1. Introduction: The Problem of Managing Natural Heritage on a Changing Planet
This article reports the findings of an exploratory study that sought to understand the complexity and challenges of managing World Natural Heritage Sites from a case study of the Shiretoko Peninsula World Heritage Site in Japan, with a specific focus on one of the keystone species (the Blakiston’s Fish Owl). The article specifically focuses on the fragmentation of landscape level connectivity pathways and the potential of using the Ecological Integrity concept for World Natural Heritage Sites (WNHS) management. World Heritage Sites are known for their Outstanding Universal Value [
1], and WNHS, in particular, contain some of the most extraordinarily beautiful landscapes, a high degree of biodiversity and geodiversity, and habitats for many endangered species [
2]. These sites therefore are important both for their scientific and social values, the latter mainly rooted in their popularity with tourists and their potential to generate economic benefits [
3]. However it has also been noted that currently nearly half of the 229 registered sites face some type of threat such as the deterioration of ecosystems resulting from the expansion of industrial activities and urbanization [
4,
5,
6]; and those threats are amplified by factors such as institutional failure, poor management execution, and conflicts [
7,
8]. This occurs despite the World Heritage recognition supposedly bringing the highest level of international recognition to the sites [
9,
10,
11]. The problem is also related to the global trend of increasing anthropogenic pressure on Protected Areas (PA) that results in the fragmentation of ecosystems and key interaction pathways between biotic and abiotic nature [
12,
13]. In their seminal work on systematic quantitative assessment of anthropogenic threats to the WNHS between 1993 and 2009, Allan et al. (2017) found that anthropogenic pressure increased in 63% of the sites during this period: the loss of forest cover and ecological integrity were notable issues in a majority of the sites [
14]. They noted that many WNHS are deteriorating rapidly and that changes are currently under-assessed, and mentioned the need for site-level case studies to complement quantitative assessments that aggregate conditions across a large number of sites. This article aims to respond to this need by providing a qualitative analysis of the situation in the Shiretoko Peninsula. The qualitative approach was taken because key indicators are not well identified for the concerned site, as well as for the aim of highlighting the complexity angle.
While quantitative studies such as Allan et al. (2017) take up spatial analysis tools and numerical indicators to describe anthropogenic impact [
14], this article presents a holistic analysis through review of literature and empirical data from observing a key species over time; and reflects on the condition of its constituent ecosystem through a discussion of ‘ecological integrity.’
Ecological Integrity (EI) is a management guideline based on the wholeness of natural processes and organisms. Parks Canada (2018) defines EI as “…a condition that is determined to be characteristic of its natural region and likely to persist, including abiotic components and the composition and abundance of native species and biological communities, rates of change and supporting processes [
15].” In their article on the topic, Parrish et al. (2003) highlighted the presence (or lack) of functioning ecological processes over appropriate spatial and temporal scales as the key facet of EI [
16]. Elsewhere, Miller and Rees (2000) described EI as an ‘umbrella term’ that encompasses both the scientific understanding of wilderness and the social process of valuing it [
17]. Westra et al. (2000) pointed out that the concept of integrity is based on ‘wholeness’, and in the case of EI, the wholeness of wild nature (i.e., natural areas and processes that are relatively unmodified by anthropogenic impact) form the most important benchmark [
18]. They also observed that for any natural area to retain its integrity, the natural processes and the organisms or agents that partake in them must be functional over time, i.e., an apparently healthy ecosystem observed at a single point in time may not provide a good indication of its ecological integrity. These points have vital ramifications for WNHS management in particular, and for protected area management in general. However it was pointed out by Brown and Williams (2016) that the EI metric to biodiversity is not without its problems, and observed that EI-based assessments may be subject to systematic bias due to the tendency to appropriate diverse data into numerical scores as well as due to observer bias at the ground level [
19]. In order to avoid this controversy, this research does not seek to convert the condition of the concerned ecosystem into categorized scores but seeks to present a more holistic and qualitative portrait of the concept of ‘integrity’ in the concerned natural heritage landscape.
The focus of this article, the Shiretoko Peninsula WNHS in Hokkaido Island of Japan comprises of marine and terrestrial ecosystems of outstanding productivity and mutual interactions. But as it is located in a highly industrialized country, the natural landscape and its inhabitants have faced constant threats from development and anthropogenic impact over the last hundred years. Currently there are four WNHS in Japan and anthropogenic pressure on ecosystems can be observed in each of them [
20]. Major drivers of anthropogenic disturbance are infrastructure building and land development, extensive deforestation during the last century, tourism and recreational development, and the lack of robust and binding conservation targets [
21,
22,
23,
24]. Although Hokkaido is generally seen as the major island least affected by urbanization and development, it nevertheless witnessed widespread land conversion, deforestation, alteration of natural landscapes, and urbanization during the past hundred years [
25,
26,
27,
28]. Logging of natural forests and conversion of large areas supporting local flora and fauna into spaces for agricultural and settlement occurred during early-to-mid 20th century in Shiretoko itself [
29,
30]. Shoyama and Braimoh (2011) drew the important conclusion that despite a major effort of reforesting logged areas, forests of Shiretoko did not recover at patch level—the mean patch size of forest at 2004 was a mere 24% compared to 1947 (the beginning of the rapid land development phase) [
31]—this finding provides an important benchmark for understanding the current condition of EI. The area was inscribed onto the World Heritage list in 2005 under Criteria
ix, x of the World Heritage Convention; and currently its natural resources are widely seen as significant capital for tourism development. The UNESCO portal on the site mentions several problems such as industrial fisheries, presence of numerous dams on the waterways, and tourism impact as factors that currently affect the integrity of the Shiretoko Peninsula WNHS [
32]. In addition, other factors such as climate change effects and over-abundance of particular species (such as the deer species
Cervus nippon) are also mentioned as potential problems [
32]. A further point of worry here is that protected areas in general are increasingly threatened by land conversion and intensive development in their surrounding landscape [
33,
34,
35,
36]. The case of Shiretoko Peninsula, in this sense, is a valuable lesson both in its immediate context as well as for its implications for conservation challenges relating to natural heritage in general.
The article is divided into the following sections. First, the conceptual basis of Ecological Integrity and its fragmentation is explained. Descriptions of the study area and methods used for this research follow. Subsequently, the main problems affecting the EI of the property seen through the lens of the Blakiston’s Fish Owl (Bubo Blakistoni) are outlined. These sections lead to a discussion highlighting the major findings and a brief conclusion summing up the study.
