Casting Light on 20th-Century Parisian Artistic Bronze: Insights from Compositional Studies of Sculptures Using Hand-Held X-ray Fluorescence Spectroscopy
Abstract
1. Introduction
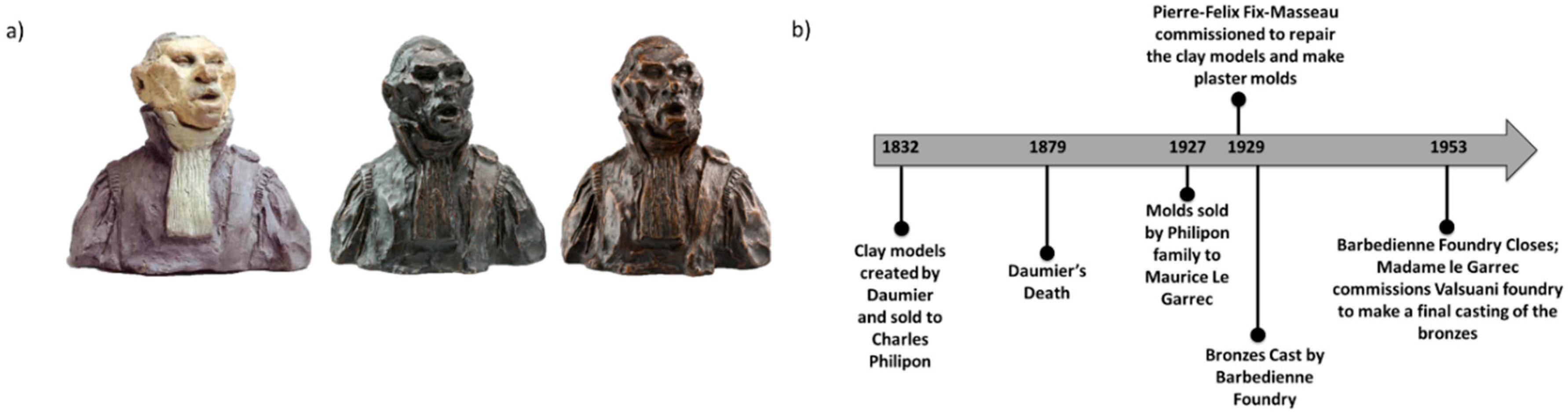
Honoré Daumier’s Busts
2. Experimental
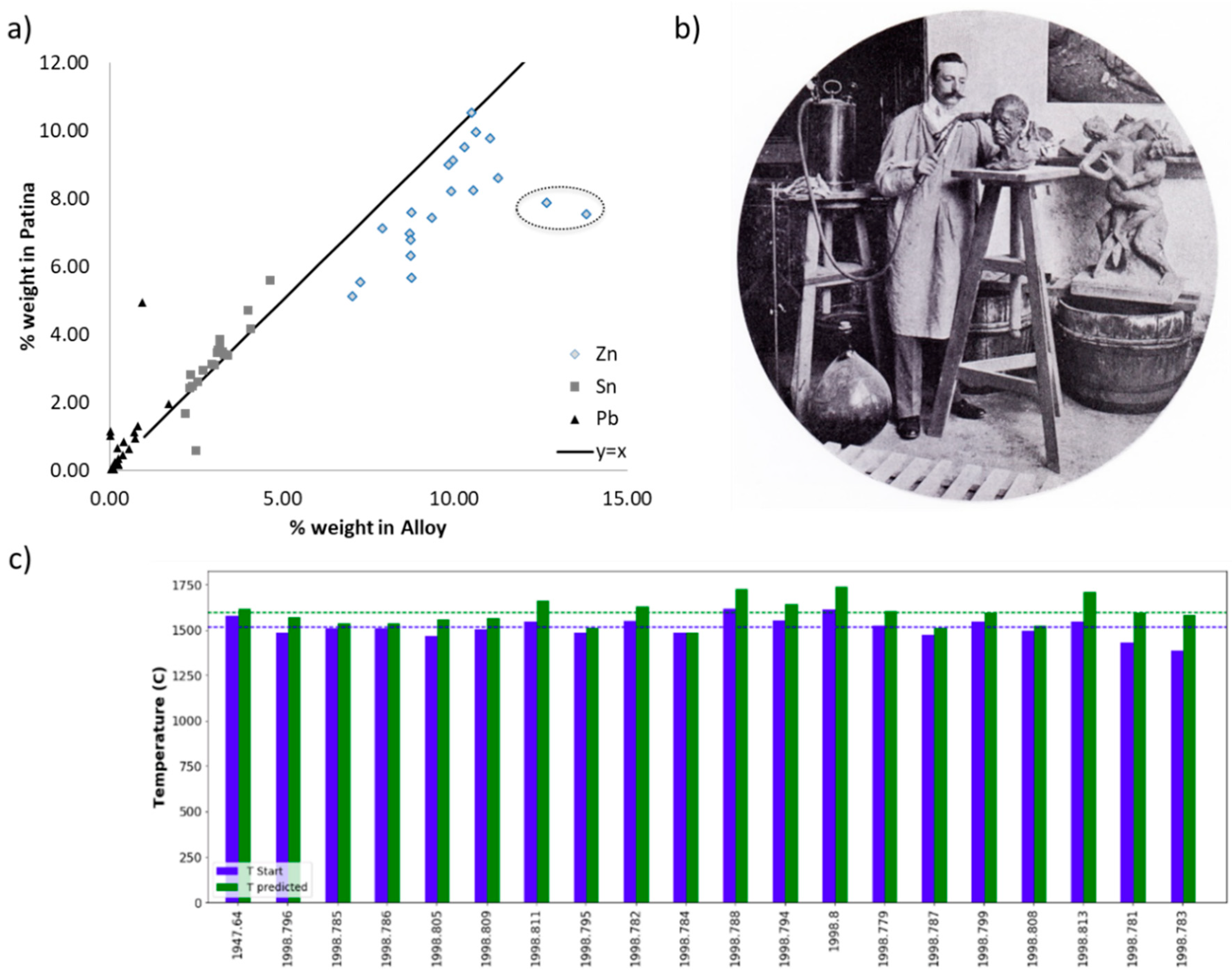
2.1. Hand-Held X-Ray Fluorescence Spectroscopy as a Semi-Quantitative Method to Determine the Elemental Composition of Bronzes
2.2. Building a Compositional Database for Artistic Bronzes
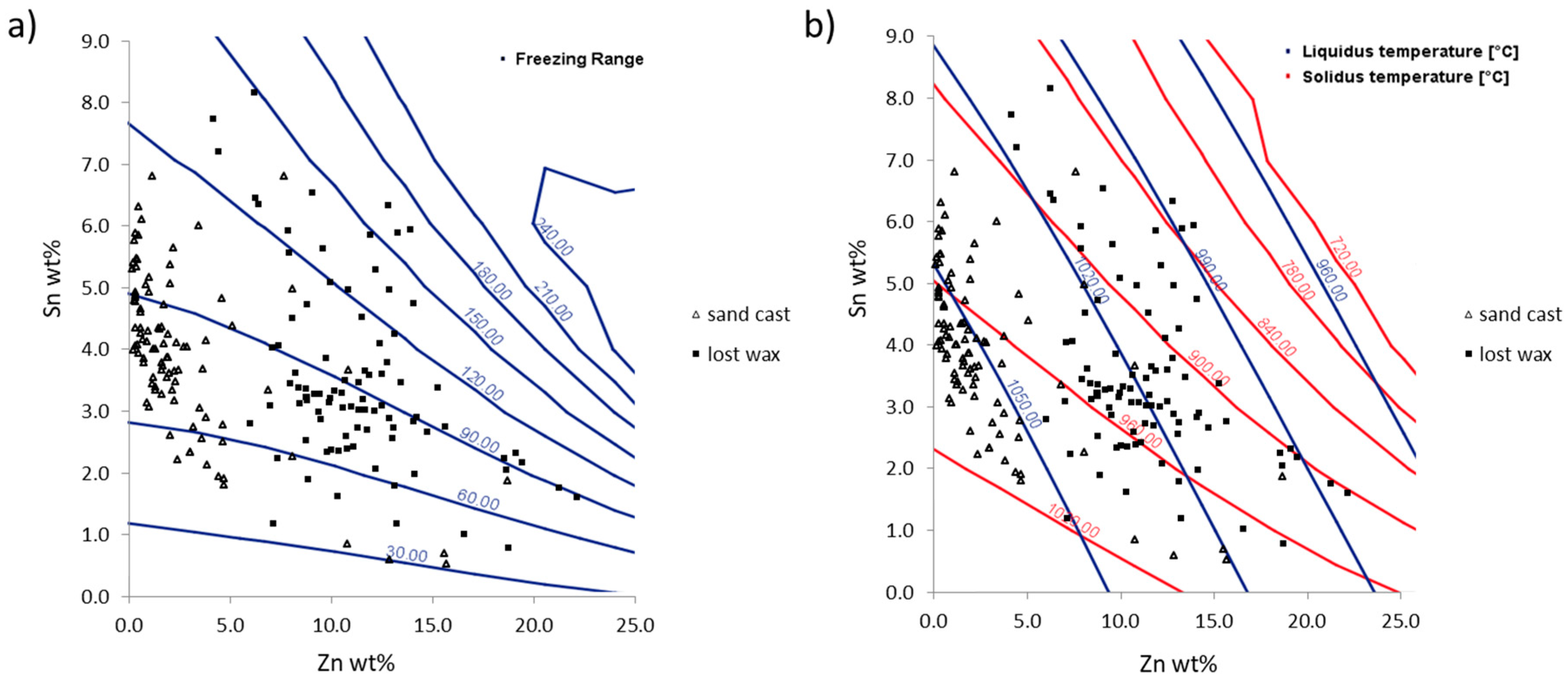
3. Interpreting Compositional Data: First Steps in Determining Foundry Fingerprints
4. In Focus: Honoré Daumier’s Bust Series
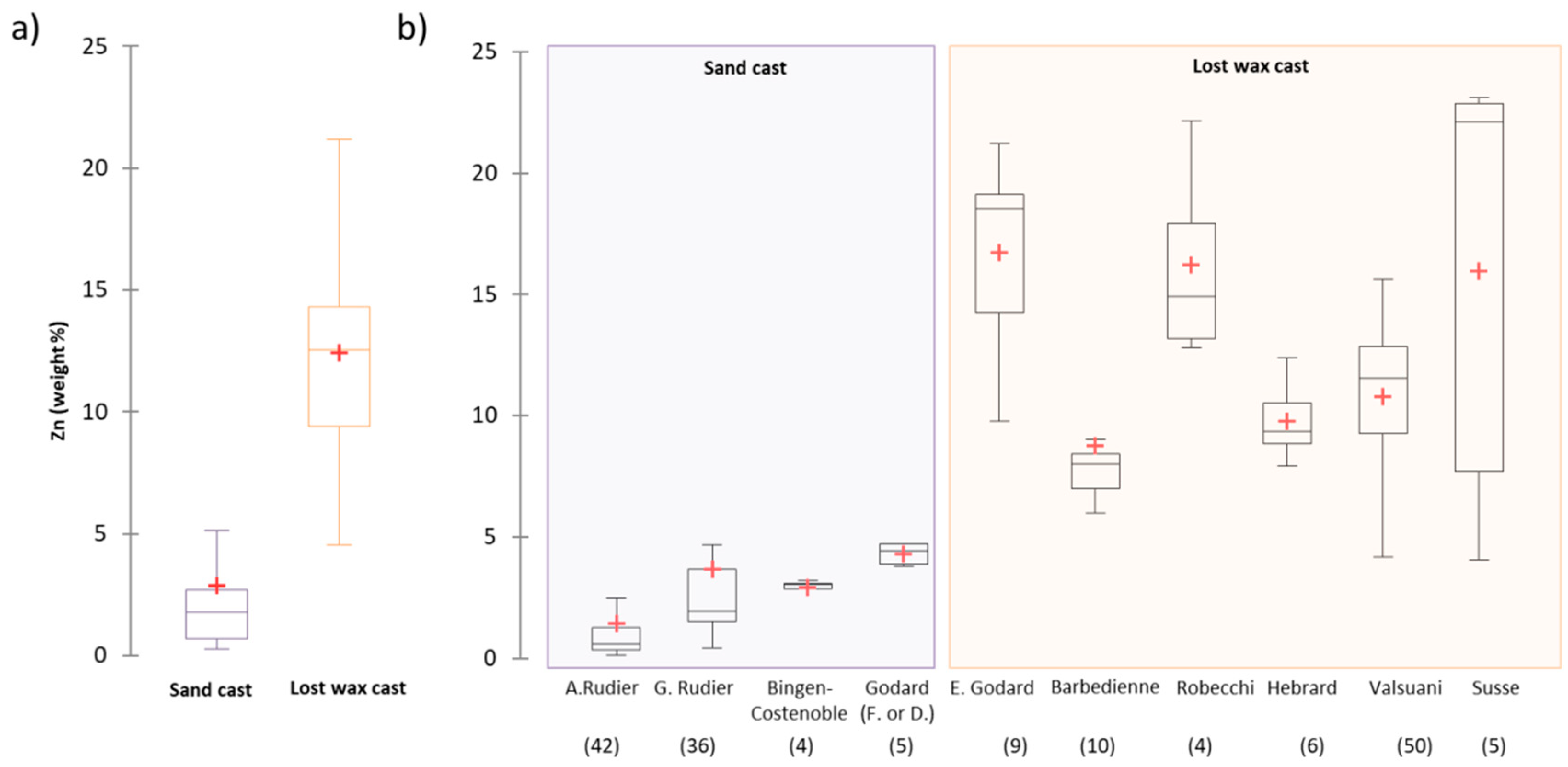
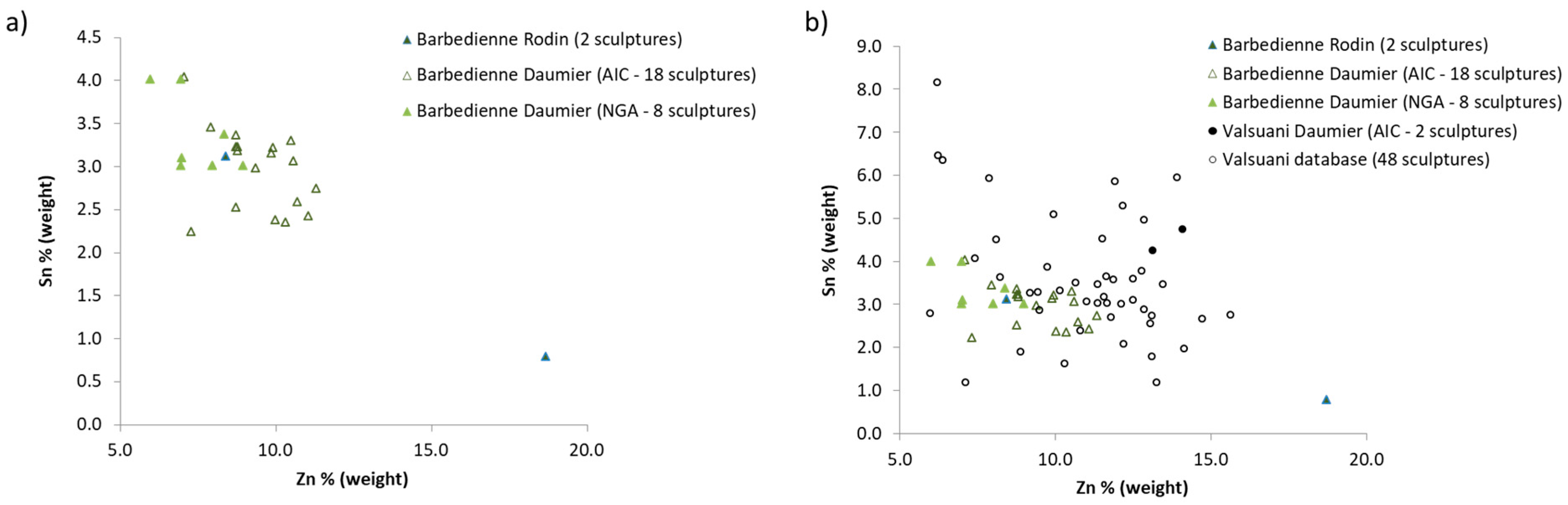
4.1. The Alloy Composition: The Fingerprint of the Foundry
4.2. Patina Technique and Materials as Supplementary Criteria of Differentiation between Foundries
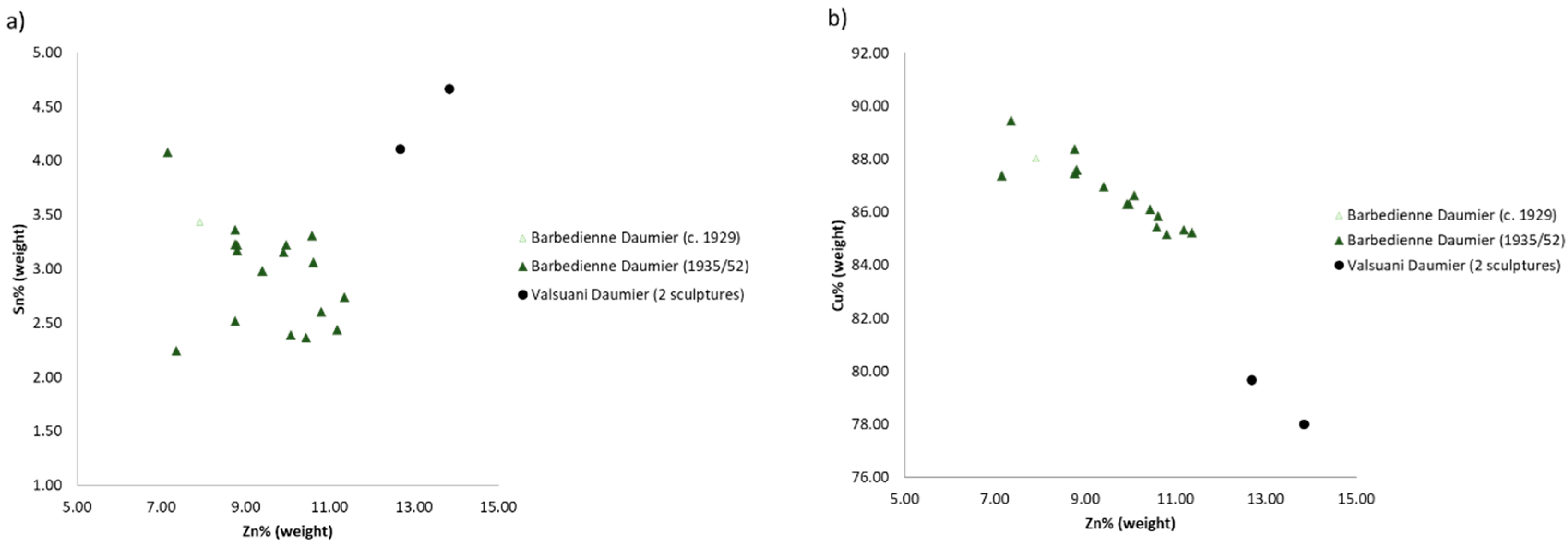
4.3. The Daumier’s Casts in the Larger Context of the Production of the Valsuani and Barbedienne Foundries
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| AIC Accession Number | Cast Period | Cast Marks | Composition | Cu (%) | Zn (%) | Sn (%) | Pb (%) | Cr (%) | Fe (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1947.64 | c. 1929 | MLG (Maurice Le Garrec) on proper left near base -E2 1/25 on underside rim, 1/25 on inside front | Patina | 89.1 | 7.1 | 3.4 | 0.0 | 0.0 | 0.1 |
| 0.7 | 0.5 | 0.1 | 0.0 | 0.0 | 0.0 | ||||
| Base | 88.0 | 7.9 | 3.4 | 0.1 | 0.0 | 0.1 | |||
| 0.4 | 0.2 | 0.4 | 0.0 | 0.0 | 0.0 | ||||
