Abstract
The simultaneous occurrence of tuberculosis (TB) and COVID-19 posed a major public health challenge, particularly in regions heavily impacted by both diseases, due to their shared effects on the lungs, immune system dysfunction, and the possibility of more severe clinical outcomes. The role of immunopathogenesis is crucial in influencing the progression of co-infection, which is marked by heightened inflammation, immune exhaustion, weakened T-cell responses, and unregulated cytokine production. To better understand the intricate interactions between host and pathogen and the immune disruptions associated with this dual epidemic, multi-omics approaches such as genomics, transcriptomics, proteomics, metabolomics, epigenomics, and microbiomics have proven to be effective methods. These comprehensive strategies provide detailed insights into the mechanisms of disease, help identify potential biomarkers, and aid in the identification of therapeutic targets. This review emphasizes the importance of immune responses and systems biology in comprehending the TB-COVID-19 syndemic and highlights the promise of multi-omics in advancing precision medicine and enhancing disease management.
1. Introduction
The deadly human pathogen M. tuberculosis (M. tb) is a leading cause of Tuberculosis mortality worldwide and currently ranked among the top ten causes of death. Approximately one-third of the world’s population retains latent tuberculosis, which can potentially be reactivated, especially in immunocompromised people [1]. In 2023, it was estimated that 10.8 million individuals contracted tuberculosis (TB) globally, comprising 6.0 million men, 3.6 million women, and 1.3 million children, and overall, 1.25 million individuals lost their lives due to tuberculosis (TB) in 2023, which includes 161,000 people who were also living with HIV [1]. Historically, tuberculosis (TB) is almost as old as humanity, as the bony TB evidence has been discovered in Egyptian mummy skeletons [2,3]. The first documented records of tuberculosis originate from ancient India and China. TB is termed ‘Rajyakshama’ in the Vedas, which means ‘to waste away’ in Sanskrit [4]. Commendable epidemiological connections were identified when it was found that troops who resided indoors in barracks contracted TB at a higher rate than those who spent a lot of time on the battlefield. As a consequence, a correlation between overcrowding and the spread of illness was observed.
During the pandemic, COVID-19 emerged as a more dangerous infectious disease than M. tb, killing more people than TB, threatening socioeconomic development, and posing a threat to the collapse of public health systems globally. A sudden increase in patients with an unidentified cause of pneumonia was diagnosed in Wuhan, Hubei province (China) [5]. Early discoveries were linked to pneumonia, with patients experiencing severe acute respiratory infection symptoms, eventually leading to respiratory failure and other significant effects. While evaluating throat swab samples from affected people, the Chinese Centers for Disease Control and Prevention (CDC) detected a new coronavirus, which the WHO named as 2019 CoV-2 [6]. The outbreak of COVID-19 pneumonia started in Wuhan in December 2019 and soon outspread throughout China and around the world. Since that date, 215 nations have been most severely affected, and the number of individuals infected with COVID-19 exceeded 16.6 million [7].
During 2020, the simultaneous occurrence of COVID-19 and tuberculosis (TB) posed a considerable global health challenge, especially in areas with a high prevalence of TB, such as India, Indonesia, Pakistan, and Bangladesh [8,9]. Both pathogens affect the respiratory system and provoke intricate immune responses characterized by inflammation, activation of immune cells, and the production of cytokines [10,11]. M. tuberculosis predominantly elicits a Th1-dominated immune response, which involves interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), essential for forming granulomas and containing the bacteria [12]. In contrast, COVID-19 infection typically results in a misregulated immune response, marked by lymphopenia, excessive production of pro-inflammatory cytokines (IL-6, IL-1β, TNF-α), and cytokine storm in severe instances [13].
The convergence of these two infections worsens immune dysfunction. Patients with TB who are co-infected with COVID-19 may undergo reactivation of latent TB, experience more severe lung damage, and face poorer clinical outcomes due to immune exhaustion and compromised T-cell responses [14,15,16]. On the other hand, COVID-19 may hasten TB progression by disrupting granulomatous structures that are essential for bacterial containment [17,18]. Both diseases undermine innate immunity, particularly affecting alveolar macrophage function and type I interferon signaling, leading to an environment conducive to pathogen multiplication [19,20]. Furthermore, co-infection complicates diagnosis and treatment, as overlapping clinical features like fever, cough, and dyspnea, along with similar radiological findings (e.g., lung infiltrates), can hinder prompt and accurate identification [7,21]. Grasping the immunopathogenic interactions between COVID-19 and M. tuberculosis is crucial for managing co-infections, refining treatment protocols, and advancing vaccination strategies, especially in TB-endemic regions [22,23].
Additionally, in order to grasp the intricate interactions between M. tb and COVID-19 during simultaneous infections, a systems-level methodology is essential, and multi-omics technologies present an effective platform for unraveling this syndemic [24]. By combining information from genomics, transcriptomics, proteomics, metabolomics, epigenomics, and microbiomics, scientists can reveal how each pathogen influences host reactions and how their intersection modifies immune, metabolic, and cellular pathways [25]. Transcriptomic analyses indicate disrupted interferon signaling and increased inflammatory responses among co-infected patients, while proteomic studies detect heightened levels of cytokines and enzymes that cause tissue damage [25,26]. Metabolomic investigations illustrate immune-metabolic exhaustion through changes in glycolysis and amino acid metabolism, and epigenomic assessments reveal chromatin alterations that influence long-term immune responses [27,28]. Additionally, shifts in the microbiome of both lung and gut indicate another layer of immune regulation [29,30]. The amalgamation of these omics layers through computational modeling and network analysis facilitates the discovery of disease-specific biomarkers and potential therapeutic targets, ultimately leading towards precision medicine strategies in addressing TB–COVID-19 co-infection [31,32].
This review highlights the critical importance of host immune responses and systems biology in understanding the intricate interactions that characterize the TB–COVID-19 syndemic. Both infections significantly alter the immune system, resulting in disrupted inflammatory pathways, modified T-cell reactions, and tissue injury that complicate health outcomes. By employing a systems biology approach, these complex host–pathogen interactions can be interpreted as interrelated phenomena rather than separate occurrences. Additionally, utilizing multi-omics techniques, including genomics, transcriptomics, proteomics, and metabolomics, provides unique opportunities to capture the evolving molecular profiles associated with co-infection. Such comprehensive analyses can aid in the identification of biomarkers for early detection, forecast disease progression, and pinpoint therapeutic targets, thereby enhancing the framework of precision medicine and ultimately improving management strategies for those affected by both infections.
1.1. M. tb and COVID-19 Co-Infection: Clinical Aspects
Co-infection with COVID-19 and M. tuberculosis (M. tb) exhibits numerous overlapping clinical and pathological characteristics, mainly because both pathogens localize within the human lungs, although they can also spread to other sites outside the lungs [33]. In the pulmonary system, both M. tb and COVID-19 replicate in mucus-secreting respiratory epithelial cells, type II alveolar cells, and macrophages, triggering vigorous innate immune responses [34,35]. Upon entering the body, both pathogens provoke the release of pro-inflammatory cytokines (e.g., IL-6, TNF-α, IFN-γ), which leads to neutrophil recruitment, damage to alveoli, and disruptions in gas exchange [36]. An overproduction of cytokines, particularly during COVID-19 infection, can hinder effective immune responses and increase the likelihood of secondary infections, including the reactivation of latent TB. Pyroptosis induced by the virus and endothelial dysfunction further intensify Th1-mediated inflammation, worsening lung damage [37].
Additionally, the co-occurrence of M. tb and COVID-19 presents unique clinical difficulties, especially in regions where TB is common. Those with active or latent TB are at heightened risk for severe COVID-19, and immunosuppressive therapies such as corticosteroids used in COVID-19 treatment can hasten TB reactivation [38]. The clinical manifestations of the disease are significantly overlapping, encompassing symptoms like fever, cough, and shortness of breath, which complicates accurate differential diagnosis. Radiological assessments revealing pulmonary infiltrates and cavitation may be linked to either infection, creating challenges in pathogen-specific identification [39]. Co-infected patients generally face worse clinical outcomes, including elevated hospitalization rates, increased ICU admissions, and higher mortality [40]. Delays in diagnosing and starting treatment for either condition further augment the risk of transmission and hinder disease control measures. Hence, integrated diagnostic approaches, simultaneous testing, and coordinated treatment plans are crucial for effectively managing this syndemic.
Recently, a group study performed for the TB/COVID-19 showed that the majority (74%) of the 767 patients in a cohort who had TB and COVID-19 co-infection had TB prior to receiving COVID-19, whereas 9.5% received COVID-19 first and 16.5% received both diagnoses in the same week. The overall mortality rate was 11.1%, with Europe having a considerably higher rate (14.2%) than non-Europe (9.2%, p = 0.03). COVID-19 alone was responsible for almost half of the deaths, while TB and COVID-19 together accounted for more than one-third. Significant mortality risks were determined by univariate analysis to include age, male gender, numerous comorbidities (diabetes, cardiovascular, chronic respiratory/renal disease), major symptoms, invasive ventilation, and hospitalization linked to COVID-19. Age, male sex, and invasive ventilation were all validated as independent predictors of death using multivariable logistic regression [41].
An another study, used TB RNA-seq data from 1181 samples from 853 people; 35 COVID-19 gene signatures from nine qualifying studies with 98 samples were analyzed. Thirteen of the 25 signatures were confirmed in two separate datasets, and they showed a significantly increased COVID-19 risk in active TB (ATB) compared to latent TB infection (p < 0.005). During severe COVID-19, macrophages enriched in BALF that expressed FCN1 and SPP1 were shown to be in circulation during ATB. Antigen presentation, platelet activation, epigenetic regulation, and ROS/RNS generation were among the shared disturbed ontologies that were enriched as the severity of COVID-19 increased. Lastly, show that the creation of blood-based diagnostic markers of co-infection may be complicated by the overlapping transcriptional responses [42].
