Multiscale Study of Interactions Between Corrosion Products Layer Formed on Heritage Cu Objects and Organic Protection Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Carboxylate Protective Treatment
2.3. Analyses Methodology
2.4. Analytical Techniques
2.4.1. µRaman Spectroscopy (µRS)
2.4.2. Scanning Electron Microscopy – Energy Dispersive Spectroscopy (SEM-EDS)
2.4.3. Scanning Auger Microscopy (SAM)
3. Results
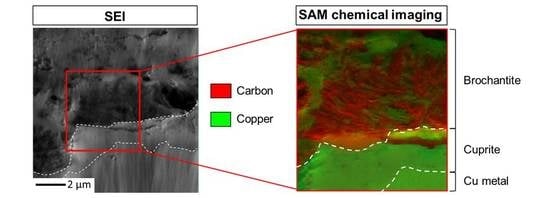
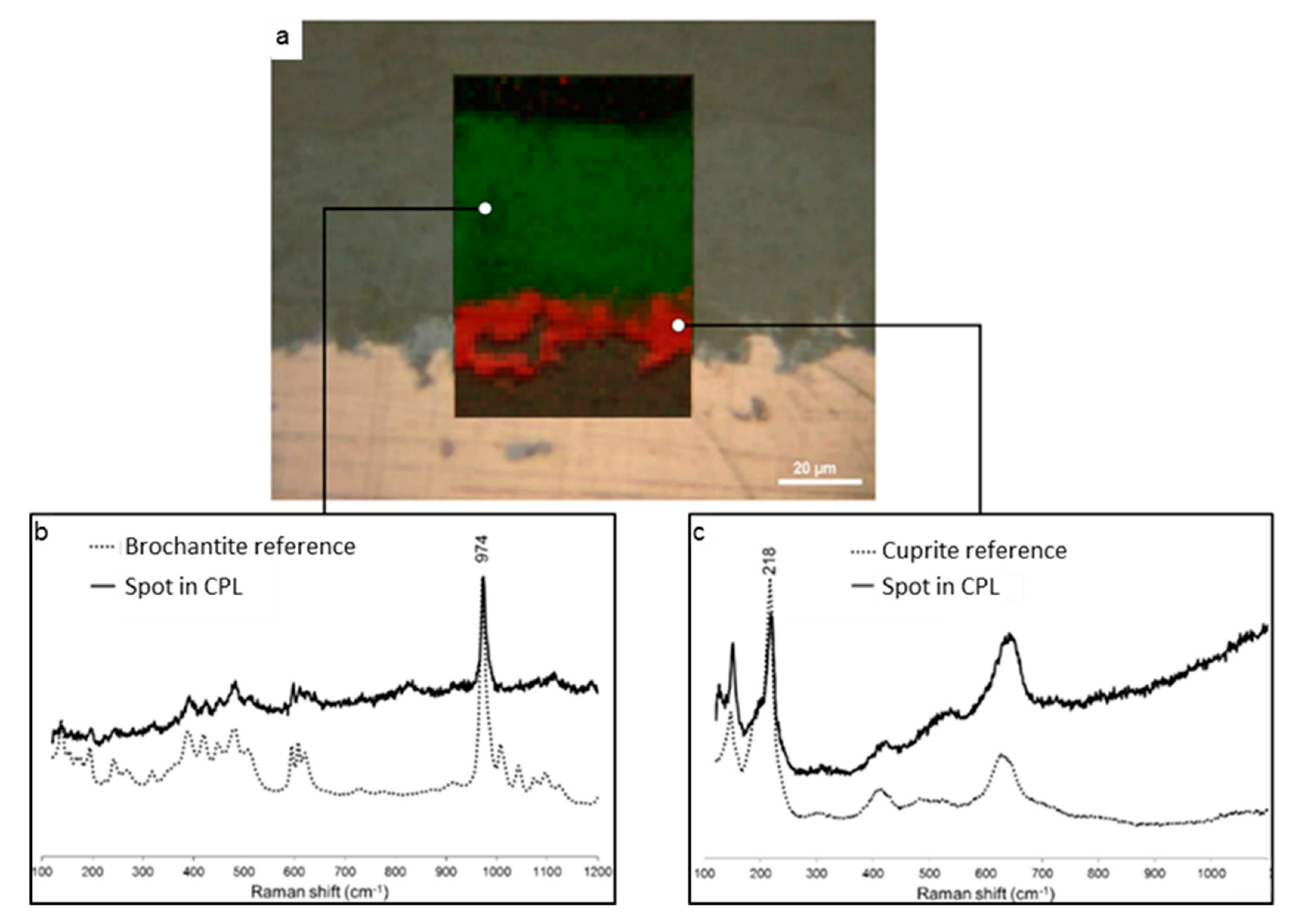
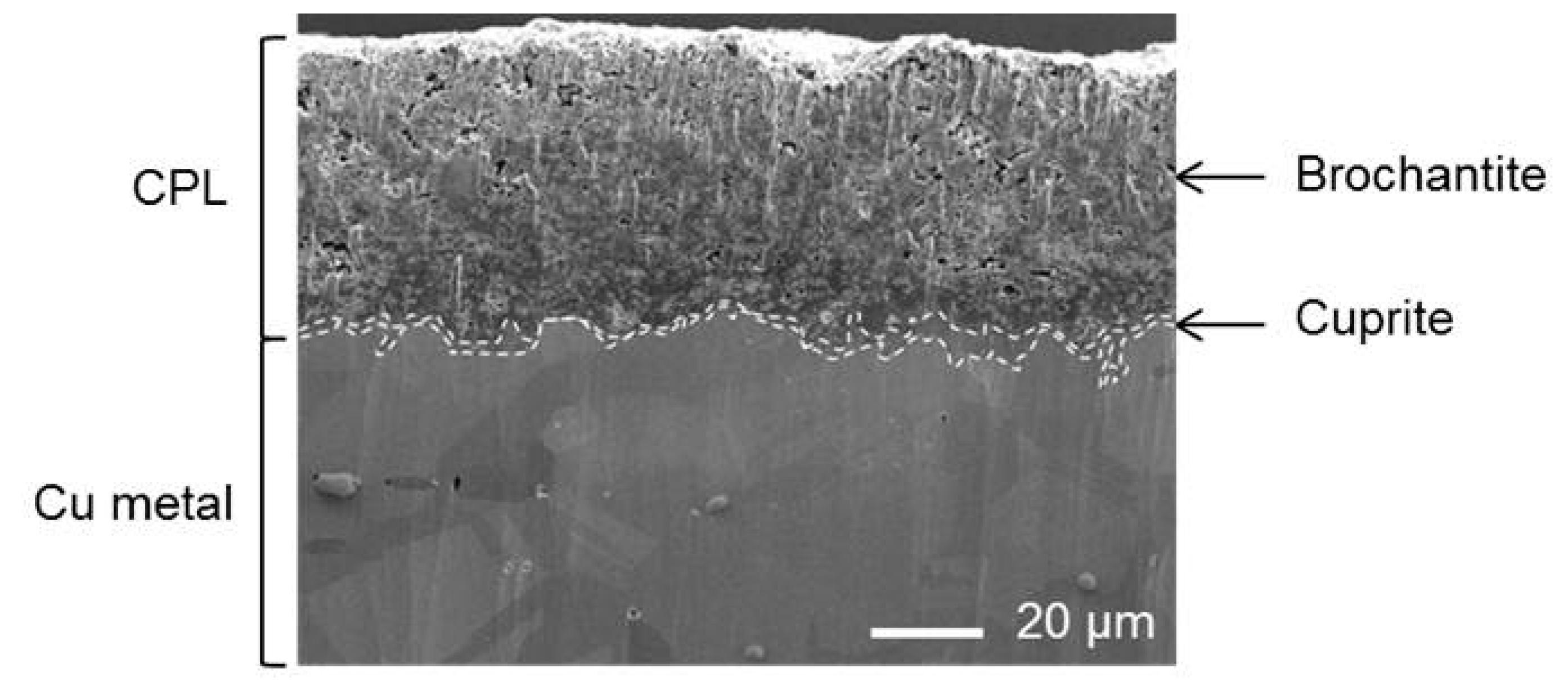
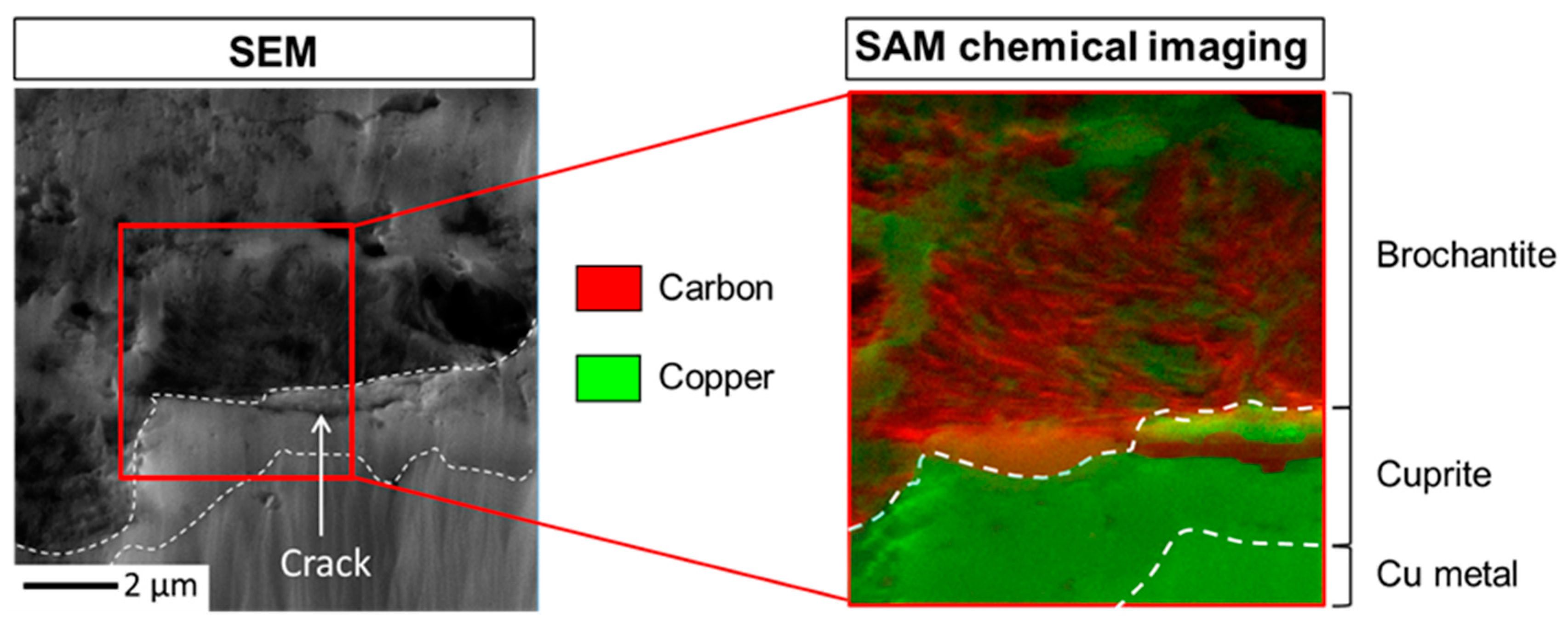
3.1. Nature and Depth Organization of the Corrosion Product Layer
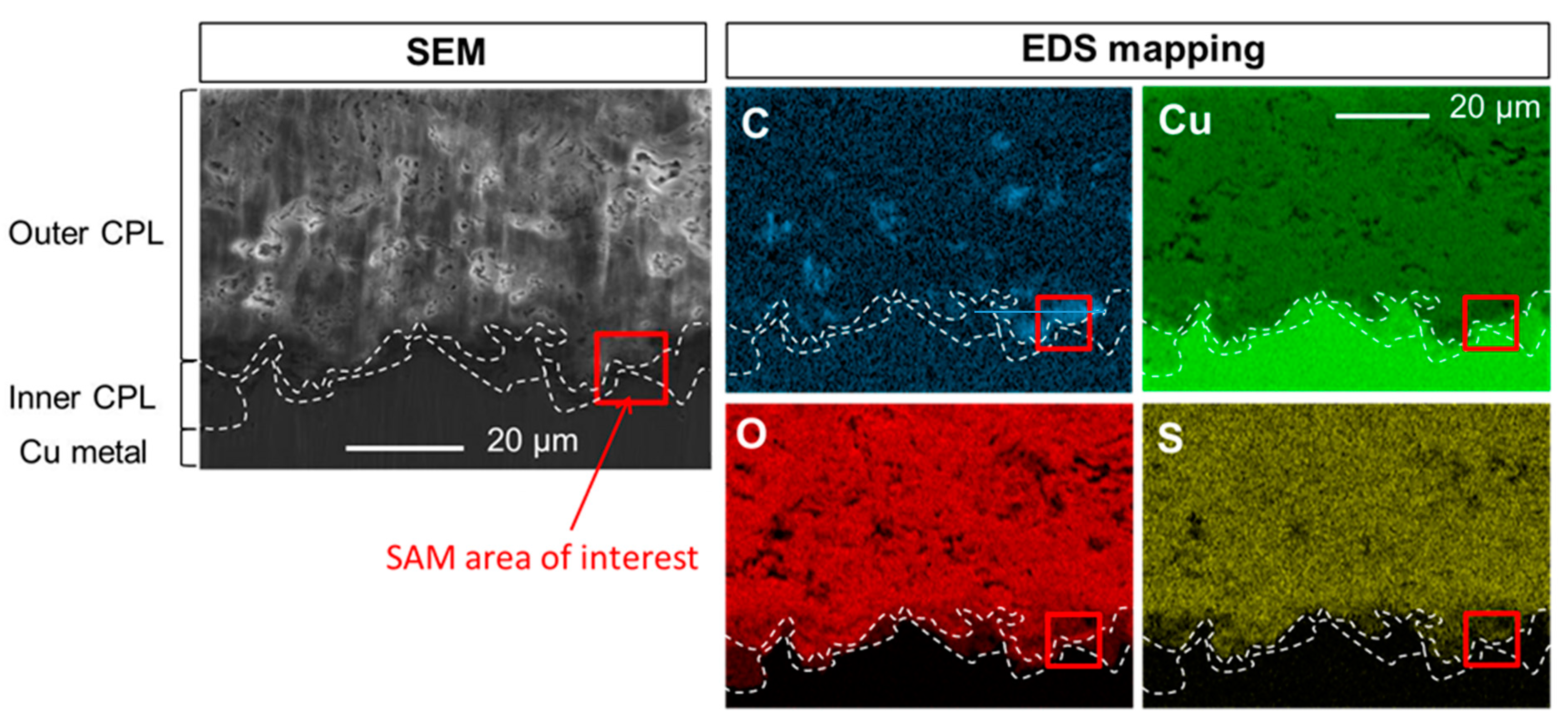
3.2. Penetration of the Carboxylate Treatment in the Corrosion Product Layer
3.2.1. Immersion Time in HC10 Solution: Thirty Minutes
3.2.2. Immersion Time in HC10 Solution: One Minute
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cano, E.; Lafuente, D. Corrosion inhibitors for the preservation of metallic heritage artefacts. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; European Federation of Corrosion (EFC) Series; Dillmann, P., Watkinson, D.E., Angelini, E., Adriaens, A., Eds.; Woodhead Publishing: Sawston/Cambridge, UK, 2013; pp. 570–594. [Google Scholar]
- Wu, X.; Chou, N.; Lupher, D.; Davis, L.C. Benzotriazoles: Toxicity and Degradation. In Proceedings of the 13th Annual Conference on Hazardous Waste Research, Snowbird, Utah, 19–21 May 1998; pp. 374–382. [Google Scholar]
