High-Salt Exposure Disrupts Cardiovascular Development in Zebrafish Embryos, Brachyodanio rerio, via Calcium and MAPK Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Husbandry
2.3. Salt Exposure Protocol
2.4. Heart Rate Measurement and Cardiac Imaging
2.5. RNA Extraction and Sequencing
2.6. RNA-Seq Data Processing and Analysis
2.7. Pathway and Functional Enrichment Analysis
2.8. Statistical Analysis
3. Results
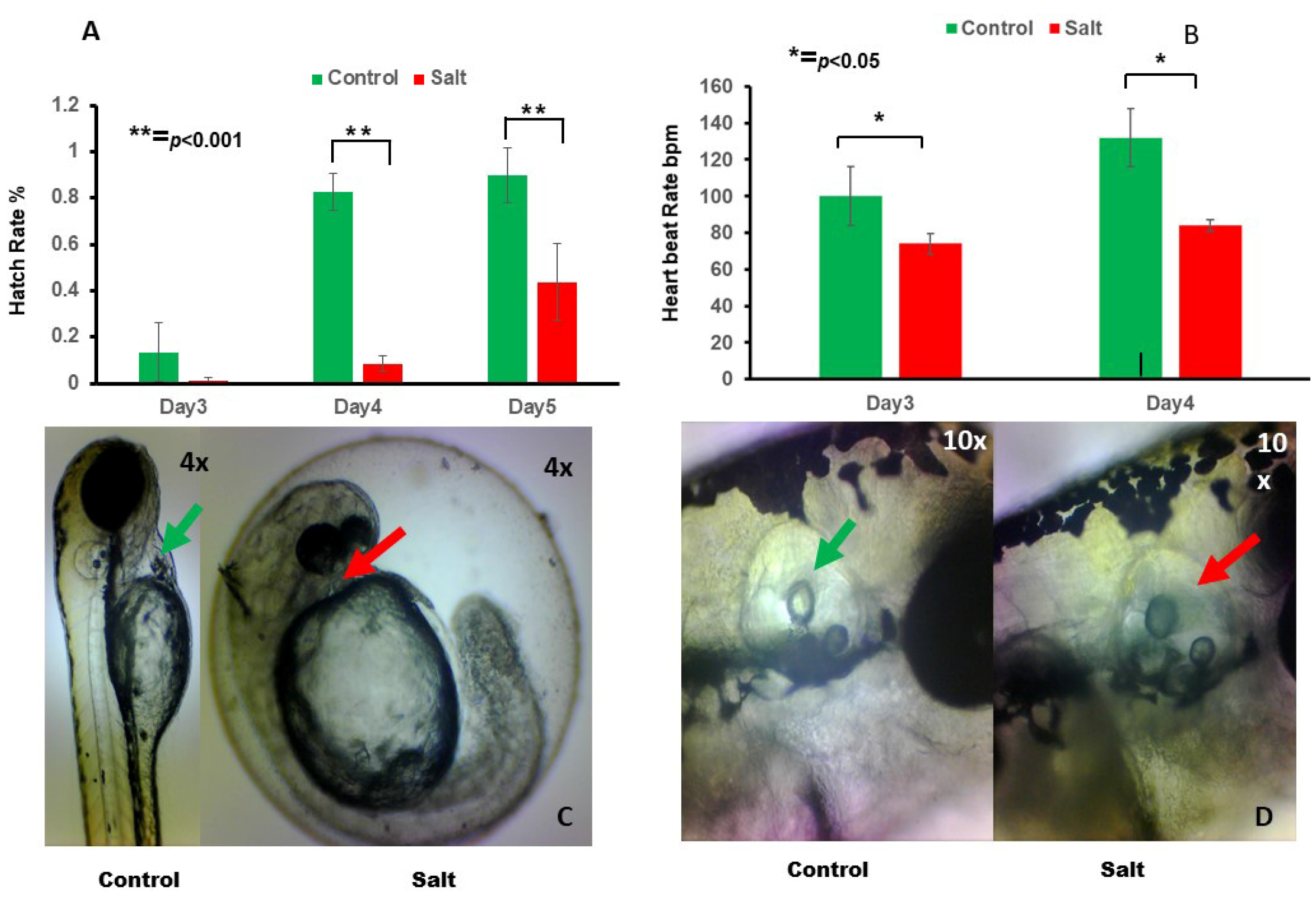
3.1. Effect of Salt on Zebrafish Embryo Development
3.1.1. The Effect of Salt on Morphology and Physiology Changes
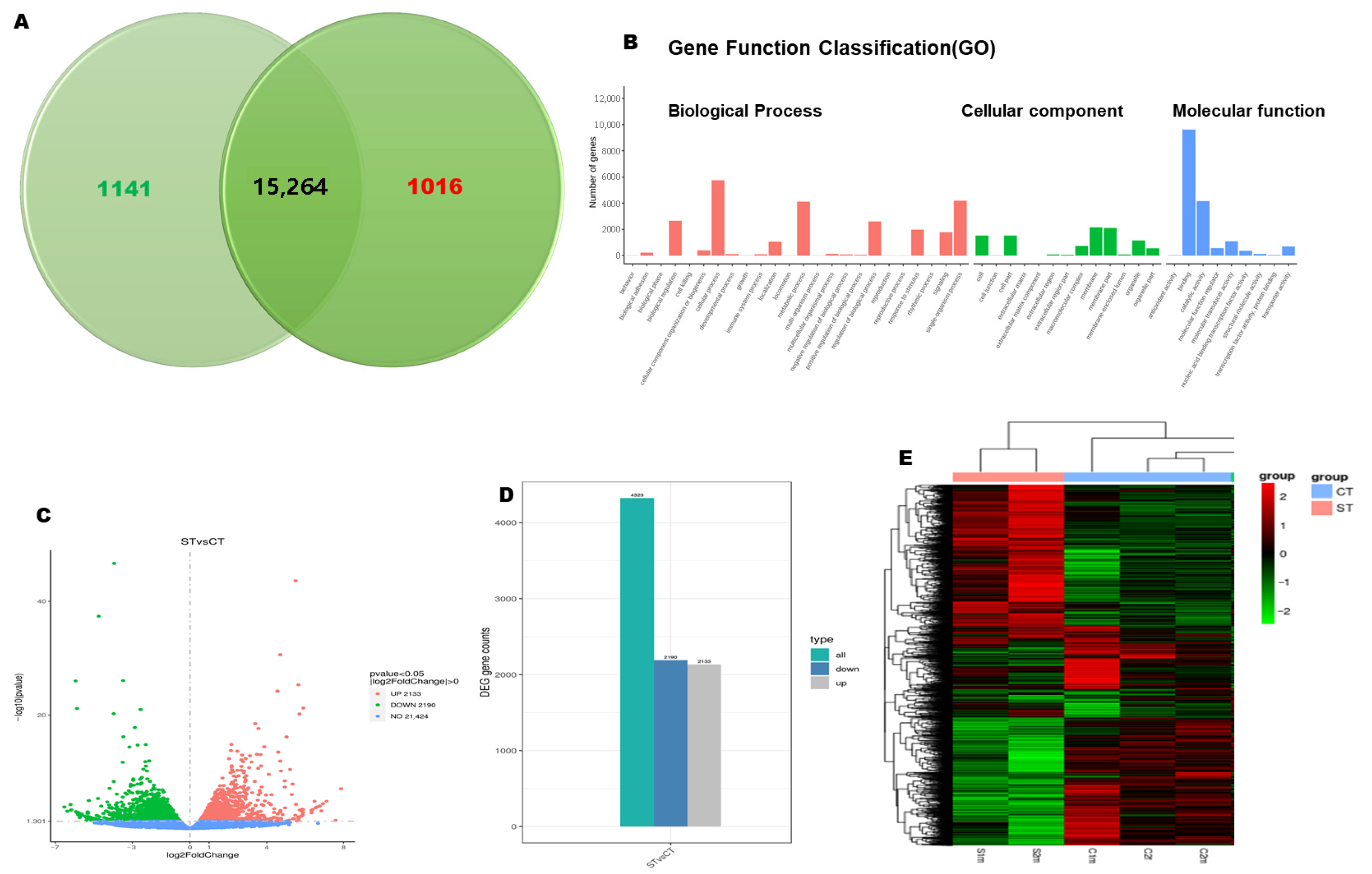
3.1.2. RNA-Seq Analysis Displayed Significant Gene Dysregulation in the Salt-Treated Embryos
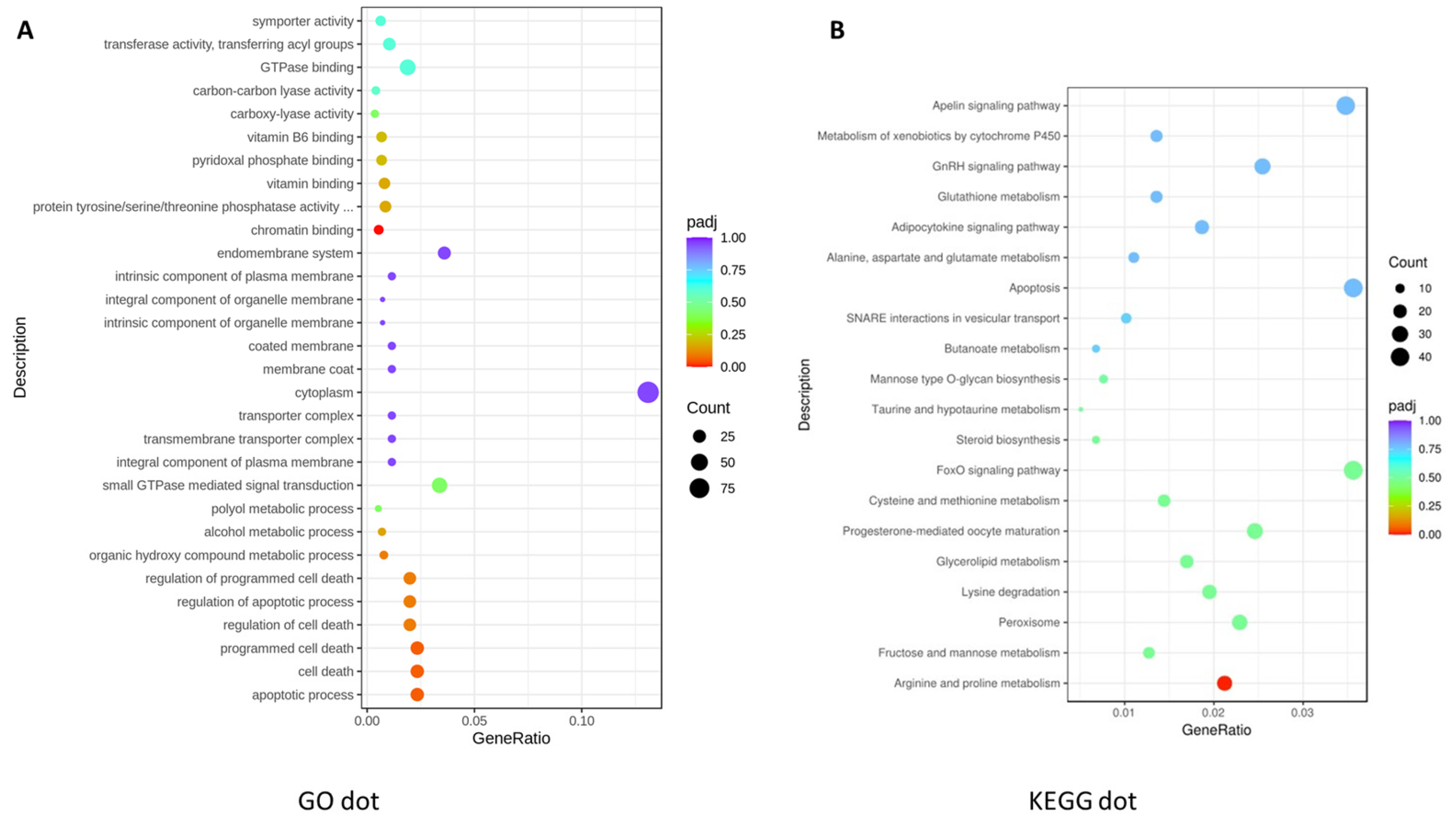
3.1.3. Functional Analysis of DEGs Using Gene Ontology (GO) Analysis
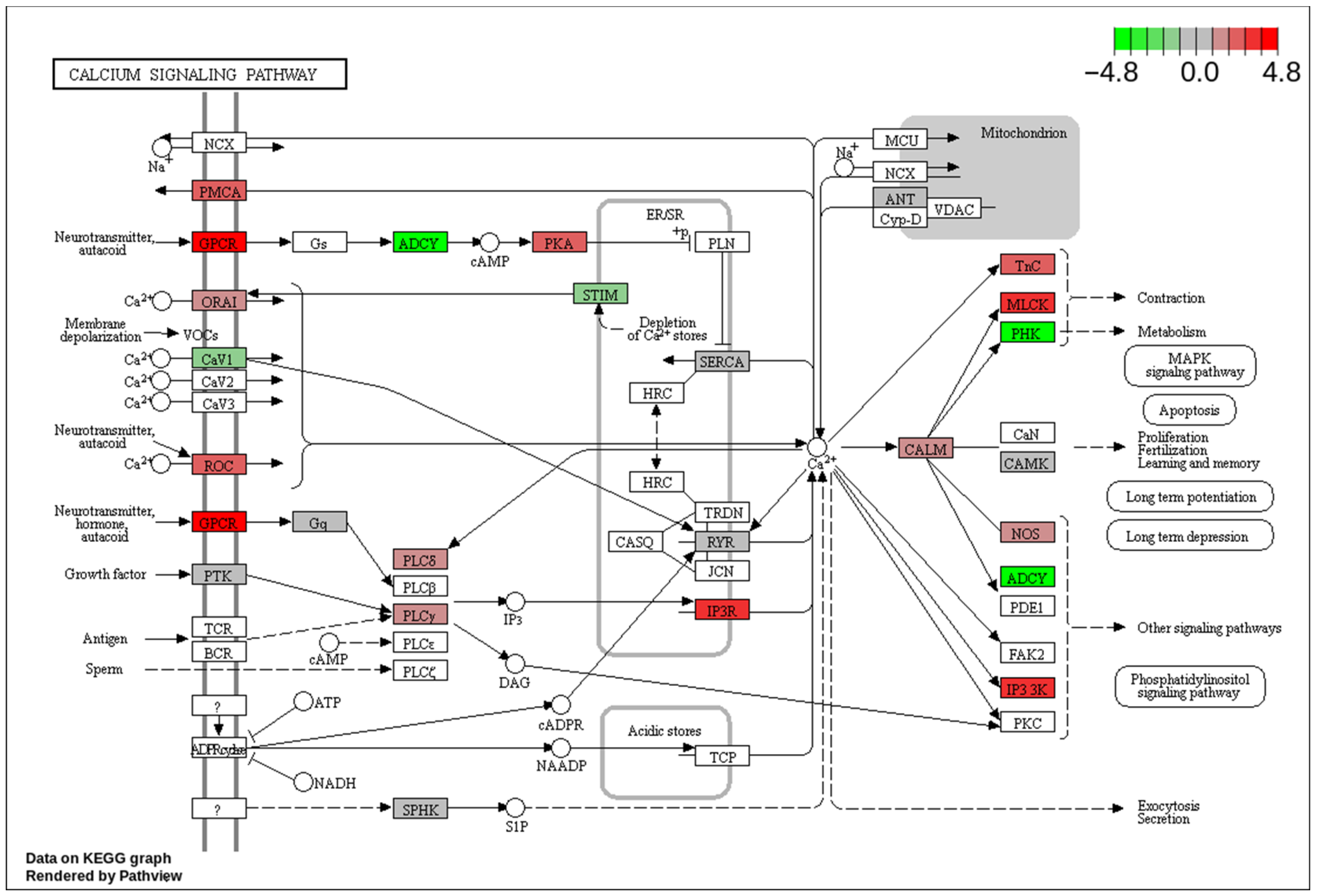
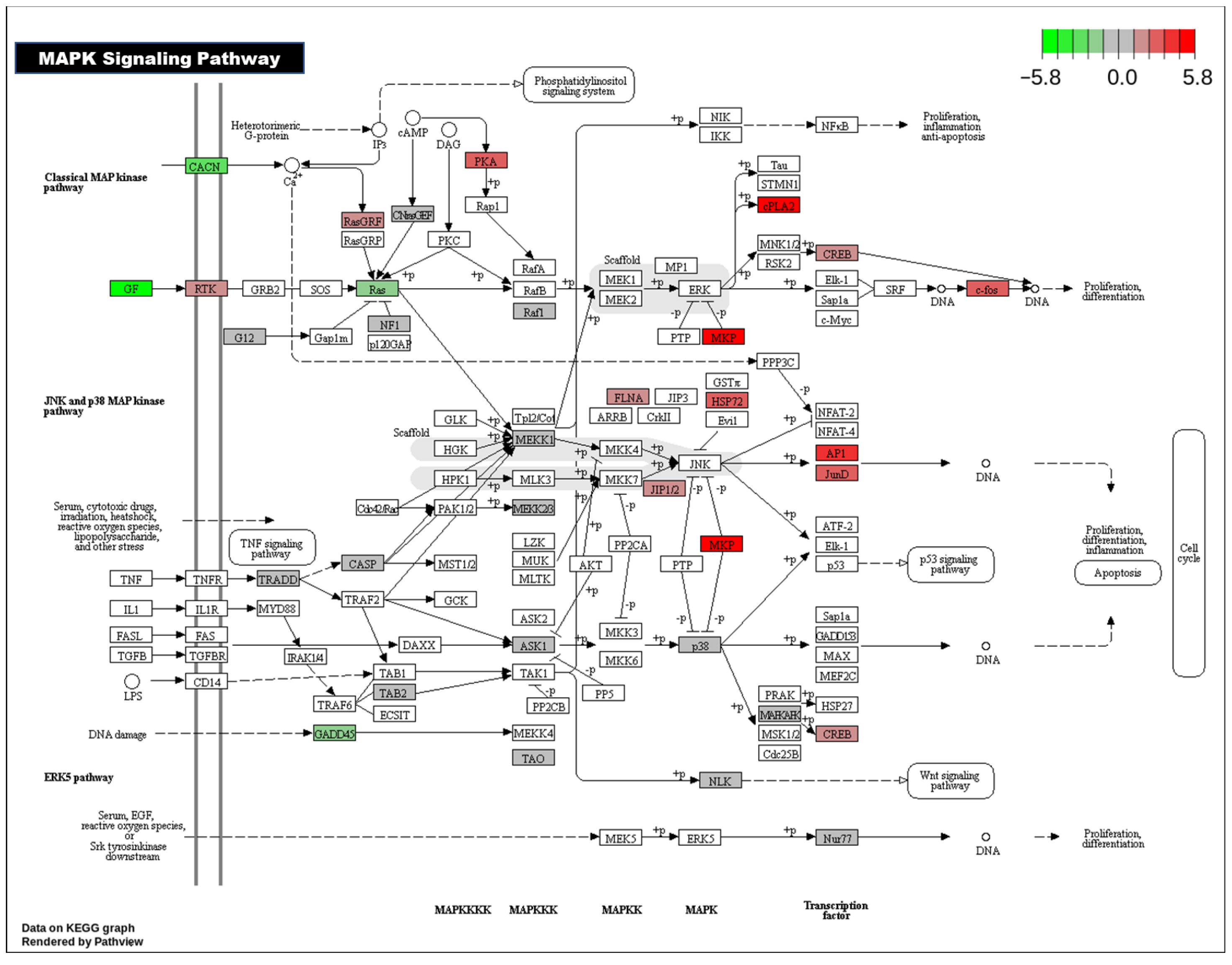
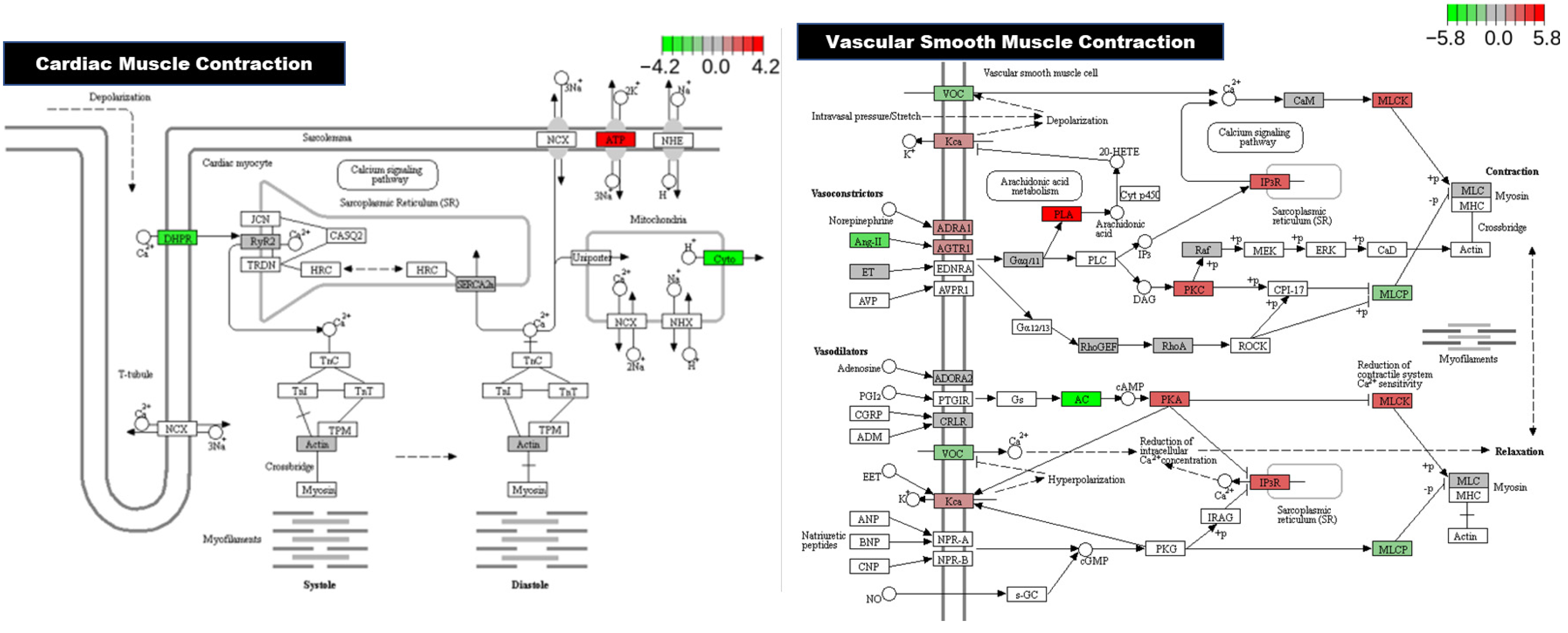
3.1.4. KEGG Pathway Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAPK | mitogen-activated protein kinase |
| hpf | hours post-fertilization |
| RNA | ribonucleic acid |
| DEGs | differentially expressed genes |
References
- Goldsborough, E., III; Osuji, N.; Blaha, M.J. Assessment of Cardiovascular Disease Risk: A 2022 Update. Endocrinol. Metab. Clin. 2022, 51, 483–509. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Campbell, N.R.C. Sodium and Cardiovascular Disease. New Engl. J. Med. 2014, 371, 2134–2139. [Google Scholar]
- Whelton, P.K.; Appel, L.J.; Sacco, R.L.; Anderson, C.A.M.; Antman, E.M.; Campbell, N.; Dunbar, S.B.; Van Horn, L.V. Sodium, Blood Pressure, and Cardiovascular Disease: Further Evidence Supporting the AHA Sodium Reduction Recommendations. Circulation 2012, 126, e86–e88. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Burnier, M.; MacGregor, G.A. Nutrition in cardiovascular disease: Salt in hypertension and heart failure. Eur. Heart J. 2011, 32, 3073–3080. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global Sodium Consumption and Death from Cardiovascular Causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef]