3. Study Area: Shiretoko as Natural Heritage Against the Backdrop of Fragmentation of Ecosystems
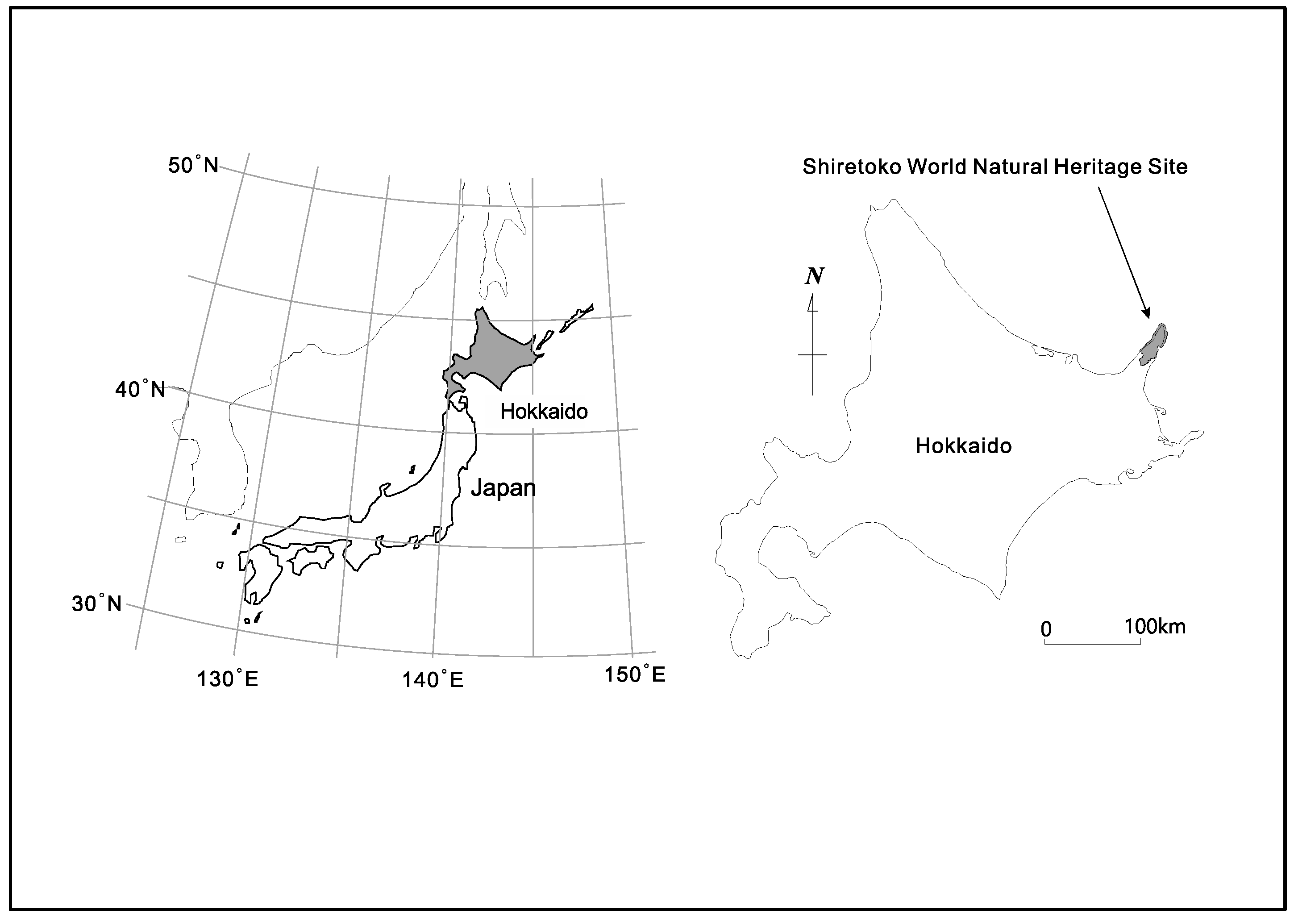
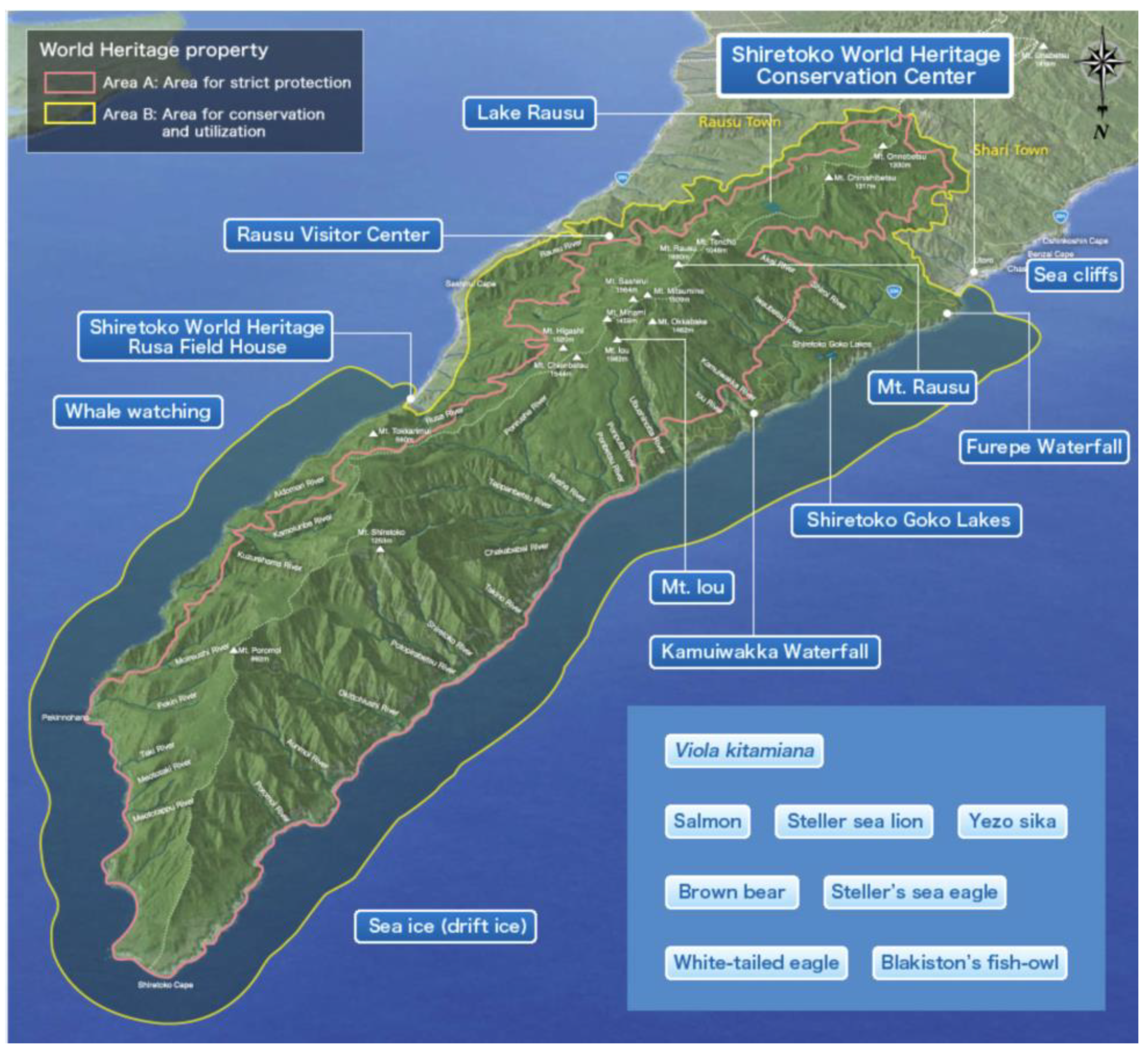
The 71,000 ha. property of Shiretoko Peninsula currently inscribed onto the WNHS list is located in the northeast of Hokkaido Island (see
Figure 1 and
Figure 2 for an outline of the WNHS property). The Outstanding Universal Value (OUV) of the property is centered on the integration of marine and terrestrial ecosystems. Marine areas surrounding Shiretoko are locations for the formation of sea ice at the most southerly latitude in the Northern Hemisphere. Sea ice formation supports a rich productivity of diatoms that form the base of a vast food web that sustains cetaceans, sea lions, the brown bear, many species of raptors, and migratory birds. The local continental shelf topography, oceanic circulation, and distant factors such as the freshwater pulse provided by the Amur River into the Sea of Okhotsk (crucial for the formation of sea ice), are important biophysical characteristics of the area. Ishikawa (2010) documented 871 plant species in the area of which 97 are endangered [
52]; and Shiretoko is either home or seasonal habitat for a number of endangered species such as the Blakiston’s Fish Owl (
B. blakistoni), Steller’s Sea Eagle (
Haliaeetus pelagicus), Steller’s Sea Lion (
Eumetopias jubatus) as well as several cetacean and seal species. In addition, the peninsular landmass provides one of the last remaining contiguous habitat range of the Brown Bear (
Ursus arctos) in the Japanese Islands. Chakraborty (2018) provided a detailed analysis of the connectivity between the geological, geomorphological and ecological components of the heritage system—and noted the important role of numerous drainage channels that connect the volcanic upland formations (mountains) to the marine environment [
21]. While under natural conditions such drainage systems are able to generate a rich variety of landform and landscape diversity through active erosion, material transport, and habitat provision, currently the rivers in the peninsula are affected by extensive anthropogenic engineering of their watercourses through damming, check weirs, and artificial embankments. These structures and local fishing practices of maximizing catch affect the natural condition of fish migration (notably salmon run consisting of the Chum, Pink, and Masu salmon among others) in such a way that the biomass transportation network throughout the peninsula is now fundamentally altered due to human impact. The WNHS State of Conservation Document of 2017 explicitly mentions check dams and related infrastructure as having considerable negative effect on fish migration and biomass/sediment transport along waterways [
32]. The report also notes the continued practice of culling of Steller’s Sea Lions due to the conflict between industrial fisheries (maximal catch) and the species’ food habit—and mentions that the sea lion population at subspecies level had previously witnessed a drastic collapse between 1977 and 2007 due to unknown reasons (unrelated to the current culling practice). Thus, despite the subsequent trend of ‘recovery’, several species remain highly vulnerable. The foregoing discussion also clarifies that despite Shiretoko Peninsula being popularly portrayed as a wild, undisturbed landscape, the heritage property and its constituent parts are under considerable pressure due to anthropogenic fragmentation.
4. A Note on Materials and Methods
This article synthesizes two phases of research: findings of a research project on the habitat and ecology of the Blaksiton’s Fish Owl (
