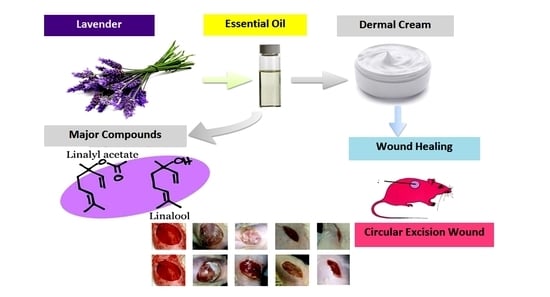
Topical Emulsion Containing Lavandula stoechas Essential Oil as a Therapeutic Agent for Cutaneous Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Lavandula stoechas Essential Oil
2.1.2. Animals
2.1.3. Drugs and Chemicals
2.2. Methods
2.2.1. Determination of Chemical Composition of Essential Oil
2.2.2. In Vivo Wound Healing Activity
Preparation of Test Samples for Bioassay
Circular Excision Wound Model
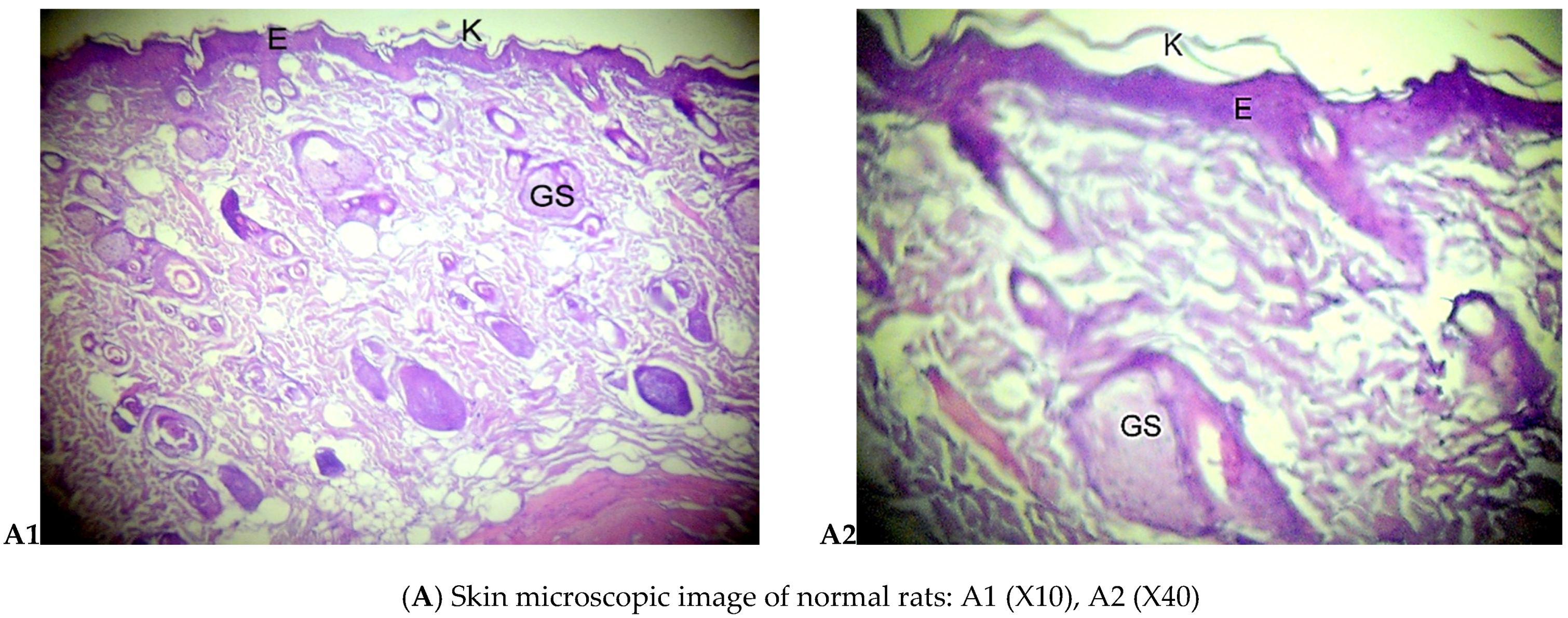
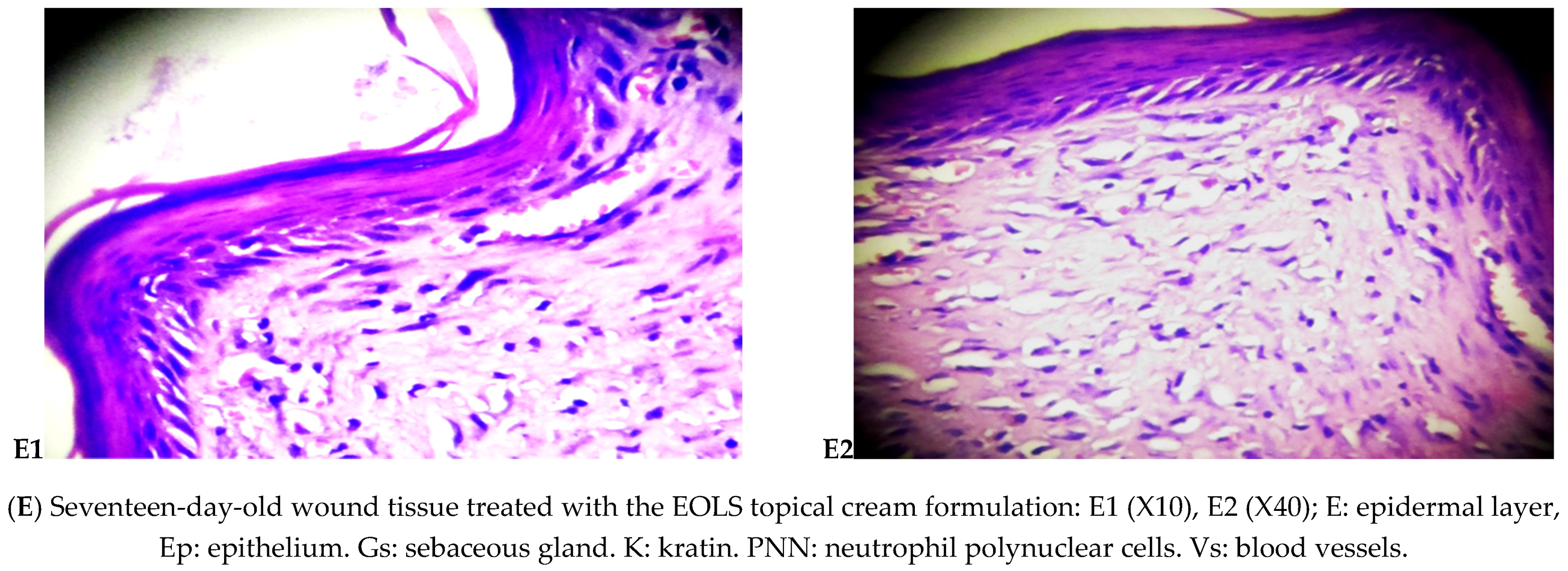
Histology Examination
2.3. Statistical Analysis
3. Results
3.1. Chemical Composition of Lavandula Stoechas Essential Oil
3.2. In Vivo Pharmacological Evaluation of Wound Healing Effect
3.2.1. Effect of Lavender Essential Oil on Percent Wound Contraction and Area
3.2.2. Histological Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| EGF | Epidermal growth factor |
| EO | Essential oil |
| EOLS | Lavandula stoechas essential oil |
| GC-MS | Gas chromatography-mass spectrometry |
| H&E | Hematoxylin and eosin |
| HD | Hydrodistillation |
| hMDM | human Monocyte-derived macrophage |
| IFNγ | Interferon gamma |
| IL-6 | Interleukin-6 |
| LNCPP | Laboratoire National de Contrôle des Produits Pharmaceutiques |
| LPS | Lipopolysaccharide |
| MDA | Malondialdehyde |
| NIST | National Institute of Standards and Technology |
| NPs | Nanoparticles |
| PAI-1 | Plasminogen activator inhibitor |
| PDGF-A | Platelet-derived growth factor subunit A |
| REEDA | Redness, edema, ecchymosis, discharge, and approximation scale |
| RT | Retention times |
| SSD | Silver sulfadiazine |
| TENS | Transcutaneous electrical nerve stimulation |
| TGF-β | Transforming growth factor-β |
| TNF-α | Tumor necrosis factor alpha |
| UVB | Ultraviolet radiation |
| VEGF | Vascular endothelial growth factor |
References
- Suleyman, H.; Demircan, B.; Karagoz, Y. Anti-inflammatory and side effects of cyclo-oxygenase inhibitors. Pharmacol. Rep. 2007, 59, 247. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Lang, G.; Buchbauer, G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flav. Fragr. J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- De Oliveira, M.L.M.; Bezerra, B.M.O.; Leite, L.O.; Girão, V.C.C.; Nunes-Pinheiro, D.C.S. Topical continuous use of Lippia sidoides Cham. essential oil induces cutaneous inflammatory response, but does not delay wound healing process. J. Ethnopharmacol. 2014, 153, 283–289. [Google Scholar] [CrossRef]
- Maver, T.; Maver, U.; Stana Kleinschek, K.; Smrke, D.M.; Kreft, S. A review of herbal medicines in wound healing. Int. J. Dermatol. 2015, 54, 740–751. [Google Scholar] [CrossRef]
- Keskin, I.; Gunal, Y.; Ayla, S.; Kolbasi, B.; Sakul, A.; Kilic, U.; Ozbek, H. Effects of Foeniculum vulgare essential oil compounds, fenchone and limonene, on experimental wound healing. Biotech. Histochem. 2017, 92, 274–282. [Google Scholar] [CrossRef]
