Experimental Fish Models in the Post-Genomic Era: Tools for Multidisciplinary Science
Abstract
1. Introduction
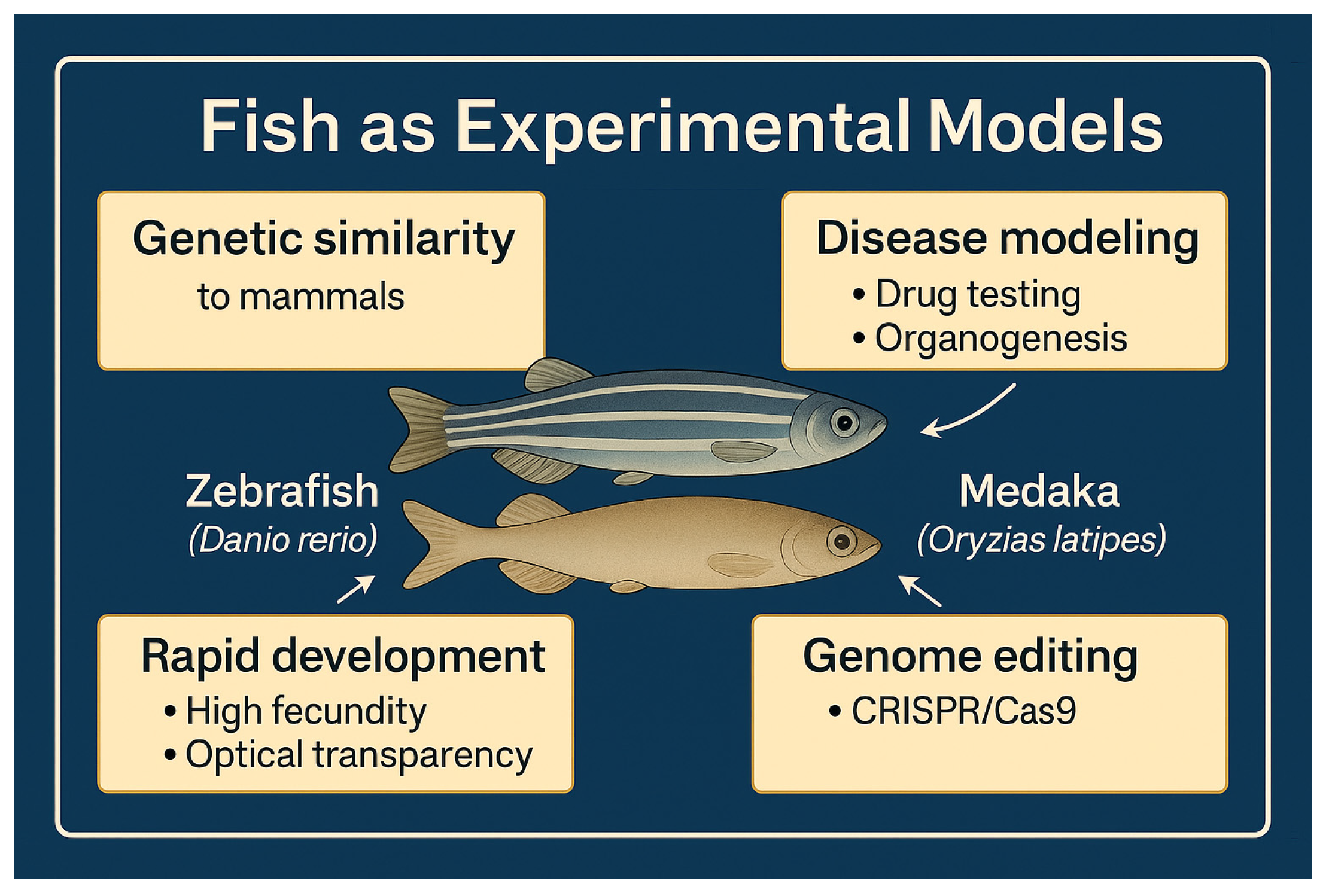
2. Main Species Used
2.1. Zebrafish (Danio rerio)
2.2. Medaka (Oryzias latipes)
2.3. Tilapia and Carp
2.4. Native or Regional Species
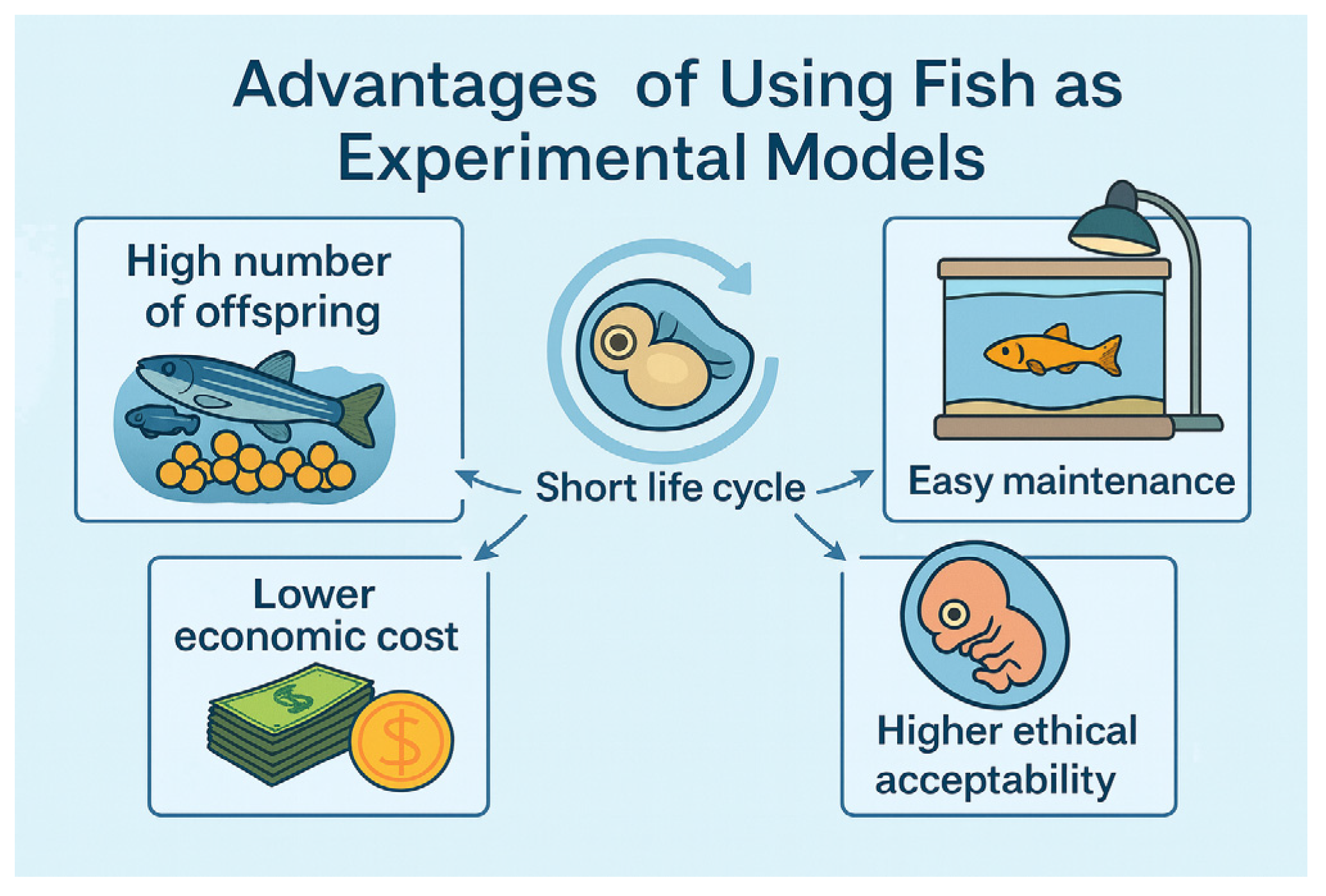
3. Advantages of Using Fish as Experimental Models
3.1. High Number of Offspring
3.2. Short Life Cycle and Rapid Development
3.3. Easy Maintenance in Laboratory Environments
3.4. Lower Economic Cost Compared to Mammals
3.5. Higher Ethical Acceptability, Especially at Early Developmental Stages (Embryos and Larvae)
4. Fields of Application for Fish as Experimental Models
4.1. Genetics and Developmental Biology
4.2. Toxicology and Ecotoxicology
4.3. Neuroscience and Behavior
4.4. Pharmacology and Drug Screening
4.5. Immunology and Pathogen Response
4.6. Modeling Human Diseases
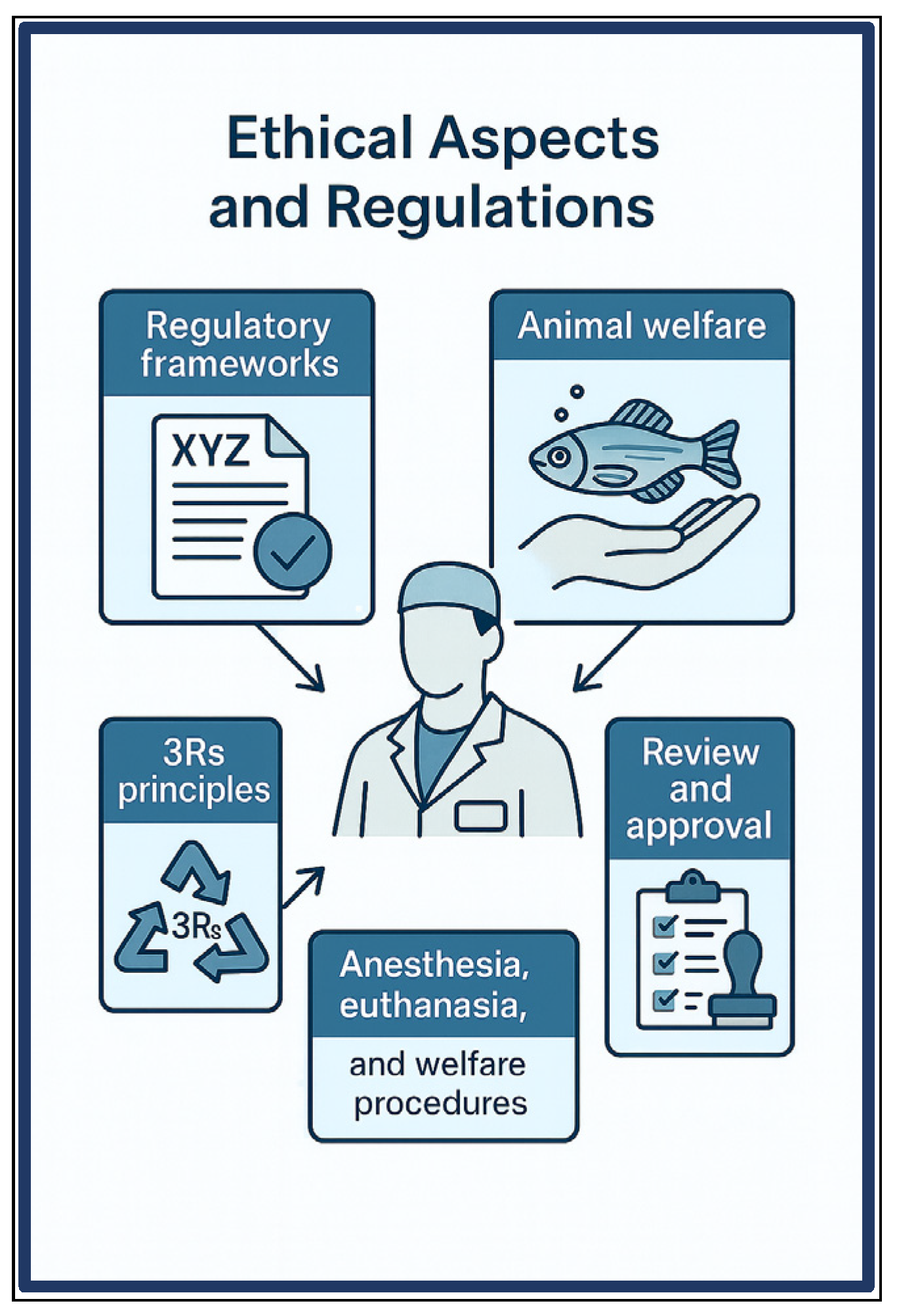
5. Ethical Aspects and Regulations
5.1. Ethical Considerations According to International Guidelines (OECD, EU Directive 2010/63)
5.2. From 3Rs to 10Rs: Ethical Principles in Fish Model Research
5.3. Anesthesia, Euthanasia, and Welfare Procedures Adapted for Fish
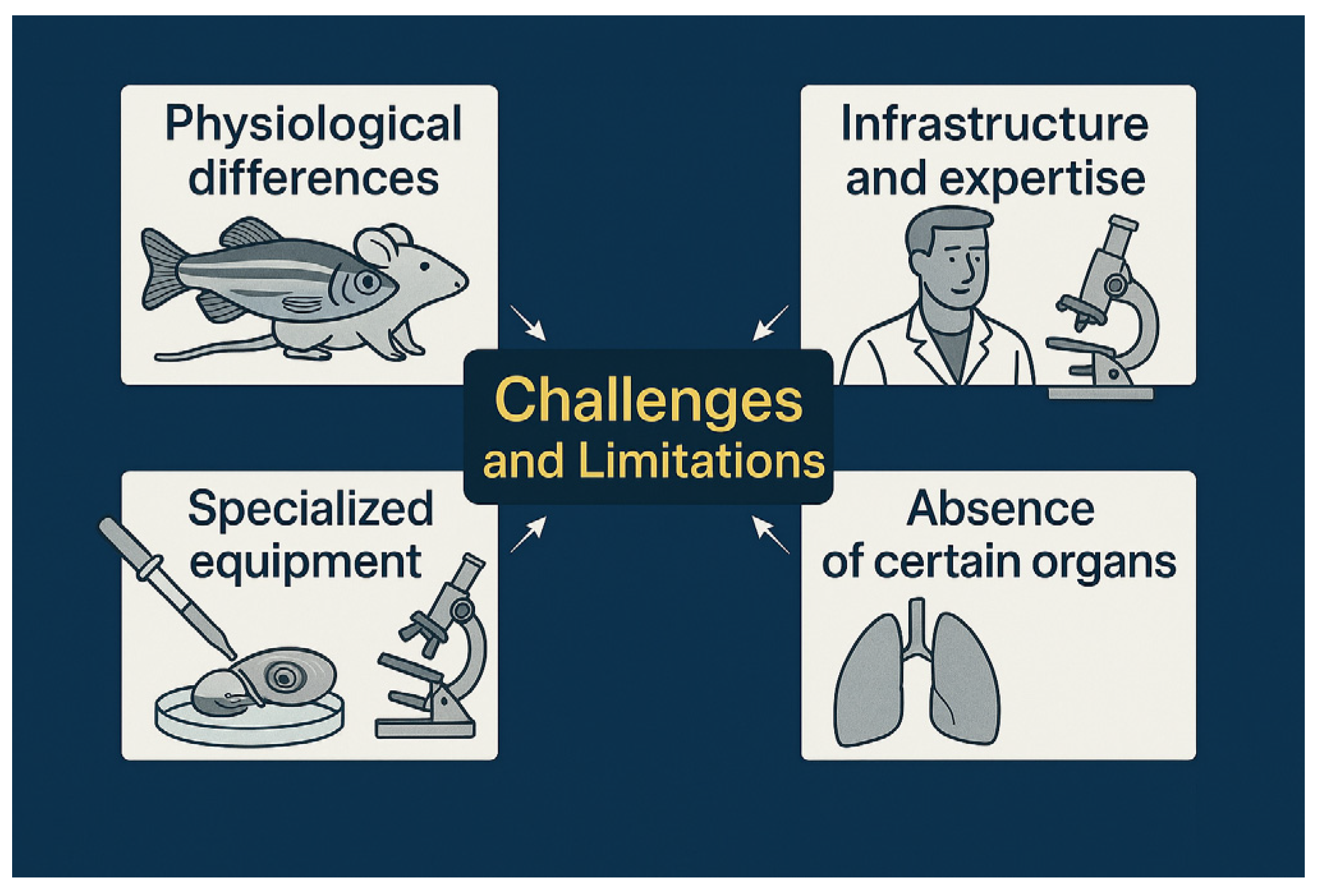
6. Challenges and Limitations
6.1. Physiological Differences That May Limit Direct Extrapolation to Mammals
6.2. Need for Infrastructure and Specialized Expertise for Proper Handling
6.3. Limitations in Studying Certain Organs (e.g., Lungs)
7. Future Perspectives
8. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APCs | Antigen-presenting cells |
| AVMA | American veterinary medical association |
| CRISPR/Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9 |
| CYP | Cytochrome P450 |
| DCs | Dendritic cells |
| EDCs | Endocrine disrupting chemicals |
| EE2 | 17α-ethinylestradiol |
| FET | Fish embryo acute toxicity |
| GFP | Green fluorescent protein |
| HTS | High-throughput screening |
| IACUC | Institutional Animal Care and Use Committee |
| Jak-STAT | Janus Kinase—Signal Transducer and Activator of Transcription pathway |
| MAPK | Mitogen-Activated Protein Kinase pathway |
| MERFISH | Multiplexed error-robust fluorescence in situ hybridization |
| MS-222 | Tricaine methanesulfonate |
| OECD | Organisation for Economic Co-operation and Development |
| OECD | Organization for Economic Co-operation and Development |
| PI3K-Akt | Phosphatidylinositol 3-Kinase—Protein Kinase B pathway |
| PPCPs | Pharmaceuticals and Personal Care Products |
| RAS | Recirculating aquaculture systems |
| RNA | Ribonucleic acid |
| scATAC-seq | Single-cell Assay for Transposase-Accessible Chromatin sequencing |
| SCHEER | Scientific Committee on Health, Environmental and Emerging Risks |
| scRNA-seq | Single-cell RNA sequencing |
| SEFI | Spatial embedded feature identification |
| TALENs | Transcription activator-like effector nucleases |
| TiLV | Tilapia lake virus |
| TLR | Toll-like receptor |
| ZFN | Zinc finger nuclease |
References
- Kirk, R.G. Recovering the Principles of Humane Experimental Technique: The 3Rs and the Human Essence of Animal Research. Sci. Technol. Hum. Values 2018, 43, 622–648. [Google Scholar] [CrossRef]
- Balls, M. It’s Time to Reconsider the Principles of Humane Experimental Technique. Altern. Lab. Anim. 2020, 48, 40–46. [Google Scholar] [CrossRef]
- Aitman, T.J.; Boone, C.; Churchill, G.A.; Hengartner, M.O.; Mackay, T.F.C.; Stemple, D.L. The Future of Model Organisms in Human Disease Research. Nat. Rev. Genet. 2011, 12, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Chiang, C.-Y.; Tsai, H.-J. Zebrafish and Medaka: New Model Organisms for Modern Biomedical Research. J. Biomed. Sci. 2016, 23, 19. [Google Scholar] [CrossRef]
- Henning, F.; Meyer, A. The Evolutionary Genomics of Cichlid Fishes: Explosive Speciation and Adaptation in the Postgenomic Era. Annu. Rev. Genom. Hum. Genet. 2014, 15, 417–441. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Volff, J.N. Genome Evolution and Biodiversity in Teleost Fish. Heredity 2005, 94, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Venkatesh, B. Rapidly Evolving Fish Genomes and Teleost Diversity. Curr. Opin. Genet. Dev. 2008, 18, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal Models of Human Disease: Zebrafish Swim into View. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Santoriello, C.; Zon, L.I. Hooked! Modeling Human Disease in Zebrafish. J. Clin. Investig. 2012, 122, 2337–2343. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E. Transparent Adult Zebrafish as a Tool for in Vivo Transplantation Analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef]
- White, R.; Rose, K.; Zon, L. Zebrafish Cancer: The State of the Art and the Path Forward. Nat. Rev. Cancer 2013, 13, 624–636. [Google Scholar] [CrossRef]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.J.; Joung, J.K. Efficient Genome Editing in Zebrafish Using a CRISPR-Cas System. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Qi, X.; Chen, J.; Chen, W.; Qiu, G.; Wu, Z.; Wu, N. CRISPR/Cas9 in Zebrafish: An Efficient Combination for Human Genetic Diseases Modeling. Hum. Genet. 2017, 136, 1–12. [Google Scholar] [CrossRef]
- Kaufmann, A.; Mickoleit, M.; Weber, M.; Huisken, J. Multilayer Mounting Enables Long-Term Imaging of Zebrafish Development in a Light Sheet Microscope. Development 2012, 139, 3242–3247. [Google Scholar] [CrossRef]
- Keller, P.J.; Schmidt, A.D.; Wittbrodt, J.; Stelzer, E.H. Reconstruction of Zebrafish Early Embryonic Development by Scanned Light Sheet Microscopy. Science 2008, 322, 1065–1069. [Google Scholar] [CrossRef]