Recent evidence indicates that the co-occurrence of coronavirus disease 2019 (COVID-19) and tuberculosis (TB) was linked to worse clinical outcomes than COVID-19 alone. With an overall fatality rate of roughly 7.1% and an in-hospital fatality rate of 11.4%, Wang et al. (2024) showed higher death, longer recovery periods, and higher hospitalization rates among co-infected patients [43]. Furthermore, Mihuta et al., 2025 showed that, especially in situations of severe or extrapulmonary TB, the mortality rates among co-infected patients varied from 7.6% to 23.6% [44]. Significant rates of secondary bacterial and fungal infections have also been reported in studies in COVID-19 populations; these rates range from 6.9% in general hospital admissions to over 40–50% in critically ill patients. These infections are primarily linked to the use of corticosteroids, comorbidities, and poorer outcomes [45,46]. These results collectively imply that immunological dysregulation and lung pathology in co-infected individuals may plausibly enhance susceptibility to secondary pathogens, warranting further clinical investigation.
1.2. COVID-19 and TB Immunopathogenesis
Several mechanisms are involved in the immunopathological responses of COVID-19 and M. tb. Some of the important mechanisms are summarized below
1.2.1. COVID-19 Immunopathogenesis
COVID-19 infection disrupts the normal immune response, resulting in a weakened immune system and uncontrolled inflammatory response. Several key symptoms are present in the patients suffering from COVID-19, including lymphopenia, dysfunction, lymphocyte activation, monocyte and granulocyte irregularities, elevated stages of inflammatory cytokines, as well as an increase in immunoglobulins (IgG). Immunological arrays of COVID-19 infection are summarized below.
Lymphocyte Dysfunction
T cell activation was investigated in multiple COVID-19 individuals, and one study encompassing 128 convalescent samples revealed that the CD8+ T cell response was more common than the CD4+ T cell response [47]. Furthermore, as compared to the mild group, virus-specific T lymphocytes from severe cases used to have a central memory phenotype and high levels of interferon (IFN-Y), tumor necrosis factor (TNF-alpha), and interleukin (IL)-2 [48]. T cells in COVID-19-positive patients also display depletion characteristics. Additionally, programmed cell death protein-1, T cell immunoglobulin domain, and mucin domain-3 levels on CD8+ cells are higher in clinically symptomatic stages than in prodromal stages, with maximal levels reported in severe granulocyte and monocyte abnormalities [49].
Lymphopenia
Lymphopenia is the hallmark feature of patients with severe COVID-19 infection. After infection, patients are more prone to develop lymphopenia, signifying a major prognosticator for severe patients [50,51]. Infected individuals also have considerably lower CD8+ T, CD4+ T, NK, and B cells [12,52,53,54]. In most severe cases, lymphocyte percentages were found to be less than 20% [55]. However, further studies revealed that in severe cases, T cell numbers, particularly CD8+ T cells, were much lower than in mild cases [56]. Although in severe conditions, the proportion of memory helper T cells (CD3+ CD4+ CD45 RO) also became reduced in comparison to the non-serious cases [57]. Collectively, these results imply that lymphopenia could be employed to assess the severity of the illness and the probability of recovery in COVID-19 patients [53]. Even while lymphopenia was found in some notable but not severe cases, it was significantly uncommon in less severe patients than in severe ones [58,59]. However, the normal range of B cells indicates that damaged B cells are not as important as compromised T or NK cells.
Abnormal Granulocytes and Agranulocytes
During acute COVID-19 infection, the count of granulocytes and monocytes is also aberrant. In severe conditions, the ratio of neutrophils to lymphocytes is much higher. Moreover, in severe cases, the number of basophils, eosinophils, and monocytes is reduced [60].
Elevated Level of Cytokines
Another important feature of severe COVID-19 is increased cytokine production, and elevated levels of cytokines such as IL-1, IL-2, IL-6, IL-7, IL-8, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon inducible protein, macrophage protein 1, inflammation, INF, and TNF alpha, etc. [61].
Antibody Dependent Cell Mediated Cytotoxicity (ADCC)
In the realm of SARS-CoV-2 infection, antibody-dependent cellular cytotoxicity (ADCC) has been recognized as a crucial element of the antiviral immune response. Antibodies specific to the virus, especially those that bind to the spike glycoprotein, are capable of attaching to infected cells and enlisting effector immune cells via Fc–FcγR interactions [62]. Natural killer (NK) cells serve as the main agents of ADCC, releasing perforin and granzymes that destroy antibody-coated cells [63]. This process aids in viral elimination and is believed to play a protective role in managing the infection. Consequently, ADCC acts as a double-edged sword in COVID-19, offering protection to the host while also potentially contributing to immune-mediated damage. While ADCC plays a critical role in clearing the virus, overactivation of NK cells has been associated with lung immunopathology and severe manifestations of COVID-19, illustrating the dual nature of ADCC in both protection and immunopathogenesis [64]. Gaining a deeper insight into ADCC could aid in refining monoclonal antibody treatments and enhancing the effectiveness of vaccination approaches by leveraging functional antibody responses [65].
1.2.2. M. tb Immunopathogenesis
Over thousands of years of co-evolution, M. tb and humans had developed a host–pathogen relationship [66]. M. tb infected one third of the world’s population, which shows how successfully the bacteria has infiltrated, survived, and spread inside its human host. However, little is known about the immunological events that occur during acute infections and human exposure. Since the majority of human studies focus on immune responses ex vivo in peripheral blood or following in vitro infection of primary cells or cell lines, it is difficult to study early disease processes at the site of disease [67]. This is primarily because there are no diagnostic tests that can reliably detect M. tb in people who have an acute infection [68].
Innate Immune Response in M. tb
In 1948, Arvid Wallgren documented the clinical symptoms of newly acquired M. tuberculosis (M. tb) infection by conducting sequential tuberculin skin tests (TST) on contacts of TB patients and recording transitions from TST-negative to TST-positive results. Numerous individuals experiencing TST conversion exhibited symptoms such as erythema nodosum, fever, and increased erythrocyte sedimentation rate (ESR), indicating that a systemic innate inflammatory response occurs prior to the development of an adaptive immune response [69,70]. These inflammatory manifestations were thought to represent a delayed-type hypersensitivity reaction, which is fundamental to the activation of M. tb-specific T cell responses [71].
Once infectious aerosol droplets are inhaled, the initial significant immunological occurrence is the absorption of bacilli by alveolar macrophages (AMs) located in the distal regions of the lungs [17]. In order to reach the alveoli, bacteria must navigate mucosal surfaces and endure both mechanical clearance and the innate immune defenses present in the upper airways. During this journey, the bacilli face heightened susceptibility to the defense mechanisms of the host [72]. Therefore, gaining insight into the primary interactions between host and pathogen at the mucosal interface, particularly those involving epithelial cells, secretory IgA, and resident immune cells, could enhance the development of preventive strategies and mucosal vaccines aimed at stopping TB infection during its earliest stages [73].
- a.
- Macrophages in M. tb phagocytosis
Aerosol macrophages are thought to play a protective role during M. tb infection. Studies using animal models have highlighted their crucial involvement in the initial phagocytosis of M. tb [74]. Determining their specific function in the human immune response to M. tb infection has been challenging; instead, we can only make educated guesses based on research conducted with cells obtained through invasive broncho-alveolar lavage (BAL), autopsy tissue sections, or lung resections, typically associated with severe disease. Consequently, isolating macrophages from their tissue context and adjacent immunological environment comprising activated cytokines and other interacting cell types complicates our understanding of how macrophages respond to in vitro M. tb infection [75]. Although this reductionist approach has its limitations, it has provided valuable insights into the differences between human alveolar macrophages (AMs) and peripheral monocytes [76].
AMs primarily utilize complement receptor 4 (CR4) during the phagocytosis process, whereas blood monocytes utilize CR1, CR3, and CR4. As a result, variations in serum concentrations and the reduction in serum heat inactivation can either enhance or diminish M. tb uptake. Additionally, AMs demonstrate greater capability than monocytes in restricting the intracellular proliferation of M. tb, and they secrete substantial amounts of TNF [77]. Notably, the production of TNF does not solely occur through phagocytosis, as uninfected AMs in the same culture also release TNF. This phenomenon does not occur when uninfected AMs are separated from infected AMs using a 0.4-µm trans well, indicating that either cell–cell contact or the presence of a soluble factor larger than 0.4 µm is essential for uninfected AMs to produce TNF [78].
- b.
- Neutrophils
In humans, peripheral neutrophilia is the defining feature of TB and a predictor of poor prognosis and morbidity [79]. The function of neutrophils in human TB has received little attention due to the absence of neutrophil involvement in murine TB and the challenges associated with studying neutrophils in vitro [80]. There was a renaissance of interest in neutrophils following the first whole-blood microarray investigation of TB patients compared to healthy controls, which revealed significant neutrophil involvement in the gene expression profile that discriminated between TB patients and controls [81].
- c.
- Innate T cells
Research into germ-line-encoded and lung-resident lymphocyte populations has recently gained attention due to the expectation that these cells can respond rapidly to M. tb infection. These lymphocytes are situated at mucosal sites within the airways, positioning them well to combat pathogens. This presents a notable advantage compared to traditional T cell responses, which need to be primed in primary lymphoid organs and subsequently differentiate into effector cells before they can reach the site of the infection [82].
- d.
- The Granuloma
Tissue-resident T cells, including mucosal-associated invariant T (MAIT) cells, demonstrate swift effector responses such as the release of cytokines and cytotoxic actions, which are essential for immediate antimicrobial protection at mucosal surfaces [83,84]. Although these cells provide protective roles during various infections, the specific contributions of airway-resident lymphocytes to the defense against M. tuberculosis (M. tb) in humans are still not fully understood [85]. In the case of tuberculosis, the formation of granulomas is a key pathological feature, creating a physical barrier that restricts access for immune cells and limits the effectiveness of anti-TB medications, ultimately allowing the bacteria to persist in a latent or subclinical state [86,87].
Adaptive Immune Responses of M. tb Infection
- a.