- Chrysant, S.G. Effects of High Salt Intake on Blood Pressure and Cardiovascular Disease: The Role of COX Inhibitors. Clin. Cardiol. 2016, 39, 240–242. [Google Scholar] [CrossRef]
- Asayama, K.; Imai, Y. The Impact of Salt Intake During and After Pregnancy. Hypertens. Res. 2018, 41, 1–5. [Google Scholar] [CrossRef]
- Oludare, G.O.; Iranloye, B.O. Implantation and Pregnancy Outcome of Sprague–Dawley Rats Fed with Low and High Salt Diet. Middle East Fertil. Soc. J. 2016, 21, 228–235. [Google Scholar] [CrossRef]
- Mao, C.; Liu, R.; Bo, L.; Chen, N.; Li, S.; Xia, S.; Chen, J.; Li, D.; Zhang, L.; Xu, Z. High-Salt Diets During Pregnancy Affected Fetal and Offspring Renal Renin—Angiotensin System. J. Endocrinol. 2013, 218, 61–73. [Google Scholar] [CrossRef]
- Ding, Y.; Lv, J.; Mao, C.; Zhang, H.; Wang, A.; Zhu, L.; Zhu, H.; Xu, Z. High-Salt Diet During Pregnancy and Angiotensin-Related Cardiac Changes. J. Hypertens. 2010, 28, 1290–1297. [Google Scholar] [CrossRef]
- Veldman, M.; Lin, S. Zebrafish as a Developmental Model Organism for Pediatric Research. Pediatr. Res. 2008, 64, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Park, C.; Lee, H.; Lugus, J.J.; Kim, S.H.; Arentson, E.; Chung, Y.S.; Gomez, G.; Kyba, M.; Lin, S.; et al. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell 2008, 2, 497–507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kudoh, T.; Tsang, M.; Hukriede, N.A.; Chen, X.; Dedekian, M.; Clarke, C.J.; Kiang, A.; Schultz, S.; Epstein, J.A.; Toyama, R.; et al. A gene expression screen in zebrafish embryogenesis. Genome Res. 2001, 11, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, A.; Nissen, R.M.; Sun, Z.; Swindell, E.C.; Farrington, S.; Hopkins, N. Identification of 315 genes essential for early zebrafish development. Proc. Natl. Acad. Sci. USA 2004, 101, 12792–12797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- White, R.J.; Collins, J.E.; Sealy, I.M.; Wali, N.; Dooley, C.M.; Digby, Z.; Stemple, D.L.; Murphy, D.N.; Billis, K.; Hourlier, T.; et al. A high-resolution mRNA expression time course of embryonic develop-ment in zebrafish. Elife 2017, 6, e30860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Parichy, D.M.; Elizondo, M.R.; Mills, M.G.; Gordon, T.N.; Engeszer, R.E. Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Dev. Dyn. 2009, 238, 2975–3015. [Google Scholar] [CrossRef]