- Weber, M.; Huisken, J. In Vivo Imaging of Cardiac Development and Function in Zebrafish Using Light Sheet Microscopy. Swiss Med. Wkly. 2015, 145, w14227. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.D.; Weinstein, B.M. In Vivo Imaging of Embryonic Vascular Development Using Transgenic Zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.S.; Hogan, B.M. Endothelial Cell Dynamics in Vascular Development: Insights from Live-Imaging in Zebrafish. Front. Physiol. 2020, 11, 842. [Google Scholar] [CrossRef]
- Hason, M.; Bartůněk, P. Zebrafish Models of Cancer—New Insights on Modeling Human Cancer in a Non-Mammalian Vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef]
- Dooley, K.; Zon, L.I. Zebrafish: A Model System for the Study of Human Disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Jenkins, J.A.; Bart, H.L., Jr.; Bowker, J.D.; Bowser, P.R.; MacMillan, J.R.; Nickum, J.G.; Rose, J.D.; Sorensen, P.W.; Whitledge, G.W.; Rachlin, J.W. Guidelines for the Use of Fishes in Research; American Fisheries Society: Bethesda, MD, USA, 2014. [Google Scholar]
- Sloman, K.A.; Bouyoucos, I.A.; Brooks, E.J.; Sneddon, L.U. Ethical Considerations in Fish Research. J. Fish Biol. 2019, 94, 556–577. [Google Scholar] [CrossRef]
- D’Angelo, L.; Lossi, L.; Merighi, A.; de Girolamo, P. Anatomical Features for the Adequate Choice of Experimental Animal Models in Biomedicine: I. Fishes. Ann. Anat.-Anat. Anz. 2016, 205, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Villéger, S.; Brosse, S.; Mouchet, M.; Mouillot, D.; Vanni, M.J. Functional Ecology of Fish: Current Approaches and Future Challenges. Aquat. Sci. 2017, 79, 783–801. [Google Scholar] [CrossRef]
- Kasahara, M.; Naruse, K.; Sasaki, S.; Nakatani, Y.; Qu, W.; Ahsan, B.; Yamada, T.; Nagayasu, Y.; Doi, K.; Kasai, Y. The Medaka Draft Genome and Insights into Vertebrate Genome Evolution. Nature 2007, 447, 714–719. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, F.R.; Ferrari, L.; Salibián, A. Biomarkers of a Native Fish Species (Cnesterodon decemmaculatus) Application to the Water Toxicity Assessment of a Peri-Urban Polluted River of Argentina. Chemosphere 2005, 59, 577–583. [Google Scholar] [CrossRef]
- PARK, Y.-S.; Chang, J.; Lek, S.; Cao, W.; Brosse, S. Conservation Strategies for Endemic Fish Species Threatened by the Three Gorges Dam. Conserv. Biol. 2003, 17, 1748–1758. [Google Scholar] [CrossRef]
- Monier, M.N.; Abd El-Naby, A.S.; Fawzy, R.M.; Samir, F.; Shady, S.H.H.; Grana, Y.S.; Albaqami, N.M.; Abdel-Tawwab, M. Growth Performance, Antioxidant, and Immune Responses of Nile Tilapia (Oreochromis niloticus) Fed on Low-Fishmeal Diets Enriched with Sodium Chloride and Its Adaptability to Different Salinity Levels. Fish Physiol. Biochem. 2025, 51, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Luo, J.; Du, J.; Liu, Q.; Xu, G.; Yang, S. Study on the Adaptability of Common Carp (Cyprinus carpio) to Diets from the Perspective of Digestion and Absorption. Aquac. Res. 2020, 51, 2495–2504. [Google Scholar] [CrossRef]
- Vilizzi, L.; Tarkan, A.S. Bioaccumulation of Metals in Common Carp (Cyprinus carpio L.) from Water Bodies of Anatolia (Turkey): A Review with Implications for Fisheries and Human Food Consumption. Environ. Monit. Assess. 2016, 188, 243. [Google Scholar] [CrossRef] [PubMed]
- Mastrochirico Filho, V.A.; Freitas, M.V.; Ariede, R.B.; Lira, L.V.; Mendes, N.J.; Hashimoto, D.T. Genetic Applications in the Conservation of Neotropical Freshwater Fish. In Biological Resources of Water; IntechOpen: London, UK, 2018; ISBN 1-78923-081-0. [Google Scholar][Green Version]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W. Freshwater Ecoregions of the World: A New Map of Biogeographic Units for Freshwater Biodiversity Conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Dubey, A.; Ghosh, N.S.; Singh, R. Zebrafish as an Emerging Model: An Important Testing Platform for Biomedical Science. J. Pharm. Negat. Results 2022, 13, 2. [Google Scholar] [CrossRef]
- Sarasamma, S.; Varikkodan, M.M.; Liang, S.-T.; Lin, Y.-C.; Wang, W.-P.; Hsiao, C.-D. Zebrafish: A Premier Vertebrate Model for Biomedical Research in Indian Scenario. Zebrafish 2017, 14, 589–605. [Google Scholar] [CrossRef]
- Teame, T.; Zhang, Z.; Ran, C.; Zhang, H.; Yang, Y.; Ding, Q.; Xie, M.; Gao, C.; Ye, Y.; Duan, M. The Use of Zebrafish (Danio rerio) as Biomedical Models. Anim. Front. 2019, 9, 68–77. [Google Scholar] [CrossRef]
- Phillips, J.B.; Westerfield, M. Zebrafish Models in Translational Research: Tipping the Scales toward Advancements in Human Health. Dis. Models Mech. 2014, 7, 739–743. [Google Scholar] [CrossRef]
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish Embryos and Larvae as Alternative Animal Models for Toxicity Testing. Int. J. Mol. Sci. 2021, 22, 13417. [Google Scholar] [CrossRef]
- Kari, G.; Rodeck, U.; Dicker, A.P. Zebrafish: An Emerging Model System for Human Disease and Drug Discovery. Clin. Pharmacol. Ther. 2007, 82, 70–80. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an Emerging Model for Studying Complex Brain Disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Varshney, G.K.; Pei, W.; LaFave, M.C.; Idol, J.; Xu, L.; Gallardo, V.; Carrington, B.; Bishop, K.; Jones, M.; Li, M. High-Throughput Gene Targeting and Phenotyping in Zebrafish Using CRISPR/Cas9. Genome Res. 2015, 25, 1030–1042. [Google Scholar] [CrossRef]