- Lis-Balchin, M. Lavender: The Genus Lavandula; CRC Press: London, UK, 2003. [Google Scholar]
- Benabdelkader, T. Biodiversité, Bioactivité et Biosynthèse des Composés Terpéniques Volatils des Lavandes Ailées, Lavandula Stoechas Sensu Lato, un Complexe D’espèces Méditerranéennes D’intérêt Pharmacologique. Ph.D. Thesis, Filière de Biologie, Université Jean Monnet-Saint-Etienne (France) en co-tutelle avec l’Ecole Normale Supérieure de Kouba, Algiers, Algeria, 2012. [Google Scholar]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Ben Djemaa, F.G.B.; Bellassoued, K.; Zouari, S.; El Feki, A.; Ammar, E. Antioxidant and wound healing activity of Lavandula aspic L. ointment. J. Tissue Viab. 2016, 25, 193–200. [Google Scholar] [CrossRef]
- Rahmati, B.; Kiasalari, Z.; Roghani, M.; Khalili, M.; Ansari, F. Antidepressant and anxiolytic activity of Lavandula officinalis aerial parts hydroalcoholic extract in scopolamine-treated rats. Pharm. Biol. 2017, 55, 958–965. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, M.; Kiani, Z.; Moezi, S.A.; Rad, G.H.M. The Effects of Lavender, Valerian, and Oxazepam on anxiety among hospitalized patients with coronary artery disease. Modern. Care J. 2018, 15, e68390. [Google Scholar] [CrossRef]
- Miraj, S. Lavandula stoechas L: A systematic review of medicinal and molecular perspectives. Der. Pharma. Lett. 2016, 8, 56–58. [Google Scholar]
- Süntar, I.; Tumen, I.; Ustün, O.; Keleş, H.; Akkol, E.K. Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J. Ethnopharmacol. 2012, 139, 533–540. [Google Scholar] [CrossRef]
- Dob, T.; Dahmane, D.; Agli, M.; Chelghoum, C. Essential oil composition of Lavandula stoechas from Algeria. Pharm. Biol. 2006, 44, 60–64. [Google Scholar] [CrossRef]
- Baali, F.; Boumerfeg, S.; Napoli, E.; Boudjelal, A.; Righi, N.; Deghima, A.; Ruberto, G. Chemical Composition and Biological Activities of Essential Oils from Two Wild Algerian Medicinal Plants: Mentha pulegium L. and Lavandula stoechas L. J. Essent. Oil Bear. Plants 2019, 22, 821–837. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Sudha, T.; Darwish, N.H.; Chader, H.; Belkadi, A.; Rajabi, M.; Mousa, S.A. A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer. Molecules 2020, 25, 3671. [Google Scholar] [CrossRef]
- Yakoubi, R.; Megateli, S.; Sadok, T.H.; Bensouici, C.; Bağci, E. A synergistic interactions of Algerian essential oils of Laurus nobilis L., Lavandula stoechas L. and Mentha pulegium L. on anticholinesterase and antioxidant activities. Biocatal. Agric. Biotechnol. 2021, 31, 101891. [Google Scholar] [CrossRef]
- Loukhaoukha, R.; Saidi, F.; Jullien, F.; Benabdelkader, T. Chemical Composition and Antibacterial Activity of Lavandula Stoechas Essential Oil and Its Main Components against Erwinia Amylovora and Pectobacterium Carotovorum Subsp. Carotovorum. Phytothérapie 2018, 16, 149–157. [Google Scholar] [CrossRef]
