Challenges and Advancements in All-Solid-State Battery Technology for Electric Vehicles
Abstract
1. Introduction
2. Overview of All-Solid-State Batteries
2.1. Properties of All-Solid-State Electrolyte Lithium-Ion Batteries
2.2. Types of Solid-State Electrolytes
3. Challenges in Integrating All-Solid-State Batteries
3.1. Manufacturing Processes
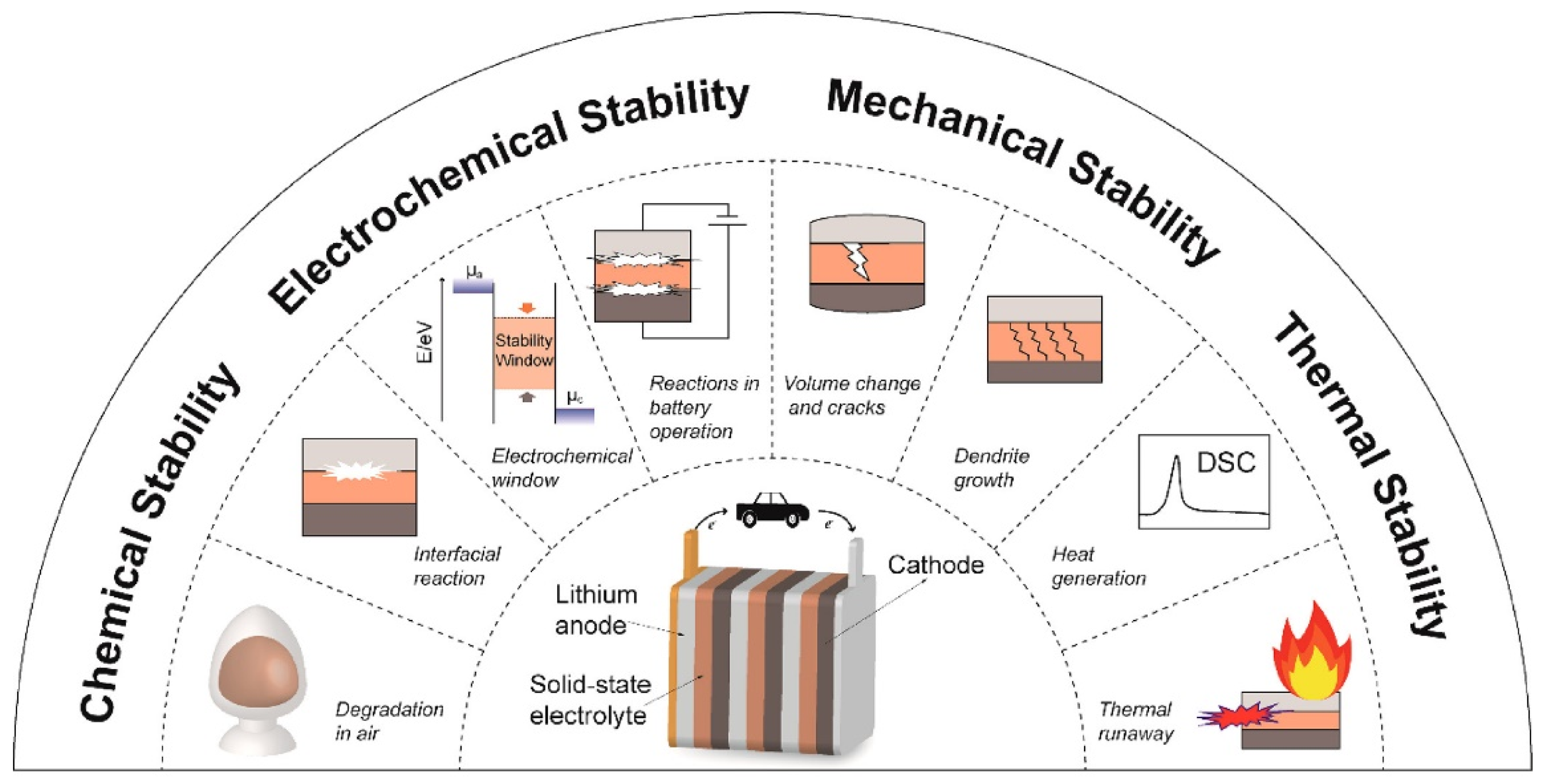
3.2. Stability
3.3. Safety Considerations
3.4. Cost and Scalability
3.5. Energy Density
4. Recent Advances
4.1. Inorganic Solid Electrolytes
4.2. Solid Polymer Electrolytes
4.3. Composite Solid Electrolytes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchie, A.G. Recent developments and likely advances in lithium rechargeable batteries. J. Power Sources 2004, 136, 285–289. [Google Scholar] [CrossRef]
- Chen, R.; Nolan, A.M.; Lu, J.; Wang, J.; Yu, X.; Mo, Y.; Chen, L.; Huang, X.; Li, H. The Thermal Stability of Lithium Solid Electrolytes with Metallic Lithium. Joule 2020, 4, 812–821. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Hu, J.; Zhang, C.; Zhang, L.; Wang, Y.; Zhang, W. Fault Diagnosis Method of Lithium-Ion Battery Leakage Based on Electrochemical Impedance Spectroscopy. IEEE Trans. Ind. Appl. 2024, 60, 1879–1889. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Chai, J.; Liu, Z.; Ma, J.; Wang, J.; Liu, X.; Liu, H.; Zhang, J.; Cui, G.; Chen, L. In Situ Generation of Poly (Vinylene Carbonate) Based Solid Electrolyte with Interfacial Stability for LiCoO2 Lithium Batteries. Adv. Sci. 2017, 4, 1600377. [Google Scholar] [CrossRef]
- Chen, S.; Wen, K.; Fan, J.; Bando, Y.; Golberg, D. Progress and future prospects of high-voltage and high-safety electrolytes in advanced lithium batteries: From liquid to solid electrolytes. J. Mater. Chem. A 2018, 6, 11631–11663. [Google Scholar] [CrossRef]
- Yoon, Y.; Park, C.; Kim, J.; Shin, D. Lattice orientation control of lithium cobalt oxide cathode film for all-solid-state thin film batteries. J. Power Sources 2013, 226, 186–190. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Li, X.; Zhao, J.; Liu, G.; Yu, W.; Dong, X.; Wang, J. Review on composite solid electrolytes for solid-state lithium-ion batteries. Mater. Today Sustain. 2023, 21, 100316. [Google Scholar] [CrossRef]