| 1998.796 | 1935–1952 | MLG on proper left near base; BRONZE underside rim proper right; 7/25 inside front proper right | Patina | 88.1 | 8.2 | 3.1 | 0.0 | 0.0 | 0.2 |
| 1.0 | 1.3 | 0.6 | 0.0 | 0.0 | 0.0 | ||||
| Base | 85.8 | 10.6 | 3.0 | 0.1 | 0.0 | 0.1 | |||
| 0.3 | 0.2 | 0.2 | 0.1 | 0.0 | 0.0 | ||||
| 1998.785 | 1935–1952 | MLG on back proper left near base with BRONZE beneath partially visible; 18/25 inside front | Patina | 86.8 | 9.1 | 2.8 | 0.6 | 0.0 | 0.3 |
| 0.9 | 0.7 | 0.2 | 0.2 | 0.0 | 0.1 | ||||
| Base | 86.6 | 10.0 | 2.4 | 0.6 | 0.0 | 0.2 | |||
| 0.3 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | ||||
| 1998.786 | 1935–1952 | MLG and BRONZE on back proper left; 17/25 inside front proper left | Patina | 86.6 | 9.0 | 3.5 | 0.3 | 0.0 | 0.3 |
| 0.4 | 0.3 | 0.2 | 0.3 | 0.0 | 0.1 | ||||
| Base | 86.3 | 9.8 | 3.1 | 0.3 | 0.0 | 0.2 | |||
| 0.5 | 0.7 | 0.3 | 0.0 | 0.0 | 0.0 | ||||
| 1998.805 | 1935–1952 | MLG and BRONZE on back proper left; 10/25 inside front | Patina | 87.7 | 8.6 | 2.9 | 0.2 | 0.0 | 0.3 |
| 1.0 | 1.0 | 0.2 | 0.1 | 0.0 | 0.0 | ||||
| Base | 85.2 | 11.3 | 2.7 | 0.3 | 0.0 | 0.2 | |||
| 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | ||||
| 1998.809 | 1935–1952 | MLG and BRONZE on proper left shoulder near base; 18/30 inside front | Patina | 86.5 | 8.2 | 3.8 | 0.6 | 0.0 | 0.5 |
| 0.9 | 0.6 | 0.1 | 0.1 | 0.0 | 0.1 | ||||
| Base | 86.3 | 9.9 | 3.2 | 0.2 | 0.0 | 0.1 | |||
| 0.1 | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 | ||||
| 1998.811 | 1935–1952 | MLG back proper left near base; BRONZE underside rim; 7/30 inside front | Patina | 89.8 | 6.3 | 3.4 | 0.0 | 0.0 | 0.2 |