- Barkat, M.; Laib, I. Antioxidant activity of the essential oil from the flowers of Lavandula stoechas. J. Pharmacogn. Phytother. 2012, 4, 96–101. [Google Scholar]
- Kokkalou, E. The constituents of the essential oil from Lavandula stoechas growing wild in Greece. Planta Med. 1988, 54, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Skoula, M.; Abidi, C.; Kokkalou, E. Essential oil variation of Lavandula stoechas L. ssp. stoechas growing wild in Crete (Greece). Biochem. Syst. Ecol. 1996, 24, 255–260. [Google Scholar] [CrossRef]
- Ristorcelli, D.; Tomi, F.; Casanova, J. 13C-NMR as a tool for identification and enantiomeric differentiation of major terpenes exemplified by the essential oil of Lavandula stoechas L. ssp. stoechas. Flav. Fragr. J. 1997, 13, 154–158. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Benzarti, A.; Marongiu, B.; Salgueiro, L. Antifungal and anti-inflammatory potential of Lavandula stoechas and Thymus herba-barona essential oils. Ind. Crops Prod. 2013, 44, 97–103. [Google Scholar] [CrossRef]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Pintado, C.S. Essential Oils of Lavandula stoechas subsp. luisieri as Antifungal Agent against Fungi from Strawberry Tree Fruit. J. Pharm. Pharmacol. 2021, 9, 98–106. [Google Scholar]
- Messaoud, C.; Chograni, H.; Boussaid, M. Chemical composition and antioxidant activities of essential oils and methanol extracts of three wild Lavandula L. species. Nat. Prod. Res. 2012, 26, 1976–1984. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Tammar, S.; Hammami, M.; Jamal Saharkhiz, M.; Debiche, N.; Marzouk, B. Essential oil composition of Lavandula dentata, L. stoechas and L. multifida cultivated in Tunisia. J. Essent. Oil Res. Bear. Plants 2012, 15, 1030–1039. [Google Scholar] [CrossRef]
- Akgün, N.A.; Akgün, M.; Dinçer, S.; Akgerman, A. Supercritical fluid extraction of Lavandula stoechas L. ssp. cariensis (Boiss.) Rozeira. J. Essent. Oil Res. 2001, 13, 143–148. [Google Scholar] [CrossRef]
- Giray, E.S.; Kırıcı, S.; Kaya, D.A.; Türk, M.; Sönmez, Ö.; Inan, M. Comparing the effect of sub-critical water extraction with conventional extraction methods on the chemical composition of Lavandula stoechas. Talanta 2008, 74, 930–935. [Google Scholar] [CrossRef]
- Kırmızıbekmez, H.; Demirci, B.; Yeşilada, E.; Başer, K.H.C.; Demirci, F. Chemical composition and antimicrobial activity of the essential oils of Lavandula stoechas L. ssp. stoechas growing wild in Turkey. Nat. Prod. Commun. 2009, 4, 1934578X0900400727. [Google Scholar]
- Gören, A.C.; Topçu, G.; Bilsel, G.; Bilsel, M.; Aydoğmusç, Z.; Pezzuto, J.M. The chemical constituents and biological activity of essential oil of Lavandula stoechas ssp. stoechas. Z. Nat. C 2002, 57, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, İ.A.; Soylu, S.; Kara, M.; Soylu, E.M. Chemical composition and antibacterial activity of essential oils isolated from medicinal plants against gall forming plant pathogenic bacterial disease agents. Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa Derg. 2020, 23, 1474–1482. [Google Scholar]