- Pillard, D.; Cornell, J.; Dufresne, D.; Hernandez, M. Toxicity of Benzotriole and Benzotriazole Derivatives to three Aquatic Species. Water Res. 2001, 35, 557–560. [Google Scholar] [CrossRef]
- Kim, J.-W.; Chang, K.-H.; Isobe, T.; Tanabe, S. Acute toxicity of benzotriazole ultraviolet stabilizers on freshwater crustacean (Daphnia pulex). J. Toxicol. Sci. 2011, 36, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Rocca, E.; Bertrand, G.; Rapin, C.; Labrune, J.C. Inhibition of copper aqueous corrosion by non-toxic linear sodium heptanoate: Mechanism and ECAFM study. J. Electroanal. Chem. 2001, 503, 133–140. [Google Scholar] [CrossRef]
- Rocca, E.; Steinmetz, J. Inhibition of lead corrosion with saturated linear aliphatic chain monocarboxylates of sodium. Corros. Sci. 2001, 43, 891–902. [Google Scholar] [CrossRef]
- Rocca, E.; Mirambet, F. Corrosion inhibitors for metallic artefacts: Temporary protection. In Corrosion of Metallic Heritage Artefacts; European Federation of Corrosion (EFC) Series; Dillmann, P., Béranger, G., Piccardo, P., Matthiesen, H., Eds.; Woodhead Publishing: Sawston/Cambridge, UK, 2007; pp. 308–334. [Google Scholar]
- Rocca, E.; Mirambet, F. Des savons métalliques pour la protection du patrimoine: Sodium carboxylates used to protect metallic artefacts. L’actualité Chim. 2007, 312–313, 65–70. [Google Scholar]
- Mirambet, F.; Reguer, S.; Rocca, E.; Hollner, S.; Testemale, D. A complementary set of electrochemical and X-ray synchrotron techniques to determine the passivation mechanism of iron treated in a new corrosion inhibitor solution specifically developed for the preservation of metallic artefacts. Appl. Phys. A Mater. Sci. Process. 2010, 99, 341–349. [Google Scholar] [CrossRef]