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A review of lithium and non-lithium based solid state batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar] [CrossRef]
- Lou, S.; Zhang, F.; Fu, C.; Chen, M.; Ma, Y.; Yin, G.; Wang, J. Interface issues and challenges in all-solid-state batteries: Lithium, sodium, and beyond. Adv. Mater. 2021, 33, 2000721. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Yu, X.; Chen, L.; Li, H. Approaching Practically Accessible Solid-State Batteries: Stability Issues Related to Solid Electrolytes and Interfaces. Chem. Rev. 2020, 120, 6820–6877. [Google Scholar] [CrossRef]
- Wang, S.; Liao, Y.; Li, S.; Cui, C.; Liang, J.; Du, G.; Tong, Z.; Yuan, L.; Zhai, T.; Li, H. Ultrathin All-Inorganic Halide Solid-State Electrolyte Membranes for All-Solid-State Li-Ion Batteries. Adv. Energy Mater. 2024, 14, 2303641. [Google Scholar] [CrossRef]
- Li, J.; Gong, Z.; Xie, W.; Yu, S.; Wei, Y.; Li, D.; Yang, L.; Chen, D.; Li, Y.; Chen, Y. Growth Process and Removal of Interface Contaminants for Garnet-Based Solid-State Lithium Metal Batteries. ACS Appl. Energy Mater. 2023, 6, 12432–12441. [Google Scholar] [CrossRef]
- Liu, D.; Lu, Z.; Lin, Z.; Zhang, C.; Dai, K.; Wei, W. Organoboron- and Cyano-Grafted Solid Polymer Electrolytes Boost the Cyclability and Safety of High-Voltage Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2023, 15, 21112–21122. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, Y.; Zhao, Z.; Shi, X.; Zhao, H.; Cheng, K.; Liu, J.; Li, L. Completely Amorphous Poly(ethylene oxide)-Based Electrolyte Enables High Ionic Conductivity for Room-Temperature All-Solid-State Lithium Metal Batteries. ACS Appl. Energy Mater. 2023, 6, 12343–12352. [Google Scholar] [CrossRef]
- Guo, S.; Kou, W.; Wu, W.; Lv, R.; Yang, Z.; Wang, J. Thin laminar inorganic solid electrolyte with high ionic conductance towards high-performance all-solid-state lithium battery. Chem. Eng. J. 2022, 427, 131948. [Google Scholar] [CrossRef]
- Xu, X.; Du, G.; Cui, C.; Liang, J.; Zeng, C.; Wang, S.; Ma, Y.; Li, H. Stabilizing the Halide Solid Electrolyte to Lithium by a β-Li3N Interfacial Layer. ACS Appl. Mater. Interfaces 2022, 14, 39951–39958. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, S.; Chen, X.; Yang, W.; Yao, X.; Hu, X.; Han, Q.; Wang, H. Tape-Casting Li0.34La0.56TiO3 Ceramic Electrolyte Films Permit High Energy Density of Lithium-Metal Batteries. Adv. Mater. 2020, 32, 1906221. [Google Scholar] [CrossRef]