| 0.5 | 0.4 | 0.1 | 0.1 | 0.0 | 0.1 | ||||
| Base | 87.5 | 8.7 | 3.3 | 0.1 | 0.0 | 0.1 | |||
| 0.4 | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 | ||||
| 1998.795 | 1935–1952 | M.L.G. and BRONZE on back, proper right; 11/25 on inside front, proper right;11/25 on underside rim, proper right | Patina | 85.9 | 9.9 | 2.6 | 1.1 | 0.1 | 0.1 |
| 0.5 | 0.4 | 0.1 | 0.1 | 0.0 | 0.1 | ||||
| Base | 85.2 | 10.6 | 2.6 | 0.7 | 0.0 | 0.6 | |||
| 0.4 | 0.3 | 0.1 | 0.0 | 0.0 | 0.4 | ||||
| 1998.782 | 1935–1952 | MLG and BRONZE on back proper right near base; 10/25 inside front | Patina | 88.7 | 6.9 | 3.6 | 0.2 | 0.0 | 0.2 |
| 0.6 | 0.8 | 0.3 | 0.0 | 0.0 | 0.0 | ||||
| Base | 87.5 | 8.7 | 3.2 | 0.2 | 0.0 | 0.2 | |||
| 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | ||||
| 1998.784 | 1935–1952 | MLG and BRONZE on back proper left near base; 17/25 inside front | Patina | 84.7 | 10.5 | 3.5 | 0.4 | 0.0 | 0.3 |
| 0.4 | 0.3 | 0.2 | 0.1 | 0.0 | 0.1 | ||||
| Base | 85.4 | 10.5 | 3.3 | 0.4 | 0.0 | 0.4 | |||
| 0.7 | 0.5 | 0.2 | 0.0 | 0.0 | 0.4 | ||||
| 1998.788 | 1935–1952 | MLG and BRONZE on back proper right near base; 15/25 inside front proper right | Patina | 90.6 | 5.5 | 1.7 | 0.8 | 0.0 | 0.9 |
| 0.3 | 0.6 | 0.3 | 0.4 | 0.0 | 0.7 | ||||
| Base | 89.4 | 7.3 | 2.2 | 0.4 | 0.0 | 0.3 | |||
| 0.4 | 0.1 | 0.1 | 0.4 | 0.0 | 0.2 | ||||
| 1998.794 | 1935–1952 | MLG and BRONZE on back proper left near base; 7/25 inside front proper right | Patina | 91.1 | 6.8 | 0.6 | 1.1 | 0.0 | 0.1 |
| 2.1 | 0.5 | 0.0 | 2.5 | 0.0 | 0.0 | ||||
| Base | 88.4 | 8.7 | 2.5 | 0.0 | 0.0 | 0.1 | |||