- Hollner, S. Developpement de Nouveaux Traitements de Protection a Base D’Acide Carboxylique Pour la Conservation D’Objets en fer du Patrimoine Culturel. Ph.D. Thesis, Henri Poincaré University, Nancy, France, 2009. [Google Scholar]
- Rocca, E.; Mirambet, F. The electrochemical techniques for the diagnosis and restoration treatments of technical and industrial heritage: Three examples of metallic artefacts. J. Solid State Electrochem. 2010, 14, 415–423. [Google Scholar] [CrossRef]
- Hollner, S.; Mirambet, F.; Rocca, E.; Reguer, S. Evaluation of new non-toxic corrosion inhibitors for conservation of iron artefacts. Corros. Eng. Sci. Technol. 2010, 45, 362–366. [Google Scholar] [CrossRef]
- Apchain, E. Apport des Traitements Carboxylates à la Protection des Alliages Cuivreux. Ph.D. Thesis, Cergy-Pontoise University, Cergy, France, 2018. [Google Scholar]
- Ingo, G.M.; Riccucci, C.; Pascucci, M.; Messina, E.; Giuliani, C.; Biocca, P.; Tortora, L.; Fierro, G.; Di Carlo, G. Combined use of FE-SEM+EDS, ToF-SIMS, XPS, XRD and OM for the study of ancient gilded artefacts. Appl. Surf. Sci. 2018, 446, 168–176. [Google Scholar] [CrossRef]
- Ingo, G.M.; Riccucci, C.; Giuliani, C.; Faustoferri, A.; Pierigè, I.; Fierro, G.; Pascucci, M.; Albini, M.; Di Carlo, G. Surface studies of patinas and metallurgical features of uncommon high-tin bronze artefacts from the Italic necropolises of ancient Abruzzo (Central Italy). Appl. Surf. Sci. 2019, 470, 74–83. [Google Scholar] [CrossRef]
