Monoterpenoids: The Next Frontier in the Treatment of Chronic Pain?
Abstract
1. Introduction
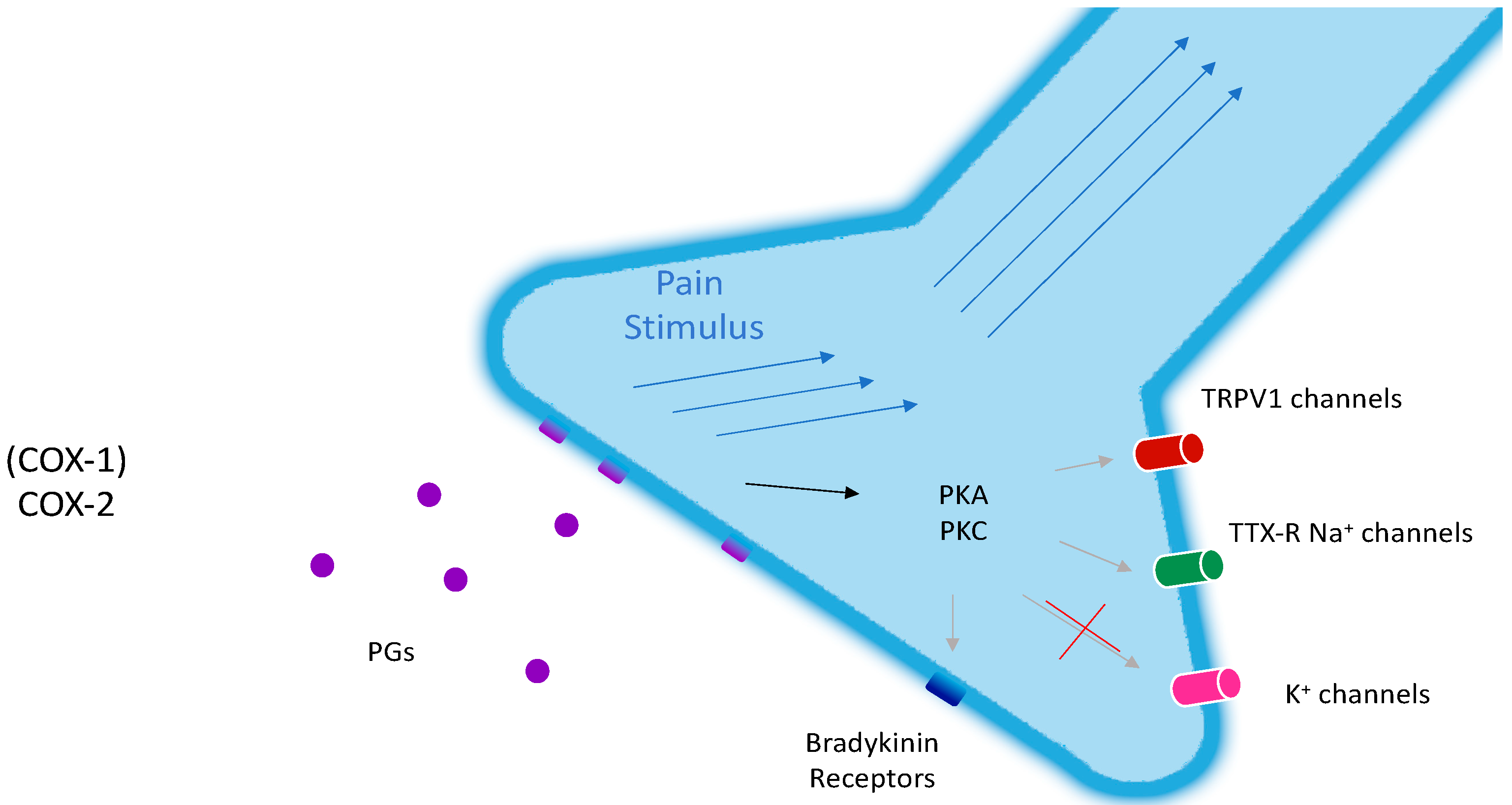
2. The Topical Management of Chronic Pain
3. Terpenes Are Suitable Candidates for Skin-Permeation Enhancement
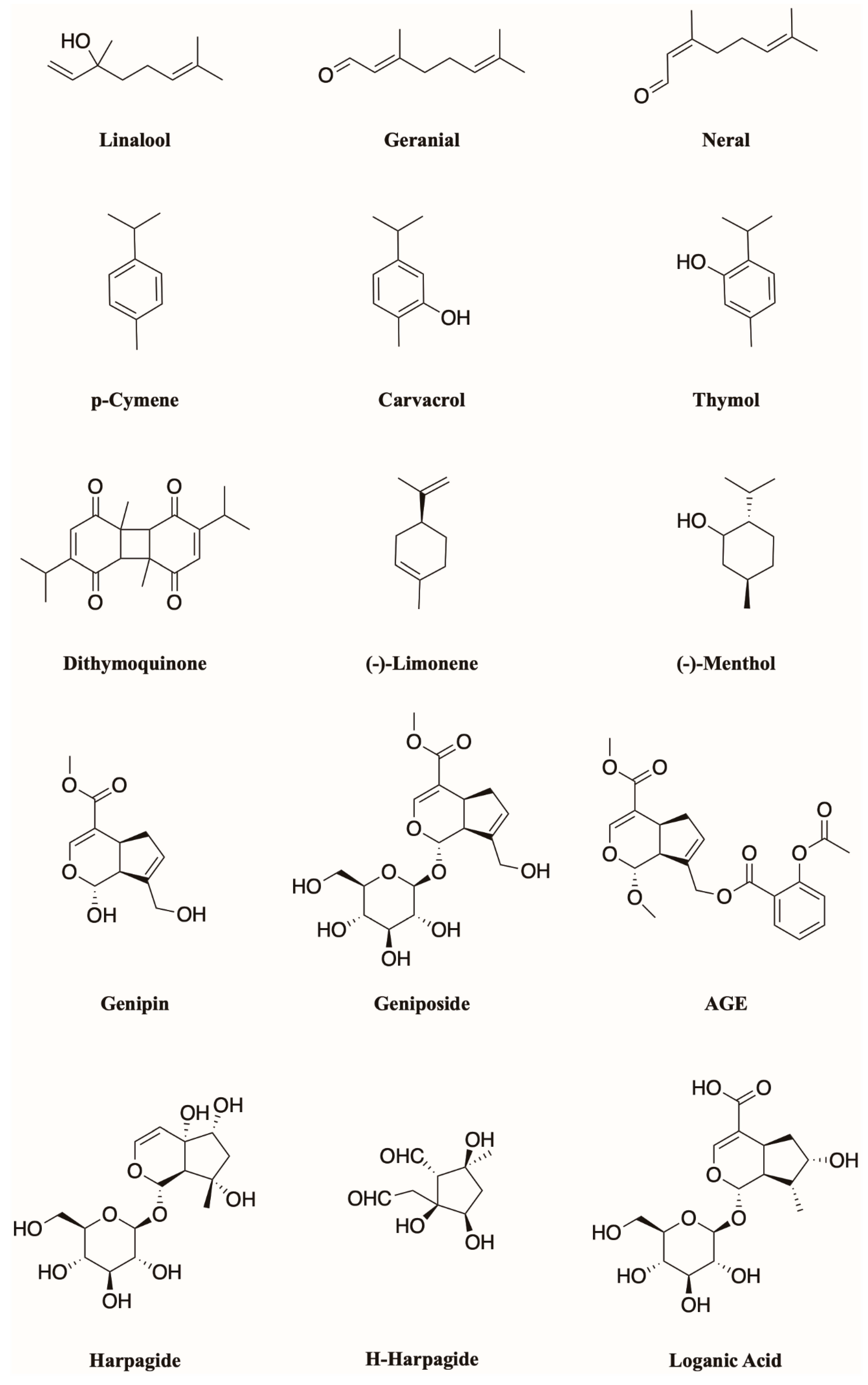
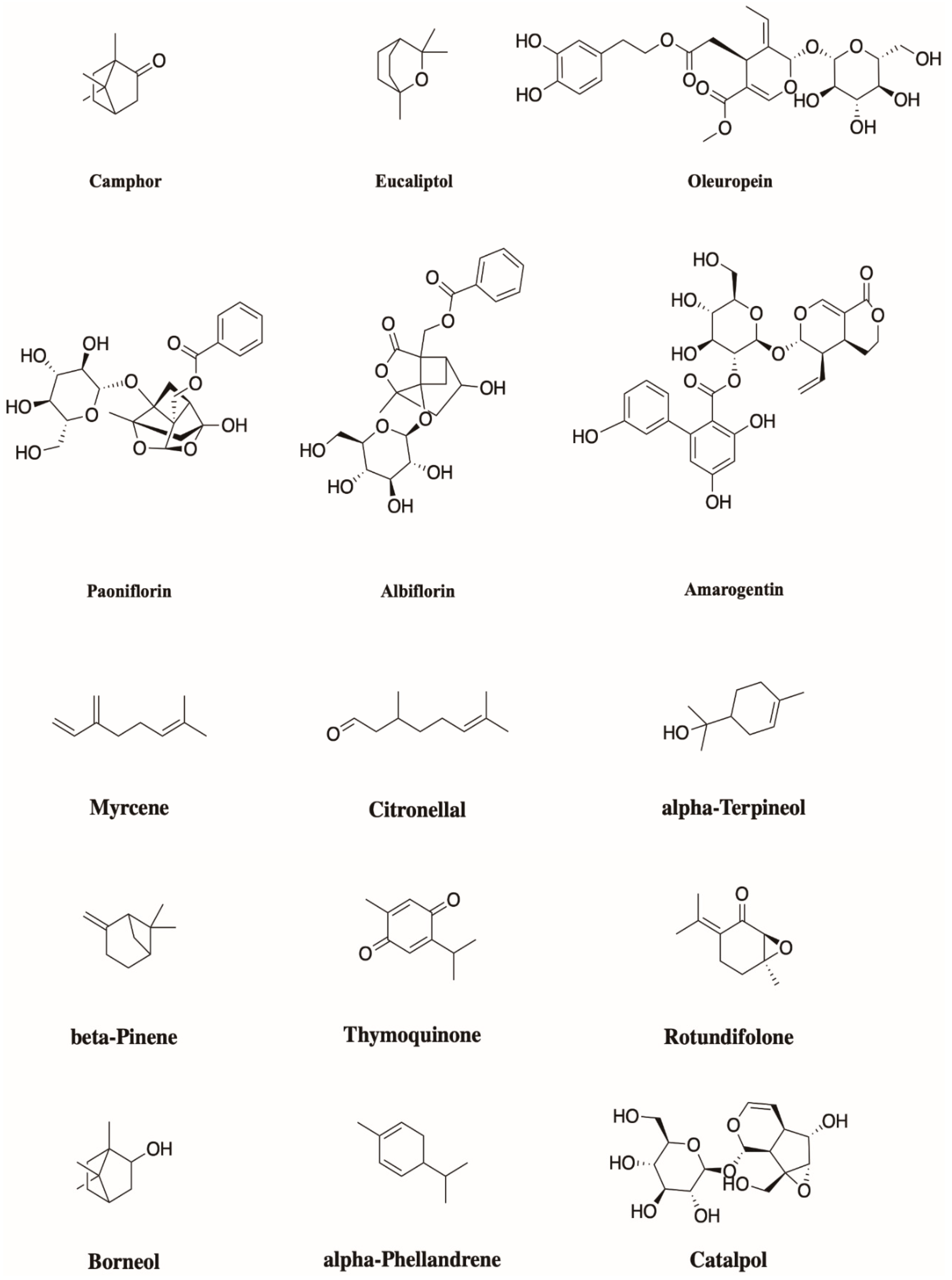
4. Monoterpenoids Have Great Potential in the Treatment of Chronic Pain
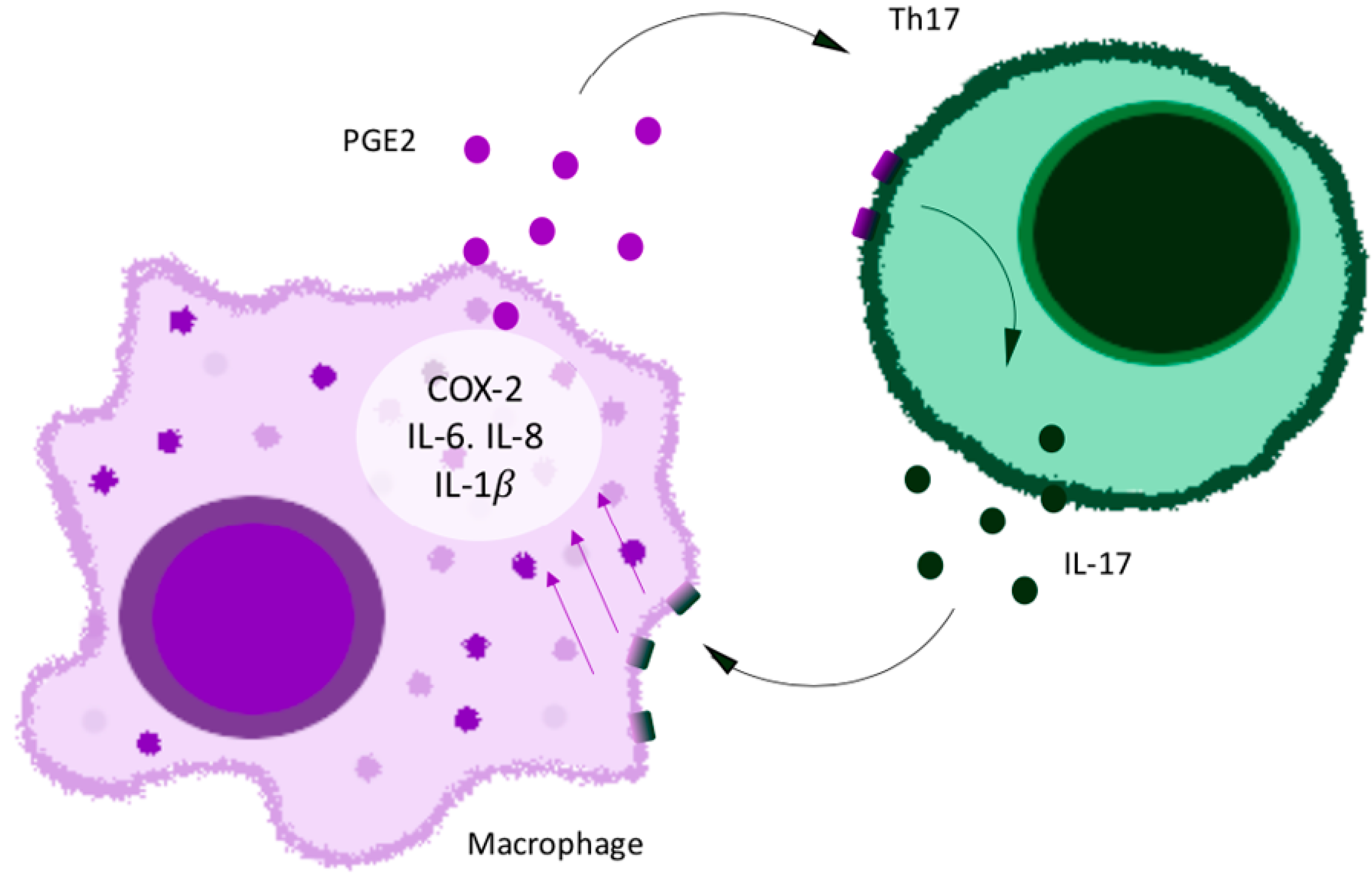
5. Pro-Inflammatory Cytokine IL-17 and Chemokines as New Targets against the Mechanisms of Pain and Chronic Pain
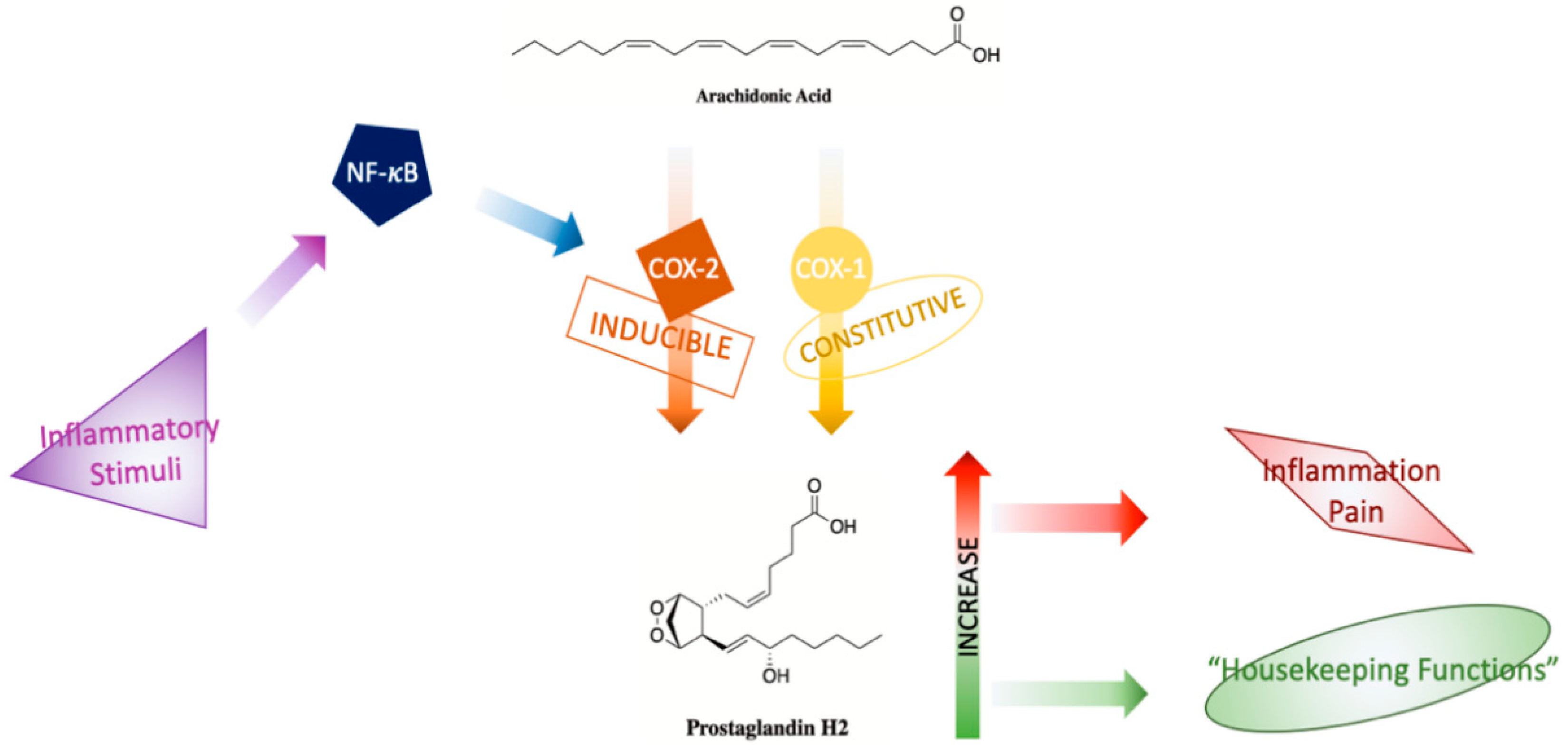
6. COX-2: Will a Target Ever Be Obsolete
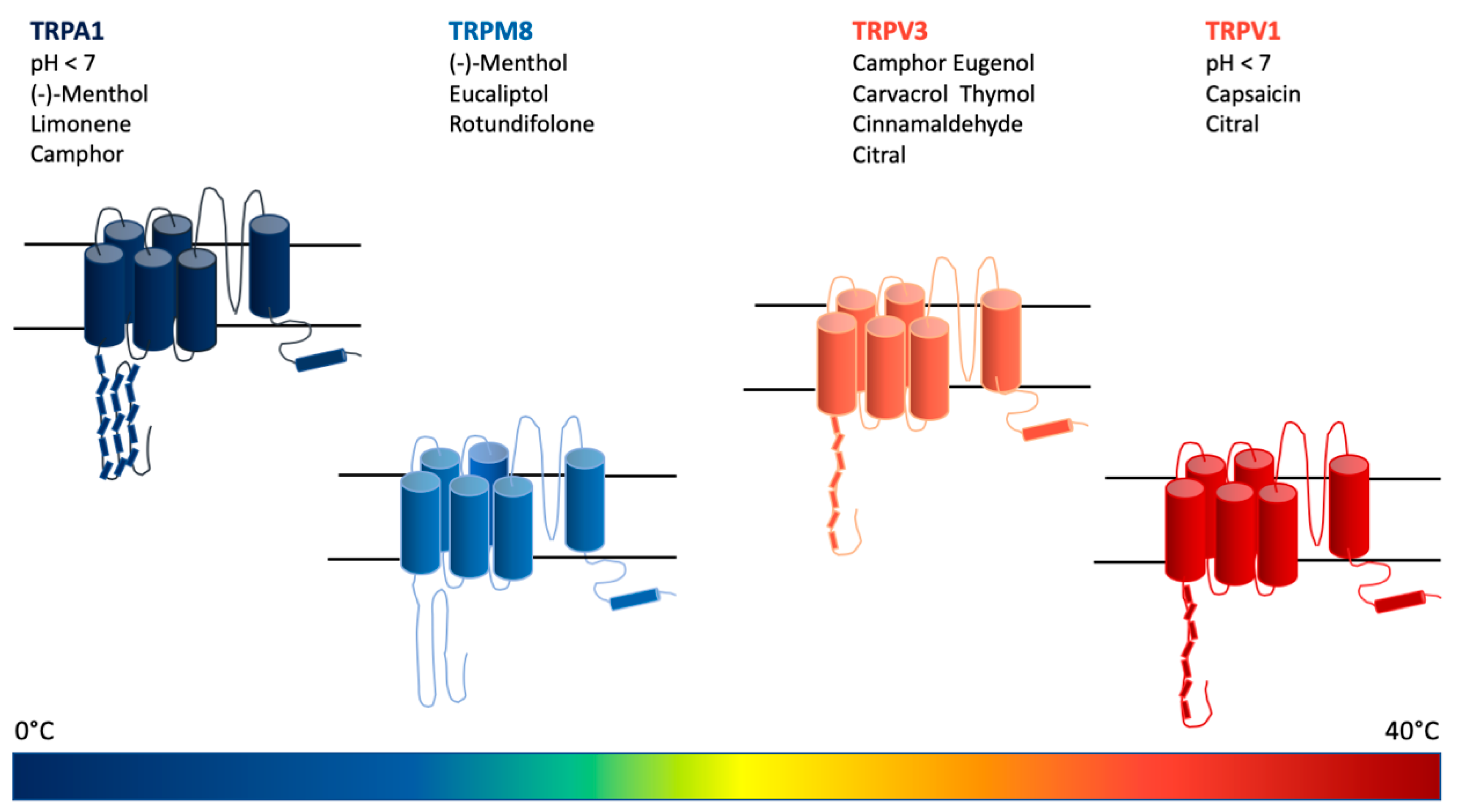
7. Monoterpenoids Show a Wide Activity Spectrum in Modulating Several Receptor Subtypes of the TRPCC Family
