Photocatalysis and Li-Ion Battery Applications of {001} Faceted Anatase TiO2-Based Composites
Abstract
:1. Introduction
2. Mechanism of Photoactivity Enhancement by {001} Facets in TiO2
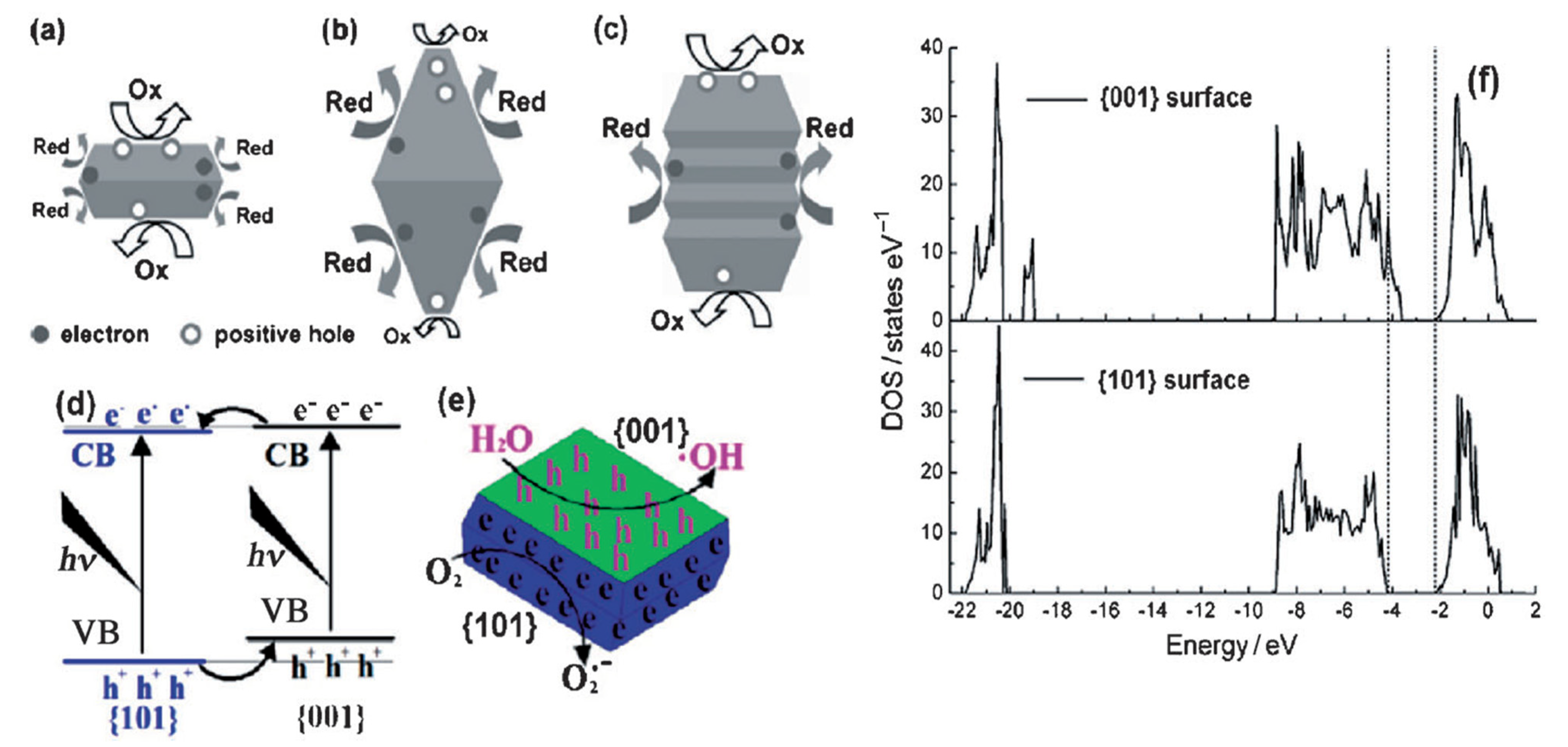
2.1. Properties of TiO2-001 Surfaces for Photocatalysis
2.1.1. Dissociative Adsorption
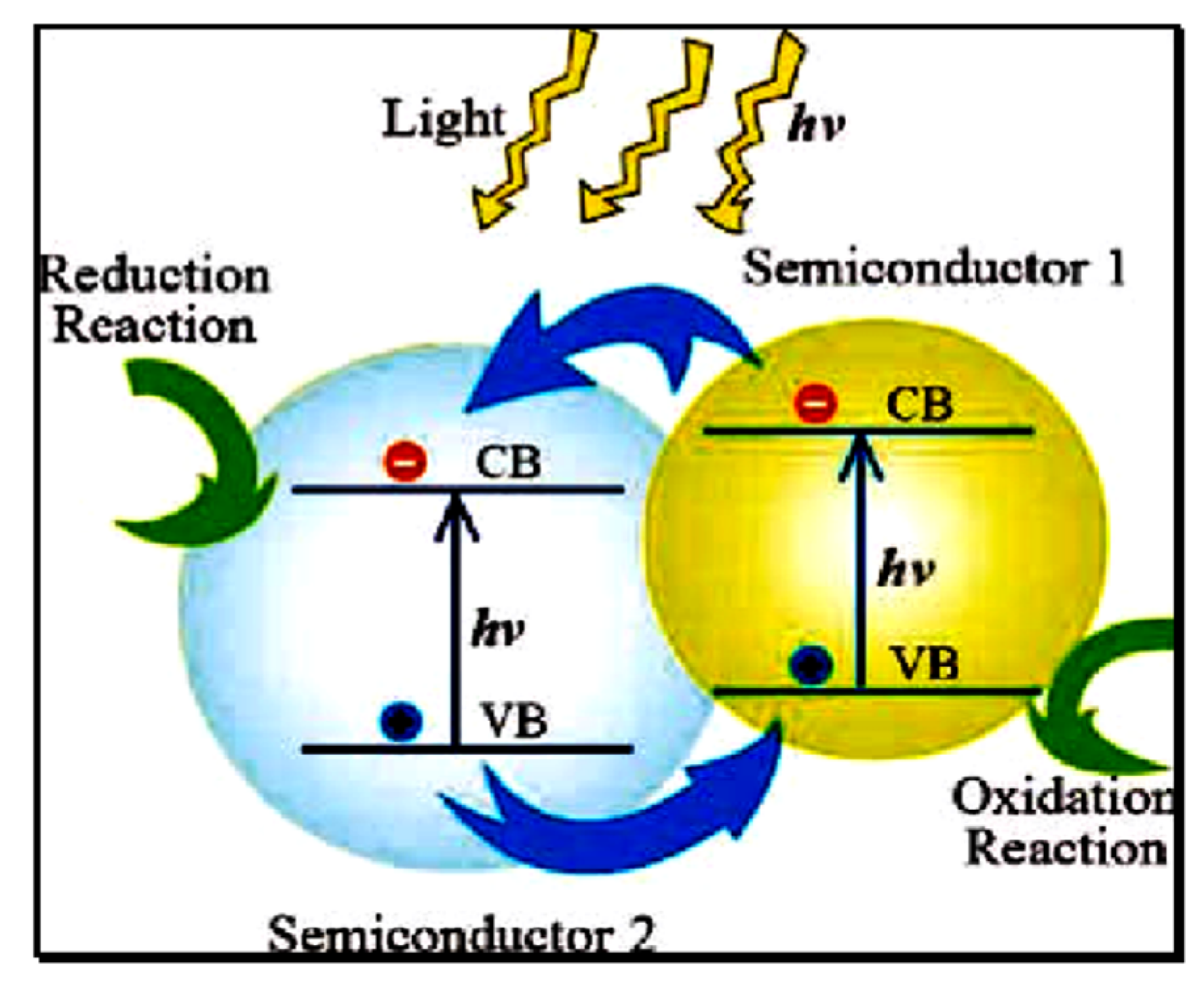
2.1.2. Charge Separation
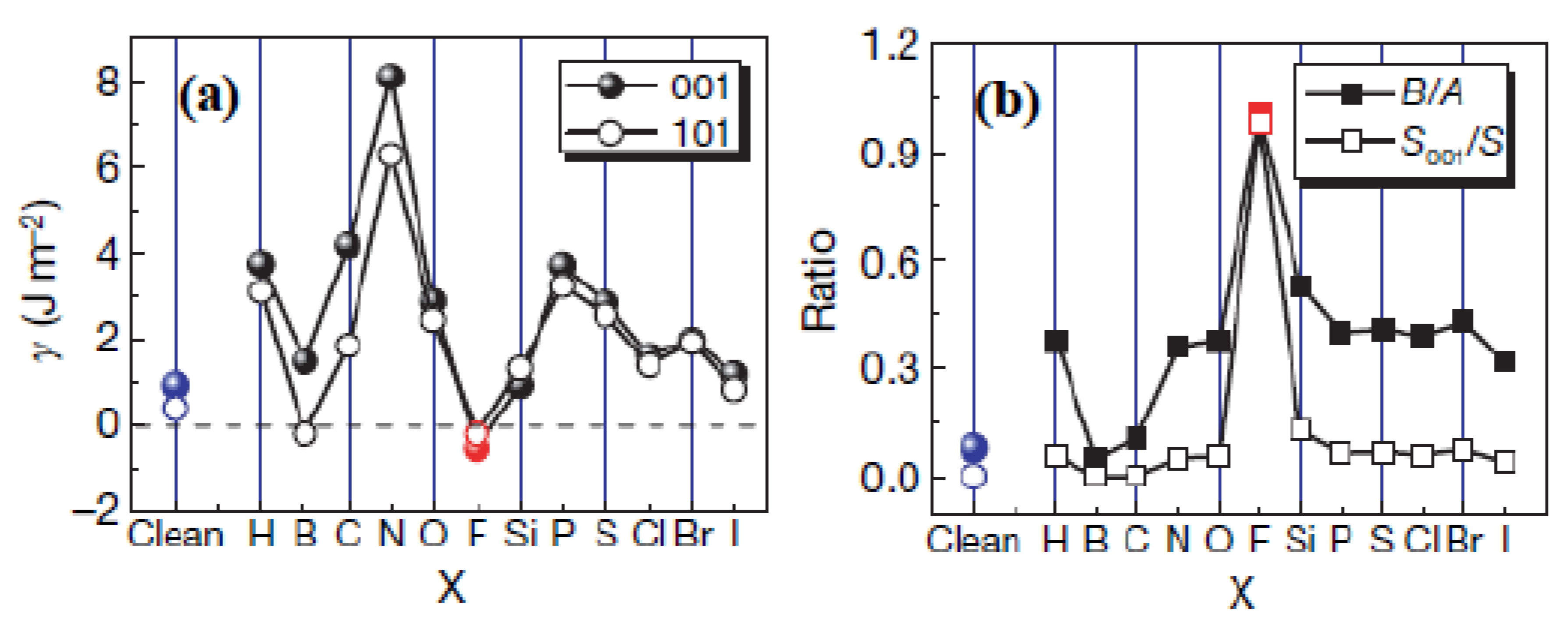
2.1.3. Optical Properties
2.1.4. Photocatalytic Selectivity
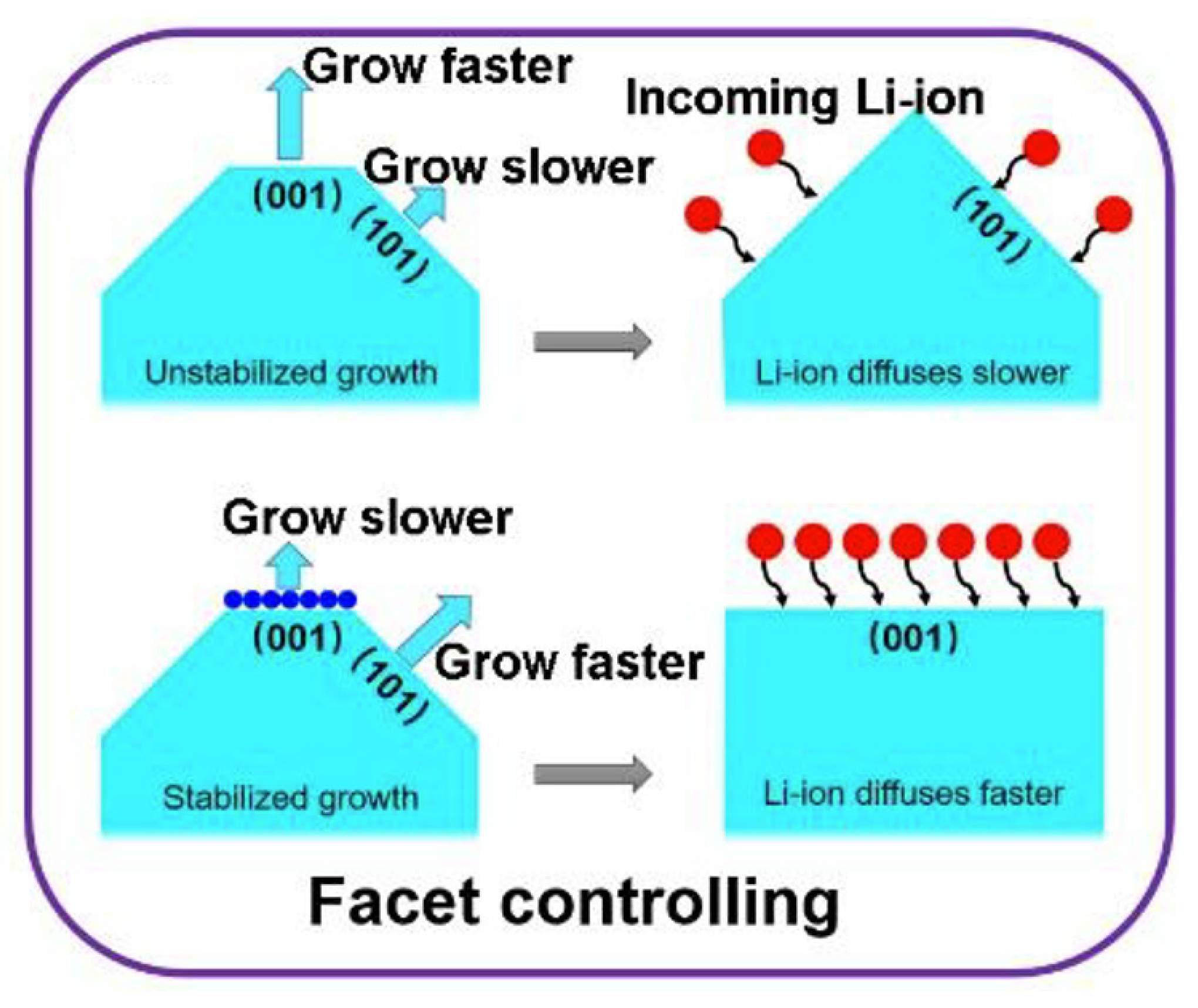
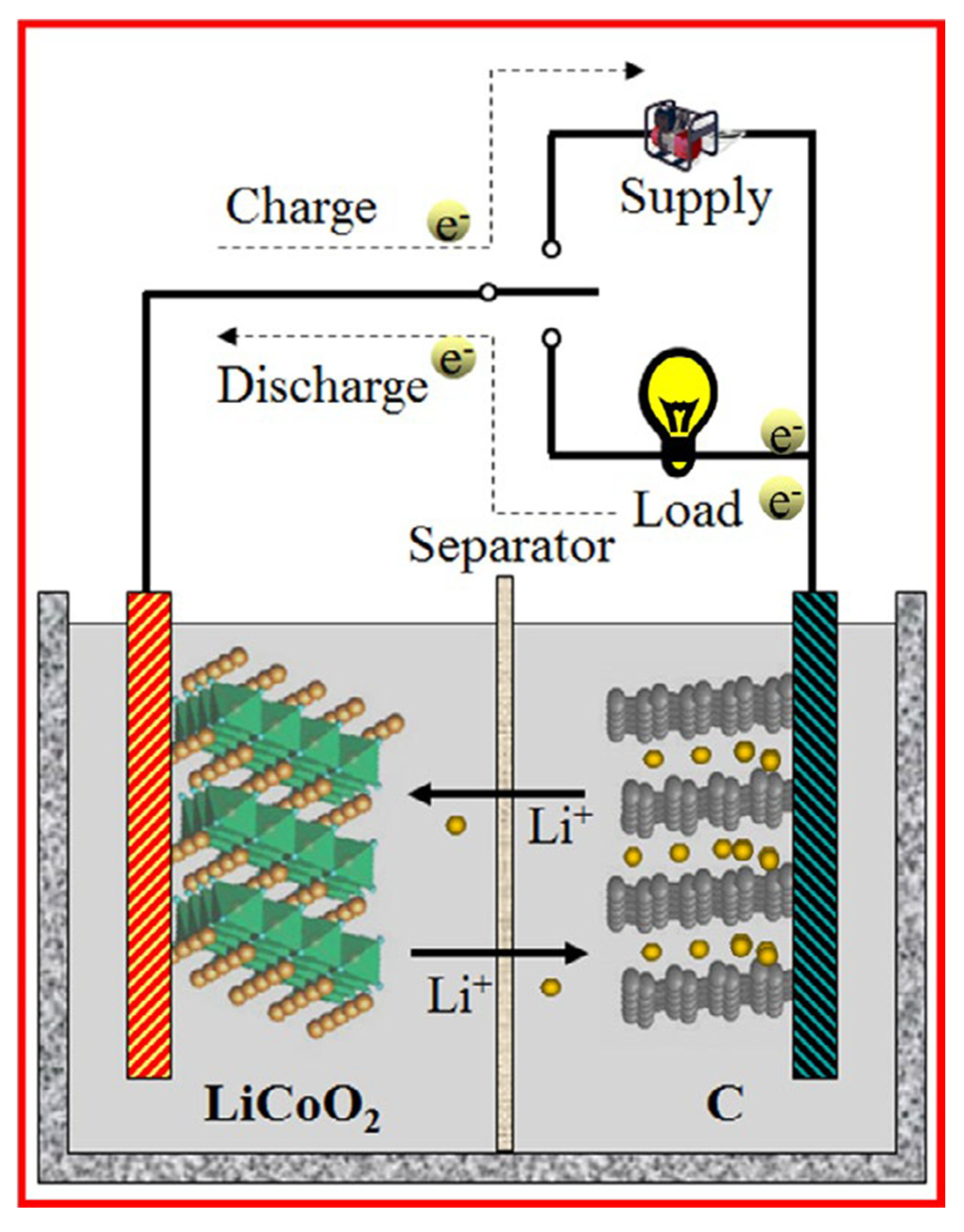
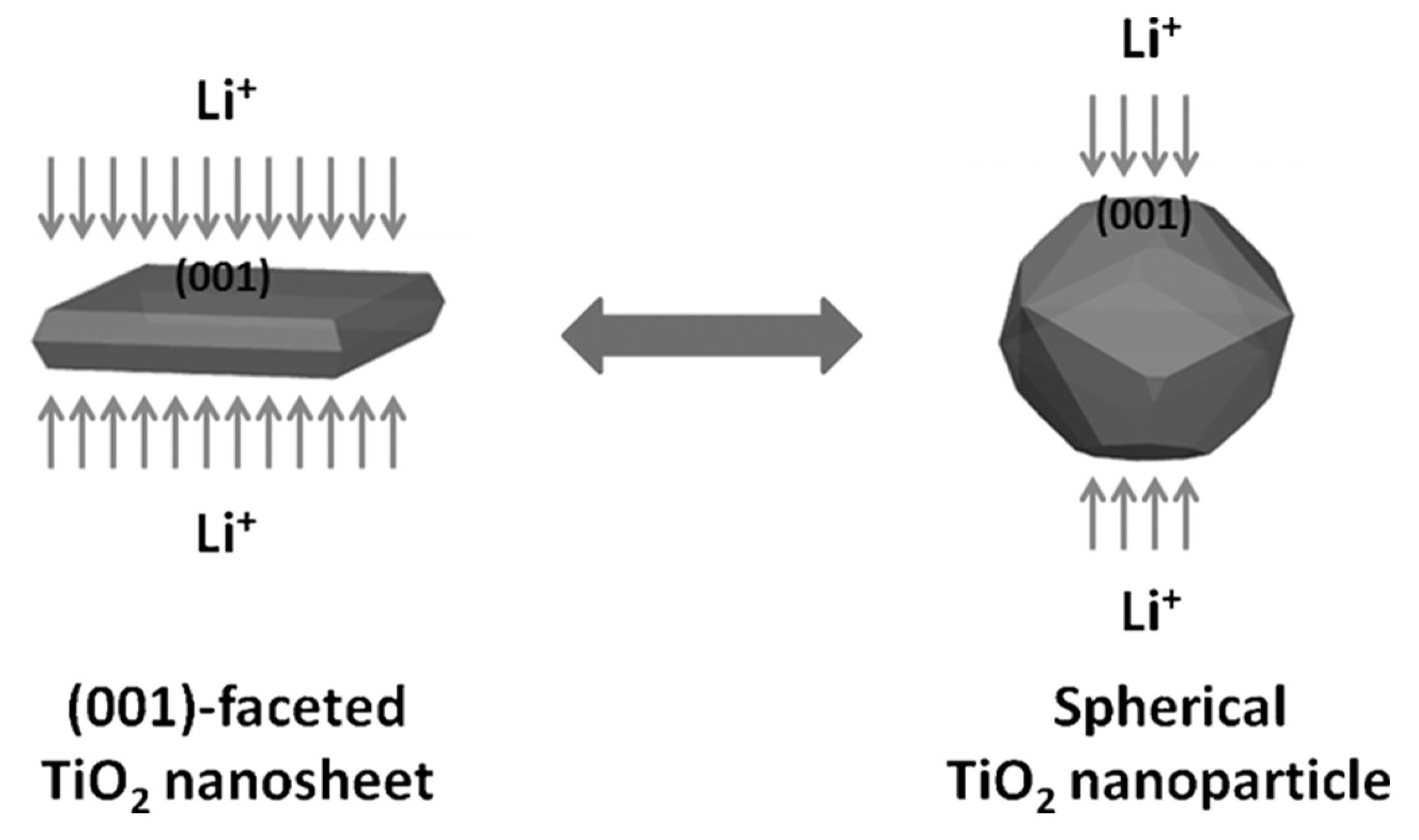
2.2. Properties of TiO2-001 Surfaces for Li-ion Battery
2.3. Methods to Modify the Properties of TiO2-001 Surfaces
2.3.1. Doping
- (a)
- Band gap narrowing: Dozzi et al. [72] found N 2p state hybrids with O 2p states in anatase TiO2 doped with nitrogen because their energies are very close, and thus the band gap of N-TiO2 is narrowed and able to absorb visible light.
- (b)
- Impurity energy level: Yalda et al. [73] stated that TiO2 oxygen sites substituted by nitrogen atom form isolated impurity energy levels above the valence ban irradiation and UV light excites electrons in both the VB and the impurity energy levels, but illumination with visible light only excites electrons in the impurity energy level.
- (c)
- Oxygen vacancies: Gonzalez-Torres et al. [74] concluded that oxygen-deficient sites formed in the grain boundaries are important for the emergence of vis-activity and nitrogen doped into oxygen-deficient sites are important as a blocker for reoxidation.
2.3.2. Coupling
- (a)
- High surface area: High specific surface area with many active sites on the surface to boost photocatalytic reactions as compared to their bulk counterpart.
- (b)
- π-conjugated structures: This leads to fast electron transfer and promote the separation of electron−hole pairs on the photocatalyst surface.
- (c)
- Excellent support matrix: Carbon-based materials can form efficient heterojunctiond by having intimate contact between them and TiO2-001 materials, such as point-to-face contact (0 D) for carbon/graphene quantum dots, line-to-face contact for carbon nanotubes (1 D) and face-to-face contact for graphene (2 D) as presented in Figure 7. This is more beneficial for the rapid charge transfer and better catalytic dispersion to enhance the photocatalytic activity [84,85].