- T cells in M. tb Infection
The adaptive immune response plays a vital role in managing M. tuberculosis (M. tb) infection, particularly through T-cell-mediated immunity [88]. Following infection, antigen-presenting cells (APCs), such as dendritic cells and macrophages, process M. tb antigens and present them on MHC molecules to naïve T cells in lymph nodes [89]. This initiates the activation of CD4+ T helper (Th1) cells, which produce interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), essential cytokines that enhance infected macrophages’ ability to eliminate or control intracellular bacilli [90,91]. CD8+ cytotoxic T lymphocytes (CTLs) also contribute by directly killing infected cells and releasing perforin and granzyme [92]. In addition, Th17 cells are involved in recruiting neutrophils to the infection site and enhancing mucosal immunity [93]. Furthermore, CD4+ T-cell responses significantly impact the outcome of M. tb infection, becoming detectable within 3–8 weeks after infection [94]. A CD4+ T-cell-mediated type IV delayed hypersensitivity reaction can be recognized by visible skin changes, appearing 24–72 h after intradermal administration of M. tb purified protein derivative (PPD) or tuberculin [tuberculin skin test (TST)] in most individuals infected with M. tb [95]. There has been considerable focus on identifying M. tb antigens that stimulate robust T-cell responses for diagnostics and vaccine development [96].
- b.
- B Cell Response in TB Defense
Because T-cell responses are dominant and M. tuberculosis (M. tb) resides within infected macrophages, antibodies have traditionally been thought to play a limited role in preventing infection upon exposure [97]. However, recent discoveries have shown that B cells and antibodies can modulate the immune response to intracellular pathogens, including M. tb, by mechanisms such as antigen presentation, cytokine secretion, and antibody-mediated effector functions [97,98]. Surprisingly, B cells and antibodies can contribute to bacterial clearance through opsonization and Fc receptor-mediated processes, promoting phagocytosis and enhancing antigen presentation to T cells. Despite these immune responses, M. tuberculosis has evolved sophisticated strategies to evade the host immune system [99]. These include inducing T-cell exhaustion, manipulating host cell death pathways, and altering antigen presentation, which allow the bacterium to persist and establish a latent infection [100]. Co-infection with COVID-19 and M. tb occurs in several immunopathological events, and is summarized in the schematic illustration in Figure 1.
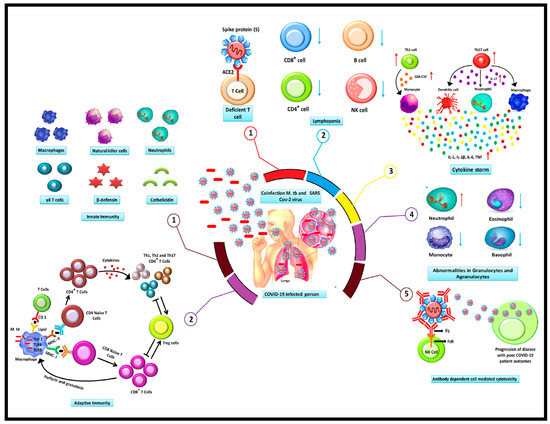
Figure 1.
A schematic illustration of the immunopathogenesis of co-infection with COVID-19 and TB.
The TB-COVID co-infection displays several immunopathological events encompassing a range of responses, including (1) the SARS-CoV-2 interaction with the ACE 2 receptors on the surface of T cells, which promotes the entry of the virus, impairing and depleting T cells. (2). The most severe manifestation of COVID-19 patients is lymphopenia, which is marked by a reduction in B and T lymphocyte levels. (3) Cytokine storm is characterized by a persistently elevated level of cytokines. Patients with COVID-19 infections produce cytokines in large quantities, which has a negative impact on the body. (4) Granulocyte and agranulocyte abnormalities may arise as a consequence of the COVID-19 infection. As opposed to monocytes, basophils, and eosinophils, neutrophils often have higher levels. (5) An antibody that targets the S protein of the SARS-CoV-2 virus facilitates virus entrance in the cells by attaching to the Fc region of the antibody with the cell surface-located Fc receptors. In contrast, when M. tb is inhaled into the lungs, the infection is treated by the body’s innate and adaptive immune response systems. Either the latent M. tb infection or the active TB infection may develop as the infection advances. During innate immune response (1) Various immune cell types, including NK cells, T cells, macrophages, neutrophils, and other soluble effectors proteins molecules participate in early innate immunity while during adaptive response (2) M. tb infection various subpopulations of T cells, consisting CD4+ and CD8+ cells, Treg cells, Th1, Th2, and Th17 cells, are responsible for regulating the adaptive immune response. These cells are proliferated and differentiated by interactions between naive CD4+ and CD8+ cells and the antigen represented by MHC class I and MHC on the surface of activated macrophages.
NK cells: Natural killer cells, Treg: T cell regulatory, Th1: T helper 1, Th2: T helper 2 Th17: T helper 17, MHC: Major histocompatibility complex, CD4+: Cluster of differentiation 4, CD8+: Cluster of differentiation 8, ACE2: Angiotensin-converting enzyme 2.
1.3. COVID-19 and Tuberculosis: Intersection
Both tuberculosis and COVID-19 are infectious diseases that impact the respiratory system. TB spreads through droplet transmission, while COVID-19 is transmitted through aerosols [101,102]. The presence of overcrowding increases the likelihood of transmission for these diseases. Individuals who are elderly, malnourished, diabetic, or have other chronic illnesses, as well as those with weakened immune systems, are more susceptible to these infections [103,104]. Table 1 describes the salient features of TB and COVID immunopathogenesis.

Table 1.
List of salient features of TB and COVID immunopathogenesis.
Table 1.
List of salient features of TB and COVID immunopathogenesis.
| Feature | Tuberculosis (TB) | COVID-19 | References |
|---|---|---|---|
| Causative Agent | Mycobacterium tuberculosis | COVID-19 | [105] |
| Primary Site of Infection | Lungs (pulmonary TB), can become systemic | Respiratory tract (lungs), can affect multiple organs | [106] |
| Innate Immune Response | Activation of macrophages and dendritic cells; formation of granulomas | Activation of epithelial cells, macrophages, and neutrophils; excessive inflammation in severe cases | [107] |
| Adaptive Immune Response | Th1-mediated immunity (IFN-γ, TNF-α), CD4+ and CD8+ T cells crucial | Early IFN response, then dysregulated T-cell response; lymphopenia common in severe cases | [108] |
| Cytokine Profile | Elevated IL-12, IFN-γ, TNF-α; chronic inflammation | High IL-6, IL-1β, TNF-α in severe cases; cytokine storm in critical patients | [109] |
| Immune Evasion Mechanisms | Inhibits phagosome-lysosome fusion; survives in macrophages | Suppresses type I IFN response; induces T-cell exhaustion and apoptosis | [110] |
| Tissue Pathology | Granuloma formation; caseous necrosis in lungs | Diffuse alveolar damage; endothelial injury and microthrombosis | [111] |
| Immune Exhaustion/Immunopathology | Chronic immune activation may lead to T-cell exhaustion | Severe cases show T-cell exhaustion and overactive innate immunity | [112] |
| Co-infection Impact | COVID-19 can exacerbate TB or lead to reactivation | TB may worsen COVID-19 outcomes due to underlying lung damage | [113] |
Infected patients on steroid treatment are prone to having additional infections due to a compromised immune system, making them vulnerable to being readily infected by external tuberculosis. Individuals who are immunocompromised and elderly, particularly those with diabetes and chronic illnesses, are at greater risk of contracting infections, wherein cell-mediated immunity plays an important role in the pathogenesis of both infectious agents [114]. Contracting COVID-19 and receiving corticosteroids can result in an external tuberculosis infection or the reactivation of a past endogenous infection [115]. A study has shown the relationships between tuberculosis and COVID-19 and found overlapping genetic markers in a meta-analysis [115]. Patients with either active or latent tuberculosis were found to be more susceptible to developing COVID-19, and those diagnosed with COVID-19 should also be screened for tuberculosis [116]. There were hypotheses that the BCG (Bacillus calmette guerin) vaccine used for protection against TB might offer some protection against COVID-19, although no definitive proof was found. Both diseases exhibit similar symptoms, such as fever, cough, fatigue, malaise, and hemoptysis, which can complicate diagnosis. For example, a study was conducted with a limited patient group and noted that TB diagnosis was more straightforward during the investigation of COVID-19 infection, and this was the first study on a cohort of TB patients with COVID-19 co-infection [117,118]. In another study, hospitalized patients with active tuberculosis were assessed, revealing that 20 out of 24 patients tested co-positive for COVID, suggesting an increased vulnerability for co-infection [51]. While these results call for more research to establish the mutual influence, numerous clinical studies have indicated that tuberculosis patients are more likely to contract the coronavirus and experience severe outcomes [7,14,43,119]. Furthermore, most of these studies presented two significant limitations: a small sample size and a lack of information on pre-existing comorbidities.
2. Multi-Omics Approaches to Dissect the TB-COVID-19 Syndemic
The phrase “omics study” refers to comprehensive biological research techniques that involve detailed analysis of various molecular types that make up cells, tissues, or organisms. The objective of these studies is to comprehend the roles, interactions, and behaviors of the diverse biological elements. Notably, a multi-omics strategy provides a high-resolution, systems-level insight into the immunopathological interactions in TB–COVID-19 co-infection. Figure 2 suggests an integrated multi-omics framework for understanding TB-COVID-19 co-infection. Additionally, here we have summarized a few of the important multi-omics studies providing insight into TB-COVID-19 infections
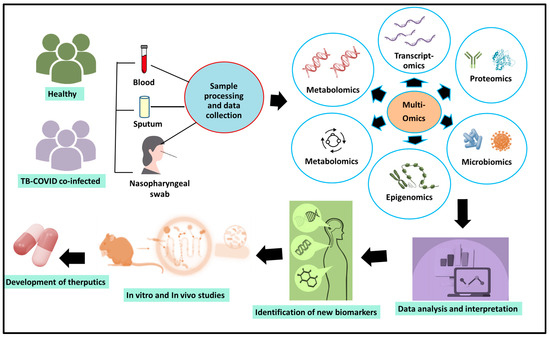
Figure 2.
A multi-omics framework for understanding TB-COVID-19 co-infection.