B. blakistoni) carried out by the second author for over two decades are combined with the findings from an intensive phase of field visits jointly carried out by the two authors between February 2017 and November 2018. The research project on the fish owl’s habitat and ecology included fish owl census, monitoring of hunting behavior, surveys of stream health (fish species) and observation of forest composition. Field research involved banding of young fish owls and monitoring of owls through installation of CCD cameras in artificial nests and radio transmitters, as well as understanding of owl behavior by following their calls across watersheds [
53,
54]. Important data such as sex ratio of the owls, life-span, productivity, territories, and food habits, were gained in the process. Those findings were tallied with the four intensive field visits jointly conducted by the two authors during 2017–2018 where key sites were revisited, landscape levels characteristics were observed, and interviews with stakeholders were carried out.
The research adopted a ‘case study’ approach [
55,
56]. The spatial unit of Shiretoko Peninsula was considered a case for analysis, and the case was analyzed both
synchronically through intensive field visits and
diachronically from the insights of previous research referred above. During the intensive field visit phase, a total of 18 interviews were conducted with key stakeholders to compare their viewpoints and to identify recent developments in the area. These stakeholders were identified from their affiliation to the WNHS management structure, from their reputation as key informants in the local community, and from their profession (in the case of guides). The interviews were open-ended in consistence with the aim of the overall qualitative framework of the study. Interview data were coded (using open and axial coding) [
57,
58] to identify relevant information and to tally information with findings from previous research. The article combines findings from these data-gathering phases with instructive literature in the field to present a holistic picture.
Analysis of EI and its anthropogenic fragmentation was based on identification of key spatial and ecological attributes and understanding of key functional aspects of the fish owl’s ecosystem. Key spatial attributes included presence or absence of conditions for feeding and breeding (such as fish and tree species) and the condition of the landscape in the fish owl territory (nature of the forest, whether the forest was degraded compared to its previous state, presence or absence of anthropogenic barriers for fish movement in rivers, and presence or absence of roads, built structures, and tourist facilities in or near the fish owl’s habitat). These attributes in turn provided insights on the functional aspects of the fish owl’s ecosystem. Overdependence on a particular food source indicated depletion of alternative food species; lack of suitable trees for nesting indicated poor breeding success (unless aided by artificial measures) as well as the absence of forest environment necessary for supporting keystone species; presence of artificial barriers on streams indicated poor stream health and fragmentation of biomass circulation as well as cascading effects on the riparian biota; presence of roads and built environments provided evidence of depletion of fine-scale landscape heterogeneity and connectivity; and proliferation of tourists or related anthropogenic disturbances indicated adverse effects on hunting and breeding of an endangered species. These factors are also interrelated and impart a cumulative effect on the ecosystem; therefore, analysis of these aspects required understanding the fish owl’s ecology as a complex and interrelated entity. Such understanding was achieved at times through triangulation of existing literature sources, previous fieldwork findings, and evidence from intensive field visits and interviews.
5. Results: Challenges for EI in a WNHS as Seen Through the Case of the Blakiston’s Fish Owl
The main findings from this research are:
- i.
Legacies of past land conversion, logging, and stream modification are pervasive and currently limit fish owl survival and breeding in a significant manner, i.e., the EI of the ecosystem has declined over time.
- ii.
