Evaluation of Photodynamic Therapy Using AuNPs@Ce6 in 3D Cultures of Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
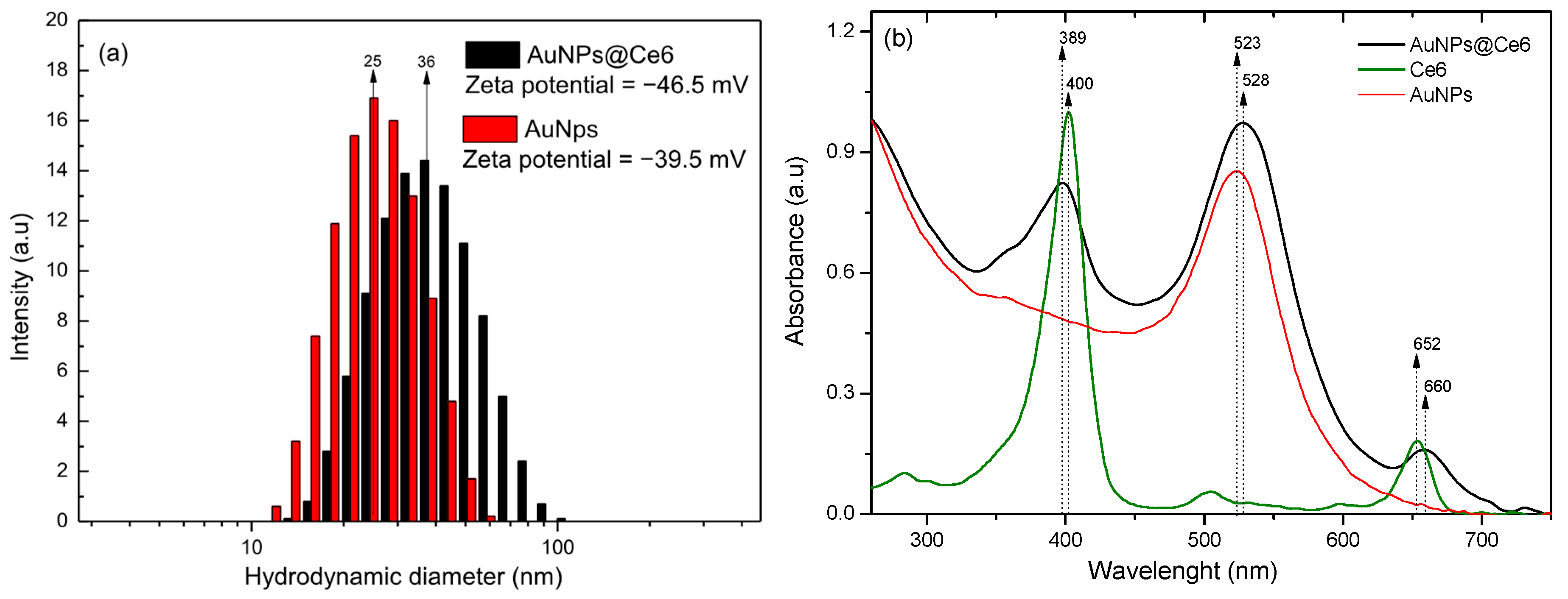
2.1. Gold Nanoparticles Synthesis
2.2. Nanostructured Photosensitizer
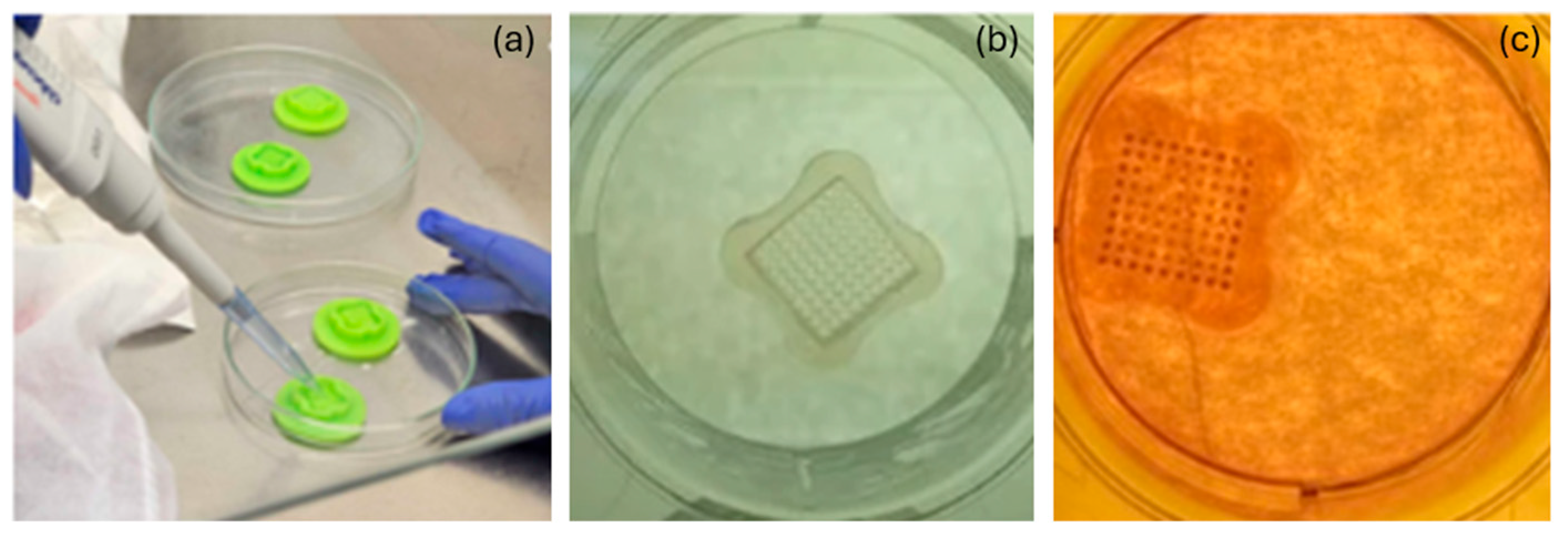
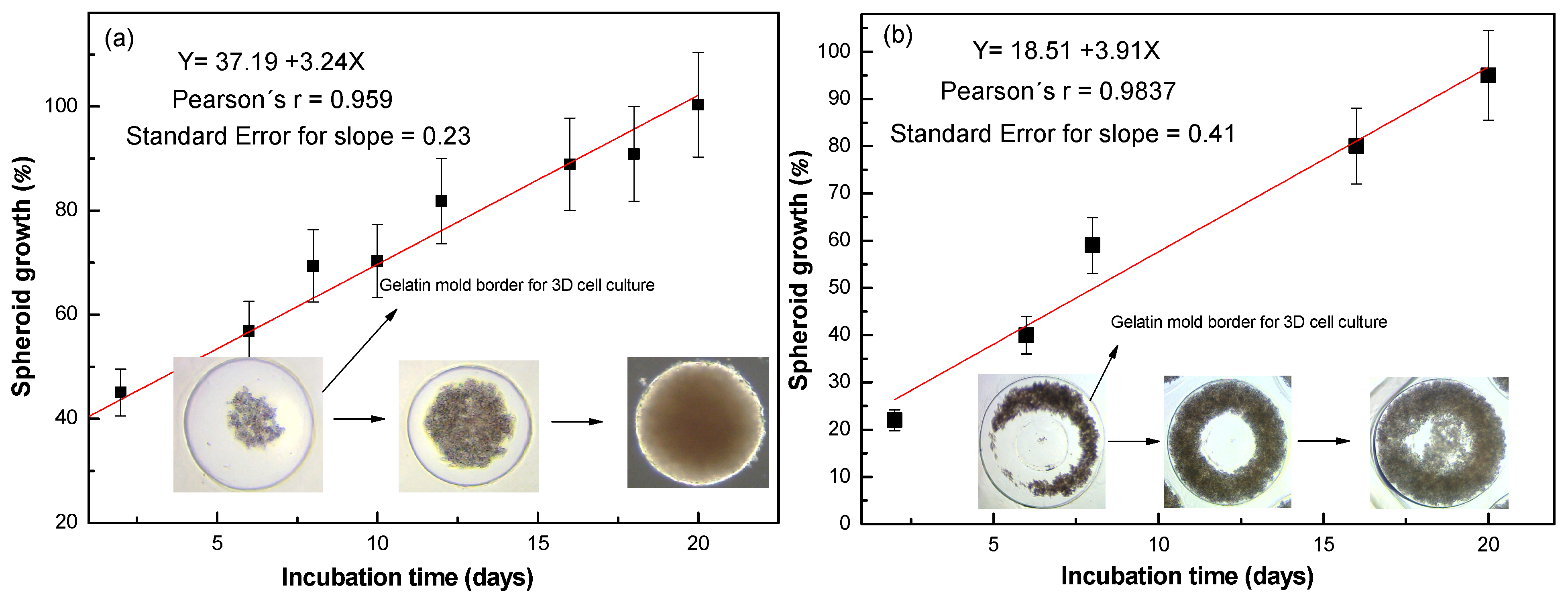
2.3. Two-Dimensional and 3D Cell Cultures
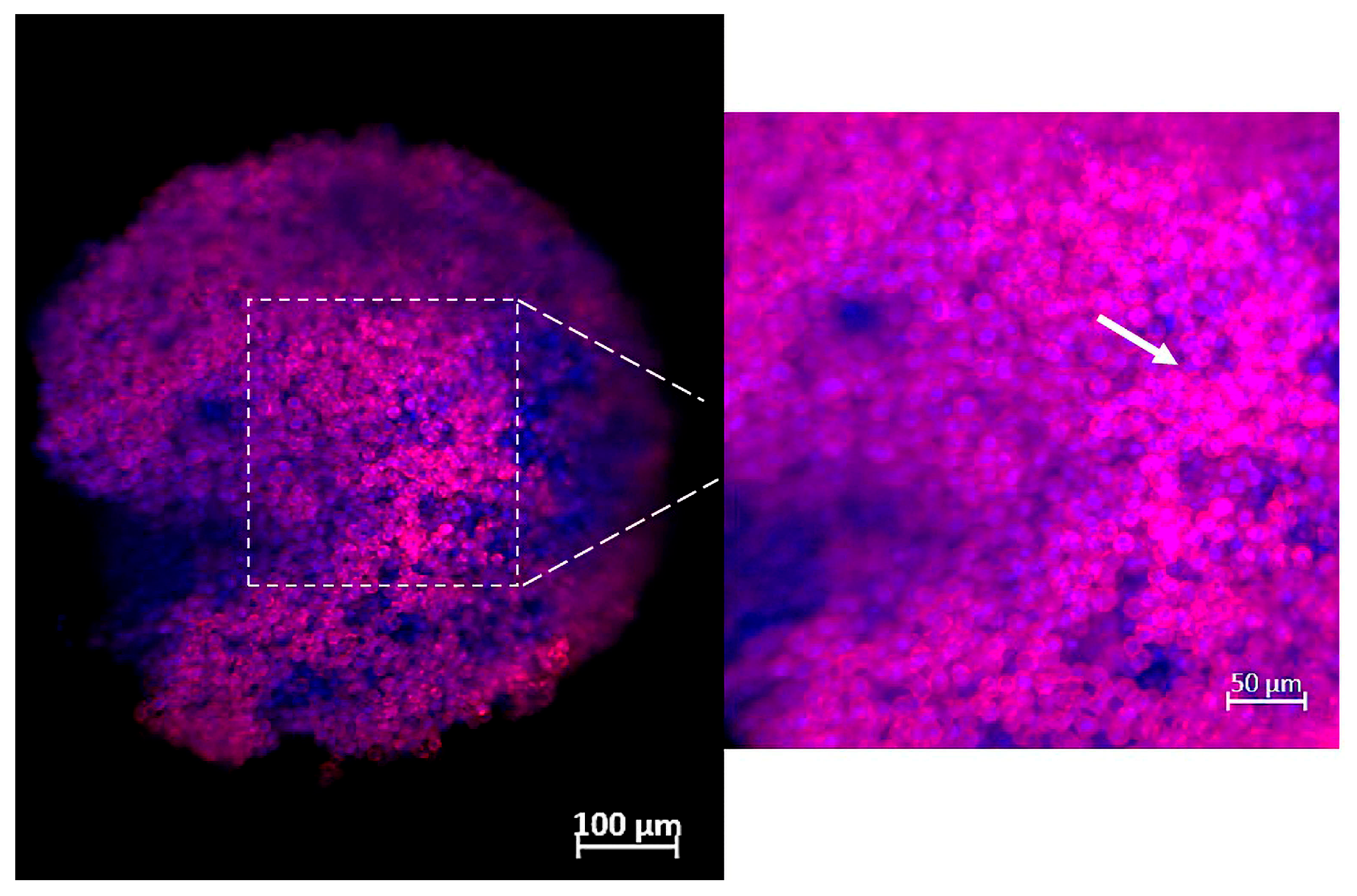
2.4. Characterization of Spheroids by Confocal Fluorescence Microscope
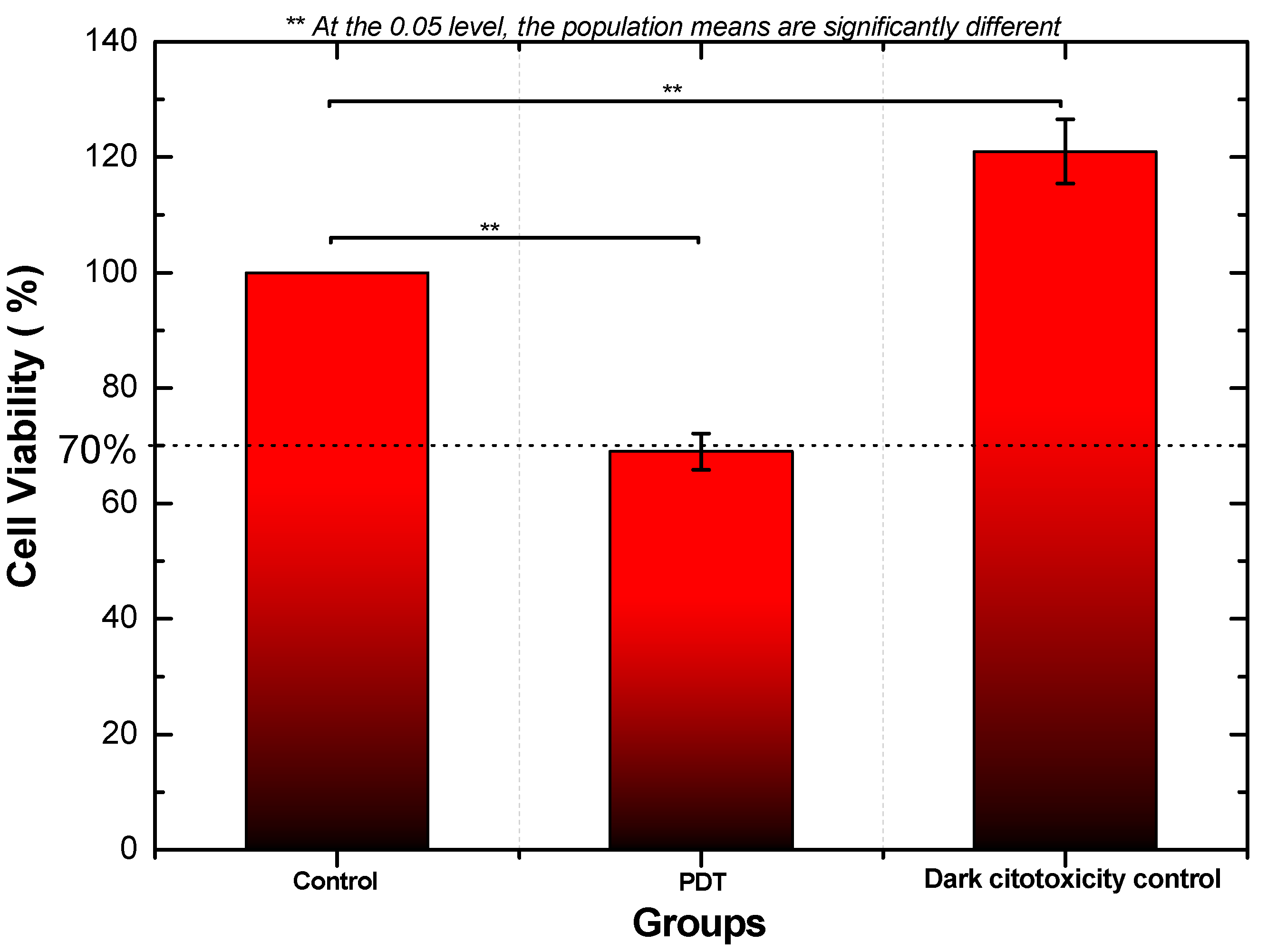
2.5. Photodynamic Therapy and Cellular Mitochondrial Activity
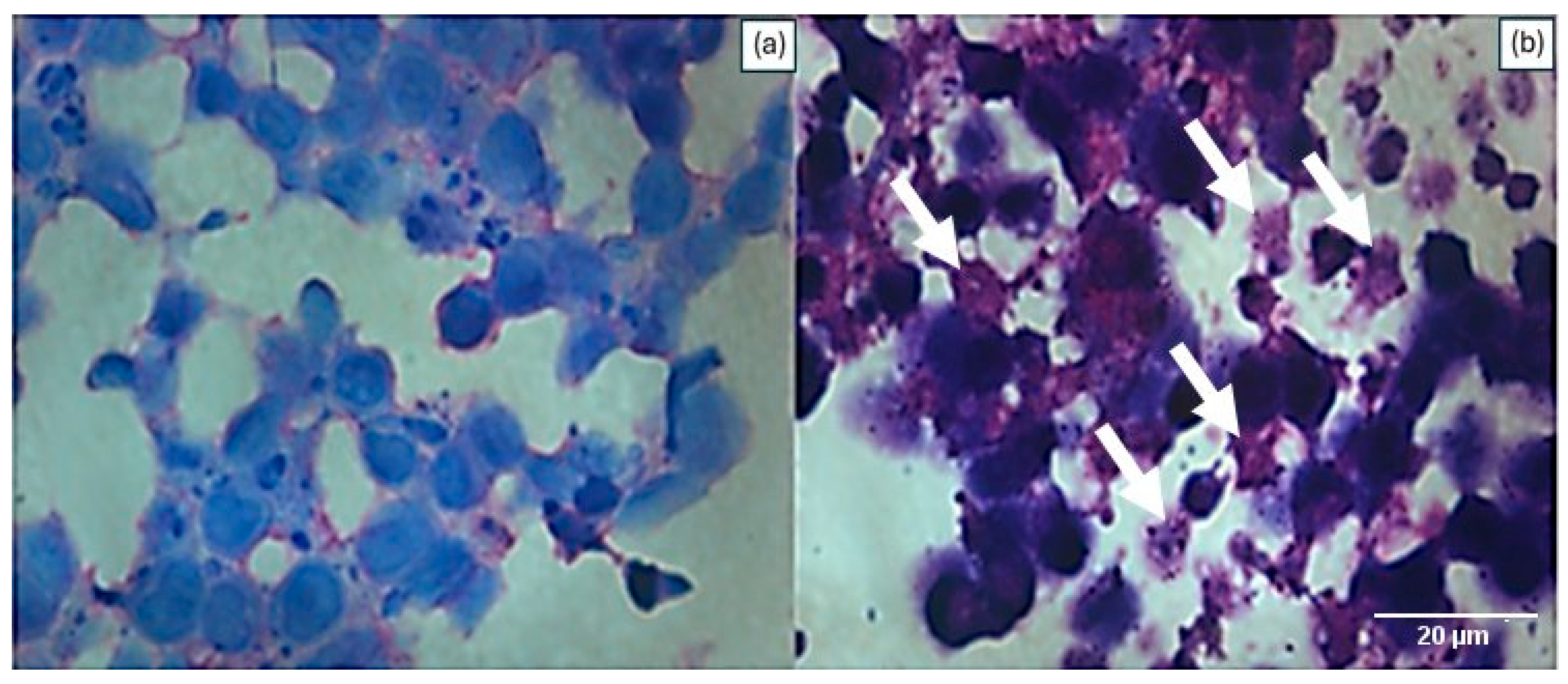
2.6. Histological Analysis of Cell Culture in 3D
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, N.K.; Bordoloi, D.; Monisha, J.; Anip, A.; Padmavathi, G.; Kunnumakkara, A.B. Cancer—An Overview and Molecular Alterations in Cancer. In Fusion Genes and Cancer; World Scientific: Singapore, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- Tao, Z.; Shi, A.; Lu, C.; Song, T.; Zhang, Z.; Zhao, J. Breast Cancer: Epidemiology and Etiology. Cell Biochem. Biophys. 2015, 72, 333–338. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment. JAMA 2019, 321, 288. [Google Scholar] [CrossRef] [PubMed]