- Driever, W.; Solnica-Krezel, L.; Schier, A.F.; Neuhauss, S.C.F.; Malicki, J.; Stemple, D.L.; Stainier, D.Y.; Zwartkruis, F.; Abdelilah, S.; Rangini, Z. A Genetic Screen for Mutations Affecting Embryogenesis in Zebrafish. Development 1996, 123, 37–46. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.A.; Peterson, R.T. Zebrafish as Tools for Drug Discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Watts, S.A.; D’Abramo, L.R. Standardized Reference Diets for Zebrafish: Addressing Nutritional Control in Experimental Methodology. Annu. Rev. Nutr. 2021, 41, 511–527. [Google Scholar] [CrossRef]
- Gonzales Jr, J.M.; Law, S.H.W. Feed and Feeding Regime Affect Growth Rate and Gonadosomatic Index of Adult Zebrafish (Danio rerio). Zebrafish 2013, 10, 532–540. [Google Scholar] [CrossRef]
- Ulloa, P.E.; Iturra, P.; Neira, R.; Araneda, C. Zebrafish as a Model Organism for Nutrition and Growth: Towards Comparative Studies of Nutritional Genomics Applied to Aquacultured Fishes. Rev. Fish Biol. Fish. 2011, 21, 649–666. [Google Scholar] [CrossRef]
- Adhish, M.; Manjubala, I. Effectiveness of Zebrafish Models in Understanding Human Diseases—A Review of Models. Heliyon 2023, 9, e14557. [Google Scholar] [CrossRef]
- Spitsbergen, J.M.; Kent, M.L. The State of the Art of the Zebrafish Model for Toxicology and Toxicologic Pathology Research—Advantages and Current Limitations. Toxicol. Pathol. 2003, 31, 62–87. [Google Scholar] [CrossRef]
- Goessling, W.; Sadler, K.C. Zebrafish: An Important Tool for Liver Disease Research. Gastroenterology 2015, 149, 1361–1377. [Google Scholar] [CrossRef]
- Wilkins, B.J.; Pack, M. Zebrafish Models of Human Liver Development and Disease. Compr. Physiol. 2011, 3, 1213–1230. [Google Scholar] [CrossRef]
- Furutani-Seiki, M.; Wittbrodt, J. Medaka and Zebrafish, an Evolutionary Twin Study. Mech. Dev. 2004, 121, 629–637. [Google Scholar] [CrossRef]
- Kirchmaier, S.; Naruse, K.; Wittbrodt, J.; Loosli, F. The Genomic and Genetic Toolbox of the Teleost Medaka (Oryzias latipes). Genetics 2015, 199, 905–918. [Google Scholar] [CrossRef]
- Naruse, K.; Fukamachi, S.; Mitani, H.; Kondo, M.; Matsuoka, T.; Kondo, S.; Hanamura, N.; Morita, Y.; Hasegawa, K.; Nishigaki, R. A Detailed Linkage Map of Medaka, Oryzias latipes: Comparative Genomics and Genome Evolution. Genetics 2000, 154, 1773–1784. [Google Scholar] [CrossRef]
- Wakamatsu, Y.; Pristyazhnyuk, S.; Kinoshita, M.; Tanaka, M.; Ozato, K. The See-through Medaka: A Fish Model That Is Transparent throughout Life. Proc. Natl. Acad. Sci. USA 2001, 98, 10046–10050. [Google Scholar] [CrossRef]
- Chowdhury, K.; Lin, S.; Lai, S.-L. Comparative Study in Zebrafish and Medaka Unravels the Mechanisms of Tissue Regeneration. Front. Ecol. Evol. 2022, 10, 783818. [Google Scholar] [CrossRef]
- Takeda, H.; Shimada, A. The Art of Medaka Genetics and Genomics: What Makes Them so Unique? Annu. Rev. Genet. 2010, 44, 217–241. [Google Scholar] [CrossRef]
- Kimura, T.; Shimada, A.; Sakai, N.; Mitani, H.; Naruse, K.; Takeda, H.; Inoko, H.; Tamiya, G.; Shinya, M. Genetic Analysis of Craniofacial Traits in the Medaka. Genetics 2007, 177, 2379–2388. [Google Scholar] [CrossRef][Green Version]
- Sampetrean, O.; Iida, S.; Makino, S.; Matsuzaki, Y.; Ohno, K.; Saya, H. Reversible Whole-Organism Cell Cycle Arrest in a Living Vertebrate. Cell Cycle 2009, 8, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Takehana, Y.; Nagai, N.; Matsuda, M.; Tsuchiya, K.; Sakaizumi, M. Geographic Variation and Diversity of the Cytochrome b Gene in Japanese Wild Populations of Medaka, Oryzias latipes. Zoolog. Sci. 2003, 20, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Senou, H.; Hosoya, K. Oryzias sakaizumii, a New Ricefish from Northern Japan (Teleostei: Adrianichthyidae). Ichthyol. Explor. Freshw. 2011, 22, 289. [Google Scholar]
- Naruse, K.; Hori, H.; Shimizu, N.; Kohara, Y.; Takeda, H. Medaka Genomics: A Bridge between Mutant Phenotype and Gene Function. Mech. Dev. 2004, 121, 619–628. [Google Scholar] [CrossRef]
- Spivakov, M.; Auer, T.O.; Peravali, R.; Dunham, I.; Dolle, D.; Fujiyama, A.; Toyoda, A.; Aizu, T.; Minakuchi, Y.; Loosli, F. Genomic and Phenotypic Characterization of a Wild Medaka Population: Towards the Establishment of an Isogenic Population Genetic Resource in Fish. G3 Genes Genomes Genet. 2014, 4, 433–445. [Google Scholar] [CrossRef]
- Myosho, T.; Takehana, Y.; Sato, T.; Hamaguchi, S.; Sakaizumi, M. The Origin of the Large Metacentric Chromosome Pair in Chinese Medaka (Oryzias sinensis). Ichthyol. Res. 2012, 59, 384–388. [Google Scholar] [CrossRef]
- Matsuda, M.; Nagahama, Y.; Shinomiya, A.; Sato, T.; Matsuda, C.; Kobayashi, T.; Morrey, C.E.; Shibata, N.; Asakawa, S.; Shimizu, N. DMY Is a Y-Specific DM-Domain Gene Required for Male Development in the Medaka Fish. Nature 2002, 417, 559–563. [Google Scholar] [CrossRef]
- Myosho, T.; Otake, H.; Masuyama, H.; Matsuda, M.; Kuroki, Y.; Fujiyama, A.; Naruse, K.; Hamaguchi, S.; Sakaizumi, M. Tracing the Emergence of a Novel Sex-Determining Gene in Medaka, Oryzias luzonensis. Genetics 2012, 191, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Takehana, Y.; Matsuda, M.; Myosho, T.; Suster, M.L.; Kawakami, K.; Shin-i, T.; Kohara, Y.; Kuroki, Y.; Toyoda, A.; Fujiyama, A. Co-Option of Sox3 as the Male-Determining Factor on the Y Chromosome in the Fish Oryzias dancena. Nat. Commun. 2014, 5, 4157. [Google Scholar] [CrossRef]
- Inoue, K.; Takei, Y. Diverse Adaptability in Oryzias Species to High Environmental Salinity. Zoolog. Sci. 2002, 19, 727–734. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-Year Retrospective Review of Global Aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.F.; Aracati, M.F.; Luporini de Oliveira, S.; Carlino-Costa, C.; Alves Rodrigues, R.; Pereira, M.R.; Borba, H.; Menegasso Mansano, C.F.; Marques Rossi, G.A.; Galindo-Villegas, J.; et al. Dietary Supplementation with Green Alga (Chlorella pyrenoidosa) Enhances the Shelf Life of Refrigerated Nile Tilapia (Oreochromis niloticus) Fillets. Foods 2025, 14, 1642. [Google Scholar] [CrossRef]