The illustration presents an elaborate multi-omics framework aimed at uncovering the intricate biological interactions involved in co-infections of tuberculosis (TB) and COVID-19. To accomplish this, a variety of clinical specimens, including blood, sputum, and nasopharyngeal swabs, were gathered from both healthy individuals and those diagnosed with co-infection of TB and COVID-19. These biospecimens can undergo systematic processing to produce raw data across several omics layers. Genomics provides insights into the genetic variations in both the host and pathogens that may impact susceptibility, disease severity, or the development of drug resistance. Transcriptomics reveals the gene expression patterns of both the host and microbes, emphasizing disrupted immune pathways and co-infection signatures. Proteomics offers comprehensive profiles of protein levels, their modifications, and signaling networks associated with the progression of disease, while metabolomics identifies metabolic alterations and biochemical imbalances stemming from the simultaneous infection. Epigenomics contributes by uncovering changes in host DNA methylation and histone modifications that could influence the regulation of immune responses, latency, or reactivation. Meanwhile, microbiomics examines the composition and behavior of host-associated microbiota, which may significantly affect immune function and the overall outcome of the disease. By integrating these varied omics datasets, it enables system-wide analyses that lead to the discovery of strong biomarkers for early diagnosis, prognosis evaluation, and treatment monitoring. Additionally, the multi-omics framework enhances both in vitro and in vivo experimental research, promoting the validation of essential molecular pathways and the identification of new therapeutic targets. Ultimately, this comprehensive approach offers a valuable perspective for understanding the TB-COVID-19 syndemic, aiding precision medicine efforts and informing public health strategies aimed at alleviating the dual impact of these infections.
2.1. Genomics
The traits and phenotype of every cell species are determined by the central dogma of the biological system, which is DNA-RNA-Protein 2011 [34,120]. Finding M. tb-specific nucleic acids through amplification techniques and identifying gene alterations through sequencing were the main goals of the initial attempts at molecular typing of M. tb.
To differentiate between M. tb and M. bovis (BCG strain), early studies used probe-based typing techniques, including PFGE (Pulse field gel electrophoresis) typing and IS6110-RFLP (IS6110 genetic biomarker-based restriction fragment length polymorphism, also known as genetic fingerprinting). Fast ligation-mediated PCR (FliP), ligation-mediated PCR (LM-PCR), and mixed-linker PCR (ML-PCR) are examples of second-generation genomic methods for M. tb molecular typing [12,34].
Technology advancements that have improved the cost-effective, high-throughput analysis of biological molecules are the primary drivers of high-throughput gene expression technologies, which have transformed medical research. Furthermore, array-based comparative genomics showed promise as a method for tracking the evolution of microorganisms, molecular epidemiology, and disease. High-density oligonucleotide microarray research showed that clinically and epidemiologically described M. tb can be used to identify trim-level genomic deletions and assess genetic variability in the wild population [121].
Additionally, an another study also reported that with enrichment in viral genome replication and immune-related pathways, this study found 96 similar differentially expressed genes (DEGs) that are shared by COVID-19 and tuberculosis (TB), revealing common processes underlying both illnesses. Ten important hub genes (such as IFI44L, ISG15, MX1, OASL, and GBP1) were identified using protein–protein interaction network analysis; several of these genes are interferon-stimulated. Analysis of regulatory networks revealed 29 miRNAs and 61 transcription factors that may be in charge of these genes. Crucially, the study suggested eleven prospective medications as possible treatments for TB–COVID-19 co-infection, including propofol, acetohexamide, etoposide, and suloctidil. All things considered, our results offer pharmacological options and molecular targets that may direct the development of future treatments for patients with COVID-19 and TB.
2.2. Role of Proteomics to Explore Tb-COVID Coinfection
Particularly in cases of co-infection, proteomics offers a powerful tool for understanding the complex pathophysiology of COVID-19 and TB [41]. Researchers can find out more about disease processes, host immune responses, and potential links between COVID-19 and M. tuberculosis by analyzing the full range of proteins produced in patient samples, such as blood, lung tissue, or bronchoalveolar lavage.
In pathogenesis studies, proteomics helps to identify host proteins that were differentially expressed and linked to inflammation, tissue damage, and immunological regulation [51]. For example, TB and COVID-19 both provoke cytokine storms, acute-phase reactions, and complement system activation, but the molecular actors and severity may vary. Granuloma formation and a cell-mediated immune response are the major causes of TB, while interferon signaling, viral defense mechanisms, and coagulopathy are major drivers for COVID-19 infection [122]. Proteomics can show how these reactions vary in co-infected people, which may result in reduced immune response, leading to increased susceptibility to co-infection by other pathogens. Additionally, proteins such as ferritin, haptoglobin, annexin A1, and SAA1 have been identified as possible biomarkers that may represent common or unique disease processes [123]. In order to comprehend the severity and development of disease, comparative proteome profiling can be leveraged to investigate contributing factors such as immunometabolic reprogramming, disturbances in oxidative stress, and tissue remodeling pathways.
Combining clinically annotated proteomics and transcriptomics data offers an opportunity to develop thorough models of TB–COVID-19 pathogenesis. These models have the capacity to assist differential diagnosis, host-directed therapy, and dual-action intervention target identification. In this systems-wide investigation, sophisticated methods like LC-MS/MS in conjunction with bioinformatics tools (e.g., MaxQuant, STRING) are crucial [124].
2.3. Transcriptomics
Investigating the pathophysiology of COVID-19 and tuberculosis (TB) requires a thorough understanding of transcriptomics, particularly when it involves identifying how host gene expression changes during single or co-infections [125]. Variations in gene expression, which represent immune activation, inflammation, tissue damage, and immune evasion tactics, can be detected by employing methods such as RNA sequencing (RNA-Seq) to analyze the entire spectrum of RNA transcripts (mRNA, non-coding RNA, etc.). COVID-19 is frequently characterized by the overexpression of innate immune receptors, pro-inflammatory cytokines like TNF-α and IL-6, and interferon-stimulated genes (ISGs). In severe cases, these cytokines are frequently associated with cytokine storm syndromes. Transcriptome profiling explained the poor prognosis of co-infected patients, which shows signs of immune exhaustion or suppression, modification of immune-regulatory networks, and worsened inflammatory gene expression.
Complement activation, antigen presentation, and cell death mechanisms (including apoptosis and pyroptosis) are often associated with dysregulated genes. Transcriptomics also helps to identify diagnostic gene signatures and prognosis markers, which can distinguish TB from COVID-19 or identify co-infection biomarkers. Integrating proteomics and clinical data provides a systems-level view of etiology and facilitates the identification of host-directed therapeutic targets [125].
2.4. Metabolomics
Metabolomics, which involves the extensive examination of small molecules in biological systems, provides crucial insights into the interactions between hosts and pathogens during co-infection with TB and COVID-19. This methodology can reveal unique metabolic patterns linked to the severity of diseases, immune dysfunction, and concurrent pathogenesis [126]. Research on tuberculosis has uncovered disturbances in lipid metabolism, energy metabolism, and amino acid synthesis, especially concerning the tryptophan–kynurenine and arginine–proline pathways, which are tied to immune suppression and the formation of granulomas [127]. In the context of COVID-19, investigations have shown that glycolysis, intermediates in the TCA cycle, and lipid profiles are dysregulated, indicating hyperinflammation and fatigue in immune cells [128,129]. TB-COVID-19 co-occurrence leads to shared disruptions in immunometabolism, such as decreased mitochondrial oxidative phosphorylation and the build-up of pro-inflammatory metabolites, which can collectively weaken the host defense mechanisms [130]. Consequently, metabolomics offers great potential for identifying biomarkers for early diagnosis, distinguishing individuals with co-infections, and informing precision treatments aimed at metabolic profiles in populations that are vulnerable to both TB and COVID-19 [131,132].
2.5. Epigenomics
Epigenomics is crucial for understanding the immune dysregulation observed in co-infections of TB and COVID-19, as it illustrates how the expression of host genes is adjusted without modifications to the DNA sequence [133]. Both M. tuberculosis (M. tb) and COVID-19 trigger epigenetic changes in immune cells, including alterations in histones, DNA methylation, and variations in non-coding RNA expression, which can either boost or inhibit immune reactions [134]. In case of TB, both latent and active infections are linked to trained immunity, wherein innate immune cells, particularly monocytes and macrophages, experience epigenetic modifications that enhance their readiness for secondary infections [135]. On the other hand, COVID-19 leads to epigenetic suppression of interferon-stimulated genes, resulting in compromised antiviral responses [136]. When these two infections occur simultaneously, the conflicting impacts on type I interferon signaling and immune cell activation can result in immune depletion, impaired T-cell memory, and lasting inflammation. New single-cell multi-omics and ATAC-seq research have been used to uncover changes in chromatin accessibility and transcription factor networks affected in co-infection scenarios, providing a more profound insight into the mechanisms of disease and potential paths for host-targeted therapies [137]. Therefore, incorporating epigenomic findings into TB and COVID-19 research is vital for identifying biomarkers, understanding long-term immune effects, and enhancing vaccination strategies in regions where both diseases are prevalent.
2.6. Microbiomics
Microbiomics, which includes the microbial communities and their genetic components, provides a vital understanding of how co-infection with TB and COVID-19 affects host immunity and disease outcomes. Research has indicated that infections caused by M. tuberculosis (M. tb) and COVID-19 can disrupt both respiratory and gut microbiota, leading to immune dysregulation [138]. In individuals with TB, the composition of lung microbiota shifts toward lower microbial diversity and increased presence of inflammatory genera like Streptococcus and Prevotella, which can influence alveolar immunity and the formation of granulomas [139]. Likewise, COVID-19 infection disturbs the gut-lung axis and local pulmonary microbiota, often resulting in dysbiosis, which is marked by a reduction in beneficial microorganisms such as Bifidobacterium and a rise in opportunistic pathogens [140]. During co-infection, these imbalances may combine to exacerbate mucosal barrier dysfunction, heighten systemic inflammation, and hinder both innate and adaptive immune responses, leading to a worsening of disease progression. Recent research suggests that the microbial metabolites, including short-chain fatty acids (SCFAs) and tryptophan derivatives, are modified in individuals with co-infection, affecting T cell activation and cytokine production [141]. Consequently, profiling the microbiome in cases of TB and COVID-19 co-infection might serve as a diagnostic approach, predict treatment outcomes, and inform microbiota-focused therapies to restore immune equilibrium and lower the risk of comorbidities. Table 2 provides the key highlights of the multi-omics study to understand TB-COVID co-infection.