- Gangi, A.; Chung, A.; Mirocha, J.; Liou, D.Z.; Leong, T.; Giuliano, A.E. Breast-conserving therapy for triple-negative breast cancer. JAMA Surg. 2014, 149, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Santonja, A.; Sánchez-Muñoz, A.; Lluch, A.; Chica-Parrado, M.R.; Albanell, J.; Chacón, J.I.; Antolín, S.; Jerez, J.M.; de la Haba, J.; de Luque, V.; et al. Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget 2018, 9, 26406. [Google Scholar] [CrossRef]
- Abramson, V.G.; Lehmann, B.D.; Ballinger, T.J.; Pietenpol, J.A. Subtyping of triple-negative breast cancer: Implications for therapy. Cancer 2015, 121, 8–16. [Google Scholar] [CrossRef]
- Wang, D.Y.; Jiang, Z.; Ben-David, Y.; Woodgett, J.R.; Zacksenhaus, E. Molecular stratification within triple-negative breast cancer subtypes. Sci. Rep. 2019, 9, 19107. [Google Scholar] [CrossRef]
- Amraee, A.; Alamzadeh, Z.; Irajirad, R.; Sarikhani, A.; Ghaznavi, H.; Ghadiri Harvani, H.; Mahdavi, S.R.; Shirvalilou, S.; Khoei, S. Theranostic RGD@Fe3O4-Au/Gd NPs for the targeted radiotherapy and MR imaging of breast cancer. Cancer Nanotechnol. 2023, 14, 61. [Google Scholar] [CrossRef]
- Vieira, P.; Jesus, V.; Cândido, M.A.; Pacheco-Soares, C.; Castilho, M.; Raniero, L. Specific nanomarkers fluorescence: In vitro analysis for EGFR overexpressed cells in triple-negative breast cancer and malignant glioblastoma. Photodiagnosis Photodyn. Ther. 2022, 39, 102997. [Google Scholar] [CrossRef]