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Premkumar, L.S. Transient receptor potential channels as targets for phytochemicals. ACS Chem. Neurosci. 2014, 5, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.M.; McAlexander, M.A.; Bíró, T.; Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Wang, X. Control of pain with topical plant medicines. Asian Pacific J. Tropical Biomed. 2015, 5, 93–95. [Google Scholar] [CrossRef]
- Adams, J. The effects of yin, yang and qi in the skin on pain. Medicines 2016, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Adams, J. Chronic pain can it be cured? J. Pharmaceut. Drug Devel. 2017, 4, 105–109. [Google Scholar]
- Fernandes, E.; Vong, C.; Quek, S.; Cheong, J.; Awal, S.; Gentry, C.; Aubdool, A.; Liang, L.; Bodkin, J.; Bevan, S.; et al. Superoxide generation and leukocyte accumulation: Key elements in the mediation of leukotriene B ₄ -induced itch by transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1. FASEB J. 2013, 27, 1664–1673. [Google Scholar] [CrossRef]
- Adams, J.D.; Guhr, S.; Villaseñor, E. Salvia mellifera—How Does It Alleviate Chronic Pain? Medicines (Basel) 2019, 6, 33. [Google Scholar]
- Adams, J. Chronic pain two cures. OBM Integ. Comp. Med. 2018, 3. [Google Scholar] [CrossRef]
- Fontaine, P.; Wong, V.; Williams, T.; Garcia, C.; Adams, J. Chemical Composition and Antinociceptive Activity of California Sagebrush (Artemisia californica). J. Pharmacog. Phytother. 2013, 5, 1–11. [Google Scholar]
- Roosterman, D.; Goerge, T.; Schneider, S.W.; Bunnett, N.W.; Steinhoff, M. Neuronal control of skin function: The skin as a neuroimmunoendocrine organ. Physiol. Rev. 2006, 86, 1309–1379. [Google Scholar] [CrossRef]
- Church, M.K.; Clough, G.F. Human skin mast cells: In vitro and in vivo studies. Ann. Allergy Asthma Immunol. 1999, 83, 471–475. [Google Scholar] [CrossRef]
- Burian, M.; Geisslinger, G. COX-dependent mechanisms involved in the antinociceptive action of NSAIDs at central and peripheral sites. Pharmacol. Ther. 2005, 107, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Moody, M.L. Topical medications in the treatment of pain. Pain Med. News 2010, 15–31. Available online: http://www.nationalsalesforce.org/wp-content/uploads/2014/08/Topical-Medications-in-the-Treatment-of-Pain.pdf (accessed on 27 May 2020).