Table 2.
Summarized highlights of a multi-omics study to understand TB-COVID co-infection.
Table 2.
Summarized highlights of a multi-omics study to understand TB-COVID co-infection.
| Muti-Omics Approach | Biological Molecule | Analyzed Molecules | Techniques for Analysis | Application in TB & COVID-19 Co-Infection | Major/Significant Findings |
|---|---|---|---|---|---|
| Genomics | DNA (genome) | Genes, mutations, SNPs | Whole Genome Sequencing (WGS), PCR, GWAS | Analyses variations and resistance genes, finds host and pathogen mutations, and determines genetic vulnerability to TB/COVID. | A study highlighted the role of gut and respiratory microbiota in regulating host immunity during viral infections such as influenza and coronavirus, affecting vulnerability, disease severity, and responses to vaccines and, thus, proposed microbiome-targeted strategies as supplementary treatments [142]. Genome-to-genome analysis in TB patients from Tanzania uncovered notable links between human genetic variants and the genomic variation of Mycobacterium tuberculosis, underscoring the co-evolution of host and pathogen and its influence on the outcomes of TB disease [143]. |
| Transcriptomics | RNA (transcriptome) | mRNA, non-coding RNA | RNA-Seq, Microarrays, qRT-PCR | detects immune response pathways, reveals changes in gene expression, and differentiates between illness phases and co-infection markers. | A study used whole-genome sequencing to explore host genetic factors influencing COVID-19 outcomes and identified variants linked to susceptibility and disease severity. The findings suggested that host genetic background plays a crucial role in determining individual responses to SARS-CoV-2 infection [144]. |
| Proteomics | Proteins (proteome) | Protein levels, modifications | Mass Spectrometry (LC-MS/MS), Western blot, ELISA | examines alterations in functional proteins, finds therapeutic targets and indicators of inflammation, and investigates immunological dysregulation in co-infection. | A review explored the growing importance of non-coding RNAs in the regulation of the immune system and the development of autoimmune diseases, emphasizing how microRNAs, lncRNAs, and circRNAs affect the differentiation, signaling, and tolerance of immune cells. It also underscored recent advancements in technology that facilitate their investigation, while acknowledging significant challenges for clinical application [145]. Another study demonstrated the importance of proteomics in investigating new and re-emerging RNA virus infections, emphasizing how proteomic strategies reveal interactions between hosts and viruses, immune responses, and possible biomarkers. It emphasizes proteomics as an effective method for comprehending viral pathogenesis and informing the development of therapies [146]. |
| Metabolomics | Small molecules/metabolites | Amino acids, lipids, nucleotides, organic acids, carbohydrates | NMR spectroscopy, LC-MS/MS, GC-MS | Identifies metabolic signatures linked to immune dysregulation in co-infection; e.g., altered tryptophan, arginine, and lipid metabolism affecting macrophage function and cytokine storms. | Tandem mass spectrometry (MS/MS) has become a valuable technique for analyzing proteins, facilitating structural analysis, the identification of biomarkers, and clinical diagnostics related to infectious diseases. Additionally, another research highlighted that proteomic, metabolomic, and epigenetic profiles can shed light on host reactions in COVID-19/TB co-infection and guide future approaches for diagnostics and treatment strategies [147,148]. |
| Epigenomics | DNA, chromatin | DNA methylation, histone modifications, chromatin accessibility | Whole-genome bisulfite sequencing (WGBS), ChIP-seq, ATAC-seq | Reveals host epigenetic reprogramming during M. tb and COVID-19 infections; explains altered immune gene expression, T cell exhaustion, and trained immunity. | Research into epigenomics has demonstrated how trained immunity affects the host’s defense mechanisms, with tuberculosis (TB) and COVID-19 exhibiting shared immune reprogramming processes. Metabolomic analysis has shown significant disruptions in immune metabolism during co-infection, characterized by changes in crucial energy and amino acid pathways. Moreover, recent studies indicate that gut microbiota has the potential to influence systemic immunity and affect the outcomes of COVID-19, suggesting it could serve as a therapeutic target. Collectively, these findings highlight the interconnected significance of epigenetic memory, metabolic control, and microbial ecology in influencing host vulnerability and the progression of diseases such as TB and COVID-19 [149]. |
| Microbiomics | Microbial nucleic acids | 16S rRNA genes, microbial DNA/RNA | 16S rRNA sequencing, whole metagenome sequencing (WMS), qPCR | Characterizes lung and gut microbiome shifts; disrupted microbiota in TB or COVID-19 affects immune modulation, secondary infections, inflammation, and recovery trajectories. | Recent studies emphasized the significance of the microbiome in influencing host responses to COVID-19 and tuberculosis. Alterations in gut microbiota were associated with dysfunctional immune responses in both infections, indicating that microbial imbalances could worsen disease progression or severity. Additionally, shifts in the lung microbiome during COVID-19 have been linked to inflammation, weakened immunity, and secondary infections, highlighting its role in respiratory diseases. More extensive reviews further endorse a two-way relationship between microbiota composition and COVID-19 outcomes, suggesting that the microbiome may serve as both a marker and a potential modulator of the disease. Together, these insights imply that targeting the microbiome could pave the way for novel therapeutic and diagnostic approaches in COVID-19 and TB [150]. |
3. Conclusions
This review describes the importance of various multi-omics strategies, i.e., genomics, transcriptomics, proteomics, metabolomics, epigenomics, and microbiomics, and their crucial role in providing critical insights into TB and COVID-19 co-infection. These evolving strategies were leveraged to track changes in immune responses, gene regulation, microbial communities, and metabolic pathways that contributed to disease severity and progression. A microbiomics study demonstrated that co-infection disrupts lung and gut microbiota, deteriorates inflammation, and causes immune dysfunction. Transcriptomic, Proteomics, and Metabolomics can be used to identify biomarkers like SAA and ferritin, which can eventually be leveraged to develop diagnosis and treatment strategies. A systems-level multi-omics framework integrates this data to understand infection mechanisms and develop targeted therapies. These approaches play a key role in overcoming diagnostic challenges that arise due to overlapping symptoms. Overall, multi-omics provides a window to improve disease management and vaccine development in endemic regions.
Although multi-omics strategies hold considerable promise in understanding the intricate relationships between co-infection with tuberculosis (TB) and COVID-19, numerous limitations persist. In the realm of TB-COVID-19 research, multi-omics strategies encounter issues such as disparate studies, small and varied participant groups, high expenses, and an absence of standardized procedures for integrating data. Difficulties in collecting biospecimens, computational capabilities, and reproducibility further hinder their broad implementation, particularly in settings with limited resources.
Author Contributions
M.C.: Original draft preparation, review, writing, editing, and compilation of manuscript; S.V.: Review, writing, and editing of manuscript; S.D.: Review, writing, and editing of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors want to thanks ANRF-NPDF fellowship India, reference no. PDF/2025/007980 to support M.C.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023; Available online: https://iris.who.int/handle/10665/373828 (accessed on 26 July 2025).
- Morse, D.; Brothwell, D.R.; Ucko, P.J. Tuberculosis in ancient Egypt. Am. Rev. Respir. Dis. 1964, 90, 524–541. [Google Scholar]
- Cave, A.J.; Demonstrator, A. The evidence for the incidence of tuberculosis in ancient Egypt. Br. J. Tuberc. 1939, 33, 142–152. [Google Scholar] [CrossRef]
- Flick, L.F. Development of Our Knowledge of Tuberculosis; Wickersham: Sheffield, UK, 1925. [Google Scholar]
- Singh, H.; Singh, J.; Khubaib, M.; Jamal, S.; Sheikh, J.A.; Kohli, S.; Rahman, S.A. Mapping the genomic landscape & diversity of COVID-19 based on >3950 clinical isolates of SARS-CoV-2: Likely origin & transmission dynamics of isolates sequenced in India. Indian J. Med. Res. 2020, 151, 474–478. [Google Scholar] [PubMed]
- WHO. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 27 July 2025).
