Riluzole Oral Suspension for the Treatment of Amyotrophic Lateral Sclerosis: Texture and Compatibility with Food Thickeners Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Nutilis Powder (Nutricia, Zutemer, Netherlands)
- Nutilis Clear (Nutricia)
- RESOURCE® ThickenUp™ (Nestlé Health Science, Vevey, Switzerland)
- Fresubin® Clear Thickener (Fresenius Kabi, Bad Humboldt, Germany)
- THICK & EASY™ Instant Food Thickener (Fresenius Kabi)
- Milli-Q grade water, analytical grade solvents/reagents (Acetonitrile, Trifluoroacetic acid, Ethanol) analytical grade laboratory glassware, disposable plastic syringes and 0.45-µm hydrophilic PTFE syringe filters were used.
2.2. Methods
2.2.1. IDDSI Flow Test (Drink Testing Methods)
2.2.2. Preparation of Samples for the HPLC Analyses of Riluzole 5 mg/mL Oral Suspension-Thickener Mixtures
2.2.3. Determination of Riluzole Assay and Related Substances by HPLC
2.2.4. Determination of Appearance and pH of Riluzole 5 mg/mL Oral Suspension-Thickener Mixtures
3. Results
3.1. IDDSI Flow Test Results
3.2. HPLC Results: Riluzole Assay of Riluzole 5 mg/mL Oral Suspension-Thickener Mixtures
3.3. HPLC Results: Riluzole-Related Substances of Riluzole 5 mg/mL Oral Suspension-Thickener Mixtures
3.4. Appearance and pH of Riluzole 5 mg/mL Oral Suspension-Thickener Mixtures and Placebo Control Samples
4. Discussion
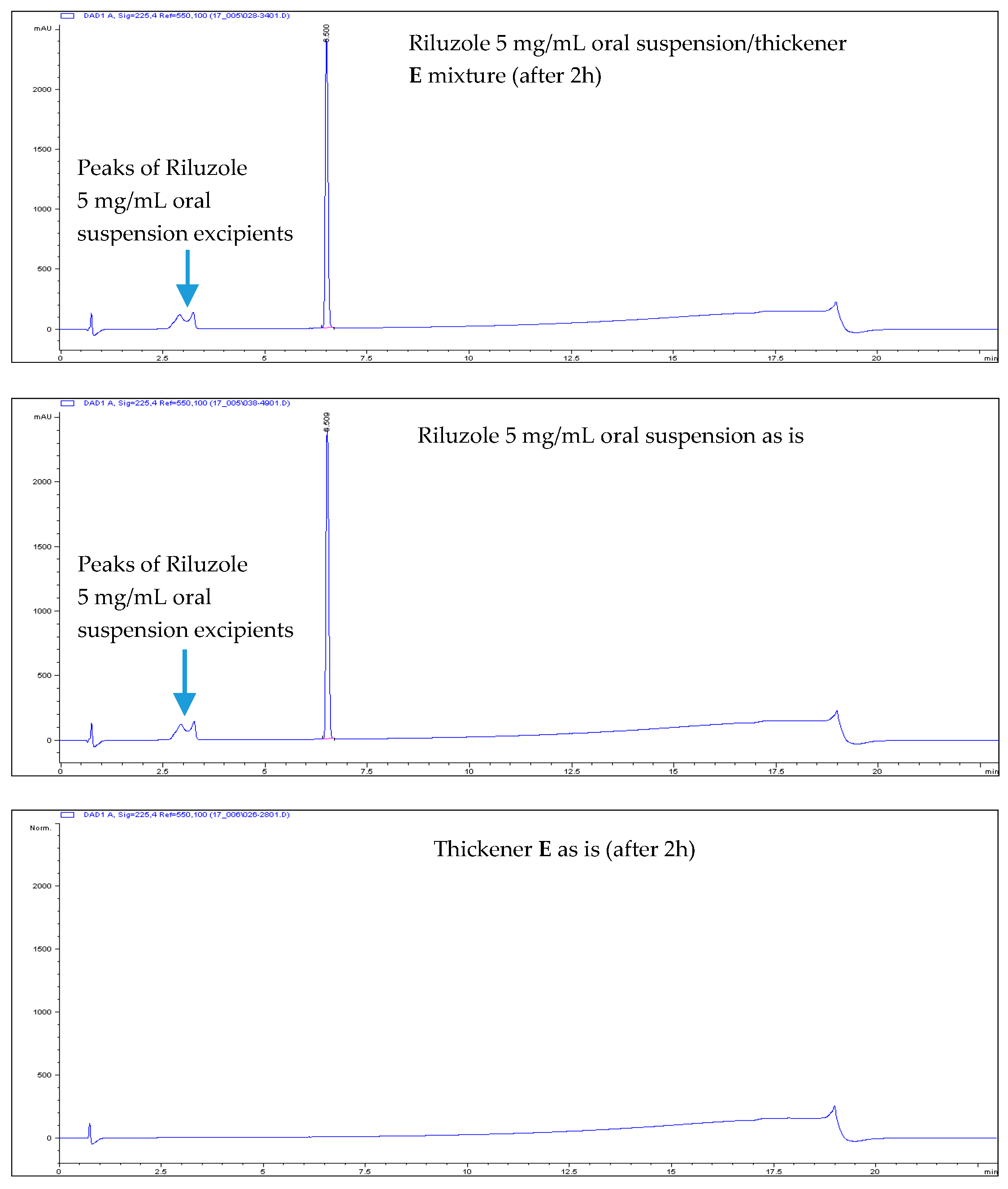
- All Riluzole HPLC assays were within the 90–110% of the label concentration, both at initial time and after two h.