- Li, C.; Zhang, M.; Li, P.; Ren, H.-R.; Wu, X.; Piao, Z.; Xiao, X.; Zhang, M.; Liang, X.; Wu, X.; et al. Self-Assembly of Ultrathin, Ultrastrong Layered Membranes by Protic Solvent Penetration. J. Am. Chem. Soc. 2024, 146, 3553–3563. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, H.; Wang, S.; Song, X.; Yao, X.; Wang, H. Perovskite Membranes with Vertically Aligned Microchannels for All-Solid-State Lithium Batteries. Adv. Energy Mater. 2018, 8, 1801433. [Google Scholar] [CrossRef]
- Koerver, R.; Aygün, I.; Leichtweiß, T.; Dietrich, C.; Zhang, W.; Binder, J.O.; Hartmann, P.; Zeier, W.G.; Janek, J. Capacity Fade in Solid-State Batteries: Interphase Formation and Chemomechanical Processes in Nickel-Rich Layered Oxide Cathodes and Lithium Thiophosphate Solid Electrolytes. Chem. Mater. 2017, 29, 5574–5582. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Bubulinca, C.; Kazantseva, N.E.; Pechancova, V.; Joseph, N.; Fei, H.; Venher, M.; Ivanichenko, A.; Saha, P. Development of All-Solid-State Li-Ion Batteries: From Key Technical Areas to Commercial Use. Batteries 2023, 9, 157. [Google Scholar] [CrossRef]
- Rui, X.; Ren, D.; Liu, X.; Wang, X.; Wang, K.; Lu, Y.; Li, L.; Wang, P.; Zhu, G.; Mao, Y.; et al. Distinct thermal runaway mechanisms of sulfide-based all-solid-state batteries. Energy Environ. Sci. 2023, 16, 3552–3563. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R Rep. 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Bo, S.H.; Kim, J.C.; Miara, L.J.; Ceder, G. Understanding interface stability in solid-state batteries. Nat. Rev. Mater. 2020, 5, 105–126. [Google Scholar] [CrossRef]
- Yang, R.; Xie, Y.; Li, K.; Li, W.; Hu, X.; Fan, Y.; Zhang, Y. Thermal characteristics of solid-state battery and its thermal management system based on flat heat pipe. Appl. Therm. Eng. 2024, 123575. [Google Scholar] [CrossRef]
- Bai, F.; Kakimoto, K.; Shang, X.; Mori, D.; Taminato, S.; Matsumoto, M.; Takeda, Y.; Yamamoto, O.; Izumi, H.; Minami, H.; et al. Water-Stable High Lithium-Ion Conducting Solid Electrolyte of Li1.4Al0.4Ge0.2Ti1.4(PO4)3–LiCl for Aqueous Lithium-Air Batteries. Front. Energy Res. 2020, 8, 00187. [Google Scholar] [CrossRef]
- Cui, G. Reasonable design of high-energy-density solid-state lithium-metal batteries. Matter 2020, 2, 805–815. [Google Scholar] [CrossRef]