- Tadolini, M.; Codecasa, L.R.; García-García, J.-M.; Blanc, F.-X.; Borisov, S.; Alffenaar, J.-W.; Andréjak, C.; Bachez, P.; Bart, P.-A.; Belilovski, E.; et al. Active tuberculosis, sequelae and COVID-19 co-infection: First cohort of 49 cases. Eur. Respir. J. 2020, 56, 2001398. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P.R. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Chan, J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001, 19, 93–129. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Fleming, J.; Yu, Y.; Gu, Y.; Liu, C. Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. medRxiv 2020. [Google Scholar] [CrossRef]
- Stochino, C.; Villa, S.; Zucchi, P.; Parravicini, P.; Gori, A.; Raviglione, M.C. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur. Respir. J. 2020, 56, 2001708. [Google Scholar] [CrossRef]
- Riou, C.; du Bruyn, E.; Stek, C.; Daroowala, R.; Goliath, R.T.; Abrahams, F.; Said-Hartley, Q.; Allwood, B.W.; Hsiao, N.Y.; Wilkinson, K.A.; et al. Relationship of SARS-CoV-2-specific CD4 response to COVID-19 severity and impact of HIV-1 and tuberculosis coinfection. J. Clin. Investig. 2021, 131, e149125. [Google Scholar] [CrossRef]
- Acharya, D.; Liu, G.; Gack, M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020, 20, 397–398. [Google Scholar] [CrossRef]
- Sia, J.K.; Rengarajan, J. Immunology of M. tuberculosis infections. Microbiol. Spectr. 2019, 7, GPP3–GPP0022. [Google Scholar] [CrossRef]
- Torrelles, J.B.; Schlesinger, L.S. Integrating lung physiology, immunology, and tuberculosis. Trends Microbiol. 2017, 25, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Iwasaki, A. Type I and Type III interferons—Induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 2020, 27, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Mohan, A.; Kohli, M. COVID-19 and tuberculosis: A double trouble. Indian, J. Tuberc. 2020, 67, S1–S2. [Google Scholar] [CrossRef]
- Yang, L.; Xiang, F.; Wang, D.; Guo, Q.; Deng, B.; Jiang, D.; Ren, H. The safety and immunogenicity of inactivated COVID-19 vaccine in old pulmonary tuberculosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 503–512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ong, C.W.M.; Migliori, G.B.; Raviglione, M.; MacGregor-Skinner, G.; Sotgiu, G.; Alffenaar, J.W.; Tiberi, S.; Adlhoch, C.; Alonzi, T.; Archuleta, S.; et al. Epidemic and pandemic viral infections: Impact on tuberculosis and the lung: A consensus by the World Association for Infectious Diseases and Immunological Disorders (WAidid), Global Tuberculosis Network (GTN), and members of the European Society of Clinical Microbiology and Infectious Diseases Study Group for Mycobacterial Infections (ESGMYC). Eur. Respir. J. 2020, 56, 2001727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burel, J.G. Transcriptomic profiling reveals immune dysregulation in COVID-19 and TB coinfection. Front. Immunol. 2021, 12, 678604. [Google Scholar]
- Yan, Q.; Wadsworth, M.H., II; He, A.; Wang, S.; Li, R.; Tran, L.M.; Zhang, L.; Yu, J.; Ng, C.T.; Park, S.M.; et al. Interferon response in COVID-19–TB coinfection: Insights from blood transcriptomics. Cell Rep. Med. 2021, 2, 100402. [Google Scholar]
- Messner, C.B.; Demichev, V.; Wendisch, D.; Michalick, L.; White, M.; Freiwald, A.; Textoris-Taube, K.; Vernardis, S.I.; Egger, A.S.; Kreidl, M.; et al. Ultra-High-Throughput Clinical Proteomics Reveals Classifiers of COVID-19 Infection. Cell Syst. 2020, 11, 11–24.e4. [Google Scholar] [CrossRef]
- Flores-Lovon, K.; Ortiz-Saavedra, B.; Cueva-Chicaña, L.A.; Aperrigue-Lira, S.; Montes-Madariaga, E.S.; Soriano-Moreno, D.R.; Bell, B.; Macedo, R. Immune responses in COVID-19 and tuberculosis coinfection: A scoping review. Front. Immunol. 2022, 13, 992743. [Google Scholar] [CrossRef]
- Mantovani, A.; Netea, M.G. Trained innate immunity, epigenetics, and COVID-19. New Engl. J. Med. 2020, 383, 1078–1080. [Google Scholar] [CrossRef]
- Cheong, J.G.; Ravishankar, A.; Sharma, S.; Parkhurst, C.N.; Grassmann, S.A.; Wingert, C.K.; Laurent, P.; Ma, S.; Paddock, L.; Miranda, I.C.; et al. Epigenetic memory of coronavirus infection in innate immune cells. Cell 2023, 186, 1290–1304.e17. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Shen, X.; Wang, A.; Hu, C.; Chen, J. The gut microbiome: A line of defense against tuberculosis development. Front. Cell. Infect. Microbiol. 2023, 13, 1149679. [Google Scholar] [CrossRef] [PubMed]
- Comberiati, P.; Di Cicco, M.; Paravati, F.; Pelosi, U.; Di Gangi, A.; Arasi, S.; Barni, S.; Caimmi, D.; Mastrorilli, C.; Licari, A.; et al. The role of gut and lung microbiota in susceptibility to tuberculosis. Int. J. Environ. Res. Public Health 2021, 18, 12220. [Google Scholar] [CrossRef] [PubMed]
- Otchere, I.D.; Aboagye, S.Y.; Arthur, P.K.; Asante-Poku, A. Viewpoint of multi-omics potential in tuberculosis research: Identifying biomarkers for biomanufacturing of efficient control tools. Front. Trop. Dis. 2024, 5, 1443248. [Google Scholar] [CrossRef]
- Imran, M.; Alzahrani, H.A.; Elmagzoub, R.M.; Rehman, Z.U.; Abd Elaleem, K.G.; Mohamed, A.S.; Elsharief, A.A.; Alhuthali, H.M. A Systems Biology Approach to Elucidating Functional and Genomic Signatures in Host-Pathogen Interactions of Triple Infections: Tuberculosis, HIV, and SARS-CoV-2. J. Pure Appl. Microbiol. 2025, 19, 1154–1173. [Google Scholar] [CrossRef]
- Mousquer, G.T.; Peres, A.; Fiegenbaum, M. Pathology of TB/COVID-19 co-infection: The phantom menace. Tuberculosis 2021, 126, 102020. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Palepu, S.; Palepu, S.; Handu, S. COVID-19 and tuberculosis co-infection—A neglected paradigm. Monaldi Arch. Chest Dis. 2020, 90, 1437. [Google Scholar] [CrossRef]
- Dheda, K.; Perumal, T.; Moultrie, H.; Perumal, R.; Esmail, A.; Scott, A.J.; Udwadia, Z.; Chang, K.C.; Peter, J.; Pooran, A.; et al. The intersecting pandemics of tuberculosis and COVID-19: Population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir. Med. 2022, 10, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Visca, D.; Ong, C.W.M.; Tiberi, S.; Centis, R. Tuberculosis and COVID-19 interaction: A review of biological, clinical and public health effects. Pulmonology 2021, 27, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Thevarajan, I.; Nguyen, T.H.O.; Koutsakos, M.; Druce, J.; Caly, L.; van de Sandt, C.E.; Jia, X.; Nicholson, S.; Catton, M.; Cowie, B.; et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat. Med. 2020, 26, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Sy, K.T.L.; Haw, N.J.L.; Uy, J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect. Dis. 2020, 52, 902–907. [Google Scholar] [CrossRef]
- TB/COVID-19 Global Study Group. Tuberculosis and COVID-19 co-infection: Description of the global cohort. Eur. Respir. J. 2022, 59, 2102538. [Google Scholar] [CrossRef]
- Sheerin, D.; Wang, X.; Johnson, W.E.; Coussens, A. Systematic evaluation of transcriptomic disease risk and diagnostic biomarker overlap between COVID-19 and tuberculosis: A patient-level meta-analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhang, L.; Zhao, J. Clinical characteristics and outcomes of COVID-19 patients with tuberculosis: A systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2024, 18, e0012136. [Google Scholar] [CrossRef]
- Muflihah, A.N.; Rahmawati, I.; Sutanto, H. Impact of tuberculosis on COVID-19 outcomes: A meta-analysis. Respir. Med. 2024, 220, 107456. [Google Scholar]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2023, 29, 22–36. [Google Scholar] [CrossRef]
- Calderón-Parra, J.; Muiño-Miguez, A.; Bendala-Estrada, A.D.; Ramos-Martínez, A.; Muñez-Rubio, E.; Fernández Carracedo, E. Grupo COVID-19 del Hospital Universitario Ramón y Cajal. Secondary infections in COVID-19 patients: A systematic review. J. Infect. 2023, 86, 135–148. [Google Scholar]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Li, C.K.; Wu, H.; Yan, H.; Ma, S.; Wang, L.; Zhang, M.; Tang, X.; Temperton, N.J.; Weiss, R.A.; Brenchley, J.M.; et al. T cell responses to whole SARS coronavirus in humans. J. Immunol. 2008, 181, 5490–5500. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+ CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020, 11, 551898. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of COVID-19 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef]
- Liguoro, I.; Pilotto, C.; Bonanni, M.; Ferrari, M.E.; Pusiol, A.; Nocerino, A.; Vidal, E.; Cogo, P. COVID-19 infection in children and newborns: A systematic review. Eur. J. Pediatr. 2020, 179, 1029–1046. [Google Scholar] [CrossRef]
- Yang, W.; Cao, Q.; Qin, L.E.; Wang, X.; Cheng, Z.; Pan, A.; Dai, J.; Sun, Q.; Zhao, F.; Qu, J.; et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020, 80, 388–393. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Zhao, X.; Liu, C.; Wang, W.; Wang, D.; Li, L. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu Province: A multicenter descriptive study. Clin. Infect. Dis. 2020, 71, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Schrepping, J.; Reusch, N.; Paclik, D.; Baßler, K.; Schlickeiser, S.; Zhang, B.; Krämer, B.; Krammer, T.; Brumhard, S.; Bonaguro, L.; et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 2020, 182, 1419–1440.e23. [Google Scholar] [CrossRef] [PubMed]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. COVID-19 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Suthar, M.S.; Zimmerman, M.G.; Kauffman, R.C.; Mantus, G.; Linderman, S.L.; Hudson, W.H.; Vanderheiden, A.; Nyhoff, L.; Davis, C.W.; Adekunle, O.; et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep. Med. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Witkowski, M.; Tizian, C.; Ferreira-Gomes, M.; Niemeyer, D.; Jones, T.C.; Heinrich, F.; Frischbutter, S.; Angermair, S.; Hohnstein, T.; Mattiola, I.; et al. Untimely TGFβ responses in COVID-19 limit antiviral functions of NK cells. Nature 2021, 600, 295–301. [Google Scholar] [CrossRef]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, F.D. FcγR-mediated COVID-19 infection of monocytes activates inflammation. Nature 2022, 606, 576–584. [Google Scholar] [CrossRef]