- Karan, T. Metabolic profile and biological activities of Lavandula stoechas L. Cell. Mol. Biol. 2018, 64, 1–7. [Google Scholar] [CrossRef]
- Cherrat, L.; Espina, L.; Bakkali, M.; Pagán, R.; Laglaoui, A. Chemical composition, antioxidant and antimicrobial properties of Mentha pulegium, Lavandula stoechas and Satureja calamintha Scheele essential oils and an evaluation of their bactericidal effect in combined processes. Innov. Food Sci. Emerg. Technol. 2014, 22, 221–229. [Google Scholar] [CrossRef]
- Khavarpour, M.; Vahdat, S.M.; Moghadamnia, A.A.; Hasanzadeh, O.; Salimi, Z.; Rahmanpour, N. Chemical composition, antibacterial and analgesic activity of Lavandula stoechas flowers from north of Iran. Int. J. Eng. 2019, 32, 1065–1073. [Google Scholar]
- Asghari, J.; Sadani, S.; Ghaemi, E.; Mazaheri Tehrani, M. Investigation of composition and antimicrobial properties of Lavandula stoechas essential oil using disk diffusion and broth microdilution. Med. Lab. J. 2016, 10, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Chebil, B.; Achouri, M.; Idrissi Hassani, L.M.; Hmamouchi, M. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J. Ethnopharmacol. 2003, 89, 165–169. [Google Scholar]
- Vokou, D.; Chalkos, D.; Karamanlidou, G.; Yiangou, M. Activation of soil respiration and shift of the microbial population balance in soil as a response to Lavandula stoechas essential oil. J. Chem. Ecol. 2002, 28, 755–768. [Google Scholar] [CrossRef]
- Danh, L.T.; Triet, N.D.A.; Zhao, J.; Mammucari, R.; Foster, N. Comparison of chemical composition, antioxidant and antimicrobial activity of lavender (Lavandula angustifolia L.) essential oils extracted by supercritical CO2, hexane and hydrodistillation. Food Bioproc. Technol. 2013, 6, 3481–3489. [Google Scholar] [CrossRef]
- Granger, R.; Passet, J.; Teulade-Arbousset, G. A propos d’une labiée cosmopolite: LaVandula stoechas L. Trav. Soc. Pharm. Montp. 1973, 33, 335–360. [Google Scholar]
- Demasi, S.; Caser, M.; Lonati, M.; Cioni, P.L.; Pistelli, L.; Najar, B.; Scariot, V. Latitude and altitude influence secondary metabolite production in peripheral alpine populations of the Mediterranean species Lavandula angustifolia Mill. Front. Plant Sci. 2018, 9, 983. [Google Scholar] [CrossRef] [Green Version]
- Hassiotis, C.N.; Ntana, F.; Lazari, D.M.; Poulios, S.; Vlachonasios, K.E. Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period. Ind. Crops Prod. 2014, 62, 359–366. [Google Scholar] [CrossRef]
- Baali, F.; Boumerfeg, S.; Boudjelal, A.; Denaro, M.; Ginestra, G.; Baghiani, A.; Trombetta, D. Wound-healing activity of Algerian Lavandula stoechas and Mentha pulegium extracts: From traditional use to scientific validation. Plant Biosyst. 2021, 1–20. [Google Scholar] [CrossRef]