- Li, X.; Deng, Y.; Li, K.; Yang, Z.; Hu, X.; Liu, Y.; Zhang, Z. Advancements in Performance Optimization of Electrospun Polyethylene Oxide-Based Solid-State Electrolytes for Lithium-Ion Batteries. Polymers 2023, 15, 3727. [Google Scholar] [CrossRef] [PubMed]
- Glynos, E.; Pantazidis, C.; Sakellariou, G. Designing all-polymer nanostructured solid electrolytes: Advances and prospects. ACS Omega 2020, 5, 2531–2540. [Google Scholar] [CrossRef]
- Jamil, S.; Wang, G.; Fasehullah, M.; Xu, M. Challenges and prospects of nickel-rich layered oxide cathode material. J. Alloys Compd. 2022, 909, 164727. [Google Scholar] [CrossRef]
- Zaman, W.; Hatzell, K.B. Processing and manufacturing of next generation lithium-based all solid-state batteries. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101003. [Google Scholar] [CrossRef]
- Ren, D.; Lu, L.; Hua, R.; Zhu, G.; Liu, X.; Mao, Y.; Rui, X.; Wang, S.; Zhao, B.; Cui, H.; et al. Challenges and opportunities of practical sulfide-based all-solid-state batteries. Etransportation 2023, 18, 100272. [Google Scholar] [CrossRef]
- Masquelier, C.; Croguennec, L. Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode Materials for Rechargeable Li (or Na) Batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.-R.; Long, T.; Ma, Q.; Ning, P.; Dong, X.-R.; Zhou, C.-S.; Wu, X.-W.; Zeng, X.-X. Borosilicate Glass-Enabled Antifracture NASICON Solid Electrolytes for Lithium-Metal Batteries. ACS Appl. Energy Mater. 2022, 5, 3734–3740. [Google Scholar] [CrossRef]
- Yang, X.; Tang, S.; Zheng, C.; Ren, F.; Huang, Y.; Fei, X.; Yang, W.; Pan, S.; Gong, Z.; Yang, Y. From Contaminated to Highly Lithiated Interfaces: A Versatile Modification Strategy for Garnet Solid Electrolytes. Adv. Funct. Mater. 2023, 33, 2209120. [Google Scholar] [CrossRef]
- Jia, M.; Zhao, N.; Huo, H.; Guo, X. Comprehensive Investigation into Garnet Electrolytes Toward Application-Oriented Solid Lithium Batteries. Electrochem. Energy Rev. 2020, 3, 656–689. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, L.; Tang, J.; Liu, X.; Liu, J.; Tang, M.; Wang, Z. Large-scale preparation of ultrathin composite polymer electrolytes with excellent mechanical properties and high thermal stability for solid-state lithium-metal batteries. Energy Storage Mater. 2023, 55, 847–856. [Google Scholar] [CrossRef]