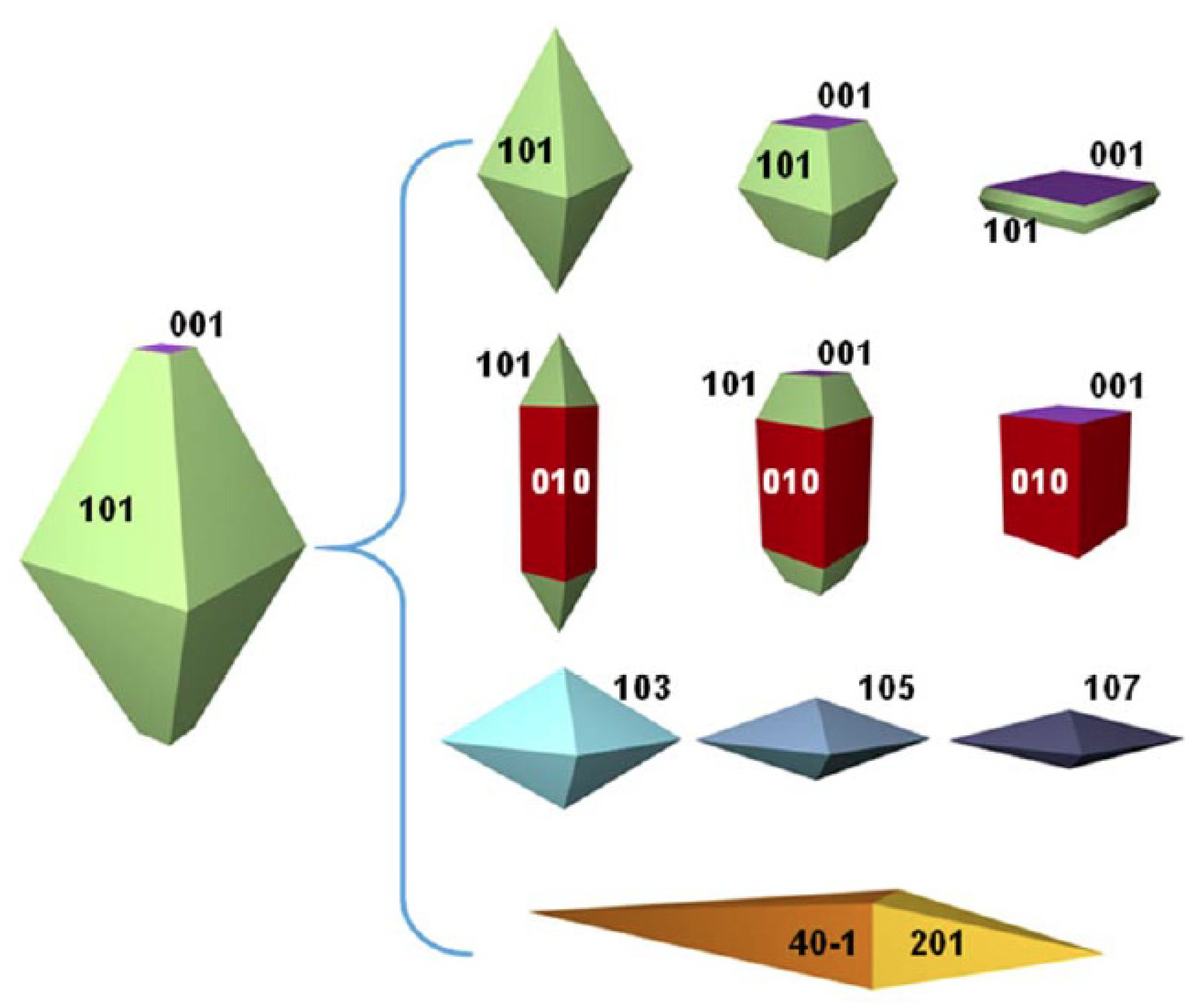
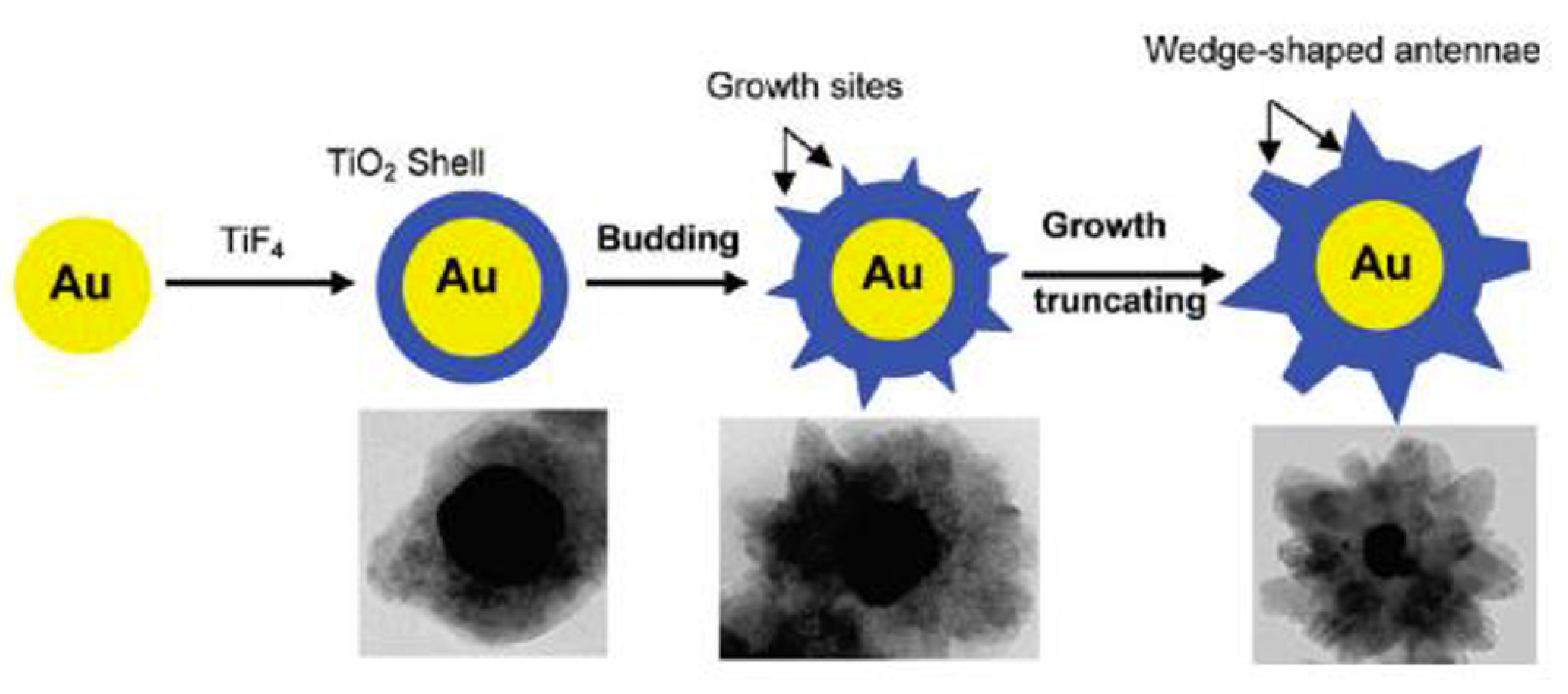
2.3.3. Crystal Growth
3. Synthesis of TiO2-001 and TiO2-001-Based Composites
3.1. Synthesis of TiO2-001 Nanomaterials
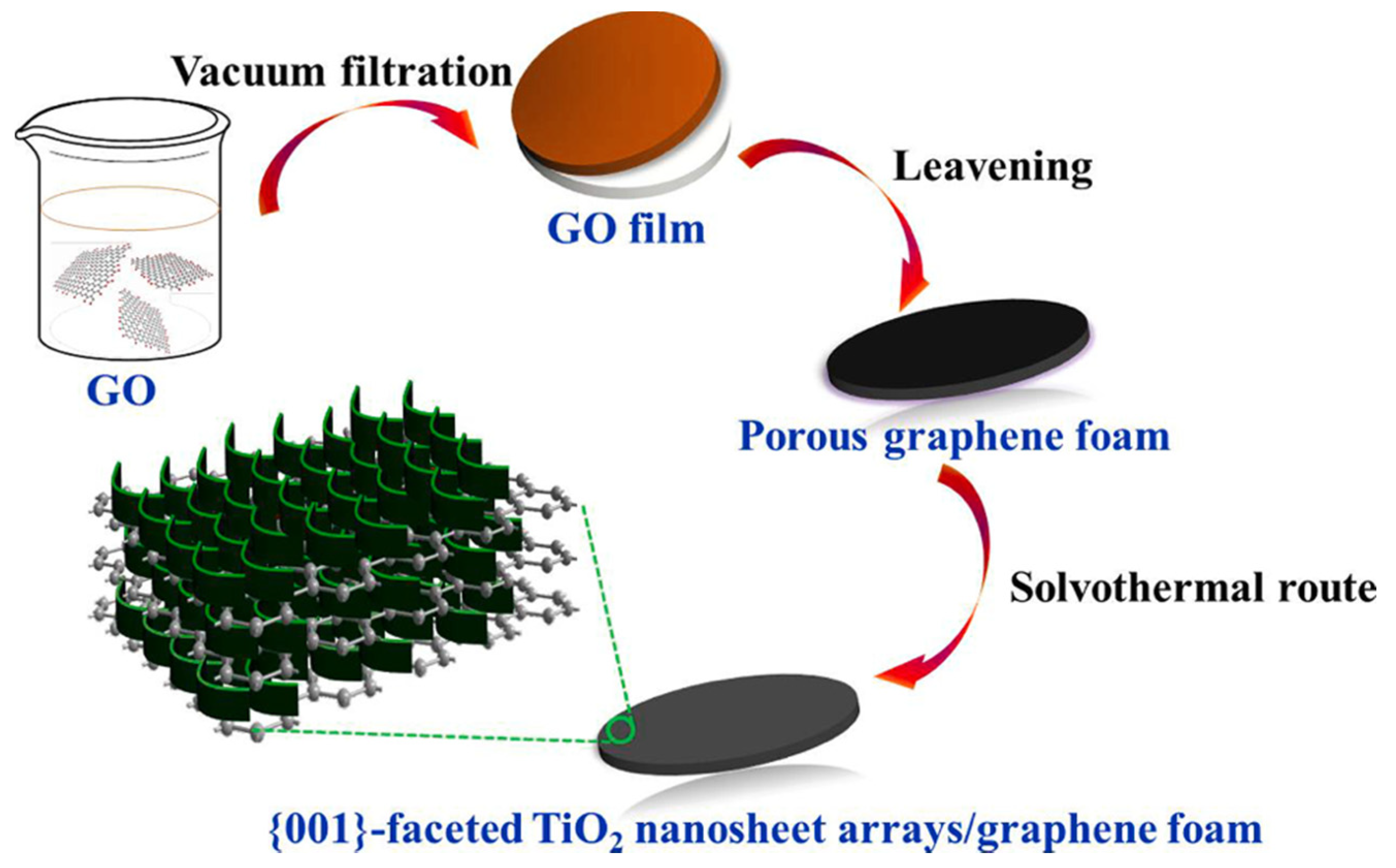
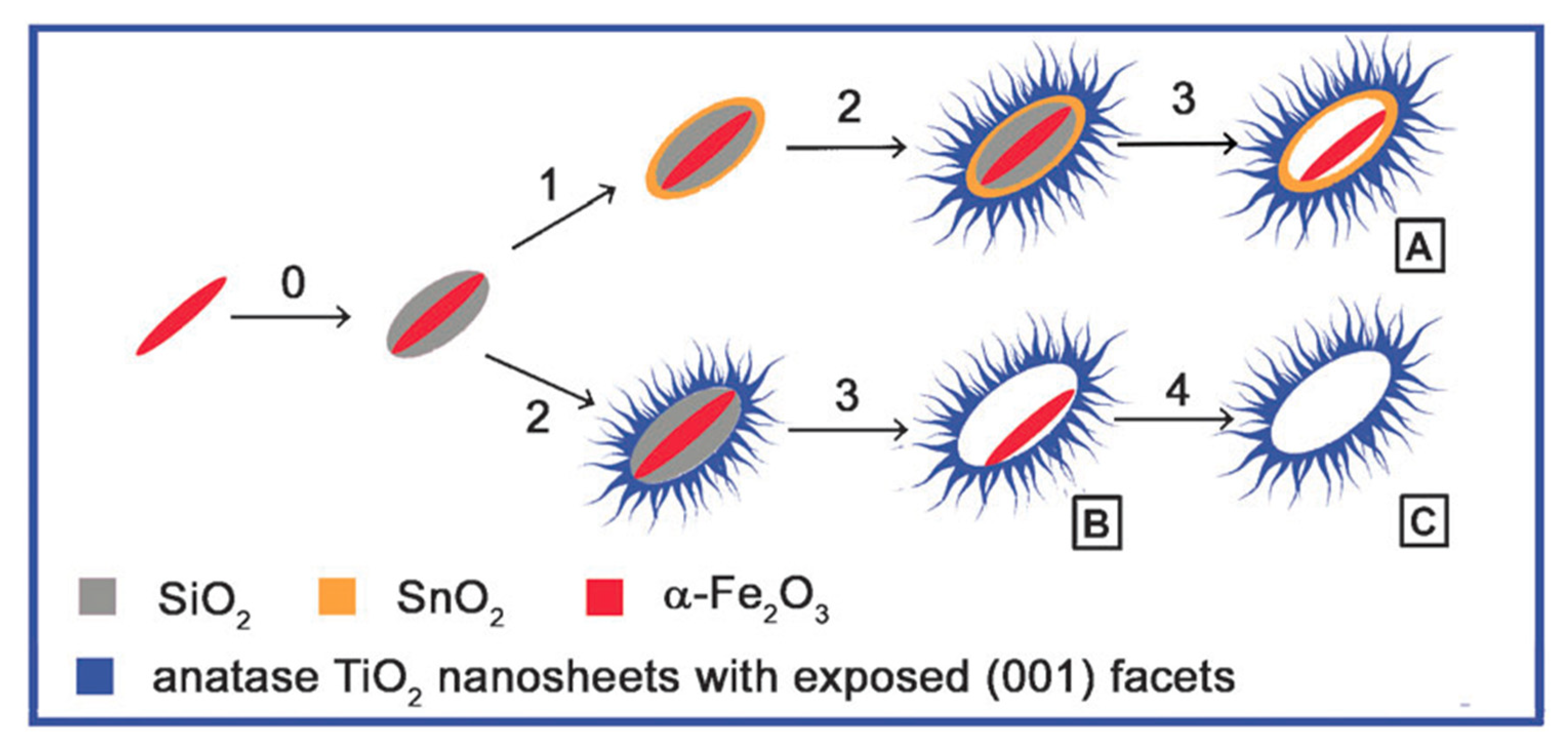
3.2. Synthesis of TiO2-001-Based Composites
4. Applications
4.1. TiO2-001-Based Composites for Photocatalytic Applications
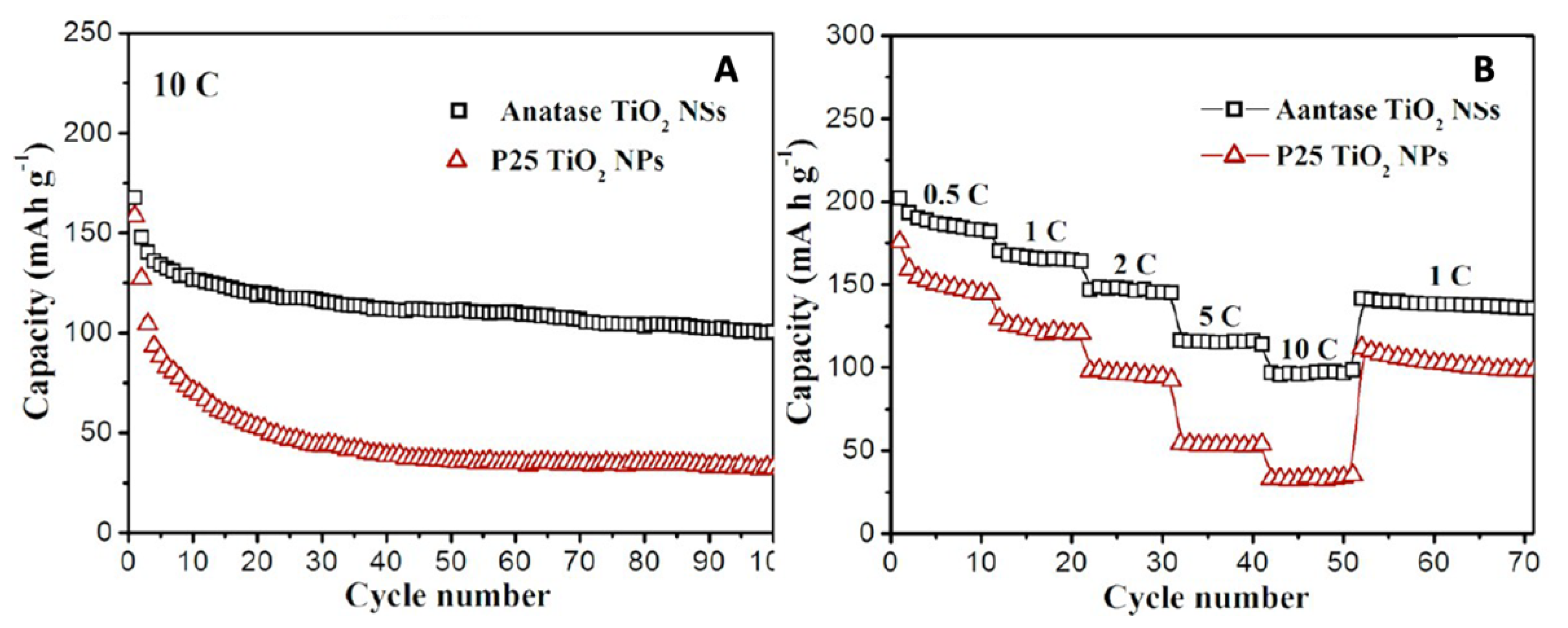
4.2. TiO2-001-Based Composites as a Li-ion Battery Anode Material
5. Conclusions and Future Scope
- Developing novel, large-scale synthesis methods
- 2.