| 0.7 | 0.2 | 0.8 | 0.1 | 0.0 | 0.0 | ||||
| 1998.800 | 1935–1952 | MLG and BRONZE on back near base; 15/25 inside front proper right | Patina | 83.5 | 5.1 | 4.7 | 4.9 | 0.0 | 1.0 |
| 0.6 | 0.6 | 0.2 | 0.7 | 0.0 | 0.3 | ||||
| Base | 87.4 | 7.0 | 4.0 | 0.9 | 0.0 | 0.3 | |||
| 0.3 | 0.2 | 0.2 | 0.2 | 0.0 | 0.2 | ||||
| 1998.779 | 1935–1952 | MLG and BRONZE on back center near base; 10/25 inside proper right | Patina | 88.8 | 7.4 | 3.1 | 0.1 | 0.0 | 0.2 |
| 0.8 | 0.8 | 0.1 | 0.0 | 0.0 | 0.0 | ||||
| Base | 87.0 | 9.3 | 3.0 | 0.3 | 0.0 | 0.2 | |||
| 0.3 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | ||||
| 1998.787 | 1935–1952 | Indecipherable stamp (may be MLG) and BRONZE on back; 11/25 on underside rim and stamped inside | Patina | 86.0 | 9.8 | 2.5 | 1.3 | 0.0 | 0.2 |
| 0.5 | 0.4 | 0.1 | 0.2 | 0.0 | 0.0 | ||||
| Base | 85.3 | 11.0 | 2.4 | 0.8 | 0.0 | 0.1 | |||
| 0.5 | 0.3 | 0.0 | 0.1 | 0.0 | 0.0 | ||||
| 1998.799 | 1935–1952 | MLG on back proper right; BRONZE underside rim proper right; 8/25 inside proper left | Patina | 88.4 | 7.6 | 3.5 | 0.0 | 0.0 | 0.1 |
| 0.7 | 0.6 | 0.1 | 0.0 | 0.0 | 0.0 | ||||
| Base | 87.6 | 8.8 | 3.2 | 0.1 | 0.0 | 0.1 | |||
| 0.2 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | ||||
| 1998.808 | 1935–1952 | MLG and BRONZE on back proper right; 11/25 underside rim front; 11/25 inside front proper right | Patina | 86.7 | 9.5 | 2.4 | 0.9 | 0.0 | 0.2 |
| 0.5 | 0.4 | 0.1 | 0.2 | 0.0 | 0.1 | ||||
| Base | 86.1 | 10.3 | 2.3 | 0.8 | 0.0 | 0.2 | |||
| 0.6 | 0.5 | 0.2 | 0.1 | 0.0 | 0.0 | ||||