- Fan, L.-Z.; He, H.; Nan, C.-W. Tailoring inorganic–polymer composites for the mass production of solid-state batteries. Nat. Rev. Mater. 2021, 6, 1003–1019. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Polizos, G.; Goswami, M.; Keum, J.K.; He, L.; Jafta, C.J.; Sharma, J.; Wang, Y.; Kearney, L.T.; Tao, R.; Li, J. Nanoscale Ion Transport Enhances Conductivity in Solid Polymer-Ceramic Lithium Electrolytes. ACS Nano 2024, 18, 2750–2762. [Google Scholar] [CrossRef] [PubMed]
| Feature | Traditional Lithium-Ion | All-Solid-State Lithium-Ion |
|---|---|---|
| Energy Density | Moderate to high | Potentially higher |
| Safety | Risk of leakage, flammability | More stable, less risk of leakage |
| Charging Speed | Moderate | Higher impedance, but potentially faster with advancement |
| Temperature Range | Limited, can degrade at high temperatures | Wider, more stable at high temperatures |
| Lifecycle | Moderate (500–1500 cycles) | Longer (Potentially 2–10× Li-Ion) |
| Manufacturing Complexity | Mature technology, well-established | Emerging, still under development |
| Cost | Relatively lower | Higher, but expected to decrease |
| Form Factor | Limited Flexibility | More design flexibility |
| Commercial Availability | Widely available | Limited, mostly in development |
| Feature | Inorganic Solid Electrolytes | Solid Polymer Electrolytes | Composite Solid Electrolytes |
|---|---|---|---|
| Electrolyte Material | Ceramic | Polymers | Combination of Ceramics and Polymers |
| Ionic Conductivity | High | Moderate | Values are potentially between those of inorganic solid electrolytes and solid polymer electrolytes based on composition |
| Electronic Conductivity | Low | Moderate | |
| Thermal Stability | Excellent | Moderate | |
| Chemical Stability | Excellent | Moderate | |
| Electrochemical Window | Wide | Narrow | |
| Durability | Brittle | Flexible | |
| Cost | Expensive | Moderate |
| Inorganic Solid Electrolyte Description | Key Findings | Impact on ASSBs |
|---|---|---|
| Sulfide-based SEs | Improved safety and high energy density; challenges include air instability, limited electrochemical stability, and interfacial failures | Strategies like H2S absorbents, element substitution, and sulfide-polymer composites improve stability; buffer layers recommended to mitigate instability; optimized microstructural design needed |