- Šimková, A.; Vojtek, L.; Halačka, K.; Hyršl, P.; Vetešník, L. The Effect of Hybridization on Fish Physiology, Immunity and Blood Biochemistry: A Case Study in Hybridizing Cyprinus carpio and Carassius Gibelio (Cyprinidae). Aquaculture 2015, 435, 381–389. [Google Scholar] [CrossRef]
- Rohlenová, K.; Morand, S.; Hyršl, P.; Tolarová, S.; Flajšhans, M.; Šimková, A. Are Fish Immune Systems Really Affected by Parasites? An Immunoecological Study of Common Carp (Cyprinus carpio). Parasit. Vectors 2011, 4, 120. [Google Scholar] [CrossRef]
- Saha, N.R.; Usami, T.; Suzuki, Y. Seasonal Changes in the Immune Activities of Common Carp (Cyprinus carpio). Fish Physiol. Biochem. 2002, 26, 379–387. [Google Scholar] [CrossRef]
- Tarnawska, M.; Augustyniak, M.; Łaszczyca, P.; Migula, P.; Irnazarow, I.; Krzyżowski, M.; Babczyńska, A. Immune Response of Juvenile Common Carp (Cyprinus carpio L.) Exposed to a Mixture of Sewage Chemicals. Fish Shellfish Immunol. 2019, 88, 17–27. [Google Scholar] [CrossRef]
- Liu, Y.; Li, E.; Xu, C.; Su, Y.; Qin, J.G.; Chen, L.; Wang, X. Brain Transcriptome Profiling Analysis of Nile Tilapia (Oreochromis niloticus) under Long-Term Hypersaline Stress. Front. Physiol. 2018, 9, 219. [Google Scholar] [CrossRef]
- Xing, S.; Li, P.; He, S.; Cao, Z.; Wang, X.; Cao, X.; Liu, B.; Chen, C.; You, H.; Li, Z.-H. Physiological Responses in Nile Tilapia (Oreochromis niloticus) Induced by Combined Stress of Environmental Salinity and Triphenyltin. Mar. Environ. Res. 2022, 180, 105736. [Google Scholar] [CrossRef]
- Copatti, C.E.; Baldisserotto, B. Osmoregulation in Tilapia: Environmental Factors and Internal Mechanisms. In Biology and Aquaculture of Tilapia; CRC Press: Boca Raton, FL, USA, 2021; pp. 104–118. ISBN 1-003-00413-X. [Google Scholar]
- Li, Y.; Yang, H.; Fu, B.; Kaneko, G.; Li, H.; Tian, J.; Wang, G.; Wei, M.; Xie, J.; Yu, E. Integration of Multi-Omics, Histological, and Biochemical Analysis Reveals the Toxic Responses of Nile Tilapia Liver to Chronic Microcystin-LR Exposure. Toxins 2024, 16, 149. [Google Scholar] [CrossRef]
- Mahboob, S.; Al-Ghanim, K.A.; Al-Balawi, H.F.; Al-Misned, F.; Ahmed, Z. Toxicological Effects of Heavy Metals on Histological Alterations in Various Organs in Nile Tilapia (Oreochromis niloticus) from Freshwater Reservoir. J. King Saud Univ.-Sci. 2020, 32, 970–973. [Google Scholar] [CrossRef]
- Scott, G.R.; Sloman, K.A. The Effects of Environmental Pollutants on Complex Fish Behaviour: Integrating Behavioural and Physiological Indicators of Toxicity. Aquat. Toxicol. 2004, 68, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.-Y.; Li, J.-X.; Hsu, T.-H.; Gong, H.-Y.; Lin, C.-Y.; Wang, J.-H.; Huang, C.-W. Identification of Genes Related to Cold Tolerance and Novel Genetic Markers for Molecular Breeding in Taiwan Tilapia (Oreochromis Spp.) via Transcriptome Analysis. Animals 2021, 11, 3538. [Google Scholar] [CrossRef]
- Rasal, K.D.; Kumar, P.V.; Risha, S.; Asgolkar, P.; Harshavarthini, M.; Acharya, A.; Shinde, S.; Dhere, S.; Rasal, A.; Sonwane, A. Genetic Improvement and Genomic Resources of Important Cyprinid Species: Status and Future Perspectives for Sustainable Production. Front. Genet. 2024, 15, 1398084. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.E.; Bianchini, A. Toxicity Tests Aiming to Protect Brazilian Aquatic Systems: Current Status and Implications for Management. J. Environ. Monit. 2011, 13, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P. Fish Bioaccumulation and Biomarkers in Environmental Risk Assessment: A Review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Loro, V.L.; Murussi, C.; Menezes, C.; Leitemperger, J.; Severo, E.; Guerra, L.; Costa, M.; Perazzo, G.X.; Zanella, R. Spatial and Temporal Biomarkers Responses of Astyanax jacuhiensis (Cope, 1894)(Characiformes: Characidae) from the Middle Rio Uruguai, Brazil. Neotrop. Ichthyol. 2015, 13, 569–578. [Google Scholar] [CrossRef][Green Version]
- Barletta, M.; Jaureguizar, A.J.; Baigun, C.; Fontoura, N.F.; Agostinho, A.A.; Almeida-Val, V.M.F.; Val, A.L.; Torres, R.A.; Jimenes-Segura, L.F.; Giarrizzo, T.; et al. Fish and Aquatic Habitat Conservation in South America: A Continental Overview with Emphasis on Neotropical Systems. J. Fish Biol. 2010, 76, 2118–2176. [Google Scholar] [CrossRef]
- Fangue, N.A.; Hofmeister, M.; Schulte, P.M. Intraspecific Variation in Thermal Tolerance and Heat Shock Protein Gene Expression in Common Killifish, Fundulus heteroclitus. J. Exp. Biol. 2006, 209, 2859–2872. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C. Thermal Tolerance and Potential Impacts of Climate Change on Coastal and Estuarine Organisms. J. Sea Res. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Lawrence, C. The Husbandry of Zebrafish (Danio rerio): A Review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and Husbandry Recommendations. Lab. Anim. 2020, 54, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book. In A Guide for the Laboratory Use of Zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Habjan, E.; Schouten, G.K.; Speer, A.; Van Ulsen, P.; Bitter, W. Diving into Drug-Screening: Zebrafish Embryos as an in Vivo Platform for Antimicrobial Drug Discovery and Assessment. FEMS Microbiol. Rev. 2024, 48, fuae011. [Google Scholar] [CrossRef]
- European Parliament and Council. Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 276, 33–79. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 21 May 2025).