- Coupling TiO2-001 with energy-harvesting materials
- 3.
- Exploring new characterization techniques
- 4.
- Theoretical study for understanding photocatalysis mechanisms
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalair, A.; Abas, N.; Saleem, M.S.; Kalair, A.R.; Khan, N. Role of energy storage systems in energy transition from fossil fuels to renewables. Energy Storage 2021, 3, e135. [Google Scholar] [CrossRef] [Green Version]
- Rogdakis, K.; Karakostas, N.; Kymakis, E. Up-scalable emerging energy conversion technologies enabled by 2D materials: From miniature power harvesters towards grid-connected energy systems. Energy Environ. Sci. 2021, 14, 3352–3392. [Google Scholar] [CrossRef]
- Detz, R.J.; Reek, J.N.H.; van der Zwaan, B.C.C. The future of solar fuels: When could they become competitive? Energy Environ. Sci. 2018, 11, 1653–1669. [Google Scholar] [CrossRef]
- Gong, J.; Li, C.; Wasielewski, M.R. Advances in solar energy conversion. Chem. Soc. Rev. 2019, 48, 1862–1864. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Xu, Y.-J. Promises and Challenges in Photocatalysis. Front. Catal. 2021, 1, 6. [Google Scholar] [CrossRef]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef] [PubMed]
- Do, H.H.; Nguyen, D.L.T.; Nguyen, X.C.; Le, T.-H.; Nguyen, T.P.; Trinh, Q.T.; Ahn, S.H.; Vo, D.-V.N.; Kim, S.Y.; Van Le, Q. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: A review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Bokare, A.; Pai, M.; Athawale, A.A. Surface modified Nd doped TiO2 nanoparticles as photocatalysts in UV and solar light irradiation. Sol. Energy 2013, 91, 111–119. [Google Scholar] [CrossRef]
- Weon, S.; Choi, E.; Kim, H.; Kim, J.Y.; Park, H.-J.; Kim, S.-M.; Kim, W.; Choi, W. Active {001} Facet Exposed TiO2 Nanotubes Photocatalyst Filter for Volatile Organic Compounds Removal: From Material Development to Commercial Indoor Air Cleaner Application. Environ. Sci. Technol. 2018, 52, 9330–9340. [Google Scholar] [CrossRef]
- Chu, L.; Qin, Z.; Yang, J.; Li, X. Anatase TiO2 Nanoparticles with Exposed {001} Facets for Efficient Dye-Sensitized Solar Cells. Sci. Rep. 2015, 5, 12143. [Google Scholar] [CrossRef]
- Tachikawa, T.; Yamashita, S.; Majima, T. Evidence for crystal-face-dependent TiO2 photocatalysis from single-molecule imaging and kinetic analysis. J. Am. Chem. Soc. 2011, 133, 7197–7204. [Google Scholar] [CrossRef]
- Du, Y.-E.; Niu, X.; Li, W.; An, J.; Liu, Y.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Microwave-Assisted Synthesis of High-Energy Faceted TiO2 Nanocrystals Derived from Exfoliated Porous Metatitanic Acid Nanosheets with Improved Photocatalytic and Photovoltaic Performance. Materials 2019, 12, 3614. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.M.; Cheng, H.-M. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef]
- Andrés, J.; Gracia, L.; Gouveia, A.F.; Ferrer, M.M.; Longo, E. Effects of surface stability on the morphological transformation of metals and metal oxides as investigated by first-principles calculations. Nanotechnology 2015, 26, 405703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martsinovich, N.; Troisi, A. How TiO2 crystallographic surfaces influence charge injection rates from a chemisorbed dye sensitiser. Phys. Chem. Chem. Phys. 2012, 14, 13392–13401. [Google Scholar] [CrossRef]
- Butburee, T.; Kotchasarn, P.; Hirunsit, P.; Sun, Z.; Tang, Q.; Khemthong, P.; Sangkhun, W.; Thongsuwan, W.; Kumnorkaew, P.; Wang, H.; et al. New understanding of crystal control and facet selectivity of titanium dioxide ruling photocatalytic performance. J. Mater. Chem. A Mater. Energy Sustain. 2019, 7, 8156–8166. [Google Scholar] [CrossRef]
- Liu, X.; Du, G.; Li, M. True Photoreactivity Origin of Ti3+-Doped Anatase TiO2 Crystals with Respectively Dominated Exposed {001}, {101}, and {100} Facets. ACS Omega 2019, 4, 14902–14912. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.-Y.; Bao, S.-H.; Guo, Y.; Jin, P. Anatase TiO2 films with dominant {001} facets fabricated by direct-current reactive magnetron sputtering at room temperature: Oxygen defects and enhanced visible-light photocatalytic behaviors. ACS Appl. Mater. Interfaces 2014, 6, 5940–5946. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, X.; Sun, H.; Ahmad, M. 3D anatase TiO2 hollow microspheres assembled with high-energy {001} facets for lithium-ion batteries. RSC Adv. 2012, 2, 7901–7905. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, Q.; Liu, J.; Qian, J.; Zhou, X. TiO2 nanoparticles embedded in hollow cube with highly exposed 001 facets: Facile synthesis and photovoltaic applications. J. Alloys Compd. 2016, 656, 863–870. [Google Scholar] [CrossRef]
- Pei, D.-N.; Gong, L.; Zhang, A.-Y.; Zhang, X.; Chen, J.-J.; Mu, Y.; Yu, H.Q. Defective titanium dioxide single crystals exposed by high-energy {001} facets for efficient oxygen reduction. Nat. Commun. 2015, 6, 8696. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Zhang, X.; Li, W.; Yan, Y.; Xi, G.; Yang, H.; Yu, H.-Q. Large-scale, ultrathin and (001) facet exposed TiO2 nanosheet superstructures and their applications in photocatalysis. J. Mater. Chem. A Mater. Energy Sustain. 2014, 2, 2040–2043. [Google Scholar] [CrossRef]
- Umar, A.A.; Md Saad, S.K.; Ali Umar, M.I.; Rahman, M.Y.A.; Oyama, M. Advances in porous and high-energy (001)-faceted anatase TiO2 nanostructures. Opt. Mater. 2018, 75, 390–430. [Google Scholar] [CrossRef]
- Di Liberto, G.; Tosoni, S.; Pacchioni, G. Nitrogen doping in coexposed (001)-(101) anatase TiO2 surfaces: A DFT study. Phys. Chem. Chem. Phys. 2019, 21, 21497–21505. [Google Scholar] [CrossRef]
- Banerjee, B.; Amoli, V.; Maurya, A.; Sinha, A.K.; Bhaumik, A. Green synthesis of Pt-doped TiO2 nanocrystals with exposed (001) facets and mesoscopic void space for photo-splitting of water under solar irradiation. Nanoscale 2015, 7, 10504–10512. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.; Balčytis, A.; Nitta, A.; Mendez-Medrano, M.-G.; Colbeau-Justin, C.; Juodkazis, S.; Othani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. B 2018, 237, 574–587. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, R.; Hu, C.; Chen, F.; Duo, S.; Hu, Q. TiO2-graphene composites with exposed {001} facets produced by a one-pot solvothermal approach for high performance photocatalyst. Phys. Chem. Chem. Phys. 2013, 15, 12963–12970. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Chen, H.; Peng, H.; Wang, Z.; Huang, W. Graphene Modified TiO2 Composite Photocatalysts: Mechanism, Progress and Perspective. Nanomaterials 2018, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Facet-dependent photocatalytic properties of TiO2-based composites for energy conversion and environmental remediation. ChemSusChem 2014, 7, 690–719. [Google Scholar] [CrossRef]
- Chen, J.S.; Lou, X.W. SnO2 and TiO2 nanosheets for lithium-ion batteries. Mater. Today 2012, 15, 246–254. [Google Scholar] [CrossRef]
- Kuang, Q.; Wang, X.; Jiang, Z.; Xie, Z.; Zheng, L. High-energy-surface engineered metal oxide micro- and nanocrystallites and their applications. Acc. Chem. Res. 2014, 47, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zang, Y.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. Meaningful comparison of photocatalytic properties of {001} and {101} faceted anatase TiO2 nanocrystals. Sci. Bull. Fac. Agric. Kyushu Univ. 2016, 61, 1003–1012. [Google Scholar]
- Yang, J.; Wu, Q.; He, S.; Yan, J.; Shi, J.; Chen, J.; Wu, M.; Yang, X. Completely oriented anatase TiO2 nanoarrays: Topotactic growth and orientation-related efficient photocatalysis. Nanoscale 2015, 7, 13888–13897. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Li, Y.; Dong, L.; Zhao, Y.; Zhang, J. A review on modification of facet-engineered TiO2 for photocatalytic CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2018, 36, 24–47. [Google Scholar] [CrossRef]
- Maisano, M.; Dozzi, M.V.; Coduri, M.; Artiglia, L.; Granozzi, G.; Selli, E. Unraveling the Multiple Effects Originating the Increased Oxidative Photoactivity of {001}-Facet Enriched Anatase TiO2. ACS Appl. Mater. Interfaces 2016, 8, 9745–9754. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Specific Facets-Dominated Anatase TiO2: Fluorine-Mediated Synthesis and Photoactivity. Catalysts 2013, 3, 455–485. [Google Scholar] [CrossRef]
- Vittadini, A.; Selloni, A.; Rotzinger, F.P.; Grätzel, M. Structure and Energetics of Water Adsorbed at TiO2 Anatase (101) and (001) Surfaces. Phys. Rev. Lett. 1998, 81, 2954–2957. [Google Scholar] [CrossRef]
- Selloni, A. Crystal growth: Anatase shows its reactive side. Nat. Mater. 2008, 7, 613–615. [Google Scholar] [CrossRef]