- Chan, C.E.Z.; Seah, S.G.K.; Chye, H.; Massey, S.; Torres, M.; Lim, A.P.C.; Wong, S.K.K.; Neo, J.J.Y.; Wong, P.S.; Lim, J.H.; et al. The Fc-mediated effector functions of a potent SARS-CoV-2 neutralizing antibody, SC31, isolated from an early convalescent COVID-19 patient, are essential for the optimal therapeutic efficacy of the antibody. PLoS ONE 2021, 16, e0253487. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front. Immunol. 2020, 11, 544639. [Google Scholar] [CrossRef]
- Gagneux, S. Host–pathogen coevolution in human tuberculosis. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 850–859. [Google Scholar] [CrossRef]
- Dye, C.; Scheele, S.; Pathania, V.; Raviglione, M.C. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. JAMA 1999, 282, 677–686. [Google Scholar] [CrossRef]
- Wallgren, A. The time-table of tuberculosis. Tubercle 1948, 29, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Sud, D.; Sharma, S.; Madan, P. Textbook of Pulmonary Medicine; Jaypee Brothers: New Delhi, India, 2006. [Google Scholar]
- Cambier, C.J.; Falkow, S.; Ramakrishnan, L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell 2014, 159, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Tait, D.R.; Hatherill, M.; Van Der Meeren, O.; Ginsberg, A.M.; Van Brakel, E.; Salaun, B.; Scriba, T.J.; Akite, E.J.; Ayles, H.M.; Bollaerts, A.; et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. New Engl. J. Med. 2019, 381, 2429–2439. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, E.; Paul, M.J.; Reljic, R.; McShane, H. Mucosal delivery of tuberculosis vaccines: A review of current approaches and challenges. Expert Rev. Vaccines 2019, 18, 1271–1284. [Google Scholar] [CrossRef]
- Cohen, S.B.; Gern, B.H.; Delahaye, J.L.; Adams, K.N.; Plumlee, C.R.; Winkler, J.K.; Urdahl, K.B. Alveolar macrophages provide an early Mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe 2018, 24, 439–446. [Google Scholar] [CrossRef]
- Keane, J.; Balcewicz-Sablinska, M.K.; Remold, H.G.; Chupp, G.L.; Meek, B.B.; Fenton, M.J.; Kornfeld, H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 1997, 65, 298–304. [Google Scholar] [CrossRef]
- Engele, M.; Stöβel, E.; Castiglione, K.; Schwerdtner, N.; Wagner, M.; Bölcskei, P.; Stenger, S. Induction of TNF in human alveolar macrophages as a potential evasion mechanism of virulent Mycobacterium tuberculosis. J. Immunol. 2002, 168, 1328–1337. [Google Scholar] [CrossRef]
- Barnes, P.F.; Leedom, J.M.; Chan, L.S.; Wong, S.F.; Shah, J.; Vachon, L.A.; Modlin, R.L. Predictors of short-term prognosis in patients with pulmonary tuberculosis. J. Infect. Dis. 1988, 158, 366–371. [Google Scholar] [CrossRef]
- Lowe, D.M.; Bandara, A.K.; Packe, G.E.; Barker, R.D.; Wilkinson, R.J.; Griffiths, C.J.; Martineau, A.R. Neutrophilia independently predicts death in tuberculosis. Eur. Respir. J. 2013, 42, 1752–1757. [Google Scholar] [CrossRef]
- Berry, M.P.; Graham, C.M.; McNab, F.W.; Xu, Z.; Bloch, S.A.; Oni, T.; Wilkinson, K.A.; Banchereau, R.; Skinner, J.; Wilkinson, R.J.; et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 2010, 466, 973–977. [Google Scholar] [CrossRef]
- Davis, J.M.; Ramakrishnan, L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 2009, 136, 37–49. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Uldrich, A.P.; McCluskey, J.; Rossjohn, J.; Moody, D.B. The burgeoning family of unconventional T cells. Nat. Immunol. 2019, 20, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Flynn, J.L. The end of the binary era: Revisiting the spectrum of tuberculosis. J. Immunol. 2015, 195, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Llibre, A.; Gomes, A.; Ferrer, M.; Rosàs, M.; Torre, A. Tissue-resident memory T cells in the lung and their role in protection against respiratory infections. Front. Immunol. 2021, 12, 715972. [Google Scholar] [CrossRef]
- Pallett, L.J.; Davies, J. The role of tissue-resident T cells in the lung. Immunology 2021, 164, 413–423. [Google Scholar] [CrossRef]
- Srivastava, S.; Ernst, J.D. Cell-to-cell transfer of M. tuberculosis antigens optimizes CD4 T cell priming. Cell Host Microbe 2014, 15, 741–752. [Google Scholar] [CrossRef]
- Flynn, J.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An essential role for interferon gamma in resistance to M. tuberculosis infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar] [CrossRef]
- Cooper, A.M. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 2009, 27, 393–422. [Google Scholar] [CrossRef]
- Lewinsohn, D.M.; Briden, A.L.; Reed, S.G.; Grabstein, K.H.; Alderson, M.R. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: The relative contribution of classical versus nonclassical HLA restriction. J. Immunol. 2000, 165, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Khader, S.A.; Bell, G.K.; Pearl, J.E.; Fountain, J.J.; Rangel-Moreno, J.; Cilley, G.E.; Shen, F.; Eaton, S.M.; Gaffen, S.L.; Swain, S.L.; et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during M. tuberculosis challenge. Nat. Immunol. 2007, 8, 369–377. [Google Scholar] [CrossRef]
- Barry, C.E.; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.; Schnappinger, D.; Wilkinson, R.; Young, D. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 2009, 7, 845–855. [Google Scholar] [CrossRef]
- Seder, R.A.; Darrah, P.A.; Roederer, M. T-cell quality in memory and protection: Implications for vaccine design. Nat. Rev. Immunol. 2008, 8, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Lindestam Arlehamn, C.S.; Sette, A. Definition of CD4 immunosubsets in tuberculosis. Curr. Top. Microbiol. Immunol. 2016, 404, 127–142. [Google Scholar] [CrossRef]
- Achkar, J.M.; Casadevall, A. Antibody-mediated immunity against tuberculosis: Implications for vaccine development. Cell Host Microbe 2013, 13, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Torrado, E.; Cooper, A.M. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010, 21, 455–462. [Google Scholar] [CrossRef]
- Maglione, P.J.; Xu, J.; Chan, J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J. Immunol. 2007, 178, 7222–7234. [Google Scholar] [CrossRef]
- Barber, D.L.; Mayer-Barber, K.D.; Feng, C.G.; Sharpe, A.H.; Sher, A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1–mediated inhibition. J. Immunol. 2011, 186, 1598–1607. [Google Scholar] [CrossRef]
- Behar, S.M.; Divangahi, M.; Remold, H.G. Evasion of innate immunity by Mycobacterium tuberculosis: Is death an exit strategy? Nat. Rev. Microbiol. 2010, 8, 668–674. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Rasmussen, J.P.; Gavin, M.A.; Rudensky, A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005, 6, 1142–1151. [Google Scholar] [CrossRef]
- Yang, M.; Chen, S.; Huang, B.; Zhong, J.-M.; Su, H.; Chen, Y.-J.; Cao, Q.; Ma, L.; He, J.; Li, X.-F.; et al. Pathological findings in the testes of COVID-19 patients: Clinical implications. Eur. Urol. Focus 2020, 6, 1124–1129. [Google Scholar] [CrossRef]
- Krupa, A. Binding of CXCL8/IL-8 to M. tuberculosis modulates the innate immune response. Mediat. Inflamm. 2015, 2015, 124762. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Denkinger, C.M.; Kik, S.V.; Rangaka, M.X.; Zwerling, A.; Oxlade, O.; Banaei, N. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin. Microbiol. Rev. 2014, 27, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L. Pathology of post primary tuberculosis of the lung: An illustrated critical review. Tuberculosis 2011, 91, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Shah, Z.; Yasmeen, N.; Baloch, Z.; Xia, X. Pathogenesis of COVID-19 and M. tuberculosis coinfection. Front. Immunol. 2022, 13, 909011. [Google Scholar] [CrossRef]
- Bostanghadiri, N.; Jazi, F.M.; Razavi, S.; Fattorini, L.; Darban-Sarokhalil, D.M. Tuberculosis and COVID-19 coinfections: A review. Front. Microbiol. 2022, 12, 747827. [Google Scholar] [CrossRef]
- Aiello, A.; Najafi-Fard, S.; Goletti, D. Initial immune response after exposure to M. tuberculosis or to COVID-19: Similarities and differences. Front. Immunol. 2023, 14, 1244556. [Google Scholar] [CrossRef]
- Najafi-Fard, S.; Aiello, A.; Navarra, A.; Cuzzi, G.; Vanini, V.; Migliori, G.B.; Gualano, G.; Cerva, C.; Grifoni, A.; Sette, A.; et al. Characterization of the immune impairment of patients with tuberculosis and COVID-19 coinfection. Int. J. Infect. Dis. 2023, 130, S34–S42. [Google Scholar] [CrossRef]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. [Google Scholar] [CrossRef]
- Lazarevic, I.; Pravica, V.; Miljanovic, D.; Cupic, M. Immune evasion of COVID-19 emerging variants: What have we learnt so far? Viruses 2021, 13, 1192. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, Y.; Liu, X.; Fu, Y.; Zhang, J.; Zhang, Y.; Zhang, L.; Wei, X.; Yang, L. Systematic review and meta-analysis of Tuberculosis and COVID-19 Co-infection: Prevalence, fatality, and treatment considerations. PLoS Neglected Trop. Dis. 2024, 18, e0012136. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Tyagi, R. The impact of COVID-19 on tuberculosis: Challenges and opportunities. Ther. Adv. Infect. Dis. 2021, 8, 20499361211016973. [Google Scholar] [CrossRef] [PubMed]