- Clark, J.M. The 3Rs in Research: A Contemporary Approach to Replacement, Reduction and Refinement. Br. J. Nutr. 2018, 120, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Leaf, R.T.; Jiao, Y.; Murphy, B.R.; Kramer, J.I.; Sorensen, K.M.; Wooten, V.G. Life-History Characteristics of Japanese Medaka Oryzias latipes. Ichthyol. Herpetol. 2011, 2011, 559–565. [Google Scholar] [CrossRef]
- Lessman, C.A. The Developing Zebrafish (Danio rerio): A Vertebrate Model for High-throughput Screening of Chemical Libraries. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 268–280. [Google Scholar] [CrossRef]
- Shima, A.; Mitani, H. Medaka as a Research Organism: Past, Present and Future. Mech. Dev. 2004, 121, 599–604. [Google Scholar] [CrossRef]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H. Zebrafish Embryos as an Alternative to Animal Experiments—A Commentary on the Definition of the Onset of Protected Life Stages in Animal Welfare Regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- Ton, C.; Lin, Y.; Willett, C. Zebrafish as a Model for Developmental Neurotoxicity Testing. Birt. Defects Res. A. Clin. Mol. Teratol. 2006, 76, 553–567. [Google Scholar] [CrossRef]
- Terekhanova, N.V.; Logacheva, M.D.; Penin, A.A.; Neretina, T.V.; Barmintseva, A.E.; Bazykin, G.A.; Kondrashov, A.S.; Mugue, N.S. Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback Gasterosteus Aculeatus. PLoS Genet. 2014, 10, e1004696. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G. Regular Care and Maintenance of a Zebrafish (Danio rerio) Laboratory: An Introduction. J. Vis. Exp. JoVE 2012, 69, e4196. [Google Scholar] [CrossRef]
- Ameen-Ali, K.E.; Allen, C. The 3Rs in Zebrafish Research. In Zebrafish: A Practical Guide to Husbandry, Welfare and Research Methodology; CABI GB: Wallingford, UK, 2024; pp. 225–250. [Google Scholar]
- Maqsood, H.M.; Jaias, I.; Mattoo, A.I.; Akram, T.; Akram, W.; Ganai, N.A. Breeding of Zebrafish and Its Life Cycle. In Zebrafish as a Model for Parkinson’s Disease; CRC Press: Boca Raton, FL, USA, 2024; pp. 29–42. [Google Scholar]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. The Use of the Zebrafish Model in Stress Research. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1432–1451. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Zon, L.I. The Art and Design of Genetic Screens: Zebrafish. Nat. Rev. Genet. 2001, 2, 956–966. [Google Scholar] [CrossRef]
- Rennekamp, A.J.; Peterson, R.T. 15 Years of Zebrafish Chemical Screening. Curr. Opin. Chem. Biol. 2015, 24, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Schartl, M. Beyond the Zebrafish: Diverse Fish Species for Modeling Human Disease. Dis. Models Mech. 2014, 7, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.; Green, J.M.; Tyler, C.R. Transgenic Fish Systems and Their Application in Ecotoxicology. Crit. Rev. Toxicol. 2015, 45, 124–141. [Google Scholar] [CrossRef]
- Mahanayak, B. Zebrafish as a Model Organism in Bio-Medical Research. Int. J. Res. Publ. Rev. 2024, 5, 3637–3646. [Google Scholar]
- Do Nascimento, L.S.; De Oliveira, S.L.; Da Costa, C.C.; Aracati, M.F.; Rodrigues, L.F.; Charlie-Silva, I.; Conde, G.; Mansano, C.F.M.; Andreani, D.I.K.; de Andrade Belo, M.A. Deleterious Effects of Polypropylene Microplastic Ingestion in Nile Tilapia (Oreochromis niloticus). Bull. Environ. Contam. Toxicol. 2023, 111, 13. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Freeman, J.L. Toxicogenomics to Evaluate Endocrine Disrupting Effects of Environmental Chemicals Using the Zebrafish Model. Curr. Genom. 2016, 17, 515–527. [Google Scholar] [CrossRef]
- Yoganantharjah, P.; Gibert, Y. The Use of the Zebrafish Model to Aid in Drug Discovery and Target Validation. Curr. Top. Med. Chem. 2017, 17, 2041–2055. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, S.; Schroeder, A.; Hu, J.; Li, K.; Zhang, B.; Dai, D.; Lee, E.B.; Xiao, R.; Li, M. Leveraging Spatial Transcriptomics Data to Recover Cell Locations in Single-Cell RNA-Seq with CeLEry. Nat. Commun. 2023, 14, 4050. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.-K.; Kim, C.-H. Zebrafish: A Powerful Model for Genetics and Genomics. Int. J. Mol. Sci. 2023, 24, 8169. [Google Scholar] [CrossRef]
- Maclean, N.; Laight, R.J. Transgenic Fish: An Evaluation of Benefits and Risks. Fish Fish. 2000, 1, 146–172. [Google Scholar] [CrossRef]
- Higashijima, S.; Okamoto, H.; Ueno, N.; Hotta, Y.; Eguchi, G. High-Frequency Generation of Transgenic Zebrafish Which Reliably Express GFP in Whole Muscles or the Whole Body by Using Promoters of Zebrafish Origin. Dev. Biol. 1997, 192, 289–299. [Google Scholar] [CrossRef]
- Lu, J.; Fang, W.; Huang, J.; Li, S. The Application of Genome Editing Technology in Fish. Mar. Life Sci. Technol. 2021, 3, 326–346. [Google Scholar] [CrossRef]
- Bedbrook, C.N.; Nath, R.D.; Nagvekar, R.; Deisseroth, K.; Brunet, A. Rapid and Precise Genome Engineering in a Naturally Short-Lived Vertebrate. eLife 2023, 12, e80639. [Google Scholar] [CrossRef] [PubMed]
- Newland, R.I.; Hu, M.; Speer, W.; Veldman, M.B. Spatial Transcriptomic Identification of Regional Responses and Ligand-Receptor Interactions during Optic Nerve Regeneration in Zebrafish. Investig. Ophthalmol. Vis. Sci. 2024, 65, 3436. [Google Scholar]
- Morrison, J.K.; DeRossi, C.; Alter, I.L.; Nayar, S.; Giri, M.; Zhang, C.; Cho, J.H.; Chu, J. Single-cell transcriptomics reveals conserved cell identities and fibrogenic phenotypes in zebrafish and human liver. Hepatol. Commun. 2022, 6, 1711. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Shu, W.; Li, P. Fluorescence In Situ Hybridization: Cell-Based Genetic Diagnostic and Research Applications. Front. Cell Dev. Biol. 2016, 4, 89. [Google Scholar] [CrossRef]
- Saraswathy, V.M.; Zhou, L.; Mokalled, M.H. Single-Cell Analysis of Innate Spinal Cord Regeneration Identifies Intersecting Modes of Neuronal Repair. Nat. Commun. 2024, 15, 6808. [Google Scholar] [CrossRef]
- Baek, S.; Tran, N.T.; Diaz, D.C.; Tsai, Y.-Y.; Acedo, J.; Lush, M.E.; Piotrowski, T. Single-Cell Transcriptome Analysis Reveals Three Sequential Phases of Gene Expression during Zebrafish Sensory Hair Cell Regeneration. Dev. Cell 2022, 57, 799–819.e6. [Google Scholar] [CrossRef]
- Shah, S.; Lubeck, E.; Schwarzkopf, M.; He, T.-F.; Greenbaum, A.; Sohn, C.H.; Lignell, A.; Choi, H.M.; Gradinaru, V.; Pierce, N.A. Single-Molecule RNA Detection at Depth by Hybridization Chain Reaction and Tissue Hydrogel Embedding and Clearing. Development 2016, 143, 2862–2867. [Google Scholar] [CrossRef]
- Xia, C.; Colognori, D.; Jiang, X.S.; Xu, K.; Doudna, J.A. Single-Molecule Live-Cell RNA Imaging with CRISPR–Csm. Nat. Biotechnol. 2025, 1–8. [Google Scholar] [CrossRef]
- Ren, J.; Luo, S.; Shi, H.; Wang, X. Spatial Omics Advances for in Situ RNA Biology. Mol. Cell 2024, 84, 3737–3757. [Google Scholar] [CrossRef]
- Bludau, O.; Weber, A.; Bosak, V.; Kuscha, V.; Dietrich, K.; Hans, S.; Brand, M. Successful Regeneration of the Adult Zebrafish Retina Is Dependent on Inflammatory Signaling. bioRxiv 2023. bioRxiv:2023.08.11.552956. [Google Scholar] [CrossRef]
- Lange, M.; Granados, A.; VijayKumar, S.; Bragantini, J.; Ancheta, S.; Kim, Y.-J.; Santhosh, S.; Borja, M.; Kobayashi, H.; McGeever, E. A Multimodal Zebrafish Developmental Atlas Reveals the State-Transition Dynamics of Late-Vertebrate Pluripotent Axial Progenitors. Cell 2024, 187, 6742–6759.e17. [Google Scholar] [CrossRef]
- Gutierrez-Triana, J.A.; Mateo, J.L.; Ibberson, D.; Ryu, S.; Wittbrodt, J. iDamIDseq and iDEAR: An Improved Method and Computational Pipeline to Profile Chromatin-Binding Proteins. Development 2016, 143, 4272–4278. [Google Scholar] [CrossRef]
- He, J.; Mo, D.; Chen, J.; Luo, L. Combined Whole-Mount Fluorescence in Situ Hybridization and Antibody Staining in Zebrafish Embryos and Larvae. Nat. Protoc. 2020, 15, 3361–3379. [Google Scholar] [CrossRef]
- OECD. Test Guideline number 236: Fish Embryo Acute Toxicity (FET) test. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2013; Volume 2. [Google Scholar]
- Socha, M.; Chyb, J.; Suder, A.; Bojarski, B. How Endocrine Disruptors Affect Fish Reproduction on Multiple Levels: A Review. Fish. Aquat. Life 2024, 32, 128–136. [Google Scholar] [CrossRef]