- Malatesti, N.; Harej, A.; Pavelić, S.K.; Lončarić, M.; Zorc, H.; Wittine, K.; Andjelkovic, U.; Josic, D. Synthesis, characterization and in vitro investigation of photodynamic activity of 5-(4-octadecanamidophenyl)-10, 15, 20-tris (N-methylpyridinium-3-yl) porphyrin trichloride on HeLa cells using low light fluence rate. Photodiagnosis Photodyn. Ther. 2016, 15, 115–126. [Google Scholar] [CrossRef]
- Muguruma, M.; Teraoka, S.; Miyahara, K.; Ueda, A.; Asaoka, M.; Okazaki, M.; Kawate, T.; Kuroda, M.; Miyagi, Y.; Ishikawa, T. Differences in drug sensitivity between two-dimensional and three-dimensional culture systems in triple-negative breast cancer cell lines. Biochem. Biophys. Res. Commun. 2020, 533, 268–274. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Navarro, J.R.G.; Werts, M.H.V. Resonant light scattering spectroscopy of gold, silver and gold–silver alloy nanoparticles and optical detection in microfluidic channels. Analyst 2013, 138, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.; Castilho, M.L.; Ferreira, I.; Ferreira-Strixino, J.; Hewitt, K.C.; Raniero, L. Synthesis and characterization of gold nanostructured