- Ord, J. Ionic Stress Prompts Premature Hatching of Zebrafish (Danio rerio) Embryos. Fishes 2019, 4, 20. [Google Scholar] [CrossRef]
- Sawant, M.S.; Zhang, S.; Li, L. Effect of salinity on development of zebrafish, Brachydanio rerio. Curr. Sci. 2001, 81, 1347–1350. [Google Scholar]
- Micou, S.; Forner-Piquer, I.; Cresto, N.; Zassot, T.; Drouard, A.; Larbi, M.; Mangoni, M.E.; Audinat, E.; Jopling, C.; Faucherre, A.; et al. High-speed optical mapping of heart and brain voltage activities in zebrafish larvae exposed to environmental contaminants. Environ. Technol. Innov. 2023, 31, 103196. [Google Scholar]
- Seli, D.A.; Prendergast, A.; Ergun, Y.; Tyagi, A.; Taylor, H.S. High NaCl Concentrations in Water Are Associated with Developmental Abnormalities and Altered Gene Expression in Zebrafish. Int. J. Mol. Sci. 2024, 25, 4104. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Noreldin, A.E.; Batiha, G.E.; Beshbishy, A.M.; Ohran, H.; Khafaga, A.F.; Othman, S.I.; Allam, A.A.; Swelum, A.A. High Salt Diet Affects the Reproductive Health in Animals: An Overview. Animals 2020, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, X.; Ren, L.; Huang, L.; Pan, J.; Yao, J.; Du, L.; Chen, D.; Chen, J. Maternal high salt-diet increases offspring’s blood pressure with dysfunction of NO/PKGI signaling pathway in heart tissue. Gynecol. Obstet. Clin. Med. 2022, 2, 69–75. [Google Scholar]
- Baldo, M.P.; Rodrigues, S.L.; Mill, J.G. High salt intake as a multifaceted cardiovascular disease: New support from cellular and molecular evidence. Heart Fail. Rev. 2015, 20, 461–474. [Google Scholar] [CrossRef]
- Bardwell, L. Mechanisms of MAPK signalling specificity. Biochem. Soc. Trans. 2006, 34, 837–841. [Google Scholar] [CrossRef]
- Muslin, A.J. MAPK signalling in cardiovascular health and disease: Molecular mechanisms and therapeutic targets. Clin. Sci. 2008, 115, 203–218. [Google Scholar] [CrossRef]