- Örn, S.; Holbech, H.; Norrgren, L. Sexual Disruption in Zebrafish (Danio rerio) Exposed to Mixtures of 17α-Ethinylestradiol and 17β-Trenbolone. Environ. Toxicol. Pharmacol. 2016, 41, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Morthorst, J.E.; Holbech, H.; Bjerregaard, P. Trenbolone Causes Irreversible Masculinization of Zebrafish at Environmentally Relevant Concentrations. Aquat. Toxicol. 2010, 98, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Frame, L.; Dickerson, R.L. Fish and Wildlife as Sentinels of Environmental Contamination. In Endocrine Disruption: Biological Bases for Health Effects in Wildlife and Humans; Oxford University Press: Oxford, UK, 2006; p. 202. [Google Scholar]
- Sedeño-Díaz, J.E.; López-López, E. Freshwater Fish as Sentinel Organisms: From the Molecular to the Population Level. In New Advances and Contributions to Fish Biology; IntechOpen Limited: London, UK, 2012; p. 151. [Google Scholar]
- Wang, Y.; Gu, W.; Xu, Z.; Lv, L.; Wang, D.; Jin, Y.; Wang, X. Comprehensive Multi-Omics Investigation of Sub-Chronic Toxicity Induced by Cadmium and Triazophos Co-Exposure in Hook Snout Carps (Opsariichthys bidens). J. Hazard. Mater. 2024, 476, 135104. [Google Scholar] [CrossRef]
- Brander, S.M.; Biales, A.D.; Connon, R.E. The Role of Epigenomics in Aquatic Toxicology. Environ. Toxicol. Chem. 2017, 36, 2565–2573. [Google Scholar] [CrossRef]
- Gerlai, R. Using Zebrafish to Unravel the Genetics of Complex Brain Disorders. Behav. Neurogenet. 2012, 12, 3–24. [Google Scholar]
- Sison, M.; Gerlai, R. Associative Learning in Zebrafish (Danio rerio) in the plus Maze. Behav. Brain Res. 2010, 207, 99–104. [Google Scholar] [CrossRef]
- Geng, Y.; Peterson, R.T. The Zebrafish Subcortical Social Brain as a Model for Studying Social Behavior Disorders. Dis. Models Mech. 2019, 12, dmm039446. [Google Scholar] [CrossRef]
- Shams, S.; Chatterjee, D.; Gerlai, R. Chronic Social Isolation Affects Thigmotaxis and Whole-Brain Serotonin Levels in Adult Zebrafish. Behav. Brain Res. 2015, 292, 283–287. [Google Scholar] [CrossRef]
- Müller, T.E.; Ziani, P.R.; Fontana, B.D.; Duarte, T.; Stefanello, F.V.; Canzian, J.; Santos, A.R.; Rosemberg, D.B. Role of the Serotonergic System in Ethanol-Induced Aggression and Anxiety: A Pharmacological Approach Using the Zebrafish Model. Eur. Neuropsychopharmacol. 2020, 32, 66–76. [Google Scholar] [CrossRef]
- Ahrens, M.B.; Li, J.M.; Orger, M.B.; Robson, D.N.; Schier, A.F.; Engert, F.; Portugues, R. Brain-Wide Neuronal Dynamics during Motor Adaptation in Zebrafish. Nature 2012, 485, 471–477. [Google Scholar] [CrossRef]
- de Vito, G.; Turrini, L.; Müllenbroich, C.; Ricci, P.; Sancataldo, G.; Mazzamuto, G.; Tiso, N.; Sacconi, L.; Fanelli, D.; Silvestri, L. Fast Whole-Brain Imaging of Seizures in Zebrafish Larvae by Two-Photon Light-Sheet Microscopy. Biomed. Opt. Express 2022, 13, 1516–1536. [Google Scholar] [CrossRef]
- Turrini, L.; Ricci, P.; Sorelli, M.; de Vito, G.; Marchetti, M.; Vanzi, F.; Pavone, F.S. Two-Photon All-Optical Neurophysiology for the Dissection of Larval Zebrafish Brain Functional and Effective Connectivity. Commun. Biol. 2024, 7, 1261. [Google Scholar] [CrossRef]
- Kedikian, X.; Faillace, M.P.; Bernabeu, R. Behavioral and Molecular Analysis of Nicotine-Conditioned Place Preference in Zebrafish. PLoS ONE 2013, 8, e69453. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.D.; Khan, K.M.; Caramillo, E.M.; Mohn, R.S.; Echevarria, D.J. Zebrafish and Conditioned Place Preference: A Translational Model of Drug Reward. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 55, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nam, H.G.; Valenzano, D.R. The Short-Lived African Turquoise Killifish: An Emerging Experimental Model for Ageing. Dis. Models Mech. 2016, 9, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R. Associative Learning in Zebrafish (Danio rerio). Methods Cell Biol. 2011, 101, 249–270. [Google Scholar] [PubMed]
- Penberthy, W.T.; Shafizadeh, E.; Lin, S. The Zebrafish as a Model for Human Disease. Front. Biosci. 2002, 7, D1439–D1453. [Google Scholar] [CrossRef]
- Goldstone, J.V.; McArthur, A.G.; Kubota, A.; Zanette, J.; Parente, T.; Jönsson, M.E.; Nelson, D.R.; Stegeman, J.J. Identification and Developmental Expression of the Full Complement of Cytochrome P450 Genes in Zebrafish. BMC Genom. 2010, 11, 643. [Google Scholar] [CrossRef]
- Yan, C.; Brunson, D.C.; Tang, Q.; Do, D.; Iftimia, N.A.; Moore, J.C.; Hayes, M.N.; Welker, A.M.; Garcia, E.G.; Dubash, T.D. Visualizing Engrafted Human Cancer and Therapy Responses in Immunodeficient Zebrafish. Cell 2019, 177, 1903–1914.e14. [Google Scholar] [CrossRef]
- Liu, Y.; Asnani, A.; Zou, L.; Bentley, V.L.; Yu, M.; Wang, Y.; Dellaire, G.; Sarkar, K.S.; Dai, M.; Chen, H.H. Visnagin Protects against Doxorubicin-Induced Cardiomyopathy through Modulation of Mitochondrial Malate Dehydrogenase. Sci. Transl. Med. 2014, 6, 266ra170. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Kim, J.-H.; Ko, D.H.; Kim, C.-H.; Hwang, J.-S.; Ahn, S.; Kim, S.Y.; Kim, C.-D.; Lee, J.-H.; Yoon, T.-J. Zebrafish as a New Model for Phenotype-based Screening of Melanogenic Regulatory Compounds. Pigment Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef]
- Delvecchio, C.; Tiefenbach, J.; Krause, H.M. The Zebrafish: A Powerful Platform for in Vivo, HTS Drug Discovery. Assay Drug Dev. Technol. 2011, 9, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Takashima, F.; Hibiya, T. An Atlas of Fish Histology: Normal and Pathological Features; Gustav Fischer Verlag: Stuttgart, Germany, 1995. [Google Scholar]
- Kleinow, K.M.; Melancon, M.J.; Lech, J.J. Biotransformation and Induction: Implications for Toxicity, Bioaccumulation and Monitoring of Environmental Xenobiotics in Fish. Environ. Health Perspect. 1987, 71, 105–119. [Google Scholar] [CrossRef][Green Version]
- Hughes, G.M.; Morgan, M. The Structure of Fish Gills in Relation to Their Respiratory Function. Biol. Rev. 1973, 48, 419–475. [Google Scholar] [CrossRef]
- Glasauer, S.M.; Neuhauss, S.C. Whole-Genome Duplication in Teleost Fishes and Its Evolutionary Consequences. Mol. Genet. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef]
- Renshaw, S.A.; Trede, N.S. A Model 450 Million Years in the Making: Zebrafish and Vertebrate Immunity. Dis. Models Mech. 2012, 5, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.S.; Renshaw, S.A. Using in Vivo Zebrafish Models to Understand the Biochemical Basis of Neutrophilic Respiratory Disease. Biochem. Soc. Trans. 2009, 37, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Rauta, P.R.; Nayak, B.; Das, S. Immune System and Immune Responses in Fish and Their Role in Comparative Immunity Study: A Model for Higher Organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef]
- Galindo-Villegas, J. The Zebrafish Disease and Drug Screening Model: A Strong Ally against Covid-19. Front. Pharmacol. 2020, 11, 680. [Google Scholar] [CrossRef]
- Zimmerman, A.M.; Moustafa, F.M.; Romanowski, K.E.; Steiner, L.A. Zebrafish Immunoglobulin IgD: Unusual Exon Usage and Quantitative Expression Profiles with IgM and IgZ/T Heavy Chain Isotypes. Mol. Immunol. 2011, 48, 2220–2223. [Google Scholar] [CrossRef] [PubMed]
- Postlethwait, J.H.; Johnson, S.L.; Midson, C.N.; Talbot, W.S.; Gates, M.; Ballinger, E.W.; Africa, D.; Andrews, R.; Carl, T.; Eisen, J.S. A Genetic Linkage Map for the Zebrafish. Science 1994, 264, 699–703. [Google Scholar] [CrossRef]
- Ransom, D.G.; Haffter, P.; Odenthal, J.; Brownlie, A.; Vogelsang, E.; Kelsh, R.N.; Brand, M.; van Eeden, F.J.; Furutani-Seiki, M.; Granato, M. Characterization of Zebrafish Mutants with Defects in Embryonic Hematopoiesis. Development 1996, 123, 311–319. [Google Scholar] [CrossRef]
- Mathias, J.R.; Perrin, B.J.; Liu, T.-X.; Kanki, J.; Look, A.T.; Huttenlocher, A. Resolution of Inflammation by Retrograde Chemotaxis of Neutrophils in Transgenic Zebrafish. J. Leukoc. Biol. 2006, 80, 1281–1288. [Google Scholar] [CrossRef]
- Dobson, J.T.; Seibert, J.; Teh, E.M.; Da’as, S.; Fraser, R.B.; Paw, B.H.; Lin, T.-J.; Berman, J.N. Carboxypeptidase A5 Identifies a Novel Mast Cell Lineage in the Zebrafish Providing New Insight into Mast Cell Fate Determination. Blood J. Am. Soc. Hematol. 2008, 112, 2969–2972. [Google Scholar] [CrossRef]
- Liang, Z.; Kong, Y.; Luo, C.; Shen, Y.; Zhang, S. Molecular Cloning, Functional Characterization and Phylogenetic Analysis of B-Cell Activating Factor in Zebrafish (Danio rerio). Fish Shellfish Immunol. 2010, 29, 233–240. [Google Scholar] [CrossRef]
- Ryo, S.; Wijdeven, R.H.; Tyagi, A.; Hermsen, T.; Kono, T.; Karunasagar, I.; Rombout, J.H.; Sakai, M.; Verburg-van Kemenade, B.L.; Savan, R. Common Carp Have Two Subclasses of Bonyfish Specific Antibody IgZ Showing Differential Expression in Response to Infection. Dev. Comp. Immunol. 2010, 34, 1183–1190. [Google Scholar] [CrossRef]
