling in Late Antiquity: A new case study from Aquileia (Italy). J. Glass Stud. 2016, 58, 69–86. [Google Scholar]
- Gliozzo, E.; Santagostino Barbone, A.; Turchiano, M.; Memmi, I.; Volpe, G. The coloured tesserae decorating the vaults of the Faragola balneum (Ascoli Satriano, Foggia, Southern Italy). Archaeometry 2012, 54, 311–331. [Google Scholar] [CrossRef]
- Henderson, J. The raw materials of early glass production. Oxf. J. Archaeol. 1985, 4, 267–291. [Google Scholar] [CrossRef]
- Lahlil, S.; Biron, I.; Cotte, M.; Susini, J. New insight on the in situ crystallization of calcium antimonate opacified glass during the Roman period. Appl. Phys. A Mater. Sci. Process. 2010, 100, 683–692. [Google Scholar] [CrossRef]
- Lahlil, S.; Biron, I.; Cotte, M.; Susini, J.; Menguy, N. Synthesis of calcium antimonate nano-crystals by the 18th dynasty Egyptian glassmakers. Appl. Phys. A Mater. Sci. Process. 2010, 98, 1–8. [Google Scholar] [CrossRef]
- Lahlil, S.; Biron, I.; Galoisy, L.; Morin, G. Rediscovering ancient glass technologies through the examination of opacifier crystals. Appl. Phys. A Mater. Sci. Process. 2008, 92, 109–116. [Google Scholar] [CrossRef]
- Maltoni, S.; Silvestri, A.; Marcante, A.; Molin, G. The transition from Roman to Late Antique glass: New insights from the Domus of Tito Macro in Aquileia (Italy). J. Archaeol. Sci. 2016, 73, 1–16. [Google Scholar] [CrossRef]
- Neri, E.; Verita, M.; Conventi, A. Glass mosaic tesserae from the 5th-6th century baptistery of San Giovanni alle Fonti, Milan, Italy. Analytical investigations. In New Light on Old Glass: Recent Research on Byzantine Mosaics and Glass; Entwistle, C., James, L., Eds.; British Museum Press: London, UK, 2013; pp. 1–10. [Google Scholar]
- Schibille, N.; Neri, E.; Ebanista, C.; Ammar, M.R.; Bisconti, F. Something old, something new: The late antique mosaics from the catacomb of San Gennaro (Naples). J. Archaeol. Sci. Rep. 2018, 20, 411–422. [Google Scholar] [CrossRef]