- Segal, L.; Etzion, S.; Elyagon, S.; Shahar, M.; Klapper-Goldstein, H.; Levitas, A.; Kapiloff, M.S.; Parvari, R.; Etzion, Y. Dock10 Regulates Cardiac Function under Neurohormonal Stress. Int. J. Mol. Sci. 2022, 23, 9616. [Google Scholar] [CrossRef]
- Li, S.; Shi, Y.; Yuan, S.; Ruan, J.; Pan, H.; Ma, M.; Huang, G.; Ji, Q.; Zhong, Y.; Jiang, T. Inhibiting the MAPK pathway improves heart failure with preserved ejection fraction induced by salt-sensitive hypertension. Biomed. Pharmacother. 2024, 170, 115987. [Google Scholar] [CrossRef]
- Thompson, E.; Hensley, J.; Taylor, R.S. Effect of High Glucose on Embryological Development of Zebrafish, Brachyodanio, Rerio through Wnt Pathway. Int. J. Mol. Sci. 2024, 25, 9443. [Google Scholar] [CrossRef]
- Lammer, E.; Carr, G.J.; Wendler, K.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp Biochem. Physiol C Toxicol Pharmacol. 2009, 149, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lee, J.E.; Dorsky, R.I. Identification of Wnt-responsive cells in the zebrafish hypothalamus. Zebrafish 2009, 6, 49–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akieda, Y.; Ogamino, S.; Furuie, H.; Ishitani, S.; Akiyoshi, R.; Nogami, J.; Masuda, T.; Shimizu, N.; Ohkawa, Y.; Ishitani, T. Cell competition corrects noisy Wnt morphogen gradients to achieve robust patterning in the zebrafish embryo. Nat. Commun. 2019, 10, 4710. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panth, N.; Park, S.-H.; Kim, H.J.; Kim, D.-H.; Oak, M.-H. Protective Effect of Salicornia europaea Extracts on High Salt Intake-Induced Vascular Dysfunction and Hypertension. Int. J. Mol. Sci. 2016, 17, 1176. [Google Scholar] [CrossRef]
- Brand, A.; Visser, M.E.; Schoonees, A.; Naude, C.E. Replacing Salt with Low-Sodium Salt Substitutes (LSSS) for Cardiovascular Health in Adults, Children, and Pregnant Women. Cochrane Database Syst. Rev. 2022, 8, CD015207. [Google Scholar] [CrossRef]
- Xu, Y.X.; Zhang, S.H.; Zhang, S.Z.; Yang, M.Y.; Zhao, X.; Sun, M.Z.; Feng, X.Z. Exposure of zebrafish embryos to sodium propionate disrupts circadian behavior and 18 metabolism-related development. Ecotoxicol. Environ. Saf. 2022, 241, 113791. [Google Scholar] [CrossRef]