- Lusby, P.E.; Coombes, A.L.; Wilkinson, J.M. A comparison of wound healing following treatment with Lavandula x allardii honey or essential oil. Phytother. Res. 2006, 20, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Vakilian, K.; Atarha, M.; Bekhradi, R.; Chaman, R. Healing advantages of lavender essential oil during episiotomy recovery: A clinical trial. Complement. Ther. Clin. Pract. 2011, 17, 50–53. [Google Scholar] [CrossRef]
- Kazemi, M.; Mohammadifar, M.; Aghadavoud, E.; Vakili, Z.; Aarabi, M.H.; Talaei, S.A. Deep skin wound healing potential of lavender essential oil and licorice extract in a nanoemulsion form: Biochemical, histopathological and gene expression evidences. J. Tissue Viability 2020, 29, 116–124. [Google Scholar] [CrossRef]
- Carbone, C.; Caddeo, C.; Grimaudo, M.A.; Manno, D.E.; Serra, A.; Musumeci, T. Ferulic Acid-NLC with Lavandula essential oil: A possible strategy for wound-healing? Nanomaterials 2020, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, F.A. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int. J. Pharm. 2019, 569, 118590. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement. Altern. Med. 2016, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Momtaz, S.; Abdolghaffari, A.; Jasemi, E.; Yaghoobvand, B.; Esmaeilzadeh, S.; Abdollahi, A.; Abdollahi, M. Evaluation of wound healing and anti-inflammatory activities of a herbal ointment consisting of Althaea officinalis, Lavandula angustifolia, and Rosa x damascena in animal excision wound model. J. Med. Plants 2020, 20, 37–49. [Google Scholar]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Pintore, G. Fibroblast proliferation and migration in wound healing by phytochemicals: Evidence for a novel synergic outcome. Int. J. Med. Sci. 2020, 17, 1030. [Google Scholar] [CrossRef] [Green Version]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Mele, E. Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef] [Green Version]
- Miastkowska, M.; Kantyka, T.; Bielecka, E.; Kałucka, U.; Kamińska, M.; Kucharska, M.; Cudzik, K. Enhanced Biological Activity of a Novel Preparation of Lavandula angustifolia Essential Oil. Molecules 2021, 26, 2458. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Beiraghdar, F.; Akbari, H.; Bekhradi, H.; Taghizadeh, M.; Sahebkar, A. A herbal cream consisting of Aloe vera, Lavandula stoechas, and Pelargonium roseum as an alternative for silver sulfadiazine in burn management. Asian Biomed. 2012, 6, 273–278. [Google Scholar]
- Hartman, D.; Coetzee, J.C. Two US practitioners’ experience of using essential oils for wound care. J. Wound Care 2002, 11, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, T.; Barakat, R.; Ragab, A.; Badria, F.; Badawy, A. Lavender-thymol as a new topical aromatherapy preparation for episiotomy: A randomised clinical trial. J. Obstet. Gynaecol. 2015, 35, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Altaei, D.T. Topical lavender oil for the treatment of recurrent aphthous ulceration. Am. J. Dent. 2012, 25, 39–43. [Google Scholar] [PubMed]