- Silvestri, A.; Tonietto, S.; Molin, G.; Guerriero, P. The palaeo-Christian glass mosaic of St. Prosdocimus (Padova, Italy): Archaeometric characterisation of tesserae with antimony-or phosphorus-based opacifiers. J. Archaeol. Sci. 2012, 39, 2177–2190. [Google Scholar] [CrossRef]
- Wypyski, M.T.; Becker, L. Glassmaking technology at Antioch: Evidence from the Atrium House Triclinium and later mosaics. In The Arts of Antioch—Art Historical and Scientific Approaches to Roman Mosaics; Becker, L., Kondoleon, C., Eds.; Worcester Art Museum: Worcester, MA, USA, 2005; pp. 115–175. [Google Scholar]
- Gliozzo, E.; Barbone, A.S.; D’Acapito, F.; Turchiano, M.; Memmi, I.T.; Volpe, G. The Sectilia Panels of Faragola (Ascoli Satriano, Southern Italy): A Multi-Analytical Study of the Green, Marbled (Green and Yellow), Blue and Blackish Glass Slabs. Archaeometry 2010, 52, 389–415. [Google Scholar] [CrossRef]
- Tesser, E.; Verità, M.; Lazzarini, L.; Falcone, R.; Saguì, L.; Antonelli, F. Glass in imitation of exotic marbles: An analytical investigation of 2nd-century AD Roman sectilia from the Gorga collection. J. Cult. Herit. 2019. [Google Scholar] [CrossRef]
- Tite, M.; Pradell, T.; Shortland, A. Discovery, production and use of tin-based opacifiers in glasses, enamels and glazes from the late iron age onwards: A reassessment. Archaeometry 2008, 50, 67–84. [Google Scholar] [CrossRef]
- Uboldi, M.; Verita, M. Scientific analyses of glasses from Late Antique and Early Medieval archeological sites in Northern Italy. J. Glass Stud. 2003, 45, 115–137. [Google Scholar]
- Neri, E.; Jackson, M.; O’Hea, M.; Gregory, T.; Blet-Lemarquand, M.; Schibille, N. Analyses of glass tesserae from Kilise Tepe: New insights into an early Byzantine production technology. J. Archaeol. Sci. Rep. 2017, 11, 600–612. [Google Scholar] [CrossRef]
- Schibille, N.; Gratuze, B.; Ollivier, E.; Blondeau, É. Chronology of early Islamic glass compositions from Egypt. J. Archaeol. Sci. 2019, 104, 10–18. [Google Scholar] [CrossRef]