- No related substances were detected, both at initial time and after two h. The only peaks detected in the HPLC chromatograms were unequivocally attributed to Placebo (i.e., the thickener itself).
- No practical pH differences were observed between Riluzole 5 mg/mL oral suspension alone and mixed with the thickeners, except for the Fresubin® Clear Thickener sample, for which a pH decrease was observed (from 7.2 to 6.4), however this pH decrease resulted to have no impact on Riluzole chemical stability.
- No significant changes in appearance were observed after two h.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ALS Therapy Development Institute. What Is ALS? Available online: https://www.als.net/what-is-als/ (accessed on 14 February 2020).
- Onesti, E.; Schettino, I.; Gori, M.C.; Frasca, V.; Ceccanti, M.; Cambieri, C.; Ruoppolo, G.; Inghilleri, M. Dysphagia in amytrophic lateral sclerosis: Impact on patient behavior, diet adaptation, and Riluzole management. Front. Neurol. 2017, 8, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühnlein, P.; Gdynia, H.J.; Sperfeld, A.D.; Lindner-Pfleghar, B.; Ludolph, A.C.; Prosiegel, M.; Riecker, A. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat. Clin. Prac. Neurol. 2008, 4, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Ruggiero, C.; Patriti, A.; Marano, L. Diagnostic Assessment and Management of Dysphagia in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 50, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Riluzole oral suspension in amyotrophic lateral sclerosis: A guide to its use. Drugs Ther. Perspect. 2016, 32, 282–286. [Google Scholar] [CrossRef]
- Healtcare-Disphagia and Dementia. Available online: https://www.premierfoodservice.co.uk/downloads (accessed on 14 February 2020).
- Hinchcliffe, M.; Smith, A. Riluzole: Real-world evidence supports significant extension of median survival times in patients with amyotrophic lateral sclerosis. Degener. Neurol. Neuromuscul. Dis. 2017, 7, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, D.; Tomlin, S. How to help if a patient can’t swallow. Pharm. J. 2011, 286, 271–274. [Google Scholar]
- Wright, D.; Chapman, N.; Miah, F.M.; Greenwall, R.; Griffith, R.; Guyon, A.; Merriman, H. Guideline on the Medication Management of Adults with Swallowing Difficulties; Medenium Group Publishing Ltd.: Berkhamsted, UK, 2015. [Google Scholar]
- Bensimon, G.; Lacomblez, L.; Meininger, V. The ALS/Riluzole Study Group A Controlled Trial of Riluzole in Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 1994, 330, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Georgoulopoulou, E.; Fini, N.; Vinceti, M.; Monelli, M.; Vacondio, P.; Bianconi, G.; Sola, P.; Paolo Nichelli, P.; Mandrioli, J. The impact of clinical factors, riluzole and therapeutic interventions on ALS survival: A population based study in Modena, Italy. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.M.; Abrahams, S.; Borasio, G.D.; de Carvalho, M.; Chio, A.; van Damme, P.; Hardiman, O.; Kollewe, K.; Morrison, K.E.; Petri, S.; et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)—Revised report of an EFNS task force. Eur. J. Neurol. 2012, 19, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Radunović, A.; Mitsumoto, H.; Leigh, P.N. Clinical care of patients with amyotrophic lateral sclerosis. Lancet Neurol. 2007, 6, 913–925. [Google Scholar] [CrossRef]
- Mandrioli, J.; Malerba, S.A.; Beghi, E.; Fini, N.; Fasano, A.; Zucchi, E.; de Pasqua, S.; Guidi, C.; Terlizzi, E.; Sette, E.; et al. Riluzole and other prognostic factors in ALS: A population-based registry study in Italy. J. Neurol. 2018, 265, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.M.; Smith, A. Riluzole 5 mg/mL oral suspension: For optimized drug delivery in amyotrophic lateral sclerosis. Drug Des. Dev. Ther. 2017, 11, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinko, P.J.; Singh, Y. Coarse Dispersions. Martin’s Physical Pharmacy and Pharmaceutical Sciences, 6th ed.; Troy, D.B., Ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2010; ISBN 978-0-7817-9766-5. [Google Scholar]
- ALS News Today. FDA Approves Tiglutik™, Liquid Form of Riluzole, for ALS Patients. Available online: https://alsnewstoday.com/2018/09/07/fda-approves-tiglutik-an-oral-suspension-of-Riluzole-to-treat-als/ (accessed on 14 February 2020).