Chorin e6 for Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2017, 18, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Filho, O.O.C.; Cândido, M.A.; Ventura, A.; Morais, F.V.; Raniero, L. Nano-Enabled Colorimetric Assay for the Detection of Paracoccidioides lutzii: Advancing Diagnostics with Nanotechnology. J. Nanotheranostics 2024, 5, 75–83. [Google Scholar] [CrossRef]
- McDonald, A.R.; Garbary, D.J.; Duckett, J.G. Rhodamine-Phalloidin Staining of F-Actin in Rhodophyta. Biotech. Histochem. 1993, 68, 91–98. [Google Scholar] [CrossRef]
- Otto, F. Chapter 11 DAPI Staining of Fixed Cells for High-Resolution Flow Cytometry of Nuclear DNA. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 1990; pp. 105–110. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 10 June 2024).
- Bursch, W.; Taper, H.S.; Lauer, B.; Schulte-Hermann, R. Quantitative histological and histochemical studies on the occurrence and stages of controlled cell death (apoptosis) during regression of rat liver hyperplasia. Virchows Arch. B Cell Pathol. 1986, 50, 153–166. [Google Scholar] [CrossRef]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin e6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef]
- Li, Z.; Pan, W.; Shi, E.; Bai, L.; Liu, H.; Li, C.; Wang, Y.; Deng, J.; Wang, Y. A Multifunctional Nanosystem Based on Bacterial Cell-Penetrating Photosensitizer for Fighting Periodontitis via Combining Photodynamic and Antibiotic Therapies. ACS Biomater. Sci. Eng. 2021, 7, 772–786. [Google Scholar] [CrossRef]
- Cândido, M.; Vieira, P.; Campos, A.; Soares, C.; Raniero, L. Gold-Coated Superparamagnetic Iron Oxide Nanoparticles Functionalized to EGF and Ce6 Complexes for Breast Cancer Diagnoses and Therapy. Pharmaceutics 2023, 15, 100. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Xie, Z.; Centurion, F.; Sun, B.; Paterson, D.J.; Tsao, S.C.; Chu, D.; Shen, Y.; Mao, G.; et al. Size-Dependent Penetration of Nanoparticles in Tumor Spheroids: A Multidimensional and Quantitative Study of Transcellular and Paracellular Pathways. Small 2023, 20, e2304693. [Google Scholar] [CrossRef]
- Ballav, S.; Jaywant Deshmukh, A.; Siddiqui, S.; Aich, J.; Basu, S. Two-Dimensional and Three-Dimensional Cell Culture and Their Applications. In Cell Culture-Advanced Technology and Applications in Medical and Life Sciences; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Z.; Chang, H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.S.