- Liu, N.; Li, K.; Li, X.; Chang, Y.; Feng, Y.; Sun, X.; Cheng, Y.; Wu, Z.; Zhang, H. Crystallographic Facet-Induced Toxicological Responses by Faceted Titanium Dioxide Nanocrystals. ACS Nano 2016, 10, 6062–6073. [Google Scholar] [CrossRef]
- Liu, N.; Chang, Y.; Feng, Y.; Cheng, Y.; Sun, X.; Jian, H.; Feng, Y.; Li, X.; Zhang, H. {101}-{001} Surface Heterojunction-Enhanced Antibacterial Activity of Titanium Dioxide Nanocrystals Under Sunlight Irradiation. ACS Appl. Mater. Interfaces 2017, 9, 5907–5915. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Jaroniec, M. Anatase TiO2 with dominant high-energy 001 facets: Synthesis, properties, and applications. Chem. Mater. 2011, 23, 4085–4093. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Yang, H.G.; Wang, X.; Cheng, L.; Lu, H.; Wang, L.; Lu, G.Q.; Cheng, H.M. Enhanced Photoactivity of Oxygen-Deficient Anatase TiO2 Sheets with Dominant {001} Facets. J. Phys. Chem. C 2009, 113, 21784–21788. [Google Scholar] [CrossRef]
- Yang, P.; Douthwaite, M.; Pan, J.; Zheng, L.; Hong, S.; Morgan, D.J.; Gao, M.; Li, D.; Feng, J.; Hutchings, G.J. Coordinately unsaturated O2c–Ti5c–O2c sites promote the reactivity of Pt/TiO2 catalysts in the solvent-free oxidation of n-octanol. Catal. Sci. Technol. 2021, 11, 4898–4910. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 2002, 26, 1167–1170. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Niu, L.; He, X. Enhanced visible-light activity of Ti3+ self-doped TiO2 with co-exposed 001 and 101 facets. Micro Nano Lett. 2018, 13, 514–517. [Google Scholar] [CrossRef]
- Di Liberto, G.; Tosoni, S.; Pacchioni, G. Role of Heterojunction in Charge Carrier Separation in Coexposed Anatase (001)-(101) Surfaces. J. Phys. Chem. Lett. 2019, 10, 2372–2377. [Google Scholar] [CrossRef]
- Murakami, N.; Kurihara, Y.; Tsubota, T.; Ohno, T. Shape-Controlled Anatase Titanium(IV) Oxide Particles Prepared by Hydrothermal Treatment of Peroxo Titanic Acid in the Presence of Polyvinyl Alcohol. J. Phys. Chem. C 2009, 113, 3062–3069. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Liu, J.; Tian, L.; Peng, T.; Zan, L. The replacement of {101} by {010} facets inhibits the photocatalytic activity of anatase TiO2. Appl. Catal. B 2013, 134–135, 60–65. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Lu, J.; Qin, X.; Zhang, X.; Dai, Y. Hierarchical TiO2 microspheres: Synergetic effect of {001} and {101} facets for enhanced photocatalytic activity. Chemistry 2011, 17, 15032–15038. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, W.; Zhou, S.; Li, Z.; Hu, H.; Tao, Y.; Zhou, S.; Zhao, X.; Kong, Y. Probing the formation and optical properties of Ti3+–TiO2 with (001) exposed crystal facet by ethanol-assisted fluorination. New J. Chem. 2021, 45, 12453–12463. [Google Scholar] [CrossRef]
- Liu, G.; Sun, C.; Yang, H.G.; Smith, S.C.; Wang, L.; Lu, G.Q.M.; Cheng, H.M. Nanosized anatase TiO2 single crystals for enhanced photocatalytic activity. Chem. Commun. 2010, 46, 755–757. [Google Scholar] [CrossRef]
- Angeline Dorothy, A.; Panigrahi, P. Tuning optical properties of TiO2 by dimension reduction: From 3D bulk to 2D sheets along {001} and {101} plane. Mater. Res. Express 2020, 6, 1250f1. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Bian, Z.; Xie, S.; Cai, C.; Wang, J.; Yang, H.; Li, H. Solvothermally controllable synthesis of anatase TiO2 nanocrystals with dominant {001} facets and enhanced photocatalytic activity. CrystEngComm 2010, 12, 2219–2224. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Jaroniec, M. Tunable photocatalytic selectivity of hollow TiO2 microspheres composed of anatase polyhedra with exposed {001} facets. J. Am. Chem. Soc. 2010, 132, 11914–11916. [Google Scholar] [CrossRef]
- Amano, F.; Yasumoto, T.; Mahaney, O.O.P.; Uchida, S.; Shibayama, T.; Terada, Y.; Ohtani, B. Highly active Titania photocatalyst particles of controlled crystal phase, size, and polyhedral shapes. Top. Catal. 2010, 53, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.S.; Tan, Y.L.; Li, C.M.; Cheah, Y.L.; Luan, D.; Madhavi, S.; Boey, F.Y.; Archer, L.A.; Lou, X.W. Constructing hierarchical spheres from large ultrathin anatase TiO2 nanosheets with nearly 100% exposed (001) facets for fast reversible lithium storage. J. Am. Chem. Soc. 2010, 132, 6124–6130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hengerer, R.; Kavan, L.; Krtil, P.; Grätzel, M. Orientation Dependence of Charge-Transfer Processes on TiO2 (Anatase) Single Crystals. J. Electrochem. Soc. 2000, 147, 1467. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Li, W.; Chen, X. Nanostructured TiO2-Based Anode Materials for High-Performance Rechargeable Lithium-Ion Batteries. Chem. Nano. Mat. 2016, 2, 764–775. [Google Scholar] [CrossRef]
- Cheng, X.-L.; Hu, M.; Huang, R.; Jiang, J.-S. HF-free synthesis of anatase TiO2 nanosheets with largely exposed and clean {001} facets and their enhanced rate performance as anodes of lithium-ion battery. ACS Appl. Mater. Interfaces 2014, 6, 19176–19183. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The effect of doping elements on photocatalytic activity. Mater. Adv. 2020, 1, 1193–1201. [Google Scholar] [CrossRef]
- Sulaiman, S.N.A.; Noh, M.Z.; Adnan, N.N.; Bidin, N.; Razak, S.N.A. Effects of photocatalytic activity of metal and non-metal doped TiO2 for Hydrogen production enhancement—A Review. J. Phys. Conf. Ser. 2018, 1027, 012006. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Mazierski, P.; Mikolajczyk, A.; Bajorowicz, B.; Malankowska, A.; Zaleska-Medynska, A.; Nadolna, J. The role of lanthanides in TiO2-based photocatalysis: A review. Appl. Catal. B 2018, 233, 301–317. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Z.; Lu, N.; Hua, R.; Dong, B. Prolonging charge-separation states by doping lanthanide-ions into {001}/{101} facets-coexposed TiO2 nanosheets for enhancing photocatalytic H2 evolution. Chin. J. Catal. 2019, 40, 413–423. [Google Scholar]
- Zhang, J.; Xi, J.; Ji, Z. Mo + N Codoped TiO2 sheets with dominant {001} facets for enhancing visible-light photocatalytic activity. J. Mater. Chem. 2012, 22, 17700–17708. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Wang, W.; Jaroniec, M. Nitrogen self-doped nanosized TiO2 sheets with exposed {001} facets for enhanced visible-light photocatalytic activity. Chem. Commun. 2011, 47, 6906–6908. [Google Scholar] [CrossRef]
- Liu, G.; Sun, C.; Smith, S.C.; Wang, L.; Lu, G.Q.M.; Cheng, H.-M. Sulfur doped anatase TiO2 single crystals with a high percentage of {001} facets. J. Colloid Interface Sci. 2010, 349, 477–483. [Google Scholar]
- Shi, J.-W.; Liu, C.; He, C.; Li, J.; Xie, C.; Yang, S.; Chen, J.-W.; Li, S.; Niu, C. Carbon-doped titania nanoplates with exposed {001} facets: Facile synthesis, characterization and visible-light photocatalytic performance. RSC Adv. 2015, 5, 17667–17675. [Google Scholar]
- Li, C.; Sun, Z.; Ma, R.; Xue, Y.; Zheng, S. Fluorine doped anatase TiO2 with exposed reactive (001) facets supported on porous diatomite for enhanced visible-light photocatalytic activity. Microporous Mesoporous Mater. 2017, 243, 281–290. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Doping TiO2 with p-block elements: Effects on photocatalytic activity. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 13–28. [Google Scholar] [CrossRef]
- Yalçın, Y.; Kılıç, M.; Çınar, Z. The Role of Non-Metal Doping in TiO2 Photocatalysis. J. Adv. Oxid. Technol. 2010, 13, 281–296. [Google Scholar] [CrossRef]
- González-Torres, J.C.; Poulain, E.; Domínguez-Soria, V.; García-Cruz, R.; Olvera-Neria, O. C-, N-, S-, and F-Doped Anatase TiO2 (101) with Oxygen Vacancies: Photocatalysts Active in the Visible Region. Int. J. Photoenergy 2018, 2018, 7506151. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.; Zhang, Y.; Chu, Z.; Long, J.; An, X.; Zhang, H.; Lin, H.; Zhang, Z.; Wang, X. Synergy of metal and nonmetal dopants for visible-light photocatalysis: A case-study of Sn and N co-doped TiO2. Phys. Chem. Chem. Phys. 2016, 18, 9636–9644. [Google Scholar] [CrossRef] [Green Version]
- Suriyachai, N.; Chuangchote, S.; Laosiripojana, N.; Champreda, V.; Sagawa, T. Synergistic Effects of Co-Doping on Photocatalytic Activity of Titanium Dioxide on Glucose Conversion to Value-Added Chemicals. ACS Omega 2020, 5, 20373–20381. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Nitrogen and sulfur co-doped TiO2 nanosheets with exposed {001} facets: Synthesis, characterization and visible-light photocatalytic activity. Phys. Chem. Chem. Phys. 2011, 13, 4853–4861. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Pan, C. N + Ni codoped anatase TiO2 nanocrystals with exposed 001 facets through two-step hydrothermal route. J. Am. Ceram. Soc. 2012, 95, 2951–2956. [Google Scholar] [CrossRef]