- Heyneman, C.A.; Lawless-Liday, C.; Wall, G.C. Oral versus topical NSAIDs in rheumatic diseases: A comparison. Drugs 2000, 60, 555–574. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Stanos, S.P.; Galluzzi, K.E. Topical therapies in the management of chronic pain. Postgrad. Med. 2013, 125, 25–33. [Google Scholar] [CrossRef]
- Subramanian, N.; Ghosal, S.K.; Moulik, S.P. Topical delivery of celecoxib using microemulsion. Acta Pol. Pharm. 2004, 61, 335–341. [Google Scholar]
- Williams, A.C.; Barry, B.W. The enhancement index concept applied to terpene penetration enhancers for human skin and model lipophilic (oestradiol) and hydrophilic (Sfluorouracil) drugs. Int. J. Pharmaceut. 1991, 74, 157–168. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.D.; Chai, Y.P.; Zhang, H.; Peng, P.; Yang, X.X. Natural Terpenes as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef]
- Sapra, B.; Jain, S.; Tiwary, A.K. Percutaneous permeation enhancement by terpenes: Mechanistic view. AAPS J. 2008, 10, 120–132. [Google Scholar] [CrossRef]
- Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A. Status of terpenes as skin penetration enhancers. Drug Discov. Today 2007, 12, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.; Schaefer, U.; Sporer, F.; Reichling, J. Comparative study on the in vitro human skin permeation of monoterpenes and phenylpropanoids applied in rose oil and in form of neat single compounds. Pharmazie 2010, 65, 102–105. [Google Scholar] [PubMed]
- Magnussona, B.M.; Runn, P.; Karlsson, K.; Koskinen, L.O.D. Terpenes and ethanol enhance the transdermal permeation of the tripeptide thyrotropin releasing hormone in human epidermis. Int. J. Pharmaceutics. 1997, 157, 113–121. [Google Scholar] [CrossRef]
- Cal, K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Med. 2006, 72, 311–316. [Google Scholar] [CrossRef]
- Shereen, A.; Yousef, E.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–167. [Google Scholar]
- Davis, E.M. Advances in the Enzymology of Monoterpene Cyclization Reactions. Compr. Nat. Prod. II 2010, 1, 585–608. [Google Scholar]
- Tchimene, M.K.; Okunji, C.O.; Iwu, M.M.; Kuete, V. Monoterpenes and Related Compounds from the Medicinal Plants of Africa. In Medicinal Plant Research in Africa; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–32. [Google Scholar]
- Guimarães, A.G.; Quintans, J.S.; Quintans, L.J. Monoterpenes with analgesic activity—A systematic review. Phytother. Res. 2013, 27, 1–15. [Google Scholar] [CrossRef]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Gouveia, D.N.; Pina, L.T.S.; Rabelo, T.K.; da Rocha Santos, W.B.; Quintans, J.S.S.; Guimaraes, A.G. Monoterpenes as Perspective to Chronic Pain Management: A Systematic Review. Curr. Drug Targets 2018, 19, 960–972. [Google Scholar] [CrossRef]
- Passos, F.F.; Lopes, E.M.; de Araújo, J.M.; de Sousa, D.P.; Veras, L.M.; Leite, J.R.; Almeida, F.R. Involvement of Cholinergic and Opioid System in γ-Terpinene-Mediated Antinociception. Evid. Based Complement. Alternat. Med. 2015, 2015, 829414. [Google Scholar] [CrossRef]
- Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: Natural inhibitors of NF-kappaB signaling with anti-inflammatory and anticancer potential. Cell Mol. Life Sci. 2008, 65, 2979–2999. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Yang, Y.J.; Li, Y.S.; Zhang, W.K.; Tang, H.B. α-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.H.; Chang, Y.C.; Jean, Y.H. Excitatory amino acid glutamate: Role in peripheral nociceptive transduction and inflammation in experimental and clinical osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.S.; Houghton, P.J.; Theobald, A.; Jenner, P.; Perry, E.K. In-vitro inhibition of human erythrocyte acetylcholinesterase by salvia lavandulaefolia essential oil and constituent terpenes. J. Pharm. Pharmacol. 2000, 52, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Painter, S.L.; Fanslow, W.C.; Ulrich, D.; Macduff, B.M.; Spriggs, M.K.; Armitage, R.J. Human IL-17: A novel cytokine derived from T cells. J. Immunol. 1995, 155, 5483–5486. [Google Scholar]
- Wilson, N.J.; Boniface, K.; Chan, J.R.; McKenzie, B.S.; Blumenschein, W.M.; Mattson, J.D.; Basham, B.; Smith, K.; Chen, T.; Morel, F.; et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007, 8, 950–957. [Google Scholar] [CrossRef]
- Zhang, X.; Angkasekwinai, P.; Dong, C.; Tang, H. Structure and function of interleukin-17 family cytokines. Protein. Cell 2011, 2, 26–40. [Google Scholar] [CrossRef]
- Yu, J.J.; Gaffen, S.L. Interleukin-17: A novel inflammatory cytokine that bridges innate and adaptive immunity. Front. Biosci. 2008, 13, 170–177. [Google Scholar] [CrossRef]
- Shalom-Barak, T.; Quach, J.; Lotz, M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J. Biol. Chem. 1998, 273, 27467–27473. [Google Scholar] [CrossRef]
- Lotz, M.; Blanco, F.J.; von Kempis, J.; Dudler, J.; Maier, R.; Villiger, P.M.; Geng, Y. Cytokine regulation of chondrocyte functions. J. Rheumatol. Suppl. 1995, 43, 104–108. [Google Scholar]
- Faour, W.H.; Mancini, A.; He, Q.W.; Di Battista, J.A. T-cell-derived interleukin-17 regulates the level and stability of cyclooxygenase-2 (COX-2) mRNA through restricted activation of the p38 mitogen-activated protein kinase cascade: Role of distal sequences in the 3’-untranslated region of COX-2 mRNA. J. Biol. Chem. 2003, 278, 26897–26907. [Google Scholar] [CrossRef] [PubMed]
- Paulissen, S.M.; van Hamburg, J.P.; Davelaar, N.; Asmawidjaja, P.S.; Hazes, J.M.; Lubberts, E. Synovial fibroblasts directly induce Th17 pathogenicity via the cyclooxygenase/prostaglandin E2 pathway, independent of IL-23. J. Immunol. 2013, 191, 1364–1372. [Google Scholar] [CrossRef]
- Boniface, K.; Bak-Jensen, K.S.; Li, Y.; Blumenschein, W.M.; McGeachy, M.J.; McClanahan, T.K.; McKenzie, B.S.; Kastelein, R.A.; Cua, D.J.; de Waal Malefyt, R. Prostaglandin E2 regulates Th17 cell diff erentiation and function through cyclicAMP and EP2/EP4 receptor signaling. J. Exp. Med. 2008, 206, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Sakata, D.; Esaki, Y.; Li, Y.; Matsuoka, T.; Kuroiwa, K.; Sugimoto, Y.; Narumiya, S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat. Med. 2009, 15, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Witowski, J.; Ksiazek, K.; Jorres, A. Interleukin-17: A mediator of inflammatory responses. Cell. Molec. Life Sci. 2004, 61, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Katz, Y.; Nadiv, O.; Beer, Y. Interleukin-17 Enhances Tumor Necrosis Factor a–Induced Synthesis of Interleukins 1, 6, and 8 in Skin and Synovial Fibroblasts. Arthritis Rheum. 2001, 44, 2176–2184. [Google Scholar] [CrossRef]
- Stamp, L.K.; Cleland, L.G.; James, M.J. Upregulation of Synoviocyte COX-2 Through Interactions with T Lymphocytes: Role of Interleukin 17 and Tumor Necrosis Factor-α. J. Rheumatol. 2004, 31, 146–1254. [Google Scholar]
- Moseley, T.T.; Rose, L.; Reddi, A.H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003, 14, 155–174. [Google Scholar] [CrossRef]