- Ingo, G.M.; Riccucci, C.; Guida, G.; Pascucci, M.; Giuliani, C.; Messina, E.; Fierro, G.; Di Carlo, G. Micro-chemical investigation of corrosion products naturally grown on archaeological Cu-based artefacts retrieved from the Mediterranean sea. Appl. Surf. Sci. 2019, 470, 695–706. [Google Scholar] [CrossRef]
- Carrasco, E.; Oujja, M.; Sanz, M.; Marco, J.F.; Castillejo, M. X-ray and ion irradiation effects on azurite, malachite and alizarin pictorial models. Microchem. J. 2018, 137, 381–391. [Google Scholar] [CrossRef]
- Kratschmer, A.; Wallinder, I.; Leygraf, C. The evolution of out copper patina. Corros. Sci. 2002, 44, 425–450. [Google Scholar] [CrossRef]
- De la Fuente, D.; Simancas, J.; Morcillo, M. Morphological study of 16-year patinas formed on copper in a wide range of atmospheric exposures. Corros. Sci. 2008, 50, 268–285. [Google Scholar] [CrossRef]
- Leygraf, C.; Wallinder, I.O.; Tidblad, J.; Graedel, T. Atmospheric Corrosion, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Morcillo, M.; Chang, T.; Chico, B.; de la Fuente, D.; Odnevall Wallinder, I.; Jiménez, J.A.; Leygraf, C. Characterisation of a centuries-old patinated copper roof tile from Queen Anne’s Summer Palace in Prague. Mater. Charact. 2017, 133, 146–155. [Google Scholar] [CrossRef]
- Fitzgerald, K.P.; Naim, J.; Skennerton, G.; Atrens, A. Atmospheric corrosion of copper and the colour, structure and composition of natural patinas on copper. Corros. Sci. 2006, 48, 2480–2509. [Google Scholar] [CrossRef]