As the fish owl is a keystone species, its current vulnerability shows the declined state of key ecological attributes of the WNHS area: specifically the deterioration of suitable forest composition to maintain rich ecosystems and modification of stream environments resulting in the rupture of upstream-downstream connectivity and a decline of fish availability upstream constitute a deterioration of the material-energy circulation in the system; and hence are major drawbacks for EI.
- iii.
Recent tourism and related infrastructure development are novel stressors that further impact the EI of the area and the situation is complicated by a low level of awareness in a section of local stakeholders.
These insights are explained in detail below along with descriptions of the ecological characteristics of B. Blakistoni.
5.1. A Brief Note on Blakiston’s Fish Owl as an Endangered Species
The Blakiston’s Fish Owl (
B. blakistoni) is the planet’s largest owl species at ~70 cm body length, upto 4.6 kg of body-weight and ~180 cm wingspan [
59] (a photo of the owl is provided in
Figure 3 above). First described by ornithologist Henry Seebohm based on a specimen collected by the English naturalist Thomas Blakiston in 1883, this raptor finds its habitat range in Russia and northeast Asia. As its name suggests, the fish owl survives on a diet that mainly consists of fish. Globally there are an estimated 1000–1900 individuals, of which around 160–180 individuals are found in Japan. The species was widespread in Hokkaido Island in early 20th century, but its numbers fell drastically during the mid-to-late 20th century due to intense logging, land conversion, river engineering, and urban development. Its habitat steadily diminished throughout this period and currently all fish owl populations exist in fragments of their former habitat range [
52]. Fish owl numbers fell to below 100 individuals in the early years of the 1990s and the species became critically endangered. The main threats faced by the species were: drastic reduction of large trees of ~1 m diameter-at-breast-height (DBH) (a crucial requirement for nesting) due to extensive logging of natural forests, fragmentation of their territories and feeding grounds by linear infrastructure intrusions, alteration of riverflow and riverine biomass distribution due to channel engineering, and overharvesting of crucial food resource such as fish. Takenaka (2018) detailed the far-reaching changes to the owl habitats and the ecology of Hokkaido in general: rivers were polluted due to paper mill effluents, potato starch factories, and coalmine tailings; fundamental change in watershed properties resulted from large dams and small check weirs for regulating water and sediment flow that currently number in thousands; and rivers were regulated by channel engineering [
53,
54]. A conservation-restoration program targeting the critically endangered fish owl began in the 1980s, which resulted in the set-up of 300 artificial nests to facilitate breeding, at present nearly 80% of breeding fish owl pairs of Hokkaido are dependent on nest boxes [
53].
Among the breeding pairs, nearly half are located within the Shiretoko Peninsula (both inside the WNHS territory and adjacent areas). There are currently 30 breeding pairs in Shiretoko of which 11 pairs inhabit the WNHS territory, making Shiretoko the most important habitat-area for conserving fish owls in Japan. In 1993 the fish owl was declared a nationally endangered species [
53], which aided the subsequent efforts to conserve the species. The second author of this article has led the fish owl conservation/breeding assistance program with support from the Ministry of the Environment and the Forestry Agency. During the stage of preparation of Shiretoko as a World Heritage nomination, the fish owl’s presence was used for appealing to both national and international audience to recognize the value of the natural environment.
5.2. The Ecology of Fish Owls
True to its name, the fish owls persist mainly on fish. In the case of Shiretoko, the species is apparently highly dependent on the Dolly Varden Char (
Salvelinus malma)—observation of food mass brought into one nest showed that
S. malma occupied 67% of that pair’s diet (a photograph of the Dolly Varden Char is given below in
Figure 4). This has implications for EI and ecosystem integrity, as observed in the following section. It appears that though the fish owls of Shiretoko mainly feed on fluvial fish, marine fish is also an important part of their diet—this is why fish owls are often observed flying into the rivermouth or coastal areas where they have higher chances of collision with vehicles and other artificial infrastructure. Fluvial fish are generally caught within a few kilometers of the nest and marine fish predation mainly occurs at late night—perhaps indicating that the owls have adapted to avoid human activities in the coast during earlier hours.
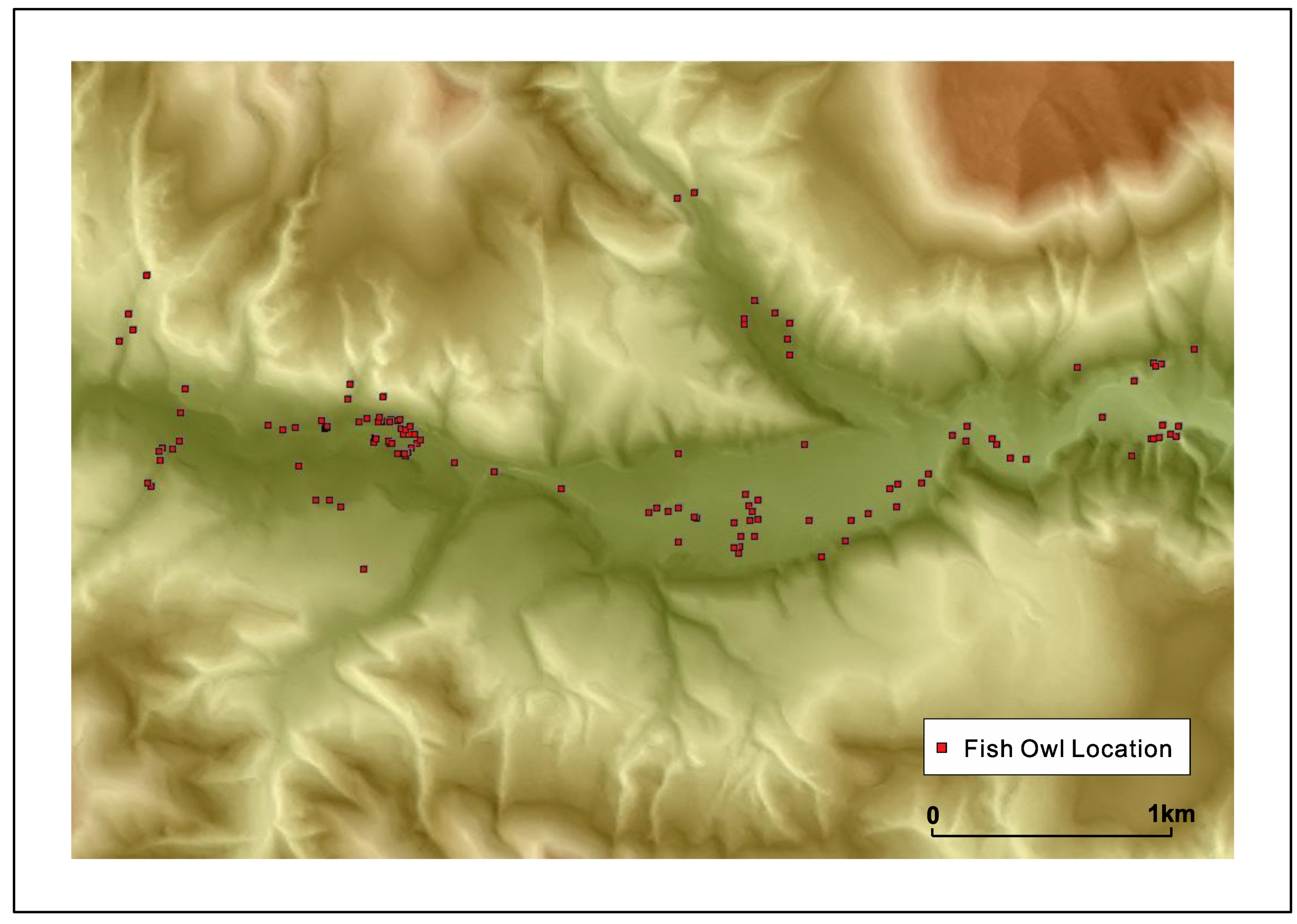
Fish owls nest in pairs and usually the pairing is long-term. A pair establishes and guards its ‘territory’. It was revealed by careful observation and tracking by transmitters that the territory of a pair can extend normally up to 10 km in Hokkaido but the range tends to be a little smaller in Shiretoko, possibly due to landform barriers of between drainage basins and better fish availability; although territory size can be influenced by the density of fish owls in an area as well owing to the highly territorial nature of these raptors. A graphic of a fish owl’s movement across a drainage basin is provided in