- Athawale, A.; Bokare, A.; Singh, H.; Nguyen, V.-H.; Sharma, A. Synthesis of Ag2O Coated TiO2 Nanoparticles by Sonochemically Activated Methods for Enhanced Photocatalytic Activities. Top. Catal. 2020, 63, 1056–1065. [Google Scholar] [CrossRef]
- Sescu, A.M.; Favier, L.; Lutic, D.; Soto-Donoso, N.; Ciobanu, G.; Harja, M. TiO2 Doped with Noble Metals as an Efficient Solution for the Photodegradation of Hazardous Organic Water Pollutants at Ambient Conditions. Water 2020, 13, 19. [Google Scholar]
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen Production by Photocatalytic Water Splitting over Pt/TiO2 Nanosheets with Exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Yan, J.; Wu, G.; Dai, W.; Guan, N.; Li, L. Synthetic Design of Gold Nanoparticles on Anatase TiO2 {001} for Enhanced Visible Light Harvesting. ACS Sustain. Chem. Eng. 2014, 2, 1940–1946. [Google Scholar] [CrossRef]
- Lyu, Z.; Liu, B.; Wang, R.; Tian, L. Synergy of palladium species and hydrogenation for enhanced photocatalytic activity of {001} facets dominant TiO2 nanosheets. J. Mater. Res. 2017, 32, 2781–2789. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Bahuguna, A.; Sharma, V.; Krishnan, V. Two-dimensional carbon-based nanocomposites for photocatalytic energy generation and environmental remediation applications. Beilstein J. Nanotechnol. 2017, 8, 1571–1600. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.; Lee, D.-K.; Kim, S.M.; Park, W.; Cho, S.Y.; Sim, U. Low Dimensional Carbon-Based Catalysts for Efficient Photocatalytic and Photo/Electrochemical Water Splitting Reactions. Materials 2019, 13, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent Advances in Graphene Based TiO2 Nanocomposites (GTiO2Ns) for Photocatalytic Degradation of Synthetic Dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Bokare, A.; Chinnusamy, S.; Erogbogbo, F. TiO2-Graphene Quantum Dots Nanocomposites for Photocatalysis in Energy and Biomedical Applications. Catalysts 2021, 11, 319. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y. Graphene-based nanocomposite anodes for lithium-ion batteries. Nanoscale 2014, 6, 11528–11552. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Bokare, A.; Singh, H.; Pai, M.; Nair, R.; Sabharwal, S.; Athawale, A.A. Hydrothermal synthesis of Ag@TiO2–Fe3O4 nanocomposites using sonochemically activated precursors: Magnetic, photocatalytic and antibacterial properties. Mater. Res. Express 2014, 1, 046111. [Google Scholar] [CrossRef]
- Liu, L.; Gu, X.; Sun, C.; Li, H.; Deng, Y.; Gao, F.; Lin, D. In situ loading of ultra-small Cu2O particles on TiO2 nanosheets to enhance the visible-light photoactivity. Nanoscale 2012, 4, 6351–6359. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Magnetic composite microspheres with exposed {001} faceted TiO2 shells: A highly active and selective visible-light photocatalyst. J. Mater. Chem. 2012, 22, 13341–13347. [Google Scholar] [CrossRef]
- Qi, L.; Yu, J.; Jaroniec, M. Preparation and enhanced visible-light photocatalytic H2-production activity of CdS-sensitized Pt/TiO2 nanosheets with exposed (001) facets. Phys. Chem. Chem. Phys. 2011, 13, 8915–8923. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Kim, M.J.; Im, S.H. Synthesis of lustering two-dimensional α-MoO3 van der Waals crystals by TiO2 assisted selective facet passivation. J. Ind. Eng. Chem. 2020, 84, 358–365. [Google Scholar] [CrossRef]
- Chen, G.; Ji, S.; Li, H.; Kang, X.; Chang, S.; Wang, Y.; Yu, G.; Lu, J.; Claverie, J.; Sang, Y.; et al. High.-Energy Faceted SnO2-Coated TiO2 Nanobelt Heterostructure for Near-Ambient Temperature-Responsive Ethanol Sensor. ACS Appl. Mater. Interfaces 2015, 7, 24950–24956. [Google Scholar] [CrossRef]
- Angel, R.D.; Durán-Álvarez, J.C.; Zanella, R. Photocatalysis. In Titanium Dioxide; Yang, D., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Nguyen, C.K.; Cha, H.G.; Kang, Y.S. Axis-Oriented, Anatase TiO2 Single Crystals with Dominant {001} and {100} Facets. Cryst. Growth Des. 2011, 11, 3947–3953. [Google Scholar] [CrossRef]
- Yang, X.H.; Li, Z.; Sun, C.; Yang, H.G.; Li, C. Hydrothermal Stability of {001} Faceted Anatase TiO2. Chem. Mater. 2011, 23, 3486–3494. [Google Scholar] [CrossRef]
- Barnard, A.S.; Curtiss, L.A. Prediction of TiO2 nanoparticle phase and shape transitions controlled by surface chemistry. Nano Lett. 2005, 5, 1261–1266. [Google Scholar] [CrossRef]
- Li, F.; Huang, Y.; Peng, H.; Cao, Y.; Niu, Y. Preparation and Photocatalytic Water Splitting Hydrogen Production of Titanium Dioxide Nanosheets. Int. J. Photoenergy 2020, 2020, 3617312. [Google Scholar] [CrossRef]
- Liu, S.; Liu, B.; Nakata, K.; Ochiai, T.; Murakami, T.; Fujishima, A. Electrospinning Preparation and Photocatalytic Activity of Porous TiO2 Nanofibers. J. Nanomater. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Yu, J.; Fan, J.; Lv, K. Anatase TiO2 nanosheets with exposed (001) facets: Improved photoelectric conversion efficiency in dye-sensitized solar cells. Nanoscale 2010, 2, 2144–2149. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.S.; Wang, Z.; Cheah, Y.L.; Madhavi, S.; Hu, X.; Lou, X.-W. TiO2 hollow spheres with large amount of exposed (001) facets for fast reversible lithium storage. J. Mater. Chem. 2011, 21, 1677–1680. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Highly reactive {001} facets of TiO2-based composites: Synthesis, formation mechanism and characterization. Nanoscale 2014, 6, 1946–2008. [Google Scholar] [CrossRef]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.-M.; Lu, G.-Q. Solvothermal synthesis and photoreactivity of anatase TiO2 nanosheets with dominant {001} facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef]
- Sayed, M.; Pingfeng, F.; Khan, H.M.; Zhang, P. Effect of Isopropanol on Microstructure and Activity of TiO2 Films with Dominant {001} Facets for Photocatalytic Degradation of Bezafibrate. Int. J. Photoenergy 2014, 2014, 490264. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Kuang, Q.; Jin, M.; Xie, Z.; Zheng, L. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef]
- Miao, J.; Liu, B. Anatase TiO2 microspheres with reactive {001} facets for improved photocatalytic activity. RSC Adv. 2012, 3, 1222–1226. [Google Scholar] [CrossRef]
- Ma, X.Y.; Chen, Z.G.; Hartono, S.B.; Jiang, H.B.; Zou, J.; Qiao, S.Z.; Yang, H.-G. Fabrication of uniform anatase TiO2 particles exposed by {001} facets. Chem. Commun. 2010, 46, 6608–6610. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Yin, M.; Wang, Z.; Yan, S.; Wan, L.; Li, Z.; Yang, H.-G. Facile synthesis of anatase TiO2 mesocrystal sheets with dominant {001} facets based on topochemical conversion. CrystEngComm 2010, 12, 3425–3429. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, Q.; Ran, J.; Mann, S. One-step hydrothermal fabrication and photocatalytic activity of surface-fluorinated TiO2 hollow microspheres and tabular anatase single micro-crystals with high-energy facets. CrystEngComm 2010, 12, 872–879. [Google Scholar] [CrossRef]
- Lai, Z.; Peng, F.; Wang, Y.; Wang, H.; Yu, H.; Liu, P.; Zhao, H. Low temperature solvothermal synthesis of anatase TiO2 single crystals with wholly {100} and {001} faceted surfaces. J. Mater. Chem. 2012, 22, 23906–23912. [Google Scholar] [CrossRef]
- Yu, H.; Tian, B.; Zhang, J. Layered TiO2 composed of anatase nanosheets with exposed {001} facets: Facile synthesis and enhanced photocatalytic activity. Chemistry 2011, 17, 5499–5502. [Google Scholar] [CrossRef]
- Han, X.; Wang, X.; Xie, S.; Kuang, Q.; Ouyang, J.; Xie, Z.; Zheng, L. Carbonate ions-assisted syntheses of anatase TiO2 nanoparticles exposed with high energy (001) facets. RSC Adv. 2012, 2, 3251–3253. [Google Scholar] [CrossRef]
- Dinh, C.-T.; Nguyen, T.-D.; Kleitz, F.; Do, T.-O. Shape-controlled synthesis of highly crystalline titania nanocrystals. ACS Nano 2009, 3, 3737–3743. [Google Scholar] [CrossRef]
- Dai, Y.; Cobley, C.M.; Zeng, J.; Sun, Y.; Xia, Y. Synthesis of anatase TiO2 nanocrystals with exposed {001} facets. Nano Lett. 2009, 9, 2455–2459. [Google Scholar] [CrossRef]
- Shan, G.-B.; Demopoulos, G.P. The synthesis of aqueous-dispersible anatase TiO2 nanoplatelets. Nanotechnology 2010, 21, 025604. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Z.; Zhao, H.; Zheng, M.; Du, P.; Zhao, J.; Fan, H. Phase control of hierarchically structured mesoporous anatase TiO2 microspheres covered with {001} facets. J. Mater. Chem. 2012, 22, 21965–21971. [Google Scholar] [CrossRef]
- Roy, N.; Sohn, Y.; Pradhan, D. Synergy of low-energy {101} and high-energy {001} TiO2 crystal facets for enhanced photocatalysis. ACS Nano 2013, 7, 2532–2540. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Chen, Q.; Li, J.; Xu, D. Controllable Synthesis of TiO2 Single Crystals with Tunable Shapes Using Ammonium-Exchanged Titanate Nanowires as Precursors. Cryst. Growth Des. 2010, 10, 2111–2115. [Google Scholar] [CrossRef]