- Graedel, T.E.; Nassau, K.; Franey, J.P. Copper patinas formed in the atmosphere-I. Introduction. Corros. Sci. 1987, 27, 639–649. [Google Scholar] [CrossRef]
- Wallinder, I.O.; Leygraf, C. A study of copper run off in an urban atmosphere. Corros. Sci. 1997, 39, 2039–2052. [Google Scholar] [CrossRef]
- Vernon, W.H.J.; Whitby, L. The open-air corrosion of copper: A chemical study of the surface patina. J. Inst. Met. 1929, 42, 181–202. [Google Scholar]
- Oesch, S.; Faller, M. Environmental effects on materials: The effect of the air pollutants SO2, NO2, NO and O3 on the corrosion of copper, zinc and aluminium. A short literature survey and results of laboratory exposures. Corros. Sci. 1997, 39, 1505–1530. [Google Scholar]
- Rice, D.W.; Peterson, P.; Rigby, E.B.; Phipps, P.B.P.; Cappell, R.J.; Tremoureux, R. Atmospheric corrosion of copper and silver. J. Electrochem. Soc. 1981, 128, 275–284. [Google Scholar] [CrossRef]
- Oesch, S.; Heimgartner, P. Environmental effects on metallic materials–results of an outdoor exposure programme running in Switzerland. Mater. Corros. 1996, 47, 425–438. [Google Scholar] [CrossRef]
- Fitzgerald, K.P.; Nairm, J.; Atrens, A. The chemistry of copper patination. Corros. Sci. 1998, 40, 2029–2050. [Google Scholar] [CrossRef]
- Vernon, W.H.J. Second experimental report to the atmospheric corrosion research committee (bristish non-ferrous metals research association. Trans. Faraday Soc. 1927, 23, 113–183. [Google Scholar] [CrossRef]
- North, R.F.; Pryor, M.J. The influence of corrosion product structure on the corrosion rate of Cu-Ni alloys. Corros. Sci. 1970, 10, 297–311. [Google Scholar] [CrossRef]