- Lundin, E.A.; Zhang, J.; Kramer, M.S.; Klebanoff, M.A.; Levine, R.J. Maternal Diet and Hypertension in Pregnancy Among Nulliparous Women. Am. J. Clin. Nutr. 2001, 73, 786–793. [Google Scholar]
- Varatharajan, S.; Dixit, S.D. Effects of Sodium Chloride on Zebrafish (Danio rerio) Embryonic Development and Analysis of the Zebrafish Embryo using ImageJ software. Int. Res. J. Biotechnol. 2023, 14, 4. [Google Scholar]
- Das, R.; Panigrahi, G.K. Complexities of salinity stress on zebrafish survivability. Toxicol. Int. 2025, 32, 57–63. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Jalil, J.E. Mitogen-Activated Protein Kinases as Biomarkers of Hypertension or Cardiac Pressure Overload. Hypertension 2010, 55, 23–25. [Google Scholar] [CrossRef]
- Streicher, J.M.; Ren, S.; Herschman, H.; Wang, Y. MAPK-Activated Protein Kinase-2 in Cardiac Hypertrophy and Cyclooxygenase-2 Regulation in Heart. Circ. Res. 2010, 106, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, R.; Liu, D. The Role of the MAPK Signaling Pathway in Cardiovascular Disease: Pathophysiological Mechanisms and Clinical Therapy. Int. J. Mol. Sci. 2025, 26, 2667. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Ding, Y.; Li, J.; Zhu, P.; Shih, Y.H.; Wang, M.; Zhang, Y.; Lin, X.; Xu, X. Inhibition of mTOR or MAPK ameliorates vmhcl/myh7 cardiomyopathy in zebrafish. JCI Insight 2021, 6, e154215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, P.; Zhong, T.P. MAPK/ERK signalling is required for zebrafish cardiac regeneration. Biotechnol. Lett. 2017, 39, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhong, S.; Wang, Y.; Wang, X.; Liu, Z.; Hu, G. Bmp4 in Zebrafish Enhances Antiviral Innate Immunity through p38 MAPK (Mitogen-Activated Protein Kinases) Pathway. Int. J. Mol. Sci. 2023, 24, 14444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Missinato, M.A.; Saydmohammed, M.; Zuppo, D.A.; Rao, K.S.; Opie, G.W.; Kühn, B.; Tsang, M. Dusp6 attenuates Ras/MAPK signaling to limit zebrafish heart regeneration. Development 2018, 145, dev157206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, Y.; Zhang, M.; Zheng, B.; Zhang, Y.; Chu, X.; Liu, Y.; Li, Z.; Han, X.; Chu, L. [8]-Gingerol exerts anti-myocardial ischemic effects in rats via modulation of the MAPK signaling pathway and L-type Ca2+ channels. Pharmacol. Res. Perspect. 