| 1998.813 | 1935–1952 | MLG proper right near rim; BRONZE underside rim proper left; 7/30 inside front proper left | Patina | 90.3 | 5.6 | 3.6 | 0.0 | 0.0 | 0.2 |
| 0.5 | 0.8 | 0.3 | 0.0 | 0.0 | 0.1 | ||||
| Base | 87.6 | 8.8 | 3.2 | 0.1 | 0.0 | 0.1 | |||
| 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | ||||
| 1998.781 | 1953–1965 | Cire Perdue Valsuani back proper right near rim; MLG back proper right and C incised | Patina | 81.1 | 7.9 | 4.2 | 1.0 | 2.9 | 2.6 |
| 2.9 | 1.0 | 0.4 | 1.2 | 0.7 | 0.4 | ||||
| Base | 79.7 | 12.7 | 4.1 | 0.0 | 1.6 | 1.7 | |||
| 1.0 | 0.4 | 0.4 | 0.0 | 0.5 | 0.4 | ||||
| 1998.783 | 1953–1965 | Cire Perdue Valsuani back proper left near rim; MLG back proper right and Mme.H. incised | Patina | 79.3 | 7.5 | 5.6 | 1.9 | 4.2 | 1.1 |
| 1.6 | 1.2 | 0.6 | 0.7 | 1.4 | 0.2 | ||||
| Base | 78.0 | 13.8 | 4.7 | 1.7 | 0.9 | 0.5 | |||
| 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 |
References and Note
- Finn, C. Rumours of war: Rudier and art bronze casting during the Second World War. Sculpt. J. 2013, 22, 128–133. [Google Scholar] [CrossRef]
- Lebon, E. Dictionnaire des Fondeurs de Bronze D’art: France, 1890–1950; Marjon: Perth, Australia, 2003. [Google Scholar]
- Rionnet, F. Le Rôle de La Maison Barbedienne (1834–1954) dans la Diffusion de la Sculpture Aux XIXe et XXe Siècles: Considérations Sur Les Bronzes D’édition et L’histoire du Goût. Ph.D. Thesis, Université Paris Sorbonne, Paris IV, France, 2006. [Google Scholar]
- Rionnet, F. Les bronzes Barbedienne: l’œuvre d’une dynastie de fondeurs (1834–1954). In Arthéna, Association Pour la Diffusion de L’histoire de L’art; IMPR: Paris, France, 2016. [Google Scholar]