- Steele, C.M.; Alsanei, W.A.; Ayanikalath, S.; Ayanikalath, S.; Barbon, C.E.A.; Chen, J.; Cichero, J.A.Y.; Coutts, K.; Dantas, R.O.; Duivestein, J.; et al. The Influence of Food Texture and Liquid Consistency Modification on Swallowing Physiology and Function: A Systematic Review. Dysphagia 2015, 30, 2–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cichero, J.A.Y. Thickening agents used for dysphagia management: Effect on bioavailability of water, medication and feelings of satiety. Nutr. J. 2013, 12, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbon, C.E.A.; Steele, C.M. Thickened Liquids for Dysphagia Management: A Current Review of the Measurement of Liquid Flow. Curr. Phys. Med. Rehabil. Rep. 2018, 6, 220–226. [Google Scholar] [CrossRef] [PubMed]
- IDDSI. Complete IDDSI Framework Testing Methods. Available online: https://iddsi.org/Documents/IDDSIFramework-TestingMethods.pdf (accessed on 14 February 2020).
- BDA/RCSLT. National Descriptors for Texture Modification in Adults. Available online: https://www.findusfoodservices.se/content/SE/Special%20Foods/Fakta%20&%20forskning/Konsistensanpassad%20mat/TMF_National_descriptors_UK.pdf (accessed on 14 February 2020).
- United Kingdom Adoption of IDDSI. Available online: https://www.bda.uk.com/resource/the-bda-announces-adoption-of-the-international-dysphagia-diet-standardisation-initiative-iddsi-framework.html (accessed on 14 February 2020).
- APSM (Association of Pharmaceutical Specials Manufacturers). Medicines Manipulation—Crushing, Opening or Splitting Tablets. Available online: http://www.apsm-uk.com/files/Crushing_tablets.pdf (accessed on 14 February 2020).