- Balla, K.M.; Lugo-Villarino, G.; Spitsbergen, J.M.; Stachura, D.L.; Hu, Y.; Banuelos, K.; Romo-Fewell, O.; Aroian, R.V.; Traver, D. Eosinophils in the Zebrafish: Prospective Isolation, Characterization, and Eosinophilia Induction by Helminth Determinants. Blood J. Am. Soc. Hematol. 2010, 116, 3944–3954. [Google Scholar] [CrossRef]
- Hu, C.-B.; Wang, J.; Hong, Y.; Li, H.; Fan, D.-D.; Lin, A.-F.; Xiang, L.-X.; Shao, J.-Z. Single-cell Transcriptome Profiling Reveals Diverse Immune Cell Populations and Their Responses to Viral Infection in the Spleen of Zebrafish. FASEB J. 2023, 37, e22951. [Google Scholar] [CrossRef] [PubMed]
- Ken, C.-F.; Chen, C.-N.; Ting, C.-H.; Pan, C.-Y.; Chen, J.-Y. Transcriptome Analysis of Hybrid Tilapia (Oreochromis Spp.) with Streptococcus Agalactiae Infection Identifies Toll-like Receptor Pathway-Mediated Induction of NADPH Oxidase Complex and Piscidins as Primary Immune-Related Responses. Fish Shellfish Immunol. 2017, 70, 106–120. [Google Scholar] [CrossRef]
- Sood, N.; Verma, D.K.; Paria, A.; Yadav, S.C.; Yadav, M.K.; Bedekar, M.K.; Kumar, S.; Swaminathan, T.R.; Mohan, C.V.; Rajendran, K.V. Transcriptome Analysis of Liver Elucidates Key Immune-Related Pathways in Nile Tilapia Oreochromis niloticus Following Infection with Tilapia Lake Virus. Fish Shellfish Immunol. 2021, 111, 208–219. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, N.; Hong, Y.; Tie, R.; Fan, D.; Lin, A.; Chen, Y.; Xiang, L.; Shao, J. Single-Cell RNA Sequencing Unveils the Hidden Powers of Zebrafish Kidney for Generating Both Hematopoiesis and Adaptive Antiviral Immunity. eLife 2024, 13, RP92424. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Hu, X.; Bai, J.; Lv, A. Multiomics Analysis Revealed miRNAs as Potential Regulators of the Immune Response in Carassius auratus Gills to Aeromonas hydrophila Infection. Front. Immunol. 2023, 14, 1098455. [Google Scholar] [CrossRef] [PubMed]
- Vivekaa, A.; Nellore, J.; Sunkar, S. Zebrafish Metabolomics: A Comprehensive Approach to Understanding Health and Disease. Funct. Integr. Genom. 2025, 25, 110. [Google Scholar] [CrossRef]
- de Andrade Belo, M.A.; Charlie-Silva, I. Teleost Fish as an Experimental Model for Vaccine Development. In Vaccine Design: Methods and Protocols, Volume 2. Vaccines for Veterinary Diseases; Springer: Berlin/Heidelberg, Germany, 2021; pp. 175–194. [Google Scholar]
- Berghmans, S.; Murphey, R.D.; Wienholds, E.; Neuberg, D.; Kutok, J.L.; Fletcher, C.D.; Morris, J.P.; Liu, T.X.; Schulte-Merker, S.; Kanki, J.P. Tp53 Mutant Zebrafish Develop Malignant Peripheral Nerve Sheath Tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 407–412. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I. Taking Human Cancer Genes to the Fish: A Transgenic Model of Melanoma in Zebrafish. Zebrafish 2005, 1, 363–368. [Google Scholar] [CrossRef]
- Ignatius, M.S.; Hayes, M.N.; Moore, F.E.; Tang, Q.; Garcia, S.P.; Blackburn, P.R.; Baxi, K.; Wang, L.; Jin, A.; Ramakrishnan, A. Tp53 Deficiency Causes a Wide Tumor Spectrum and Increases Embryonal Rhabdomyosarcoma Metastasis in Zebrafish. eLife 2018, 7, e37202. [Google Scholar] [CrossRef]
- Park, S.W.; Davison, J.M.; Rhee, J.; Hruban, R.H.; Maitra, A.; Leach, S.D. Oncogenic KRAS Induces Progenitor Cell Expansion and Malignant Transformation in Zebrafish Exocrine Pancreas. Gastroenterology 2008, 134, 2080–2090. [Google Scholar] [CrossRef]
- Santoriello, C.; Deflorian, G.; Pezzimenti, F.; Kawakami, K.; Lanfrancone, L.; d’Adda di Fagagna, F.; Mione, M. Expression of H-RASV12 in a Zebrafish Model of Costello Syndrome Causes Cellular Senescence in Adult Proliferating Cells. Dis. Models Mech. 2009, 2, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.S.; Garg, R.; Mohanty, A.; Garg, P.; Ramisetty, S.K.; Mirzapoiazova, T.; Soldi, R.; Sharma, S.; Kulkarni, P.; Salgia, R. Recent Advancement in Breast Cancer Research: Insights from Model Organisms—Mouse Models to Zebrafish. Cancers 2023, 15, 2961. [Google Scholar] [CrossRef]
- Bournele, D.; Beis, D. Zebrafish Models of Cardiovascular Disease. Heart Fail. Rev. 2016, 21, 803–813. [Google Scholar] [CrossRef]
- Gore, A.V.; Pillay, L.M.; Venero Galanternik, M.; Weinstein, B.M. The Zebrafish: A Fintastic Model for Hematopoietic Development and Disease. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e312. [Google Scholar] [CrossRef] [PubMed]
- Kulkeaw, K.; Sugiyama, D. Zebrafish Erythropoiesis and the Utility of Fish as Models of Anemia. Stem Cell Res. Ther. 2012, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-T.; Hsieh, C.-S.; Chang, S.-N.; Chuang, E.Y.; Ueng, K.-C.; Tsai, C.-F.; Lin, T.-H.; Wu, C.-K.; Lee, J.-K.; Lin, L.-Y. Genome-Wide Screening Identifies a KCNIP1 Copy Number Variant as a Genetic Predictor for Atrial Fibrillation. Nat. Commun. 2016, 7, 10190. [Google Scholar] [CrossRef]
- Dina, C.; Bouatia-Naji, N.; Tucker, N.; Delling, F.N.; Toomer, K.; Durst, R.; Perrocheau, M.; Fernandez-Friera, L.; Solis, J.; PROMESA investigators. Genetic Association Analyses Highlight Biological Pathways Underlying Mitral Valve Prolapse. Nat. Genet. 2015, 47, 1206–1211. [Google Scholar] [CrossRef]
- Asimaki, A.; Kapoor, S.; Plovie, E.; Karin Arndt, A.; Adams, E.; Liu, Z.; James, C.A.; Judge, D.P.; Calkins, H.; Churko, J. Identification of a New Modulator of the Intercalated Disc in a Zebrafish Model of Arrhythmogenic Cardiomyopathy. Sci. Transl. Med. 2014, 6, 240ra74. [Google Scholar] [CrossRef]
- Louw, J.J.; Nunes Bastos, R.; Chen, X.; Verdood, C.; Corveleyn, A.; Jia, Y.; Breckpot, J.; Gewillig, M.; Peeters, H.; Santoro, M.M. Compound Heterozygous Loss-of-Function Mutations in KIF20A Are Associated with a Novel Lethal Congenital Cardiomyopathy in Two Siblings. PLoS Genet. 2018, 14, e1007138. [Google Scholar] [CrossRef] [PubMed]
- Elmonem, M.A.; Khalil, R.; Khodaparast, L.; Khodaparast, L.; Arcolino, F.O.; Morgan, J.; Pastore, A.; Tylzanowski, P.; Ny, A.; Lowe, M. Cystinosis (Ctns) Zebrafish Mutant Shows Pronephric Glomerular and Tubular Dysfunction. Sci. Rep. 2017, 7, 42583. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Ebarasi, L.; Hultenby, K.; Tryggvason, K.; Betsholtz, C. Podocin-Green Fluorescence Protein Allows Visualization and Functional Analysis of Podocytes. J. Am. Soc. Nephrol. 2011, 22, 1019–1023. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Weeks, O.; Mijatovic, V.; Teumer, A.; Huffman, J.E.; Tromp, G.; Fuchsberger, C.; Gorski, M.; Lyytikäinen, L.-P. SOS2 and ACP1 Loci Identified through Large-Scale Exome Chip Analysis Regulate Kidney Development and Function. J. Am. Soc. Nephrol. 2017, 28, 981–994. [Google Scholar] [CrossRef]
- Hinkes, B.; Wiggins, R.C.; Gbadegesin, R.; Vlangos, C.N.; Seelow, D.; Nürnberg, G.; Garg, P.; Verma, R.; Chaib, H.; Hoskins, B.E. Positional Cloning Uncovers Mutations in PLCE1 Responsible for a Nephrotic Syndrome Variant That May Be Reversible. Nat. Genet. 2006, 38, 1397–1405. [Google Scholar] [CrossRef]
- Gee, H.Y.; Sadowski, C.E.; Aggarwal, P.K.; Porath, J.D.; Yakulov, T.A.; Schueler, M.; Lovric, S.; Ashraf, S.; Braun, D.A.; Halbritter, J. FAT1 Mutations Cause a Glomerulotubular Nephropathy. Nat. Commun. 2016, 7, 10822. [Google Scholar] [CrossRef]
- Rosa, J.T.; Laizé, V.; Gavaia, P.J.; Cancela, M.L. Fish Models of Induced Osteoporosis. Front. Cell Dev. Biol. 2021, 9, 672424. [Google Scholar] [CrossRef] [PubMed]
- Underwood, W.; Raymond, A. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; Retrieved March; American Veterinary Medical Association: Schaumburg, IL, USA, 2020; Volume 2013, pp. 2020–2021. [Google Scholar]
- Huntingford, F.A.; Adams, C.; Braithwaite, V.A.; Kadri, S.; Pottinger, T.G.; Sandøe, P.; Turnbull, J.F. Current Issues in Fish Welfare. J. Fish Biol. 2006, 68, 332–372. [Google Scholar] [CrossRef]
- Borges, T.; De Jong, W.; Testai, E.; Vighi, M.; Bakkers, J.; Fiedler, W.; Hawkins, P.; Ohnesorge, N.; Parker, M.; Schroeder, J. SCHEER (Scientific Committee on Health, Environmental and Emerging Risks), Opinion on the Revision of Annexes III and IV of Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes Regarding Accommodation Parameters and Methods of Killing for Zebrafish, and Accommodation Parameters for Passerine Birds, Adopted on 25 September 2023; European Commission: Brussels, Belgium, 2023; pp. 1–90. [Google Scholar] [CrossRef]
- Canedo, A.; Saiki, P.; Santos, A.L.; da Carneiro, K.S.; de Souza, A.M.; Qualhato, G.; da Brito, R.S.; Mello-Andrade, F.; Rocha, T.L. Zebrafish (Danio rerio) Meets Bioethics: The 10Rs Ethical Principles in Research. Ciênc. Anim. Bras. 2022, 23, e-70884. [Google Scholar] [CrossRef]