- Tite, M.; Watson, O.; Pradell, T.; Matin, M.; Molina, G.; Domoney, K.; Bouquillon, A. Revisiting the beginnings of tin-opacified Islamic glazes. J. Archaeol. Sci. 2015, 57, 80–91. [Google Scholar] [CrossRef]
- Moretti, C.; Salerno, C.S.; Ferroni, S.T. Ricette Vetrarie Muranesi: Gasparo Brunoro e il Manoscritto di Danzica; Nardini Florence: Florence, Italy, 2004. [Google Scholar]
- Wainwright, I.N.; Taylor, J.M.; Harley, R.D. Lead antimonate yellow. In Artists’ Pigments: A Handbook of Their History and Characteristics; National Gallery of Art; Cambridge University Press: Washington DC, USA, 1986; Volume 1, pp. 219–254. [Google Scholar]
- Freestone, I.C. Laboratory studies of the Portland Vase. J. Glass Stud. 1990, 32, 103–107. [Google Scholar]
- Freestone, I.C.; Stapleton, C. Composition technology and production of coloured glasses from Roman mosaic vessels. In Glass of the Roman World; Bayley, J., Freestone, I.C., Jackson, C., Eds.; Oxbow: Oxford, UK, 2015; pp. 61–76. [Google Scholar]
- Gedzevičiūtė, V.; Welter, N.; Schüssler, U.; Weiss, C. Chemical composition and colouring agents of Roman mosaic and millefiori glass, studied by electron microprobe analysis and Raman microspectroscopy. Archaeol. Anthropol. Sci. 2009, 1, 15–29. [Google Scholar] [CrossRef]
- Paynter, S.; Jackson, C. Clarity and brilliance: Antimony in colourless natron glass explored using Roman glass found in Britain. Archaeol. Anthropol. Sci. 2018, 1–19. [Google Scholar] [CrossRef]
- Roberts, P.; Gudenrath, W.; Tatton-Brown, V.; Whitehouse, D. Roman Cameo Glass in the British Museum; British Museum Publications Limited: London, UK, 2010. [Google Scholar]
- Sablerolles, Y.; Henderson, J.; Dijkman, W. Early medieval glass bead-making in Maastricht (Jodenstraat 30), The Netherlands. An archaeological and scientific investigation. In Perlen: Archäologie, Techniken, Analysen; Akten des Internationalen Perlensymposiums in Mannheim vom 11. bis 14. November 1994; von Freeden, U., Wiczorek, A., Eds.; Habelt: Bonn, Germany, 1997; pp. 225–226, 293–314. [Google Scholar]