- Koca Kutlu, A.; Çeçen, D.; Gürgen, S.G.; Sayın, O.; Çetin, F. A comparison study of growth factor expression following treatment with transcutaneous electrical nerve stimulation, saline solution, povidone-iodine, and lavender oil in wounds healing. Evid. Based Complement. Alternat. Med. 2013. [Google Scholar] [CrossRef]
- Sheikhan, F.; Jahdi, F.; Khoei, E.M.; Shamsalizadeh, N.; Sheikhan, M.; Haghani, H. Episiotomy pain relief: Use of Lavender oil essence in primiparous Iranian women. Complement. Ther. Clin. Pract. 2012, 18, 66–70. [Google Scholar] [CrossRef]
- Harpreet, K.; Monika, K.B. A study to assess the effectiveness of lavender oil versus povidine iodine on healing of episiotomy wound among postnatal mothers. Indian J. Public Health Res. Dev. 2016, 7, 3. [Google Scholar]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-based colloidal carriers for peptide and protein delivery—Liposomes versus lipid nanoparticles. Int. J. Nanomed. 2007, 2, 595–607. [Google Scholar]
- Han, X.; Beaumont, C.; Stevens, N. Chemical composition analysis and in vitro biological activities of ten essential oils in human skin cells. Biochim. Open 2017, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]






| Ingredients | Quantity (%) |
|---|---|
| Lipophilic phase | |
| Almond oil | 12–20 |
| Beeswax | 3–5 |
| Stearic acid | 6–8 |
| Cetylic alcohol | 0.2–2 |
| Stearyl alcohol | 0.2–1 |
| Ceteareth-20 | 0.2–2 |
| Lavandula stoechas essential oil | 0.5 |
| Hydrophilic phase | |
| Deionized water | 60–70 |
| Octyldodecanol | 1–2 |
| Glycerin | 3–5 |
| Xanthan Gum | 0.1–0.3 |
| Trolamine | 0.5 |
| RT b | Compounds a | % |
|---|---|---|
| 8.842 | α-Pinene | 0.44 |
| 10.158 | Myrcene | 7.62 |
| 10.808 | L-Limonene | 2.84 |
| 11.000 | trans-β-Ocimene | 4.79 |
| 11.191 | cis-β-Ocimene | 5.70 |
| 11.328 | γ-Terpinene | 0.27 |
| 12.204 | Linalool | 24.87 |
| 12.763 | Camphor | 0.13 |
| 13.303 | Terpineol-4 | 5.15 |
| 13.476 | α-Terpineol | 1.92 |
| 14.481 | Linalyl acetate | 19.10 |
| 15.865 | Neryl acetate | 1.86 |
| 16.128 | Geranyl acetate | 3.73 |
| 16.640 | trans-Caryophyllene | 6.35 |
| 17.062 | β-Farnesene | 7.17 |
| 17.673 | β-Bisabolene | 0.17 |
| 17.861 | δ-Cadinene | 0.10 |
| 18.570 | Caryophyllene oxide | 0.20 |
| 19.246 | δ-Cadinol | 0.14 |
| Monoterpene hydrocarbons | 21.66 | |
| Oxygenated monoterpenes | 56.76 | |
| Sesquiterpene hydrocarbons | 13.79 | |
| Oxygenated sesquiterpenes | 0.34 | |
| Total identified | 92.55 |
| Country | Plant Material | Extraction Method | Major Compounds (%) | References |
|---|---|---|---|---|
| Algeria | Dried and finely powdered aerial parts (leaves and flowers) Flowering period | Hydrodistillation (HD) | Fenchone = 31.6 Camphor = 22.4 p-Cymene = 6.5 | Dob et al. [18] |