2021, 9, e00852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, M.; Xie, X.; Chen, H.; Xiong, Q.; Tong, R.; Peng, C.; Peng, F. Aconitine induces cardiotoxicity through regulation of calcium signaling pathway in zebrafish embryos and in H9c2 cells. J. Appl. Toxicol. 2020, 40, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhou, C.; Hou, L.; Xu, K.; Zhang, Y.; Wang, X.; Li, J.; Liu, K.; Xia, Q. Narcissin induces developmental toxicity and cardiotoxicity in zebrafish embryos via Nrf2/HO-1 and calcium signaling pathways. J. Appl. Toxicol. 2024, 44, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Kong, H.; Shen, C.; She, G.; Tian, S.; Liu, H.; Cui, L.; Zhang, Y.; He, Q.; Xia, Q.; et al. Dimethyl phthalate induced cardiovascular developmental toxicity in zebrafish embryos by regulating MAPK and calcium signaling pathways. Sci. Total Environ. 2024, 926, 171902. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, S.; Miao, N. Transcriptional responses of fluxapyroxad-induced dysfunctional heart in zebrafish (Danio rerio) embryos. Environ. Sci. Pollut. Res. Int. 2022, 29, 90034–90045. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Z.; Meng, Y.; Wei, Y.; Xu, Z.; Ma, J.; Zhong, K.; Cao, Z.; Liao, X.; Lu, H. Famoxadone-cymoxanil induced cardiotoxicity in zebrafish embryos. Ecotoxicol. Environ. Saf. 2020, 205, 111339. [Google Scholar] [CrossRef] [PubMed]
- Hofsteen, P.; Robitaille, A.M.; Strash, N.; Palpant, N.; Moon, R.T.; Pabon, L.; Murry, C.E. ALPK2 Promotes Cardiogenesis in Zebrafish and Human Pluripotent Stem Cells. iScience 2018, 2, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jiang, X.; Fan, H.; Zhang, M.; Wang, Y.; Liu, J.; Li, L.; Yang, Y. Inhibition of circALPK2 Enhances Proliferation and Therapeutic Potential of Human Pluripotent Stem Cell-Derived Cardiomyocytes in Myocardial Infarction. Stem Cell Res. Ther. 2025, 16, 107. [Google Scholar] [CrossRef]