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Wojtkowiak, J.; Neilson, A.; Gillies, R.J. Metabolic profiling of healthy and cancerous tissues in 2D and 3D. Sci. Rep. 2017, 7, 15285. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Boyer, C.J.; Ballard, D.H.; Barzegar, M.; Winny Yun, J.; Woerner, J.E.; Ghali, G.E.; Boktor, M.; Wang, Y.; Alexander, J.S. High-throughput scaffold-free microtissues through 3D printing. 3D Print. Med. 2018, 4, 9. [Google Scholar] [CrossRef]
- Dias-Netipanyj, M.F.; Cowden, K.; Sopchenski, L.; Cogo, S.C.; Elifio-Esposito, S.; Popat, K.C.; Soares, P. Effect of crystalline phases of titania nanotube arrays on adipose derived stem cell adhesion and proliferation. Mater. Sci. Eng. C 2019, 103, 109850. [Google Scholar] [CrossRef]
- Buranaamnuay, K. The MTT assay application to measure the viability of spermatozoa: A variety of the assay protocols. Open Vet. J. 2021, 11, 251–269. [Google Scholar] [CrossRef]
- Chan, J.K.C. The Wonderful Colors of the Hematoxylin–Eosin Stain in Diagnostic Surgical Pathology. Int. J. Surg. Pathol. 2014, 22, 12–32. [Google Scholar] [CrossRef]
- Tidwell, T.R.; Røsland, G.V.; Tronstad, K.J.; Søreide, K.; Hagland, H.R. Metabolic flux analysis of 3D spheroids reveals significant differences in glucose metabolism from matched 2D cultures of colorectal cancer and pancreatic ductal adenocarcinoma cell lines. Cancer Metab. 2022, 10, 9. [Google Scholar] [CrossRef]