- Ackerman, P.A.; Morgan, J.D.; Iwama, G.K. Les Anesthésiques. In Information Additionnelle au Sujet des Lignes Directrices du CCPA Sur le Soin et L’Utilisation des Poissons en Recherche, en Enseignement et dans les Tests; Conseil Canadien de Protection des Animaux: Ottawa, ON, Canada, 2005; Volume 2005, pp. 1–25. [Google Scholar]
- Neiffer, D.L.; Stamper, M.A. Fish Sedation, Anesthesia, Analgesia, and Euthanasia: Considerations, Methods, and Types of Drugs. ILAR J. 2009, 50, 343–360. [Google Scholar] [CrossRef]
- Dang, Z.; van der Ven, L.T.; Kienhuis, A.S. Fish Embryo Toxicity Test, Threshold Approach, and Moribund as Approaches to Implement 3R Principles to the Acute Fish Toxicity Test. Chemosphere 2017, 186, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Lammer, E.; Carr, G.J.; Wendler, K.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T. Is the Fish Embryo Toxicity Test (FET) with the Zebrafish (Danio rerio) a Potential Alternative for the Fish Acute Toxicity Test? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Zahl, I.H.; Samuelsen, O.; Kiessling, A. Anaesthesia of Farmed Fish: Implications for Welfare. Fish Physiol. Biochem. 2012, 38, 201–218. [Google Scholar] [CrossRef]
- Posner, L.P.; Harms, C.A.; Smith, S.A. Sedation, Anesthesia, Analgesia and Euthanasia. In Fish Diseases and Medicine; CRC Press: Boca Raton, FL, USA, 2019; pp. 283–304. [Google Scholar]
- Readman, G.D.; Owen, S.F.; Knowles, T.G.; Murrell, J.C. Species Specific Anaesthetics for Fish Anaesthesia and Euthanasia. Sci. Rep. 2017, 7, 7102. [Google Scholar] [CrossRef]
- Brønstad, A. Good Anesthesia Practice for Fish and Other Aquatics. Biology 2022, 11, 1355. [Google Scholar] [CrossRef]
- Nordgreen, J.; Tahamtani, F.M.; Janczak, A.M.; Horsberg, T.E. Behavioural Effects of the Commonly Used Fish Anaesthetic Tricaine Methanesulfonate (MS-222) on Zebrafish (Danio rerio) and Its Relevance for the Acetic Acid Pain Test. PLoS ONE 2014, 9, e92116. [Google Scholar] [CrossRef]
- Huang, W.-C.; Hsieh, Y.-S.; Chen, I.-H.; Wang, C.-H.; Chang, H.-W.; Yang, C.-C.; Ku, T.-H.; Yeh, S.-R.; Chuang, Y.-J. Combined Use of MS-222 (Tricaine) and Isoflurane Extends Anesthesia Time and Minimizes Cardiac Rhythm Side Effects in Adult Zebrafish. Zebrafish 2010, 7, 297–304. [Google Scholar] [CrossRef]
- Harms, C.A.; Lewbart, G.A.; Swanson, C.R.; Kishimori, J.M.; Boylan, S.M. Behavioral and Clinical Pathology Changes in Koi Carp (Cyprinus carpio) Subjected to Anesthesia and Surgery with and without Intra-Operative Analgesics. Comp. Med. 2005, 55, 221–226. [Google Scholar] [PubMed]
- Matthews, M.; Varga, Z.M. Anesthesia and Euthanasia in Zebrafish. ILAR J. 2012, 53, 192–204. [Google Scholar] [CrossRef]
- Wallace, C.K.; Bright, L.A.; Marx, J.O.; Andersen, R.P.; Mullins, M.C.; Carty, A.J. Effectiveness of Rapid Cooling as a Method of Euthanasia for Young Zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 58–63. [Google Scholar]
- Valentim, A.M.; van Eeden, F.J.; Strähle, U.; Olsson, I.A.S. Euthanizing Zebrafish Legally in Europe: Are the Approved Methods of Euthanizing Zebrafish Appropriate to Research Reality and Animal Welfare? EMBO Rep. 2016, 17, 1688–1689. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an Animal Model for Biomedical Research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Tavares, B.; Lopes, S.S. The Importance of Zebrafish in Biomedical Research. Acta Med. Port. 2013, 26, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Bailone, R.L.; Fukushima, H.C.S.; Ventura Fernandes, B.H.; De Aguiar, L.K.; Corrêa, T.; Janke, H.; Grejo Setti, P.; Roça, R.D.O.; Borra, R.C. Zebrafish as an Alternative Animal Model in Human and Animal Vaccination Research. Lab. Anim. Res. 2020, 36, 13. [Google Scholar] [CrossRef]
- Supriya, R.H.; Reshma, K.J.; Sreekanth, G.B. Fish Models in Experimental Pharmacology: On the Mark or off the Mark. Curr. Sci. 2022, 123, 1199–1206. [Google Scholar] [CrossRef]
- Matrone, G.; Tucker, C.S.; Denvir, M.A. Cardiomyocyte Proliferation in Zebrafish and Mammals: Lessons for Human Disease. Cell. Mol. Life Sci. 2017, 74, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Chia, K.; Klingseisen, A.; Sieger, D.; Priller, J. Zebrafish as a Model Organism for Neurodegenerative Disease. Front. Mol. Neurosci. 2022, 15, 940484. [Google Scholar] [CrossRef]
- Sanches, P.H.G.; de Melo, N.C.; Porcari, A.M.; de Carvalho, L.M. Integrating Molecular Perspectives: Strategies for Comprehensive Multi-Omics Integrative Data Analysis and Machine Learning Applications in Transcriptomics, Proteomics, and Metabolomics. Biology 2024, 13, 848. [Google Scholar] [CrossRef]
- Qian, X.; Ba, Y.; Zhuang, Q.; Zhong, G. RNA-Seq Technology and Its Application in Fish Transcriptomics. Omics J. Integr. Biol. 2014, 18, 98–110. [Google Scholar] [CrossRef]
- Collins, F.S.; Morgan, M.; Patrinos, A. The Human Genome Project: Lessons from Large-Scale Biology. Science 2003, 300, 286–290. [Google Scholar] [CrossRef]
- Valenzano, D.R.; Benayoun, B.A.; Singh, P.P.; Zhang, E.; Etter, P.D.; Hu, C.-K.; Clément-Ziza, M.; Willemsen, D.; Cui, R.; Harel, I. The African Turquoise Killifish Genome Provides Insights into Evolution and Genetic Architecture of Lifespan. Cell 2015, 163, 1539–1554. [Google Scholar] [CrossRef]
- Liu, L.; Yang, T.; Jiang, Q.; Sun, J.; Gu, L.; Wang, S.; Li, Y.; Chen, B.; Zhao, D.; Sun, R. Integrated Transcriptomic and Proteomic Analysis Reveals Potential Targets for Heart Regeneration. Biomol. Biomed. 2023, 23, 101. [Google Scholar] [CrossRef]
- Liu, C.; Li, R.; Li, Y.; Lin, X.; Zhao, K.; Liu, Q.; Wang, S.; Yang, X.; Shi, X.; Ma, Y. Spatiotemporal Mapping of Gene Expression Landscapes and Developmental Trajectories during Zebrafish Embryogenesis. Dev. Cell 2022, 57, 1284–1298.e5. [Google Scholar] [CrossRef]
- Sun, K.; Liu, X.; Xu, R.; Liu, C.; Meng, A.; Lan, X. Mapping the Chromatin Accessibility Landscape of Zebrafish Embryogenesis at Single-Cell Resolution by SPATAC-Seq. Nat. Cell Biol. 2024, 26, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Korsunsky, I.; Millard, N.; Fan, J.; Slowikowski, K.; Zhang, F.; Wei, K.; Baglaenko, Y.; Brenner, M.; Loh, P.; Raychaudhuri, S. Fast, Sensitive and Accurate Integration of Single-Cell Data with Harmony. Nat. Methods 2019, 16, 1289–1296. [Google Scholar] [CrossRef]
- Moreno-Mateos, M.A.; Fernandez, J.P.; Rouet, R.; Vejnar, C.E.; Lane, M.A.; Mis, E.; Khokha, M.K.; Doudna, J.A.; Giraldez, A.J. CRISPR-Cpf1 Mediates Efficient Homology-Directed Repair and Temperature-Controlled Genome Editing. Nat. Commun. 2017, 8, 2024. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A. Search-and-Replace Genome Editing without Double-Strand Breaks or Donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Schartl, M.; Wilde, B.; Laisney, J.A.; Taniguchi, Y.; Takeda, S.; Meierjohann, S. A Mutated EGFR Is Sufficient to Induce Malignant Melanoma with Genetic Background-Dependent Histopathologies. J. Investig. Dermatol. 2010, 130, 249–258. [Google Scholar] [CrossRef]
- Hartmann, N.; Englert, C. A Microinjection Protocol for the Generation of Transgenic Killifish (Species: Nothobranchius furzeri). Dev. Dyn. 2012, 241, 1133–1141. [Google Scholar] [CrossRef]
- Yergeau, D.A.; Cornell, C.N.; Parker, S.K.; Zhou, Y.; Detrich III, H.W. Bloodthirsty, an RBCC/TRIM Gene Required for Erythropoiesis in Zebrafish. Dev. Biol. 2005, 283, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Crozier, L.G.; Hutchings, J.A. Plastic and Evolutionary Responses to Climate Change in Fish. Evol. Appl. 2014, 7, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, S.; McKenzie, D.J.; Nilsson, G.E. Models Projecting the Fate of Fish Populations under Climate Change Need to Be Based on Valid Physiological Mechanisms. Glob. Change Biol. 2017, 23, 3449–3459. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlino-Costa, C.; Belo, M.A.d.A. Experimental Fish Models in the Post-Genomic Era: Tools for Multidisciplinary Science. J 2025, 8, 39. https://doi.org/10.3390/j8040039
Carlino-Costa C, Belo MAdA. Experimental Fish Models in the Post-Genomic Era: Tools for Multidisciplinary Science. J. 2025; 8(4):39. https://doi.org/10.3390/j8040039
Chicago/Turabian StyleCarlino-Costa, Camila, and Marco Antonio de Andrade Belo. 2025. "Experimental Fish Models in the Post-Genomic Era: Tools for Multidisciplinary Science" J 8, no. 4: 39. https://doi.org/10.3390/j8040039
APA StyleCarlino-Costa, C., & Belo, M. A. d. A. (2025). Experimental Fish Models in the Post-Genomic Era: Tools for Multidisciplinary Science. J, 8(4), 39. https://doi.org/10.3390/j8040039