- Barnett, N.; Parmar, P. How to tailoring medication formulations for patients with dysphagia. Pharm. J. 2016, 297. [Google Scholar] [CrossRef]
- Stegemann, S.; Gosch, M.; Breitkreutz, J. Swallowing dysfunction and dysphagia is an unrecognized challenge for oral drug therapy. Int. J. Pharm. 2012, 430, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Royal Pharmaceutical Society. Pharmaceutical Issues when Crushing, Opening or Splitting Oral Dosage Forms. June 2011. Available online: https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Support/toolkit/pharmaceuticalissuesdosageforms-%282%29.pdf (accessed on 14 February 2020).
- Kelly, J.; Wright, D.; Wood, J. Medicine administration errors in patients with dysphagia in secondary care: A multi-centre observational study. J. Adv. Nurs. 2011, 67, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Leder, S.B.; Judson, B.L.; Sliwinski, E.; Madson, L. Promoting Safe Swallowing When Puree is Swallowed without Aspiration but Thin Liquid is Aspirated: Nectar is Enough: Original Article. Dysphagia 2013, 28, 58–62. [Google Scholar] [CrossRef] [PubMed]
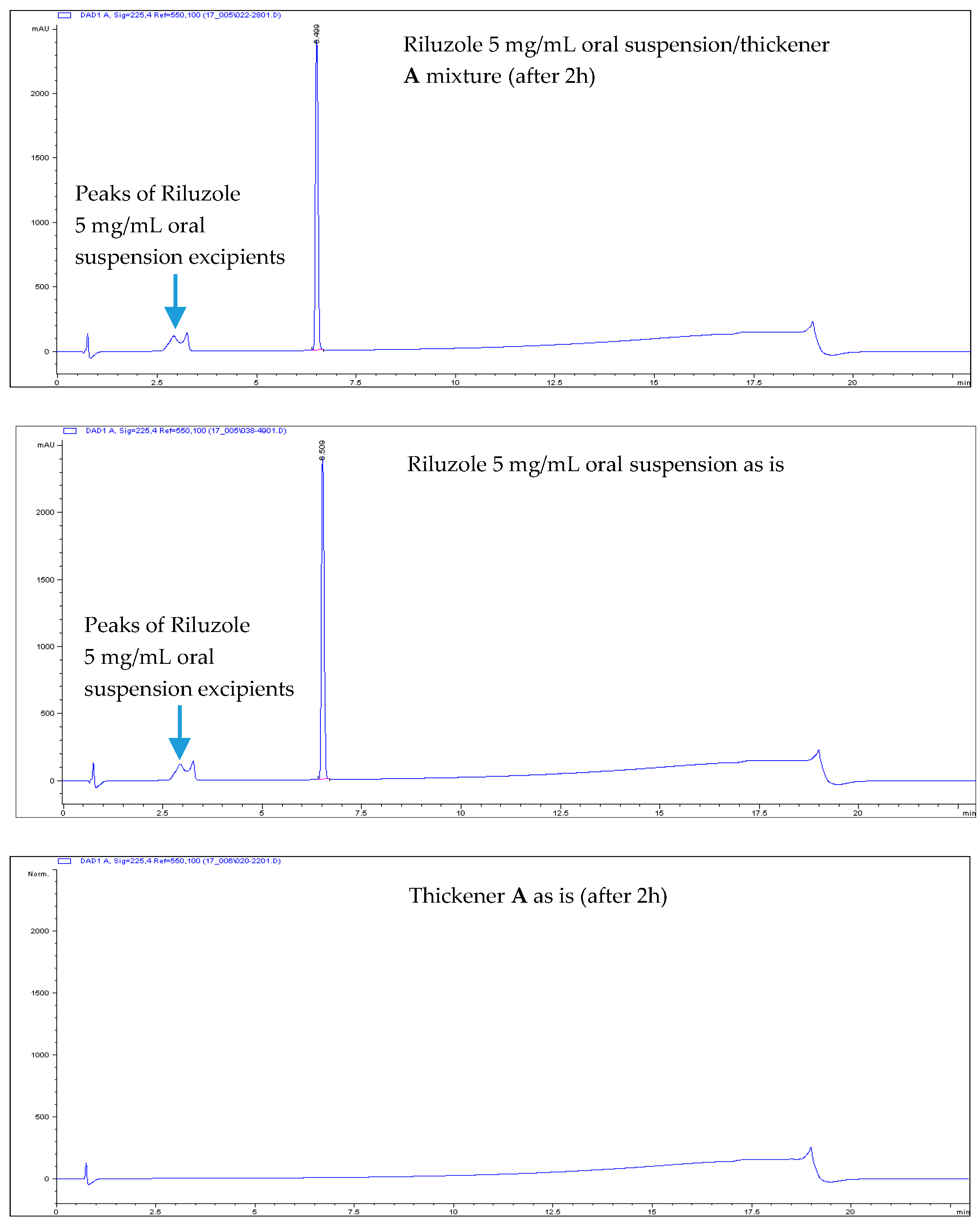
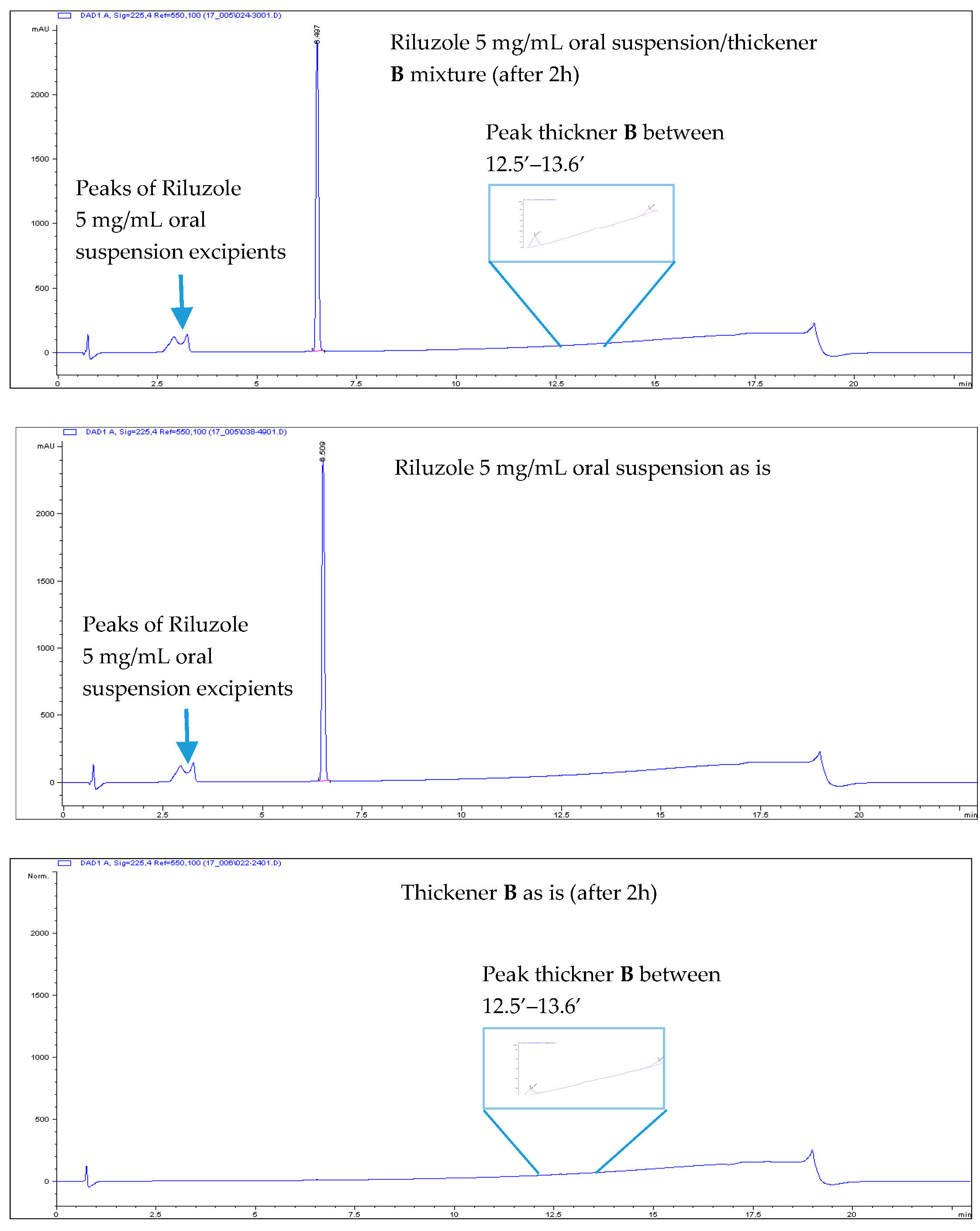
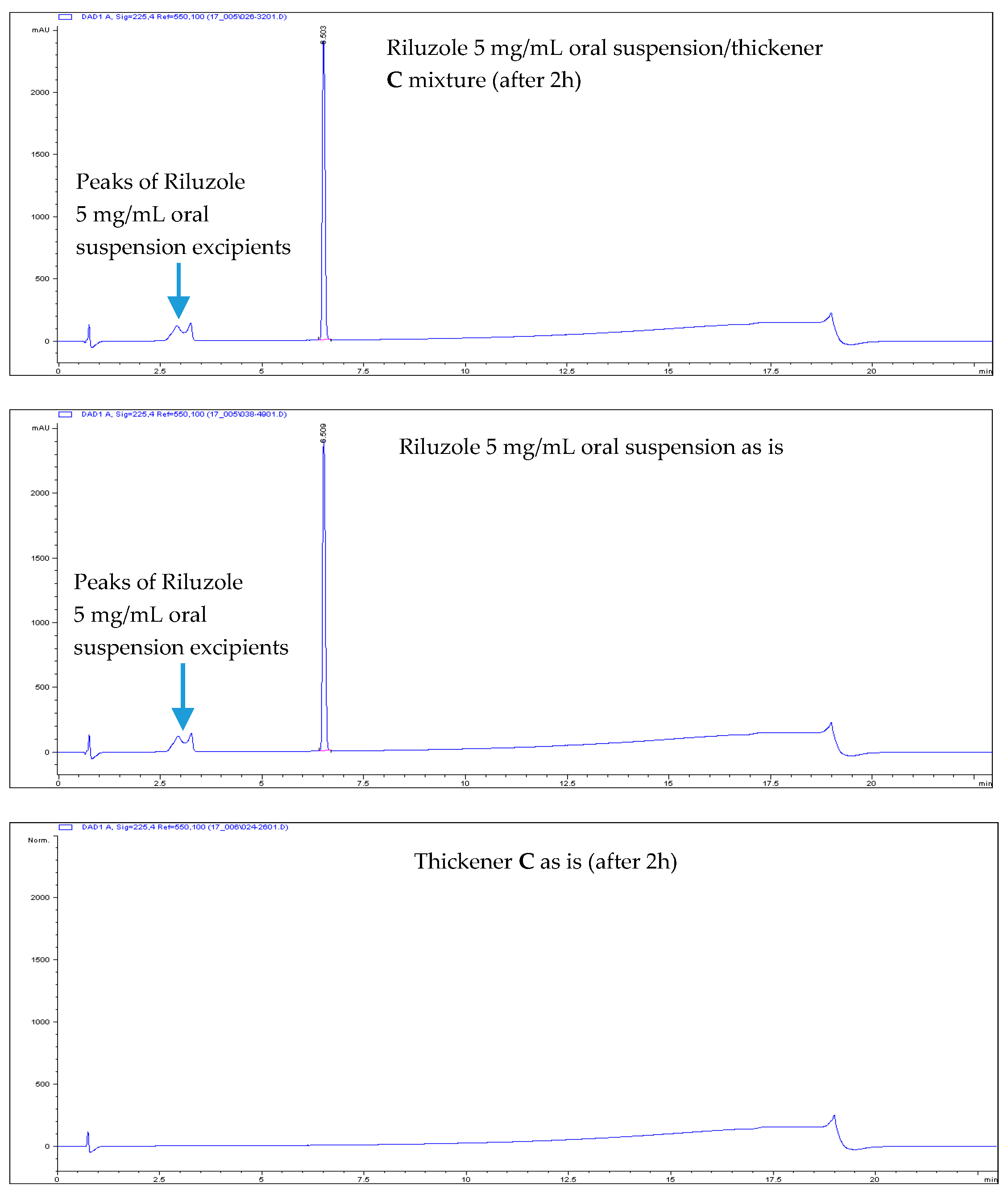
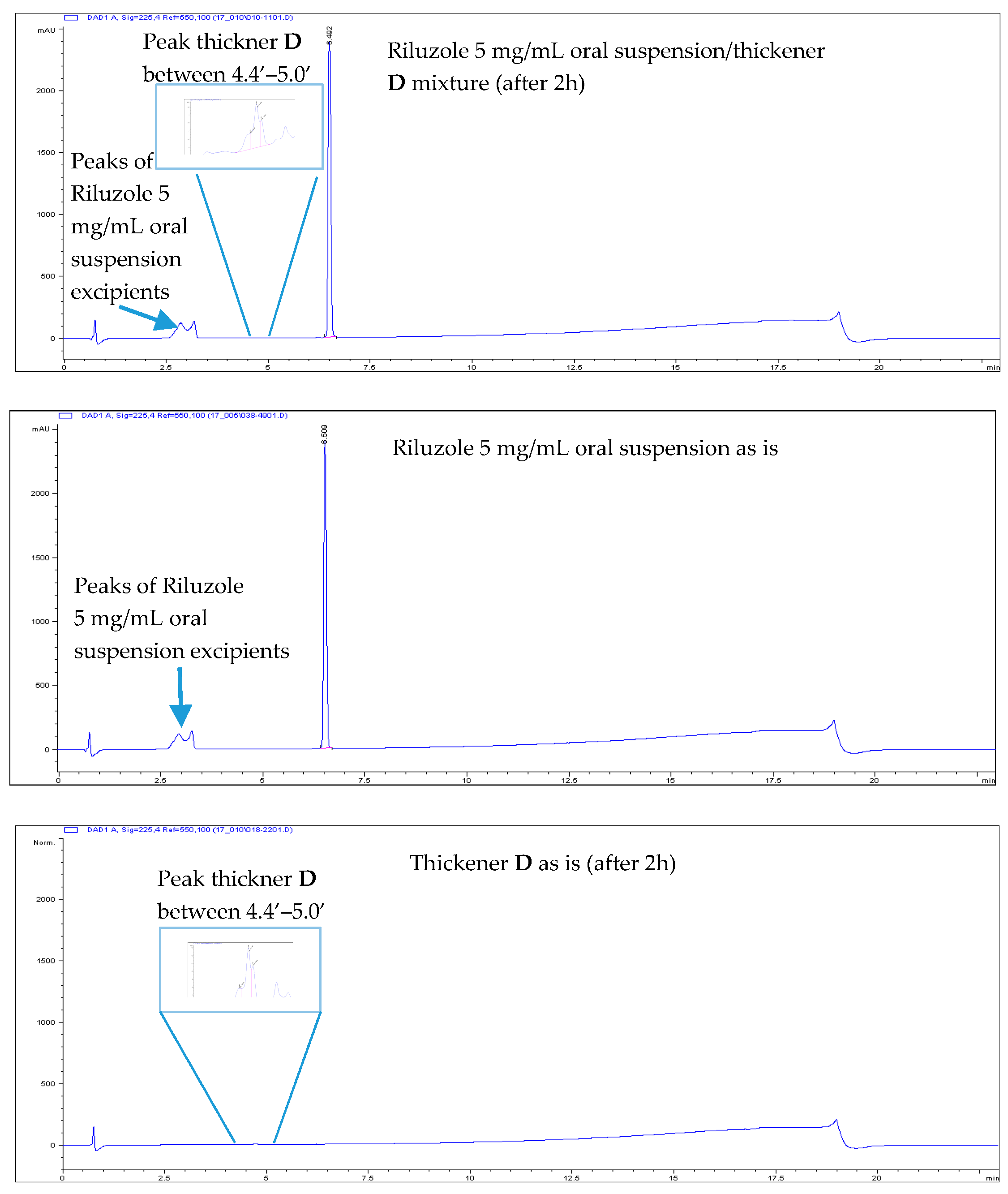
| Thickening Agent | Stage 1 (Syrup) | Stage 2 (Custard) | Stage 3 (Pudding) |
|---|---|---|---|
| Nutilis Powder | 2–3 scoops (1) per 200 mL H2O | 3–4 scoops per 200 mL H2O | 4–5 scoops per 200 mL H2O |