- Verità, M. Perle vitree dalle necropoli longobarde in Italia. Natura dei materiali e tecniche di lavorazione. In Archeologia Medievale a Trezzo Sull’Adda. Il Sepolcreto Longobardo e l’Oratorio di San Martino. Le chiese di Santo Stefano e San Michele in Sallianense; Contributi di Archeologia; V&P Vita e Pensiero: Milano, Italy, 2012; pp. 355–378. [Google Scholar]
- Drünert, F.; Palamara, E.; Zacharias, N.; Wondraczek, L.; Möncke, D. Ancient Roman nano-technology: Insight into the manufacture of mosaic tesserae opacified by calcium antimonate. J. Eur. Ceram. Soc. 2018, 38, 4799–4805. [Google Scholar] [CrossRef]
- Schibille, N.; Boschetti, C.; Valero Tévar, M.Á.; De Juan Ares, J.; Veron, E. The colour palette of the mosaics in the Roman villa of Noheda (Spain). Minerals 2020, 10, 272. [Google Scholar] [CrossRef]
- Montanari, F.; Miselli, P.; Leonelli, C.; Boschetti, C.; Henderson, J.; Baraldi, P. Calibration and use of the heating microscope for indirect evaluation of the viscosity and meltability of archeological glasses. Int. J. Appl. Glass Sci. 2014, 5, 161–177. [Google Scholar] [CrossRef]
- Jobson, J.D. Applied Multivariate Data Analysis Volume I: Regression and Experimental Design; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Gualtieri, A.F.; Andreozzi, G.B.; Giacobbe, C.; Lusvardi, G.; Viti, C. Structural and spectroscopic characterization of anorthite synthesized from secondary raw materials. Periodico di Mineralogia 2011, 80, 231–245. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Montazerian, M.; Gulbiten, O.; Mauro, J.C.; Zanotto, E.D.; Yue, Y. Understanding Glass through Differential Scanning Calorimetry. Chem. Rev. 2019, 119, 7848–7939. [Google Scholar] [CrossRef] [PubMed]
- Lara, C.; Pascual, M.; Duran, A. Glass-forming ability, sinterability and thermal properties in the systems RO–BaO–SiO2 (R = Mg, Zn). J. Non-Cryst. Solids 2004, 348, 149–155. [Google Scholar] [CrossRef]