Figure 5 below in order to illustrate the dimensions of its territory. From the second author’s sampling of fish biomass across 33 river basins in Hokkaido, it was established that fish owls would require presence of 25 salmonid fish/100 m
2 over the feeding area, which roughly corresponds to the excess of 1000 gm./100 m
2 of fish biomass in the minimum. It was also found that rivers with a high density of
S. malma supported a higher density of fish owls compared to streams where
Salvelinus leucomaenis (White-spotted Char) and
Oncorhynchus masou masou (Masu salmon) were dominant. As Kitano (1995) described,
S. malma is a relict species from the last glacial maximum restricted to colder mountainous streams [
60], and this raises the possibility that the fish owls of Shiretoko are uniquely adapted to this species. It is also likely that human modification of watercourses that once supported high density of anadromous fish are in a state of higher disturbance and therefore inhibit fish owl survival, leading to the adaptation of the species to colder mountainous stream ecology in remote areas.
Fish owls require large cavities in trees to nest and therefore their intergenerational survival is closely related to the composition of the forest. Large deciduous trees are ideal for the species and from past records and current monitoring it was identified that deciduous varieties such as the Japanese Elm (
Ulmus davidiana var. japonica), Mizunara Oak (
Quercus crispula Blume), Manchurian Elm (
Ulmus laciniata), and Katsura (
Cercidiphyllum japonicum) are preferred. Photographs of these key tree species are given in
Figure 6 below. As the species nests in large cavities, comparatively older trees are preferred, this implies that old-growth or undisturbed riparian forests were crucial for supporting a high number of fish owls in Hokkaido before the twentieth century economic development and deforestation; and that deforestation, especially logging of older forest tracts, was a main cause behind the swift decline of the fish owl population. Along with the reduction of such forest cover, watershed characteristics were also drastically changed due to human modification of watercourses, and this led a lasting impact on the fish owl’s ecology.
5.3. Current Stressors in the Fish Owl’s Habitat
The biggest current threats for the fish owl ecology are: (i) anthropogenic modification of riparian forests and (ii) the fragmentation of rivers in the WNHS area.
As mentioned above, large deciduous trees are vital for the intergenerational continuity of the species. However, the forests of Shiretoko had experienced logging on an extensive scale merely fifty years ago and have not recovered enough, largely due to the slow natural process of forest maturity. In the WNHS area the key deciduous species of
U. davidiana, U. laciniata, Q. crispula, and
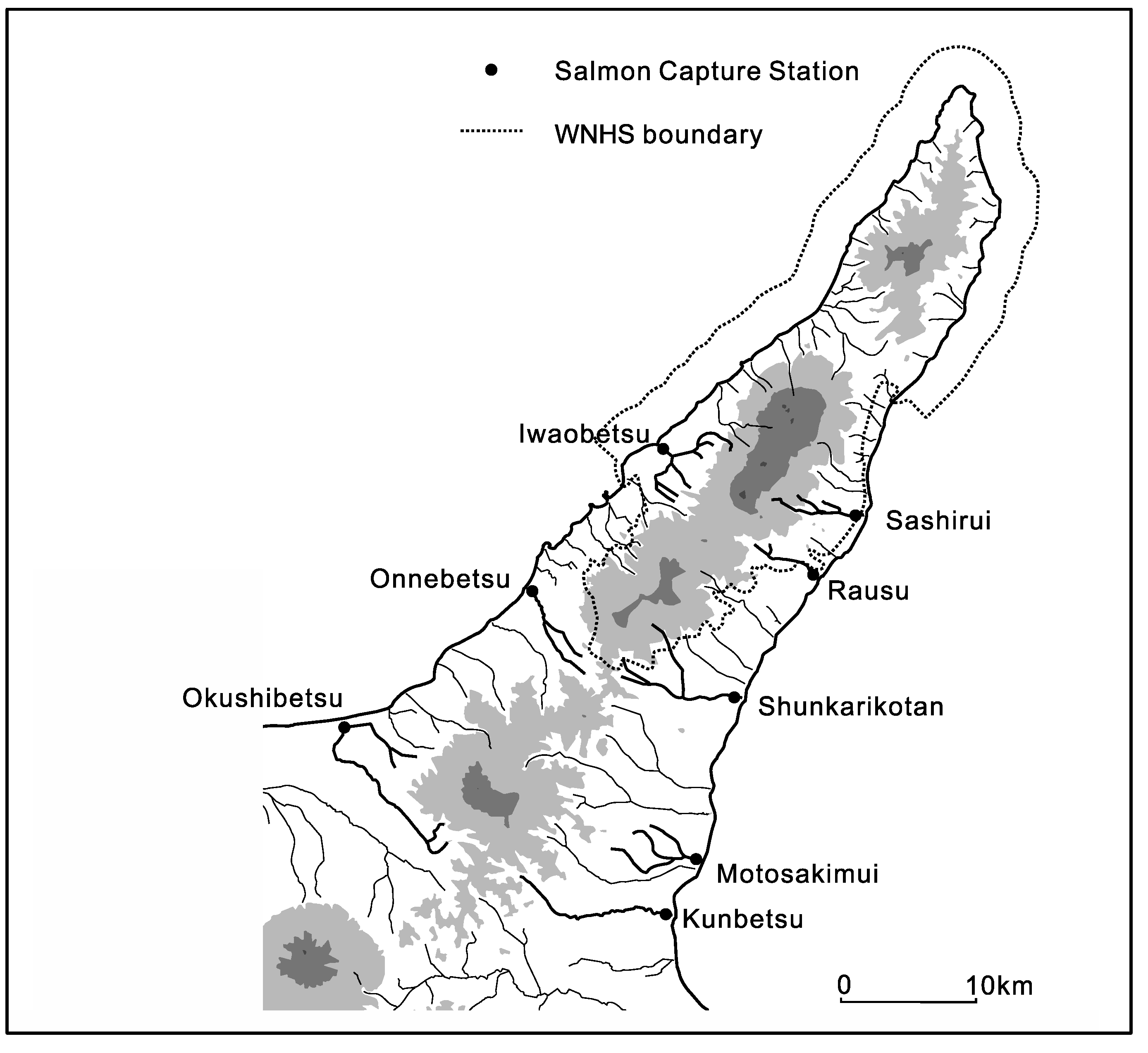
C. japonicum cover a mere 12–28% of the forested area and trees over 1 m DBH are rare. Nearly all drainage basins have some form of anthropogenic impact and upstream-downstream connectivity is fragmented. A total of 8 artificial salmon capture stations are located in and around the WNHS, of which 3 are located within the WNHS territory (a graphic of their locations is provided in
Figure 7 below). Thus the radial drainage that connects ecosystems across the WNHS and trophic levels within ecosystems is constrained and the circulation of material and energy occurs far below the natural level necessary to support these highly complex systems. Without any meaningful protection of prey species and the safeguarding of their migration pathways, conservation of the apex species is likely to remain a highly challenging task. For an instance; the important prey species of
S. malma currently faces a novel threat from an increase of sport fishers in the area. Under the Japanese legal system, although national park land is protected, rivers do not have protected status and therefore can be exploited relatively freely. As noted above,
S. malma is concentrated in the colder streams of Shiretoko, and an increase of amateur fishing activity is likely to have a strong impact on the species density in the streams. As the size of the streams is small, the effect of fishing can appear suddenly and in a drastic manner—which was observed at several sites. Although specific data is not currently available, based on repeated observations we fear that such change is already underway in some rivers of this area.
While the WNHS itself is protected, its surrounding areas are not; and this poses another significant threat for species like fish owls that locate themselves at the top of the local food chain. Roads connecting Shiretoko to surrounding areas have increasingly expanded in terms of surface area and were straightened to allow speedier traffic. In order to accommodate larger and straighter roads—which are partially in response to facilitate tourist access—coastal landfilling, tunnel construction, stabilization of erosion-prone hillslopes (facing the coast), construction of concrete breakwater, and logging of large trees have taken place since the 2005 inscription. The WNHS Visitor Center and the adjacent tourist facility in Shari Town are located on a large stretch of landfilled site on the bay that erased the fine-scale structure of the coastline of that section. As mentioned earlier the fish owls of Shiretoko also predate on marine fish for a substantial part of their diet and these developments affect their hunting behavior adversely. An increase of roads and vehicular traffic has also brought new risks. In 2018, two fish owls were involved in vehicular collisions; one of the birds died while the other was unable to return to the wild.