| Nutilis Clear | 1 scoop (1) per 200 mL H2O | 3 scoops per 200 mL H2O | 6 scoops per 200 mL H2O |
| RESOURCE® ThickenUp™ | 1 brimming spoon (2) per 100 mL H2O | 1.5 brimming spoons per 100 mL H2O | 2 brimming spoons per 100 mL H2O |
| Fresubin® Clear Thickener | 1 levelled scoop (3) per 100 mL H2O | 3 levelled scoops per 100 mL H2O | 6 levelled scoops per 100 mL H2O |
| THICK & EASY™ Instant Food Thickener | 1 scoop (4) per 100 mL H2O | 1.5 scoops per 100 mL H2O | 2 scoops per 100 mL H2O |
| Composition | Nutilis Powder (A) | Nutilis Clear (B) | RESOURCE® ThickenUp™ (C) | Fresubin® Clear Thickener (D) | THICK & EASY™ Instant Food Thickener (E) |
|---|---|---|---|---|---|
| Riluzole 5 mg/mL oral suspension (g) | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Thickening agent (g) | 0.174 | 0.2088 | 0.1566 | 0.1827 | 0.1566 |
| Syringe Type | Syringe n° | Replicate n° | Riluzole 5 mg/mL Oral Suspension Lot 1521601 (Development Lot) | |||
|---|---|---|---|---|---|---|
| Operator n° 1 | Operator n° 2 | |||||
| Volume of Liquid (mL) Remaining into the Syringe after 10 s | IDDSI Level | Volume of Liquid (mL) Remaining into the Syringe after 10 s | IDDSI Level | |||
| BD Plastipak™ 10 mL (*) | (1) | 1 | 4.0–4.5 mL | 2 | 4.0–4.5 mL | 2 |
| 2 | 4.0–4.5 mL | 2 | 4.0–4.5 mL | 2 | ||
| 3 | 4.0–4.5 mL | 2 | 4.0–4.5 mL | 2 | ||
| (2) | 1 | 5.0–5.5 mL | 2 | 4.5–5.0 mL | 2 | |
| 2 | 5.0–5.5 mL | 2 | 4.5–5.0 mL | 2 | ||
| 3 | 5.0–5.5 mL | 2 | 4.5–5.0 mL | 2 | ||
| (3) | 1 | 4.0–4.5 mL | 2 | 3.5–4.0 mL | 1 | |