- Li, T.; Tian, B.; Zhang, J.; Dong, R.; Wang, T.; Yang, F. Facile Tailoring of Anatase TiO2 Morphology by Use of H2O2: From Microflowers with Dominant {101} Facets to Microspheres with Exposed {001} Facets. Ind. Eng. Chem. Res. 2013, 52, 6704–6712. [Google Scholar] [CrossRef]
- Amano, F.; Prieto-Mahaney, O.-O.; Terada, Y.; Yasumoto, T.; Shibayama, T.; Ohtani, B. Decahedral Single-Crystalline Particles of Anatase Titanium(IV) Oxide with High Photocatalytic Activity. Chem Mater. 2009, 21, 2601–2603. [Google Scholar] [CrossRef]
- Jung, M.-H.; Chu, M.-J.; Kang, M.G. TiO2 nanotube fabrication with highly exposed (001) facets for enhanced conversion efficiency of solar cells. Chem. Commun. 2012, 48, 5016–5018. [Google Scholar] [CrossRef]
- Lee, W.-J.; Sung, Y.-M. Synthesis of Anatase Nanosheets with Exposed (001) Facets via Chemical Vapor Deposition. Cryst. Growth Des. 2012, 12, 5792–5795. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Wang, X.; Cheng, L.; Pan, J.; Lu, G.Q.M.; Cheng, H.-M. Visible light responsive nitrogen doped anatase TiO2 sheets with dominant {001} facets derived from TiN. J. Am. Chem. Soc. 2009, 131, 12868–12869. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Dai, G.; Xiang, Q.; Jaroniec, M. Fabrication and enhanced visible-light photocatalytic activity of carbon self-doped TiO2 sheets with exposed {001} facets. J. Mater. Chem. 2011, 21, 1049–1057. [Google Scholar] [CrossRef]
- Wu, X.-F.; Song, H.-Y.; Yoon, J.-M.; Yu, Y.-T.; Chen, Y.-F. Synthesis of core-shell Au@TiO2 nanoparticles with truncated wedge-shaped morphology and their photocatalytic properties. Langmuir 2009, 25, 6438–6447. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Wen, Y.; Du, L.; Zeng, W.; Shi, Y.; Zhang, F.; Zhu, G.; Xu, X.; Wang, Y. Highly dispersive {001} facets-exposed nanocrystalline TiO2 on high quality graphene as a high performance photocatalyst. J. Mater. Chem. 2012, 22, 7484–7491. [Google Scholar] [CrossRef]
- Chinnusamy, S.; Kaur, R.; Bokare, A.; Erogbogbo, F. Incorporation of graphene quantum dots to enhance photocatalytic properties of anatase TiO2. MRS Commun. 2018, 8, 137–144. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Enhanced photocatalytic H2-production activity of graphene-modified titania nanosheets. Nanoscale 2011, 3, 3670–3678. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Z.; Zhou, Y.; Liu, L. Anatase TiO2 nanocrystals with exposed {001} facets on graphene sheets via molecular grafting for enhanced photocatalytic activity. Nanoscale 2012, 4, 613–620. [Google Scholar] [CrossRef]
- Wang, W.-S.; Wang, D.-H.; Qu, W.-G.; Lu, L.-Q.; Xu, A.-W. Large Ultrathin Anatase TiO2 Nanosheets with Exposed {001} Facets on Graphene for Enhanced Visible Light Photocatalytic Activity. J. Phys. Chem. C 2012, 116, 19893–19901. [Google Scholar] [CrossRef]
- Jiang, B.; Tian, C.; Pan, Q.; Jiang, Z.; Wang, J.-Q.; Yan, W.; Honggang, F. Enhanced Photocatalytic Activity and Electron Transfer Mechanisms of Graphene/TiO2 with Exposed {001} Facets. J. Phys. Chem. C 2011, 115, 23718–23725. [Google Scholar] [CrossRef]
- Qiu, B.; Xing, M.; Zhang, J. Mesoporous TiO2 nanocrystals grown in situ on graphene aerogels for high photocatalysis and lithium-ion batteries. J. Am. Chem. Soc. 2014, 136, 5852–5855. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Zhang, Q.; Zhu, J.; Zhao, P. Rational design of {001}-faceted TiO2 nanosheet arrays/graphene foam with superior charge transfer interfaces for efficient photocatalytic degradation of toxic pollutants. Sep. Purif. Technol. 2021, 265, 118444. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Bai, H.; Du, C.; Wei, D.; Su, Y.; Wang, Y.; Yang, L. Incorporation of reduced graphene oxide into faceted flower-like {001} TiO2 for enhanced photocatalytic activity. R. Soc. Open Sci. 2018, 5, 180613. [Google Scholar] [CrossRef] [Green Version]
- Thien, G.S.H.; Omar, F.S.; Blya, N.I.S.A.; Chiu, W.S.; Lim, H.N.; Yousefi, R.; Sheini, F.-J.; Huang, N.-M. Improved Synthesis of Reduced Graphene Oxide-Titanium Dioxide Composite with Highly Exposed 001 Facets and Its Photoelectrochemical Response. Int. J. Photoenergy 2014, 2014, 650583. [Google Scholar] [CrossRef]
- Sui, Y.; Wu, L.; Zhong, S.; Liu, Q. Carbon quantum dots/TiO2 nanosheets with dominant (001) facets for enhanced photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 480, 810–816. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, F.; Lin, C.; Wang, Z.L. Direct growth of TiO2 nanosheet arrays on carbon fibers for highly efficient photocatalytic degradation of methyl orange. Adv. Mater. 2012, 24, 4761–4764. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zheng, H.; Yang, H.; Hao, L.; Wen, L.; Xu, T.; Wu, S. mpg-C3N4/anatase TiO2 with reactive {001} facets composites to enhance the photocatalytic activity of organic dye degradation. RSC Adv. 2016, 6, 54215–54225. [Google Scholar] [CrossRef]
- Wang, W.; Ni, Y.R.; Lu, C.H.; Xu, Z.Z. Coupling {001} Facets Dominated Anatase TiO2 Nanosheets with CNTs for Efficient Photocatalyst. Adv. Mater. Res. 2014, 853, 111–115. [Google Scholar] [CrossRef]
- Chen, J.S.; Chen, C.; Liu, J.; Xu, R.; Qiao, S.Z.; Lou, X.W. Ellipsoidal hollow nanostructures assembled from anatase TiO2 nanosheets as a magnetically separable photocatalyst. Chem Commun. 2011, 47, 2631–2633. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, J.; Yan, F.; Wang, X.; Huang, F. A situ hydrothermal synthesis of SrTiO3/TiO2 heterostructure nanosheets with exposed (001) facets for enhancing photocatalytic degradation activity. Appl. Surf. Sci. 2014, 319, 68–74. [Google Scholar] [CrossRef]
- Li, S.; Zhu, G.; Jia, Y.; Pan, L.; Nie, J.; Rao, F.; Gao, J.; Zhang, F.; Kwon, N.; Liu, C. TiO2 with exposed (001) facets/Bi4O5Br2 nanosheets heterojunction with enhanced photocatalytic for NO removal. Nanotechnology 2020, 31, 254002. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Godin, R.; Moss, B.; Kafizas, A.; Zafeiratos, S.; Durrant, J.-R.; Petit, C. Titanium dioxide/carbon nitride nanosheet nanocomposites for gas phase CO2 photoreduction under UV-visible irradiation. Appl. Catal. B 2019, 242, 369–378. [Google Scholar] [CrossRef]
- Wang, S.; Xu, M.; Peng, T.; Zhang, C.; Li, T.; Hussain, I.; Wang, J.; Tan, B. Porous hypercrosslinked polymer-TiO2-graphene composite photocatalysts for visible-light-driven CO2 conversion. Nat. Commun. 2019, 10, 676. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Self-assembly of nitrogen-doped TiO2 with exposed {001} facets on a graphene scaffold as photo-active hybrid nanostructures for reduction of carbon dioxide to methane. Nano Res. 2014, 7, 1528–1547. [Google Scholar] [CrossRef]
- Liu, X.; Cong, R.; Cao, L.; Liu, S.; Cui, H. The structure, morphology and photocatalytic activity of graphene–TiO2 multilayer films and charge transfer at the interface. New J. Chem. 2014, 38, 2362–2367. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef]
- Manthiram, A. An Outlook on Lithium Ion Battery Technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A Mater. Energy Sustain. 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Diouf, B.; Pode, R. Potential of lithium-ion batteries in renewable energy. Renew. Energy 2015, 76, 375–380. [Google Scholar] [CrossRef]
- Dufo-López, R.; Cortés-Arcos, T.; Artal-Sevil, J.S.; Bernal-Agustín, J.L. Comparison of Lead-Acid and Li-Ion Batteries Lifetime Prediction Models in Stand-Alone Photovoltaic Systems. NATO Adv. Sci. Inst. Ser. E Appl. Sci. 2021, 11, 1099. [Google Scholar]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Pradeep, N.; Sivasenthil, E.; Janarthanan, B.; Sharmila, S. A Review of Anode Material for Lithium Ion Batteries. J. Phys. Conf. Ser. 2019, 1362, 012026. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, J.; Lu, G.; Li, W.; Tao, Q.; Shi, C.; Jin, H.; Chen, G.; Wang, S. Fundamentals of Electrolytes for Solid-State Batteries: Challenges and Perspectives. Front. Mater. 2020, 7, 111. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Recent Progress of TiO2-Based Anodes for Li Ion Batteries. J. Nanomater. 2016, 2016, 8123652. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Wang, Z.; He, M.; Hou, Z.; Chen, X. TiO2 Nanomaterials as Anode Materials for Lithium-Ion Rechargeable Batteries. Energy Technol. 2015, 3, 801–814. [Google Scholar] [CrossRef]
- Song, T.; Paik, U. TiO2 as an active or supplemental material for lithium batteries. J. Mater. Chem. A Mater. Energy Sustain. 2015, 4, 14–31. [Google Scholar] [CrossRef]