- Van Beelen, A.J.; Teunissen, M.B.; Kapsenberg, M.L.; de Jong, E.C. Interleukin-17 in inflammatory skin disorders. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 374–381. [Google Scholar] [CrossRef]
- Acosta-Rodriguez, E.V.; Rivino, L.; Geginat, J.; Jarrossay, D.; Gattorno, M.; Lanzavecchia, A.; Sallusto, F.; Napolitani, G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007, 8, 639–646. [Google Scholar] [CrossRef]
- Shabgah, A.G.; Fattahi, E.; Shahneh, F.Z. Interleukin-17 in human inflammatory diseases. Postepy Dermatol. Alergol. 2014, 31, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, Y.; Nakae, S.; Saijo, S.; Ishigame, H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol. Rev. 2008, 226, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.J. IL-17 cytokine/receptor families: Emerging targets for the modulation of inflammatory responses. Expert Opin. Ther. Pat. 2003, 13, 287–303. [Google Scholar] [CrossRef]
- Botelho, M.A.; Nogueira, N.A.; Bastos, G.M.; Fonseca, S.G.; Lemos, T.L.; Matos, F.J.; Montenegro, D.; Heukelbach, J.; Rao, V.S.; Brito, G.A. Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz. J. Med. Biol. Res. 2007, 40, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Games, E.; Guerreiro, M.; Santana, F.R.; Pinheiro, N.M.; de Oliveira, E.A.; Lopes, F.D.; Olivo, C.R.; Tibério, I.F.; Martins, M.A.; Lago, J.H.; et al. Structurally Related Monoterpenes p-Cymene, Carvacrol and Thymol Isolated from Essential Oil from Leaves of Lippia sidoides Cham. (Verbenaceae) Protect Mice against Elastase-Induced Emphysema. Molecules 2016, 21, 1390. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- De Bock, M.; Hodgkinson, S.; Cutfield, W.; Schlothauer, R. Methods and Uses of an Extract from Olive Leaf in Management of Type 2 Diabetes. U.S. Patent WO2014038962A1, 13 August 2015. [Google Scholar]
- Qabaha, K.; Al-Rimawi, F.; Qasem, A.; Naser, S.A. Oleuropein Is Responsible for the Major Anti-Inflammatory Effects of Olive Leaf Extract. J. Med. Food 2018, 21, 302–305. [Google Scholar] [CrossRef]
- Larussa, T.; Oliverio, M.; Suraci, E.; Greco, M.; Placida, R.; Gervasi, S.; Marasco, R.; Imeneo, M.; Paolino, D.; Tucci, L.; et al. Oleuropein Decreases Cyclooxygenase-2 and Interleukin-17 Expression and Attenuates Inflammatory Damage in Colonic Samples from Ulcerative Colitis Patients. Nutrients 2017, 9, 391. [Google Scholar] [CrossRef]
- Citová, I.; Ganzera, M.; Stuppner, H.; Solich, P. Determination of gentisin, isogentisin, and amarogentin in Gentiana lutea L. by capillary electrophoresis. J. Sep. Sci. 2008, 31, 195–200. [Google Scholar] [CrossRef]
- Ray, S.; Majumder, H.K.; Chakravarty, A.K.; Mukhopadhyay, S.; Gil, R.R.; Cordell, G.A. Amarogentin, a naturally occurring secoiridoid glycoside and a newly recognized inhibitor of topoisomerase I from Leishmania donovani. J. Nat. Prod. 1996, 59, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Wölfle, U.; Haarhaus, B.; Schempp, C.M. Amarogentin Displays Immunomodulatory Effects in Human Mast Cells and Keratinocytes. Mediat. Inflamm. 2015, 2015, 630128. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Taguchi, H. The Constituents of Gardenia jasminoides Geniposide and Genipin-gentiobioside. Chem. Pharm. Bull. 1973, 21, 2684–2688. [Google Scholar] [CrossRef]
- Yu, S.X.; Du, C.T.; Chen, W.; Lei, Q.Q.; Li, N.; Qi, S.; Zhang, X.J.; Hu, G.Q.; Deng, X.M.; Han, W.Y.; et al. Genipin inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Sci. Rep. 2015, 5, 17935. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; He, J.L.; Li, W.M.; Yang, Z.; Wang, Y.X.; Yin, J.; Du, Y.G.; Yu, C. Geniposide inhibits interleukin-6 and interleukin-8 production in lipopolysaccharide-induced human umbilical vein endothelial cells by blocking p38 and ERK1/2 signaling pathways. Inflamm. Res. 2010, 59, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.M.; Wu, H.; Li, H.; Chen, J.; Chen, J.Y.; Hu, S.L.; Shen, C. Effects and mechanisms of Geniposide on rats with adjuvant arthritis. Int. Immunopharmacol. 2014, 20, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Wang, P.; Xue, Y. Paeoniflorin attenuates Aβ1-42-induced inflammation and chemotaxis of microglia in vitro and inhibits NF-κB- and VEGF/Flt-1 signaling pathways. Brain Res. 2015, 1618, 149–158. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, Z.; Wang, Y.; Dai, X.; Zhang, J.; Xue, D. Paeoniflorin exerts a nephroprotective effect on concanavalin A-induced damage through inhibition of macrophage infiltration. Diagn. Pathol. 2015, 10, 120. [Google Scholar] [CrossRef]
- Gong, W.G.; Lin, J.L.; Niu, Q.X.; Wang, H.M.; Zhou, Y.C.; Chen, S.Y.; Liang, G.W. Paeoniflorin diminishes ConA-induced IL-8 production in primary human hepatic sinusoidal endothelial cells in the involvement of ERK1/2 and Akt phosphorylation. Int. J. Biochem. Cell Biol. 2015, 62, 93–100. [Google Scholar] [CrossRef]
- Chen, T.; Guo, Z.; Jiao, X.; Jia, B.; Zhang, Y.; Li, J.; Huang, X.; Liu, H. Peoniflorin suppresses tumor necrosis factor-α induced chemokine production in human dermal microvascular endothelial cells by blocking nuclear factor-κB and ERK pathway. Arch. Dermatol. Res. 2011, 303, 351–360. [Google Scholar] [CrossRef]
- Dai, X.; Wang, L.; Jia, X.; Chang, Y.; Wu, H.; Wang, C.; Wei, W. Paeoniflorin regulates the function of human peripheral blood mononuclear cells stimulated by rhIL-1β by up-regulating Treg expression. Immunopharmacol. Immunotox. 2015, 37, 252–257. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Wang, J.; Wang, C.; Yang, Z.; Zhu, Y.; Zhang, J. Paeoniflorin and Albiflorin Attenuate Neuropathic Pain via MAPK Pathway in Chronic Constriction Injury Rats. Evid. Based Complement. Alternat. Med. 2016, 2016, 8082753. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Khoo, C.S.; Hennell, J.R.; Pearson, J.L.; Jarouche, M.; Halstead, C.W.; Bensoussan, A. LC determination of albiflorin and paeoniflorin in Bai Shao (Paeonia lactiflora) as a raw herb and dried aqueous extract. J. AOAC Int. 2009, 92, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xu, H.; Wu, J.; Qu, C.; Sun, F.; Xu, S. Linalool inhibits cigarette smoke-induced lung inflammation by inhibiting NF-κB activation. Int. Immunopharmacol. 2015, 29, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, V.; Kumar Singh, A.; Acharya, V. A systematic approach to prioritize drug targets using machine learning, a molecular descriptor-based classification model, and high-throughput screening of plant derived molecules: A case study in oral cancer. Mol. Biosyst. 2015, 11, 3362–3377. [Google Scholar] [CrossRef]
- Brunton, L.; Chubner, B.; Knollmann, B. Goodman & Gilman’s The Pharmacological Basis Of Therapeutics, 12th ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenses 1 and 2. Ann. Rev. Pharmacol. Tox. 1998, 38, 97–120. [Google Scholar] [CrossRef]
- Bollettino d’Informazione Sui Farmaci. 2000, Lug-Ago. Available online: http://www.quadernidellasalute.it/imgs/C_17_pubblicazioni_217_allegato.pdf (accessed on 27 May 2020).