- Ganio, M.; Leonard, A.; Salvant Plisson, J.; Walton, M. From sculptures to foundries: Elemental analysis to determine the provenance of Modern bronzes. In Proceedings of the 15ème journées d’étude de la SFIIC-ICOMOS, Paris, France, 4–5 December 2014; pp. 136–145. [Google Scholar]
- Day, J.; Stenger, J.; Eremin, K.; Khandekar, N.; Budny, V. Gaston Lachaise’s Bronze Sculptures in the Fogg Museum. J. Am. Inst. Conserv. 2010, 49, 1–26. [Google Scholar] [CrossRef]
- Boulton, A. The Making of Matisse’s Bronzes. MCI Topics in Museum ConservationSuitland, MD. 2007. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwjB-ImcjczgAhWAxIsBHRyqCP4QFjAAegQIABAC&url=https%3A%2F%2Fwww.si.edu%2Fmci%2Fdownloads%2Ftopics%2FBoulton.pdf&usg=AOvVaw3m2vYw28wcR_S7JxNtruRI (accessed on 11 January 2019).
- Considine, B.B. Conserving Outdoor Sculpture: The Stark Collection at the Getty Center; Getty Conservation Institute: Los Angeles, CA, USA, 2010. [Google Scholar]
- Young, M.; Dunand, D. Comparing Compositions of Modern Cast Bronze Sculptures: Optical Emission Spectroscopy Versus X-ray Fluorescence Spectroscopy. JOM 2015, 67, 1646–1658. [Google Scholar] [CrossRef]
- Barbour, D.; Deming Glinsman, L. Facture: Conservation, Science, Art History-August Rodin’s Lifetime Bronze Sculptures in the Simpson Collection; National Gallery of Art: Washington, DC, USA, 2013. [Google Scholar]
- Wasserman, J.L. Daumier Sculpture: A Critical and Comparative Study; Distributed by New York Graphic Society: Norwalk, CA, USA, 1969. [Google Scholar]
- Lindsay, S.G.; Barbour, D.; Sturman, S. Edgar Degas Sculpture; National Gallery of Art: Washington, DC, USA, 2010; pp. 54–81. [Google Scholar]