In addition to these physical barriers, fish owls and other animal species are also probably affected by tourism related activities. Currently an estimated 2.5 million visits occur in the Shiretoko Peninsula annually. Most tourism activities are concentrated at the peripheral sections of the terrestrial part of the WNHS, but the marine part of the property is widely utilized by sightseeing boats. Visiting the ‘five lakes’ area in Shari, hiking mountains in the central highland, kayaking/canoeing in nearshore waters, and boat cruises that offer glimpses of marine wildlife, are major tourism themes. Owing to heavy snowfall in winter, tourist activities are concentrated in summer to autumn, although drift ice watching tours and snowshoe walks in winter forests are popular as well. Tourism foci are contrasting in the two administrative units of Shari and Rausu Towns: most tourism attractions in Shari are terrestrial but in Rausu—which is also a major local fishing port—tourism packages include marine wildlife watching (cetacean and seal species in summer and birds in winter). Although impact on the natural environment from individual tours is likely to be low, there are worrying aspects such as artificial feeding of fishing eagles in the winter, isolated incidents of tourists throwing food to lure animals for photography, and eagerness of some photographers to approach bears. All of these activities potentially disrupt feeding and movement patterns of animals. In addition, cruise boats often venture close to rocky ledges used by a number of bird species for nesting and feeding, and the existence of a large number of similar tour packages possibly imparts an amount of stress to the local environment. There has been a rapid increase of guided tours and wildlife viewing packages in the area starting around the time when the property was being prepared for WNHS nomination. A number of guides currently engage in searching fish owls with high-powered searchlights in the night—as a part of the nighttime wildlife viewing tours that are gaining popularity. Some guides also take visitors to areas known as fish owl feeding spots and try to locate fish owls with searchlights, this is also likely to disturb the hunting success and feeding behavior of the species. There are even instances of tourists trying to search owls in the night with their own searchlights to avoid paying guiding charge. Apart from these, an inn in Rausu Town engages in baiting a fish owl pair with fish in an artificially lighted location to allow photographing opportunities. Although this practice is currently limited to a single facility, the precedent set in this manner is not ideal for managing an endangered and sensitive species in a WNHS. The interviews revealed a generally low capacity of tourism stakeholders to manage human-wildlife conflict, dependence on middle-aged or older visitors from urban areas, a tendency to facilitate easy access, limited linguistic skills of guides inhibiting meaningful interaction with international visitors (who are coincidentally more likely to approach wildlife), lack of experience in guides in terms of familiarity with other WNHS, and a strong preference of retaining as many tourists as possible. There was also the important revelation that though nominally entrusted with the management of the WNHS, the national park managers are often seen as ‘outsiders’, with local actors such as fishermen and tourism business owners having a strong influence over real management. A table showing information with open and axial coding, along with the interview topic guide, are available as supplementary files.
6. Discussion: Insights from Ecological Integrity for Better Management of WNHS
The concept of EI allows us to think beyond species level to trophic and assemblage levels in ecosystems. Seen from this perspective, the fish owl case study offers several important insights regarding the current level of fragmentation and better management pathways for WNHS.
In this exploratory study, the EI concept was mainly used for describing the state of fragmentation in the trophic levels and species assemblages that are key for the long-term survival of resident keystone species such as fish owls. It was noted that two guiding questions regarding EI are: whether the most critical threats confronting the biological resources are changing severity or geographic scope in response to conservation strategies; and whether the ecosystems, communities, or species that are the foci of conservation occur with sufficient size, with appropriately functioning ecological processes, and with sufficiently natural composition, structure, and function to persist over the long term [
16]. From the case study three overarching findings regarding ecosystem integrity can be provided: (i) the reduction of diversity in the prey species over time; (ii) the reduction of ecological functions of streams due to stream engineering; and (iii) the loss of appropriate habitat structure for both the predator and prey species. These conditions have not changed significantly since the WNHS inscription, and in some cases they face further and novel disturbances.