- Texier, A. La sculpture monumentale en cuivre et alliages cuivreux. In Monumental. Revue Scientifique et Technique des Monuments Historiques; Editions du Patrimoine: Paris, France, 2010. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
L’héronde, M.; Bouttemy, M.; Mercier-Bion, F.; Neff, D.; Apchain, E.; Etcheberry, A.; Dillmann, P. Multiscale Study of Interactions Between Corrosion Products Layer Formed on Heritage Cu Objects and Organic Protection Treatments. Heritage 2019, 2, 2640-2651. https://doi.org/10.3390/heritage2030162
L’héronde M, Bouttemy M, Mercier-Bion F, Neff D, Apchain E, Etcheberry A, Dillmann P. Multiscale Study of Interactions Between Corrosion Products Layer Formed on Heritage Cu Objects and Organic Protection Treatments. Heritage. 2019; 2(3):2640-2651. https://doi.org/10.3390/heritage2030162
Chicago/Turabian StyleL’héronde, Maëva, Muriel Bouttemy, Florence Mercier-Bion, Delphine Neff, Emilande Apchain, Arnaud Etcheberry, and Philippe Dillmann. 2019. "Multiscale Study of Interactions Between Corrosion Products Layer Formed on Heritage Cu Objects and Organic Protection Treatments" Heritage 2, no. 3: 2640-2651. https://doi.org/10.3390/heritage2030162
APA StyleL’héronde, M., Bouttemy, M., Mercier-Bion, F., Neff, D., Apchain, E., Etcheberry, A., & Dillmann, P. (2019). Multiscale Study of Interactions Between Corrosion Products Layer Formed on Heritage Cu Objects and Organic Protection Treatments. Heritage, 2(3), 2640-2651. https://doi.org/10.3390/heritage2030162