- Martines-Arano, H.; García-Pérez, B.E.; Vidales-Hurtado, M.A.; Trejo-Valdez, M.; Hernández-Gómez, L.H.; Torres-Torres, C. Chaotic Signatures Exhibited by Plasmonic Effects in Au Nanoparticles with Cells. Sensors 2019, 19, 4728. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.L.; Jesus Viviane, P.S.; Vieira, P.F.A.; Hewitt, K.C.; Raniero, L. Chlorin e6-EGF conjugated gold nanoparticles as a nanomedicine based therapeutic agent for triple negative breast cancer. Photodiagnosis Photodyn. Ther. 2021, 33, 102186. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Jesus, V.P.; Vieira, P.F.A.; Cintra, R.C.; Sant’Anna, L.B.; Zezell, D.M.; Castilho, M.L.; Raniero, L. Triple-negative breast cancer treatment in xenograft models by bifunctional nanoprobes combined to photodynamic therapy. Photodiagnosis Photodyn. Ther. 2022, 38, 102796. [Google Scholar] [CrossRef] [PubMed]
- Urzì, O.; Gasparro, R.; Costanzo, E.; De Luca, A.; Giavaresi, G.; Fontana, S.; Alessandro, R. Three-Dimensional Cell Cultures: The Bridge between In Vitro and In Vivo Models. Int. J. Mol. Sci. 2023, 24, 12046. [Google Scholar] [CrossRef]