| LLTO (Li0.34La0.56TiO3) | Freestanding ceramic electrolyte film with 25 μm thickness reduces internal resistance | Enhances energy density, power output, and charging rates; suitable for high-performance applications like EVs |
| ZrO2 nanowires & Li3InCl6 | Ultrathin membranes (25 μm) created using solution-infusion method | Reduces internal resistance, increases energy density by up to 300%; ensures thermal stability and safety |
| NASICON with borosilicate glass (BG) | Enhanced mechanical properties and density; fracture strength 74 MPa, relative density 97.17% | Excellent cycling stability with LiFePO4 cathodes and Li anodes; discharge specific capacity of 154.5 mAh g−1, near 100% Coulombic efficiency after 100 cycles |
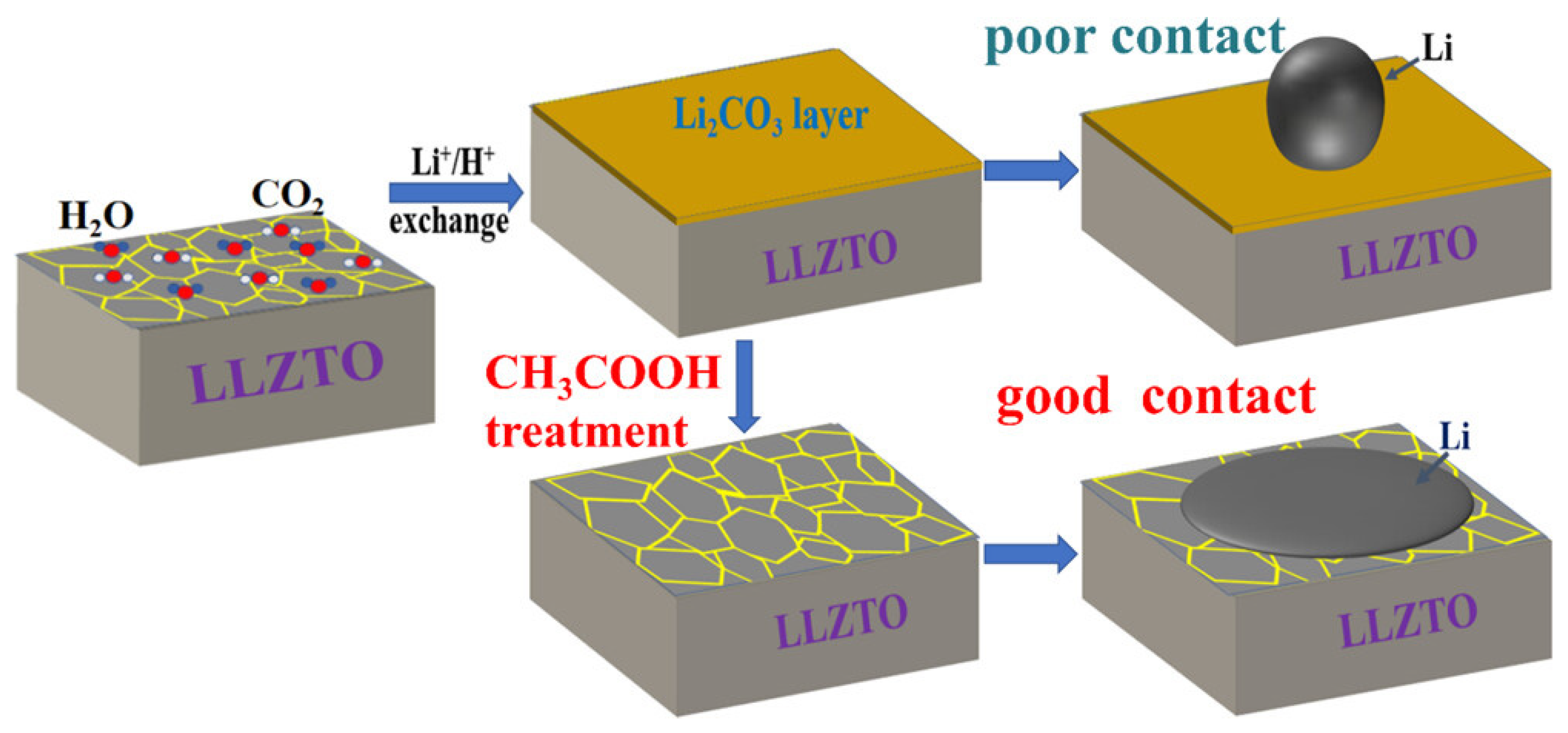
| Garnet-based SEs | High energy density and safety, but issues with Li2CO3 contaminants | Innovative approach using acetic acid (CH3COOH) to remove contaminants, reducing interface resistance from 5542 to 5 Ω cm2 |
| LLZO nanosheets | Thin, defect-free, freestanding LLZO laminar electrolytes with ultrahigh ionic conductance | High energy density (340 Wh kg−1), compressive strength (3.2 GPa); excellent cycling stability, discharge specific capacity of 143.2 mAh g−1 after 200 cycles |
| Solid Polymer Electrolyte Description | Key Findings | Impact on ASSBs |
|---|---|---|
| GP-LiF-SN solid electrolyte | Combines amorphous poly(ethylene oxide) with glass fiber reinforcement, nano-LiF, and succinonitrile (SN) additives; SN contributes to mechanical robustness, safety, and ionic conductivity | Enhanced mechanical stability and electrochemical performance; improved interfacial contact between electrolytes and electrodes; promising for practical ASSB applications |
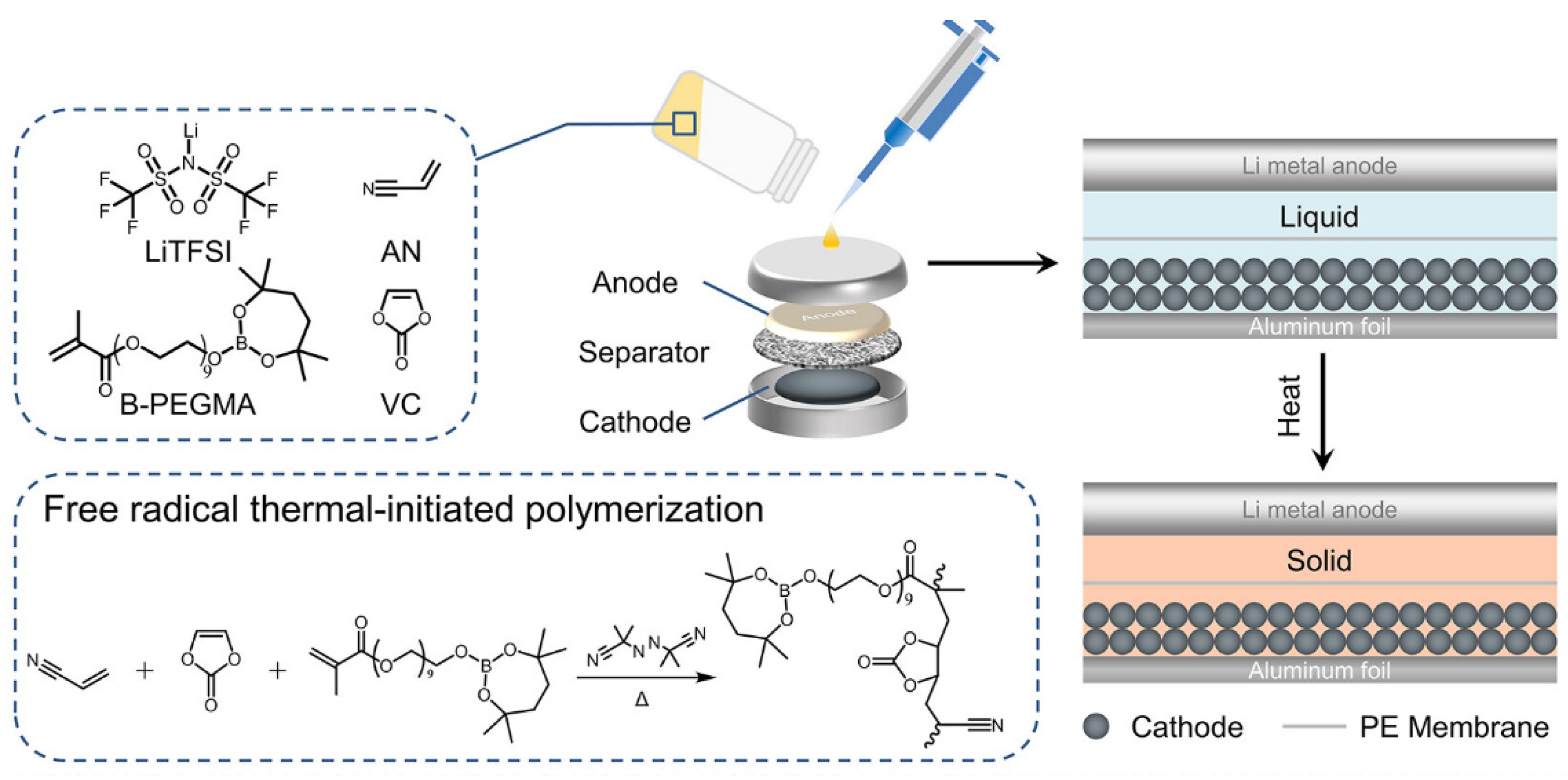
| PVNB solid polymer electrolyte | Developed through in situ polymerization via thermally initiated free-radical reaction; boron and cyano groups immobilize anion, forming stable interfaces | Improved cycling stability and flame-retardant properties; cells maintain 80% capacity after 1000 cycles at 5C; stable interfaces prevent electrolyte decomposition |