- Jeong, T.Y.; Kim, T.S.; Kim, S.D. Toxicological responses of zebrafish (Danio rerio) to high NaCl concentrations: Influence on embryonic development. Chemosphere 2013, 90, 958–964. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Liu, L.; Xu, Y.; Sun, P.; Li, H. Sodium chloride-induced developmental toxicity and cardiac effects in zebrafish embryos. Environ. Toxicol. Pharmacol. 2020, 80, 103474. [Google Scholar] [CrossRef]
- Guh, Y.J.; Lin, C.H.; Hwang, P.P. Osmoregulation in zebrafish: Ion transport mechanisms and functional regulation. EXCLI J. 2015, 14, 627–659. [Google Scholar] [CrossRef]
| Gene | Biological Process | Molecular Function |
|---|---|---|
| mgea5 | Glycoprotein metabolic process | Enables beta-N-acetylglucosaminidase activity |
| arrdc3b | Protein transport | Arrestin domain involved in protein transport |
| ppdpfb | Cell differentiation in nervous system, pancreatic system, and pronephric duct | Pancreatic progenitor cell differentiation and proliferation factor b |
| slc16a9b | Solute carrier, integral component of plasma membrane | Monocarboxylic acid transmembrane transport |
| slc16a6b | Solute carrier, integral component of plasma membrane | Monocarboxylic acid transmembrane transport |
| h1f0 | Negative regulation of DNA recombination and nucleosome positioning, chromosome condensation | Enable double stranded DNA binding activity and nucleosomal DNA binding activity |
| h1fx | Chromosome condensation, negative regulation of DNA recombination and nucleosome positioning | Enable double stranded DNA binding activity and nucleosomal DNA binding activity |
| histh1l | Chromosome condensation, negative regulation of DNA recombination and nucleosome positioning | Enable double stranded DNA binding activity and nucleosomal DNA binding activity |
| ino80e | Brain development | Ino80 complex component located in the nucleus |
| gpcpd1 | Glycerophospholipid catabolic process, lipid metabolism | Glyceophosphocholine and phosphodiesterase activity |
| Gene | Biological Process | Molecular Function |
|---|---|---|
| si:ch211-153b23.3 | Regulation of translational fidelity | Nucleotidyltransferase activity and tRNA binding activity |