| 2 | 4.0–4.5 mL | 2 | 3.5–4.0 mL | 1 | ||
| 3 | 4.0–4.5 mL | 2 | 3.5–4.0 mL | 1 | ||
| IDDSI level AVERAGE (**) | - | 2 | - | 2 | ||
| Syringe Type | Syringe n° | Replicate n° | Riluzole 5 mg/mL Oral Suspension Lot 16,100 (Commercial Lot) | |||
|---|---|---|---|---|---|---|
| Operator n° 1 | Operator n° 2 | |||||
| Volume of Liquid (mL) Remaining into the Syringe after 10 s | IDDSI Level | Volume of Liquid (mL) Remaining into the Syringe after 10 s | IDDSI Level | |||
| BD Plastipak™ 10 mL (*) | (1) | 1 | 5.5–6.0 mL | 2 | 5.5–6.0 mL | 2 |
| 2 | 5.5–6.0 mL | 2 | 5.5–6.0 mL | 2 | ||
| 3 | 5.5–6.0 mL | 2 | 5.5–6.0 mL | 2 | ||
| (2) | 1 | 6.5–7.0 mL | 2 | 5.0–5.5 mL | 2 | |
| 2 | 6.5–7.0 mL | 2 | 5.0–5.5 mL | 2 | ||
| 3 | 6.5–7.0 mL | 2 | 6.0–6.5 mL | 2 | ||
| (3) | 1 | 5.5–6.0 mL | 2 | 5.0–5.5 mL | 2 | |
| 2 | 5.5–6.0 mL | 2 | 5.0–5.5 mL | 2 | ||
| 3 | 5.5–6.0 mL | 2 | 5.0–5.5 mL | 2 | ||
| IDDSI level AVERAGE (**) | - | 2 | - | 2 | ||
| Sample | Riluzole Individual Assay mg/mL (% l.c.) (*) | Riluzole Average Assay mg/mL (% l.c.) (*) | |
|---|---|---|---|
| Control (Riluzole 5 mg/mL oral suspension) | Control— sample 1 | 4.96 (99.2) | 4.99 (99.8) |
| Control— sample 2 | 5.01 (100.2) | ||
| A: Nutilis Powder—Riluzole 5 mg/mL oral suspension mixture | Time 0— sample 1 | 4.93 (98.6) | 4.97 (99.4) |
| Time 0— sample 2 | 5.00 (100.0) | ||
| After 2 h— sample 1 | 5.06 (101.2) | 5.06 (101.2) | |
| After 2 h— sample 2 | 5.05 (101.0) | ||
| B: Nutilis Clear—Riluzole 5 mg/mL oral suspension mixture | Time 0— sample 1 | 5.12 (102.4) | 5.11 (102.2) |