- Gómez-Mercader, A.; Monzón-Atienza, L.; Montero, D.; Bravo, J.; Acosta, F. Fish Cell Spheroids, a Promising in Vitro Model to Mimic In Vivo Research: A Review. Cells 2024, 13, 1818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura, A.; Gonçalves, G.C.; Soares, C.P.; Sant’anna, L.B.; Marmo, V.L.M.; Sibelino, S.K.; Raniero, L. Evaluation of Photodynamic Therapy Using AuNPs@Ce6 in 3D Cultures of Triple-Negative Breast Cancer. J 2025, 8, 43. https://doi.org/10.3390/j8040043
Ventura A, Gonçalves GC, Soares CP, Sant’anna LB, Marmo VLM, Sibelino SK, Raniero L. Evaluation of Photodynamic Therapy Using AuNPs@Ce6 in 3D Cultures of Triple-Negative Breast Cancer. J. 2025; 8(4):43. https://doi.org/10.3390/j8040043
Chicago/Turabian StyleVentura, Aveline, Giulia Capizzani Gonçalves, Cristina Pacheco Soares, Luciana Barros Sant’anna, Vitor Luca Moura Marmo, Sônia Khouri Sibelino, and Leandro Raniero. 2025. "Evaluation of Photodynamic Therapy Using AuNPs@Ce6 in 3D Cultures of Triple-Negative Breast Cancer" J 8, no. 4: 43. https://doi.org/10.3390/j8040043
APA StyleVentura, A., Gonçalves, G. C., Soares, C. P., Sant’anna, L. B., Marmo, V. L. M., Sibelino, S. K., & Raniero, L. (2025). Evaluation of Photodynamic Therapy Using AuNPs@Ce6 in 3D Cultures of Triple-Negative Breast Cancer. J, 8(4), 43. https://doi.org/10.3390/j8040043