| irg1l | Immunoresponse; inflammatory response to wounding and bacterium expressed in cloaca, liver, oral region, and pharynx | Aconitate decarboxylase activity in mitochondria |
| SLC6A6 (2 of 2) | Taurine transport component in plasma membrane | Taurine/sodium symporter activity |
| slc38a2 | Amino acid transmembrane transport and sodium ion transport | L-glutamine transmembrane transporter activity |
| si:dkey-162h11.3 | Protein coding in nucleoplasm | Involved in protein coding |
| slc6a6a | Taurine transport component in plasma membrane | Taurine/sodium symporter activity |
| si:ch211-153b23.4 | Apoptotic process | Intracellular signal transduction |
| slc22a16 | Organic cation transport, transmembrane transport | Organic cation transmembrane transporter activity |
| plekhs1 | Protein coding, mild elevation of blood glucose levels and insulin resistance | Protein coding activity |
| alpk2 | Epicardium morphogenesis, heart development, negative regulation of Wnt signaling pathway | ATP binding activity, protein serine kinase activity, protein serine/threonine kinase activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, E.; Hensley, J.; Taylor, R.S. High-Salt Exposure Disrupts Cardiovascular Development in Zebrafish Embryos, Brachyodanio rerio, via Calcium and MAPK Signaling Pathways. J 2025, 8, 26. https://doi.org/10.3390/j8030026
Thompson E, Hensley J, Taylor RS. High-Salt Exposure Disrupts Cardiovascular Development in Zebrafish Embryos, Brachyodanio rerio, via Calcium and MAPK Signaling Pathways. J. 2025; 8(3):26. https://doi.org/10.3390/j8030026
Chicago/Turabian StyleThompson, Ebony, Justin Hensley, and Renfang Song Taylor. 2025. "High-Salt Exposure Disrupts Cardiovascular Development in Zebrafish Embryos, Brachyodanio rerio, via Calcium and MAPK Signaling Pathways" J 8, no. 3: 26. https://doi.org/10.3390/j8030026
APA StyleThompson, E., Hensley, J., & Taylor, R. S. (2025). High-Salt Exposure Disrupts Cardiovascular Development in Zebrafish Embryos, Brachyodanio rerio, via Calcium and MAPK Signaling Pathways. J, 8(3), 26. https://doi.org/10.3390/j8030026