| Air dried aerial parts | HD | Fenchone = 50.29 Camphor = 14.02 Bornyl acetate = 5.60 | Baali et al. [19] | |
| Aerial parts (leaves, stems, flowers) | Alembic steam distillation. | 1,8-Cineol = 61.36 β-Pinene = 13.83 α-Pinene = 4.75 | Boukhatem et al. [20] | |
| Dried plants (leaves) | HD | Fenchone = 25.48 Camphor = 24.44 Pulegone = 5.81 | Yakoubi et al. [21] | |
| Dried flowers | HD | Fenchone = 40.78 Camphor = 9.76 Myrtenyl acetate = 8.94 Bornyl acetate = 5.1 | Loukhaoukha et al. [22] | |
| Dried flowers | HD | Linalyl acetate = 15.26 Linalool = 10.68 1,8-Cineol = 10.25 γ-Terpinene = 11.2 | Barkat and Laib [23] | |
| Crete (Greece) | Dried aerial part | Steam distillation | Fenchone = 30.85 | Kokkalou [24] |
| Air dried aerial part (leaves, inflorescences) | HD | Fenchone = 44.8 1,8-Cineol = 16.7 α-Cadinol = 7.4 Camphor = 6.2 α-Pinene = 2.2 | Skoula et al. [25] | |
| Corsica (France) | Fresh material | HD | Fenchone = 31.6–75.5 Camphor = 9.1–28.4 1,8-Cineol = 17.8 | Ristorcelli et al. [26] |
| Sardinia (Italy) | Air-dried aerial part | HD | Fenchone = 37 Camphor = 27.3 Bornyl acetate = 6.2 1,8-Cineol = 6 | Zuzarte et al. [27] |
| Portugal | Aerial parts (leaves and flowers) of L. stoechas subsp. Luisieri | HD | Dormancy stage trans-α-Necrodyl acetate = 12.58 Fenchone = 5.97 trans-α-Necrodol = 5.22 Flowering stage trans-α-Necrodyl acetate = 26.90 trans-α-Necrodol = 13.02 Lavandulyl acetate = 6.53 | Domingues et al. [28] |
| Tunisia | Air-dried aerial parts (stems, leaves) Vegetative stage. | HD | Fenchone = 34.3 Camphor = 27.4 Lavandulyl acetate = 5.6 | Messaoud et al. [29] |
| Air-dried aerial parts | HD | Linalyl acetate = 64.30–7.55 Linalool = 20.25–3.21 β-Thuyone = 8.97–0.99 | Msaada et al. [30] | |
| Turkey | Flowers | CO2 Supercritical fluid extraction | Camphor = 58.8 Fenchone = 33 α-Pinene = 3.5 α-Cadinol = 0.2 | Akgün et al. [31] |
| Flowers | Solvent extraction (Soxhlet) | Camphor = 41.3 Fenchone = 31.3 α-Cadinol = 11.7 α-Pinene = 2.6 | ||
| Dried flowers (flowering stage) | Sub-critical water extraction | Camphor = 29.64 Fenchone = 26.93 1,8-Cineol = 4.38 | Giray et al. [32] | |
| Ultrasound assisted extraction | Camphor = 41.09 Fenchone = 34.23 Myrtenyl acetate = 4.97 | |||
| HD | Fenchone = 32.03 Camphor = 14.71 Myrtenyl acetate = 11.70 1,8-Cineol = 7.67 | |||
| Air dried leaves and flowers | HD | Leaves α-Fenchone = 41.9 ± 1.2 1,8-Cineol = 15.6 ± 0.8 Camphor = 12.1 ± 0.5 Viridiflorol = 4.1 ± 0.4 Flowers α-Fenchone = 39.2 ± 0.9 Myrtenyl acetate = 9.5 ± 0.4 α-Pinene = 6.1 ± 0.09 Camphor = 5.9 ± 0.05 1,8-Cineol = 3.8 ± 0.1 | Kırmızıbekmez et al. [33] | |
| Leaves | HD | Pulegone = 40.37 Menthol = 18.09 Menthone = 12.57 Eucalyptol = 3.9 | Gören et al. [34] | |
| Air dried leaves | HD | 1,8-Cineol = 35.5 Camphor = 20.2 α-Thujone = 15.9 | Bozkurt et al. [35] | |
| Aerial part (flowering season) | HD | Camphor = 48.1 Fenchone = 30.5 Muurolol = 5.72 | Karan et al. [36] | |