- Chen, L.C.; Ashcroft, D.M. Risk of myocardial infarction associated with selective COX-2 inhibitors: Meta-analysis of randomised controlled trials. Pharmacoepidemiol. Drug Saf. 2007, 16, 762–772. [Google Scholar] [CrossRef]
- Coricello, A.; El-Magboub, A.; Luna, M.; Ferrario, A.; Haworth, I.S.; Gomer, C.J.; Aiello, F.; Adams, J.D. Rational drug design and synthesis of new α-Santonin derivatives as potential COX-2 inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 993–996. [Google Scholar] [CrossRef]
- Garavito, R.; Mulichak, A. The structure of mammalian cyclooxygneases. Ann. Rev. Biophys. Biomolecular Structures. 2003, 32, 183–206. [Google Scholar] [CrossRef]
- Picot, D.; Loll, P.J.; Garavito, R.M. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature 1994, 367, 243–249. [Google Scholar] [CrossRef]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Khazdair, M.R. The Protective Effects of Nigella sativa and Its Constituents on Induced Neurotoxicity. J. Toxicol. 2015, 2015, 841823. [Google Scholar] [CrossRef] [PubMed]
- Cirino, I.C.; Menezes-Silva, S.M.; Silva, H.T.; de Souza, E.L.; Siqueira-Júnior, J.P. The Essential Oil from Origanum vulgare L. and Its Individual Constituents Carvacrol and Thymol Enhance the Effect of Tetracycline against Staphylococcus aureus. Chemotherapy 2014, 60, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; Carvalho, M.D.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K. Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the Inflammatory Response. Evid. Based Complement. Alternat. Med. 2012, 2012, 657026. [Google Scholar] [CrossRef]
- Landa, P.; Kokoska, L.; Pribylova, M.; Vanek, T.; Marsik, P. In vitro Anti-inflammatory Activity of Carvacrol: Inhibitory Effect on COX-2 Catalyzed Prostaglandin E2 Biosynthesis. Arch. Pharmaceut. Res. 2009, 32, 75–78. [Google Scholar] [CrossRef]
- Marsik, P.; Kokoska, L.; Landa, P.; Nepovim, A.; Soudek, P.; Vanek, T. In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1- and -2-catalyzed prostaglandin E2 biosyntheses. Planta. Med. 2005, 71, 739–742. [Google Scholar] [CrossRef]
- Shukla, S.; Bafna, K.; Sundar, D.; Thorat, S.S. The bitter barricading of prostaglandin biosynthesis pathway: Understanding the molecular mechanism of selective cyclooxygenase-2 inhibition by amarogentin, a secoiridoid glycoside from Swertia chirayita. PLoS ONE 2014, 9, e90637. [Google Scholar] [CrossRef]
- Ramírez-Cisneros, M.; Rios, M.Y.; Aguilar-Guadarrama, A.B.; Rao, P.P.; Aburto-Amar, R.; Rodríguez-López, V. In vitro COX-1 and COX-2 enzyme inhibitory activities of iridoids from Penstemon barbatus, Castilleja tenuiflora, Cresentia alata and Vitex mollis. Bioorg. Med. Chem. Lett. 2015, 25, 4505–4508. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, S.; Gao, W.; Zhu, D. Iridoid glycosides from Scrophularia ningpoensis. Phytochem 1999, 50, 101–104. [Google Scholar] [CrossRef]
- Lin, S.; Jiang, S.; Li, Y.; Zeng, J.; Zhu, D. Two novel iridoids from Scrophularia buergeriana. Tet. Lett. 2000, 41, 1069–1071. [Google Scholar] [CrossRef]
- Qi, J.; Chen, J.J.; Cheng, Z.H.; Zhou, J.H.; Yu, B.Y.; Qiu, S.X. Iridoid glycosides from Harpagophytum procumbens D.C. (devil’s claw). Phytochemistry 2006, 67, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, L.; Jia, Q.; Xu, J.; Wang, R.; Wang, Z.; Wu, Y.; Li, Y. Effects of b-glucosidase hydrolyzed products of harpagide and harpagoside on cyclooxygenase-2 (COX-2) in vitro. Bioorg. Med. Chem 2011, 19, 4882–4886. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Wu, H.; Zhang, Y.Z.; Wang, R.; Wang, W.Y.; Wang, W.; Li, S.P.; Dai, L.; Zhang, Z.R. Design, synthesis and preliminary evaluation of the anti-inflammatory of the specific selective targeting druggable enzymome cyclooxygenase-2 (COX-2) small molecule. Pharm. Biol. 2016, 54, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Parenti, A.; De Logu, F.; Geppetti, P.; Benemei, S. What is the evidence for the role of TRP channels in inflammatory and immune cells? Br. J. Pharmacol. 2016, 173, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Mickle, A.D.; Shepherd, A.J.; Mohapatra, D.P. Sensory TRP channels: The key transducers of nociception and pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 73–118. [Google Scholar]
- Southall, M.D.; Li, T.; Gharibova, L.S.; Pei, Y.; Nicol, G.D.; Travers, J.B. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J. Pharmacol. Exp. Ther. 2003, 304, 217–222. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Biro, T.; Acs, G.; Acs, P.; Modarres, S.; Blumberg, P.M. Recent advances in understanding of vanilloid receptors: A therapeutic target for treatment of pain and inflammation in skin. J. Investig. Dermatol. Symp. Proc. 1997, 2, 56–60. [Google Scholar] [CrossRef]
- Nishijima, C.M.; Ganev, E.G.; Mazzardo-Martins, L.; Martins, D.F.; Rocha, L.R.; Santos, A.R.; Hiruma-Lima, C.A. Citral: A monoterpene with prophylactic and therapeutic anti-nociceptive effects in experimental models of acute and chronic pain. Eur. J. Pharmacol. 2014, 736, 16–25. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M. Camphor (Cinnamomum camphora), a traditional remedy with the history of treating several diseases. IJCRI 2013, 4, 86–89. [Google Scholar] [CrossRef]