| Time 0— sample 2 | 5.10 (102.0) | ||
| After 2 h— sample 1 | 5.11 (102.2) | 5.12 (102.4) | |
| After 2 h— sample 2 | 5.13 (102.6) | ||
| C: RESOURCE® ThickenUp™ —Riluzole 5 mg/mL oral suspension mixture | Time 0— sample 1 | 4.89 (97.8) | 4.93 (98.6) |
| Time 0— sample 2 | 4.96 (99.2) | ||
| After 2 h— sample 1 | 4.94 (98.8) | 4.96 (99.2) | |
| After 2 h— sample 2 | 4.98 (99.6) | ||
| D: Fresubin® Clear Thickener—Riluzole 5 mg/mL oral suspension mixture | Time 0— sample 1 | 4.99 (99.8) | 5.03 (100.7) |
| Time 0— sample 2 | 5.07 (101.4) | ||
| After 2 h— sample 1 | 4.93 (98.6) | 4.95 (98.9) | |
| After 2 h— sample 2 | 4.96 (99.2) | ||
| E: THICK & EASY™ Instant Food Thickener—Riluzole 5 mg/mL oral suspension mixture | Time 0— sample 1 | 4.96 (99.2) | 4.97 (99.4) |
| Time 0— sample 2 | 4.97 (99.4) | ||
| After 2 h— sample 1 | 5.03 (100.6) | 5.01 (100.2) | |
| After 2 h— sample 2 | 4.98 (99.6) | ||
| Sample | Initial (time 0) | After 2 h | ||
|---|---|---|---|---|
| Appearance | pH | Appearance | pH | |
| Control (Riluzole 5 mg/mL oral suspension) | Slightly brown, opaque homogenous suspension | 8.19 | Slightly brown, opaque homogenous suspension | 8.18 |
| A: Nutilis Powder—Riluzole 5 mg/mL oral suspension mixture | Slightly brown, opaque homogenous mixture | 8.02 | Slightly brown, opaque homogenous mixture | 7.99 |
| B: Nutilis Clear—Riluzole 5 mg/mL oral suspension mixture | Slightly brown, opaque homogenous mixture | 8.01 | Slightly brown, opaque homogenous mixture | 7.86 |