- Vittiglio, G.; Janssens, K.; Vekemans, B.; Adams, F.; Oost, A. A compact small-beam XRF instrument for in-situ analysis of objects of historical and/or artistic value. Spectrochim. Acta Part B At. Spectrosc. 1999, 54, 1697–1710. [Google Scholar] [CrossRef]
- Cartechini, L.; Rinaldi, R.; Kockelmann, W.; Bonamore, S.; Manconi, D.; Borgia, I.; Rocchi, P.; Brunetti, B.; Sgamellotti, A. Non-destructive characterization of compositional and textural properties of Etruscan bronzes: A multi-method approach. Appl. Phys. A Mater. Sci. Process. 2006, 83, 631–636. [Google Scholar] [CrossRef]
- Heginbotham, A.; Bassett, J.; Bourgarit, D.; Eveleigh, C.; Glinsman, L.; Hook, D.; Smith, D.; Speakman, R.J.; Shugar, A.; Van Langh, R. The Copper CHARM Set: A New Set of Certified Reference Materials for the Standardization of Quantitative X-Ray Fluorescence Analysis of Heritage Copper Alloys. Archaeometry 2015, 57, 856–868. [Google Scholar] [CrossRef]
- Heginbotham, A.; Solé, V. CHARMed PyMca, Part I: A Protocol for Improved Inter-laboratory Reproducibility in the Quantitative ED-XRF Analysis of Copper Alloys. Archaeometry 2017, 59, 714–730. [Google Scholar] [CrossRef]
- Campanella, L.; Ferretti, M.; Polese, C.; García, C.R.; Sabatini, I. Non-destructive approach to multilayer objects: XRF analysis of gilt and enamelled metals of the medieval cross of Rosciolo. Procedia Chem. 2013, 8, 284–291. [Google Scholar] [CrossRef]
- A.I.o. Chicago. Available online: http://www.artic.edu/aic/collections/ (accessed on 27 December 2018).