- Sherkheli, M.A.; Vogt-Eisele, A.K.; Weber, K.; Hatt, H. Camphor modulates TRPV3 cation channels activity by interacting with critical pore-region cysteine residues. Pak. J. Pharm. Sci. 2013, 26, 431–438. [Google Scholar] [PubMed]
- Vogt-Eisele, A.K.; Weber, K.; Sherkheli, M.A.; Vielhaber, G.; Panten, J.; Gisselmann, G.; Hatt, H. Monoterpenoid agonists of TRPV3. Br. J. Pharmacol. 2007, 151, 530–540. [Google Scholar] [CrossRef]
- Caceres, A.I.; Liu, B.; Jabba, S.V.; Achanta, S.; Morris, J.B.; Jordt, S.E. Transient Receptor Potential Cation Channel Subfamily M Member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br. J. Pharmacol. 2017, 174, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Rahman, L.; Verma, R.; Chauhan, A.; Yadav, A.; Singh, A. Essential Oil Composition of Menthol Mint (Mentha arvensis) and Peppermint (Mentha piperita) Cultivars at Different Stages of Plant Growth from Kumaon Region of Western Himalaya. Open Access J. Med. Aromatic Plants. 2010, 1, 13–18. [Google Scholar]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Amaral, R.G.; Fonseca, C.S.; Silva, T.K.; Andrade, L.N.; França, M.E.; Barbosa-Filho, J.M.; de Sousa, D.P.; Moraes, M.O.; Pessoa, C.; Carvalho, A.A.; et al. Evaluation of the cytotoxic and antitumour effects of the essential oil from Mentha x villosa and its main compound, rotundifolone. J. Pharm. Pharmacol. 2015, 67, 1100–1106. [Google Scholar] [CrossRef]
- Silva, D.F.; de Almeida, M.M.; Chaves, C.G.; Braz, A.L.; Gomes, M.A.; Pinho-da-Silva, L.; Pesquero, J.L.; Andrade, V.A.; Leite, M.e.F.; de Albuquerque, J.G.; et al. TRPM8 Channel Activation Induced by Monoterpenoid Rotundifolone Underlies Mesenteric Artery Relaxation. PLoS ONE 2015, 10, e0143171. [Google Scholar] [CrossRef]
- Sherkheli, M.A.; Vogt-Eisele, A.K.; Bura, D.; Beltrán Márques, L.R.; Gisselmann, G.; Hatt, H. Characterization of selective TRPM8 ligands and their structure activity response (S.A.R) relationship. J. Pharm. Pharm. Sci. 2010, 13, 242–253. [Google Scholar] [CrossRef]
- Skerratt, S. Recent Progress in the Discovery and Development of TRPA1 Modulators. Prog. Med. Chem. 2017, 56, 81–115. [Google Scholar]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denliger, B.; Fajardo, O.; Manenschijn, F.P.; Talavera, A.; Kichko, T.; Navia, B.; et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.; Gershenzon, J.; Nielson, E.E.; Froehlich, J.E.; Croteau, R. Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol. 1999, 120, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Dubin, A.; Bursulaya, B.; Viswanath, V.; Jegla, T.; Patapoutian, A. Identification of the Transmembrane Domain Five as a Critical Molecular Determinant of Menthol Sensitivity in Mammalian TRPA1 Channels. J. Neurosci. 2008, 24, 9640–9651. [Google Scholar] [CrossRef] [PubMed]
- Alpizar, Y.; Gees, M.; Sanchez, A.; Apetrei, A.; Voets, T.; Nilius, B.; Talavera, K. Bimodal effects of cinnamaldehyde and camphor on mouse TRPA1. Eur. J. Physiol. 2013, 465, 853–864. [Google Scholar] [CrossRef]
- Kaimoto, T.; Hatakeyama, Y.; Tkahashi, K.; Imagawa, T.; Tominaga, M.; Ohta, T. Involvement of transient receptor potential A1 channel in algesic and analgesic actions of the organic compound limonene. Eur. J. Pain 2016, 20, 1155–1165. [Google Scholar] [CrossRef]
- Oz, M.; Lozon, Y.; Sultan, A.; Yang, K.H.; Galadari, S. Effects of monoterpenes on ion channels of excitable cells. Pharmacol. Ther. 2015, 152, 83–97. [Google Scholar] [CrossRef]
- Mélik Parsadaniantz, S.; Rivat, C.; Rostène, W.; Réaux-Le Goazigo, A. Opioid and chemokine receptor crosstalk: A promising target for pain therapy? Nat. Rev. Neurosci. 2015, 16, 69–78. [Google Scholar] [CrossRef]
- Kumamoto, E.; Fujita, T. Differential Activation of TRP Channels in the Adult Rat Spinal Substantia Gelatinosa by Stereoisomers of Plant-Derived Chemicals. Pharmaceuticals 2016, 9, 46. [Google Scholar] [CrossRef]
- Mastrangelo, F.; Frydas, I.; Ronconi, G.; Kritas, S.K.; Tettamanti, L.; Caraffa, A.; Ovidio, D.C.; Younes, A.; Gallenga, C.E.; Conti, P. Low-grade chronic inflammation mediated by mast cells in fibromyalgia: Role of IL-37. J. Biol. Regul. Homeost Agents. 2018, 32, 195–198. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perri, F.; Coricello, A.; Adams, J.D. Monoterpenoids: The Next Frontier in the Treatment of Chronic Pain? J 2020, 3, 195-214. https://doi.org/10.3390/j3020016
Perri F, Coricello A, Adams JD. Monoterpenoids: The Next Frontier in the Treatment of Chronic Pain? J. 2020; 3(2):195-214. https://doi.org/10.3390/j3020016
Chicago/Turabian StylePerri, Filomena, Adriana Coricello, and James D. Adams. 2020. "Monoterpenoids: The Next Frontier in the Treatment of Chronic Pain?" J 3, no. 2: 195-214. https://doi.org/10.3390/j3020016
APA StylePerri, F., Coricello, A., & Adams, J. D. (2020). Monoterpenoids: The Next Frontier in the Treatment of Chronic Pain? J, 3(2), 195-214. https://doi.org/10.3390/j3020016