- Yu, W.-B.; Hu, Z.-Y.; Jin, J.; Yi, M.; Yan, M.; Li, Y.; Wang, H.-E.; Gao, H.-X.; Mai, L.-Q.; Hasan, T.; et al. Unprecedented and highly stable lithium storage capacity of (001) faceted nanosheet-constructed hierarchically porous TiO2/rGO hybrid architecture for high-performance Li-ion batteries. Natl. Sci. Rev. 2020, 7, 1046–1058. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, Y.; Xia, T.; Murowchick, J.; Liu, G.; Chen, X. Lithium-ion battery performance of (001)-faceted TiO2 Nanosheets vs. Spherical TiO2 Nanoparticles. Energy Technol. 2014, 2, 376–382. [Google Scholar] [CrossRef]
- Sun, C.H.; Yang, X.H.; Chen, J.S.; Li, Z.; Lou, X.W.; Li, C.; Smith, S.-C.; Lu, G.-Q.; Yang, H.-G. Higher charge/discharge rates of lithium-ions across engineered TiO2 surfaces leads to enhanced battery performance. Chem. Commun. 2010, 46, 6129–6131. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.S.; Wei, X.; Lou, X.W.; Liu, X.-W. Sandwich-like, stacked ultrathin titanate nanosheets for ultrafast lithium storage. Adv. Mater. 2011, 23, 998–1002. [Google Scholar] [CrossRef]
- Tao, H.-C.; Fan, L.-Z.; Yan, X.; Qu, X. In situ synthesis of TiO2–graphene nanosheets composites as anode materials for high-power lithium ion batteries. Electrochim. Acta 2012, 69, 328–333. [Google Scholar] [CrossRef]
- Zheng, P.; Liu, T.; Su, Y.; Zhang, L.; Guo, S. TiO2 nanotubes wrapped with reduced graphene oxide as a high-performance anode material for lithium-ion batteries. Sci. Rep. 2016, 6, 36580. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.S.; Luan, D.; Li, C.M.; Boey, F.Y.C.; Qiao, S.; Lou, X.W. TiO2 and SnO2@TiO2 hollow spheres assembled from anatase TiO2 nanosheets with enhanced lithium storage properties. Chem. Commun. 2010, 46, 8252–8254. [Google Scholar] [CrossRef]
- Chen, J.S.; Wen, X.; Lou, D. Unusual rutile TiO2 nanosheets with exposed (001) facets. Chem. Sci. 2011, 2, 2219–2223. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.S.; David Lou, X.W. One-dimensional hierarchical structures composed of novel metal oxide nanosheets on a carbon nanotube backbone and their lithium-storage properties. Adv. Funct. Mater. 2011, 21, 4120–4125. [Google Scholar] [CrossRef]
- Liu, G.; Yin, L.-C.; Pan, J.; Li, F.; Wen, L.; Zhen, C.; Cheng, H.-M. Greatly Enhanced Electronic Conduction and Lithium Storage of Faceted TiO2 Crystals Supported on Metallic Substrates by Tuning Crystallographic Orientation of TiO2. Adv. Mater. 2015, 27, 3507–3512. [Google Scholar] [CrossRef]
- Chen, J.S.; Liu, H.; Qiao, S.Z.; Lou, X.W. Carbon-supported ultra-thin anatase TiO2 nanosheets for fast reversible lithium storage. J. Mater. Chem. 2011, 21, 5687–5692. [Google Scholar] [CrossRef]




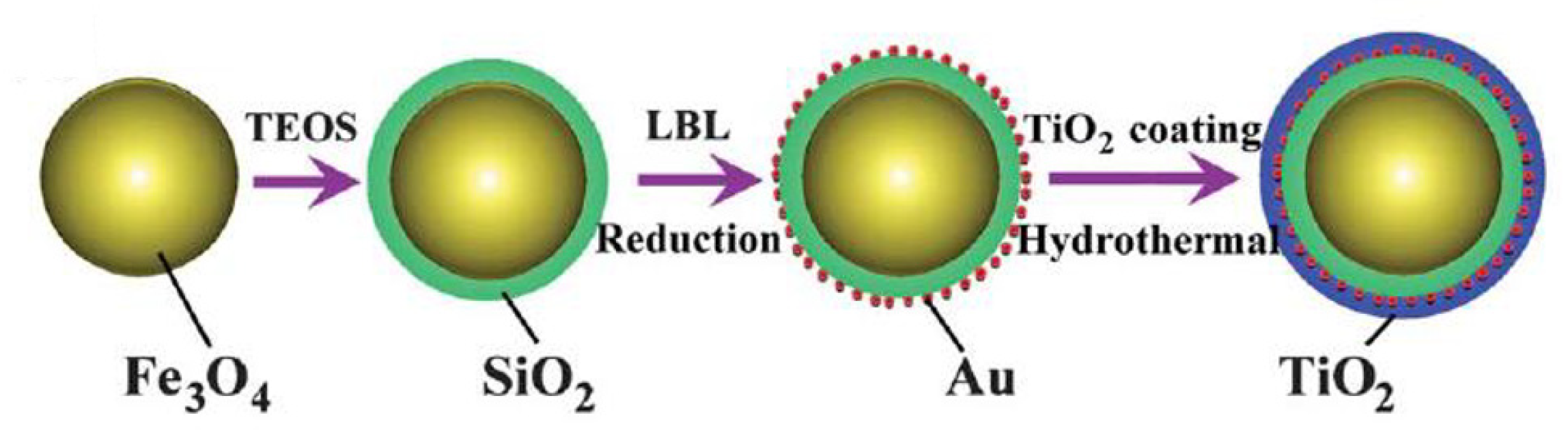
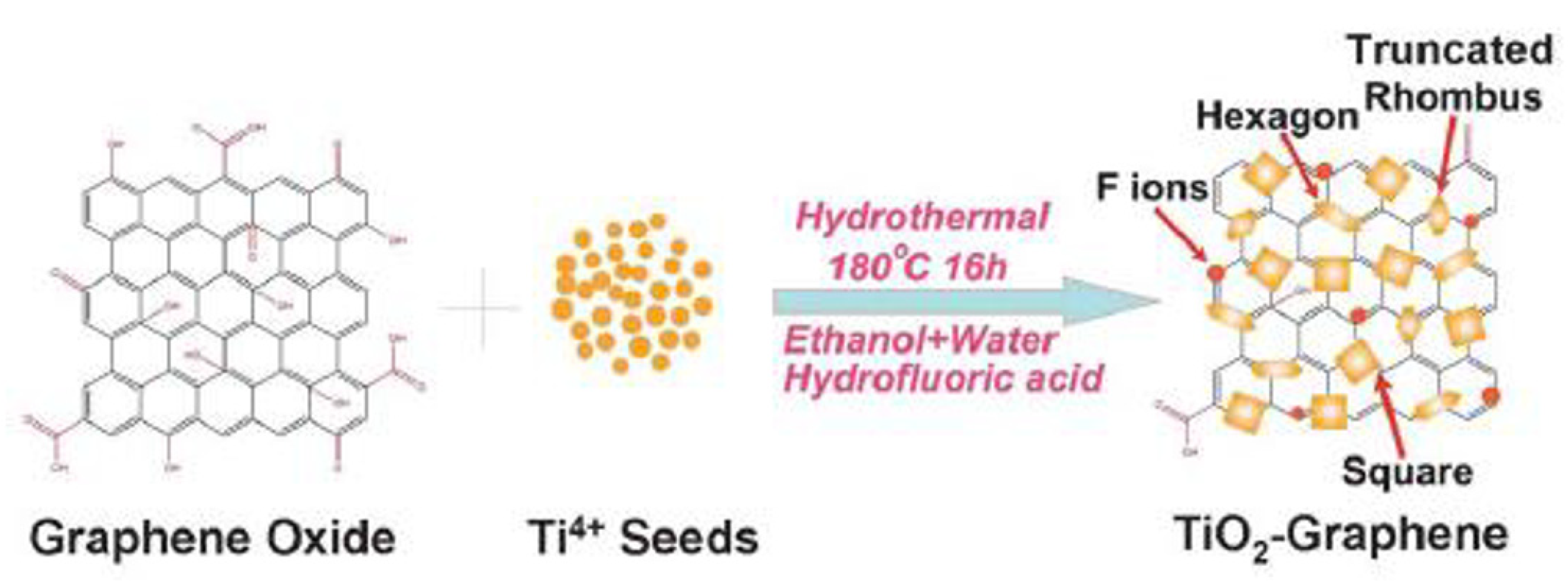
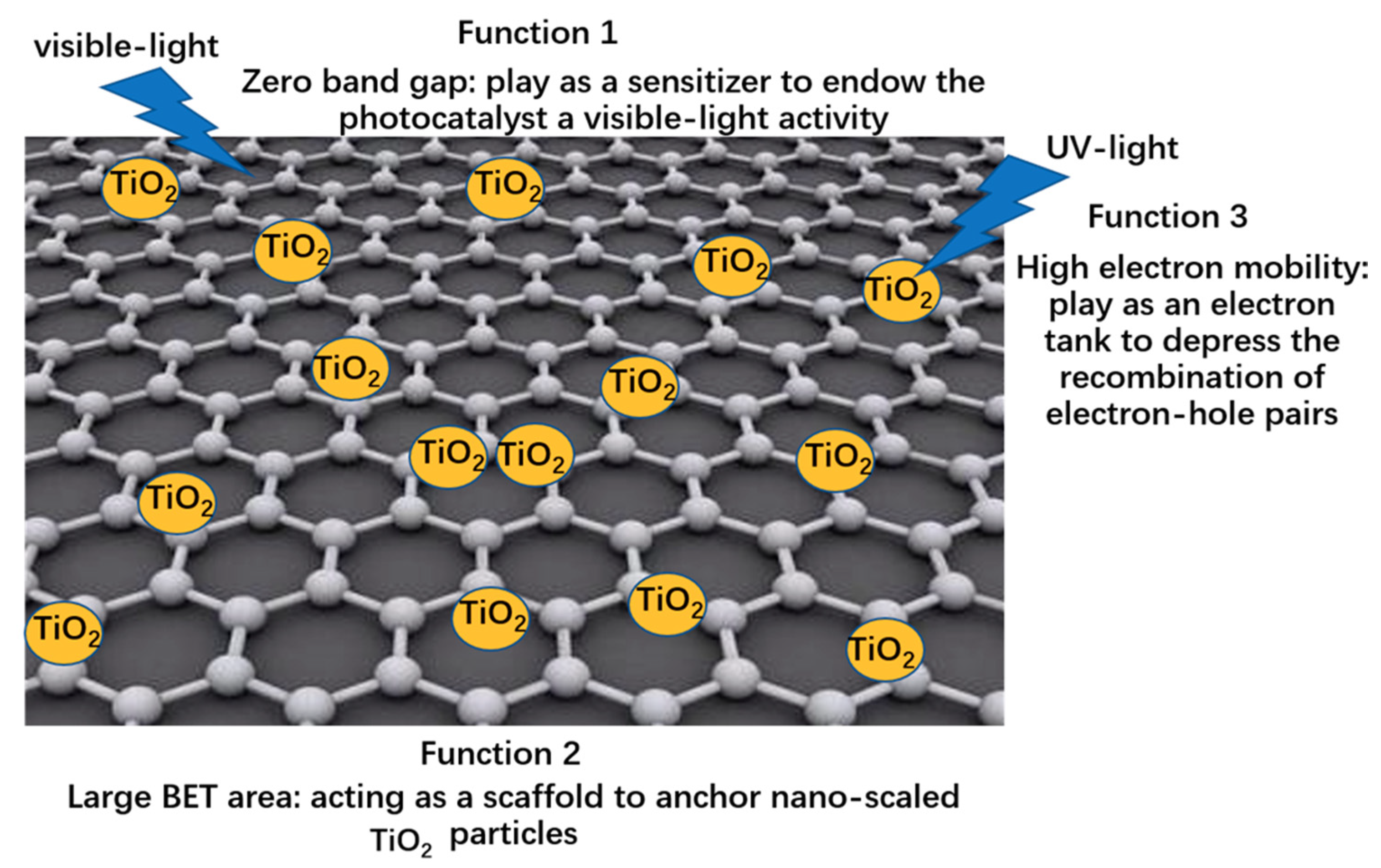
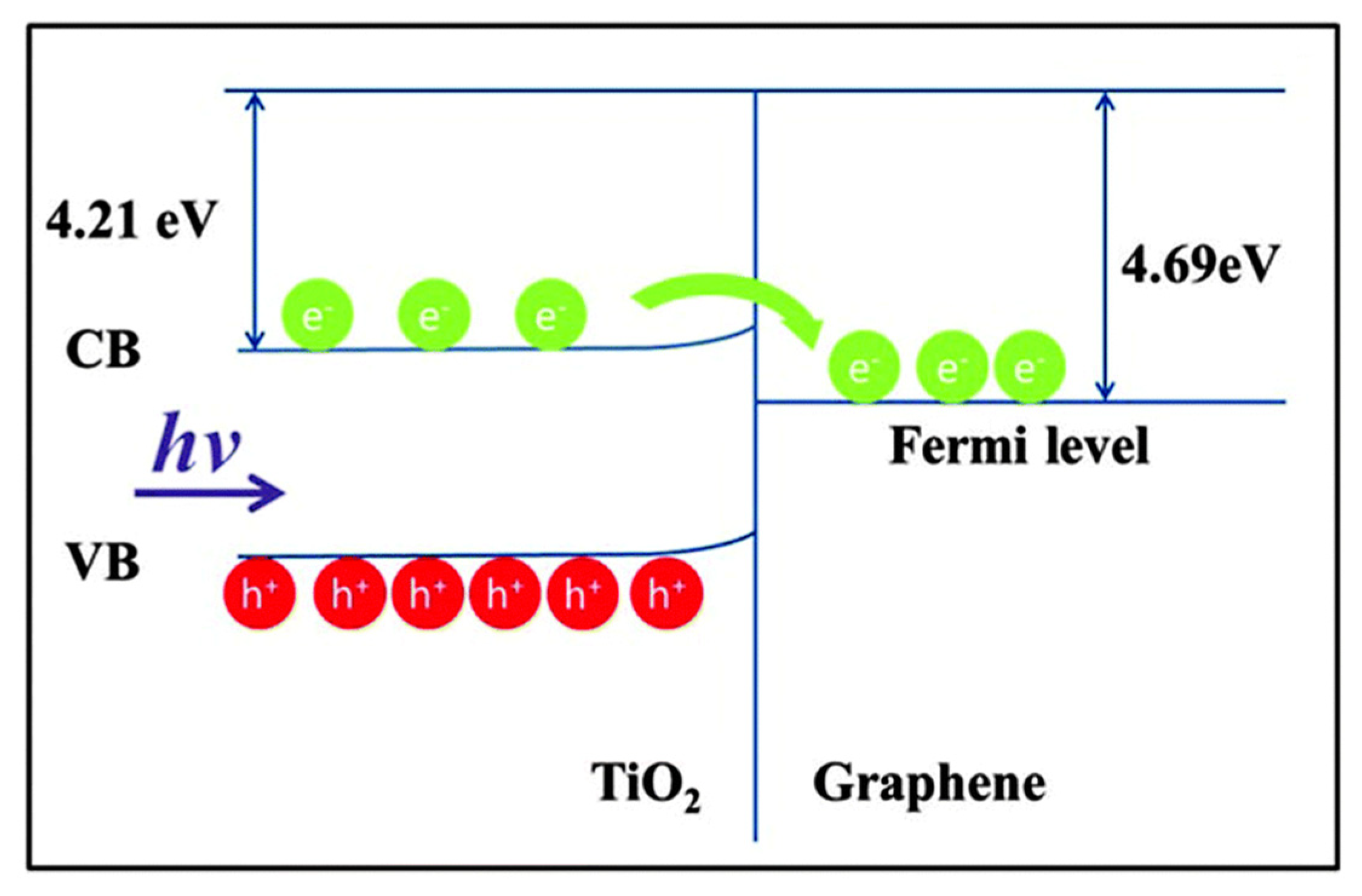



| Precursor | Capping Agent | Synthesis Route | Solvent | % Of 001 Facets | Reference |
|---|---|---|---|---|---|
| TiF4 | HF | Hydrothermal | Water | 47 | [43] |
| Titanium butoxide | HF | Hydrothermal | Water | 89 | [107] |
| TiF4 | HCl | Hydrothermal | Water | >90 | [108] |
| TiF4 | Disodium EDTA | Hydrothermal | Water | 22.8 | [109] |
| TiCl4 | NH4F and 2-propanol | Solvothermal | Alcohol/water | - | [110] |
| TiOSO4 | NH4HF2 | Solvothermal | Water | - | [111] |
| TiCl4 | NaBF4 and NaF | Solvothermal | HCl/water | 55% | [112] |
| (NH4)2TiF6 | H3BO3 and 2-propanol | Solvothermal | H3BO3 and 2-propanol | [113] |
| Precursor | Capping Agent | Synthesis Route | Solvent | Reference |
|---|---|---|---|---|
| TiO2 powder | Oleic acid Oleyamine | Solvothermal | Water vapor | [115] |
| Amorphous TiO2 | Polyvinyl pyrrolidone with acetic acid | Electrospun followed by hydrothermal method | 9.6% | [116] |
| TiCl4 | Ethylene glycol | Hydrothermal | 18% | [117] |
| K-titanate wires | (NH4)2(CO3)) | Hydrothermal | 60% | [114] |
| Ti-isopropoxide | DETA -and isopropyl alcohol | Solvothermal | ~100% | [58] |
| Tetrabutyl titanate | H2SO4 | Hydrothermal | ~100% | [118] |
| Ti-isopropoxide | DEA-TBAH | Hydrothermal | - | [119] |
| Ammonium titanate nanowires | HMTA | Hydrothermal | - | [120] |
| TiCl3 | H2O2 | Hydrothermal | - | [121] |
| Catalysts | % Of 001 Facets | Synthesis Methods (TiO2 Composites) | Applications | Ref |
|---|---|---|---|---|
| TiO2 on graphene sheets | 61 | Fluorine-mediated microwave–hydrothermal (ethanol–water) | H2 production | [130] |
| TiO2 on graphene sheets | 64 | Fluorine-mediated solvothermal method | Degradation of RhB | [131] |
| Graphene–TiO2 composites | ~100% | Fluorine-mediated solvothermal method | Degradation of methylene blue | [132] |
| Graphene–TiO2 | - | Flurine-mediated solvothermal (methnol–water) | H2 production | [133] |
| Graphene–TiO2 | - | Solvothermal method without any capping agent | Degradation of methylene blue | [28] |
| Graphene aerogels–TiO2 | 50% | Glucose-mediated hydrothermal method | Degradation of methyl orange | [134] |
| Graphene foam–TiO2 | - | Fluorine-mediated solvothermal method (ethanol–water) | Degradation of Cr (IV), methyl orange and phenol | [135] |
| RGO–TiO2 | - | Fluorine-mediated solvothermal method | Degradation of basic violet | [136] |
| RGO–TiO2 | - | (DETA)-mediated by solvothermal method | Photo-electrochemical current | [137] |
| Carbon dots–TiO2 | - | Fluorine-mediated hydrothermal method | H2 production | [138] |
| Carbon fibres–TiO2 | 40−92 | Fluorine-mediated hydrothermal method | Degradation of methyl orange | [139] |
| Mesoporous-carbon nitride C3N4-TiO2 | 80.6 | TiO2 fluorine-mediated hydrothermal, composite-mechanical stirring | Degradation of methylene blue | [140] |
| CNTs–TiO2 | 75% | Fluorine-mediated hydrothermal method | Degradation of RhB | [141] |
| Fe3O4 @TiO2 core-shell | - | Combination of template and solvothermal method | Degradation of methylene blue | [142] |
| SrTiO3–TiO2 | - | Fluorine-mediated hydrothermal method | Degradation of RhB dye | [143] |
| Bi4O5Br2–TiO2 | - | TiO2: Fluorine-mediated hydrothermal method Composite: Electrostatic self-assembly | NO removal | [144] |
| Au/TiO2 | 58−77 | TiO2: Fluorine-mediated hydrothermal method Au photodeposition: green method using citrate ions | Photocurrent density | [82] |
| Pt/TiO2 | 75 | TiO2: Fluorine-mediated hydrothermal method Pt deposition: Photochemical reduction | Hydrogen Production | [81] |
| PdO–TiO2 | - | TiO2: Fluorine-mediated hydrothermal method PdO deposition: Wet impregnation | Degradation of methylene blue and photocurrent measurement | [83] |
| Carbon nitrides–TiO2 | - | Fluorine-mediated hydrothermal method | Photoreduction of CO2 to CO | [145] |
| Polymer–TiO2–Graphene | Sonochemically assisted fluorine mediated solvothermal method | Photoreduction of CO2 to CO | [146] | |
| N-doped TiO2–graphene | Fluorine-mediated solvothermal method | Photoreduction of CO2 to CH4 | [147] |
| Catalysts | Synthesis Method | Capacity at Rate (mAh g−1) | Cycling Performance (mAh g−1) | Ref |
|---|---|---|---|---|
| Graphene–TiO2- sandwiched structure | Ionic liquid-mediated solvothermal method | 321/1 C | 155 at 10 C | [165] |
| Graphene aerogels–TiO2 | Glucose-mediated hydrothermal method | 605/0.59 C | 50 cycles at 0.59 C | [134] |
| rGO–TiO2 hybrid | Fluorine-mediated hydrothermal method | 250/1 C | 160, 500 cycles at 10 C | [162] |
| TiO2@graphene nanosheets | Wet chemical method | 306 | 400 cycles | [166] |
| rGO@TiO2 nanotubes | Electrostatic interaction and high temp reduction | 263/0.5 C | 500 cycles at 0.5 C | [167] |
| SnO2@TiO2 | Nanotemplate method | 264.8/1 C | 100 cycles at 10 C | [168] |
| MoO3–rutile TiO2 | Fluorine-mediated hydrothermal method | 330/2 C | 100 cycles at 10 C | [169] |
| TiO2–NSs@CNT: | DETA-mediated hydrothermal synthesis | 395/1 C | 120 cycles at 1C | [170] |
| Cu–TiO2 (001) interface | Fluorine-mediated hydrothermal method | 168/1 C | [171] | |
| Carbon supported TiO2 | DETA-mediated solvothermal synthesis | 172.6/1 C | 100 cycles at 1 C | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokare, A.; Erogbogbo, F. Photocatalysis and Li-Ion Battery Applications of {001} Faceted Anatase TiO2-Based Composites. J 2021, 4, 500-530. https://doi.org/10.3390/j4030038
Bokare A, Erogbogbo F. Photocatalysis and Li-Ion Battery Applications of {001} Faceted Anatase TiO2-Based Composites. J. 2021; 4(3):500-530. https://doi.org/10.3390/j4030038
Chicago/Turabian StyleBokare, Anuja, and Folarin Erogbogbo. 2021. "Photocatalysis and Li-Ion Battery Applications of {001} Faceted Anatase TiO2-Based Composites" J 4, no. 3: 500-530. https://doi.org/10.3390/j4030038
APA StyleBokare, A., & Erogbogbo, F. (2021). Photocatalysis and Li-Ion Battery Applications of {001} Faceted Anatase TiO2-Based Composites. J, 4(3), 500-530. https://doi.org/10.3390/j4030038