| Morocco | Dried aerial part (leaves) | HD | 10s,11s-himachala-3(12),4-diene = 23.62 Cubenol = 16.19 Methyl eugenol = 6.19 | Cherrat et al. [37] |
| Iran | Air-dried flowers | HD | Linalool = 35.69 Borneol = 14.99 1,8-Cineol = 11.45 Camphor = 4.32 4-Terpineol = 3.72 | Khavarpour et al. [38] |
| Shade dried flowers | Steam distillation | Camphor = 71.8 1,8-Cineol = 4.08 Linalool = 3.77 Borneol = 3.19 | Asghari et al. [39] |
| Wound Healing Processes | Healing Phases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Groups | S | U | RE | FP | CD | PMN | NV | I | P | R |
| Vehicle | +++ | ++ | −/+ | +++ | ++ | ++ | ++ | + | +++ | −/+ |
| EOLS | ++ | + | ++ | ++ | ++ | + | ++ | + | ++ | ++ |
| Madecassol® | +/++ | − | +++ | + | +++ | −/+ | + | + | + | ++ |
| Authors | Country | Plant Species | Objectives | Main Results |
|---|---|---|---|---|
| Baali et al. [46] | Algeria | Lavandula stoechas |
|
|
| Kazemi et al. [49] | Iran | Lavandula Angustifolia |
|
|
| Carbone et al. [50] | Italy | Lavandula intermedia |
|
|
| Sofi et al. [51] | India | Lavandula Angustifolia |
|
|
| Mori et al. [52] | Japan | Lavandula angustifolia |
|
|
| Ben Djemaa et al. [13] | Tunisia | Lavandula aspic |
|
|
| Lusby et al. [47] | Australia | Lavandula allardii |
|
|
| Momtaz et al. [53] | Iran | Lavandula angustifolia |
|
|
| Addis et al. [54] | Italy | Lavandula stoechas |
|
|
| Hajiali et al. [55] | Italy | Lavandulaangustifolia |
|
|
| Miastkowska et al. [56] | Poland | Lavandula angustifolia |
|
|
| Panahi et al. [57] | Iran | Lavandula stoechas | Investigation of the ability of herbal combination cream containing lavender and rose-scented geranium EOs and aloe vera gel in the improvement of symptoms in patients with superficial second-degree burns, in comparison with silver sulfadiazine (SSD) 1% cream. |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukhatem, M.N.; Chader, H.; Houche, A.; Oudjida, F.; Benkebaili, F.; Hakim, Y. Topical Emulsion Containing Lavandula stoechas Essential Oil as a Therapeutic Agent for Cutaneous Wound Healing. J 2021, 4, 288-307. https://doi.org/10.3390/j4030023
Boukhatem MN, Chader H, Houche A, Oudjida F, Benkebaili F, Hakim Y. Topical Emulsion Containing Lavandula stoechas Essential Oil as a Therapeutic Agent for Cutaneous Wound Healing. J. 2021; 4(3):288-307. https://doi.org/10.3390/j4030023
Chicago/Turabian StyleBoukhatem, Mohamed Nadjib, Henni Chader, Aicha Houche, Faiza Oudjida, Fatma Benkebaili, and Yahia Hakim. 2021. "Topical Emulsion Containing Lavandula stoechas Essential Oil as a Therapeutic Agent for Cutaneous Wound Healing" J 4, no. 3: 288-307. https://doi.org/10.3390/j4030023
APA StyleBoukhatem, M. N., Chader, H., Houche, A., Oudjida, F., Benkebaili, F., & Hakim, Y. (2021). Topical Emulsion Containing Lavandula stoechas Essential Oil as a Therapeutic Agent for Cutaneous Wound Healing. J, 4(3), 288-307. https://doi.org/10.3390/j4030023