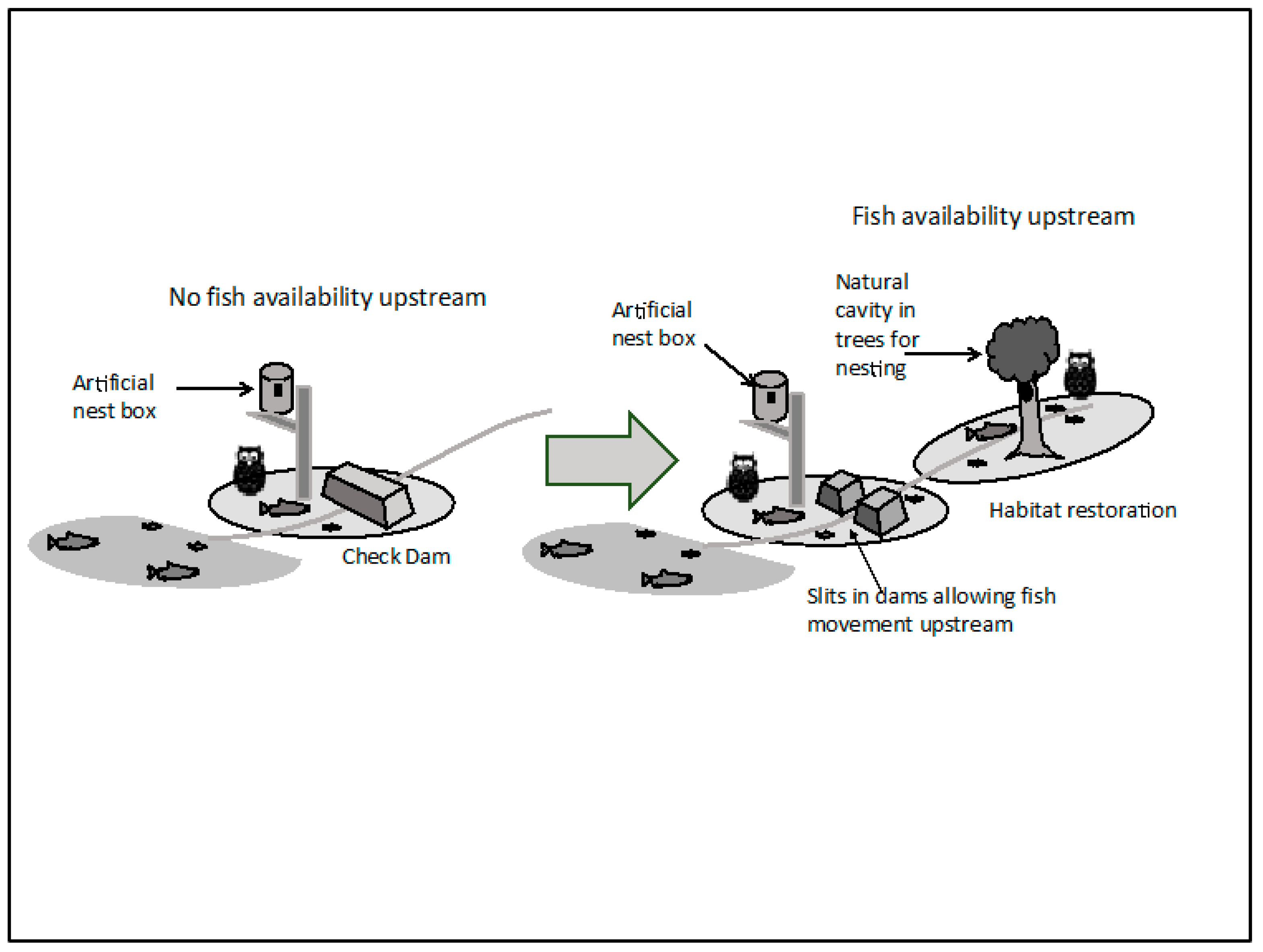
While the current owl population in Hokkaido shows a modest recovery compared to the 1980s, 80% of the owl pairs are dependent on artificial nest-boxes, and in some cases artificial feeding is necessary for increasing the productivity and survival of newborn owls. While artificial feeding is useful for preventing untimely death of owl chicks, it has the detrimental effect of discouraging young owls from dispersing from their natal territories—and over the years this has resulted in a higher ratio of inbreeding in Hokkaido in general. It is also noteworthy that the retraction of fish owl habitat in the twentieth century also contributed to the inbreeding problem. While in Shiretoko better availability of fish in the streams ensured that artificial feeding was not required for owl conservation, artificial nest-boxes had to be installed as deforestation had reduced suitable natural nesting opportunities drastically. The low-productivity of fish owls of Shiretoko reflects this situation. However, the presence of a facility that currently engages in baiting fish owls for facilitating animal photography carries the risk that in the future owls near this location will be able to outperform others in breeding success, resulting in unnatural genetic distribution. The recovery of suitable habitat is an urgent need; but the process to regenerate mature broadleaf forests is a slow one—and is still in its early stages. Similarly, restoration of stream biota and key ecosystem processes such as natural water-sediment transport regime is crucial. Some efforts to create ‘slits’ in existing silt-check dams and construction of fish ladders are aimed at improvement of biomass circulation at the watershed level—but rivers in Shiretoko, and Hokkaido in general, remain far-too-much constrained to allow near-natural biomass circulation. Thus, the two notable aspects of fragmentation that currently affect the EI of fish owl ecosystem—and in turn the terrestrial ecosystem components of the WNHS are:
- (i)
the fragmentation of the forests and the change in their composition into a state where key species are not able to breed, hunt, and disperse at appropriate scales; and
- (ii)
the fragmentation of biomass circulation, notably salmonid run in the rivers that support the biodiversity of the WNHS.
As Shoyama and Braimoh (2011) demonstrated; forests of the area still show a legacy of the twentieth century deforestation and land development and occupy considerably smaller patches compared to the pre-cultivation stage [
31]. This fragmented state of the habitat poses a fundamental challenge for restoring the ecology of keystone species such as fish owls. It is important to add that as fish owls live for several decades, the species typically shows a ‘time-lag’ in its response to habitat conditions. This was observed in the case of their population shrinkage—while the cultivation of Hokkaido began in the early 20th century, fish owl populations fell to critical levels several decades later in the 1980s–1990s. Similarly, habitat recovery measures will not translate to immediate reduction of threats at the species level. Especially without suitable corridors and habitats for dispersal, the current condition of dependence on artificial feeding and problem of inbreeding will continue. This situation requires the restoration of mature broadleaf forests throughout Hokkaido Island, and especially in and around the Shiretoko Peninsula.