- Zenaro, E.; Donini, M.; Dusi, S. Induction of Th1/Th17 immune response by Mycobacterium tuberculosis: Role of dectin-1, mannose receptor, and DC-SIGN. J. Leukoc. Biol. 2009, 86, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, L.; Creti, R.; Palma, C.; Pantosti, A. Bacterial coinfections in COVID-19: An underestimated adversary. Ann. Dell’istituto Super. Di Sanita 2020, 56, 359–364. [Google Scholar]
- Ahamad, N.; Gupta, S.; Parashar, D. Using omics to study leprosy, tuberculosis, and other mycobacterial diseases. Front. Cell. Infect. Microbiol. 2022, 12, 792617. [Google Scholar] [CrossRef]
- Jagielski, T.; Minias, A.; van Ingen, J.; Rastogi, N.; Brzostek, A.; Żaczek, A.; Dziadek, J. Methodological and clinical aspects of the molecular epidemiology of M. tuberculosis and other mycobacteria. Clin. Microbiol. Rev. 2016, 29, 239–290. [Google Scholar] [CrossRef]
- Kato-Maeda, M.; Rhee, J.T.; Gingeras, T.R.; Salamon, H.; Drenkow, J.; Smittipat, N.; Small, P.M. Comparing genomes within the species Mycobacterium tuberculosis. Genome Res. 2001, 11, 547–554. [Google Scholar] [CrossRef]
- Cioboata, R.; Biciusca, V.; Olteanu, M.; Vasile, C.M. COVID-19 and tuberculosis: Unveiling the dual threat and shared solutions perspective. J. Clin. Med. 2023, 12, 4784. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.; Tong, Y.; Cao, L.; Zeng, L.; Xu, Y.; Zhao, X. Whole-cell response of coronavirus-infected BMDCs through proteomic and transcriptomic analyses. Front. Immunol. 2025, 16, 1513952. [Google Scholar] [CrossRef]
- He, J.; Zhou, X.; Zhang, D.; Cai, Y.; Pan, H.; Huang, T.; He, F. Investigation of the relationship between chronic hepatitis B and tuberculosis using bioinformatics and systems biology approaches. Front. Med. 2025, 12, 1519216. [Google Scholar] [CrossRef]
- Gajula, S.N.R.; Khairnar, A.S.; Jock, P.; Kumari, N.; Pratima, K.; Munjal, V.; Sonti, R. LC-MS/MS: A sensitive and selective analytical technique to detect COVID-19 protein biomarkers in the early disease stage. Expert Rev. Proteom. 2023, 20, 5–18. [Google Scholar] [CrossRef]
- Rajczewski, A.T.; Jagtap, P.D.; Griffin, T.J. An overview of technologies for MS-based proteomics-centric multi-omics. Expert Rev. Proteom. 2022, 19, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Sheerin, D.; Peton, N.; Vo, W.; Allison, C.C.; Wang, X.; Johnson, W.E.; Coussens, A.K. Immunopathogenic overlap between COVID-19 and tuberculosis identified from transcriptomic meta-analysis and human macrophage infection. Iscience 2022, 25, 104464. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Subbian, S. Critical determinants of cytokine storm and type I interferon response in COVID-19 pathogenesis. Clin. Microbiol. Rev. 2021, 34, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J., 3rd; Parida, S.K.; Maertzdorf, J.; Black, G.F.; Repsilber, D.; Telaar, A.; Kaufmann, S.H. Correction: Biomarkers of inflammation, immunosuppression and stress with active disease are revealed by metabolomic profiling of tuberculosis patients. PLoS ONE 2012, 7, 10–1371. [Google Scholar] [CrossRef]
- Frediani, J.K.; Jones, D.P.; Tukvadze, N.; Uppal, K.; Sanikidze, E.; Kipiani, M.; Ziegler, T.R. Plasma metabolomics in human pulmonary tuberculosis disease: A pilot study. PLoS ONE 2014, 9, e108854. [Google Scholar] [CrossRef]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Guo, T. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell 2020, 182, 59–72. [Google Scholar] [CrossRef]
- Thomas, T.; Stefanoni, D.; Reisz, J.A.; Nemkov, T.; Bertolone, L.; Francis, R.O.; D’Alessandro, A. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 2020, 5, e140327. [Google Scholar] [CrossRef]
- Wang, K.; Khoramjoo, M.; Srinivasan, K.; Gordon, P.M.; Mandal, R.; Jackson, D.; Oudit, G.Y. Sequential multi-omics analysis identifies clinical phenotypes and predictive biomarkers for long COVID. Cell Rep. Med. 2023, 4, 101254. [Google Scholar] [CrossRef]
- Collins, J.M.; Siddiqa, A.; Jones, D.P.; Liu, K.; Kempker, R.R.; Nizam, A.; Ziegler, T.R. Tryptophan catabolism reflects disease activity in human tuberculosis. JCI Insight 2020, 5, e137131. [Google Scholar] [CrossRef]
- Preez, I.D.; Luies, L.; Loots, D.T. Metabolomics biomarkers for tuberculosis diagnostics: Current status and future objectives. Biomark. Med. 2017, 11, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Khadela, A.; Chavda, V.P.; Postwala, H.; Shah, Y.; Mistry, P.; Apostolopoulos, V. Epigenetics in Tuberculosis: Immunomodulation of Host Immune Response. Vaccines 2022, 10, 1740. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.-C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef]
- Netea, M.G.; van der Meer, J.W.M. Trained immunity: A tool for reducing susceptibility to and the severity of COVID-19 infection. Cell 2020, 181, 969–977. [Google Scholar] [CrossRef]
- Corley, M.J.; Pang, A.P.; Dody, K.; Mudd, P.A.; Patterson, B.K.; Seethamraju, H.; Bram, Y.; Peluso, M.J.; Torres, L.; Iyer, N.S.; et al. Genome-wide DNA methylation profiling of peripheral blood reveals epigenetic signatures associated with severe COVID-19. J. Leukoc. Biol. 2021, 110, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, E.; Reynolds, G.; Botting, R.A.; Calero-Nieto, F.J.; Morgan, M.; Tuong, Z.K.; Bach, K.; Sungnak, W.; Worlock, K.B.; Yoshida, M.; et al. The cellular immune response to COVID-19 deciphered by single-cell multi-omics across three UK centres. Nature 2021, 599, 506–514. [Google Scholar] [CrossRef]
- Naidoo, C.C.; Nyawo, G.R.; Wu, B.G.; Walzl, G.; Warren, R.M. The microbiome and tuberculosis: A review. Tuberculosis 2021, 126, 102038. [Google Scholar] [CrossRef]
- Li, S.; Zhuo, M.; Huang, X.; Huang, Y.; Zhou, J.; Xiong, D.; Li, J.; Liu, Y.; Pan, Z.; Li, H.; et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ 2020, 8, e9574. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Deriu, E.; Pezoldt, J.; Lochner, M. Host–microbiota interactions in viral infections: Lessons from influenza and coronavirus. Immunity 2021, 54, 1584–1599. [Google Scholar] [CrossRef]
- Xu, Z.M.; Zwyer, M.; Hiza, H.; Schmidiger, S.; Sasamalo, M.; Reinhard, M.; Doetsch, A.; Borrell, S.; Naret, O.; Rüeger, S.; et al. Genome-to-genome analysis reveals associations between human and mycobacterial genetic variation in tuberculosis patients from Tanzania. BMC Med. Genom. 2025, 18, 99. [Google Scholar] [CrossRef]
- Wang, F.; Huang, S.; Gao, R.; Zhou, Y.; Lai, C.; Li, Z.; Liu, L. Initial whole-genome sequencing and analysis of the host genetic contribution to COVID-19 severity and susceptibility. Cell Discov. 2020, 6, 83. [Google Scholar] [CrossRef]
- Kumar, D.; Sahoo, S.S.; Chauss, D.; Kazemian, M.; Afzali, B. Non-coding RNAs in immunoregulation and autoimmunity: Technological advances and critical limitations. J. Autoimmun. 2023, 134, 102982. [Google Scholar] [CrossRef] [PubMed]
- Sperk, M.; van Domselaar, R.; Rodriguez, J.E.; Mikaeloff, F.; Sá Vinhas, B.; Saccon, E.; Sönnerborg, A.; Singh, K.; Gupta, S.; Végvári, Á.; et al. Utility of Proteomics in Emerging and Re-Emerging Infectious Diseases Caused by RNA Viruses. J. Proteome Res. 2020, 19, 4259–4274. [Google Scholar] [CrossRef] [PubMed]
- Neagu, A.N.; Jayathirtha, M.; Baxter, E.; Donnelly, M.; Petre, B.A.; Darie, C.C. Applications of Tandem Mass Spectrometry (MS/MS) in Protein Analysis for Biomedical Research. Molecules 2022, 27, 2411. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C.; Luies, L.; Loots, D.T.; Williams, A.A. The metabolic consequences of HIV/TB co-infection. BMC Infect. Dis. 2023, 23, 536. [Google Scholar] [CrossRef]
- Carrero Longlax, S.; Koster, K.J.; Kamat, A.M.; Lozano, M.; Lerner, S.P.; Hannigan, R.; Nishiguchi, T.; Abhimanyu Sheikh, D.; Ladki, M.; Portillo, A. BCG-Induced DNA Methylation Changes Improve Coronavirus Disease 2019 Vaccine Immunity Without Decreasing the Risk for Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Open Forum Infect. Dis. 2025, 12, ofaf007. [Google Scholar] [CrossRef]
- Sun, G.; Wang, Q.; Shan, X.; Kuerbanjiang, M.; Ma, R.; Zhou, W.; Sun, L.; Li, Q. Metabolomics and lipidomics of plasma biomarkers for tuberculosis diagnostics using UHPLC-HRMS. Front. Cell. Infect. Microbiol. 2025, 15, 1526740. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khatiwada, S.; Subedi, A. Lung microbiome and coronavirus disease 2019 (COVID-19): A narrative review. Int. J. Infect. Dis. 2021, 103, 476–482. [Google Scholar] [CrossRef]
- Mulder, W.J.M.; Ochando, J.; Joosten, L.A.B.; Fayad, Z.A.; Netea, M.G. Epigenomic approaches to understand trained immunity in TB and COVID-19. Cell Host Microbe 2021, 29, 728–731. [Google Scholar] [CrossRef]
- Nardelli, C.; Gentile, I.; Setaro, M.; di Domenico, C.; di Dato, M.T.; Rizzo, M.R. Microbiota and COVID-19: Possible link and implications. Int. J. Mol. Sci. 2021, 22, 4474. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, X.X.; Li, J.C. Biomarker discovery for tuberculosis using metabolomics. Front. Mol. Biosci. 2023, 10, 1099654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pacis, A.; Nédélec, Y.; Barreiro, L.B.; Gagneux, S. Epigenetic immune memory in mycobacterial infection. Trends Immunol. 2019, 40, 900–915. [Google Scholar] [CrossRef]
- Madden, K.; Liang, Y.C.; Rajabalee, N.; Alvarez, G.G.; Sun, J. Surveying the Epigenetic Landscape of Tuberculosis in Alveolar Macrophages. Infect. Immun. 2022, 90, e00522-21. [Google Scholar] [CrossRef]
- Sengupta, S.; Pattanaik, K.P.; Mishra, S.; Sonawane, A. Epigenetic orchestration of host immune defences by Mycobacterium tuberculosis. Microbiol. Res. 2023, 273, 127400. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).