| C: RESOURCE® ThickenUp™—Riluzole 5 mg/mL oral suspension mixture | Slightly brown, opaque homogenous mixture | 7.99 | Slightly brown, opaque homogenous mixture | 8.03 |
| D: Fresubin® Clear Thickener—Riluzole 5 mg/mL oral suspension mixture | Slightly brown, opaque homogenous mixture | 7.21 | Slightly brown, opaque homogenous mixture | 6.40 |
| E: THICK & EASY™ Instant Food Thickener—Riluzole 5 mg/mL oral suspension mixture | Slightly brown, opaque homogenous mixture | 8.05 | Slightly brown, opaque homogenous mixture | 7.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, G.; Artico, R.; Barbareschi, D. Riluzole Oral Suspension for the Treatment of Amyotrophic Lateral Sclerosis: Texture and Compatibility with Food Thickeners Evaluation. J 2020, 3, 275-288. https://doi.org/10.3390/j3030021
Colombo G, Artico R, Barbareschi D. Riluzole Oral Suspension for the Treatment of Amyotrophic Lateral Sclerosis: Texture and Compatibility with Food Thickeners Evaluation. J. 2020; 3(3):275-288. https://doi.org/10.3390/j3030021
Chicago/Turabian StyleColombo, Giuseppe, Roberta Artico, and Daniele Barbareschi. 2020. "Riluzole Oral Suspension for the Treatment of Amyotrophic Lateral Sclerosis: Texture and Compatibility with Food Thickeners Evaluation" J 3, no. 3: 275-288. https://doi.org/10.3390/j3030021
APA StyleColombo, G., Artico, R., & Barbareschi, D. (2020). Riluzole Oral Suspension for the Treatment of Amyotrophic Lateral Sclerosis: Texture and Compatibility with Food Thickeners Evaluation. J, 3(3), 275-288. https://doi.org/10.3390/j3030021