- Butler, R.; Lindsay, S.G.; Luchs, A. European Sculpture of the Nineteenth Century; National Gallery of Art: Washington, DC, USA, 2000. [Google Scholar]
- Young, M.L.; Schnepp, S.; Casadio, F.; Lins, A.; Meighan, M.; Lambert, J.B.; Dunand, D.C. Matisse to Picasso: A compositional study of modern bronze sculptures. Anal. Bioanal. Chem. 2009, 395, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Bezur, A.; Casadio, F. The analysis of porcelain using handheld and portable X-ray fluorescence spectrometers. In Handheld XRF for Art and Archaeology; Cornell University Press: Ithaca, NY, USA, 2012; Volume 3, p. 249. [Google Scholar]
- Solé, V.A.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Kosinski, D.M.; Fisher, J.M.; Nash, S.A.; Matisse, H. Matisse: Painter as Sculptor; Yale University Press: Connecticut, CT, USA, 2007. [Google Scholar]
- Center for Scientific Studies in the Arts. Bronze Alloy Master Table. Available online: https://scienceforart.northwestern.edu/projects-proposals/projects/bronze-sculptures.html (accessed on 11 January 2019).
- Dussubieux, L. Laser Ablation—Inductively Coupled Plasma—Mass Spectrometry Analysis of Ancient Copper Alloy Artifacts; ACS Publications: Washington, DC, USA, 2007. [Google Scholar]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Pearson Education Limited: Essex, UK, 2000. [Google Scholar]
- Rich, J.C. The Materials and Methods of Sculpture; Courier Corporation: North Chelmsford, MA, USA, 1988. [Google Scholar]
- Lebon, E. Fonte au Sable-Fonte à Cire Perdue: Histoire D’une Rivalité; Editions Ophrys: Paris, France, 2012; Volume 5. [Google Scholar]
- De Morveau, L.-B.G. Annales de Chimie, ou, Recueil de Mémoires Concernant la Chimie et Les Arts Qui en Dépendent; chez Joseph de Boffe: London, UK, 1801; Volume 1. [Google Scholar]
- Bastien, P. Flowability and Viscosity; American Foundrymen’s Society: Schaumburg, IL, USA, 1962. [Google Scholar]
- Kaufman, L.; Ågren, J. CALPHAD, first and second generation–Birth of the materials genome. Scr. Mater. 2014, 70, 3–6. [Google Scholar] [CrossRef]
- Kaufman, L.; Bernstein, H. Computer Calculation of Phase Diagrams with Special Reference to Refractory Metals; Academic Press: Cambridge, MA, USA, 1970. [Google Scholar]
- Andersson, J.-O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar]
- Zhan, L.; Qiu, Z.; Xu, Z. Separating zinc from copper and zinc mixed particles using vacuum sublimation. Sep. Purif. Technol. 2009, 68, 397–402. [Google Scholar] [CrossRef]
- Kearns, T.; Martinon-Torres, M.; Rehren, T. Metal to mould: Alloy identification in experimental casting moulds using XRF. Hist. Metall. 2010, 44, 48–58. [Google Scholar]
- Dungworth, D. A note on the analysis of crucibles and moulds. Hist. Metall. 2000, 34, 83–86. [Google Scholar]
- Vautel, C. Un fondeur artiste. La Vie Illustrée 1904.
- Lacombe, M.S.; Debonliez, M.M.G.; Malepeyre, F. Nouveau Manuel Complet du Bronzage des Métaux et Du Plâtre; 1887 Reprint; Chez Leonce Laget: Paris, France, 1979. [Google Scholar]
- Michel, J. Coloration des Métaux: Bronzage-Patinage-Oxydation-Marbrage-Irisation-Nielle-Teinture; Desforges: Paris, France, 1953. [Google Scholar]
- Rama, J.P. Le Bronze D’art et Ses Techniques; Edition, H. Vial: Dourdan, France, 1988. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pouyet, E.; Ganio, M.; Motlani, A.; Saboo, A.; Casadio, F.; Walton, M. Casting Light on 20th-Century Parisian Artistic Bronze: Insights from Compositional Studies of Sculptures Using Hand-Held X-ray Fluorescence Spectroscopy. Heritage 2019, 2, 732-748. https://doi.org/10.3390/heritage2010047
Pouyet E, Ganio M, Motlani A, Saboo A, Casadio F, Walton M. Casting Light on 20th-Century Parisian Artistic Bronze: Insights from Compositional Studies of Sculptures Using Hand-Held X-ray Fluorescence Spectroscopy. Heritage. 2019; 2(1):732-748. https://doi.org/10.3390/heritage2010047
Chicago/Turabian StylePouyet, Emeline, Monica Ganio, Aisha Motlani, Abhinav Saboo, Francesca Casadio, and Marc Walton. 2019. "Casting Light on 20th-Century Parisian Artistic Bronze: Insights from Compositional Studies of Sculptures Using Hand-Held X-ray Fluorescence Spectroscopy" Heritage 2, no. 1: 732-748. https://doi.org/10.3390/heritage2010047
APA StylePouyet, E., Ganio, M., Motlani, A., Saboo, A., Casadio, F., & Walton, M. (2019). Casting Light on 20th-Century Parisian Artistic Bronze: Insights from Compositional Studies of Sculptures Using Hand-Held X-ray Fluorescence Spectroscopy. Heritage, 2(1), 732-748. https://doi.org/10.3390/heritage2010047