- Wiesenberg, F. Experimentelle Archäologie: Römische Glasöfen: Rekonstruktion und Betrieb einer Glashütte nach römischem Vorbild in der Villa Borg: “Borg Furnace Project 2013”. 2014. Available online: http://openarchaeology.info/bibliography/experimentelle-archaologie-romische-glasofen-rekonstruktion-und-betrieb-einer-glashutte (accessed on 25 June 2020).
- Shelby, J.E. Introduction to Glass Science and Technology; Royal Society of Chemistry: Cambridge, UK, 2005. [Google Scholar]
- Mee, M.; Davies, B.C.; Orman, R.G.; Thomas, M.F.; Holland, D. Antimony and silicon environments in antimony silicate glasses. J. Solid State Chem. 2010, 183, 1925–1934. [Google Scholar] [CrossRef]
- Nakagawa, K.; Izumitani, T. Metastable phase separation and crystallization of Li2O Al2O3 SiO3 glasses: Determination of miscibility gap from the lattice parameters of precipitated β-quartz solid solution. J. Non-Cryst. Solids 1972, 7, 168–180. [Google Scholar] [CrossRef]
- Boisen, M.B., Jr.; Gibbs, G.V.; Bukowinski, M.S.T. Framework silica structures generated using simulated annealing with a potential energy function based on an H 6 Si 2 O 7 molecule. Phys. Chem. Miner. 1994, 21, 269–284. [Google Scholar] [CrossRef]
- Vogel, W. Glass Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1994. [Google Scholar]


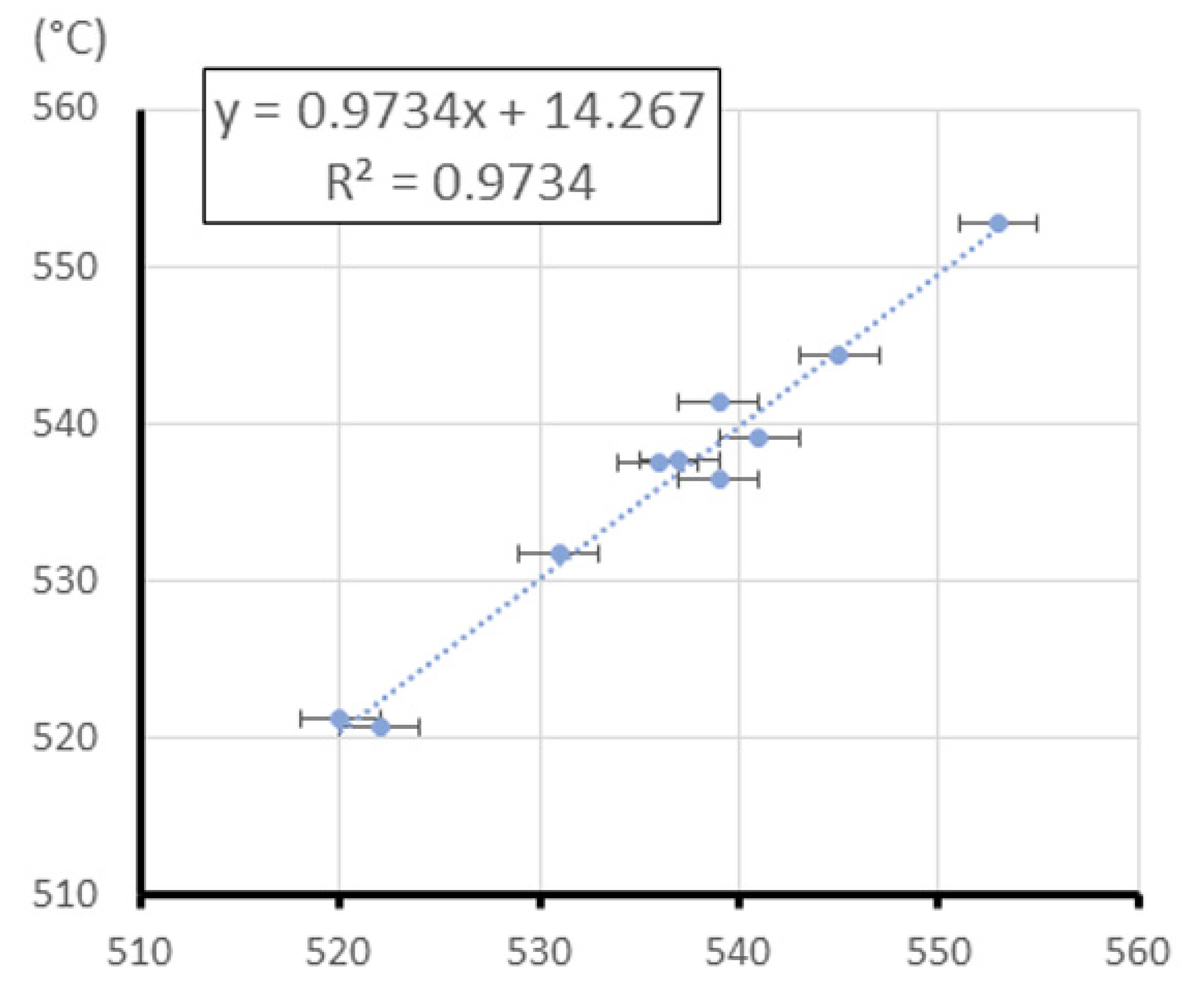
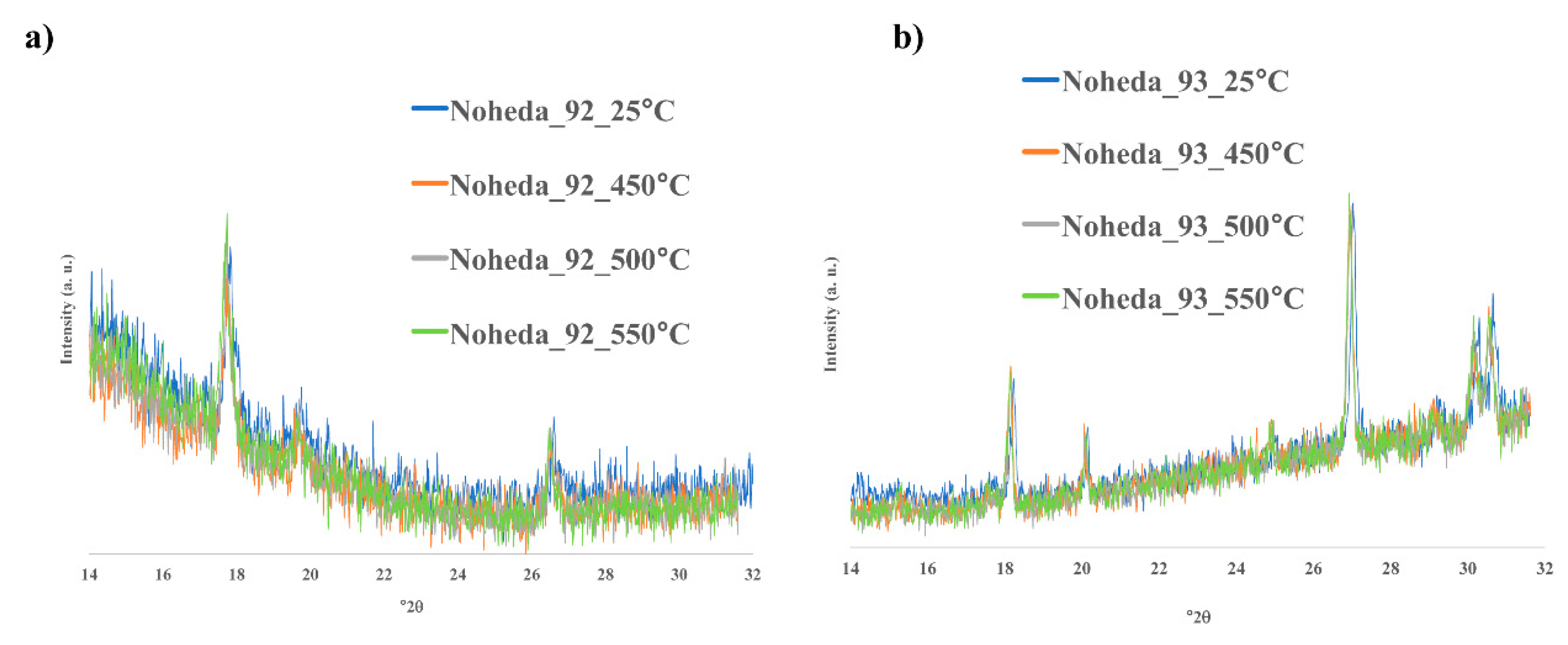

| Sample | Na2O | MgO | Al2O3 | SiO2 | P2O5 | Cl | K2O | CaO | TiO2 | MnO | Fe2O3 | CuO | Sb2O3 | PbO | First Peak (Tg) | Second Peak (Tg) | Third Peak (Tm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noheda 071 | 15.8 | 3.09 | 1.97 | 66.1 | 0.03 | 0.85 | 0.44 | 6.13 | 0.07 | 0.03 | 0.44 | 0.00 | 4.80 | 0.02 | 545 | 619 | - |
| Noheda 076 | 14.7 | 4.03 | 1.68 | 62.7 | 0.02 | 0.90 | 0.43 | 6.71 | 0.06 | 0.01 | 0.34 | 0.00 | 8.09 | 0.16 | 539 | - | - |
| Noheda 077 | 13.3 | 0.35 | 2.48 | 66.8 | 0.10 | 0.84 | 0.34 | 6.76 | 0.05 | 0.03 | 0.31 | 0.00 | 7.10 | 1.43 | 553 | 620 | 1154 |
| Noheda 080 | 16.8 | 0.40 | 1.96 | 65.5 | 0.02 | 1.10 | 0.49 | 5.43 | 0.08 | 0.10 | 0.43 | 0.01 | 5.06 | 2.47 | 522 | 609 | 1132 |