| Ultrathin solid polymer membranes | Protic solvent-penetration induced self-assembly technique produces membranes below 5 μm with tensile strength of 556.6 MPa; thinnest SSE at 3.4 μm thickness | Reduced internal resistance and increased ionic conductivity; high mechanical strength; allows Li-S batteries to cycle over a thousand times at a high rate of 1C |
| Composite Electrolyte Description | Key Findings | Impact on ASSBs |
|---|---|---|
| Ultrathin, flexible composite polymer electrolyte (PLP-HP) | Combined scraping and hot-pressing process; PEO/LiTFSI electrolyte impregnated into a PTFE matrix; thicknesses as low as 6 μm | Significantly reduced internal resistance; enhanced battery performance; stable cycling for over 900 h at 60 °C without lithium dendrite growth; LiFePO4/Li full cell cycles over 500 times with high Coulombic efficiency |
| Flexible, solvent-free polymer electrolytes | Incorporating lithium salts into polymer matrix with garnet-type ceramic electrolytes; higher Li+ conductivity while ensuring stability | Enhanced lithium-ion transport and electrochemical stability; crucial for high-performance batteries; improved understanding of ion transport mechanisms |
| Hybrid polymer-ceramic electrolytes (e.g., LLZO in polymer matrices) | Established correlations among composite structures, polymer dynamics, and lithium-ion transport; integration addresses brittleness and processing difficulties | High ionic conductivity and mechanical flexibility; addresses brittleness and processing difficulties; key for high-performance and commercial applications of ASSBs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, R.; Mittal, V.; Precilla, A.M. Challenges and Advancements in All-Solid-State Battery Technology for Electric Vehicles. J 2024, 7, 204-217. https://doi.org/10.3390/j7030012
Shah R, Mittal V, Precilla AM. Challenges and Advancements in All-Solid-State Battery Technology for Electric Vehicles. J. 2024; 7(3):204-217. https://doi.org/10.3390/j7030012
Chicago/Turabian StyleShah, Rajesh, Vikram Mittal, and Angelina Mae Precilla. 2024. "Challenges and Advancements in All-Solid-State Battery Technology for Electric Vehicles" J 7, no. 3: 204-217. https://doi.org/10.3390/j7030012
APA StyleShah, R., Mittal, V., & Precilla, A. M. (2024). Challenges and Advancements in All-Solid-State Battery Technology for Electric Vehicles. J, 7(3), 204-217. https://doi.org/10.3390/j7030012