Secondly; most rivers in the WNHS have some type of artificial modification (including dams, check weirs, rivermouth weirs, artificial embankments, and channel engineering). This situation poses a significant barrier for the movement of anadromous fish species. Without the restoration of salmonid migration in the rivers of the WNHS, it is likely that the apex predator species will remain vulnerable. It should also be kept in mind that in the case of Shiretoko, the fish owl’s successful survival is largely due to the availability of fish species such as
S. malma—but as this particular fish is adapted to colder stream temperatures, it will likely fare poorly if climate change results in increased warming of the streams. Besides, as reported earlier, the recent tendency of amateur fishers to catch
S. malma—which is allowed to continue due to the lack of provisions to stop fishing in rivers—carries the added risk of reducing the species density in Shiretoko’s streams. From this point of view, it is clear that the food source of the fish owl is in a vulnerable state due to over-reliance on a single species (
S. malma) which itself is under considerable stress. With reference to the point made by Woodley (2010) [
38], it can be said that the trophic levels do not show the desired level of integrity due to unavailability of a diversity of prey species in the rivers; and the implication for the integrity of nutrient cycling is also negative. While the ‘State of Conservation’ document of 2017 mentions river engineering and aquaculture as having negative effect on natural salmonid run in the area [
32], in our opinion the problem is under-stated, and swift and effective measures are required to restore the natural fish cycle. Allowing fish and biomass to pass artificial barriers by designing slits in check dams or removing them whenever appropriate will be a meaningful step for restoring the resource flow across drainage basins; this is illustrated in
Figure 8 below. There is an urgent need for river management to shift from artificial modification of watercourses in order to safeguard infrastructure, profit, and human interest to a more holistic vision of managing rivers in a world heritage site for their ecological functions.
It is also an added cause for concern that the WNHS inscription has apparently led to emergence of some novel stressors in this already fragmented environment. Construction of roads, tunnels, and infrastructure (outside the WNHS area but nevertheless in locations that are used by species such as the fish owl) to accommodate tourist demand; as well as increased pressure on the species from the behavior of tourist and tour guides, are two prominent examples of new stressors. Larger roads bring in speedier traffic, thereby increasing the chance of mortality by accident; and road, tunnel, and buildings erase the fine scale heterogeneity in the landscape that supports the interaction between predator and prey species. The practice of chasing fish owls with searchlight, taking visitors to fish owl feeding areas in the night, and baiting fish owls for photography enthusiasts all constitute worrying signs indicating that this fragile species is being exploited as a tourism resource with potentially adverse impacts on its hunting behavior.
Therefore, from this case study it can be posited that while the fish owl remains a primary conservation focus in this WNHS, the ecological processes required for its long-term survival and the ecosystem within which the species is located do not occur with sufficiently natural composition, structure, and functional attributes; and therefore the ecosystem within which the species is found lacks integrity. While Shiretoko sells ‘connectivity’ between ecosystems as a mainstay for its claim of Outstanding Universal Value, upon close inspection it become clear that connectivities between species, communities, and ecosystems remain in a fragmented and fragile state. The conceptual framework of EI gives us vital insights about the nature of this fragmentation, and is useful even in cases where precise data on the scale and state of fragmentation is not available. The following recommendations are drawn from these insights for the management of this WNHS:
- (i)
Seen in its role of a ‘keystone’ species, the fish owl is an indicator of the state of the ecosystem it fits into. Habitat shrinkage, dependence on artificial nests, and lack of diversity in food (prey species) are clear signs that the environment in the WNHS (particularly riparian forest composition, stream ecological health, and spatial connectivity) is in a highly fragmented state. This situation requires proactive forest and stream restoration; particularly restoration of forests at suitable patch levels and with suitable tree species, and rehabilitation of natural stream functions.
- (ii)
As the fish owl is nocturnal and sensitive, it is difficult to monitor the species directly, but it will be useful to monitor the health of its food chain by observing the condition of its prey species and stressors on those species. As mentioned already, the food chain remains disrupted due to artificial alteration of salmonid species’ migration routes and aquaculture. While fishing of salmonid species cannot realistically be prohibited as they bring significant profit; it is evident that overharvesting of these species has resulted in poor stream health and fragmentation of trophic level integrity. Besides, salmonid migration is important for many other species such as U. arctos and fishing eagles. Urgent attention is therefore required to restore salmonid migration in the streams of WNHS.
- (iii)
It will be necessary to protect prey or lower trophic level species (fish in this case) in order to safeguard the EI of sensitive ecosystems. Currently there is no restriction on fishing in the rivers of the WNHS and its adjacent areas—this situation has resulted in a highly vulnerable situation of trophic level connectivity. It will be required to develop local ‘codes of conduct’ to prohibit overharvesting of crucial prey species, if national level legal protection is not available.
- (iv)
WNHS managers should proactively push back against development projects in adjacent areas as ecological integrity and species health cannot be managed by solely protecting the WNHS (the ‘kernel’ site) itself but requires protection and rehabilitation of landscapes surrounding the WNHS, particularly in the cases where it is clear that the species that are the foci of conservation use those areas for feeding, migration, or dispersal. In cases such as this particular WNHS this would entail the restriction of coastal development and protection of vegetation at the roadsides that provides crucial cover for species movement. Kormos et al. (2016) and Allan et al. (2017) pointed out the urgent need for utilizing existing mechanisms such as the ‘buffer zone’; and argued that expansion of appropriate buffers can be useful for protecting ecological integrity and promoting connectivity in and between ecosystems [
36,
61]. Our findings from the Shiretoko WNHS support this argument: the existing buffer zone around the property should be expanded based on ecological principles and its capacity to mediate anthropogenic stress.
- (v)
It would be necessary to establish corridors for species movement and dispersal in areas around the heritage site. This in turn will require the understanding that the environment constrained within the WNHS cannot retain its integrity over the long term without connection to the broader landscape. Appropriate long-term plans of establishing ecological corridors for species movement as well as for movement of abiotic components such as water or silt that have crucial ecosystem functions are therefore required to address the problem of ecological integrity.
The broader significance of the findings is twofold: they help to generate relevant knowledge for effective safeguarding of natural heritage through the World Heritage Convention, and they provide instructive insights for effective management of natural heritage landscapes in general. Both Kormos et al. (2016) and Allan et al. (2017) noted that currently there are significant gaps in the World Heritage Convention that makes its efficacy to safeguard wilderness a limited one, and that there is a need to upscale heritage conservation by integrating sites [
36,
61]. EI, as analyzed in this study, can be a pertinent conceptual tool in this regard. Finally, a number of studies have highlighted the potential of EI to inform sustainable management of natural areas and raise social awareness [
62,
63]; and the insights gained from this WNHS are useful for designing robust natural heritage conservation schemes that can deliver meaningful results against the general backdrop of pervasive anthropogenic fragmentation of the planet’s biophysical heritage.