| Noheda 084 | 15.9 | 3.62 | 1.90 | 63.9 | 0.04 | 0.68 | 0.46 | 6.89 | 0.08 | 0.02 | 0.57 | 0.00 | 5.17 | 0.64 | 539 | - | 1129 |
| Noheda 086 | 15.9 | 2.57 | 1.97 | 65.4 | 0.04 | 0.71 | 0.50 | 7.02 | 0.08 | 0.02 | 0.52 | 0.00 | 5.14 | 0.01 | 536 | - | - |
| Noheda 092 | 17.1 | 0.40 | 1.95 | 65.4 | 0.03 | 1.25 | 0.52 | 5.27 | 0.08 | 0.10 | 0.43 | 0.01 | 4.90 | 2.34 | 520 | 613 | 1146 |
| Noheda 093 | 14.6 | 1.51 | 1.69 | 64.7 | 0.08 | 0.88 | 0.51 | 6.01 | 0.07 | 0.04 | 0.45 | 0.02 | 9.23 | 0.03 | 541 | - | - |
| Noheda 099 | 16.9 | 0.40 | 2.02 | 67.6 | 0.03 | 0.94 | 0.50 | 5.25 | 0.09 | 0.07 | 0.49 | 0.01 | 4.50 | 1.08 | 531 | 616 | 1122 |
| Noheda 106 | 13.2 | 0.39 | 2.06 | 66.3 | 0.18 | 0.63 | 0.56 | 5.95 | 0.05 | 0.62 | 0.36 | 0.01 | 7.88 | 1.68 | 537 | 589 | 1155 |
| Coefficient | Standard Error | |
|---|---|---|
| MgO | 4.9168 | ±1.5874 |
| Al2O3 | 52.0224 | ±7.8020 |
| CaO | −6.4054 | ±2.9516 |
| Sb2O3 | 3.6050 | ±0.6639 |
| PbO | −6.3829 | ±1.3941 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschetti, C.; Leonelli, C.; Rosa, R.; Romagnoli, M.; Valero Tévar, M.Á.; Schibille, N. Preliminary Thermal Investigations of Calcium Antimonate Opacified White Glass Tesserae. Heritage 2020, 3, 549-560. https://doi.org/10.3390/heritage3020032
Boschetti C, Leonelli C, Rosa R, Romagnoli M, Valero Tévar MÁ, Schibille N. Preliminary Thermal Investigations of Calcium Antimonate Opacified White Glass Tesserae. Heritage. 2020; 3(2):549-560. https://doi.org/10.3390/heritage3020032
Chicago/Turabian StyleBoschetti, Cristina, Cristina Leonelli, Roberto Rosa, Marcello Romagnoli, Miguel Ángel Valero Tévar, and Nadine Schibille. 2020. "Preliminary Thermal Investigations of Calcium Antimonate Opacified White Glass Tesserae" Heritage 3, no. 2: 549-560. https://doi.org/10.3390/heritage3020032
APA StyleBoschetti, C., Leonelli, C., Rosa, R., Romagnoli, M., Valero Tévar, M. Á., & Schibille, N. (2020). Preliminary Thermal Investigations of Calcium Antimonate Opacified White Glass Tesserae. Heritage, 3(2), 549-560. https://doi.org/10.3390/heritage3020032







